Abstract
Introduction
In 2012, the estimated global prevalence of pre-diabetes was 280 million, and the prevalence is expected to rise to 400 million by 2030. Oat-based foods are a good source of beta-glucans, which have been shown to lower postprandial blood glucose. Studies to evaluate the effectiveness of the long-term intake of beta-glucan-enriched bread as part of a habitual diet among individuals with pre-diabetes are needed. Therefore, we designed a multicentre intervention study in adults with pre-diabetes to investigate the effects of consumption of an oat-derived beta-glucan-enriched bread as part of a normal diet on glycated haemoglobin (HbA1c) in comparison to consumption of whole-grain wheat bread.
Methods and analysis
The CarbHealth trial is a multicentre double-blind randomised controlled 16-week dietary intervention trial in participants 40–70 years of age with a body mass index of ≥27 kg/m2 and HbA1c of 35–50 mmol/mol. The study is conducted at four universities located in Norway, Sweden and Germany and uses intervention breads specifically designed for the trial by Nofima AS. The aim is to recruit 250 participants. The primary outcome is the difference in HbA1c between the intervention and the control groups. The main analysis will include intervention group, study centre and baseline HbA1c as independent variables in an analysis of covariance model.
Ethics and dissemination
The study protocol was approved by respective ethical authorities in participating countries. The results of the study will be communicated through publication in international scientific journals and presentations at (inter)national conferences.
Trial registration number
Keywords: General diabetes, PUBLIC HEALTH, Clinical trials
Strengths and limitations of this study.
The multicentre study takes advantage of the expertise of different groups, thus adding microbiota research, chronotype and continuous glucose measurements, as well as consumer acceptance to the study outcomes.
Furthermore, collaboration with food technologists that were able to design, produce and extensively characterise a beta-glucan-enriched bread is an additional strength of the multicentre study.
The intervention bread contains >4 g beta-glucan per 30 g available carbohydrate and qualifies for an European Food Safety Authority health claim on reduction of postprandial glycaemic response.
Due to logistics, breads had to be provided frozen, which is known to reduce bread quality and could lower consumer acceptance.
Introduction
The prevalence of type 2 diabetes mellitus (T2D) has increased drastically over the last 35 years and is expected to continue to rise.1 2 Impaired glucose tolerance (IGT) and impaired fasting glycaemia (IGF) are intermediate conditions between normal glucose metabolism and T2D and are often referred to as pre-diabetes. In 2012, the International Diabetes Federation estimated the global prevalence of pre-diabetes to 280 million, which is expected to rise to 400 million by 2030.3 Persons with pre-diabetes are at high risk of developing T2D, and it is estimated that 70% of those with pre-diabetes may develop T2D within 10 years.3 4 Glycated haemoglobin (HbA1c) is used as a measure of glycaemic control since HbA1c reflects average plasma glucose over the previous 8–12 weeks. Common diagnostic criteria of pre-diabetes is an intermediate HbA1c of 42–47 mmol/mol.5 The causes and aetiology of IGT and IGF are not fully understood, but there are strong links to obesity, age, ethnicity as well as heredity, and nutrition.6–8 Cereal grain products, especially bread, are staple foods in European diets and cereals are the main source of carbohydrate, plant protein, dietary fibre and total energy worldwide.9 High whole-grain and cereal fibre intake have consistently been associated with lower risks of T2D.10 Hence, replacing refined grains with dietary fiber-rich whole grains is regarded as a major strategy to improve public health.11 Oat-based or barley-based foods are a good source of mixed-linkage beta-glucans, that is, viscous forming dietary fiber, which have been shown to improve postprandial blood glucose. This has been endorsed through authorised health claims by the European Food Safety Authority. However, few studies have investigated the long-term effect of breads enriched with beta-glucans on HbA1c and thereby the risk of developing T2D.12–14 The existing studies are of small sample sizes and use a high amount of test food (eight servings per day=320 g bread), thus not reflecting average consumption conditions. A recent study investigated the efficacy of beta-glucans on blood lipid profile and fasting plasma glucose in a cross-over study on 83 subjects, showing significant effects on blood lipid profile but not on fasting blood glucose. However, the participants were euglycaemic, and the dose of beta-glucan was comparably low (3 g/day) and provided under strictly controlled conditions.15 Hence, there is a need for studies to evaluate the effectiveness of the long-term intake of feasible amounts of bread enriched in beta-glucan as part of a habitual diet on diabetes risk factors, particularly among individuals at elevated risk.
