Abstract
Abstract
Reliable immunoassays are essential to early predict and monitor vaccine efficacy against SARS-CoV-2. The performance of an Interferon Gamma Release Assay (IGRA, QuantiFERON® SARS-CoV-2), and a current anti-spike serological test, compared to a plaque reduction neutralization test (PRNT) taken as gold standard were compared. Eighty vaccinated individuals, whose 16% had a previous history of COVID-19, were included in a longitudinal prospective study and sampled before and two to four weeks after each dose of vaccine. In non-infected patients, 2 doses were required for obtaining both positive IGRA and PRNT assays, while serology was positive after one dose. Each dose of vaccine significantly increased the humoral and cellular response. By contrast, convalescent subjects needed a single dose of vaccine to be positive on all 3 tests. Both IGRA and current serology assay were found predictive of a positive titer of neutralizing antibodies that is correlated with vaccine protection. Patients over 65 or 80 years old had a significantly reduced response. The response tended to be better with the heterologous scheme (vs. homologous) and with the mRNA-1273 vaccine (vs. BNT162b2) in the homologous group, in patients under 55 and under 65 years old, respectively. Finally, decrease intensity or absence of IGRA response and to a less extent of anti-spike serology were also correlated to reinfection which has occurred during the follow up. In conclusion, both IGRA and current anti-spike serology assays could be used at defined thresholds to monitor the vaccine response against SARS-CoV-2 and to simply identify non-responding individuals after a complete vaccination scheme.
Capsule summary
Two available specific tests (IGRA and anti-spike antibodies) could early assess the vaccine-induced immunity against SARS-CoV-2 at the individual scale, to potentially adapt the vaccination scheme in non-responder patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-022-01354-x.
Keywords: IGRA, SARS-CoV-2, neutralization, antibody, vaccination
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for more than 6.2 million deaths reported to the World Health Organization (WHO) until the end of May 2022. Several vaccines were rapidly developed and approved for the prevention of COVID-19. More than 11 billion vaccine doses have been administered according to the WHO [1]. Three of these vaccines are currently administered worldwide and target the surface spike (S) protein. Two mRNA vaccines, BNT162b2 (trade name Comirnaty®, by Pfizer-BioNTech) and mRNA-1273 (trade name COVID-19 vaccine Moderna®, then Spikevax®, by Moderna) use the modified sequence of the S protein [2]. The short-term efficacy of the primary vaccination (median follow-up of 2 months) observed in phase 3 clinical trials was 95.0% 7 days after two doses of BNT162b2 (30 µg administered 21 days apart) and 94.1% 14 days after two doses of mRNA-1273 (100 µg administered 28 days apart), to prevent infection (homologous vaccination scheme). Second doses for both vaccines are extendable to 6 weeks [3, 4]. Long-term efficacy of the primary vaccination with BNT162b2 (6 months of follow-up) was 91.3% in preventing COVID-19 illness starting at 7 days after the second dose, and 96.7% against severe COVID-19 [5]. It was 93.2% for mRNA-1273 (median follow-up of 5.3 months) in preventing COVID-19 illness 14 days after the second dose, 98.2% in preventing severe disease, and 63.0% in preventing asymptomatic infection [6]. The third vaccine is based on recombinant chimpanzee adenovirus expressing the full-length S protein, ChAdOx1 nCoV-19 (AZD1222, trade name Vaxzevria®, by Oxford-AstraZeneca) [7]. Clinical trial showed an overall efficacy of the primary vaccination using a standard dose of 5 × 1010 viral particles after variable intervals (two doses administered at intervals of 4–12 weeks) of 70.4%, which increased to 90.0% after the first dose was halved [8]. In France, ChAdOx1 nCoV-19 was first recommended for people under 55 years old (because of initially unclear efficacy at higher ages), and then indicated for people over 55 years old in March 2021 (due to a potentially higher risk of thromboembolic events in younger population, but the anti-PF4 antibody hypothesis was not sufficient to provoke clinically evident thrombosis) [9, 10]. For this population aged under 55 who received a first dose of ChAdOx1 nCoV-19, the French High Authority of Health (HAS) then recommended to complete the vaccination scheme with a mRNA vaccine within 12 weeks after the first injection (heterologous vaccination scheme) [11]. Later, at the end of 2021, the HAS recommended the administration of a third dose of vaccine (corresponding to a booster dose of mRNA vaccine), with a delay of 6 months after the primary vaccination, subsequently shortened to 3 months, in people over the age of 18, due to the increase in the number of cases of infection linked to the delta variant and the expansion of the omicron variant [12].
To early predict and monitor the effectiveness of a vaccination scheme against COVID-19 on an individual scale, reliable high-throughput immunoassays measuring the specific adaptive immune response are of high interest. Neutralizing antibodies (NAb) are currently the best correlate of protection (CoP) against SARS-CoV-2 infection [13–18]. Previous studies showed a significant correlation between anti-spike immunoglobulins G (IgG) antibodies (measured by routine commercial tests) and neutralizing titers, suggesting that IgG antibodies might serve as a correlate of neutralization [17, 19]. Vaccination induces a combined adaptive humoral and cellular immune responses with both high titers of NAb beyond the levels observed in convalescent patients, and substantial T cell responses with T helper type 1 (TH1) CD4+ T cells and CD8+ T cells, both secreting predominantly IFNγ [2, 15, 17, 20]. However, vaccines were designed against the initial SARS-CoV-2 virus that emerged in 2019. With the rapid spread of SARS-CoV-2 variants of concern (VOC), including 19A (B.38), alpha (B.1.1.7), beta (B.1.351), delta (B.1.617), and omicron (B.1.1.529) variants, numerous studies have evaluated the impact of key mutations in the spike protein on the neutralizing activity with plaque reduction neutralization tests (PRNT) or pseudovirus-based assays [21–45].
In this context, the performance of the QuantiFERON SARS-CoV-2 Starter Set® for Research Use Only (QFN SARS-CoV-2, Qiagen) which is an Interferon Gamma (IFNγ) Release Assay (IGRA), and a serological assay (Architect SARS-CoV-2 IgG II Quant®, Abbott) were evaluated, in comparison with a PRNT as the gold standard method. We aimed to assess the ability of QFN SARS-CoV-2 and anti-spike serology to predict effective neutralization on the 19A, alpha, beta, delta, and omicron VOC. In a second time, we compared the results obtained by the 3 immunological assays between different patient cohorts, to show the differences in vaccine responses according to status of convalescent for COVID-19, age, vaccination scheme (homologous vs. heterologous), and type of mRNA vaccine (BNT162b2 vs. mRNA-1273) in the homologous group.
Methods
Cohort Description
Eighty individuals with complete vaccination scheme were included in a prospective, single-center, longitudinal cohort study conducted in the University Hospital of Saint-Etienne (France). Individuals included were either healthcare workers (HCWs) belonging to the COVIMMUNITY cohort, or elderly patients belonging to the PROOF cohort. Sixty-seven participants were included as COVID-19 negative (never infected) if they were asymptomatic, with a history of negative virological or serological testing, a 2-dose vaccination scheme, and at least the post-second dose sample collected (corresponding to a complete primary vaccination scheme). Thirteen individuals were included as COVID-19 positive (convalescent) if they had COVID-19 infection confirmed by a positive SARS-CoV-2 RT-PCR test between March and November 2020, a single-dose vaccination scheme between 3 and 6 months after their infection, and at least the post-vaccination sample collected (corresponding to a complete primary vaccination scheme).
Three vaccines were used in non-infected subjects, BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and AZD1222 (Oxford-AstraZeneca) with different immunization schemes: homologous BNT/BNT, homologous mRNA-1273/mRNA-1273, and heterologous scheme; in addition, one patient received a homologous AZD1222/AZD1222 combination. Twenty-seven of them had a third dose of vaccine (booster dose of BNT162b2) more than 6 months after their second dose. Convalescent subjects received a single BNT or mRNA-1273 dose between 3 and 6 months after their infection.
