For over five decades, the allergen provocation test has served as a gold standard model of allergic mechanisms underlying asthma and allergic rhinitis, and more recently has been used to understand the interactions between both airway compartments [1–4]. As an indirect stimulus, allergen challenge allows investigation of subsequent changes in respiratory physiology and inflammatory pathways in sensitised individuals and the effect of interventions [5–8].
Abstract
The allergen provocation test is an established model of allergic airway diseases, including asthma and allergic rhinitis, allowing the study of allergen-induced changes in respiratory physiology and inflammatory mechanisms in sensitised individuals as well as their associations. In the upper airways, allergen challenge is focused on the clinical and pathophysiological sequelae of the early allergic response, and is applied both as a diagnostic tool and in research settings. In contrast, bronchial allergen challenge has almost exclusively served as a research tool in specialised research settings with a focus on the late asthmatic response and the underlying type 2 inflammation. The allergen-induced late asthmatic response is also characterised by prolonged airway narrowing, increased nonspecific airway hyperresponsiveness and features of airway remodelling including the small airways, and hence allows the study of several key mechanisms and features of asthma. In line with these characteristics, allergen challenge has served as a valued tool to study the cross-talk of the upper and lower airways and in proof-of-mechanism studies of drug development. In recent years, several new insights into respiratory phenotypes and endotypes including the involvement of the upper and small airways, innovative biomarker sampling methods and detection techniques, refined lung function testing as well as targeted treatment options further shaped the applicability of the allergen provocation test in precision medicine. These topics, along with descriptions of subject populations and safety, in line with the updated Global Initiative for Asthma 2021 document, will be addressed in this review.
Short abstract
Allergen provocation of the airways identifies novel inflammatory pathways and guides our understanding about efficacy of new therapies. Testing of the upper or lower airways may affect the other compartment but cannot be substituted for each other. https://bit.ly/3FuT1qt
Introduction
For over five decades, the allergen provocation test has served as a gold standard model of allergic mechanisms underlying asthma and allergic rhinitis, and more recently has been used to understand the interactions between both airway compartments [1–4]. As an indirect stimulus, allergen challenge allows investigation of subsequent changes in respiratory physiology and inflammatory pathways in sensitised individuals and the effect of interventions [5–8].
In the upper airways, nasal allergen challenge (NAC) is presently used both as a diagnostic tool and for research purposes with a focus on the early allergic response, symptom scores and the release of acute pro-inflammatory mediators [5, 6]. In contrast, in the lower airways, while occupational agents are well established as diagnostic and research tools for occupational asthma, challenge with allergen extracts has almost exclusively been used in specialised research settings, focusing on the sequelae associated with the late asthmatic response (LAR), i.e. the underlying type 2 (T2) inflammation, prolonged airway narrowing and increased nonallergic airway hyperresponsiveness (AHR): all pathognomonic features of asthma [7, 9–11].
As an extension of the European Respiratory Society/American Thoracic Society Task Force position document on indirect challenges [12] and our previous expert review [7], this review discusses additional frequently used, standardised allergen challenge methods of the airways, and updates existing methodologies according to recent insights about the development of T2 biologics for precision medicine of asthma and allergy, as well as study populations and safety aspects, in line with the Global Initiative for Asthma (GINA) 2021 document [13].
Bronchoprovocation and drug development
Provoked models of bronchoconstriction and airway inflammation have significantly contributed to our understanding of asthma pathophysiology and the development of targeted drugs [14]. T2 asthma has recently been recognised as a relevant asthma endotype [15]. Through characterisation and standardisation of clinical trial end-points such as the early asthmatic response (EAR), LAR, allergen provocative concentration/dose causing 20% fall (PC20/PD20) in forced expiratory volume in 1 s (FEV1), shift in allergen-induced PC20/PD20 methacholine/histamine, and sputum eosinophils and derived biomarkers, allergen bronchoprovocation has been used to assess the potential utility and clinical efficacy of T2 biologics with an overall good predictive value [16–22]. Indeed, a wide variety of therapeutics within many different inflammatory pathways have been tested, including those with broad anti-inflammatory properties, those targeting T2 pathways, bronchodilators and other more specific targets (supplementary material). Through comprehensive testing, efficacy data applicable to other disease indications can be generated (figure 1) [23, 24].
FIGURE 1.
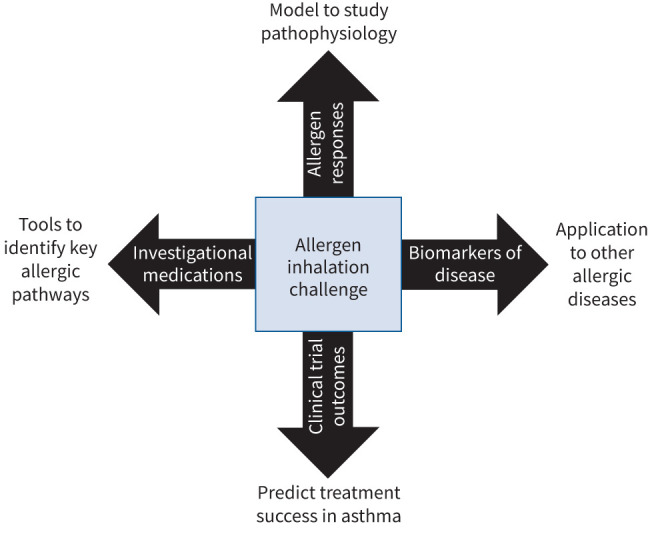
Applications of allergen bronchoprovocation.
Allergen bronchoprovocation and asthma mechanisms
More recent investigations have further characterised the inflammatory response following inhaled allergen and included whole-blood RNA biomarker panels that are predictive of the LAR [25]. Identification of different inflammatory phenotypes in sputum during the LAR [26–28] and examination of the kinetics of T2 inflammatory cytokines and chemokines in sputum [11] together with the observed increase in effector cells such as dendritic cells in bone marrow and trafficking of these cells into the airway [29] should help to further elucidate the cellular and molecular mechanisms underlying allergen-induced airway responses and promote the development of targeted treatments for asthma.
T2 biomarker assessments and allergen bronchoprovocation
During bronchoprovocation, biomarkers can be used to readout various mechanisms involved in the allergic airway response. The allergen-induced airway inflammation can be explored either locally via upper airway samplings or lower airway samplings including exhaled air, induced sputum or bronchoscopy, or systemically in peripheral blood [10, 30, 31]. Examination of the lipid metabolism pathway demonstrates enrichment of significant genes and metabolites post-challenge [32], with leukotrienes and prostaglandin D2 increased [33, 34]. In the case of these lipid mediators, urine sampling has been particularly useful [35–37]. Primarily, T2 inflammatory biomarkers have been shown to be involved in the allergic response, with a main focus on eosinophils and eosinophil-related biomarkers and cytokines, e.g. exhaled nitric oxide fraction (FENO), alarmins (interleukin (IL)-25, IL-33, thymic stromal lymphopoietin), IL-4, IL-5 and IL-13 [38–41] as well as eosinophil activation markers [42, 43]. Furthermore, neutrophils [43], basophils and mast cells [44] are altered in response to allergen challenges. More recently, the proportions of other cell types in sputum have been shown to alter following allergen challenge, highlighting the dynamic nature of inflammatory subtypes in asthma [26, 28, 29, 45–48]. In addition, changes in RNA profiles of T2 inflammation have been defined in sputum cells [27, 45, 49]. One should be aware that many of the aforementioned biomarkers are responsive to corticosteroid treatment, which lowers their respective levels [7, 50].
Non-T2 pathways and allergen bronchoprovocation
The airway response to inhaled allergen is mainly described as a T2 response but non-T2 inflammation is also described. Non-T2 asthma may have different underlying mechanisms and presently there is no consensus on signature biomarkers, but a main hallmark is neutrophilic rather than eosinophilic inflammation [51–53], with increased airway neutrophil numbers and their pro-inflammatory products neutrophil elastase and myeloperoxidase observed after allergen bronchoprovocation [54]. Several other cell types and cytokines, including T-helper (Th)1, Th17, T-regulatory (Treg) cells, and IL-1β, IL-6, IL-8, IL-17, interferon-γ, tumour necrosis factor-α and neutrophil extracellular traps, are believed to contribute to mechanisms of non-T2 pathobiology [53]. Allergen bronchoprovocation has documented the activation of a subset of circulating mature neutrophils [55], an increase in Th17 cells and IL-17A in blood [56], an increase in blood Th17/Treg cell ratio specifically in dual responders [57], and a parallel diminished percentage of sputum Treg cells [58] and an increase in IL-6 [59] following allergen bronchoprovocation. Targeted treatment using a single dose of tocilizumab (an IL-6 receptor blocker), however, was not able to prevent allergen-induced bronchoconstriction [60]. Markers of remodelling have also been detected post-allergen challenge, including increased myofibroblasts in endobronchial biopsies [61] and metalloproteinase-9 in sputum [62].
The innate immune system may play an important role in the development of allergen-induced airway inflammation with a number of potential signalling pathways. The epithelial cell-derived alarmins are upregulated after allergen challenge [63, 64], and are widely recognised as important upstream orchestrators of both T1 and T2 inflammation. Epithelial cell triggers can also drive inflammation through innate receptors such as protease-activated receptors (PAR2) and Toll-like receptors including TLR4 and TLR7; intranasal administration of a TLR7 antagonist significantly reduced allergen-induced LAR, demonstrating the potential of these pathways to support the development of asthmatic responses [65, 66]. Bronchial vasculature may be activated after an allergen challenge via increased lung homing of endothelial progenitor cells [67]. Apart from the inflammatory pathways, it has also been suggested that airway obstruction could be driven by structural changes within the airways, such as airway smooth muscle hypertrophy [68].
Microbiome
The human microbiome is an exciting new area of study. Defined as a characteristic microbial community living in a normally well-defined environment with distinct physio-chemical properties, the microbiome interacts with the host immune system and may drive future development of inflammatory and allergic disorders [69, 70]. The pulmonary microbiome consists of microorganisms such as viruses, bacteria and fungi. Although we have some insight about which bacteria are present in health and disease, we are only beginning to learn how the microbiota and disbalance interact with the local and systemic immune system and responses to allergen [71, 72]. Inhaled corticosteroids (ICSs) are known to suppress the microorganisms of the lungs [73], potentially leading to an exponential growth of other organisms resulting in pathology on a macroscopic scale. Allergic diseases show a dramatic increase in prevalence in recent decades, with influencing factors before and in the first years of life, such as breastfeeding, (air) pollution and exposure to (domestic) animals considered to be major contributors. These factors in turn might influence epigenetic mechanisms and thereby increase or decrease the chance of (pulmonary) allergy and altered immune response. Studying interactions of the microbiome with controlled allergen challenges might help in finding medications to stabilise and create a microbial homeostasis of the respiratory system.
Methodology of allergen bronchoprovocation
Presently, there are three standardised protocols with inhaled allergen [7]. Two protocols use (relatively) high-dose allergen, applying the incremental or titrated step-up method or the single-bolus method. Despite a different approach to achieve EAR, both methods yield comparable allergen-induced airway responses [74–76]. The third protocol is a repeat low-dose allergen bronchoprovocation. All methods are extensively described in our previous expert review [7].