Therefore, we designed a multicentre intervention study in adults with a moderate to high risk of developing T2D, that is, persons with pre-diabetes to evaluate the long-term effects of regular consumption of an oat-derived beta-glucan-enriched bread, as part of a normal diet on HbA1c, in comparison to consumption of a whole-grain wheat bread. Furthermore, exploratory analysis will be performed assessing effects on fasting blood glucose and serum lipid profile, body weight, hepatic steatosis markers, 24-hour glucose profiles, gastric emptying, changes in microbiota, but also consumer acceptance and attrition rates.
Methods/design
The CarbHealth trial is a multicentre double-blind randomised controlled 16-week dietary intervention trial in participants with high normal HbA1c concentrations. The study is conducted at four university centres at (1) University of Bergen, Bergen, Norway; (2) Chalmers University of Technology, Gothenburg, Sweden; (3i) Paderborn University, Paderborn, Germany; and (4) Leipzig University, Leipzig, Germany. Intervention breads were specifically produced for the study by Nofima (Ås, Norway). This study was initiated in July 2019 and the recruitment started in July 2021. The trial is expected to be finalised by summer 2023.
Ethics and dissemination
The study protocol was approved by the respective ethic authorities (Swedish Ethical Review Authority, Sweden (protocol DNR 2021–02584), ethical committee of Paderborn University, Paderborn (approved 13 July 2021), ethical committee of the medical faculty of the University of Leipzig, Leipzig (316/21-ek) and regional committees for Medical and Health Research Ethics, Norway (REC Nord, ref. 106931)). The study is registered in the public trial registry Clinicaltrials.gov. The results of the study will be communicated through publication in international scientific journals and presentations at (inter)national conferences.
Patient and public involvement
Participants and public were not involved in designing this study. Results will be presented to participants at the end of the trial. Participants will receive information on allocated group, HbA1c, blood lipids and body composition.
Experimental design
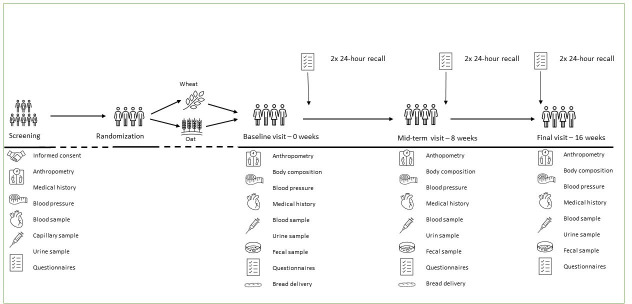
Prior to the intervention, potential participants take part in a prescreening evaluation to assess eligibility for inclusion, either by phone, or using an online questionnaire. If eligible, a screening visit is booked. At the subsequent screening visit, non-fasting blood samples are drawn and analysed locally for HbA1c, liver enzymes and safety markers. Furthermore, height, body weight, waist circumference and blood pressure are measured. Participants complete a medical history questionnaire including assessment of prescribed and non-prescribed medication in relation to exclusion criteria. If enrolled, clinical visits take place in weeks 0, 8 and 16. During the baseline visit, the intermediate visit at 8 weeks and the final visit at 16 weeks, measurements of body weight, body composition, waist circumference and blood pressure are made; fasting blood samples are drawn; and participants provide frozen faecal samples. In two centres (Gothenburg and Paderborn), continuous glucose monitoring (CGM) measurements are performed covering 1 week at baseline and at the end of the study. Participants are asked to maintain habitual diets and levels of physical activity during the study period. This is monitored by six in-study 24-hour dietary recalls which are not preannounced to the participants and by physical activity questionnaires at the study visits. An overview of the study design is presented in figure 1. During the intervention period, participants are instructed to replace their usually consumed bread with the study breads. The participants are asked to consume at least three slices of the presliced intervention bread or the presliced control bread on at least 6 days/week for 16 weeks.
Figure 1.
Flowchart of the study visits in the CarbHealth multicentre study.
Study bread
The ingredients for the breads are shown in table 1, and the calculated nutrient composition is shown in table 2. The two breads were matched for starch and fat content on a slice basis (table 2). The daily portion of three slices of the beta-glucan-enriched bread provides 286 kcal, 16.6 g dietary fibre and 6 g of beta-glucan. Three slices of the wheat bread provide 244 kcal, 5 g dietary fibre and 0.02 g beta-glucan per day (table 2). Both breads were developed and distributed by Nofima AS, Norway. The breads were baked at Åpent Bakeri, Oslo, Norway. The bread is provided frozen in vacuum packs of six slices and free of charge to the participants.
