Abstract
Background and Aims:
Barrett’s esophagus (BE) is the only recognized precursor for esophageal adenocarcinoma. Helicobacter pylori (H. pylori) infection is a major contributing factor towards upper gastrointestinal diseases, but its relationship with BE remains controversial. Some previous studies suggested that H. pylori infection negatively correlated with BE, while others did not. This may be attributed to the difference in the selection of control groups among studies. The present meta-analysis aims to clarify their association by combining all available data from well-designed studies.
Methods:
The PubMed, EMBASE, and Cochrane Library databases were searched. Odds ratios (ORs) with 95% confidence intervals (CIs) were pooled by a random-effects model. Heterogeneity was evaluated using the Cochran’s Q test and I2 statistics. Meta-regression, subgroup, and leave-one-out sensitivity analyses were employed to explore the sources of heterogeneity.
Results:
Twenty-four studies with 1,354,369 participants were included. Meta-analysis found that patients with BE had a significantly lower prevalence of H. pylori infection than those without (OR = 0.53, 95% CI = 0.45–0.64; p < 0.001). The heterogeneity was statistically significant (I² = 79%; p < 0.001). Meta-regression, subgroup, and leave-one-out sensitivity analyses did not find any source of heterogeneity. Meta-analysis of 7 studies demonstrated that CagA-positive H. pylori infection inversely correlated with BE (OR = 0.25, 95% CI = 0.15–0.44; p = 0.000), but not CagA-negative H. pylori infection (OR = 1.22, 95% CI = 0.90–1.67; p = 0.206). Meta-analysis of 4 studies also demonstrated that H. pylori infection inversely correlated with LSBE (OR = 0.39, 95% CI = 0.18–0.86; p = 0.019), but not SSBE (OR = 0.73, 95% CI = 0.30–1.77; p = 0.484).
Conclusion:
H. pylori infection negatively correlates with BE. More experimental studies should be necessary to elucidate the potential mechanisms in future.
Keywords: Barrett’s esophagus, esophageal adenocarcinoma, gastroesophageal reflux disease, Helicobacter pylori, meta-analysis
Introduction
Barrett’s esophagus (BE), a precancerous lesion of esophageal adenocarcinoma (EAC), is defined as the squamous epithelium replaced by specialized intestinal metaplasia (IM). 1 It is often asymptomatic and can only be diagnosed by endoscopy. 2 Risk factors of BE include age greater than 50 years, male, smoking, white race, gastroesophageal reflux disease (GERD), high body mass index (BMI), and hiatal hernia. 3 Its prevalence is approximately 10% to 15% in patients with GERD.4,5
Helicobacter pylori (H. pylori), a gram-negative microaerophilic bacterium, influences at least 50% of the world’s population. 6 At present, H. pylori is the most common causative agent of infection-associated cancers, accounting for 5.5% of the global cancer burden. 7 The importance of H. pylori in upper gastrointestinal pathology is undoubted, because it can lead to gastric and duodenal ulcers, mucosa-associated tissue lymphoma, non-Hodgkin’s lymphoma of the stomach, and gastric adenocarcinoma. 8 Nevertheless, the relationship between H. pylori and esophagus diseases, especially BE, remains controversial. 9 Some studies have reported that H. pylori is a risk factor for BE,10,11 while others have inferred that H. pylori has a protective effect on BE12,13 and that H. pylori infection may not be associated with BE.14,15 Until now, several meta-analyses have explored their association. However, among these meta-analyses, not all control subjects have undergone endoscopy, which may cause missed diagnoses of GERD or peptic ulcer, thereby overestimating or underestimating the effect of H. pylori infection on BE. Therefore, we have comprehensively collected the most recent data from well-designed studies and conducted a meta-analysis to analyze the correlation between H. pylori and BE.
Methods
The meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PRISMA checklist is shown in Supplementary Material .
Registration
The meta-analysis was registered in PROSPERO with a registration number of CRD42021241042.
Literature search
All relevant papers regarding the prevalence of H. pylori infection in patients with and without BE were searched in the PubMed, EMBASE, and Cochrane Library databases. The search was performed using the following terms: (‘Helicobacter pylori’ OR ‘H pylori’ OR ‘H. pylori’ OR ‘Helicobacter’ OR ‘Campylobacter Pylori’) AND (‘Barrett esophagus’ OR ‘Barrett’ OR ‘Barrett metaplasia’ OR ‘Barrett oesophagus’). The last search was conducted on November 11, 2021. There was no language restriction.
Selection criteria
Inclusion criteria were as follows: (1) observational (case-control or cross-sectional) studies, (2) all participants should undergo upper gastrointestinal endoscopy, (3) studies should compare the prevalence of H. pylori infection between participants with and without BE, and (4) H. pylori infection should be confirmed by histology, rapid urease test, culture, stool antigen test, and/or serology.
Exclusion criteria were as follows: (1) duplicated studies; (2) reviews and meta-analyses; (3) case reports; (4) guidelines, consensus, or reports; (5) editorials, comments, letters, or notes; (6) experimental or animal studies; (7) studies which did not explore the association between H. pylori infection and BE; (8) participants had peptic ulcer or gastric cancer; (9) participants in control groups did not undergo upper gastrointestinal endoscopy; (10) participants in control groups had GERD; (11) overlapping participants among studies; and (12) absence of relevant data.
Data extraction
The following data were extracted from the included studies: first author, publication year, country, study design, number of participants in case and control groups, the prevalence of H. pylori infection in the 2 groups, methods and biopsy sites for detecting H. pylori infection, diagnostic criteria for BE, diagnostic timing of BE, whether age and gender were matched between participants with and without BE, whether participants with a history of H. pylori eradication therapy were excluded, whether participants who used proton pump inhibitors (PPIs) within at least 2 weeks before detection of H. pylori infection were excluded, and the prevalence of H. pylori infection according to the segment lengths of BE and status of cytotoxin-associated gene A (CagA).