In non-infected patients, four blood samples were drawn for immunological assays: prior vaccination (T0), prior second dose (T1, 2–4 weeks after the first dose of mRNA-1273 or BNT and 4–12 weeks after the first dose of AZD1222), 2–4 weeks after the second dose (T2), and 2–4 weeks after the third dose (T3). In convalescent subjects, blood samples were drawn prior vaccination (T0) and then 2–10 weeks after the single dose (T1). The study protocol is illustrated in Supplementary Fig. 1.
All the patients were informed of the study and its protocol and voluntarily agreed to participate by providing written consent. Ethics approval was obtained from the national review board and the study was registered on ClinicalTrials.gov (COVIMMUNITY NCT04648709; PROOF NCT00759304).
IGRA Immunoassay
IGRA (QFN, QuantiFERON SARS-CoV-2 Starter Set® for Research Use Only, Qiagen) was used according to manufacturer’s recommendations. Venous blood was collected, and 1 ml (1 ml) was added in each of the 4 tubes of the kit and incubated with antigens. Two antigen tubes, SARS-CoV-2 Ag1 and Ag2, used a combination of epitopes specific to SARS-CoV-2 S protein to stimulate CD4+ cells [receptor binding domain (RBD) and S1 epitopes] and both CD4+ and CD8+ cells (RBD, S1 and S2 epitopes), respectively. A negative control tube (Nil tube) and a positive control tube (Mitogen tube) were tested at the same time for each test to validate the interpretation. After stimulation, plasma samples were used for the determination of IFNγ level in international units per ml (IU/ml) using QuantiFERON enzyme-linked immunosorbent assay (ELISA), according to manufacturer’s recommendations. Positive response was defined according to initial data from the manufacturer as a value of IFNγ level at least 0.15 IU/ml greater than the background value from the Nil tube, for Ag1 or Ag2 tube. We also chose to use the sum of IFNγ levels of Ag1 and Ag2 with a positive threshold at 0.25 IU/ml (according to receiver operating characteristic (ROC) analysis) to have a unique interpretation of the test and which combines the 2 results.
Anti-spike Serology
S-specific IgG (directed against the RBD of the S1 subunit of the S protein) concentrations in serum specimens were measured using the Architect SARS-CoV-2 IgG II Quant® assay, which is a chemiluminescent microparticle immunoassay, according to manufacturer’s recommendations. A concentration greater than or equal to 60 arbitrary units (AU)/ml or 8.5 binding antibody units (BAU)/ml was defined as positive (supplier’s threshold).
PRNT Neutralization Assay
A plaque reduction neutralization test (PRNT) was used for the detection and titration of neutralizing antibodies. A tenfold dilution of each serum specimen in culture medium was first heated for 30 min at 56 °C to avoid complement-linked reduction of viral activity. Serial twofold dilutions (tested in duplicate) of the serum specimens in culture medium were mixed in equal volume with the live SARS-CoV-2 virus. After gentle shaking and a contact of 30 min at room temperature in plastic microplates, 150 µl of the mix was transferred into 96-well microplates covered with Vero E6 cells (American Type Culture Collection (ATCC), CRL-1586, not authenticated but regularly tested for mycoplasma contamination). The plates were incubated at 37 °C in a 5% CO2 atmosphere. Infection efficiency was evaluated by microscopy 5 days later when the cytopathic effect of the virus control reached 100–500 TCID50 (median culture infectious dose) per 150 µl. Neutralization was recorded if more than 50% of the cells present in the well were preserved. The neutralizing titer was expressed as the inverse of the higher serum dilution that exhibited neutralizing activity; a threshold of 20 was used (PRNT50 titer ≥ 20). All experiments were performed in a biosafety level 3 laboratory. The different viral strains that were used were sequenced and deposited at GISAID (https://www.gisaid.org/) (accession numbers EPI_ISL_1707038 19A (B.38 lineage); EPI_ISL_1707039 Alpha (B.1.1.7 lineage); EPI_ISL_768828 Beta (B.1.351 lineage); EPI_ ISL_1904989 Delta (B.1.617.2 lineage); and EPI_ISL_7608613 Omicron (B.1.1.529 lineage)).
Statistical Analysis
GraphPad Prism version 8 was used for statistical analysis, with a significance level (alpha) of 0.05, and curve fitting. A two-tailed Mann–Whitney U test was used to calculate p-values for continuous, non-parametric variables. For comparing more than one population, Kruskal–Wallis test was used with Dunn’s multiple comparisons test. Fisher’s exact test was used for contingency. XLSTAT 2021 was used for receiver operating characteristic (ROC) analysis. It consisted of calculating the area under the curve (AUC), and the sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) for each threshold value of the QuantiFERON test and serology to predict a neutralizing titer greater than or equal to 20. A GraphPad calculator was used to quantify the degree of agreement by Cohen’s kappa.
Results
Characteristics of Study Population
Among the participants, 67 (84%) were naive for COVID-19 (non-infected) and 13 (16%) had a history of COVID-19 (convalescent) in 2020 between March and November (Table 1). The mean (± SD) age in both groups was 57 (± 23) and 51 (± 17) respectively, and about 84% of participants were women in both groups. Our study contained a large proportion (39%, n = 26) of patients aged over 65 among non-infected people, with a majority being at least 80 years old (92%, n = 24). In the non-infected population, nearly 84% of patients received a homologous BNT/BNT or mRNA-1273/mRNA-1273 combination. The other patients followed a heterologous scheme (a first AZD1222 dose followed by an mRNA-1273 or a BNT boost). Twenty-seven subjects aged under 65 had a third dose of vaccine (booster dose of BNT162b2) more than 6 months after their second dose. Four patients who did not respond after their second dose had their third dose earlier than other patients: a 50-year-old woman with an autoimmune disease (2 and a half months later the second dose) and 3 elderly patients (1 month later the second dose). Convalescent subjects received a single BNT or mRNA-1273 dose. Between Jan 15, 2021, and Apr 22, 2022, 160 blood samples from the 67 non-infected participants, and 23 blood samples from the 13 convalescent participants were collected.
Table 1.
Baseline characteristics of the study population
| Total population: n = 80 subjects | Non-infected n = 67 (83.8%) |
Convalescent n = 13 (16.2%) |
||
|---|---|---|---|---|
| Sex | ||||
| Female n(%) | 56 | (83.6) | 11 | (84.6) |
| Male n(%) | 11 | (16.4) | 2 | (15.4) |
| Age (years) | ||||
| ≤ 45 n(%) | 26 | (38.8) | 6 | (46.2) |
| 46–65 n(%) | 15 | (22.4) | 5 | (38.4) |
| ≥ 66 n(%) | 26 | (38.8) | 2 | (15.4) |
| Median [IQR] | 52 | [34–84] | 54 | [36–60] |
| Type of primary vaccination | ||||
| Homologous BNT162b2 n(%) | 38 | (56.7) | 6 | (46.2) |
| Homologous mRNA-1273 n(%) | 18 | (26.9) | 7 | (53.8) |
| Homologous AZD1222 n(%) | 1 | (1.5) | / | / |
| Heterologous AZD1222/BNT162b2 n(%) | 1 | (1.5) | / | / |
| Heterologous AZD1222/mRNA-1273 n(%) | 9 | (13.4) | / | / |
| Sampling time | ||||
| T0 n(%) | 16 | (23.9) | 10 | (76.9) |
| T1 n(%) | 46 | (68.7) | 13 | (100.0) |
| T2 n(%) | 67 | (100.0) | / | / |
| T3 n(%) | 31 | (46.2) | / | / |
| Total | 160 | 23 | ||
Type of vaccine: BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), AZD1222 (Oxford-AstraZeneca); sampling time: T0, before vaccination; T1, 2–4 weeks after the first dose; T2, 2–4 weeks after the second dose; T3, 2–4 weeks after the third dose.