Table 1.
Ingredients for beta-glucan and control bread
| Ingredients | Supplier | Beta-glucan bread (%) |
Control bread (%) |
| Rapeseed oil | Idun Industri AS, Norway | 0.7 | 4.7 |
| Dry yeast | Idun Industri AS, Norway | 0.7 | 0.6 |
| Salt | GC Rieber AS, Norway | 1.0 | 1.0 |
| Sieved white wheat flour | Lantmännen Cerealia, Norway | 21.9 | 18.7 |
| Whole-grain wheat flour | Lantmännen Cerealia, Norway | 0 | 37.5 |
| Water | Oslo kommune, Norway | 53.8 | 37.5 |
| SWEOAT Bran BG14 Bakery | Swedish Oat fibre, Sweden | 21.9 | 0 |
| Coatec sorbic acid (E200) | RAPS GmbH Co. KG, Germany | 0.05 | 0.05 |
Table 2.
Macronutrient composition of test breads
| g/slice | g/day (three slices) | kcal/day | |
| Beta-glucan bread (intervention) | |||
| Starch | 12.2 | 36.7 | 146.7 |
| Fat | 2.1 | 6.3 | 56.8 |
| Beta-glucan | 2 | 6 | |
| Protein | 4.1 | 12.4 | 49.4 |
| Fibre | 5.5 | 16.5 | 32.9 |
| Salt | 0.6 | 1.8 | |
| Moisture | 24.8 | 74.4 | |
| Total | 50.7 | 152.1 | 285.9 |
| Whole grain Wheat bread (Control) | |||
| Starch | 12.3 | 36.9 | 148 |
| Fat | 2.1 | 6.3 | 56 |
| Beta-glucan | 0.02 | 0.06 | |
| Protein | 2.5 | 7.5 | 29.9 |
| Fibre | 1.6 | 4.8 | 9.8 |
| Salt | 0.4 | 1.2 | |
| Moisture | 11.4 | 34.2 | |
| Total | 30.3 | 90.9 | 243.7 |
Eligibility criteria
The eligibility criteria were designed to reach people with pre-diabetes; hence, we invite men and women with body mass index (BMI) of ≥27 kg/m2, aged 40–70 years. Additional inclusion criteria: HbA1c 35–50 mmol/mol, signed informed consent, regular bread eater and having freezer capacity for at least two loaves of bread. Exclusion criteria are type 1 diabetes mellitus or pharmacologically treated T2D, non-fasting blood glucose of >11.1 mmol/L, urine glucose of ≥180 mg/dL or protein excretion as indicated by dipstick (+++, Combur 10 test strips (Roche Diagnostics)), food allergies or intolerances preventing consumption of the study breads, pregnancy, lactation or planning a pregnancy during the study period, systolic blood pressure of ≥160 mm Hg or diastolic blood pressure ≥100 mm Hg at screening,16 history of stomach or gastrointestinal conditions (ie, inflammatory bowel disease and Crohn’s disease) history of myocardial infarction, heart failure, stroke, heart attack or cancer within 3 years prior to screening, use of antidiabetic agents or insulin and history of alcohol abuse. Use of other medications or over-the-counter drugs or dietary supplements is allowed if the dose has been stable for a minimum of 3 months prior to the study. Any medication used will be recorded as will any changes of use. Initiation of medication to treat diabetes during the study is a reason for withdrawal.
Recruitment
In general, participants are recruited via leaflets, press releases, newspaper ads, ads in social media and blackboard flyers. Study specific websites are developed in Bergen and Paderborn, with the possibility for online registration. If a potential participant is interested, prescreening will be conducted over the phone or using an online questionnaire to assess eligibility, and if eligible, a screening visit is scheduled.
Informed consent procedure
All participants screened for eligibility are provided written information about the study prior to the first study visit. At the first study visit, the participants receive additional oral information on the study and are given the opportunity to ask questions to the research personnel before signing written informed consent. The study will not carry on and no samples will be drawn until the participant gives consent in writing. The participants have the right to withdraw their consent at any time, and to request that their biological samples and data be destroyed.
