Highlights
-
•
It is the first time that different ultrasonic application methods based on the nucleation mechanisms were proposed.
-
•
The introduction of ultrasound was demonstrated to have the abilities to alter and disrupt the molecular self-assembly of solute in solution.
-
•
Ultrasound pretreatment strategy was conducted to break the original molecular interactions to alter the nucleated form.
-
•
For crystallization system that the solute molecular self-associates can’t give sufficient information to predict the nucleated polymorph, the method of introducing continuous ultrasonic irradiation in the nucleation stage was proposed.
Keywords: Polymorph control, Ultrasound crystallization, Molecular self-assembly
Abstract
Molecular self-assembly plays a vital role in the nucleation process and sometimes determines the nucleation outcomes. In this study, ultrasound technology was applied to control polymorph nucleation. For the first time, different ultrasonic application methods based on the nucleation mechanisms have been proposed. For PZA-water and DHB-toluene systems that the molecular self-assembly in solution resembles the synthon in crystal structure, ultrasound pretreatment strategy was conducted to break the original molecular interactions to alter the nucleated form. When the solute molecular self-associates can’t give sufficient information to predict the nucleated polymorph like INA-ethanol system, the method of introducing continuous ultrasonic irradiation in the nucleation stage was applied. The induction of ultrasound during nucleation process can break the original interactions firstly by shear forces and accelerate the occurrence of nucleation to avoid the reorientation and rearrangement of solute molecules. These strategies were proved to be effective in polymorph control and have a degree of applicability.
1. Introduction
Most solid substances in nature exist in the form of crystals, especially for pharmaceuticals. Due to various crystallization conditions, the same pharmaceutical can engender completely different crystals, namely polymorphic phenomenon of drugs [1], [2], [3], [4]. Polymorphism is an aptitude of a substance to crystalize in different crystalline forms which have unique molecular conformation and arrangements in the crystal lattice [5]. It has been reported that more than 80 % of pharmaceuticals have polymorph phenomenon [6].The study of polymorphs of active pharmaceutical ingredients (API) have a crucial implication on the exploitation of new drugs, the optimal control of crystallization process, the patent protection and improvement of physicochemical properties [2], [7]. So how to realize the control of target polymorph has been gathered more and more focus from researchers.
Currently, the conventional polymorph regulation strategy involves adjusting experimental parameters such as solvents [8], [9], supersaturation [10], [11], temperature [12], [13], or adding crystal seed [14], [15], etc. However, these regulation strategies can’t achieve desired results for some drugs, as covering all possible crystallization conditions is not practicable. So researchers have tried to design polymorph control strategies to obtain specific crystallization outcomes. Designed methods appeared, for instance: template-induced crystallization [16], [17], magnetic-induced crystallization [18], ionic liquid-induced crystallization [19], ultrasonic-induced crystallization [20], [21], [22], [23], [24], [25], and nanoconfinement crystallization [26], [27]. Among them, introducing ultrasound in the process of crystallization has been widely applied as an effective means of nucleation outcomes control. The introduction of ultrasound can effectively enhance the micromixing [28], promote nucleation [29], reduce the induction time [29], [30], the metastable zone width [31], [32] and the agglomeration [33] and promote uniform particle size distribution [25], [32], due to the energy provided by ultrasound and the cavitation generated in the solution. As one of the alternative methods of traditional polymorph control, ultrasound-assisted crystallization has attracted extensive attentions. There are two common interpretations of ultrasound effects on polymorph regulation. Some studies have confirmed that ultrasound could reduce the nucleation barrier of a certain crystal form by influencing the intermolecular hydrogen bond interaction in solution to promote the nucleation of this polymorph [24], [34], [35]. Other studies confirmed that ultrasound could regulate the polymorphs at the nucleation stage by altering the nucleation kinetics of different polymorphs [25], [30], [31]. Fang [22] found that the ultrasound could provide enough energy to overcome the critical energetic barrier for nucleation, and eventually promote spontaneous nucleation of theophylline form V. Kaur Bhangu [25] and Ike [24] found that the cavitation effect generated by ultrasound can reduce the induction time and increase supersaturation, which tends to promote the nucleation of metastable form. Ultrasound could regulate the polymorphs of some organic compounds, however, this method is not always effective for all compounds in nucleation control [36], [37], [38].