High-dose allergen bronchoprovocation
Procedure
Titrated, relatively high-dose, allergen inhalation tests date back to the 1950s [1, 2, 77, 78] when exact methodological details were frequently lacking. Current standardised methods are based on those originally described by Hargreave and coworkers in 1974 [79], extensively outlined in our previous expert review [7]. Allergen bronchoprovocation is conducted for research purposes and can occasionally lead to severe airflow obstruction and/or systemic allergic response [79], therefore qualified staff experienced in allergen challenge and a safe starting concentration are required. In the titrated protocol, the starting concentration should be at least three doubling concentrations below the allergen predicted PC20 calculated using the skin test (with the same allergen) end-point and PC20/PD20 methacholine/histamine [80] or based on the PC20/PD20 methacholine/histamine only (modified formula) [81, 82]. Incremental allergen concentrations are inhaled at 12–15 min intervals until the EAR has been reached [79]. For within-subject repeatability, any reliable jet [7] or vibrating mesh [83] nebuliser and reproducible inhalation method is acceptable.
In the single-bolus protocol, a titrated allergen challenge is initially performed to identify the cumulative dose required to produce an EAR [74]. Repeat challenges are conducted using this single (cumulative) high dose of allergen by counted deep breath inhalations from a dosimeter. Although shortening the procedure by ∼30 min, inhalation of a single high dose of allergen has been reported to produce a greater EAR than the same (cumulative) dose of allergen administered incrementally over 20 to ≥30 min [74]. Another study did not find such a difference with respect to drop in lung function but showed increased release of cysteinyl leukotrienes in cumulative as opposed to single-dose challenge [84].
Airway response measurements
Airway response is measured by FEV1 every 10–15 min for the first hour post-allergen challenge and every 30 min for the second hour to capture the EAR, then hourly (and in case of a steep decline in FEV1, more frequently) up to ≥7 h to capture the LAR. The EAR can be defined as the maximum percentage fall in FEV1 during the first hour or the area under the curve (AUC) from 0 to 2 h (or 0 to 3 h) and the LAR as the maximum percentage fall over 3–7 h (or >7 h) or the 3–7 h (or >7 h) AUC. Changes in nonallergic AHR are assessed by measuring the shift in PC20/PD20 methacholine/histamine 24 h after allergen challenge. Isolated EAR investigations can also be assessed and quantitated using the allergen PC20/PD20 dose shift [81, 83, 85]. More sensitive lung function measurements, including impulse oscillometry (IOS), body plethysmography and carbon monoxide diffusion capacity, show more extensive involvement of small airways in dual responders (EAR+LAR) compared with isolated EAR responders (EAR-only) [86, 87]. IOS measurements may help to further differentiate responders based on baseline physiology and to detect initial airway response to allergen (or other stimuli) prior to changes in spirometry, thus supporting safety during the procedure.
Repeat testing
The EAR and LAR are reproducible outcomes when the test is properly standardised [9, 88, 89]. For studies addressing the LAR and sequelae, repeat tests should be performed after a washout of at least 2 weeks and strive to use the same dose of allergen administered in the same manner whenever possible. Occasionally an earlier-reached EAR can hinder this approach. To ensure safety and data integrity, subjects should meet asthma stability criteria (symptoms, lung function and PC20/PD20 methacholine/histamine) pre-challenge [7]. For studies addressing only the EAR PC20/PD20, the appropriate allergen dose should be administered [81] to quantitate the treatment effect. In such studies, an LAR can be prevented by administration of a single moderate-to-high dose of ICS after the EAR [90]. While the EAR method has several logistical advantages such as less time, fewer eligibility criteria and a greater ability to discriminate large changes, it fails to capture the clinically more relevant LAR and sequelae.
Safety
Incremental allergen inhalations inducing a gradual decline in FEV1 during the EAR are preferred. Irrespective of the inhalation method, allergen should only be administered in asthmatic subjects where pre-challenge clinical stability is confirmed [7]. Although linear in theory due to geometrical dose increments, the allergen dose–response curve steepens as a 15–20% fall in FEV1 is approached. The possibility of excessive airway narrowing (i.e. a decrease in FEV1 ≥50%) underscores the requirement for an experienced clinician and medications to treat severe bronchoconstriction or anaphylaxis (bronchodilators, corticosteroids, antihistamines, epinephrine, oxygen and cardiopulmonary resuscitation equipment) to be immediately available. To minimise exposure of laboratory personnel to aerosolised allergen, good ventilation, exhaust hoods and other protective measures are required [7].
Selection of subjects
Inhaled allergen bronchoprovocation tests have mainly been conducted in subjects with mild, clinically stable asthma in specialised research settings [7] for the purpose of studying mechanisms of allergic airway disease [10] and in the context of clinical trials to support understanding of precision medicine [38]. Current research allergen bronchoprovocation methodologies are not recommended as clinical tests in asthmatic patients, except in rare cases where guidelines of some countries permit these for diagnostic purposes in occupational settings. Neither a safe starting allergen dose [91] nor the safety of allergen-induced bronchoconstriction have been established in patients with more severe asthma, considering such patients would require withholding of their medications with the risk of losing asthma control or have effects of these medications confound interpretation of the test.
Inhalation of a provocative allergen in subjects with mild allergic asthma, while generally safe and well tolerated, can lead to significant acute bronchoconstriction, prolonged airway responses (i.e. a clinically relevant exacerbation) and, in rare cases, anaphylaxis [92]. Careful tracking of spirometry and follow-up with availability of medical assistance after the test needs to be applied in all settings. Safety begins with subject selection: individuals with (very) mild well-controlled asthma requiring infrequent use of rescue medication and with no or relatively little resting airflow limitation, i.e. baseline FEV1 ≥70% predicted. Since asthma medications interfere with allergen challenge end-points (table 1), a key inclusion criterion is the requirement for mild allergic asthma with infrequent use of a short-acting bronchodilator (short-acting β-agonist (SABA)) only. However, as per the revised GINA 2021 document, individuals who experience infrequent asthma symptoms are now recommended “as-needed” low-dose ICS associated with rescue medication (i.e. bronchodilators) [13]. Subjects should, however, be able to maintain good asthma control and optimal lung function despite fulfilling washout requirements indicated in table 1 [7, 13]. Washout periods are consistent with at least five half-lives of the drug plus an estimate of the duration of drug effect. It is unethical and unsafe to discontinue maintenance medications in more severe asthma to meet these washout requirements for allergen bronchoprovocation.
TABLE 1.
Recommended washout periods for medications and other general measures before and during allergen inhalation challenge testing in clinical research settings
| Recommended washout or avoidance period before start of study | Recommended washout or avoidance period during the study | |
| Medication | ||
| Immunosuppressants (e.g. methotrexate, dactinomycin, mercaptopurine) | 4 weeks washout | Prohibited during study |
| Systemic corticosteroids (intravenous/intramuscular/oral) | 12 weeks washout; up to one burst of OCS in the previous 5 years | Prohibited during study |
| Inhaled corticosteroids | 4–6 weeks washout from regular daily dosing | Permitted if used acutely to rescue after allergen challenge or to stabilise asthma during the study if needed, provided dosing is acute (3–5 days maximum) and 1 week washout is applied before subsequent study visits |
| Nasal corticosteroids | 4 weeks washout from seasonal dosing | Permitted if used at a constant dose throughout the study and subject meets all eligibility criteria while dosing |
| Dermal corticosteroids | 4 weeks washout from maintenance therapy on large body surfaces (larger than ∼10 cm2) | Permitted if infrequent use on small body surface |
| Allergen immunotherapy for challenging allergen | Full exclusion | Prohibited during study |
| Allergen immunotherapy for nonchallenging allergen | 12–16 weeks washout | Permitted if ongoing throughout the study and at least 12–16 weeks on stable oral or subcutaneous dose |
| SABA (e.g. salbutamol, albuterol, terbutaline) | No washout required | Permitted with ≥6 h washout before allergen challenge |
| SAMA (e.g. ipratropium bromide, oxitropium bromide) | No washout required | Permitted with ≥12 h washout before allergen challenge |
| LABA (e.g. salmeterol, formoterol) | 2 weeks washout | Prohibited during study |
| Ultra-LABA (e.g. abediterol, indacaterol, olodaterol, vilanterol) | 2 weeks washout | Prohibited during study |
| LAMA (e.g. aclidinium bromide, tiotropium bromide, glycopyrronium bromide, umeclidinium bromide) | 2–3 weeks washout | Prohibited during study |
| Leukotriene modulators including LTRAs (e.g. montelukast, zafirlukast, pranlukast, zileuton) | 2 weeks washout | Prohibited during study |
| PDE inhibitors (e.g. Daxas, ensifentrine) | 2 weeks washout | Prohibited during study |
| Xanthines (e.g. caffeine, theobromine) | No washout required | Permitted with 4–12 h washout before allergen challenge |
| Cromoglycate/nedocromil | 2–4 weeks washout | Prohibited during study |
| Theophylline | 4 weeks washout | Prohibited during study |
| Oral anti-inflammatories: salicylates (e.g. acetylsalicylic acid), acetaminophen, other NSAIDs (e.g. ibuprofen, naproxen, diclofenac) | No washout required | Permitted with 7 days washout before allergen challenge |
| Short-acting antihistamines | No washout required | Permitted with 3 days washout before allergen challenge |
| Intermediate-acting antihistamines | No washout required | Permitted with 4 days washout before allergen challenge |
| Biologics (registered or experimental) | 3–6 months washout or five half-lives | Prohibited during study |
| Living vaccines | 3 months washout | Prohibited during study |
| Other vaccines (e.g. vector, mRNA/DNA vaccines) | 4 weeks washout | Prohibited during study |
| Chemotherapy | Full exclusion | Prohibited during study |
| Investigational medications | 3–6 months washout or five half-lives | Prohibited during study |
| Interfering factor | ||
| Major surgery | 3–6 months washout | Subjects with planned major surgery should not be enrolled on the study |
| Lower respiratory tract viral infection; upper respiratory tract common cold | 6 weeks; 3 weeks washout | Allergen challenge should not be conducted within 6 weeks of a lower respiratory tract infection |
| Strenuous exercise | 72 h delay | No strenuous exercise (e.g. marathon) during the study or 1–2 weeks after an allergen challenge test, depending on recovery |
| Alcohol-containing beverages | 48–72 h washout | Prohibited during study |
| Party drugs | 72 h washout | Prohibited during study |
| Hard drugs (e.g. heroin, cocaine, amphetamine, LSD, ecstasy) | Full exclusion for current or history of use | Prohibited during study |
| Increased allergen exposure (e.g. contact with animals, pollen season, moving houses) | 3–4 weeks washout | Prohibited during study |
| Increased environmental triggers (e.g. cigarette smoke, pollution) | 72 h washout | Prohibited during study |
OCS: oral corticosteroid; SABA: short-acting β-agonist; SAMA: short-acting muscarinic antagonist; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; LTRA: leukotriene receptor antagonist; PDE: phosphodiesterase; NSAID: non-steroidal anti-inflammatory drug; LSD: lysergic acid diethylamide.
Medication washout, withhold and rescue
Clinical trials utilising allergen bronchoprovocation tests are often complex, long lasting and require multiple challenges with adequate washout periods (table 1). The timeframe between two subsequent allergen challenges is 2–3 weeks minimum, and studies typically span the cold and flu season to avoid the pollen seasons in pollen-allergic participants. Lengthy study durations and potential viral exposure could increase the risk of exacerbation, and ICS may be required for subject safety and wellbeing. Situations where an exacerbation with or without the use of ICS occurring within the required washout period should be dealt with on a case-by-case basis with the goal of postponing a scheduled visit until the appropriate washout can be achieved. Subjects requiring oral corticosteroid (OCS) rescue during a bronchoprovocation study should be withdrawn with the focus on regaining asthma control.
