Pulmonary hypertension (PH) is a hemodynamic disorder syndrome defined as a mean resting pulmonary artery pressure higher than 20 mmHg to 25 mmHg, which continues to cause high mortalities and low survival rates (1).Current clinical classification divides PH into five major groups, among which the precise diagnosis and classification of Group 1 (pulmonary arterial hypertension, PAH) caused by narrowed/thickened/stiff lung arteries and Group 3 due to lung disease remains an unmet challenge (2). Most of PH treatments are based on Group 1 studies, but specific therapy has no significant effects for Group 3 patients in clinic (3). Thus, developing easy, precise, and noninvasive biomarkers to identify Group 1 and 3 PH would be of significant clinical benefit to avoid delayed or inefficient treatment(4). Here, we report a new methodology that leverages high-throughput RNA modification profiling platform based on LC-MS/MS, that simultaneously identifies and quantifies multiple RNA modifications (5), to determine if it is possible to distinguish Group 1/3 PH patients from control individuals according to RNA modification signatures (PH patient recruitment following the updated guideline of the 6th Word Symposium for Pulmonary Hypertension (1)). As a result, we successfully developed a diagnostic signature composed of 7 RNA modifications (m1A, m1G, m2G, m5C, m5U, Am, and Im), which efficiently classifies patients with Group 3 PH separate from both controls and patients with Group 1 PH in the discovery and validation cohorts.
The discovery cohort was composed of four controls, eight subjects with Group 1 PH, and three subjects with Group 3 PH (Figure 1A). Peripheral blood of these subjects was collected from the ulnar vein followed by RNA extraction. RNA was digested to ribonucleosides using benzonase nuclease, phosphodiesterase nuclease and alkaline phosphatase I. RNA modifications were detected and quantified using a high-throughput LC-MS/MS based approach we established (5). The data from LC-MS/MS were acquired by Xcalibur Workstation software and modified ribonucleoside concentrations were quantified using Xcalibur QuanBrowser. Using this approach, we efficiently identify and quantify 18 modified nucleosides, including ψ (psi), Am, Cm, Um, Gm, I, m7G, m2G, m5U, m5C, m1A, m22G, m1G, ac4C, m5Um, m3U, Im, and m6A. Indeed, principal component analysis on the RNA modification profiles revealed a distinct pattern