Abstract
Introduction
Malignant tumors of the small bowel are rare. The jejunum, ileum, and duodenum represent the most common sites of intestinal leiomyosarcoma (LMS). Herein, we present a case of a 65-year-old patient having ileal LMS successfully treated with surgical resection.
Presentation of case
A 65-year-old patient, with no comorbidities, presented with chronic and paroxysmal abdominal pain.
Upper endoscopy and colonoscopy showed no abnormalities. Thoracoabdominal computed tomography (CT) revealed an ileal lobulated, heterogeneously enhancing solid mass measuring 6 cm.
Laparotomy was performed. Findings showed a lobulated ileal mass. We made an enlarged ileal resection with end-to-end anastomosis. The postoperative course was uneventful.
Histology and IHC stains concluded into ileal LMS. No relapse of the disease was noted during the 4-month follow-up.
Clinical discussion
Ileal LMS is a rare tumor originating from the smooth muscle cells within the muscularis mucosa or muscularis propria. CT colonography (CTC) and magnetic resonance enterography (MRE) represent good options to aid the diagnosis. Histologically, LMS often has a comparable morphological appearance to GISTs.
IHC is essential to differentiate those tumors. Surgery is the only curative treatment.
The prognosis is poor knowing that those tumors are discovered at advanced stages.
Conclusion
Ileal LMS is a rare tumor originating from the smooth muscle cells. It has a comparable morphological appearance to GISTs. Immunohistochemistry is essential to confirm the diagnosis. Surgery is the only curative treatment. The prognosis is poor.
Keywords: Leiomyosarcoma, Small bowel, Ileal neoplasms, Case report
Highlights
-
•
Small bowel leiomyosarcoma (LMS) wall is an extremely rare tumor.
-
•
LMS originates from the smooth muscle cells.
-
•
Immunohistochemistry is essential to confirm the diagnosis.
-
•
Those tumors have a low response rate to chemotherapy.
-
•
Surgery is the only curative treatment.
1. Introduction and importance
Malignant tumors of the small bowel are rare. They account for less than 5 % of all gastrointestinal cancers [1]. Malignant small bowel tumors are carcinoids (44,3 %), adenocarcinomas (32,6 %), lymphomas (14,7 %), gastrointestinal stromal tumors (GISTs) (7,2 %), and leiomyosarcomas (1,2 %) [2]. Thus, small bowel leiomyosarcoma is extremely rare. The jejunum (32 %), ileum (25,2 %), and duodenum (12,6 %) represent the most common sites [3]. LMSs arise in the submucosa and bulge out the mucosa and serosa [4]. In the early stages, those tumors remain asymptomatic. They are not visualized by usual upper and lower endoscopies. Thus, there are delays in diagnosis making the prognosis worse. Herein, we present a case of a 65-year-old patient having ileal leiomyosarcoma, having chronic anemia, successfully treated with surgical resection.
This work has been reported in line with the SCARE 2020 criteria [5].
2. Presentation of a case
A 65-year-old patient, with no comorbidities, presented with chronic and paroxysmal abdominal pain. He had no other digestive signs. He had no familial neoplasm history.
On examination, he was pale and afebrile. He had periumbilical tenderness. No palpable mass in the abdomen was noted. Rectal touch was normal.
Laboratory tests revealed white blood cells 9.3 × 103/μL, platelets 315 × 103/μL, hypochromic microcytic anemia with Hemoglobin 8.5 g/dL.
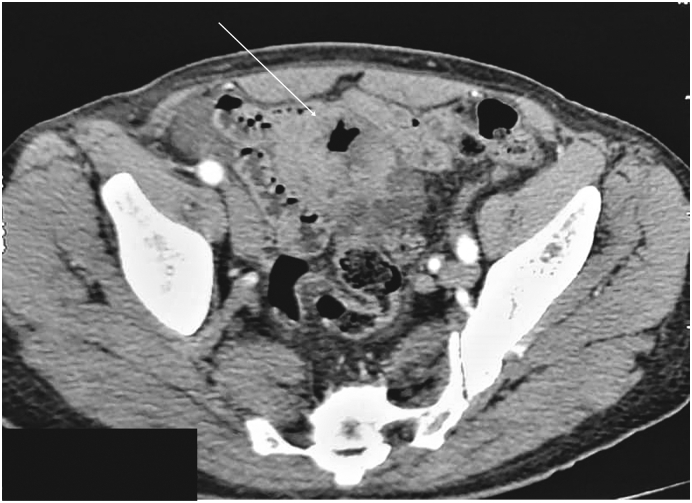
Upper endoscopy and colonoscopy showed no abnormalities. Thoracoabdominal computed tomography (CT) revealed an ileal lobulated, heterogeneously enhancing solid mass measuring 65 × 61 × 54 mm, with no regional lymph nodes or free fluid. There were no metastases (Fig. 1).