After allergen bronchoprovocation, a SABA is usually administered to reverse existing bronchoconstriction such that the FEV1 returns to within 10% of the pre-challenge value. In the case of a partial reversal, a short-acting muscarinic antagonist (SAMA) and/or low-dose ICS may be added (e.g. budesonide 200 µg, 1–2 times during the next 2–3 days post-challenge). Prior to leaving the unit, subjects should be provided with appropriate rescue (e.g. SABA±SAMA) and controller (e.g. ICS) medications with instructions to use if required, and a 24 h emergency contact, keeping in mind use of these medications could require subsequent tests to be aborted if withhold periods (i.e. continuation criteria) cannot be met.
Low-dose repeated allergen bronchoprovocation
Procedure
Repeated low-dose allergen bronchoprovocation has been introduced as a standardised method to mimic natural allergen exposure [93, 94]. Small doses of allergen titrated to cause minimal bronchoconstriction are inhaled once daily over 1–2 weeks [95–101]. The allergen dose is selected from an initial incremental dose challenge (usually as the provocative dose causing an early drop in FEV1 of 5% (PD5)) [95, 98, 100, 101]. Methacholine or histamine challenge to measure airway responsiveness and induced sputum collection are performed before and after the low-dose challenge period. Spirometry recordings as well as FENO measurements are performed daily, and subjects record home monitoring of lung function, asthma symptom scores and short-acting β2-agonist usage. A 2-week washout is recommended between subsequent challenge periods [95, 102]. The procedure generates a distinct and consistent increase in nonspecific AHR [93, 96, 97, 99, 102, 103], as well as increases in airway eosinophils [95, 96, 99, 100, 102, 103] and FENO [99–102] that are more pronounced than after a single high-dose allergen bronchoprovocation inducing a dual asthmatic response [95, 100–103]. Notably, the increase in AHR occurs in the relative absence of asthma symptoms.
Implementation
Because this bronchoprovocation model induces robust readouts such as increased AHR and many signs of T2 inflammation with minimal bronchoconstriction and symptoms, it has received interest as a valuable tool to investigate early events of importance for the development of symptomatic and possibly persistent asthma. In addition, this model has been useful in evaluating drug effects in mild asthma, particularly for ICS [99, 102, 103]. However, it is not as widely used as high-dose bronchoprovocation, most likely because of the greater number of visits required for the subjects. In the current search to understand how different biological treatments affect asthmatic airway inflammation and related sequelae, it might be of value to use repeated low-dose allergen bronchoprovocation again. The emergence of sensitive omics technologies may offer new opportunities to define mechanisms by measurements in blood, sputum, exhaled breath and urine during the development of the response [104].
Segmental allergen bronchoprovocation
Procedure
Segmental allergen challenge (SAC) is an invasive, bronchoscopic research procedure which allows direct investigation of the inflammatory response following local allergen instillation [105]. Compared with inhaled bronchoprovocation, SAC allows a more precise dosing with one or more agents, and the procedure has been shown to be safe and easy to control [106]. In both mild and moderate asthma, SAC allows reproducible readouts as well as simultaneous control lavage within individual subjects [107]. A drawback is the requirement of two bronchoscopic procedures: one for allergen instillation and one ∼24 h post-challenge for the evaluation of the inflammatory response.
Implementation
SAC allows study of direct effects of allergen on the inflammatory airway response within lower lung segments. In addition to biopsy, lavage, wash or brushings for cellular and molecular components, SAC allows visualisation of the airway response with imaging techniques which may substitute the second bronchoscopy [108, 109]. In contrast to inhaled bronchoprovocation, the overall physiological response in the airways may be suppressed as a result of procedural medications and, given the localised administration of allergen, may not evoke a full airway response, which precludes studying the relationship between the allergen-driven inflammation and airway physiology.
Occupational bronchoprovocation
Specific inhalation challenges (SICs) with occupational agents are well established as useful diagnostic and research tools for occupational asthma [110] and hypersensitivity pneumonitis [111]. The main purpose for which occupational challenges are being used is to establish an aetiological diagnosis in occupational asthma and hypersensitivity pneumonitis, as well as to investigate the pathogenic mechanisms underlying these diseases.
Originally, SIC was an empirical approach in which a patient was asked to reproduce his/her working environment [112]. Currently, SIC is a standardised test, in which the respiratory effects of an agent present in the workplace are assessed ideally under controlled conditions, including the generation of a low and stable concentration of the agent [113]. SIC is considered the reference standard test to confirm occupational asthma and it is essential to include the assessment of airway inflammation in the procedure [113, 114]. Exposure to occupational agents can be produced in various ways, depending on the physical state of the agent suspected of causing occupational asthma [113], and the method of bronchoprovocation is quite different for high-molecular versus low-molecular agents, particularly for safety reasons as the latter can induce an isolated late response. A 2014 European Respiratory Society Task Force consensus statement on SIC in the diagnosis of occupational asthma provides practical recommendations and reviews the interpretation and limitations of these tests [115].
Nasal allergen challenge
Standardisation of NAC
NAC is a simple and safe method used both as a research tool to study allergen-induced pathophysiology and drug interventions in allergic individuals [5, 30], and as a diagnostic tool in clinical practice [116, 117]. Topical application of increasing doses of an allergen extract or compound in solution to the nasal mucosa of sensitised individuals induces an IgE-mediated immune response, which rapidly evokes key symptoms of allergic rhinitis including itching, sneezing, nasal congestion and/or rhinorrhoea. The occurrence and severity of symptoms and underlying mechanisms can be captured by standardised composite symptom scores and nasal patency assessments (table 2).
TABLE 2.
Clinical assessments of nasal allergen challenge (NAC) testing
| Method | Notes | References |
| Symptom scores | Lebel composite symptom score/TNSS±ocular symptoms; typically, four domains, each scored 0–3: itch, sneezing, running/secretions, blockage; total 0–12 | [118, 119] |
| Visual analogue scale, 0–10 cm Likert scale, individual symptoms (blockage, itch, running/secretions, sneezing) or overall score | ||
| Scored by the participant/patient; simple to use, but subjective (except for sneezes if these are counted by the assessor) | ||
| Sneezing is primarily a feature of the early-phase response, so this tends to skew the score upwards during the 5–15 min immediately post-challenge | ||
| Relatively blunt tool for capturing late-phase responses where blockage alone is often the predominant symptom | ||
| PNIF (L·min−1) | In-Check nasal inspiratory flow meter; scale 30–370 L·min−1 (Clement Clarke, Harlow, UK) in combination with a well-fitting anaesthetic face mask | [120–126] |
| Standardised normal range | ||
| Good intraindividual reproducibility and response to NAC and to decongestants | ||
| Cheap, quick and easy measurement, but user dependent | ||
| Acoustic rhinometry | Sound waves are passed into the nostril through a closed tube; they reflect off the nasal passage walls and are then sampled by a microphone and mathematically converted into a graph of the cross-sectional area of the nasal passage against the distance into the nose from the nares | [127] |
| Good for demonstrating cross-sectional area (a surrogate for obstruction) of the anterior portion of the nose | ||
| Responsive to NAC and decongestant use | ||
| Technical and expensive compared with PNIF with a limitation to the anterior portion of the nose, but user independent | ||
| Rhinomanometry | Anterior (or posterior) recording of pressure–flow relationship using one or both nostrils during active breathing cycle (or breath holding if passive rather than active) | [127, 128] |
| Response to nasal challenge and to decongestant use | ||
| Technically demanding method requiring expensive equipment compared with PNIF, difficult to standardise, but user independent |
TNSS: total nasal symptom score; PNIF: peak nasal inspiratory flow.
Ocular symptoms (irritation, itching and tearing) are common during the acute phase [129], while a more generalised response (e.g. exanthema) is rare. Pharyngeal symptoms (itching and swelling) can usually be avoided by applying allergen to the nose after the subject has breathed in. In line with the bidirectional cross-talk between both airway compartments, one report suggests NAC may induce both an EAR and/or LAR in subjects with pre-existing AHR [130]. Hence, in these individuals, lung function measurements and therapeutic measures should be considered for safety reasons. Asthma medication can usually be continued, especially if NAC is used for upper airway diagnostic purposes.
While most of the acute-phase symptoms subside within ∼15–30 min, nasal congestion may persist for several hours post-NAC. Prolonged congestion serves as a clinical marker of the local late-phase response, and is associated with both upper and lower airway inflammation and AHR. Analytes from the upper airways can be collected using exhalates, nasal lavages, mucosal brushings or curettage, absorbent strips and sponges. For RNA-based analysis, apart from biopsies, minimally invasive scrapings can be used (table 3).
TABLE 3.
Changes in upper airway and systemic biomarkers after nasal allergen challenge (NAC)
| Sample source | Biomarker | Notes | Reference |
| Exhaled air | nNO | nNO measured by a chemiluminescence analyser (CLD88sp; Ecomedics, Duernten, Switzerland) at baseline and 7 and 24 h post-NAC; large intersubject variability, although good intrasubject performance, showing significant increase at 24 h post-NAC | [131] |
| Nasal lavage with saline | Cytology | Manual or automated counting of eosinophil influx post-NAC; shown to increase post-allergen challenge and differentiate between allergic rhinitic subjects and controls | [5, 132] |
| Nasal lining fluid | Mediators | Direct sampling with absorbent strips or sponges produces concentrated sample of lower volume | [119, 133] |
| Tryptase ng·mL−1 | Highly stable, measured by ImmunoCAP (Thermo Fisher Scientific, Waltham, MA, USA); peaks 5–10 min after nasal challenge | [119, 134] | |
| Histamine ng·mL−1 | Less stable than tryptase; elevated during early-phase response; may be second peak during late-phase response | [135] | |
| Cysteinyl leukotrienes pg·mL−1 | Elevated during early-phase response; level correlated with clinical symptom scores after NAC | [136] | |
| T2 cytokines pg·mL−1 | Measurable by commercial multiplex immunoassay; IL-4, IL-5, IL-13 elevated from 6 h post-grass pollen NAC; inhibited by allergen immunotherapy | [119, 133] | |
| T2 chemokines pg·mL−1 | Eotaxin, RANTES elevated from 4–6 h post-NAC | [128, 137] | |
| Eosinophil activation markers ng·mL−1 | ECP (measurable by ImmunoCAP), MBP, EDN; elevated at 6–8 h post-NAC; also reported at 24 h post-NAC | [138, 139] | |
| Neuropeptides pg·mL−1 | Substance P, CGRP, VIP increased during the early-phase reaction | [140] | |
| Markers of plasma leakage/transudate µg·mL−1 | α2-macroglobulin, albumin elevated in early- and late-phase response to NAC | [141, 142] | |
| Markers of glandular secretion µg·mL−1 | Lactoferrin, lysozyme | [143] | |
| Fluid metabolomics | Differences in metabolic pathways and metabolite levels seen in patients with N-ERD versus controls; yet to be studied in allergic subjects post-NAC | [144] | |
| Nasal cytology brushing | Nasal mucosal gene expression | Nasal scrapes 8 h post-NAC and EEC show similar patterns of altered expression of mucosal biology and transcriptional regulation genes; nasal ACE2 receptor expression reduced after NAC | [145–147] |
| Nasal curettage | Cytology | Nasal curettage 24 h post-NAC show increases in mucosal ILC2 cells | [148] |
| Nasal biopsy | Cytology | Compartmental assessment of NAC-induced inflammatory cells | [149–153] |
| Peripheral blood | Mediators | Serum cytokines (IL-5, IL-13) increased at 8 h post-NAC and EEC | [145] |
| Allergen-specific T-cells | Assayed by flow cytometry; levels increased after both EEC and NAC exposures | [145] | |
| Basophils | IgE-dependent basophil activation in vitro increased following cat NAC | [154] |
nNO: nasal nitric oxide; T2: type 2; IL: interleukin; ECP: eosinophil cationic protein; MBP: major basic protein; EDN: eosinophil-derived neurotoxin; CGRP: calcitonin gene-related peptide; VIP: vasoactive intestinal peptide; N-ERD: nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; EEC: environmental exposure chamber; ACE2: angiotensin-converting enzyme 2; ILC2: innate lymphoid cell type 2.