Significant Increase in Vaccine Response After Each Dose Measured by the 3 Tests
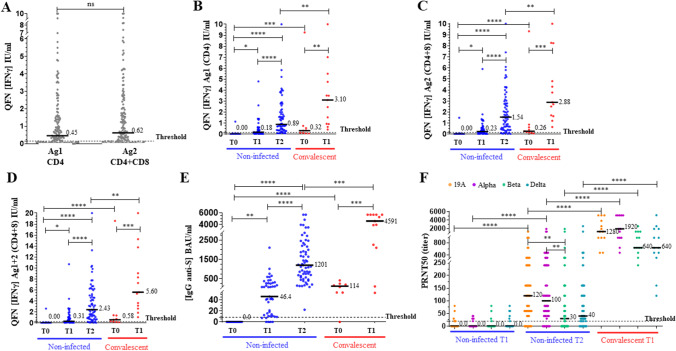
Comparison of the three immunoassays was done at each sample time (T0, T1, T2) during the primary vaccination (Fig. 1). For IGRA, IFNγ release in Ag1 tube (IFNγ Ag1) stimulating CD4+ cells was significantly correlated with that in Ag2 tube (IFNγ Ag2) stimulating both CD4+ and CD8+ cells (Spearman r = 0.97; 0.96–0.98; p < 0.0001, Supplementary Fig. 2). Figure 1A shows that IFNγ Ag2 level (mean ± SD: 1.50 ± 2.09 IU/ml) was not significantly different than IFNγ Ag1 level (1.20 ± 1.84 IU/ml) (Mann Whitney test, p = 0.10, n = 154). QFN test could therefore be interpreted with the result of IFNγ Ag1 as well as IFNγ Ag2 or the IFNγ Ag1 + 2 sum which combines the 2 results to have a unique interpretation of the test. Cellular response measured by QFN test (Fig. 1B–D) and humoral response measured by serological assay (Fig. 1E) increased significantly 2–4 weeks after the first dose of vaccine (T1) in non-infected people (Kruskal–Wallis test, p < 0.05 and p < 0.01, respectively). However, QFN Ag1 + 2 test remained negative (IFNγ < 0.25 IU/ml) for 40% of the patients at this time, while serological assay was positive (IgG ≥ 8.5 BAU/ml) for 87% of the patients (Supplementary Table 1). Two to four weeks after the second dose (T2), 83.5% and 100% of the patients had a positive QFN Ag1 + 2 and a positive serology, respectively, with significantly higher levels (p < 0.0001). Two doses were required to measure a positive response with a NAb titer (PRNT50) greater than or equal to 20 (Fig. 1F) in more than 80% of patients for the 19A strain and the alpha VOC. The NAb titer was not significantly different between these two strains. A positive NAb titer was obtained only in 56% and 76% for the beta and delta VOC, respectively, and it was reduced compared to 19A and alpha strains.
Fig. 1.
Humoral and cellular immunity monitoring in non-infected and convalescent individuals. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. The different sample times are: T0, before vaccination; T1, 2–4 weeks after the first dose; T2, 2–4 weeks after the second dose. A All plasmas samples (n = 154) were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) with both tubes separately (Ag1 and Ag2). Data show the concentration of IFNγ (IU/ml) measured by QFN ELISA, and are expressed as dot plots, one dot corresponding to one patient (ns: no statistical significance, p > 0.05), with a positive threshold at 0.15 IU/ml (supplier’s threshold). B, C, D Plasmas samples were assayed for QFN Ag1, QFN Ag2, and QFN Ag1 + 2, respectively. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient. Positive threshold for QFN Ag1 + 2 is 0.25 IU/ml (determined by ROC analysis). E Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). F Sera were assayed for their capacity to neutralize the infection of Vero E6 cells by different SARS-CoV-2 strains, as indicated. Data show the PRNT50 titer and are expressed as dot plots, one dot corresponding to one patient. Positive threshold corresponds to the limit of detection at 20. Statistical significance is shown (Kruskal–Wallis’s test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05)
In convalescent individuals before vaccination (T0), QFN Ag1 + 2 and serology assay were positive for 66.7% and 87.5% of the patients, respectively, in the same way as in non-infected patients at T1. The response increased significantly 2–10 weeks after vaccination at T1 for both QFN Ag1 + 2 and serology assay (p < 0.01 and p < 0.001, respectively), with 100% of positive results. These levels were significantly higher than those in non-infected patients at T2 (p < 0.01 and p < 0.001, respectively). These results were consistent with those of the neutralization for the four viral strains, with NAb titers significantly higher than those in non-infected patients at T2 (p < 0.0001). In convalescent individuals, the NAb titer was also not significantly different between 19A and alpha strains, but reduced for the beta and delta VOC.
Validation of Analytical Performance of QFN and Serology to Predict Neutralizing Activity
Performance of QFN test and serological assay (at the suppliers’ thresholds) has been assessed in comparison to neutralization (positive threshold: PRNT50 ≥ 20) to predict vaccine effectiveness on the 19A, alpha, beta, and delta strains, after the second dose (Table 2). Results in non-infected individuals (n = 67) show that QFN Ag1, Ag2, and Ag1 + 2 tests’ performances were equivalent, with an average Se > 98% for the 4 strains and an average Sp of 55% for 19A (n = 12 negative PRNT50), 52% for alpha VOC (n = 11 negative PRNT50), 24% for beta VOC (n = 29 negative PRNT50), and 30% for delta VOC (n = 16 negative PRNT50). All the serological results (n = 63) were positive; therefore Se was 100%. By contrast, since serology did not detect any negative PRNT50, Sp was 0% and NPV could not be calculated. Globally, QFN and serology had the same Se, but QFN had better Sp and PPV compared to serology (at the supplier’s threshold of 8.5 BAU/ml). Agreement and Cohen’s kappa were also higher with QFN (Table 2). After the third dose, PRNT was performed on the 19A, delta, and omicron strains. All the 27 subjects were positive for QFN, serology, PRNT for 19A and delta strains, and 23/27 (85%) for the omicron strain.
Table 2.