Randomisation
Participants are randomised into one of the two intervention groups (1:1 allocation) using block randomisation with random block lengths, stratified by sex. The web-based randomisation is configured by the Biostatistics and Data Management Group of the Clinical Trials Unit at the University Medical Centre Göttingen, Germany, a third party not otherwise involved in the clinical trial. To reduce performance bias, at each of the four sites, a person who is not member of the research team is responsible for the randomisation. All other research personnel at the sites are blinded for the allocation group.
Dietary assessment
The dietary assessment follows the principle of 24-hour dietary recalls using country-specific food composition data. Practical conduction differs slightly between centres:
Bergen, Paderborn and Leipzig: Dietary intakes are assessed by six 24-hour recalls (24 hours) at the beginning (weeks 0–2), middle (weeks 7–9) and end of the study (weeks 15–16) using the validated tool ‘myfood24’ (https://www.myfood24.org/). The 24 hours are performed at unannounced times to attenuate the observer effect. The German version of myfood24 is based on German Food Code and Nutrient Database (Bundeslebensmittelschlüssel V.3.02) for generic food items and the database of the Dortmund Nutritional and Anthropometric Longitudinally Designed study for branded food items.17 18 The Norwegian version is based on the Norwegian Food Composition Table for generic food items and supplemented with food composition data for missing Norwegian dishes from other sources.19
Gothenburg: As Myfood24 is not available based on Swedish food composition data, dietary intake is assessed by six 24 hours on study site and over phone. Three 24 hours are performed at site by a trained dietitian using images of portion sizes from The Swedish Food Agency. Additionally, three 24 hours are performed over the phone when the participants estimate portion sizes using a standard kitchen measure (eg, dL measure and slices). To determine the nutritional composition of the intake, the DietistNet Pro software (Kost och Näringsdata AB, Bromma, Sweden) is used, which is based on Swedish Food Composition Database.
Clinical assessments
Visits include collection of blood and urine samples, assessment of blood pressure and anthropometric data including waist circumference, body weight and height, as well as body composition (described under ‘anthropometric measurements’). Participants are instructed to not eat or drink (except maximum 0.5 L of non-carbonated water) 10 hours prior to the visits. Additionally, participants are instructed to avoid alcohol consumption, smoking and use of other tobacco products, and vigorous physical activity 12 hours prior to the clinical visit.
Consumer acceptance
Participants evaluate the bread on day 1 and on week 8 of the study. The questionnaire is adapted from a previously established method.20 Participants rate their hunger, acceptability and expected satiation on a 9-point scale. The participants rate expected satiety on a 6-point scale. Participants describe the bread via a check-all-that-apply question20 21: ‘Choose all the attributes/terms that you think apply to this bread’, using 28 hedonic and descriptive attributes and 16 usage and attitude attributes. Terms are randomised within groups and across participants. They answer two consumption questions: ‘In which meals do you consume bread?’ and ‘How many bread slices do you eat on a typical day?’
Data for Norway and Sweden were collected through online forms in EyeQuestion (Logic8 BV, The Netherlands) and stored in a secure server owned by Nofima. Participants from Germany filled in the questionnaire on paper.
Blood collection and analysis
During study visits, fasting blood samples are taken from an antecubital vein and placed in tubes containing either a clot activator or lithium heparin (Becton-Dickinson, Eysins, Switzerland) to obtain serum or plasma. Serum tubes are stored at room temperature for 30 min and then centrifuged at 1300 g at 4°C for 10 min. Plasma tubes are immediately centrifuged except for one tube for HbA1c measurements. EDTA–plasma, serum and Li–heparin plasma are immediately refrigerated/kept on ice, processed and aliquoted into microtubes. Whole blood, plasma and serum aliquots are frozen at −20°C within 2 hours of sample collection and transferred into −80°C within 24 hours. Blood samples are sent on dry ice to the Department of Medical Biochemistry and Pharmacology at Haukeland University Hospital, Bergen, every 6 weeks for analyses of HbA1c and secondary outcomes.
Analytical methods
Blood glucose is measured by a validated, portable system at room temperature (HemoCue Glucose 201 RT system (HemoCue AB, Ängelholm, Sweden) at all centres. All other blood or serum measurements will be done at Haukeland University Hospital, Bergen (certified laboratory NSEN-ISO 15189), in frozen samples stored at −80°C. HbA1c is measured in EDTA whole blood samples which have been stored at maximum for 8 weeks by high-performance liquid chromatography (HPLC), (BioRad, Hercules, California, USA). Liver enzymes and plasma lipids are measured using standard methods on a Cobas c702 autoanalyser. Liver enzymes are measured photometrically according to the International Federation of Clinical Chemistry (IFCC) method (Roche Diagnostics, Mannheim, Germany). Serum triacylglycerides and total cholesterol are measured with an enzymatic colorimetric method. Low-density lipoprotein cholesterol (LDL-C) is measured photometrically, and high-density lipoprotein cholesterol (HDL-C) is measured by a homogeneous enzymatic colorimetric method.