Drugs which could interfere with challenge outcomes (topical and systemic corticosteroids, antihistamines, decongestants, antileukotrienes and topical cromones) need to be stopped. Specific drug washout periods are in line with those for bronchial challenges (table 1), with a special notice that intranasal corticosteroids and antihistamines must be stopped. Topical and systemic decongestants should be avoided 3 days pre-challenge. Standardisation of allergen concentration and major allergen content (including batch reproducibility) remains difficult, largely due to the use of numerous concentration units across different manufacturers, rather than µg·µL−1 major allergen.
Bilateral NAC
A European Academy of Allergy and Clinical Immunology Task Force convened and reached consensus on the best practice of NAC for clinical use being carried out as bilateral challenge with a volume of 0.1 mL per nostril applied via nasal spray (ideally as two 50 µL actuations) [6]. Single-dose rather than dose-titration challenges were generally recommended. Both subjective (symptom scores or visual analogue scale scores) and objective (peak nasal inspiratory flow, acoustic rhinometry and rhinomanometry) outcomes should be recorded, with defined “strong” thresholds for a positive challenge in either outcome or a combination of both at more modest levels. Clinical indications for NAC are broad, including cases of discordant history and sensitisation results, allergen selection for immunotherapy, and suspected occupational causes [117]. A 4-week post-exposure window was agreed on for testing to seasonal allergens, but difficulty remains concerning acceptable levels of background exposure to perennial allergens when these are being tested.
Exposure rooms
Environmental exposure chambers (EECs) are clinical facilities allowing for controlled exposure of subjects to inhaled substances in an enclosed environment, mimicking natural exposure under tightly regulated conditions [155]. Several different agents have been tested in these chambers, including allergens, chemicals, endotoxins and airborne pollutants. EECs allow several subjects to be studied at the same time, and have been utilised for investigation of the pathophysiology of respiratory allergic diseases and to assess the efficacy of new therapies for allergy and asthma, including allergen-specific immunotherapy [155–158]. There are few EECs available worldwide due to the need for costly and sophisticated equipment, qualified personnel, and validated procedures. However, there is growing interest including from regulatory authorities. Future goals include harmonisation of protocols among centres, standardisation of dosage and reactivity of allergens used, and reduction in costs of these facilities [155, 159].
Readouts and biomarkers of NAC
Although the NAC and EEC should not be used interchangeably [145], both challenge techniques can be complemented with various clinical readouts and biomarker samplings. Readouts include subjective composite symptoms and visual analogue scale scores, typically covering itching, sneezing, nasal congestion and rhinorrhoea [119]. Objective measures include peak nasal inspiratory flow, acoustic rhinometry and rhinomanometry (table 2).
The upper airways allow several (semi-)invasive samplings for biomarkers to study the kinetics of the immunological mechanisms underlying the acute- and late-phase upper airway response to allergen (table 3). Upper airway biomarkers include volatile products such as nasal nitric oxide [131], although reliability may be an issue [160]. Samples for cytology are collected by nasal lavage, biopsy or brushings/scrapings and soluble mediators from nasal lining fluid, following nasal lavage or direct absorption of nasal fluid with filter strips or sponges. Analytes include early-phase mediators such as histamine, tryptase, chymase, prostaglandin D2 and leukotrienes [134–136, 161], and T2 chemokines and cytokines, with IL-5, IL-4 and IL-13 elevated during the late phase [119]. Additionally, activation markers, neuropeptides, markers of vascular leakage and local antibodies have also been successfully quantified from upper airway samplings (table 3). Nasal brushings/scrapings provide material for PCR and transcriptomics [145–147], and biopsy followed by immunohistochemical staining and/or in situ hybridisation has previously demonstrated influx of T2-associated cells and cytokines [162, 163]. Finally, systemic markers including cytokines, antibodies and allergen-specific T-cells have been measured in blood [145].
Due to upregulation of inflammatory mechanisms, in a crossover clinical study, a washout period of at least 2 weeks is required between repeat NAC [145, 164].
United airways
Bidirectional communication between the upper and lower airways
Asthma and rhinitis are common disorders that often coexist, representing respiratory manifestations of the same (systemic) inflammatory process [165], referred to as “one airway disease” within the context of the “united airways concept” [166]. Apart from shared pathophysiology, there is ample evidence of cross-talk between both airway compartments, i.e. challenging one compartment induces inflammatory responses both locally and also within the other compartment [167, 168]. NAC induces upregulation of inflammatory mechanisms and eosinophilia in both the upper and lower airways in nonasthmatic subjects with seasonal allergic rhinitis [3, 169]. Conversely, allergen instillation into a lung segment of nonasthmatic subjects with allergic rhinitis induces both an inflammatory response locally, systemically (in blood) and in the upper airway mucosa [4]. Consequently, international guidelines advocate proactive diagnosis and treatment of both airway compartments to achieve optimal disease control [13, 170]. Despite similarities between both airway compartments and in airway responses, data derived from NAC cannot substitute for data acquired from a bronchoprovocation test.
Allergen extracts
General information
Allergen extracts are marketed for clinical testing of IgE-mediated allergic disease and in many countries package monographs do not include the inhaled route into the lower airways. Hence, studies incorporating allergen bronchoprovocation may require regulatory approval and the associated oversight, including reporting of serious adverse reactions. For regulatory purposes, a serious adverse reaction to allergen bronchoprovocation can be defined as a fall in FEV1 of >50% from baseline, requiring treatment with ICS or OCS and relievers, or anaphylaxis or hospitalisation.
Manufacturing of allergen extracts is regulated by organisations including the Center for Biologics Evaluation and Research and the Committee for Medicinal Products for Human Use. Some extracts are standardised by comparing the potency (concentration) to a reference, while nonstandardised extracts are simply based on protein nitrogen content or weight per volume without establishing a relationship between concentration and biological activity. Both standardised and nonstandardised extracts have become increasingly difficult to source.
Selection of extracts
Allergen extracts should be sourced from a reliable supplier prepared according to Good Manufacturing Practice [171]. The magnitude and characteristics of allergic asthmatic responses may vary according to the type of allergen, and the same allergen extract, batch and dose should be used for an individual subject throughout a clinical trial. Natural exposure to indoors allergens such as house dust mite is thought to prime a more marked airway eosinophilia than seasonal allergens in atopic subjects with or without asthma [98]. High-dose allergen bronchoprovocation was shown to induce a stronger LAR with house dust mite compared with grass pollen [172, 173], yet in a different study, similar inflammatory patterns have been shown [26]. Proteolytic activity in some extracts such as house dust mite may influence airway responses [174].
Conclusions and take-home messages
Allergen exposure to the airways is a common environmental trigger of asthma and allergic rhinitis. Allergen bronchoprovocation is an effective model of asthma for evaluation of the efficacy of various treatment options along the T2 pathway with the ability to link noninvasive biomarkers to measures of physiology. The test is primarily a research tool and the methods described here are not appropriate for common clinical practice. In contrast, bronchoprovocation with occupational agents is a useful diagnostic test that can be conducted for clinical purposes. NAC can be also used as a diagnostic test for allergy and occupational substances, as well as a research tool.
Challenging the upper or lower airway compartment may affect the other compartment, thus NACs may have implications for safety in subjects with inadequately controlled asthma. Despite this united airway response, upper and lower airway tests cannot be substituted for each other. When used in a controlled setting, allergen provocation of the airways identifies novel inflammatory pathways, and guides our understanding about the efficacy of new therapies and precision medicine.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02782-2021.Supplement (292KB, pdf)
Shareable PDF
Footnotes
G.M. Gauvreau declares grants from AstraZeneca, Biohaven, Novartis and Genentech; consulting fees from AstraZeneca, Biohaven, Novartis, Sterna Biologicals and Certior Consulting; and payment or honoraria from AstraZeneca, Genzyme and Genentech, all in the 36 months prior to manuscript submission. B.E. Davis declares no competing interests. G. Scadding declares support from ALK-Abelló (provision of active and placebo medication for the GRASS clinical trial referenced in the article) and the Immune Tolerance Network (NIAID, NIH, USA; sponsor of the GRASS clinical trial) related to the present manuscript; as well as payment for lectures from ALK-Abelló in 2020 and 2021; and support from GlaxoSmithKline for attendance at the 2021 European Academy of Allergy and Clinical Immunology meeting. L-P. Boulet declares research grants for participation in multicentre studies or research projects proposed by the investigator from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron; royalties from UptoDate and Taylor & Francis; lecture fees from AstraZeneca, Covis, GlaxoSmithKline, Novartis, Merck and Sanofi, all in the 36 months prior to manuscript submission; and the following unpaid contributions: Chair of the Global Initiative for Asthma (GINA) Board of Directors; President of the Global Asthma Organisation (Interasma); Member of the Canadian Thoracic Society Respiratory Guidelines Committee; and Laval University Chair on Knowledge Transfer, Prevention and Education in Respiratory and Cardiovascular Health. L. Bjermer declares no competing interests. A. Chaker declares grants or contracts, via Technical University Munich, from EIT Health (European Institute of Technology), BMBF (German Federal Ministry of Education and Research), AstraZeneca, Sanofi-Genzyme, Roche, GlaxoSmithKline and ALK-Abelló; payments or honoraria, via Technical University Munich, from ALK-Abelló, Allergopharma, AstraZeneca, GlaxoSmithKline, Immunotek, Novartis, Regeneron, Sanofi-Genzyme, Leti, Zeller and Bencard; travel reimbursement to attend European Academy of Allergy and Clinical Immunology meetings (2018 and 2021 from the European Academy of Allergy and Clinical Immunology; 2019 from ALK-Abelló); patents, via Technical University Munich, relating to allergen-specific immunotherapy and treatment of SARS-CoV-2 infection; participation, via Technical University Munich, on a data safety monitoring board or advisory board for ALK-Abelló, Allergopharma, AstraZeneca, GlaxoSmithKline, Immunotek, Novartis, Regeneron and Sanofi-Genzyme; and the following unpaid roles: Past Chair of the Allergy, Clinical Immunology and Environmental Medicine Section of the German ENT Society; board member at large of the German Allergy Society (DGAKI); scientific advisor/board member to the German Society for Applied Allergy (AeDA); board member of the Allergen Immuntherapy Interest Group, European Academy of Allergy and Clinical Immunology. D.W. Cockcroft reports the following research grants in the 36 months prior to manuscript submission: research grant Dept of Medicine University of Saskatchewan (Effect of tiotropium on airway response to allergen); Canadian Society of Allergy and Clinical Immunology (The effect of deep inhalation on mannitol responsiveness); AstraZeneca (A phase 2a double blind randomised parallel group placebo controlled multicentre study to evaluate the effect of AZD8154 administered via nebuliser once daily on allergen-induced inflammation in subjects with mild allergic asthma challenged with inhaled allergen); AllerGen NCE (Methacholine challenge comparison of a jet nebuliser and vibrating mesh nebuliser); Novartis (Inhaled CSJ117 in adult asthmatics with mild atopic asthma). B. Dahlén declares payment to their institution in 2004 for a clinical trial by AstraZeneca, related to the present work; and grants to support the Karolinska Severe Asthma Centre from GlaxoSmithKline and Novartis; consulting fees from Teva, AstraZeneca and Sanofi; and payment for lectures from AstraZeneca and Sanofi, all in the 36 months prior to manuscript submission. W. Fokkens declares grants to their institution from ALK, Mylan, Allergy Therapeutics, GlaxoSmithKline, Sanofi, Novartis and Chordate; consulting fees from Sanofi and Bioinspire; payment or honoraria from Sanofi, GlaxoSmithKline and Novartis; and participation on a data safety monitoring board or advisory board for Lyra, Sanofi and GlaxoSmithKline, all in the 36 months prior to manuscript submission; and that they are Secretary General of the European Rhinologic Society. P. Hellings declares no competing interests. N. Lazarinis declares no competing interests. P.M. O'Byrne reports grants from AstraZeneca, GlaxoSmithKline, Biohaven, Merck, Bayer and Novartis; consulting fees from AstraZeneca, Covis, GlaxoSmithKline, Amgen and Novartis; and payment or honoraria from AstraZeneca, Covis and Novartis, all in the 36 months prior to manuscript submission; and that they are a Section Editor of the European Respiratory Journal. E. Tufvesson declares no competing interests. S. Quirce declares consulting fees and payment or honoraria from GlaxoSmithKline, Sanofi and AstraZeneca; and payment or honoraria from Novartis, Chiesi, Mundipharma and Teva, all in the 36 months prior to manuscript submission. M. Van Maaren declares payment or honoraria from ALK-Abelló, Takeda and CSL Behring, in the 36 months prior to manuscript submission; and an unpaid role as Chairman of the Dutch Society of Allergology and Clinical Immunology since 2020. F. H. de Jongh declares an unpaid role as European Respiratory Society Assessment Director. Z. Diamant reports that until May 2020 they worked as Research Director Respiratory & Allergy at a CRO (QPS-NL), which received funding from biotech and several pharma companies for conduct of phase I–II clinical studies (drug development); and declares consulting fees from ALK-Abelló, AstraZeneca, Antabio, GlaxoSmithKline, HAL Allergy, QPS-NL and Sanofi-Genzyme; and payment or honoraria from BMR, Boehringer Ingelheim, European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), Merck Sharp & Dohme and Sanofi-Genzyme, all in the 36 months prior to manuscript submission; and unpaid roles as the European Academy of Allergy and Clinical Immunology Asthma Section Chair (2017–2019) and EUFOREA Asthma Expert Panel Chair (since 2020).