Study quality assessment
The quality of included case-control studies was assessed by the Newcastle-Ottawa Scale (NOS), which includes study selection (4 points), comparability (2 points), and exposure (3 points). The maximum NOS score is 9. A score of 0–3, 4–6, and 7–9 represents low, moderate, and high quality, respectively.
The quality of included cross-sectional studies was assessed with 11 items formulated by the Agency for Healthcare Research and Quality (AHRQ), which are answered with "yes", "no", or "unclear". The maximum AHRQ score is 11. A score of 0–3, 4–7, and 8–11 represents low, moderate, and high quality, respectively.
Statistical analyses
All statistical analyses were performed using Review Manager software (Version 5.4, Cochrane collaboration, the Nordic Cochrane Center, Copenhagen, Denmark) and Stata software (Version 12.0, Stata Corp, College Station, USA). We pooled the odds ratios (ORs) and 95% confidence intervals (CIs) by using a random-effects model. Forest plots were constructed for a visual display of ORs of individual studies. The Cochrane Q test and I² statistics were employed to assess the heterogeneity. I2 > 50% and/or p < 0.1 were considered to have statistically significant heterogeneity. Leave-one-out sensitivity analyses were assessed by sequentially omitting 1 study each time. Subgroup analyses were performed according to study design (case-control vs cross-sectional), publication year (before 2010 vs after 2010), region (Asia vs Europe vs America), country (Eastern vs Western), methods for detecting H. pylori (histology/rapid urease test vs serology vs ⩾ 2 diagnostic methods), sample size (<10,000 vs >10,000), number of biopsy sites for detecting H. Pylori (1 vs ⩾ 2), diagnostic criteria for BE (IM vs CM), diagnostic timing of BE (newly vs previously diagnosed), whether age and gender were matched between patients with and without BE (matched vs unmatched), participants with a history of H. pylori eradication therapy (excluded vs not excluded), and participants who used PPIs within at least 2 weeks before detection of H. pylori infection (excluded vs not excluded). The interaction between subgroups was tested. Meta-regression analyses were also employed according to the variables mentioned above. Publication bias among the included studies was checked by the Egger test. p < 0.1 was considered as a statistically significant publication bias. In addition, the prevalence of H. pylori infection according to different segment lengths of BE (LSBE vs SSBE) and status of CagA (CagA-positive vs CagA-negative) were also compared.
Results
Study selection
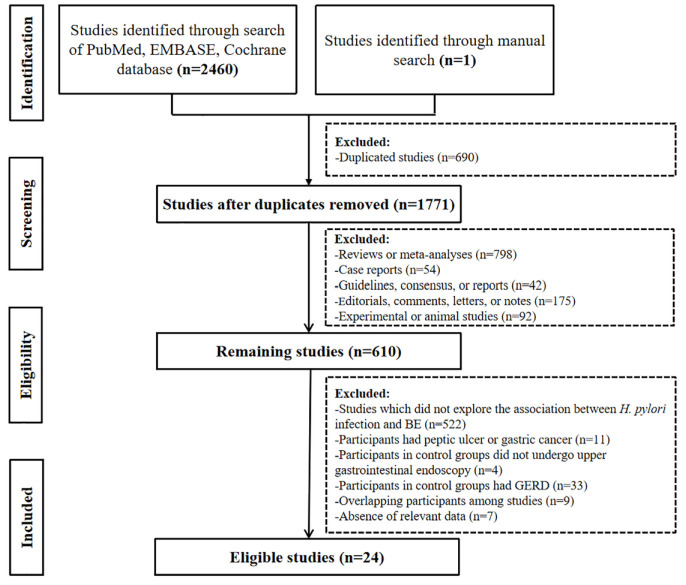
Our initial search identified 2,422 studies from the PubMed, EMBASE, and Cochrane Library databases, and 1 study from hand-searching. Finally, 24 studies with 1,354,369 participants were included (Figure 1). Notably, 2 of them had overlapping participants, but explored different variables. One had a larger sample size, but just provided the data in LSBE participants; 16 and the other had a smaller sample size, but specifically compared the prevalence of H. pylori infection according to different segment lengths of BE. 17
Figure 1.
A flowchart of study inclusion.
Study characteristics
Characteristics of the included studies were shown in Table 1. Among them, 14 studies were case-control studies, and 9 were cross-sectional studies. They were published between 1988 and 2020. Five studies were performed in Asia,16,19,22,25,27 11 in Europe,20,23,26,28–30,32–34,36,37 and 7 in America.12,18,21,24,31,35,38
Table 1.
Summary of baseline characteristics of the prevalence of H. pylori infection in patients with and without BE.