Dietary compliance
Compliance is assessed based on the evaluation of the 24 hours and a precoded compliance journal kept by the participant during the study. Participants are instructed to tick off the number of slices consumed on each day. Compliance is a secondary outcome, and sensitivity analysis will be performed based on compliance journals and 24 hours.
Anthropometric assessments
Body weight is measured during all study visits including screening, with the participants wearing light clothing (eg, underwear and t-shirt) and no shoes. Body weight is noted to the nearest 0.1 kg in the case report form. Height is measured using a Seca Stadiometer, model 217 or using the mBCA 515 integrated stadiometer. Height is measured once at screening without shoes to the nearest 0.1 cm. Waist circumference is measured twice on each occasion with a Seca 201 cm tape measurer to the nearest 0.1 cm according to WHO standards. The average of the two measurements is used for data analysis. Body composition (whole body mass, fat mass and lean body mass) is measured at all study visits by bioelectrical impedance analysis using Seca mBCA 515 (in Bergen, Paderborn and Leipzig). The measurements are performed in the morning after a 10-hour fast and in accordance with a standardised protocol.
Gothenburg: Body composition is measured using dual-energy X-ray absorptiometry (DEXA) (iDXA; GE Medical Systems, Madison, Wisconsin, USA) using the software Core V.18.0. The DEXA scan is conducted in the morning after an overnight fast, with the participants wearing light clothing.
Twenty-four-hour CGM
A CGM is used (Gothenburg and Paderborn) to obtain 24 hours of continuous interstitial glucose concentration data on two occasions for 7 days each between baseline and week 1 and between week 15 and week 16. CGM data are used to calculate 24 hours of interstitial glucose peak, mean, coefficient of variation and total area under the curve. In addition, glucose response to bread consumption is evaluated separately for morning and evening meals.
The devices differ between centres and the specific devises are described as follows.
In Paderborn, a Dexcom G6 CGM (DexCom, San Diego, USA) is used. The glucose oxidase sensor is inserted into the upper part of the non-dominant arm or the abdominal area at least 5 cm away from the umbilicus to obtain an interstitial glucose measurement. Self-monitoring glucose readings (finger sticks) are performed with a blood sugar monitoring device (Contour Next One, Ascencia Diabetes Care, USA)two times a day (morning and evening). The sensor does not need to be calibrated.
In Gothenburg, an Abbott FreeStyle Libre Pro iQ CGM (Chicago, Illinois, USA) is used. The glucose oxidase sensor is inserted into the back of the upper part of the non-dominant arm. Participants can wear the sensor for up to 14 days and it does not need to be calibrated.
Fecal samples
Spot faecal samples are collected at baseline and at weeks 8 and 16. Collection is voluntary, and non-delivery of faecal samples is not a reason for exclusion. The samples will be collected in specific devises (Easy Sampler Collection Kit, GP Medical Devices ApS, Denmark). The participants are instructed to collect the faecal sample within 72 hours of the clinical visit and are instructed to keep the sample in a household freezer until delivery to the study centre. The participants are instructed to keep the sample in a freezer bag with cooling blocks during transportation to the study centre. At the study centre, the samples are transferred to −80°C within 24 hours. Samples are analysed for the composition of the gut microbiota to provide possible mechanistic explanations underlying differential responses of participants of selected outcomes. All faecal microbiota analysis will be performed at Chalmers University of Technology (Gothenburg, Sweden).
Questionnaires
Participants complete several questionnaires during the study period. All questionnaires have been validated and are available in respective languages.
For physical activity assessment, participants complete the International Physical Activity Questionnaire (IPAQ) at baseline and at weeks 8 and 16. The IPAQ is used to estimate daily inactivity and physical activity and has been shown to have adequate validity and reliability in various nationalities.22
For chronotype, the Munich Chronotype Questionnaire (MCTQ) is administered at baseline and at weeks 8 and 16 to estimate chronotype and sleep behaviour during the trial. Chronotype is defined as the midpoint of sleep on free days corrected for sleep debt on workdays. Chronotype is also verified by use of an accelerometer in weeks 1 and 16 (Paderborn).23
For subjective health and well-being assessment, the 12-item Short Form Health Survey (RAND SF-12) is used at baseline, week 8 and 16. The questionnaire is used to evaluate perceived physical and emotional well-being.