between the control and Group 3 PH subjects, with the Group 1 PH subjects largely falling in between (Figure 1B), suggesting there is significant potential discriminative power embedded in the RNA modification information. Further detailed analyses prioritized five upregulated (m1A, m1G, m2G, m5C, and m5U) and two downregulated (Am and Im) RNA modification species in the Group 3 PH patients (Figure 1C) identifying an RNA modification signature.
Figure 1. The performance of the RNA modification signature in discovery and validation cohorts.
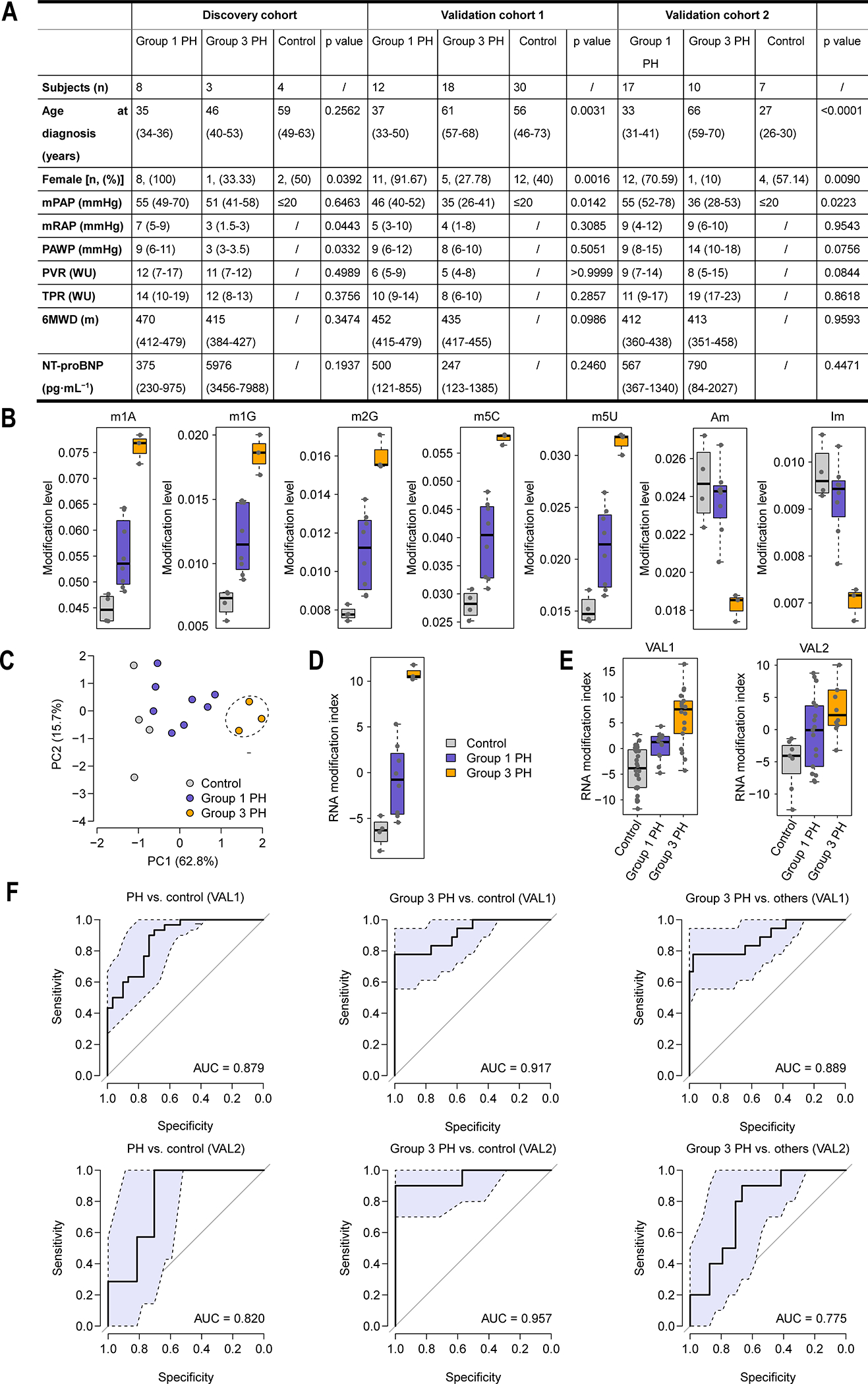
(A) Patient characteristics of the discovery and validation cohorts. Values are expressed as n, median (interquartile range) or n (%), unless otherwise stated. mPAP mmHg: Mean pulmonary arterial pressure mmHg; mRAP mmHg: Mean right atrial pressure mmHg; PAWP mmHg: Pulmonary arterial wedge pressure mmHg; PVR wood: Pulmonary vascular resistance Wood units; TPR wood: Total pulmonary resistance Wood units; 6MWD: 6-min walk distance; NT-pro BNP: N-terminal pro-brain natriuretic peptide. The t-test was performed for statistical analysis, except age at diagnosis were analyzed by ANOVA test and Chi-Squared test was used for gender comparison. (B) Principal component analysis on the RNA modification data. PC1: the first principal component; PC2: the second principal component. Each dot represents one human subject in the discovery cohort. (C) The RNA modification signature. (D) Comparison of the RNA modification index among the control, Group 1 PH, and Group 3 PH subjects in the discovery cohort. Mean ± standard deviation: −6.48±1.60 for control, −0.83±3.90 for Group 1 PH, and 10.84±0.84 for Group 3 PH. (E) Comparison of the RNA modification index among the control, Group 1 PH, and Group 3 PH subjects in two validation cohorts. Mean ± standard deviation: −3.90±4.16 for control, 0.48±2.72 for Group 1 PH, and 6.19±5.41 for Group 3 PH in VAL1 and −5.22±4.09 for control, −0.43±5.71 for Group 1 PH, and 4.39±6.41 for Group 3 PH in VAL2. (F) The ROC curve of the RNA modification index in distinguishing between the PH and control subjects, between the Group 3 PH and control subjects, and between the Group 3 PH patients and other subjects (controls and Group 1 subjects) in the validation cohorts. The light blue area indicates the confidence interval of the ROC curve. VAL1: the first validation cohort; VAL2: the second validation cohort.