| First author | Country | Study design |
BE on endoscopy | Prevalence of H. pylori infection |
Methods for detection of H. pylori | Biopsy sites for detecting H. pylori | Diagnostic criteria for BE | Diagnostic timing of BE | Age and gender matched between 2 groups | Exclusion criteria for participants | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE | No BE | BE | No BE | History of H. pylori eradication therapy | Using PPIs within at least 2 weeks before detection of H. pylori infection | ||||||||
| Turner et al. 18 | USA | Cross-sectional | 52186 | 1207207 | 2332/ 52186 |
126351/ 1207207 |
H | Antral, corpus, and angus | IM | NA | No | No | No |
| Zhang et al. 19 | China | Case-control | 40 | 40 | 15/40 | 23/40 | H, R | Esophageal and antral | CM | NA | No | No | No |
| Xu et al. 20 | UK | Cross-sectional | 38 | 202 | 3/38 | 34/202 | R, S | NA | IM | NA | No | No | No |
| Tan et al. 21 | USA | Cross-sectional | 329 | 1856 | 68/329 | 606/ 1856 |
H | Antral, corpus, and cardia | IM | Previously | No | No | No |
| Chen et al. 22 | China | Case-control | 161 | 644 | 42/148 | 261/588 | R | Antral | IM | NA | Yes | No | Yes |
| Dore et al. 23 | Italy | Cross-sectional | 133 | 1772 | 49/131 | 669/ 1772 |
H, R, U | Antral and corpus | IM | Newly | No | No | Yes |
| Rubenstein et al. 12 | USA | Case-control | 150 | 177 | 25/150 | 46/177 | S | NA | IM | Newly | No | No | No |
| Sonnenberg et al. 24 | USA | Cross-sectional | 2510 | 76475 | 144/ 2510 |
9356/ 76475 |
H | Antral, corpus, and angus | IM | Previously | No | No | No |
| Abe et al. 16 | Japan | Case-control | 36 | 108 | 4/36 | 80/108 | H, S, R | Antral, corpus | CM | Newly | Yes | Yes | Yes |
| Rajendra et al. 25 | Malaysia | Case-control | 55 | 53 | 29/55 | 32/53 | H, S, R | Antral, corpus, and cardia | IM | Previously | No | Yes | Yes |
|
§
lnomata et al. 17 |
Japan | Case-control | 36 | 80 | 3/36 | 57/80 | H, S, R | Antral, corpus | IM | NA | No | Yes | Yes |
| Loffeld et al. 26 | Netherlands | Cross-sectional | 307 | 5341 | 55/179 | 1550/ 3975 |
H, C | Antral | NA | Previously | No | No | No |
| Rajendra et al. 27 | Malaysia | Case-control | 123 | 1741 | 46/123 | 604/ 1741 |
H, R | Antral and corpus | IM | NA | No | Yes | Yes |
| Ackermark et al. 28 | Netherlands | Cross-sectional | 51 | 62 | 21/51 | 39/62 | S | NA | IM | NA | No | No | No |
| Laheij et al. 29 | Netherlands | Cross-sectional | 23 | 528 | 6/23 | 281/528 | H, C, R | Antral and corpus | CM | NA | No | Yes | No |
| Rugge et al. 30 | Italy | Case-control | 53 | 53 | 19/53 | 36/53 | H, S, P | Antral, corpus, and incisural | IM | NA | Yes | Yes | Yes |
| Vaezi et al. 31 | USA | Case-control | 83 | 60 | 28/83 | 25/60 | H, S | Antral, corpus | CM | Previously | No | Yes | No |
| Loffeld et al. 32 | Netherlands | Cross-sectional | 36 | 454 | 14/36 | 248/454 | H, S, R, C | Antral | CM | Newly | No | Yes | Yes |
| Vieth et al. 33 | Germany | Case-control | 1054 | 712 | 562/ 1054 |
468/712 | H | Antral and corpus | IM | Previously | No | No | No |
| Kiltz et al. 34 | Germany | Case-control | 35 | 320 | 8/35 | 91/320 | R, S | Antrum and corpus | CM | Previously | No | Yes | No |
| Vicari et al. 35 | USA | Case-control | 48 | 57 | 15/48 | 26/57 | H, S | Antral, fundus, and cardia | CM | Previously | No | No | No |
| Werdmuller et al. 36 | Netherlands | Case-control | 13 | 399 | 3/13 | 204/399 | H, R, C, S | Antral | NA | NA | No | No | No |
| Newton et al. 37 | UK | Case-control | 16 | 25 | 4/16 | 9/25 | H, R | Antral and fundus | CM | Previously | No | No | Yes |
| Paull et al. 38 | USA | Case-control | 26 | 26 | 10/26 | 11/26 | H | Gastric and esophageal | CM | Previously | Yes | No | No |
Study only in the analysis for BE segment length.
BE, Barrett’s esophagus; C, culture; CM, columnar metaplasia; H, histology; IM, intestinal metaplasia; PCR, polymerase chain reaction; PPIs, proton pump inhibitors; S, serology; SA, stool antigen; R, rapid urease test; U, urea breath test.
Study quality
Among the case-control studies, 8 and 6 were of moderate and high quality, respectively (Supplementary Table 1). Among the cross-sectional studies, 3 and 6 were of moderate and high quality, respectively (Supplementary Table 2).
Meta-analyses in overall patients
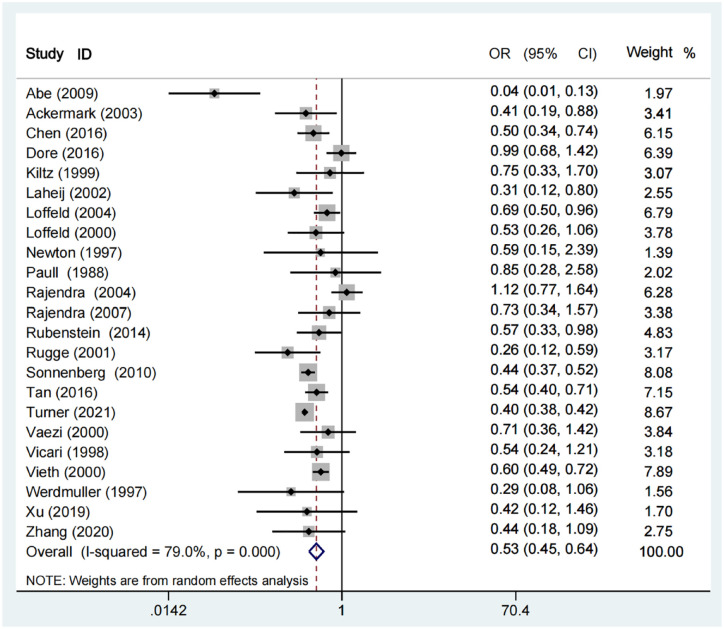
Meta-analysis demonstrated a significantly lower prevalence of H. pylori infection in patients with BE than those without (OR = 0.53, 95% CI = 0.45–0.64; p < 0.001). The heterogeneity was statistically significant (I²=79%; p < 0.001) (Figure 2).
Figure 2.
Forest plots showing the association of H. pylori infection with BE.
Results of sensitivity analyses were shown in Supplementary Table 3. After omitting each study, the heterogeneity remained statistically significant.