Performance of QFN and serology assay at the suppliers’ thresholds compared to neutralization in non-infected individuals after the second dose
| Neutralization (PRNT50 ≥ 20) | ||||
|---|---|---|---|---|
| 19A | Alpha | Beta | Delta | |
| Sensitivity (95% CI) | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
1.00 (0.93–1.00) 0.98 (0.90–1.00) 1.00 (0.93–1.00) 1.00 (0.93–1.00) |
0.98 (0.90–1.00) 0.96 (0.88–0.99) 0.98 (0.90–1.00) 1.00 (0.93–1.00) |
1.00 (0.91–1.00) 1.00 (0.91–1.00) 1.00 (0.91–1.00) 1.00 (0.90–1.00) |
0.96 (0.87–0.99) 0.94 (0.84–0.98) 0.96 (0.87–0.99) 1.00 (0.93–1.00) |
| Specificity (95% CI) | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
0.58 (0.32–0.81) 0.50 (0.25–0.75) 0.58 (0.32–0.81) 0.00 (0.00–0.24) |
0.55 (0.28–0.79) 0.45 (0.21–0.72) 0.55 (0.28–0.79) 0.00 (0.00–0.26) |
0.24 (0.12–0.42) 0.25 (0.13–0.43) 0.24 (0.12–0.42) 0.00 (0.00–0.12) |
0.33 (0.15–0.58) 0.25 (0.10–0.50) 0.31 (0.14–0.56) 0.00 (0.00–0.20) |
| PPV (95% CI) | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
0.92 (0.82–0.96) 0.90 (0.80–0.95) 0.92 (0.82–0.96) 0.81 (0.70–0.89) |
0.92 (0.82–0.96) 0.90 (0.80–0.95) 0.92 (0.82–0.96) 0.83 (0.71–0.90) |
0.63 (0.50–0.74) 0.64 (0.52–0.75) 0.63 (0.50–0.74) 0.56 (0.43–0.67) |
0.83 (0.72–0.91) 0.80 (0.68–0.88) 0.81 (0.70–0.89) 0.76 (0.64–0.85) |
| NPV (95% CI) | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
1.00 (0.65–1.00) 0.86 (0.49–0.99) 1.00 (0.65–1.00) / |
0.86 (0.49–0.99) 0.71 (0.36–0.95) 0.86 (0.49–0.99) / |
1.00 (0.65––1.00) 1.00 (0.65–1.00) 1.00 (0.65–1.00) / |
0.71 (0.36–0.95) 0.57 (0.25–0.84) 0.71 (0.36–0.95) / |
| Fisher’s exact test | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
p < 0.0001 (****) p < 0.0001 (****) p < 0.0001 (****) p > 0.9999 (ns) |
p < 0.0001 (****) p < 0.0009 (***) p < 0.0001 (****) p > 0.9999 (ns) |
p = 0.0020 (**) p = 0.0015 (**) p = 0.0020 (**) p > 0.9999 (ns) |
p = 0.0053 (**) p = 0.0532 (ns) p = 0.0074 (**) p > 0.9999 (ns) |
| Agreement | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
92.4% 89.4% 92.4% 81.0% |
90.9% 87.9% 90.9% 82.5% |
66.7% 68.2% 66.7% 55.6% |
81.8% 77.3% 80.3% 76.2% |
| Cohen’s kappa (95% CI) | ||||
|
QFN Ag1 (IFNγ ≥ 0.15 IU/ml) QFN Ag2 (IFNγ ≥ 0.15 IU/ml) QFN Ag1 + 2 (IFNγ ≥ 0.25 IU/ml) Serology (IgG ≥ 8.5 BAU/ml) |
0.70 (0.45–0.94) 0.58 (0.30–0.85) 0.70 (0.45–0.94) 0.00 (0.00–0.00) |
0.62 (0.34–0.89) 0.49 (0.19–0.79) 0.62 (0.34–0.89) 0.00 (0.00–0.00) |
0.26 (0.09–0.43) 0.28 (0.10–0.45) 0.26 (0.09–0.43) 0.00 (0.00–0.00) |
0.36 (0.09–0.64) 0.24 (0.00–0.48) 0.34 (0.07–0.60) 0.00 (0.00–0.00) |
QFN QuantiFERON SARS-CoV-2 Starter Set®, IgG G-immunoglobulins, PPV positive predictive value, NPV negative predictive value, / data not available.
Then, ROC analysis was performed to evaluate overall performance and to find more specific thresholds for QFN and serology in non-infected patients after the second dose. The ROC curves are presented in Supplementary Fig. 3. Table 3 shows that a first threshold at 0.25 IU/ml for IFNγ Ag1 + 2 and at 150 BAU/ml for S-specific IgG could be used to screen for non-responder patients for 19A strain with a Se of 100% and a NPV of 100%, respectively. Threshold 2 at 1.20 IU/ml for IFNγ Ag1 + 2 and 850 BAU/ml for IgG could be used to better predict a PRNT50 titer ≥ 20 for 19A and alpha strains, with a Sp of 92% and a PPV of 98% for both. Threshold 3 was proposed to increase Sp and PPV for the beta and delta VOC. The ROC curves obtained after the third dose are presented in Supplementary Fig. 4. These could only be obtained for the omicron variant since the neutralizing titers were all positive for the 19A and delta strains. Thereby, after the third dose, the first threshold calculated after the second dose (0.25 IU/ml for IFNγ Ag1 + 2 and 150 BAU/ml for S-specific IgG) could be also used to predict a PRNT50 titer ≥ 20 for 19A and delta strains. For the omicron strain, the AUC obtained for QFN was low, so its predictive ability was weak, and its corresponding cut-off could not be calculated. However, anti-spike serology could be used to screen for responder patients with a positive threshold at 500 BAU/ml (Se of 100% and VPP of 85%).
Table 3.
Proposed thresholds to improve the performances of QFN and serology assay compared to neutralization in non-infected individuals after the second dose
| ROC analysis | Neutralization (PRNT50 ≥ 20) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19A | Alpha | Beta | Delta | ||||||||||||||
| Se | Sp | PPV | NPV | Se | Sp | PPV | NPV | Se | Sp | PPV | NPV | Se | Sp | PPV | NPV | ||
| QFN Ag1 | |||||||||||||||||
| Threshold 1 | 0.15 IU/ml | 1.00 | 0.58 | 0.92 | 1.00 | 0.98 | 0.55 | 0.92 | 0.86 | 1.00 | 0.24 | 0.63 | 1.00 | 0.96 | 0.33 | 0.83 | 0.71 |
| Threshold 2 | 0.50 IU/ml | 0.80 | 0.92 | 0.98 | 0.50 | 0.78 | 0.91 | 0.98 | 0.46 | 0.78 | 0.48 | 0.66 | 0.64 | 0.76 | 0.63 | 0.86 | 0.46 |
| Threshold 3 | 2.00 IU/ml | 0.43 | 0.97 | 0.94 | 0.57 | 0.34 | 1.00 | 1.00 | 0.33 | ||||||||
| QFN Ag2 | |||||||||||||||||
| Threshold 1 | 0.15 IU/ml | 0.98 | 0.50 | 0.90 | 0.86 | 0.96 | 0.45 | 0.90 | 0.71 | 1.00 | 0.25 | 0.64 | 1.00 | 0.94 | 0.25 | 0.80 | 0.57 |
| Threshold 2 | 0.70 IU/ml | 0.80 | 0.92 | 0.98 | 0.50 | 0.78 | 0.91 | 0.98 | 0.46 | 0.81 | 0.52 | 0.68 | 0.68 | 0.76 | 0.63 | 0.86 | 0.46 |
| Threshold 3 | 3.00 IU/ml | 0.46 | 0.93 | 0.90 | 0.57 | 0.28 | 0.94 | 0.93 | 0.30 | ||||||||
| QFN Ag1 + 2 | |||||||||||||||||
| Threshold 1 | 0.25 IU/ml | 1.00 | 0.58 | 0.92 | 1.00 | 0.98 | 0.55 | 0.92 | 0.86 | 1.00 | 0.24 | 0.63 | 1.00 | 0.96 | 0.31 | 0.81 | 0.71 |
| Threshold 2 | 1.20 IU/ml | 0.83 | 0.92 | 0.98 | 0.55 | 0.82 | 0.91 | 0.98 | 0.50 | 0.84 | 0.48 | 0.67 | 0.70 | 0.80 | 0.63 | 0.87 | 0.50 |