For alcohol abuse, the four-question validated Cut Down, Annoyed, Guilty and Eye Opener questionnaire will be administered at screening visit to assess the risk of alcohol abuse.
Study outcomes
The primary outcome is the difference in glycaemic control measured by HbA1c after 16 weeks between the intervention and control groups. The trial has defined the following secondary outcomes: (1) difference in fasting capillary blood glucose after 16 weeks; (2) difference in fat mass (kg) and lean body mass (kg); (3) difference in blood lipids (LDL-C, HDL-C and triglycerides); (4) difference in fatty liver index (based on serum liver enzyme activities (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), changes in faecal microbiota composition; and (5) consumer acceptance at baseline and 8 weeks. Furthermore, the following exploratory investigations will be undertaken: (1) difference in postprandial response to morning and evening meals with the two breads based on CGM data (Paderborn and Gothenburg only), (2) if the individual chronotype of a person influences bread consumption and metabolic health, (3) analysis of plasma and faecal metabolome and short chain fatty acids in faecal and blood samples, and (4) sensitivity analysis of compliance to the protocol measured with 24-hour recalls and compliance journal.
Data analysis and sample size estimates
Intention-to-treat analysis will be performed following a statistical analysis plan that was set up a priori.
The primary outcome is the difference in HbA1c between the intervention and the control group at the end of the study. The main analysis will be a linear regression model adjusted for study centre and baseline HbA1c. The results will be presented as the mean difference in HbA1c (95% CIs) between groups at the end of study, with corresponding p values. Missing data will be handled with multiple imputation methods, and the analysis will be accompanied by a complete case analysis. To evaluate the theoretical range of uncertainty due to missing outcome data, best–worst and worst–best sensitivity analyses will be performed.24 The best–worst-case scenario will be constructed by assuming all dropouts in the intervention group to have an HbA1c at 16 weeks of the study centre-specific intervention group mean −2 SD, and all dropouts in the control group to have an Hba1c of study centre specific control group mean +2 SD, and vice versa. The same analysis plan will apply to secondary outcome variables. Exploratory post hoc analysis will include stratified analyses by sex, by BMI (27–30, >30 kg/m2), by chronotype (based on MCTQ), and by centre to explore potential country-specific and centre-specific differences. Product terms between the intervention and the stratification variable will be included in the models to evaluate potential interactions. Statistical analyses will be performed using R, and the linear regression models will be fitted with the lm() function.
The statistical power calculation is based on the difference in HbA1c between the two groups (intervention and control) at the end of the study, as the estimated effect. We expect the starting HbA1c concentration to be approximately 41 mmol/mol with an SD of 6 mmol/mol and expect a reduction to 38 mmol/mol in the intervention group with small changes in the control group. Power calculation is based on this between-group difference in the HbA1c concentrations, with an SD of 6 mmol/mol (assuming similar SD in both groups) and at a power of 90% and at a significance level of 0.05. To allow for 45% dropouts and ensure the conditions are met, the aim was to recruit 250 participants into the entire multicentre study: 125 in each treatment group. Assumptions and estimates for the power calculation were taken from dietary intervention studies,14 25 and the calculation was made with a paired-t test using the power.t.test () function from R. The effect size was chosen to be moderate as this is a single-food intervention study.
Discussion
The CarbHealth trial aims to evaluate effectiveness of a beta-glucan-enriched bread, that is, whether habitual consumption of a bread containing beta-glucan (>4 g oat beta-glucan per 30 g available carbohydrate) versus a control wheat bread with 66% whole grain wheat under everyday conditions will affect long-term glycaemic control among persons at risk of T2D over a period of 16 weeks.
The CarbHealth trial is a pragmatic trial investigating food typically consumed. Participants replace their habitual bread with study bread, instead of adding or removing food items. Studies have shown that people are conservative regarding dietary changes26; thus, swapping a healthier alternative for habitually consumed foods may be easier than changing food habits. A bread with 66% whole-grain wheat was chosen as the control bread instead of refined wheat bread to reflect dietary habits in Northern European countries. Since the quality of bread and the associated metabolic effects varies substantially,27 use of a medium to low glycaemic index-bread rich in beta-glucan may substantially benefit metabolic health. While complex interventions have been found to substantially reduce progression to diabetes among persons with pre-diabetes,28 many patients may not be prepared for such complex changes; thus, exchange of bread with a healthier alternative may be more feasible.