Results of subgroup analyses were shown in Table 2. Regardless of study design, publication year, region, country, diagnostic methods of H. pylori infection, sample size, number of biopsy sites for detecting H. pylori, diagnostic criteria for BE, diagnostic timing of BE, age and gender matched between 2 groups, participants with a history of H. pylori eradication therapy, and participants who used PPIs within at least 2 weeks before detection of H. pylori infection, meta-analyses demonstrated a significantly lower prevalence of H. pylori infection in patients with BE than those without. The interaction between subgroups was only significant in the subgroup analysis according to the sample size (p = 0.002), but not in others.
Table 2.
Results of subgroup analyses regarding the association of H. pylori infection with BE.
| Groups | No. Studies |
OR (95%CI) | Heterogeneity | Pinteraction | |
|---|---|---|---|---|---|
| I2 (%) | p value | ||||
| Total | 23 | 0.53 (0.45-0.64; p < 0.001) | 79% | <0.001 | |
| Study design | 0.940 | ||||
| Case-control | 14 | 0.53 (0.39–0.71; p < 0.001) | 67% | <0.001 | |
| Cross-sectional | 9 | 0.52 (0.42–0.65; p < 0.001) | 79% | <0.001 | |
| Publication year | 0.870 | ||||
| After 2010 | 8 | 0.51 (0.42–0.63; p < 0.001) | 76% | <0.001 | |
| Before 2010 | 15 | 0.53 (0.40–0.70; p < 0.001) | 66% | <0.001 | |
| Region | 0.220 | ||||
| Asia | 5 | 0.43 (0.20–0.93; p = 0.030) | 88% | <0.001 | |
| Europe | 11 | 0.58 (0.46–0.73; p < 0.001) | 39% | 0.090 | |
| America | 7 | 0.46 (0.40–0.53; p < 0.001) | 46% | 0.080 | |
| Country | 0.580 | ||||
| Eastern | 5 | 0.43 (0.20–0.93; p = 0.030) | 88% | <0.001 | |
| Western | 18 | 0.54 (0.45–0.64; p < 0.001) | 72% | <0.001 | |
| Diagnostic methods of H. Pylori | 0.900 | ||||
| Histology/RUT | 6 | 0.48 (0.41–0.58; p < 0.001) | 77% | <0.001 | |
| Serology | 3 | 0.50 (0.33–0.76; p = 0.001) | 0% | 0.770 | |
| ⩾ 2 diagnostic methods | 14 | 0.53 (0.38–0.74; p < 0.001) | 71% | <0.001 | |
| Sample size | 0.002 | ||||
| > 10000 | 2 | 0.40 (0.39–0.42; p < 0.001) | 0% | 0.330 | |
| < 10000 | 21 | 0.56 (0.46–0.68; p < 0.001) | 61% | <0.001 | |
| Number of biopsy sites for detecting H. Pylori | 0.610 | ||||
| 1 | 4 | 0.58 (0.46–0.73; p < 0.001) | 0% | 0.400 | |
| ⩾ 2 | 16 | 0.54 (0.43–0.67; p < 0.001) | 84% | <0.001 | |
| Diagnostic criteria for BE | 0.410 | ||||
| IM | 12 | 0.56 (0.45–0.69; p < 0.001) | 85% | <0.001 | |
| CM | 9 | 0.44 (0.27–0.73; p = 0.001) | 64% | 0.005 | |
| Diagnostic timing of BE | 0.450 | ||||
| Newly diagnosed | 4 | 0.39 (0.15–0.98; p < 0.001) | 89% | <0.001 | |
| Previously diagnosed | 11 | 0.55 (0.48–0.63; p < 0.001) | 17% | 0.280 | |
| Age and gender matched between 2 groups | 0.160 | ||||
| Matched | 4 | 0.28 (0.10–0.76; p < 0.001) | 84% | <0.001 | |
| Unmatched | 19 | 0.58 (0.48–0.69; p < 0.001) | 79% | <0.001 | |
| Participants with a history of H. pylori eradication therapy | 0.570 | ||||
| Excluded | 8 | 0.45 (0.25–0.81; p = 0.008) | 82% | <0.001 | |
| Not excluded | 15 | 0.54 (0.45–0.64; p < 0.001) | 75% | <0.001 | |
| Participants who used PPIs within at least 2 weeks before detection of H. pylori infection | 0.910 | ||||
| Excluded | 8 | 0.50 (0.29–0.83; p = 0.008) | 83% | <0.001 | |
| Not excluded | 15 | 0.51 (0.44–0.60; p < 0.001) | 63% | <0.001 | |
BE, Barrett’s esophagus; CM, columnar metaplasia; IM, intestinal metaplasia; OR, odds ratio; PPIs, proton pump inhibitors; RUT, rapid urease test.
Meta-regression analyses did not find any source of heterogeneity (Supplementary Table 4). Egger test showed significant evidence of publication bias (p = 0.043).
Meta-analyses regarding association of CagA status with BE
Seven studies compared the prevalence of H. pylori infection according to different status of CagA (Supplementary Table 5). There was a significantly lower prevalence of CagA-positive H. pylori infection in patients with BE than those without BE (OR = 0.25, 95% CI = 0.15–0.44; p = 0.000). The heterogeneity was not statistically significant (I² = 42%; P = 0.111) (Supplementary Figure 1). By contrast, the prevalence of CagA-negative H. pylori infection was statistically similar between patients with and without BE (OR = 1.22, 95% CI = 0.90–1.67; p = 0.206). The heterogeneity was not statistically significant (I² = 0%; p = 0.881) (Supplementary Figure 2).
Meta-analyses regarding association of H. pylori with BE segment lengths
Four studies compared the prevalence of H. pylori infection according to different segment lengths of BE (Supplementary Table 6). There was a significantly lower prevalence of H. pylori infection in patients with LSBE than those without BE (OR = 0.39, 95% CI = 0.18–0.86; p = 0.019). The heterogeneity was statistically significant (I² = 66%; p = 0.033) (Supplementary Figure 3). By contrast, the prevalence of H. pylori infection was statistically similar between patients with SSBE and those without BE (OR = 0.73, 95% CI = 0.30–1.77; p = 0.484). The heterogeneity was statistically significant (I² = 76%; p = 0.005) (Supplementary Figure 4). It is inappropriate to conduct meta-regression analyses to explore the sources of heterogeneity since only 4 studies were included. 39
Discussion
This is an updated meta-analysis which more comprehensively searched relevant studies to explore the relationship between H. pylori infection and BE. Our study found that the prevalence of H. pylori infection was significantly lower in patients with BE than those without, suggesting that H. pylori infection is inversely related to BE.