| Threshold 3 | 4.60 IU/ml | 0.46 | 0.93 | 0.90 | 0.57 | 0.36 | 0.94 | 0.95 | 0.32 | ||||||||
| Serology | |||||||||||||||||
| Threshold 1 | 150 BAU/ml | 1.00 | 0.42 | 0.88 | 1.00 | 1.00 | 0.46 | 0.90 | 1.00 | 1.00 | 0.15 | 0.52 | 1.00 | 1.00 | 0.33 | 0.83 | 1.00 |
| Threshold 2 | 850 BAU/ml | 0.77 | 0.92 | 0.98 | 0.48 | 0.75 | 0.91 | 0.98 | 0.44 | 0.80 | 0.57 | 0.70 | 0.70 | 0.75 | 0.73 | 0.90 | 0.48 |
| Threshold 3 | 2500 BAU/ml | 0.20 | 0.89 | 0.70 | 0.47 | 0.21 | 1.00 | 1.00 | 0.28 | ||||||||
ROC receiver operating characteristic, Se sensitivity, Sp specificity, PPV positive predictive value, NPV negative predictive value, QFN QuantiFERON SARS-CoV-2 Starter Set®
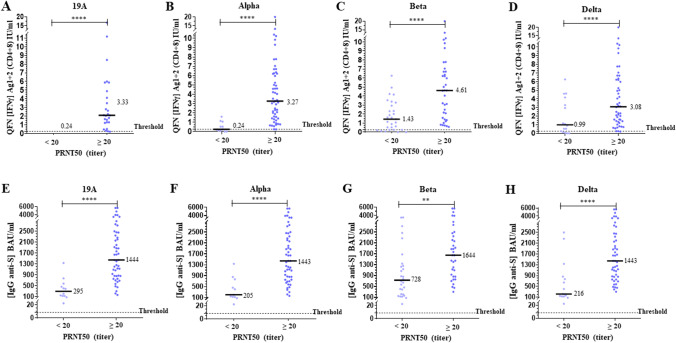
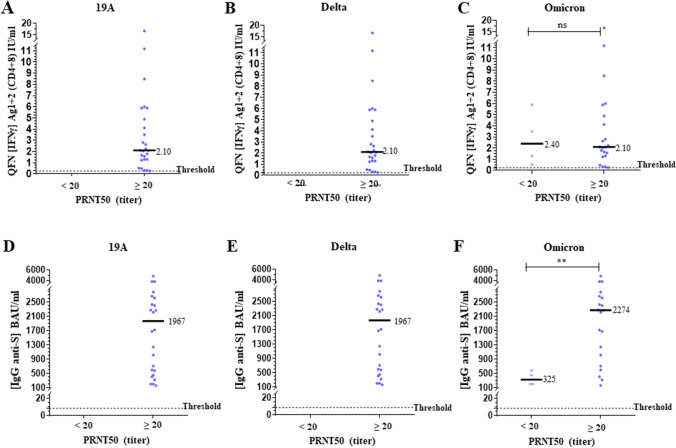
Comparison of NAb titers with QFN and serology results after the second dose is presented in Fig. 2. The levels of IFNγ Ag1 + 2 and IgG corresponding to a positive NAb titer (≥ 20) were significantly higher than those corresponding to a negative titer (p < 0.0001 for both). The positive correlations were significant (p < 0.0001), but the intensity was low for QFN (Spearman r = 0.48 for 19A strain) and moderate for serological assay (Spearman r = 0.63 for 19A strain) (Supplementary Fig. 5). After the third dose, comparison of NAb titers with QFN and serology is presented in Fig. 3 and correlations between assays are presented in Supplementary Fig. 6. The 4 subjects with a negative PRNT50 for omicron had a significantly lower S-specific IgG level, whereas the level of IFNγ Ag1 + 2 is not significantly different compared to patients with a positive PRNT50. Significant IgG anti-S antibody levels (> 150 BAU/ml) were also associated with stronger IGRA responses after the second and third dose (Supplementary Fig. 7).
Fig. 2.
Comparison of neutralization, QFN, and anti-spike IgG in non-infected individuals after the second dose. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. A–D Plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient, with a positive threshold at 0.25 IU/ml. E–H Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). A–H All results are divided into 2 groups of corresponding neutralization titers (PRNT50), and according to each strain of virus tested, as indicated. Statistical significance is shown (Kruskal–Wallis’s test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05)
Fig. 3.
Comparison of neutralization, QFN and anti-spike IgG in non-infected individuals after the third dose. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. A-C Plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient, with a positive threshold at 0.25 IU/ml. D–F Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). A-F All results are divided into 2 groups of corresponding neutralization titers (PRNT50), and according to each strain of virus tested, as indicated. No statistical significance (ns, p > 0.05) and statistical significance are shown (Kruskal–Wallis’s test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05)
Reduced Immunogenicity of Vaccination in Elderly
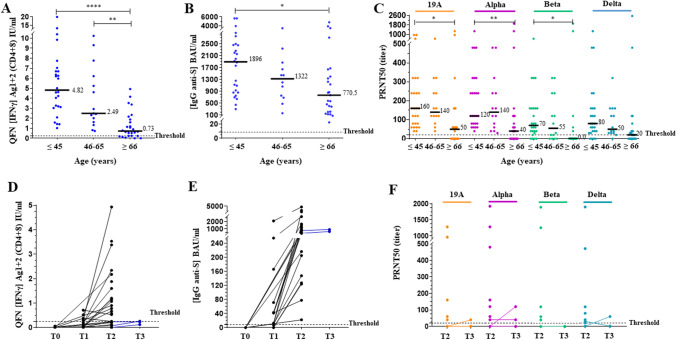
After the second dose, patients ≥ 66 years of age had significantly lower IFNγ Ag1 + 2 levels, IgG levels and NAb titers on 19A, alpha, and beta strains compared to patients ≤ 45 years (p < 0.0001, p < 0.05, p < 0.05, respectively) (Fig. 4A–C). No significant difference was observed between ≤ 45 and 46–65 years old (p = 0.64, p = 0.78, p > 1.00, respectively). A total of 100% of patients ≤ 65 years of age were positive for all the 3 assays, with IFNγ Ag1 + 2 ≥ 0.25 IU/ml, IgG ≥ 150 BAU/ml, and PRNT50 ≥ 20 (for 19A and alpha strains) (Supplementary Table 2). The first threshold for each test was sufficient to predict a positive NAb titer (on 19A and alpha strains) in patients ≤ 65 years of age, with a PPV of 100% and a Se of 100% at this age (Table 3). Only 50 to 60% of patients ≥ 66 years of age were positive for QFN and PRNT50 and 100% were positive for serology (Supplementary Table 2). All negative patients were ≥ 80 years old. Thresholds 2 and 3 were calculated from the results of these patients aged ≥ 80 to better predict a positive NAb titer at this age (Table 3).
Fig. 4.
Elderly patients have a significantly lower vaccine response. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. A Plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient, with a positive threshold at 0.25 IU/ml. B Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). C Sera were assayed for their capacity to neutralize the infection of Vero E6 cells by different SARS-CoV-2 strains, as indicated. Data show the PRNT50 titer and are expressed as dot plots, one dot corresponding to one patient. Positive threshold corresponds to the limit of detection at 20. All results obtained 2–4 weeks after the second dose are divided into 3 groups of corresponding age, as indicated. D-F Evolution of the results of each test in elderly patients aged over 80 (n = 24) according to the number of doses received (T0, before vaccination; T1/T2/T3, 2–4 weeks after the first/second/third dose). Three patients had a third dose. Statistical significance is shown (Kruskal–Wallis’s test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05)
Evolution of the response after each dose of BNT162b2 vaccine in elderly patients aged ≥ 80 is shown in Fig. 4D–F. After the first dose, only 5 in 14 patients (35.7%) responded to the QFN and 11 (78.6%) had a positive serology. After the second dose, all had a positive serology, while 70.8% and 50% responded to the QFN and to the neutralization test (19A and alpha strains), respectively. Of these non-responder patients, 3 had a third dose approximately 1 month after their second dose.