References
- 1.Booij-Noord H, de Vries K, Sluiter HJ, et al. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin Allergy 1972; 2: 43–61. doi: 10.1111/j.1365-2222.1972.tb01267.x [DOI] [PubMed] [Google Scholar]
- 2.Pepys J, Hargreave FE, Chan M, et al. Inhibitory effects of disodium cromoglycate on allergen-inhalation tests. Lancet 1968; 2: 134–137. doi: 10.1016/S0140-6736(68)90419-4 [DOI] [PubMed] [Google Scholar]
- 3.Braunstahl GJ, Overbeek SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol 2001; 107: 469–476. doi: 10.1067/mai.2001.113046 [DOI] [PubMed] [Google Scholar]
- 4.Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med 2001; 164: 858–865. doi: 10.1164/ajrccm.164.5.2006082 [DOI] [PubMed] [Google Scholar]
- 5.Boot JD, Chandoesing P, de Kam ML, et al. Applicability and reproducibility of biomarkers for the evaluation of anti-inflammatory therapy in allergic rhinitis. J Investig Allergol Clin Immunol 2008; 18: 433–442. [PubMed] [Google Scholar]
- 6.Augé J, Vent J, Agache I, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy 2018; 73: 1597–1608. doi: 10.1111/all.13416 [DOI] [PubMed] [Google Scholar]
- 7.Diamant Z, Gauvreau GM, Cockcroft DW, et al. Inhaled allergen bronchoprovocation tests. J Allergy Clin Immunol 2013; 132: 1045–1055. doi: 10.1016/j.jaci.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 8.Killian D, Cockcroft DW, Hargreave FE, et al. Factors in allergen-induced asthma: relevance of the intensity of the airways allergic reaction and non-specific bronchial reactivity. Clin Allergy 1976; 6: 219–225. doi: 10.1111/j.1365-2222.1976.tb01900.x [DOI] [PubMed] [Google Scholar]
- 9.Gauvreau GM, Watson RM, Rerecich TJ, et al. Repeatability of allergen-induced airway inflammation. J Allergy Clin Immunol 1999; 104: 66–71. doi: 10.1016/S0091-6749(99)70115-6 [DOI] [PubMed] [Google Scholar]
- 10.Gauvreau GM, El-Gammal AI, O'Byrne PM. Allergen-induced airway responses. Eur Respir J 2015; 46: 819–831. doi: 10.1183/13993003.00536-2015 [DOI] [PubMed] [Google Scholar]
- 11.Zuiker RG, Ruddy MK, Morelli N, et al. Kinetics of TH2 biomarkers in sputum of asthmatics following inhaled allergen. Eur Clin Respir J 2015; 2: 28319. doi: 10.3402/ecrj.v2.28319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallstrand TS, Leuppi JD, Joos G, et al. ERS technical standard on bronchial challenge testing: pathophysiology and methodology of indirect airway challenge testing. Eur Respir J 2018; 52: 1801033. doi: 10.1183/13993003.01033-2018 [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
- 14.O'Byrne PM, Gauvreau GM, Brannan JD. Provoked models of asthma: what have we learnt? Clin Exp Allergy 2009; 39: 181–192. doi: 10.1111/j.1365-2222.2008.03172.x [DOI] [PubMed] [Google Scholar]
- 15.Levine SJ, Wenzel SE. Narrative review: the role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann Intern Med 2010; 152: 232–237. doi: 10.7326/0003-4819-152-4-201002160-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leckie MJ, At B, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000; 356: 2144–2148. doi: 10.1016/S0140-6736(00)03496-6 [DOI] [PubMed] [Google Scholar]
- 17.Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 18.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 1997; 155: 1828–1834. doi: 10.1164/ajrccm.155.6.9196082 [DOI] [PubMed] [Google Scholar]
- 19.Fahy JV, Cockcroft DW, Boulet LP, et al. Effect of aerosolized anti-IgE (E25) on airway responses to inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med 1999; 160: 1023–1027. doi: 10.1164/ajrccm.160.3.9810012 [DOI] [PubMed] [Google Scholar]
- 20.Boulet LP, Gauvreau G, Boulay ME, et al. The allergen bronchoprovocation model: an important tool for the investigation of new asthma anti-inflammatory therapies. Allergy 2007; 62: 1101–1110. doi: 10.1111/j.1398-9995.2007.01499.x [DOI] [PubMed] [Google Scholar]
- 21.Gauvreau GM, Boulet L-P, Cockcroft DW, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med 2011; 183: 1007–1014. doi: 10.1164/rccm.201008-1210OC [DOI] [PubMed] [Google Scholar]
- 22.Wenzel S, Wilbraham D, Fuller R, et al. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 2007; 370: 1422–1431. doi: 10.1016/S0140-6736(07)61600-6 [DOI] [PubMed] [Google Scholar]
- 23.Maurer M, Giménez-Arnau AM, Sussman G, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med 2019; 381: 1321–1332. doi: 10.1056/NEJMoa1900408 [DOI] [PubMed] [Google Scholar]
- 24.Gauvreau GM, Arm JP, Boulet LP, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol 2016; 138: 1051–1059. doi: 10.1016/j.jaci.2016.02.027 [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Shannon CP, Kim YW, et al. Novel blood-based transcriptional biomarker panels predict the late-phase asthmatic response. Am J Respir Crit Care Med 2018; 197: 450–462. doi: 10.1164/rccm.201701-0110OC [DOI] [PubMed] [Google Scholar]
- 26.Revez JA, Killian KJ, O'Byrne PM, et al. Sputum cytology during late-phase responses to inhalation challenge with different allergens. Allergy 2018; 73: 1470–1478. doi: 10.1111/all.13415 [DOI] [PubMed] [Google Scholar]
- 27.Zuiker RG, Tribouley C, Diamant Z, et al. Sputum RNA signature in allergic asthmatics following allergen bronchoprovocation test. Eur Clin Respir J 2016; 3: 31324. doi: 10.3402/ecrj.v3.31324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R, Smith SG, Salter B, et al. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am J Respir Crit Care Med 2017; 196: 700–712. doi: 10.1164/rccm.201612-2427OC [DOI] [PubMed] [Google Scholar]
- 29.El-Gammal A, Oliveria JP, Howie K, et al. Allergen-induced changes in bone marrow and airway dendritic cells in subjects with asthma. Am J Respir Crit Care Med 2016; 194: 169–177. doi: 10.1164/rccm.201508-1623OC [DOI] [PubMed] [Google Scholar]
- 30.Diamant Z, Boot JD, Mantzouranis E, et al. Biomarkers in asthma and allergic rhinitis. Pulm Pharmacol Ther 2010; 23: 468–481. doi: 10.1016/j.pupt.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 31.Robinson D, Hamid Q, Bentley A, et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol 1993; 92: 313–324. doi: 10.1016/0091-6749(93)90175-F [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Yamamoto M, Kam SH, et al. Gene-metabolite expression in blood can discriminate allergen-induced isolated early from dual asthmatic responses. PLoS One 2013; 8: e67907. doi: 10.1371/journal.pone.0067907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochenek G, Nizankowska E, Gielicz A, et al. Plasma 9alpha,11beta-PGF2, a PGD2 metabolite, as a sensitive marker of mast cell activation by allergen in bronchial asthma. Thorax 2004; 59: 459–464. doi: 10.1136/thx.2003.013573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamant Z, Timmers MC, van der Veen H, et al. The effect of MK-0591, a novel 5-lipoxygenase activating protein inhibitor, on leukotriene biosynthesis and allergen-induced airway responses in asthmatic subjects in vivo. J Allergy Clin Immunol 1995; 95: 42–51. doi: 10.1016/S0091-6749(95)70151-6 [DOI] [PubMed] [Google Scholar]
- 35.Daham K, James A, Balgoma D, et al. Effects of selective COX-2 inhibition on allergen-induced bronchoconstriction and airway inflammation in asthma. J Allergy Clin Immunol 2014; 134: 306–313. doi: 10.1016/j.jaci.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 36.O'Sullivan S, Roquet A, Dahlén B, et al. Urinary excretion of inflammatory mediators during allergen-induced early and late phase asthmatic reactions. Clin Exp Allergy 1998; 28: 1332–1339. doi: 10.1046/j.1365-2222.1998.00368.x [DOI] [PubMed] [Google Scholar]
- 37.Roquet A, Dahlén B, Kumlin M, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med 1997; 155: 1856–1863. doi: 10.1164/ajrccm.155.6.9196086 [DOI] [PubMed] [Google Scholar]
- 38.Boulet LP, Côté A, Abd-Elaziz K, et al. Allergen bronchoprovocation test: an important research tool supporting precision medicine. Curr Opin Pulm Med 2021; 27: 15–22. doi: 10.1097/MCP.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 39.Bjermer L, Gauvreau GM, Postma DS, et al. Methacholine challenge tests to demonstrate therapeutic equivalence of terbutaline sulfate via different Turbuhaler. Pulm Pharmacol Ther 2017; 44: 1–6. doi: 10.1016/j.pupt.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell PD, Salter BM, Oliveria JP, et al. IL-33 and its receptor ST2 after inhaled allergen challenge in allergic asthmatics. Int Arch Allergy Immunol 2018; 176: 133–142. doi: 10.1159/000488015 [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Smith SG, Du W, et al. Interleukin-25 and eosinophils progenitor cell mobilization in allergic asthma. Clin Transl Allergy 2018; 8: 5. doi: 10.1186/s13601-018-0190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kämpe M, Stolt I, Lampinen M, et al. Patients with allergic rhinitis and allergic asthma share the same pattern of eosinophil and neutrophil degranulation after allergen challenge. Clin Mol Allergy 2011; 9: 3. doi: 10.1186/1476-7961-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmekel B, Venge P. Markers for eosinophils and T-lymphocytes as predictors of late asthmatic response. Allergy 1993; 48: 17 Suppl., 94–97. doi: 10.1111/j.1398-9995.1993.tb04708.x [DOI] [PubMed] [Google Scholar]
- 44.Gauvreau GM, Lee JM, Watson RM, et al. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med 2000; 161: 1473–1478. doi: 10.1164/ajrccm.161.5.9908090 [DOI] [PubMed] [Google Scholar]
- 45.Salter BM, Nusca G, Tworek D, et al. Expression of activation markers in circulating basophils and the relationship to allergen-induced bronchoconstriction in subjects with mild allergic asthma. J Allergy Clin Immunol 2016; 137: 936–938. doi: 10.1016/j.jaci.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 46.Tworek D, Smith SG, Salter BM, et al. IL-25 receptor expression on airway dendritic cells after allergen challenge in subjects with asthma. Am J Respir Crit Care Med 2016; 193: 957–964. doi: 10.1164/rccm.201509-1751OC [DOI] [PubMed] [Google Scholar]