Seven previous meta-analyses were performed to explore the relationship between H. pylori infection and BE. However, their conclusions were inconsistent. The meta-analysis by Wang et al. 40 demonstrated no association of H. pylori infection with BE; by comparison, 6 others reported a significantly lower prevalence of H. pylori infection in patients with BE than those without.41–46
Our current meta-analysis has several advantages as compared to previous ones. First, there was a more comprehensive collection of eligible studies by expanding the search strategy and updating the final search date. Second, a larger number of subgroup analyses were planned to further explore the association between H. pylori infection and BE according to the 12 prespecified co-variates. More importantly, the interaction between subgroups was also tested to infer whether the protective effect of H. pylori infection on BE differs among subgroups, which has not been performed in 7 previous meta-analyses yet. Third, meta-regression analyses, an important statistical method for analyzing the source of heterogeneity, were performed in our meta-analysis, but have not been done in previous meta-analyses yet. Fourth, the selection of eligible participants in our meta-analysis is more reasonable and rigorous. In details, all participants included should have undergone upper gastrointestinal endoscopy; all participants included should not have been diagnosed with gastric cancer or peptic ulcer; and the participants included in control groups should not have been diagnosed with GERD. Such considerations are very important to eliminate the effects of these potential confounding factors on the reliability of our findings. By comparison, these selection criteria have not been employed by previous meta-analyses yet. More importantly, a remarkable negative correlation between H. pylori infection and BE has been observed in all of our subgroup analyses, strengthening the robustness of our conclusion.
Currently, the pathogenesis of BE is primarily attributed to frequently transient lower esophageal sphincter (LES) relaxation, which leads to gastric acid reflux, resulting in esophageal mucosa injury.47,48 The protective effect of H. pylori infection on BE may be explained by its secondary reduction in gastric acid secretion and reflux.
First, there are several possible mechanisms that H. pylori infection protects against BE by reducing gastric acid secretion, as follows: (1) fatty acids produced by H. pylori can directly inhibit parietal cell function, and then reduce gastric acid secretion; 49 (2) cytokines, such as TNF-α and IL-1ß, resulting from corpus-predominant gastritis caused by H. pylori infection, can also inhibit parietal cell function, thereby reducing gastric acid secretion;50,51 (3) long-term colonization of H. pylori in stomach can cause atrophic gastritis, which reduces the number of parietal cell, further decreasing gastric acid secretion; and (4) ghrelin can protect gastric mucosa 52 and promote gastric acid secretion. 53 H. pylori infection causes corpus gastritis and/or gastric atrophic changes,54,55 leading to the loss of ghrelin producing cells and reduction of plasma ghrelin concentrations, which finally aggravates gastric mucosal damage and reduces gastric acid secretion.
Second, there are some potential mechanisms that H. pylori infection protects against BE by reducing gastric acid reflux, as follows: (1) elevated serum gastrin levels in antrum-predominant H. pylori gastritis may increase LES pressure, and then reduce gastric acid reflux; 56 and (2) ghrelin may promote food intake. 57 A decreased level of ghrelin secondary to H. pylori infection is associated with a reduction of BMI with a lower intra-abdominal pressure which decreases gastric acid reflux. 58 In addition, bile acids in combination with low pH may induce oxidative stress and DNA damage in esophageal cells, resulting in the activation of anti-apoptotic pathways and increased inflammatory response on esophageal tissues. 59 By contrast, reduced bile acids reflux, which can result from increased LES pressure and decreased intra-abdominal pressure caused by H. pylori infection, may play an important role in the protective effect on BE.60–63
Third, H. pylori DNA may directly down-regulate the inflammatory stimulation of type 1 interferon and IL-12 on esophageal mucosa, and then delay the progression of BE.64,65
Our meta-analysis demonstrated a significant association of BE with CagA-positive H. pylori infection, but not CagA-negative H. pylori infection. CagA can encode a high-molecular-weight immunodominant antigen that can induce IL-8 production.66–68 CagA-positive strains are also associated with enhanced local inflammatory response.69,70 Hence, CagA-positive H. pylori infection induces more severe gastric inflammation and multifocal atrophic gastritis, finally resulting in a reduction of gastric acid secretion.71,72
Our meta-analysis also found a significant association of H. pylori infection with LSBE, but not SSBE. Unfortunately, there is no direct evidence used to explain this phenomenon. It seems obvious that SSBE has a lower degree of gastric acid exposure than LSBE.73,74 Accordingly, it is hypothesized that SSBE may be less affected by a reduction of gastric acid secretion and reflux attributed to H. pylori infection compared with LSBE. It should be acknowledged that only 4 small studies explored the prevalence of H. pylori infection according to different segment lengths of BE. Thus, such statistical results may be unpowered.
Our meta-analysis had several other limitations. First, most of the included studies were retrospective, leading to the selection bias and recall bias. Second, the heterogeneity among studies was significant, in spite of leave-one-out sensitivity and meta-regression analyses. Third, 2 studies29,37 performed biopsies at different sites to assess H. pylori infection and provided more than 1 H. pylori infection rate. Only the H. pylori infection in the antrum was selected for the present meta-analysis, which may underestimate the prevalence of H. pylori infection.75,76 Forth, the absence of detailed information on race or ethnicity may compromise from estimating the prevalence of H. pylori infection in different racial or ethnic groups of BE patients. Fifth, the definitions of BE proposed by current practice guidelines and consensus are different among countries. IM is a required criterion for BE according to the American College of Gastroenterology clinical guideline published 2016, 77 but not the Chinese consensus published 2017. 78 Considering that currently available data regarding association of H. pylori with BE are from West, more well-designed studies should be conducted in China.