Fig. 1.
CT scan in the axial plane showing lobulated ileal mass.
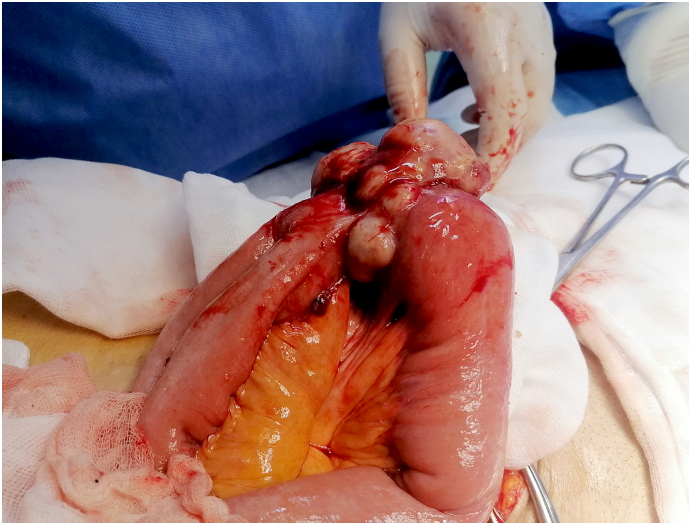
The diagnosis of GIST was considered. Laparotomy was planned and performed by a 9-year-experience surgeon. There were no peritoneal, and hepatic metastases. There were no ascites. Findings showed a lobulated ileal mass of 6 cm invading two ileal loops (Fig. 2). We performed an enlarged resection carrying the two ileal loops of the ileum with end-to-end anastomosis. The resected specimen measured 80 cm. The postoperative course was uneventful.
Fig. 2.
Intraoperative view.
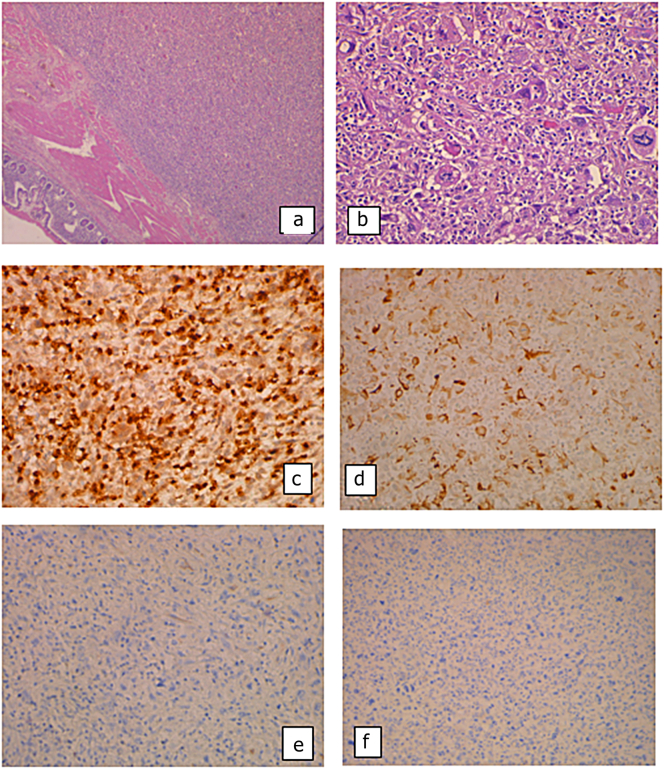
In the histological examination, we found an ileal location of a spindle-cell malignant mesenchymal proliferation. The tumor cells are provided with very atypical, pleomorphic, and mitotic nuclei. In the immunohistochemistry, they express H-Caldesmon and Desmin and were negative for CD117 and Dog1 (Fig. 3).
Fig. 3.
(a) Ileal location of a spindle-cell malignant mesenchymal proliferation (HE × 25); (b) the tumor cells are provided with very atypical, pleomorphic, and mitotic nuclei (HE × 100); they express H-Caldesmon (IHC × 100) (c) and Desmin (d) (IHC × 100) and were negative for CD117 (e) and Dog1 (f) (IHC × 100).
The diagnosis of high-grade leiomyosarcoma was confirmed. In the multidisciplinary meeting, we decided not to perform adjuvant chemotherapy.