We next computed an RNA modification index for each subject in the discovery cohort using the aforementioned RNA modification signature. This corresponded to a linear combination of the modifications within the signature:
Where I is the RNA modification index; wi is the weight of modification i within the modification signature (wi = 1 for m1A, m1G, m2G, m5C, and m5U; wi = −1 for Am and Im); ei denotes the modification level of modification i; and μi and τi are the mean and standard deviation of the level of modification i across all the samples, respectively. As a result, we found that the RNA modification index was significantly higher in the Group 3 PH patients than in the controls and Group 1 PH patients; and the index of the Group 1 PH patients is significantly higher than that of the controls (t-test: P < 0.05) (Figure 1D).
To further assess the performance of the RNA modification signature, two independent validation cohorts (30 controls, 12 Group 1 PH subjects, and 18 Group 3 PH subjects in the first validation cohort [VAL1], which was based on fresh whole blood; 7 controls, 17 Group 1 PH subjects, and 10 Group 3 PH subjects in the second cohort [VAL2], which was based on EDTA-anticoagulant blood) were investigated (Figure 1A). Our results showed that, in both validation cohorts, the RNA modification index in the Group 3 and Group 1 PH patients was significantly higher than in the controls (t-test: P < 0.05). The index of Group 3 PH patients was also significantly higher than in the Group 1 PH patients in VAL1 and marginally higher in VAL2 (t-test: P < 0.05 in VAL1 and P = 0.066 in VAL2) (Figure 1E). The area under the receiver operating characteristic (ROC) curve (AUC) was 0.879 in VAL1 and 0.820 in VAL2 between the controls and PH patients, 0.917 in VAL1 and 0.957 in VAL2 between the controls and Group 3 PH patients, and 0.889 in VAL1 and 0.775 in VAL2 between the Group 3 PH patients and the other subjects (Figure 1F). These results suggest that our RNA modification index can distinguish Group 3 patients from Group 1 and controls in both validation cohorts. Moreover, we found that the RNA modification index in fresh blood performed better than that in EDTA-anticoagulant blood samples. Thus, our data support the conclusion that this RNA modification signature has a strong classification power for PH diagnosis, especially in fresh whole blood.
In summary, we have identified an RNA modification signature in peripheral blood, which efficiently distinguishes between controls and patients with Group 1/3 PH. Moreover, blood-based RNA modification signatures could be harnessed as a noninvasive biomarker for classifying other PH subgroups and potentially other diseases. Further, while whole-blood RNA levels have been reported to linked to disease diagnosis, our blood-based RNA modification signature, combined with RNA expression profiles, might be a powerful detection biomarker to facilitate precision medicine, which will require systematic studies in future investigations.
Fundings:
This work was funded by National Key Research and Development Program of China (2019YFA0802600 and 2018YFC1004500 to Y.Z., 2016YFA0500903 and 2017YFC1001401 to E.D., 2019YFE0119400 to H.T.), Natural Science Foundation of China (82122027 and 32171110 to Y.Z., 31671568 to E.D., 81970052, 81770059 and 82170057 to H.T.), and the National Institutes of Health (P01HL146369 and P01HL134610 to S.M.B).
Reference:
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prins KW, Duval S, Markowitz J, Pritzker M, Thenappan T. Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deano RC, Glassner-Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg-Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013; 173: 887–893. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, Pahima M, Liu Y, Yan M, Cao Z, Lei X, Cao Y, Peng H, Liu S, Wang Y, Zheng H, Woolsey R, Quilici D, Zhai Q, Li L, Zhou T, Yan W, Lyko F, Zhang Y, Zhou Q, Duan E, Chen Q. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nature cell biology 2018; 20: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]