Our findings indicate an inverse relationship between H. pylori infection and BE. Accordingly, it appears that H. pylori eradication is not beneficial for the prevention of BE, which is contrary to the recommendations of current practice guidelines and consensus regarding management of H. pylori infection. 6 Indeed, other evidence also suggest the benefits of H. pylori infection on decreasing the risk of EAC, 79 especially Barrett’s adenocarcinoma, 80 and allergic diseases. 81 Therefore, we should re-evaluate the necessity of eliminating this pathogen globally, especially in the West, where gastric adenocarcinoma and peptic ulcer disease are much less prevalent.
Conclusion
The current study suggests that H. pylori infection is inversely related to BE. More well-designed prospective cohort studies are required to confirm our findings in future, and experimental studies should also be necessary to elucidate the potential mechanisms.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221117971 for Association of Barrett’s esophagus with Helicobacter pylori infection: a meta-analysis by Shaoze Ma, Xiaozhong Guo, Chunmei Wang, Yue Yin, Guangqin Xu, Hongxin Chen and Xingshun Qi in Therapeutic Advances in Chronic Disease
Acknowledgments
None.
Abbreviations: BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; GERD, gastroesophageal reflux disease; BMI, body mass index; H. pylori, Helicobacter pylori; PPIs, proton pump inhibitors; CagA, cytotoxin-associated gene A; NOS, Newcastle-Ottawa Scale; AHRQ, Agency for Healthcare Research and Quality; ORs, odds ratios; CIs, confidence intervals; CM, columnar metaplasia; IM, intestinal metaplasia; LES, lower esophageal sphincter; TNF, tumor necrosis factor; IL, interleukin.
ORCID iD: Xingshun Qi  https://orcid.org/0000-0002-9448-6739
https://orcid.org/0000-0002-9448-6739
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shaoze Ma, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China; Graduate School, Dalian Medical University, Dalian, China.
Xiaozhong Guo, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China.
Chunmei Wang, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China; Graduate School, Jinzhou Medical University, Jinzhou, China.
Yue Yin, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China; Graduate School, Jinzhou Medical University, Jinzhou, China.
Guangqin Xu, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China; Graduate School, Dalian Medical University, Dalian, China.
Hongxin Chen, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China; Graduate School, Liaoning University of Traditional Chinese Medicine, Shenyang, China.
Xingshun Qi, Department of Gastroenterology, General Hospital of Northern Theater Command, No. 83 Wenhua Road, Shenyang 110840, Liaoning Province, China.
Declarations
Ethics approval and consent to participate: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The requirement for approval is waived due to the nature of this article.
Consent for publication: Not applicable.
Author contributions: Shaoze Ma: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Xiaozhong Guo: Formal analysis; Validation; Writing – review & editing.
Chunmei Wang: Formal analysis; Writing – review & editing.
Yue Yin: Formal analysis; Writing – review & editing.
Guangqin Xu: Formal analysis; Writing – review & editing.
Hongxin Chen: Formal analysis; Writing – review & editing.
Xingshun Qi: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Full datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]
- 2. Kuipers EJ, Spaander MC. Natural history of Barrett’s esophagus. Dig Dis Sci 2018; 63: 1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc 2019; 90: 707–717. [DOI] [PubMed] [Google Scholar]
- 4. Sharma P. Clinical practice. Barrett’s Esophagus. N Engl J Med 2009; 361: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 5. Badreddine RJ, Wang KK. Barrett’s esophagus: pathogenesis, treatment, and prevention. Gastrointest Endosc Clin N Am 2008; 18: 495–512ix. [DOI] [PubMed] [Google Scholar]
- 6. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 7. Noto JM, Peek RM., Jr. Helicobacter pylori: an overview. Methods Mol Biol 2012; 921: 7–10. [DOI] [PubMed] [Google Scholar]
- 8. Pohl D, Keller PM, Bordier V, et al. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol 2019; 25: 4629–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boltin D, Niv Y, Schütte K, et al. Review: helicobacter pylori and non-malignant upper gastrointestinal diseases. Helicobacter 2019; 24(Suppl. 1): e12637. [DOI] [PubMed] [Google Scholar]
- 10. Ferrández A, Benito R, Arenas J, et al. CagA-positive Helicobacter pylori infection is not associated with decreased risk of Barrett’s esophagus in a population with high H. Pylori Infection Rate. BMC Gastroenterol 2006; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henihan RD, Stuart RC, Nolan N, et al. Barrett’s esophagus and the presence of Helicobacter pylori. Am J Gastroenterol 1998; 93: 542–546. [DOI] [PubMed] [Google Scholar]
- 12. Rubenstein JH, Inadomi JM, Scheiman J, et al. Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 2014; 12: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corley DA, Kubo A, Levin TR, et al. Helicobacter pylori infection and the risk of Barrett’s oesophagus: a community-based study. Gut 2008; 57: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng S, Cui Y, Xiao YL, et al. Prevalence of erosive esophagitis and Barrett’s esophagus in the adult Chinese population. Endoscopy 2009; 41: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 15. Abbas Z, Hussainy AS, Ibrahim F, et al. Barrett’s oesophagus and Helicobacter pylori. J Gastroenterol Hepatol 1995; 10: 331–333. [DOI] [PubMed] [Google Scholar]
- 16. Abe Y, Iijima K, Koike T, et al. Barrett’s esophagus is characterized by the absence of Helicobacter pylori infection and high levels of serum pepsinogen I concentration in Japan. J Gastroenterol Hepatol 2009; 24: 129–134. [DOI] [PubMed] [Google Scholar]