Nutrition studies often lack statistical power hampering firm conclusions.29 30 Multicentre studies may offer a strategy to overcome this shortcoming. Central production and distribution of the study breads by a third research partner (Nofima) enables this approach. Furthermore, the multicentre design employed in CarbHealth allows to study the metabolic effects and the effectiveness of bread against the background of different bread consumption patterns in the participating countries and to analyse differences in general dietary practices and potential acceptability. The multicentre study also takes advantage of the expertise of different groups, thus adding microbiota research, chronotype and continuous glucose measurements as well as consumer acceptance to the study outcomes. Furthermore, collaboration with food technologists that were able to produce a beta-glucan-enriched bread is an additional strength of the multicentre study. Additional strengths of the CarbHealth study include strong design features such as randomisation, double blinding and provision of bread. One limitation of the study is that, due to logistics, breads had to be provided frozen, which is known to reduce bread quality and could lower consumer acceptance which could result in a lower compliance.
There may be important implications from this research regardless of the findings. If a beneficial effect was supported by evidence of a positive effect on long-term blood glucose levels among persons with pre-diabetes, public health efforts should be taken to make beta-glucan-enriched bread available in European countries. This should be accompanied by efforts to increase the awareness, particularly among persons at risk of T2D, of a simple and effective replacement. Conversely, if a beneficial effect was not supported, this could suggest that the bread is either not sufficiently enriched with beta-glucans, the beta-glucan has suboptimal physiological characteristics, for example, solubility, prefrozen bread may not be the optimal matrix for beta-glucan or that the reduction in postprandial glycaemic response achieved with a similar beta-glucan-enriched bread31 does not translate into strong long-term benefits for blood glucose control compared with a whole-grain wheat bread under everyday conditions. Notwithstanding, exploratory subgroup analyses will allow insights into factors determining responsiveness (eg, compliance, meal context, consumer acceptance in different countries/centre and sex). Similarly, secondary analyses will inform whether any potential effect or lack of effectiveness extends to other metabolic parameters.
Hence, the results of the CarbHealth study will provide important information on the public health relevance of a beta-glucan-enriched bread for reduction of post-prandial glycaemic response in persons with pre-diabetes.
Supplementary Material
Footnotes
Contributors: TH wrote the first the first draft of the manuscript. JD is the chief investigator of this trial. JD, along with RL, AB, AK, and SB: research question, study design, acquisition of data, obtaining the funding, implementation of the study protocol, critical revision and final approval of the manuscript. TH, HRR, IR, LMT, AS, US, KM, KP, AR, VL and PV: feedback of study design, implementation of study protocol, acquisition of data, critical revision and final approval of the manuscript.
Funding: This project received funding from the Research Council of Norway, Formas Research Council of Sweden, the Federal Ministry of Education and Research (Germany) under the umbrella of the European Joint Programming Initiative 'A Healthy Diet for a Healthy Life' and of the ERA-NET Cofund HDHL INTIMIC (GA N° 727565 of the EU Horizon 2020 Research and Innovation Programme (grant number N/A). The funding agencies did not play any part in designing the clinical trial.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Swedish Ethical Review Authority, Sweden (protocol DNR 2021-02584), the ethical committee of Paderborn University, Paderborn (approved 13 July 2021), the ethics committee of the medical faculty of the University of Leipzig, Leipzig (316/21-ek) and the regional committees for Medical and Health Research Ethics, Norway (REC Nord, ref. 106931). The participants gave informed consent to participate in the study before taking part.
References
- 1.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6:1246–58. 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes Federation diabetes atlas. 157. 9th ed. Diabetes Research and Clinical Practice, 2019: 107843. [DOI] [PubMed] [Google Scholar]
- 3.Perreault L, Færch K. Approaching pre-diabetes. J Diabetes Complications 2014;28:226–33. 10.1016/j.jdiacomp.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization WHO . Diabetes, 2020. Available: https://www.who.int/news-room/fact-sheets/detail/diabetes [Accessed 28 Sept 2020].