- 47.Oliveria JP, El-Gammal AI, Yee M, et al. Changes in regulatory B-cell levels in bone marrow, blood, and sputum of patients with asthma following inhaled allergen challenge. J Allergy Clin Immunol 2018; 141: 1495–1498. doi: 10.1016/j.jaci.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 48.Oliveria JP, Campbell H, Beaudin S, et al. Characterization of IgE memory B cell subsets following whole lung allergen challenge in patients with mild asthma. Am J Respir Crit Care Med 2014; 189: A2237. [Google Scholar]
- 49.Gauvreau GM, Hessel EM, Boulet LP, et al. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am J Respir Crit Care Med 2006; 174: 15–20. doi: 10.1164/rccm.200601-057OC [DOI] [PubMed] [Google Scholar]
- 50.Gauvreau GM, Doctor J, Watson RM, et al. Effects of inhaled budesonide on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 1996; 154: 1267–1271. doi: 10.1164/ajrccm.154.5.8912734 [DOI] [PubMed] [Google Scholar]
- 51.Duvall MG, Krishnamoorthy N, Levy BD. Non-type 2 inflammation in severe asthma is propelled by neutrophil cytoplasts and maintained by defective resolution. Allergol Int 2019; 68: 143–149. doi: 10.1016/j.alit.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 52.Hudey SN, Ledford DK, Cardet JC. Mechanisms of non-type 2 asthma. Curr Opin Immunol 2020; 66: 123–128. doi: 10.1016/j.coi.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020; 75: 311–325. doi: 10.1111/all.13985 [DOI] [PubMed] [Google Scholar]
- 54.Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir Res 2011; 12: 140. doi: 10.1186/1465-9921-12-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekstedt S, Stenberg H, Tufvesson E, et al. The potential role of CD16high CD62Ldim neutrophils in the allergic asthma. Allergy 2019; 74: 2265–2268. doi: 10.1111/all.13861 [DOI] [PubMed] [Google Scholar]
- 56.Naji N, Smith SG, Gauvreau GM, et al. T helper 17 cells and related cytokines after allergen inhalation challenge in allergic asthmatics. Int Arch Allergy Immunol 2014; 165: 27–34. doi: 10.1159/000367789 [DOI] [PubMed] [Google Scholar]
- 57.Singh A, Yamamoto M, Ruan J, et al. Th17/Treg ratio derived using DNA methylation analysis is associated with the late phase asthmatic response. Allergy Asthma Clin Immunol 2014; 10: 32. doi: 10.1186/1710-1492-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinoshita T, Baatjes A, Smith SG, et al. Natural regulatory T cells in isolated early responders compared with dual responders with allergic asthma. J Allergy Clin Immunol 2014; 133: 696–703. doi: 10.1016/j.jaci.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 59.Esnault S, Khosravi M, Kelly EA, et al. Increased IL-6 and potential IL-6 trans-signalling in the airways after an allergen challenge. Clin Exp Allergy 2021; 51: 564–573. doi: 10.1111/cea.13832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Revez JA, Bain LM, Watson RM, et al. Effects of interleukin-6 receptor blockade on allergen-induced airway responses in mild asthmatics. Clin Transl Immunology 2019; 8: e1044. doi: 10.1002/cti2.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelly MM, O'Connor TM, Leigh R, et al. Effects of budesonide and formoterol on allergen-induced airway responses, inflammation, and airway remodeling in asthma. J Allergy Clin Immunol 2010; 125: 349–356. doi: 10.1016/j.jaci.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 62.Boulay ME, Prince P, Deschesnes F, et al. Metalloproteinase-9 in induced sputum correlates with the severity of the late allergen-induced asthmatic response. Respiration 2004; 71: 216–224. doi: 10.1159/000077418 [DOI] [PubMed] [Google Scholar]
- 63.Al-Sajee D, Sehmi R, Hawke TJ, et al. Expression of IL-33 and TSLP and their receptors in asthmatic airways after inhaled allergen challenge. Am J Respir Crit Care Med 2018; 198: 805–807. doi: 10.1164/rccm.201712-2468LE [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol 2018; 200: 2253–2262. doi: 10.4049/jimmunol.1701455 [DOI] [PubMed] [Google Scholar]
- 65.Leaker BR, Singh D, Lindgren S, et al. Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: a double-blind, randomised, parallel-group study. Respir Res 2019; 20: 288–288. doi: 10.1186/s12931-019-1252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammad H, Chieppa M, Perros F, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009; 15: 410–416. doi: 10.1038/nm.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imaoka H, Punia N, Irshad A, et al. Lung homing of endothelial progenitor cells in humans with asthma after allergen challenge. Am J Respir Crit Care Med 2011; 184: 771–778. doi: 10.1164/rccm.201102-0272OC [DOI] [PubMed] [Google Scholar]
- 68.Tliba O, Panettieri RA. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol 2019; 143: 1287–1294. doi: 10.1016/j.jaci.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015; 17: 690–703. doi: 10.1016/j.chom.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 70.Chotirmall SH, Burke CM. Aging and the microbiome: implications for asthma in the elderly? Expert Rev Respir Med 2015; 9: 125–128. doi: 10.1586/17476348.2015.1002473 [DOI] [PubMed] [Google Scholar]
- 71.Klein M, Colas L, Cheminant MA, et al. Der p 2.1 Peptide abrogates house dust mites-induced asthma features in mice and humanized mice by inhibiting DC-mediated T cell polarization. Front Immunol 2020; 11: 565431. doi: 10.3389/fimmu.2020.565431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lund J, Bartel S, Chung J, et al. House dust mite (HDM)-induced “asthma” phenotypes differ in 4 mouse strains – link to the microbiome? ERJ Open Res 2019; 5: Suppl. 2, PP106. doi: 10.1183/23120541.lungscienceconference-2019.PP106 [DOI] [Google Scholar]
- 73.Martin MJ, Zain NMM, Hearson G, et al. The airways microbiome of individuals with asthma treated with high and low doses of inhaled corticosteroids. PLoS One 2020; 15: e0244681. doi: 10.1371/journal.pone.0244681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor DA, Harris JG, O'Connor BJ. Comparison of incremental and bolus dose inhaled allergen challenge in asthmatic patients. Clin Exp Allergy 2000; 30: 56–63. doi: 10.1046/j.1365-2222.2000.00657.x [DOI] [PubMed] [Google Scholar]
- 75.Diamant Z, Sidharta PN, Singh D, et al. Setipiprant, a selective CRTH2 antagonist, reduces allergen-induced airway responses in allergic asthmatics. Clin Exp Allergy 2014; 44: 1044–1052. doi: 10.1111/cea.12357 [DOI] [PubMed] [Google Scholar]
- 76.Frølund L, Svendsen UG, Nielsen NH, et al. Bronchial allergen challenge: comparison between two different methods of provocation. Clin Allergy 1987; 17: 439–448. doi: 10.1111/j.1365-2222.1987.tb02038.x [DOI] [PubMed] [Google Scholar]
- 77.Tiffeneau R. Hypersensibilité cholinergo-histaminique pulmonaire de l'asthmatique; relation avec l'hypersensibilité allergénique pulmonaire. [Cholinergic-histaminic pulmonary hypersensitivity of asthma; relationship with pulmonary allergen hypersensitivity.] Acta Allergol Suppl 1958; 5: 187–221. [PubMed] [Google Scholar]
- 78.Herxheimer H. Bronchial obstruction induced by allergens, histamine and acetyl-beta-methylcholinechloride. Int Arch Allergy Appl Immunol 1951; 2: 27–39. doi: 10.1159/000227898 [DOI] [PubMed] [Google Scholar]
- 79.Robertson DG, Kerigan AT, Hargreave FE, et al. Late asthmatic responses induced by ragweed pollen allergen. J Allergy Clin Immunol 1974; 54: 244–254. doi: 10.1016/0091-6749(74)90067-0 [DOI] [PubMed] [Google Scholar]
- 80.Cockcroft DW, Murdock KY, Kirby J, et al. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis 1987; 135: 264–267. [DOI] [PubMed] [Google Scholar]
- 81.Cockcroft DW, McParland CP, Britto SA, et al. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993; 342: 833–837. doi: 10.1016/0140-6736(93)92695-P [DOI] [PubMed] [Google Scholar]
- 82.Ravensberg AJ, van Rensen EL, Grootendorst DC, et al. Validated safety predictions of airway responses to house dust mite in asthma. Clin Exp Allergy 2007; 37: 100–107. doi: 10.1111/j.1365-2222.2006.02617.x [DOI] [PubMed] [Google Scholar]
- 83.Cockcroft DW, Davis BE, Blais CM, et al. Use of a vibrating mesh nebulizer for allergen challenge. Allergy Asthma Clin Immunol 2019; 15: 73. doi: 10.1186/s13223-019-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumlin M, Dahlén B. The challenge procedure influences the extent of allergen-induced urinary excretion of leukotriene E4. Clin Exp Allergy 2000; 30: 585–589. doi: 10.1046/j.1365-2222.2000.00738.x [DOI] [PubMed] [Google Scholar]
- 85.Davis BE, Todd DC, Cockcroft DW. Effect of combined montelukast and desloratadine on the early asthmatic response to inhaled allergen. J Allergy Clin Immunol 2005; 116: 768–772. doi: 10.1016/j.jaci.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 86.Naji N, Keung E, Kane J, et al. Comparison of changes in lung function measured by plethymography and IOS after bronchoprovocation. Respir Med 2013; 107: 503–510. doi: 10.1016/j.rmed.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 87.Stenberg H, Diamant Z, Ankerst J, et al. Small airway involvement in the late allergic response in asthma. Clin Exp Allergy 2017; 47: 1555–1565. doi: 10.1111/cea.13036 [DOI] [PubMed] [Google Scholar]
- 88.Inman MD, Watson R, Cockcroft DW, et al. Reproducibility of allergen-induced early and late asthmatic responses. J Allergy Clin Immunol 1995; 95: 1191–1195. doi: 10.1016/S0091-6749(95)70075-7 [DOI] [PubMed] [Google Scholar]
- 89.Dente FL, Bacci E, di Franco A, et al. Reproducibility of early and late asthmatic responses to allergen challenge in a large group of asthmatics. Respir Med 2000; 94: 441–447. doi: 10.1053/rmed.1999.0760 [DOI] [PubMed] [Google Scholar]