- 17. Inomata Y, Koike T, Ohara S, et al. Preservation of gastric acid secretion may be important for the development of gastroesophageal junction adenocarcinoma in Japanese people, irrespective of the H. Am J Gastroenterol 2006; 101: 926–933. [DOI] [PubMed] [Google Scholar]
- 18. Turner KO, Genta RM, Sonnenberg A. The meaning of incidental goblet cells at the gastroesophageal junction. Dig Dis Sci 2021; 66: 1588–1592. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Tan XP, Sun XZ, et al. Experimental study of the effect of helicobacter pylori infection on barrett esophagus and its correlation with immune function. Jundishapur Journal of Microbiology 2020; 13: 1–6. [Google Scholar]
- 20. Xu Y, Miremadi A, Link A, et al. Feasibility of combined screening for upper gastrointestinal adenocarcinoma risk by serology and Cytosponge testing: the SUGAR study. J Clin Pathol 2019; 72: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan MC, Murrey-Ittmann J, Nguyen T, et al. Risk profiles for Barrett’s esophagus differ between new and prevalent, and long- and short-segment cases. PLoS ONE 2016; 11: e0169250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CC, Hsu YC, Lee CT, et al. Central obesity and H. pylori infection influence risk of Barrett’s esophagus in an Asian population. PLoS ONE 2016; 11: e0167815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dore MP, Pes GM, Bassotti G, et al. Risk factors for erosive and non-erosive gastroesophageal reflux disease and Barrett’s esophagus in Nothern Sardinia. Scand J Gastroenterol 2016; 51: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 24. Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology 2010; 139: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 25. Rajendra S, Ackroyd R, Robertson IK, et al. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter 2007; 12: 177–183. [DOI] [PubMed] [Google Scholar]
- 26. Loffeld RJ, van der Putten AB. Helicobacter pylori and gastro-oesophageal reflux disease: a cross-sectional epidemiological study. Neth J Med 2004; 62: 188–191. [PubMed] [Google Scholar]
- 27. Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett’s esophagus: the long and short of it all. Dig Dis Sci 2004; 49: 237–242. [DOI] [PubMed] [Google Scholar]
- 28. Ackermark P, Kuipers EJ, Wolf C, et al. Colonization with cagA-positive Helicobacter pylori strains in intestinal metaplasia of the esophagus and the esophagogastric junction. Am J Gastroenterol 2003; 98: 1719–1724. [DOI] [PubMed] [Google Scholar]
- 29. Laheij RJ, Van Rossum LG, De Boer WA, et al. Corpus gastritis in patients with endoscopic diagnosis of reflux oesophagitis and Barrett’s oesophagus. Aliment Pharmacol Ther 2002; 16: 887–891. [DOI] [PubMed] [Google Scholar]
- 30. Rugge M, Russo V, Busatto G, et al. The phenotype of gastric mucosa coexisting with Barrett’s oesophagus. J Clin Pathol 2001; 54: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaezi MF, Falk GW, Peek RM, et al. CagA-positive strains of Helicobacter pylori may protect against Barrett’s esophagus. Am J Gastroenterol 2000; 95: 2206–2211. [DOI] [PubMed] [Google Scholar]
- 32. Loffeld RJ, Werdmuller BF, Kuster JG, et al. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett’s esophagus. Digestion 2000; 62: 95–99. [DOI] [PubMed] [Google Scholar]
- 33. Vieth M, Masoud B, Meining A, et al. Helicobacter pylori infection: protection against Barrett’s mucosa and neoplasia. Digestion 2000; 62: 225–231. [DOI] [PubMed] [Google Scholar]
- 34. Kiltz U, Baier J, Schmidt WE, et al. Barrett’s metaplasia and Helicobacter pylori infection. Am J Gastroenterol 1999; 94: 1985–1986. [DOI] [PubMed] [Google Scholar]
- 35. Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 1998; 115: 50–57. [DOI] [PubMed] [Google Scholar]
- 36. Werdmuller BF, Loffeld RJ. Helicobacter pylori infection has no role in the pathogenesis of reflux esophagitis. Dig Dis Sci 1997; 42: 103–105. [DOI] [PubMed] [Google Scholar]
- 37. Newton M, Bryan R, Burnham WR, et al. Evaluation of Helicobacter pylori in reflux oesophagitis and Barrett’s oesophagus. Gut 1997; 40: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paull G, Yardley JH. Gastric and esophageal Campylobacter pylori in patients with Barrett’s esophagus. Gastroenterology 1988; 95: 216–218. [DOI] [PubMed] [Google Scholar]
- 39. Higgins J, Thompson S, Deeks J, et al. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy 2002; 7: 51–61. [DOI] [PubMed] [Google Scholar]
- 40. Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol 2009; 104: 492–500; quiz 491–501. [DOI] [PubMed] [Google Scholar]
- 41. Gisbert JP, Pajares JM. [Prevalence of Helicobacter pylori infection in gastroesophageal reflux disease and Barrett’s esophagus]. Med Clin (Barc) 2002; 119: 217–223. [DOI] [PubMed] [Google Scholar]
- 42. Rokkas T, Pistiolas D, Sechopoulos P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol 2007; 5: 1413–71417. [DOI] [PubMed] [Google Scholar]
- 43. Fischbach LA, Nordenstedt H, Kramer JR, et al. The association between Barrett’s esophagus and Helicobacter pylori infection: a meta-analysis. Helicobacter 2012; 17: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Erőss B, Farkas N, Vincze Á, et al. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: a meta-analysis and systematic review. Helicobacter 2018; 23: e12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Z, Shaheen NJ, Whiteman DC, et al. Helicobacter pylori infection is associated with reduced risk of Barrett’s esophagus: an analysis of the Barrett’s and esophageal adenocarcinoma consortium. Am J Gastroenterol 2018; 113: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 46. Du YL, Duan RQ, Duan LP. Helicobacter pylori infection is associated with reduced risk of Barrett’s esophagus: a meta-analysis and systematic review. BMC Gastroenterol 2021; 21: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steele D, Baig KKK, Peter S. Evolving screening and surveillance techniques for Barrett’s esophagus. World J Gastroenterol 2019; 25: 2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kahrilas PJ. GERD pathogenesis, pathophysiology, and clinical manifestations. Cleve Clin J Med 2003; 70(Suppl. 5): S4–S19. [DOI] [PubMed] [Google Scholar]