No relapse of the disease was noted during the 4-month follow-up.
3. Clinical discussion
We reported a rare case of a locally advanced ileal LMS treated successfully with surgical resection. The main weaknesses of our work are the short follow-up period and the performance of a CT scan instead of a CT colonography scan.
LMS most commonly originates in the retroperitoneal space, uterus, vascular wall, and soft tissues. Ileal LMS is a rare tumor originating from the smooth muscle cells within the muscularis mucosa or muscularis propria [3].
The highest incidence of LMS is observed in the sixth decade. There is a small preponderance of males [3].
The symptoms are not specific. Patients can present chronic abdominal pain, chronic anemia, and recurrent melena [6].
Upper endoscopy and colonoscopy are not performant in assessing the diagnosis. Thus, those tumors are discovered at advanced stages as in our case.
CT colonography (CTC) and magnetic resonance enterography (MRE) represent good options to aid the diagnosis [7]. CTC is more accessible and provides a better resolution [8]. CTC acquires better soft-tissue contrast. It is also more performant to detect small mucosal lesions. It has the advantage of avoiding radiation. Wireless capsule endoscopy (WCE) represents another diagnostic option. It is very accurate in detecting smaller superficial lesions [9], [10]. Enteroscopy is another endoscopic option. This method, however, requires substantial experience and can only be performed in expert centers.
Rockey DC et al. found that WCE is more performant in diagnosing smaller lesions [11].
Those endoscopic methods have the disadvantage of only evaluating the intestinal lumen. Thus, extraluminal growth and metastases can't be correctly evaluated.
Despite advances in imaging, determining the difference between benign and malignant tumors before surgery remains extremely challenging [12]. Therefore, the definitive diagnosis can only be confirmed after histological examination and IHC, the case presented here being a prime example.
Histologically, LMS often has a comparable morphological appearance to GISTs. It presents as a smooth muscle cell malignant neoplasm with high mitotic counts, necrosis, and cytological atypia [13]. LMS is usually composed of elongated cells with abundant cytoplasm [14], [15], [16].
IHC is essential to differentiate those tumors. LMS are distinguished from GISTs by the negativity of CD 117, DOG-1, and CD 34 and the positivity of SMA and Desmin [6], [17].
The Tumor-Node-Metastasis classification for soft tissue sarcomas is used to stage small bowel LMS [17].
In a review of 321 different localizations of LMS, Blanchard DK et al. found that 36 % of the patients had metastases: 65 % in the liver, 15 % in other gastrointestinal localizations, and 4 % in the lungs [18].
Surgery is the only curative treatment. There are no data available on the effect of radiotherapy in small bowel LMS. Those tumors have a low response rate to chemotherapy [19]. For metastatic tumors, metastasectomy, if feasible, should always be considered [20].
Knowing that those tumors are discovered at advanced stages, the prognosis is poor. Though it's more favorable than small bowel adenocarcinomas [12].
Tumor size and histological grade represent independent prognostic factors for disease-specific survival. The five-year survival ranges from 10 to 48 % [3]. We highlight the importance of performing CTC and MRE in patients with chronic abdominal pain, weight loss, or recurrent melena to diagnose intestinal LMS at earlier stages.
In summary, we reported a case of ileal LMS in a 65-year-old patient, presenting anemia and chronic abdominal pain. Ileal resection was performed. No relapses were registered during the 4-month follow-up. Further studies with bigger sample sizes and systematic reviews can aid in proposing clear guidelines for those rare tumors.
4. Conclusion
Ileal LMS is a rare tumor originating from the smooth muscle cells. It has a comparable morphological appearance to GISTs. Immunohistochemistry is essential to confirm the diagnosis. The prognosis is poor. Chemotherapy is not efficient. Surgery is the only curative treatment and should be proposed whenever the tumor is resectable.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not required.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Mahdi Bouassida and Hazem Beji did the conception and design of the work, the data collection, and the data analysis and interpretation.
Mohamed Fadhel Chtourou and Saloua Nechi did the critical revision of the article.
Abir Chaabene and Hassen Touinsi did the final approval of the version to be published.
Registration of research studies
Not applicable.
Guarantor
Dr. Mahdi Bouassida.
Dr. Hazem Beji.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
No conflicts of interest.