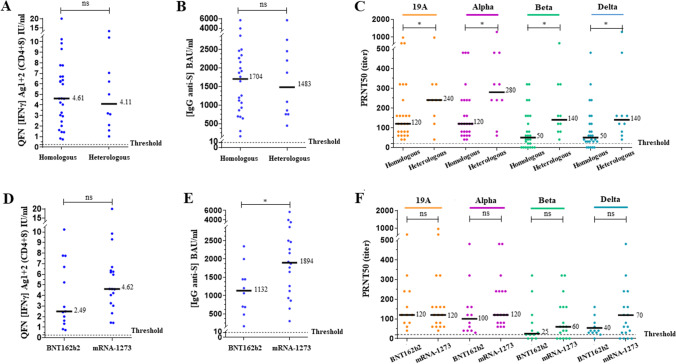
We then compared those assays between the homologous and heterologous schemes in patients < 55 years (Fig. 5A–C) and between BNT162b2 and mRNA-1273 in patients ≤ 65 years (Fig. 5D–F). No significant difference was observed between the homologous and heterologous schemes in response to either QFN (p = 0.96) or anti-S IgG concentration (p = 0.99). By contrast, higher NAb titers were observed with the heterologous scheme (p < 0.05). QFN and serology did not correlate with the higher neutralizing activity. A positive NAb titer on the beta and delta VOCs was obtained in 100% of patients with the heterologous scheme (Supplementary Table 2). In the homologous group, a trend of a higher QFN response was observed with Moderna vs Pfizer. A significant difference in anti-S IgG concentration was shown (p < 0.05). Slightly higher NAb titers were also observed with the mRNA-1273 vaccine on the alpha, beta, and delta VOCs, but not significantly.
Fig. 5.
Variation in response according to vaccination scheme in non-infected individuals after 2 doses in the same age group. The median of each subgroup is represented by a black line with the specified value. Positive threshold of each test is represented by a dotted line. A, D Plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one patient, with a positive threshold at 0.25 IU/ml. B, E Sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one patient. Positive threshold is 8.5 BAU/ml (supplier’s threshold). C, F Sera were assayed for their capacity to neutralize the infection of Vero E6 cells by different SARS-CoV-2 strains, as indicated. Data show the PRNT50 titer and are expressed as dot plots, one dot corresponding to one patient. Positive threshold corresponds to the limit of detection at 20. A–C All results obtained 2–4 weeks after the second dose in non-infected patients aged under 55 years are divided into 2 groups of corresponding vaccination scheme, as indicated. Homologous scheme includes patients with identical prime and boost dose of BNT162b2 or mRNA-1273 vaccine. Heterologous scheme includes patients with a prime dose of AZD1222 followed by an mRNA boost. D–F All results obtained 2–4 weeks after the second dose in non-infected patients aged under 65 years are divided into 2 groups of corresponding mRNA vaccine type in the homologous scheme, as indicated. No statistical significance (ns, p > 0.05) and statistical significance are shown (Kruskal–Wallis’s test, *p < 0.05)
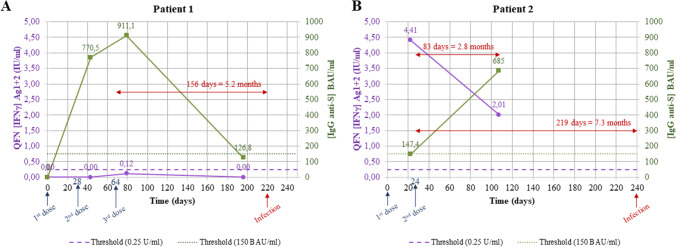
In our cohort of 67 non-infected patients, two of them were infected with the delta VOC more than 5 months after their second dose (Fig. 6). Patient 1 was an 87-year-old man who received 3 doses of BNT162b2 given 1 month apart. His QFN remained negative even after 3 doses. His anti-S IgG decreased over the 5 months after his third dose and fell below the screening threshold 1 about 20 days before his infection. Patient 2 was a 46-year-old woman who had 2 doses of BNT162b2 and became infected more than 7 months after her second dose.
Fig. 6.
Examples of follow-up and infection in two patients after vaccination. Time is represented in days with each occurrence (injection of a dose of vaccine or infection) indicated by an arrow. On the left y-axis, plasmas samples were assayed for QuantiFERON SARS-CoV-2 Starter Set® (QFN) Ag1 + 2. Data show the concentration of IFNγ (IU/ml), and are expressed as dot plots, one dot corresponding to one sample with its indicated result, for the same patient. Positive threshold is 0.25 IU/ml (dotted line). On the right x-axis, sera were assayed for S (RBD)-specific IgG. Data show the concentration (BAU/ml) of immunoglobulins, and are expressed as dot plots, one dot corresponding to one sample with its indicated result, for the same patient. Positive threshold is 150 BAU/ml (dotted line). A Results for patient 1, an 87-year-old man who received 3 doses of BNT162b2 given 1 month apart. B Results for patient 2, a 46-year-old woman who had 2 doses of BNT162b2 given 1 month apart
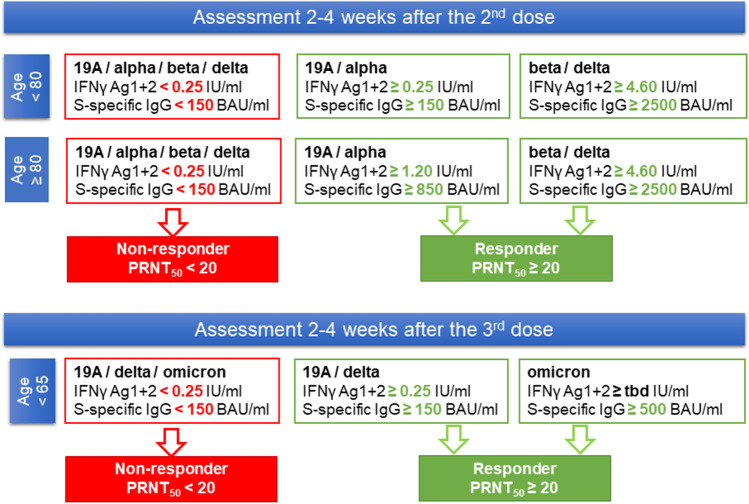
In summary, QFN and serology could be used at the thresholds describe above to predict a positive NAb titer, according to age in non-previously infected patients (Fig. 7).
Fig. 7.
Adaptation of the vaccine strategy according to the age and the results of QFN and serology. This decision tree shows a possible interpretation of the results of QFN (IFNγ Ag1 + 2) and serology (S (RBD)-specific IgG) according to the proposed thresholds. After the third dose, threshold of QFN for omicron could not be calculated due to its poor predictive ability in the population tested (tbd = to be determined)
Discussion
Emerging evidence suggests that both humoral and cellular responses to COVID-19 infection and vaccination should be considered complementary and mounting of both types of responses could be associated with the effective immunity against SARS-CoV-2. In our study, we demonstrated for the first time that QFN and anti-spike IgG above our cut-off values strongly correlate with NAb responses potentially associated with protection against breakthrough infection. Indeed, the induction of NAb response, as measured by PRNT, is currently the better correlate of protection (CoP) [13–17]. Overall vaccine response in non-infected patients was significantly higher after two doses than that observed after a single dose, as previously described [46]. Convalescent individuals were all positive for all 3 tests after their single dose of vaccine and had significantly higher results than those of non-infected patients after two doses, as previously described [47, 48]. Our thresholds were very predictive of an effective vaccination in convalescent individuals, reflected by the high titers of NAb. In non-infected subjects, all patients < 80 years old were positive for all three tests (except for certain patients without NAb on the beta and delta VOCs) and patients aged ≥ 80 had a significantly lower vaccine response. Lustig et al. showed that lower Ab levels and NAb titers were consistently associated with older age (≥ 66 years), with high IgG levels but less neutralizing or taking longer to become neutralizing [17]. Only higher NAb titers were observed with heterologous vaccination. For homologous vaccination, in people ≤ 65 years of age, higher QFN levels and NAb titers on alpha, beta, and delta VOCs were observed with the mRNA-1273 vaccine compared to BNT162b2, with significantly higher concentrations of IgG, as previously described [49]. Heterologous vaccination induced significantly higher serum neutralization activity and increased the cellular immune response [16, 19, 50]. However, the enhancer effect of the second dose has been described with homologous mRNA vaccination. Furthermore, the shorter interval between the two mRNA doses than between ChAdOx1-prime and mRNA-boost vaccination might have contributed to the higher immunogenicity of the heterologous regimen [19, 50].