- 49. Beil W, Birkholz C, Wagner S, et al. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K(+)-ATPase. Gut 1994; 35: 1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hackelsberger A, Günther T, Schultze V, et al. Role of aging in the expression of Helicobacter pylori gastritis in the antrum, corpus, and cardia. Scand J Gastroenterol 1999; 34: 138–143. [DOI] [PubMed] [Google Scholar]
- 51. Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998; 42: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology 2003; 144: 353–359. [DOI] [PubMed] [Google Scholar]
- 53. Date Y, Nakazato M, Murakami N, et al. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 2001; 280: 904–907. [DOI] [PubMed] [Google Scholar]
- 54. Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol 2008; 14: 6327–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tatsuguchi A, Miyake K, Gudis K, et al. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol 2004; 99: 2121–2127. [DOI] [PubMed] [Google Scholar]
- 56. Kandulski A, Malfertheiner P. Helicobacter pylori and gastroesophageal reflux disease. Curr Opin Gastroenterol 2014; 30: 402–407. [DOI] [PubMed] [Google Scholar]
- 57. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–198. [DOI] [PubMed] [Google Scholar]
- 58. Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology 2007; 133: 34–41; quiz 311. [DOI] [PubMed] [Google Scholar]
- 59. Dvorak K, Payne CM, Chavarria M, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut 2007; 56: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pfaffenbach B, Hullerum J, Orth KH, et al. Bile and acid reflux in long and short segment Barrett’s esophagus, and in reflux disease. Z Gastroenterol 2000; 38: 565–570. [DOI] [PubMed] [Google Scholar]
- 61. Richter J. Do we know the cause of reflux disease. Eur J Gastroenterol Hepatol 1999; 11(Suppl. 1): S3–S9. [PubMed] [Google Scholar]
- 62. Castell DO, Murray JA, Tutuian R, et al. Review article: the pathophysiology of gastro–oesophageal reflux disease – oesophageal manifestations. Aliment Pharmacol Ther 2004; 20(Suppl. 9): 14–25. [DOI] [PubMed] [Google Scholar]
- 63. Bozymski EM. Pathophysiology and diagnosis of gastroesophageal reflux disease. Am J Hosp Pharm 1993; 50(4 Suppl. 1): S4–S6. [PubMed] [Google Scholar]
- 64. Luther J, Owyang SY, Takeuchi T, et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 2011; 60: 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moons LM, Kusters JG, van Delft JH, et al. A pro-inflammatory genotype predisposes to Barrett’s esophagus. Carcinogenesis 2008; 29: 926–931. [DOI] [PubMed] [Google Scholar]
- 66. Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microb Pathog 2016; 93: 44–55. [DOI] [PubMed] [Google Scholar]
- 67. Zeng B, Chen C, Yi Q, et al. N-terminal region of Helicobacter pylori CagA induces IL-8 production in gastric epithelial cells via the β1 integrin receptor. J Med Microbiol 2020; 69: 457–464. [DOI] [PubMed] [Google Scholar]
- 68. Fazeli Z, Alebouyeh M, Rezaei Tavirani M, et al. Helicobacter pylori CagA induced interleukin-8 secretion in gastric epithelial cells. Gastroenterol Hepatol Bed Bench 2016; 9(Suppl. 1): S42–S46. [PMC free article] [PubMed] [Google Scholar]
- 69. Crabtree JE, Taylor JD, Wyatt JI, et al. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet 1991; 338: 332–335. [DOI] [PubMed] [Google Scholar]
- 70. Valmaseda Pérez T, Gisbert JP, Pajares García JM. Geographic differences and the role of cagA gene in gastroduodenal diseases associated with Helicobacter pylori infection. Rev Esp Enferm Dig 2001; 93: 471–480. [PubMed] [Google Scholar]
- 71. Sozzi M, Valentini M, Figura N, et al. Atrophic gastritis and intestinal metaplasia in Helicobacter pylori infection: the role of CagA status. Am J Gastroenterol 1998; 93: 375–379. [DOI] [PubMed] [Google Scholar]
- 72. Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, et al. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst 1995; 87: 1777–1780. [DOI] [PubMed] [Google Scholar]
- 73. Zentilin P, Reglioni S, Savarino V. Pathophysiological characteristics of long- and short-segment Barrett’s oesophagus. Scand J Gastroenterol Suppl 2003: 40–43. [DOI] [PubMed] [Google Scholar]
- 74. Nandurkar S, Talley NJ. Barrett’s esophagus: the long and the short of it. Am J Gastroenterol 1999; 94: 30–40. [DOI] [PubMed] [Google Scholar]
- 75. Talebi Bezmin Abadi A. Diagnosis of helicobacter pylori using invasive and noninvasive approaches. J Pathog 2018; 2018: 9064952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nowak JA. Limitation of histology for detecting Helicobacter pylori. Gastrointest Endosc 1995; 41: 175–177. [DOI] [PubMed] [Google Scholar]
- 77. Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s Esophagus. Am J Gastroenterol 2016; 111: 30–50; quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. The Chinese consensus for screening, diagnosis and management of Barrett’s esophagus and early adenocarcinoma (2017, Wanning). Zhonghua Nei Ke Za Zhi 2017; 56: 701–711. [DOI] [PubMed] [Google Scholar]
- 79. Xie FJ, Zhang YP, Zheng QQ, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol 2013; 19: 6098–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weston AP, Badr AS, Topalovski M, et al. Prospective evaluation of the prevalence of gastric Helicobacter pylori infection in patients with GERD, Barrett’s esophagus, Barrett’s dysplasia, and Barrett’s adenocarcinoma. Am J Gastroenterol 2000; 95: 387–394. [DOI] [PubMed] [Google Scholar]
- 81. Ness-Jensen E, Langhammer A, Hveem K, et al. Helicobacter pylori in relation to asthma and allergy modified by abdominal obesity: the HUNT study in Norway. World Allergy Organ J 2019; 12: 100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221117971 for Association of Barrett’s esophagus with Helicobacter pylori infection: a meta-analysis by Shaoze Ma, Xiaozhong Guo, Chunmei Wang, Yue Yin, Guangqin Xu, Hongxin Chen and Xingshun Qi in Therapeutic Advances in Chronic Disease