References
- 1.Mazzotta E., Lauricella S., Carannante F., Mascianà G., Caricato M., Capolupo G.T. Ileo-ileal intussusception caused by small bowel leiomyosarcoma: A rare case report. Int J Surg Case Rep. 2020;72:52–55. doi: 10.1016/j.ijscr.2020.05.049. Epub 2020 May 29. PMID: 32506030; PMCID: PMC7283087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilimoria K.Y., Bentrem D.J., Wayne J.D., et al. Small bowel cancer in the United States. Ann. Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Arts R., Bosscha K., Ranschaert E., Vogelaar J. Small bowel leiomyosarcoma: a case report and literature review. Turk. J. Gastroenterol. 2012 Aug;23(4):381–384. doi: 10.4318/tjg.2012.0406. PMID: 22965511. [DOI] [PubMed] [Google Scholar]
- 4.Sato T., Akahoshi K., Tomoeda N., Kinoshita N., Kubokawa M., Yodoe K., Hiraki Y., Oya M., Yamamoto H., Ihara E. Leiomyosarcoma of the stomach treated by endoscopic submucosal dissection. Clin. J. Gastroenterol. 2018 Aug;11(4):291–296. doi: 10.1007/s12328-018-0838-4. Epub 2018 Mar 2 PMID: 29500609. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. International Journal of Surgery. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Coco C., Rizzo G., Manno A., Mattana C., Verbo A. Surgical treatment of small bowel neoplasms. Eur. Rev. Med. Pharmacol. Sci. 2010;14(April (4)):327–333. [PubMed] [Google Scholar]
- 7.Jarman B.T. Small bowel imaging. Surg Clin North Am. 2011;91(February (1)):109–125. doi: 10.1016/j.suc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Fidler J.F., Guimaraes L., Einstein D.M. MR imaging of the small bowel. RadioGraphics. 2009;29:1811–1825. doi: 10.1148/rg.296095507. [DOI] [PubMed] [Google Scholar]
- 9.Triester S.L., Leighton J.A., Leontiadis G.I., et al. A metaanalysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am. J. Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 10.Jabr F.I., Skeik N. A leiomyosarcoma of the small bowels causing obscure gastrointestinal bleeding diagnosed by capsule endoscopy. J Med Liban. 2010;58(October–December (4)):238–240. [PubMed] [Google Scholar]
- 11.Rockey D.C. Occult and obscure gastrointestinal bleeding: causes and clinical management. Nat Rev Gastroenterol Hepatol. 2010;7:265–279. doi: 10.1038/nrgastro.2010.42. [DOI] [PubMed] [Google Scholar]
- 12.Luis J., Ejtehadi F., Howlett D.C., Donnellan I.M. Leiomyosarcoma of the small bowel: Report of a case and review of the literature. Int J Surg Case Rep. 2015;6C:51–54. doi: 10.1016/j.ijscr.2014.11.009. Epub 2020 May 29. PMID: 32506030; PMCID: PMC7283087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzel T., Mech K., Mazurkiewicz M., Dabrowski B., Lech G., Chaber A., Słodkowski M. A very rare case of a small bowel leiomyosarcoma leading to ileocaecal intussusception treated with a laparoscopic resection: a case report and a literature review. World J. Surg. Oncol. 2016;14(February (1)):48. doi: 10.1186/s12957-016-0798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M., Sarlomo-Rikala M., Sobin L.H., Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am. J. Surg. Pathol. 2000;24:211–222. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M., Kopczynski J., Makhlouf H.R., Sarlomo-Rikala M., Gyorffy H., Burke A., et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum. Am. J. Surg. Pathol. 2003;27:625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hilal L., Barada K., Mukherji D., Temraz S., Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med. Oncol. 2016;33:20. doi: 10.1007/s12032-016-0730-3. [DOI] [PubMed] [Google Scholar]
- 17.Edge S.B., Byrd D.R., Compton C.C., et al. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th ed. Springer; New York: 2010. p. 285. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard D.K., Budde J.M., Hatch G.F., 3rd, et al. Tumors of the small intestine. World J. Surg. 2000;24:421–429. doi: 10.1007/s002689910067. [DOI] [PubMed] [Google Scholar]
- 19.Serrano C., George S. Leiomyosarcoma. Hematol. Oncol. Clin. North Am. 2013 Oct;27(5):957–974. doi: 10.1016/j.hoc.2013.07.002. Epub 2013 Aug 26 PMID: 24093170. [DOI] [PubMed] [Google Scholar]
- 20.Gill S.S., Heuman D.M., Mihas A.A. Small intestinal neoplasms. J. Clin. Gastroenterol. 2001;33:267–282. doi: 10.1097/00004836-200110000-00004. [DOI] [PubMed] [Google Scholar]