Individuals with low QFN (< 0.25 IU/ml) and low anti-spike IgG (< 150 BAU/ml) have no NAb (< 20) and required a supplementary dose rapidly. Individuals with high QFN (≥ 1.20 IU/ml) and high anti-spike IgG (≥ 850 BAU/ml) after their second dose are good responders. However, several studies have shown a persistent but a significant declining humoral immunity at 3 months, with a significant decrease in the titer of NAb during the 8 months’ post-vaccination or post-infection. It predicts a significant decrease in protection against infection, but which remains preserved against severe infection [16, 51–53]. Combination of assays measuring both humoral and cellular responses is complementary since each component of SARS-CoV-2 immune memory exhibited distinct kinetics [16, 54–56]. To predict efficacy on the beta and delta VOCs, the threshold should be increased to 4.60 IU/ml and 2500 BAU/ml after the second dose in non-infected patients. Previous studies have indeed shown that the efficacy of NAb against the alpha VOC was unchanged regardless of the vaccine considered, while it was reduced on the beta and delta VOCs, both in sera from convalescent patients and from vaccinated patients [21–26, 29–31, 34, 36, 53, 57]. The need for a dose of mRNA vaccine in convalescent patients to obtain neutralizing activity against the beta VOC, by stimulating memory B cells has been also described [28, 38]. Sera from individuals vaccinated with mRNA vaccines still had a neutralizing activity on the delta VOC, with a predictive efficacy higher than that of ChAdOx1 [22, 23, 34, 53]. Concerning the omicron variant, our study showed that few patients had no neutralizing response against this variant after the third dose in a small group of individuals, while they all had a response against the delta variant. Indeed, several studies showed that vaccine efficacy against the omicron variant was lower, with the lack of humoral response after two doses and a reduced and time-limited response after the booster dose [40–43, 45, 58]. T cell response seemed to be preserved, as we also observed in our study with 100% positivity of QFN [40, 59]. Moreover, a recent study showed that the vaccine effectiveness against symptomatic disease caused by the BA.2 sub-lineage seemed to be preserved [60].
Few studies demonstrated that the NAb titers and the anti-S and anti-RBD IgG levels were strongly correlated with COVID-19 risk and vaccine efficacy, and therefore proposed the use of post-immunization Ab levels as the basis for a CoP [13, 16, 18, 61, 62]. Khoury et al. estimated the NAb titer for 50% protection against infection to be 20.2% of the mean convalescent level and that it was significantly lower (3% of the mean convalescent level) against severe infection [16]. Corbett et al. confirmed in nonhuman primates that mRNA-1273 vaccine-induced Ab responses are a mechanistic CoP against infection and protection [13]. Feng et al. demonstrated that increasing ChAdOx1 vaccine-induced Ab responses were strongly associated with lower risk of symptomatic infection (mild disease) in humans 28 days after the second dose. They estimated that 80% efficacy against symptomatic infection was achieved with 264 BAU/ml for anti-S IgG and 506 BAU/ml for anti-RBD [18]. The anti-RBD level obtained in this study is between the two thresholds calculated in our study, at 150 and 850 BAU/ml to predict a PRNT50 ≥ 20 in patients aged < 80 and ≥ 80 years, respectively. However, in this study, elderly patients represented a very small proportion, neutralization was only performed on the alpha VOC, and only the homologous scheme by ChAdOx1 was studied. Thus, CoP may variate according to age profile, disease severity (symptomatic or asymptomatic infection, mild or severe disease), type of virus variant, type of vaccine scheme, and measurement method. Further studies are needed to establish a CoP for each of these determinants of vaccine response, comparing them against the risk of having symptomatic infection in real life.
Protection may require low levels of NAb and might involve other immune effector mechanisms including non-NAb, T cells and innate immune mechanisms [63–65]. Indeed, in our study, the lower Sp and PPV of QFN compared to the PRNT on the beta and delta VOC could also indicate the need to monitor both humoral and cellular immunity. McMahan et al. showed that the depletion of CD8+ T cells in convalescent macaques partially abrogated the protective efficacy of natural immunity against challenge with SARS-CoV-2, which suggests a role for cellular immunity in the context of waning or subprotective antibody titers [65]. Moreover, a negligible impact of the mutations found on the variants B.1.1.7, B.1.351, and P.1 on the CD4+ and CD8+ responses in convalescent- or mRNA-vaccinated subjects has been reported [66]. Furthermore, measuring the cellular response could better predict the vaccine efficacy in some immunocompromised patients. Indeed, studies have shown that patients who did not have B cells (and therefore did not produce Ab) produced a positive IFNγ response after vaccination [67, 68]. Due to the ability of QFN to predict a neutralizing activity, it could be considered as a useful and user-friendly tool for monitoring vaccine responses and COVID-19 management of various (including high risk) populations including decision making regarding booster vaccine doses. The main limitation of our study is the small cohort with a lack of representation of the population aged over 80 to assess the vaccine response against the omicron variant after the third dose. Another technical limitation is the use of VeroE6 cells for our PRNT assay, which could lead to mutations or deletions in the multibasic cleavage site in the S protein as previously described [69].
Our study is the first to have shown the possible interest of QFN in addition to anti-S serology in the prediction of the neutralizing activity of Ab on different viral strains and in the adaptation of the vaccine strategy at the individual level. With the actual emergence of the Omicron VOC, a combination of Ab and T cell response will be probably strongly required to evaluate vaccine protection in the population. A proper design to establish new cutoffs for these assays would also need at least one validation cohort, to test that the proposed cutoffs are generalizable beyond this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Qiagen for the support. We thank also all the patients from the PROOF cohort and all the health care workers for their participation in this clinical study.
Author Contribution
MV, AB, LW, AH, AC, DH, FR, EBN, BP and SP performed the research; MV, TB, BP, EBN, SP designed the research study. MV, AB, SP analyzed the data. MV, SP wrote the paper. All authors approved the final version of the manuscript.
Data Availability
Data is available upon request to the corresponding author.
Declarations
Ethical Approval Consent to Participate
All the patients were informed of the study and its protocol and voluntarily agreed to participate by providing written consent. Ethics approval was obtained from the national review board and the study was registered on ClinicalTrials.gov (COVIMMUNITY NCT04648709; PROOF NCT00759304).
Consent for Publication
All the authors consent for the publication.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. [cited 2022 May 26]. Available from: https://covid19.who.int.
- 2.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;0(0):null. [DOI] [PMC free article] [PubMed]
- 6.El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;0(0):null. [DOI] [PMC free article] [PubMed]
- 7.PINHO AC. EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU. European Medicines Agency. 2021 [cited 2021 Oct 9]. Available from: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu.
- 8.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avis n° 2021.0018/AC/SEESP du 19 mars 2021 du collège de la Haute Autorité de santé sur la place du vaccin AstraZeneca dans la stratégie vaccinale suite à l’avis de l’agence européenne des médicaments concernant des évènements indésirables survenus dans plusieurs pays européens chez des personnes vaccinées [Internet]. Haute Autorité de Santé. [cited 2021 Sep 10]. Available from: https://www.has-sante.fr/jcms/p_3244283/fr/avis-n-2021-0018/ac/seesp-du-19-mars-2021-du-college-de-la-haute-autorite-de-sante-sur-la-place-du-vaccin-astrazeneca-dans-la-strategie-vaccinale-suite-a-l-avis-de-l-agence-europeenne-des-medicaments-concernant-des-evenements-indesirables-survenus-dans-plusieurs-pays-europeens-chez-des-personnes-vaccinees.