- 5.International Expert Committee . International expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–34. 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Diabetes Federation IDF . IDF diabetes atlas, 2017. 8th ed, 2017. [PubMed] [Google Scholar]
- 7.Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815–25. 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 8.Ley SH, Hamdy O, Mohan V, et al. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007. 10.1016/S0140-6736(14)60613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poutanen KS, Kårlund AO, Gómez-Gallego C, et al. Grains – a major source of sustainable protein for health. Nutr Rev 2022;80:1648–63. 10.1093/nutrit/nuab084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393:434–45. 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DS, Hu FB, Tappy L, et al. Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ 2018;361:k2340. 10.1136/bmj.k2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pick ME, Hawrysh ZJ, Gee MI, et al. Barley bread products improve glycemic control of type 2 subjects. Int J Food Sci Nutr 1998;49:71–8. 10.3109/09637489809086406 [DOI] [Google Scholar]
- 13.Östman EM, Frid AH, Groop LC, et al. A dietary exchange of common bread for tailored bread of low glycaemic index and rich in dietary fibre improved insulin economy in young women with impaired glucose tolerance. Eur J Clin Nutr 2006;60:334–41. 10.1038/sj.ejcn.1602319 [DOI] [PubMed] [Google Scholar]
- 14.Tessari P, Lante A. A multifunctional bread rich in beta glucans and low in starch improves metabolic control in type 2 diabetes: a controlled trial. Nutrients 2017;9. 10.3390/nu9030297. [Epub ahead of print: 17 Mar 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicero AFG, Fogacci F, Veronesi M, et al. A randomized placebo-controlled clinical trial to evaluate the medium-term effects of oat fibers on human health: the beta-glucan effects on lipid profile, glycemia and inTestinal health (belt) study. Nutrients 2020;12. 10.3390/nu12030686. [Epub ahead of print: 03 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams B, Mancia G, Spiering W. ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of cardiology (ESC) and the European Society of hypertension (ESH). Eur Heart J 2018;39:3021–104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 17.Koch SAJ, Conrad J, Cade JE, et al. Validation of the web-based self-administered 24-h dietary recall myfood24-Germany: comparison with a weighed dietary record and biomarkers. Eur J Nutr 2021;60:4069–82. 10.1007/s00394-021-02547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch SAJ, Conrad J, Hierath L, et al. Adaptation and evaluation of Myfood24-Germany: a web-based self-administered 24-h dietary recall for the German adult population. Nutrients 2020;12. 10.3390/nu12010160. [Epub ahead of print: 06 Jan 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvesen L, Engeset D, Øverby NC, et al. Development and evaluation of image-series for portion size estimation in dietary assessment among adults. J Nutr Sci 2021;10:e3. 10.1017/jns.2020.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen QC, Wahlgren MB, Almli VL, et al. Understanding the role of dynamic texture perception in consumers’ expectations of satiety and satiation. A case study on barley bread. Food Qual Prefer 2017;62:218–26. 10.1016/j.foodqual.2017.06.006 [DOI] [Google Scholar]
- 21.Varela P, Ares G, profiling S. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res Int 2012;48:893–908. 10.1016/j.foodres.2012.06.037 [DOI] [Google Scholar]
- 22.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 23.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90. 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- 24.Jakobsen JC, Gluud C, Wetterslev J, et al. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol 2017;17:162. 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole RE, Boyer KM, Spanbauer SM, et al. Effectiveness of prediabetes nutrition shared medical appointments: prevention of diabetes. Diabetes Educ 2013;39:344–53. 10.1177/0145721713484812 [DOI] [PubMed] [Google Scholar]
- 26.Shepherd R, Shepherd R. Resistance to changes in diet. Proc Nutr Soc 2002;61:267–72. 10.1079/PNS2002147 [DOI] [PubMed] [Google Scholar]
- 27.Goletzke J, Atkinson FS, Ek KL, et al. Glycaemic and insulin index of four common German breads. Eur J Clin Nutr 2016;70:808–11. 10.1038/ejcn.2016.9 [DOI] [PubMed] [Google Scholar]
- 28.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 29.Kroeger CM, Garza C, Lynch CJ, et al. Scientific rigor and credibility in the nutrition research landscape. Am J Clin Nutr 2018;107:484–94. 10.1093/ajcn/nqx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vorland CJ, Brown AW, Dawson JA, et al. Errors in the implementation, analysis, and reporting of randomization within obesity and nutrition research: a guide to their avoidance. Int J Obes 2021;45:2335–46. 10.1038/s41366-021-00909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieder A, Knutsen SH, Sainz Fernandez A, et al. At a high dose even partially degraded beta-glucan with decreased solubility significantly reduced the glycaemic response to bread. Food Funct 2019;10:1529–39. 10.1039/C8FO02098A [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.