- 90.Cockcroft DW, McParland CP, O'Byrne PM, et al. Beclomethasone given after the early asthmatic response inhibits the late response and the increased methacholine responsiveness and cromolyn does not. J Allergy Clin Immunol 1993; 91: 1163–1168. doi: 10.1016/0091-6749(93)90319-B [DOI] [PubMed] [Google Scholar]
- 91.Cockcroft DW, Davis BE, Boulet LP, et al. The links between allergen skin test sensitivity, airway responsiveness and airway response to allergen. Allergy 2005; 60: 56–59. doi: 10.1111/j.1398-9995.2004.00612.x [DOI] [PubMed] [Google Scholar]
- 92.Gauvreau GM, Boulet LP, Davis B, et al. Safety of allergen inhalation challenge in allergic asthmatic subjects. Am J Respir Crit Care Med 2009; 179: Suppl., A2849. doi: 10.1164/rccm.200809-1395ED [DOI] [Google Scholar]
- 93.Ihre E, Zetterström O. Increase in non-specific bronchial responsiveness after repeated inhalation of low doses of allergen. Clin Exp Allergy 1993; 23: 298–305. doi: 10.1111/j.1365-2222.1993.tb00326.x [DOI] [PubMed] [Google Scholar]
- 94.Leckie MJ. Chronic allergen challenge as an experimental model: necessary, significant or useful? Clin Exp Allergy 2000; 30: 1191–1193. doi: 10.1046/j.1365-2222.2000.00917.x [DOI] [PubMed] [Google Scholar]
- 95.Sulakvelidze I, Inman MD, Rerecich T, et al. Increases in airway eosinophils and interleukin-5 with minimal bronchoconstriction during repeated low-dose allergen challenge in atopic asthmatics. Eur Respir J 1998; 11: 821–827. doi: 10.1183/09031936.98.11040821 [DOI] [PubMed] [Google Scholar]
- 96.de Blay F, Krieger P, Spirlet F, et al. Repeated inhalation of low doses of cat allergen that do not induce clinical symptoms increases bronchial hyperresponsiveness and eosinophil cationic protein levels. Int Arch Allergy Immunol 1999; 120: 158–165. doi: 10.1159/000024234 [DOI] [PubMed] [Google Scholar]
- 97.Palmqvist M, Pettersson K, Sjöstrand M, et al. Mild experimental exacerbation of asthma induced by individualised low-dose repeated allergen exposure. A double-blind evaluation. Respir Med 1998; 92: 1223–1230. doi: 10.1016/S0954-6111(98)90425-5 [DOI] [PubMed] [Google Scholar]
- 98.Boulay ME, Boulet LP. Lower airway inflammatory responses to repeated very-low-dose allergen challenge in allergic rhinitis and asthma. Clin Exp Allergy 2002; 32: 1441–1447. doi: 10.1046/j.1365-2745.2002.01508.x [DOI] [PubMed] [Google Scholar]
- 99.de Kluijver J, Evertse CE, Schrumpf JA, et al. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med 2002; 166: 294–300. doi: 10.1164/rccm.2112097 [DOI] [PubMed] [Google Scholar]
- 100.de Kluijver J, Evertse CE, Sont JK, et al. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med 2003; 168: 1174–1180. doi: 10.1164/rccm.200212-1520OC [DOI] [PubMed] [Google Scholar]
- 101.Ihre E, Gyllfors P, Gustafsson LE, et al. Early rise in exhaled nitric oxide and mast cell activation in repeated low-dose allergen challenge. Eur Respir J 2006; 27: 1152–1159. doi: 10.1183/09031936.06.00142905 [DOI] [PubMed] [Google Scholar]
- 102.Dahlén B, Lantz AS, Ihre E, et al. Effect of formoterol with or without budesonide in repeated low-dose allergen challenge. Eur Respir J 2009; 33: 747–753. doi: 10.1183/09031936.00095508 [DOI] [PubMed] [Google Scholar]
- 103.Gauvreau GM, Sulakvelidze I, Watson RM, et al. Effects of once daily dosing with inhaled budesonide on airway hyperresponsiveness and airway inflammation following repeated low-dose allergen challenge in atopic asthmatics. Clin Exp Allergy 2000; 30: 1235–1243. doi: 10.1046/j.1365-2222.2000.00860.x [DOI] [PubMed] [Google Scholar]
- 104.Wheelock CE, Goss VM, Balgoma D, et al. Application of ‘omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J 2013; 42: 802–825. doi: 10.1183/09031936.00078812 [DOI] [PubMed] [Google Scholar]
- 105.Makker HK, Montefort S, Holgate S. Investigative use of fibreoptic bronchoscopy for local airway challenge in asthma. Eur Respir J 1993; 6: 1402–1408. [PubMed] [Google Scholar]
- 106.Julius P, Lommatzsch M, Kuepper M, et al. Safety of segmental allergen challenge in human allergic asthma. J Allergy Clin Immunol 2008; 121: 712–717. doi: 10.1016/j.jaci.2007.08.058 [DOI] [PubMed] [Google Scholar]
- 107.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765. doi: 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 108.Vogel-Claussen J, Renne J, Hinrichs J, et al. Quantification of pulmonary inflammation after segmental allergen challenge using turbo-inversion recovery-magnitude magnetic resonance imaging. Am J Respir Crit Care Med 2014; 189: 650–657. doi: 10.1164/rccm.201310-1825OC [DOI] [PubMed] [Google Scholar]
- 109.Mendoza DP, Kohli P, Nance JW, et al. Lung parenchymal and airway changes on CT imaging following allergen challenge and bronchoalveolar lavage in atopic and asthmatic subjects. Ann Transl Med 2020; 8: 862. doi: 10.21037/atm-20-1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarlo SM. Laboratory challenge testing for occupational asthma. J Allergy Clin Immunol 2003; 111: 692–694. doi: 10.1067/mai.2003.1374 [DOI] [PubMed] [Google Scholar]
- 111.Quirce S, Vandenplas O, Campo P, et al. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 2016; 71: 765–779. doi: 10.1111/all.12866 [DOI] [PubMed] [Google Scholar]
- 112.Pepys J, Hutchcroft BJ. Bronchial provocation tests in etiologic diagnosis and analysis of asthma. Am Rev Respir Dis 1975; 112: 829–859. [DOI] [PubMed] [Google Scholar]
- 113.Vandenplas O, Malo JL. Inhalation challenges with agents causing occupational asthma. Eur Respir J 1997; 10: 2612–2629. doi: 10.1183/09031936.97.10112612 [DOI] [PubMed] [Google Scholar]
- 114.Quirce S, Lemière C, de Blay F, et al. Noninvasive methods for assessment of airway inflammation in occupational settings. Allergy 2010; 65: 445–458. doi: 10.1111/j.1398-9995.2009.02274.x [DOI] [PubMed] [Google Scholar]
- 115.Vandenplas O, Suojalehto H, Aasen TB, et al. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J 2014; 43: 1573–1587. doi: 10.1183/09031936.00180313 [DOI] [PubMed] [Google Scholar]
- 116.Eguiluz-Gracia I, Testera-Montes A, González M, et al. Safety and reproducibility of nasal allergen challenge. Allergy 2019; 74: 1125–1134. doi: 10.1111/all.13728 [DOI] [PubMed] [Google Scholar]
- 117.Cullinan P, Vandenplas O, Bernstein D. Assessment and management of occupational asthma. J Allergy Clin Immunol Pract 2020; 8: 3264–3275. doi: 10.1016/j.jaip.2020.06.031 [DOI] [PubMed] [Google Scholar]
- 118.Lebel B, Bousquet J, Morel A, et al. Correlation between symptoms and the threshold for release of mediators in nasal secretions during nasal challenge with grass-pollen grains. J Allergy Clin Immunol 1988; 82: 869–877. doi: 10.1016/0091-6749(88)90092-9 [DOI] [PubMed] [Google Scholar]
- 119.Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods 2012; 384: 25–32. doi: 10.1016/j.jim.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 120.Volstad I, Olafsson T, Steinsvik EA, et al. Minimal unilateral peak nasal inspiratory flow correlates with patient reported nasal obstruction. Rhinology 2019; 57: 436–443. doi: 10.4193/Rhin19.178 [DOI] [PubMed] [Google Scholar]
- 121.Pfaar O, Nell MJ, Boot JD, et al. A randomized, 5-arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy 2016; 71: 967–976. doi: 10.1111/all.12860 [DOI] [PubMed] [Google Scholar]
- 122.Pfaar O, van Twuijver E, Boot JD, et al. A randomized DBPC trial to determine the optimal effective and safe dose of a SLIT-birch pollen extract for the treatment of allergic rhinitis: results of a phase II study. Allergy 2016; 71: 99–107. doi: 10.1111/all.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ottaviano G, Scadding GK, Coles S, et al. Peak nasal inspiratory flow; normal range in adult population. Rhinology 2006; 44: 32–35. [PubMed] [Google Scholar]
- 124.Chusakul S, Phannaso C, Sangsarsri S, et al. House-dust mite nasal provocation: a diagnostic tool in perennial rhinitis. Am J Rhinol Allergy 2010; 24: 133–136. doi: 10.2500/ajra.2010.24.3441a [DOI] [PubMed] [Google Scholar]
- 125.Ottaviano G, Lund VJ, Nardello E, et al. Comparison between unilateral PNIF and rhinomanometry in healthy and obstructed noses. Rhinology 2014; 52: 25–30. doi: 10.4193/Rhino13.037 [DOI] [PubMed] [Google Scholar]
- 126.Ottaviano G, Fokkens WJ. Measurements of nasal airflow and patency: a critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016; 71: 162–174. doi: 10.1111/all.12778 [DOI] [PubMed] [Google Scholar]
- 127.Vogt K, Bachmann-Harildstad G, Lintermann A, et al. The new agreement of the international RIGA consensus conference on nasal airway function tests. Rhinology 2018; 56: 133–143. doi: 10.4193/Rhin17.084 [DOI] [PubMed] [Google Scholar]
- 128.Dumitru AF, Shamji M, Wagenmann M, et al. Petasol butenoate complex (Ze 339) relieves allergic rhinitis-induced nasal obstruction more effectively than desloratadine. J Allergy Clin Immunol 2011; 127: 1515–1521. doi: 10.1016/j.jaci.2011.02.045 [DOI] [PubMed] [Google Scholar]
- 129.Callebaut I, Spielberg L, Hox V, et al. Conjunctival effects of a selective nasal pollen provocation. Allergy 2010; 65: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 130.Pelikan Z. Asthmatic response induced by nasal challenge with allergen. Int Arch Allergy Immunol 2009; 148: 330–338. doi: 10.1159/000170387 [DOI] [PubMed] [Google Scholar]
- 131.Boot JD, de Kam ML, Mascelli MA, et al. Nasal nitric oxide: longitudinal reproducibility and the effects of a nasal allergen challenge in patients with allergic rhinitis. Allergy 2007; 62: 378–384. doi: 10.1111/j.1398-9995.2007.01328.x [DOI] [PubMed] [Google Scholar]
- 132.de Graaf-in t Veld C, Garrelds IM, Koenders S, et al. Relationship between nasal hyperreactivity, mediators and eosinophils in patients with perennial allergic rhinitis and controls. Clin Exp Allergy 1996; 26: 903–908. doi: 10.1046/j.1365-2222.1996.d01-395.x [DOI] [PubMed] [Google Scholar]