- 10.Terpos E, Politou M, Ntanasis-Stathopoulos I, Karalis V, Merkouri E, Fotiou D, et al. High prevalence of Anti-PF4 antibodies following ChAdOx1 nCov-19 (AZD1222) Vaccination even in the absence of thrombotic events. Vaccines (Basel) 2021;9(7):712. doi: 10.3390/vaccines9070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avis n° 2021.0027/AC/SEESP du 8 avril 2021 du collège de la Haute Autorité de santé concernant le type de vaccin à utiliser pour la seconde dose chez les personnes de moins de 55 ans ayant reçu une première dose du vaccin AstraZeneca (nouvellement appelé VAXZEVRIA) contre la covid-19. Haute Autorité de Santé. [cited 2021 Sep 10]. Available from: https://www.has-sante.fr/jcms/p_3260361/fr/avis-n-2021-0027/ac/seesp-du-8-avril-2021-du-college-de-la-haute-autorite-de-sante-concernant-le-type-de-vaccin-a-utiliser-pour-la-seconde-dose-chez-les-personnes-de-moins-de-55-ans-ayant-recu-une-premiere-dose-du-vaccin-astrazeneca-nouvellement-appele-vaxzevria-contre-la-covid-19.
- 12.Avis n° 2022.0006/SESPEV du 13 janvier 2022 du collège de la Haute Autorité de santé sur la modification du décret du 1er juin 2021 relative aux schémas vaccinaux reconnus dans le cadre du passe sanitaire. Haute Autorité de Santé. [cited 2022 May 26]. Available from: https://www.has-sante.fr/jcms/p_3309646/fr/avis-n-2022-0006/sespev-du-13-janvier-2022-du-college-de-la-haute-autorite-de-sante-sur-la-modification-du-decret-du-1er-juin-2021-relative-aux-schemas-vaccinaux-reconnus-dans-le-cadre-du-passe-sanitaire.
- 13.Corbett KS, Nason MC, Flach B, Gagne M, O’Connell S, Johnston TS, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science. 373(6561):eabj0299. [DOI] [PMC free article] [PubMed]
- 14.Koup RA, Donis RO, Gilbert PB, Li AW, Shah NA, Houchens CR. A government-led effort to identify correlates of protection for COVID-19 vaccines. Nat Med. 2021;27(9):1493–1494. doi: 10.1038/s41591-021-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54(9):2133–2142.e3. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 17.Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;29:1–9. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Infectious Diseases (except HIV/AIDS); 2020 [cited 2021 Aug 18]. Available from: 10.1101/2020.12.09.20245175.
- 21.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–5. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 22.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet. 2021;398(10296):207–209. doi: 10.1016/S0140-6736(21)01462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–5. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 24.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351–62. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384(20):1885–98. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. Immunology. 2021 [cited 2021 Sep 18]. Available from: 10.1101/2021.01.25.427948.
- 31.Muik A, Wallisch AK, Sänger B, Swanson KA, Mühl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371(6534):1152–3. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021;27(4):620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- 33.Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. Neutralizing antibodies against SARS-CoV-2 Variants after infection and vaccination. JAMA. 2021;325(18):1896–1898. doi: 10.1001/jama.2021.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Azman AS, Lu W, Sun R, Zheng N, Ge S, et al. Prediction of vaccine efficacy of the Delta variant. medRxiv. 2021;2021.08.26.21262699.
- 35.Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622–5. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 36.Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–539.e3. doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The CITIID-NIHR BioResource COVID-19 Collaboration. The COVID-19 Genomics UK (COG-UK) Consortium. Collier DA, De Marco A, Ferreira IATM, Meng B, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136–41. doi: 10.1038/s41586-021-03412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skelly DT, Harding AC, Gilbert-Jaramillo J, Knight ML, Longet S, Brown A, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun. 2021;12(1):5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu K, Choi A, Koch M, Elbashir S, Ma L, Lee D, et al. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. Microbiology. 2021 [cited 2021 Aug 18]. Available from: 10.1101/2021.04.13.439482. [DOI] [PMC free article] [PubMed]
- 40.GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7(69):eabo2202. 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed]
- 41.Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans JP, Zeng C, Qu P, Faraone J, Zheng YM, Carlin C, et al. Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe. 2022 [cited 2022 May 26]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9035359/ [DOI] [PMC free article] [PubMed]
- 44.Liwsrisakun C, Pata S, Laopajon W, Takheaw N, Chaiwong W, Inchai J, et al. Neutralizing antibody and T cell responses against SARS-CoV-2 variants of concern following ChAdOx-1 or BNT162b2 boosting in the elderly previously immunized with CoronaVac vaccine. Immun Ageing. 2022;19(1):1–13. doi: 10.1186/s12979-022-00279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gils MJ, Lavell A, van der Straten K, Appelman B, Bontjer I, Poniman M, et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: A prospective cohort study. PLoS Med. 2022;19(5):e1003991. doi: 10.1371/journal.pmed.1003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iheanacho CO, Eze UIH, Adida EA. A systematic review of effectiveness of BNT162b2 mRNA and ChAdOx1 adenoviral vector COVID-19 vaccines in the general population. Bull Natl Res Cent. 2021;45(1):150. doi: 10.1186/s42269-021-00607-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 Antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021 [cited 2021 Sep 24]; Available from:10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed]
- 50.Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21(9):1212–3. [DOI] [PMC free article] [PubMed]
- 51.Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, et al. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: a third dose is expected. J Infect. 2021 [cited 2021 Oct 1]; Available from: https://www.sciencedirect.com/science/article/pii/S0163445321004333.
- 52.Terpos E, Trougakos IP, Karalis V, Ntanasis-Stathopoulos I, Gumeni S, Apostolakou F, et al. Kinetics of Anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with BNT162b2; a prospective study in 283 health workers. Cells. 2021;10(8):1942. doi: 10.3390/cells10081942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDade TW, Demonbreun AR, Sancilio A, Mustanski B, D’Aquila RT, McNally EM. Durability of antibody response to vaccination and surrogate neutralization of emerging variants based on SARS-CoV-2 exposure history. Sci Rep. 2021;11(1):17325. doi: 10.1038/s41598-021-96879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12(1):1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 [cited 2021 Sep 19];371(6529). Available from: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed]
- 57.Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suryawanshi RK, Chen IP, Ma T, Syed AM, Brazer N, Saldhi P, et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. 2022;18:1–3. doi: 10.1038/s41586-022-04865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung MK, Jeong SD, Noh JY, Kim DU, Jung S, Song JY, et al. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat Microbiol. 2022;16:1–9. doi: 10.1038/s41564-022-01123-x. [DOI] [PubMed] [Google Scholar]
- 60.Kirsebom F, et al. COVID-19 Vaccine effectiveness against the Omicron BA.2 variant in England. 2022;22(7):931–3. [DOI] [PMC free article] [PubMed]
- 61.Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert PB, Montefiori DC, McDermott A, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. Infectious Diseases (except HIV/AIDS). 2021 [cited 2021 Sep 14]. Available from: 10.1101/2021.08.09.21261290.
- 63.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarke A, Sidney J, Methot N, Zhang Y, Dan JM, Goodwin B, et al. Negligible impact of SARS-CoV-2 variants on CD4 + and CD8 + T cell reactivity in COVID-19 exposed donors and vaccinees. Immunology. 2021 [cited 2021 Sep 18]. Available from: 10.1101/2021.02.27.433180.
- 67.Malipiero G, Moratto A, Infantino M, D’Agaro P, Piscianz E, Manfredi M, et al. Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol Res. 2021;21:1–8. doi: 10.1007/s12026-021-09226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferguson J, Murugesan K, Banaei N, Liu A. Interferon-gamma release assay testing to assess COVID-19 vaccination response in a SARS-CoV-2 seronegative patient on rituximab: a case report. Int J Infect Dis. 2021;110:229–231. doi: 10.1016/j.ijid.2021.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mautner L, Hoyos M, Dangel A, Berger C, Ehrhardt A, Baiker A. Replication kinetics and infectivity of SARS-CoV-2 variants of concern in common cell culture models. Virol J. 2022;26(19):76. doi: 10.1186/s12985-022-01802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request to the corresponding author.