- 133.Nicholson GC, Kariyawasam HH, Tan AJ, et al. The effects of an anti-IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J Allergy Clin Immunol 2011; 128: 800–807. doi: 10.1016/j.jaci.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 134.Castells M, Schwartz LB. Tryptase levels in nasal-lavage fluid as an indicator of the immediate allergic response. J Allergy Clin Immunol 1988; 82: 348–355. doi: 10.1016/0091-6749(88)90005-X [DOI] [PubMed] [Google Scholar]
- 135.Naclerio RM, Proud D, Togias AG, et al. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med 1985; 313: 65–70. doi: 10.1056/NEJM198507113130201 [DOI] [PubMed] [Google Scholar]
- 136.Creticos PS, Peters SP, Adkinson NF, et al. Peptide leukotriene release after antigen challenge in patients sensitive to ragweed. N Engl J Med 1984; 310: 1626–1630. doi: 10.1056/NEJM198406213102502 [DOI] [PubMed] [Google Scholar]
- 137.Sim TC, Reece LM, Hilsmeier KA, et al. Secretion of chemokines and other cytokines in allergen-induced nasal responses: inhibition by topical steroid treatment. Am J Respir Crit Care Med 1995; 152: 927–933. doi: 10.1164/ajrccm.152.3.7545059 [DOI] [PubMed] [Google Scholar]
- 138.Bascom R, Pipkorn U, Proud D, et al. Major basic protein and eosinophil-derived neurotoxin concentrations in nasal-lavage fluid after antigen challenge: effect of systemic corticosteroids and relationship to eosinophil influx. J Allergy Clin Immunol 1989; 84: 338–346. doi: 10.1016/0091-6749(89)90418-1 [DOI] [PubMed] [Google Scholar]
- 139.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 2006; 118: 1133–1141. doi: 10.1016/j.jaci.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 140.Mosimann BL, White MV, Hohman RJ, et al. Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol 1993; 92: 95–104. doi: 10.1016/0091-6749(93)90043-F [DOI] [PubMed] [Google Scholar]
- 141.Meyer P, Persson CG, Andersson M, et al. Alpha2-macroglobulin and eosinophil cationic protein in the allergic airway mucosa in seasonal allergic rhinitis. Eur Respir J 1999; 13: 633–637. doi: 10.1183/09031936.99.13363399 [DOI] [PubMed] [Google Scholar]
- 142.Proud D, Riker DK, Togias A. Reproducibility of nasal allergen challenge in evaluating the efficacy of intranasal corticosteroid treatment. Clin Exp Allergy 2010; 40: 738–744. doi: 10.1111/j.1365-2222.2010.03466.x [DOI] [PubMed] [Google Scholar]
- 143.Raphael GD, Jeney EV, Baraniuk JN, et al. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest 1989; 84: 1528–1535. doi: 10.1172/JCI114329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haimerl P, Bernhardt U, Schindela S, et al. Inflammatory macrophage memory in nonsteroidal anti-inflammatory drug-exacerbated respiratory disease. J Allergy Clin Immunol 2021; 147: 587–599. doi: 10.1016/j.jaci.2020.04.064 [DOI] [PubMed] [Google Scholar]
- 145.Larson D, Patel P, Salapatek AM, et al. Nasal allergen challenge and environmental exposure chamber challenge: a randomized trial comparing clinical and biological responses to cat allergen. J Allergy Clin Immunol 2020; 145: 1585–1597. doi: 10.1016/j.jaci.2020.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol 2020; 146: 203–206. doi: 10.1016/j.jaci.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahuja SK, Manoharan MS, Harper NL, et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol 2017; 139: 844–854. doi: 10.1016/j.jaci.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 148.Xie Y, Ju X, Beaudin S, et al. Effect of intranasal corticosteroid treatment on allergen-induced changes in group 2 innate lymphoid cells in allergic rhinitis with mild asthma. Allergy 2021; 76: 2797–2808. doi: 10.1111/all.14835 [DOI] [PubMed] [Google Scholar]
- 149.Godthelp T, Holm AF, Fokkens WJ, et al. Dynamics of nasal eosinophils in response to a nonnatural allergen challenge in patients with allergic rhinitis and control subjects: a biopsy and brush study. J Allergy Clin Immunol 1996; 97: 800–811. doi: 10.1016/S0091-6749(96)80158-8 [DOI] [PubMed] [Google Scholar]
- 150.Godthelp T, Fokkens WJ, Kleinjan A, et al. Antigen presenting cells in the nasal mucosa of patients with allergic rhinitis during allergen provocation. Clin Exp Allergy 1996; 26: 677–688. doi: 10.1111/j.1365-2222.1996.tb00594.x [DOI] [PubMed] [Google Scholar]
- 151.Muller B, Reinartz SM, van Egmond D, et al. Mono-allergic and poly-allergic rhinitis patients have comparable numbers of mucosal Foxp3+CD4+ T lymphocytes. Rhinology 2014; 52: 260–266. doi: 10.4193/Rhino13.223 [DOI] [PubMed] [Google Scholar]
- 152.Eguíluz-Gracia I, Bosco A, Dollner R, et al. Rapid recruitment of CD14+ monocytes in experimentally induced allergic rhinitis in human subjects. J Allergy Clin Immunol 2016; 137: 1872–1881. doi: 10.1016/j.jaci.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 153.Thornton MA, Akasheh N, Walsh MT, et al. Eosinophil recruitment to nasal nerves after allergen challenge in allergic rhinitis. Clin Immunol 2013; 147: 50–57. doi: 10.1016/j.clim.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 154.Scadding GW, Eifan A, Penagos M, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy 2015; 45: 613–623. doi: 10.1111/cea.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pfaar O, Calderon MA, Andrews CP, et al. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future – an EAACI Position Paper. Allergy 2017; 72: 1035–1042. doi: 10.1111/all.13133 [DOI] [PubMed] [Google Scholar]
- 156.Pfaar O, Zieglmayer P. Allergen exposure chambers: implementation in clinical trials in allergen immunotherapy. Clin Transl Allergy 2020; 10: 33. doi: 10.1186/s13601-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hossenbaccus L, Steacy LM, Walker T, et al. Utility of environmental exposure unit challenge protocols for the study of allergic rhinitis therapies. Curr Allergy Asthma Rep 2020; 20: 34. doi: 10.1007/s11882-020-00922-8 [DOI] [PubMed] [Google Scholar]
- 158.Jacobs RL, Harper N, He W, et al. Effect of confounding cofactors on responses to pollens during natural season versus pollen challenge chamber exposure. J Allergy Clin Immunol 2014; 133: 1340–1346. doi: 10.1016/j.jaci.2013.09.051 [DOI] [PubMed] [Google Scholar]
- 159.Ellis AK, Jacobs RL, Tenn MW, et al. Clinical standardization of two controlled allergen challenge facilities: the Environmental Exposure Unit and the Biogenics Research Chamber. Ann Allergy Asthma Immunol 2019; 122: 639–646. doi: 10.1016/j.anai.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 160.Struben VM, Wieringa MH, Feenstra L, et al. Nasal nitric oxide and nasal allergy. Allergy 2006; 61: 665–670. doi: 10.1111/j.1398-9995.2006.01096.x [DOI] [PubMed] [Google Scholar]
- 161.Belkowski SM, Boot JD, Mascelli MA, et al. Cleaved secretory leucocyte protease inhibitor as a biomarker of chymase activity in allergic airway disease. Clin Exp Allergy 2009; 39: 1179–1186. doi: 10.1111/j.1365-2222.2009.03247.x [DOI] [PubMed] [Google Scholar]
- 162.KleinJan A, McEuen AR, Dijkstra MD, et al. Basophil and eosinophil accumulation and mast cell degranulation in the nasal mucosa of patients with hay fever after local allergen provocation. J Allergy Clin Immunol 2000; 106: 677–686. doi: 10.1067/mai.2000.109621 [DOI] [PubMed] [Google Scholar]
- 163.Varney VA, Jacobson MR, Sudderick RM, et al. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis 1992; 146: 170–176. doi: 10.1164/ajrccm/146.1.170 [DOI] [PubMed] [Google Scholar]
- 164.Vancheri C, Mastruzzo C, Armato F, et al. Intranasal heparin reduces eosinophil recruitment after nasal allergen challenge in patients with allergic rhinitis. J Allergy Clin Immunol 2001; 108: 703–708. doi: 10.1067/mai.2001.118785 [DOI] [PubMed] [Google Scholar]
- 165.Meltzer EO. The relationships of rhinitis and asthma. Allergy Asthma Proc 2005; 26: 336–340. [PubMed] [Google Scholar]
- 166.van Drunen CM, Fokkens WJ. Basophils and mast cells at the centre of the immunological response. Allergy 2006; 61: 273–275. doi: 10.1111/j.1398-9995.2006.01069.x [DOI] [PubMed] [Google Scholar]
- 167.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 2003; 111: 1171–1183. doi: 10.1067/mai.2003.1592 [DOI] [PubMed] [Google Scholar]
- 168.Hellings PW, Prokopakis EP. Global airway disease beyond allergy. Curr Allergy Asthma Rep 2010; 10: 143–149. doi: 10.1007/s11882-010-0107-1 [DOI] [PubMed] [Google Scholar]
- 169.Beeh KM, Beier J, Kornmann O, et al. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: correlation with plasma interleukin-5. Clin Exp Allergy 2003; 33: 475–482. doi: 10.1046/j.1365-2222.2003.01632.x [DOI] [PubMed] [Google Scholar]
- 170.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy 2008; 63: Suppl. 8, 8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 171.Diamant Z, van Maaren M, van Wijk RG, et al. Guidance for the regulatory status of allergen extracts in clinical trials. Eur Respir J 2015; 46: 1223–1225. doi: 10.1183/13993003.00729-2015 [DOI] [PubMed] [Google Scholar]
- 172.Hatzivlassiou M, Grainge C, Kehagia V, et al. The allergen specificity of the late asthmatic reaction. Allergy 2010; 65: 355–358. doi: 10.1111/j.1398-9995.2009.02184.x [DOI] [PubMed] [Google Scholar]
- 173.Boulet LP, Gauvreau G, Boulay ME, et al. Allergen-induced early and late asthmatic responses to inhaled seasonal and perennial allergens. Clin Exp Allergy 2015; 45: 1647–1653. doi: 10.1111/cea.12587 [DOI] [PubMed] [Google Scholar]
- 174.Harris J, Mason DE, Li J, et al. Activity profile of dust mite allergen extract using substrate libraries and functional proteomic microarrays. Chem Biol 2004; 11: 1361–1372. doi: 10.1016/j.chembiol.2004.08.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02782-2021.Supplement (292KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02782-2021.Shareable (381.2KB, pdf)