In general, nucleation outcomes could be determined by kinetic (supersaturations, cooling rates, etc) factors and molecular self-assembly. The chemical and physical properties of different pharmaceuticals are various, which makes corresponding nucleation control factors distinct even under the same crystallization conditions. Therefore, attempts to adjust the nucleation of certain pharmaceutical form by regulating the molecular arrangements and self-assembly in solution would fail, if the nucleation process of this certain pharmaceutical form was kinetically controlled, and vice versa. This also explained why the mechanisms of polymorph control by template-induced crystallization differed for different compounds, which was attributed to changing induction time or regulating the molecular self-assembly before nucleation [39], [40], [41]. Different crystallization conditions may give rise to changes of nucleation mechanisms and relevant nucleation kinetics and thermodynamics, which would further influence the key control impactors of the nucleation and thus the formation of polymorphs [42], [43].
The previous reports about polymorph control by ultrasound-induced crystallization indicates that the effects of ultrasound on polymorphism were triggered by disturbing molecular arrangements [24], [34], [35] or altering the nucleation kinetics [29], [30], [32]. As reported, for different crystallization systems with introduction of ultrasound, the mechanisms of polymorph regulation are distinct. Therefore, the strategies of ultrasound application should be customized according to different crystallization systems in order to control polymorph nucleation. To the best of the authors knowledge, there’s few reports study the different effects of ultrasound application based on the nucleation mechanisms of various crystallization systems.
Pyrazinamide-water and isonicotinamide-ethanol crystallization systems were chosen to explore the similarities and differences of ultrasound addition methods in polymorph regulation. Scheme 1 shows the molecular structures of PZA and INA. Crystallization of PZA in water was thermodynamically controlled in which the molecular assemblies in solution mirrors the structure of nucleated form [44], [45]. The nucleation of INA in ethanol was greatly affected by the kinetic factors of cooling rate and supersaturation [46]. In this paper, two crystallization systems with similar solute molecule structures but different nucleation regulation factors were chosen. And different ultrasonic application methods were proposed based on the nucleation mechanisms of these two crystallization systems to control the nucleation of target polymorphs.
Scheme 1.

Molecular structures of PZA and INA.
2. Experimental
2.1. Materials
Pyrazinamide (PZA), Isonicotinamide (INA), 2,6-dihydroxybenzoic acid (DHB), ethanol and toluene were supplied by Shanghai Chemistry Reagent Co. (China). Distilled-deionized water was prepared in our laboratory. All the materials were used without further purification.
2.2. Apparatus
The ultrasonic (sonicating) bath (200 W, Qs5, Kun shan ultrasonic instrument Co., ltd), generating a fixed and continuous 40 kHz ultrasonic field was chosen as the ultrasonic source in this work. A thermostatic water bath (CF41, Julabo, Germany) was employed during the cooling experiment. An electronic analytic balance (ML204, Mettler Toledo, Switzerland) with an accuracy of ± 0.0001 g was employed to measure all the masses.
2.3. PZA-water experiment
The concentration of PZA in water is 35. 82 mg ml−1 which is saturated at 45 °C. PZA solution was prepared in a magnetically stirred water bath at 50 °C for 30 min to obtain a homogeneous solution. The hot solution was filtered into preheated 50 mL vials via a polytetrafluoroethylene (PTFE) filter with 0.45 μm pores, the filtration process removes some insoluble impurities that may act as nucleation sites. Each vial contained 20 g solvent and the corresponding amount of PZA, and the vial was tightly capped during the cooling crystallization to avoid the evaporation of solvent.
The vial contained prepared solution is pretreated with ultrasound at the powers of 100, 160 and 200 W at 45 °C for 5 min, respectively. Then, vails containing solution with ultrasound pretreatment were placed in a jacket crystallizer vessel connected with a thermostatic water bath with a cooling rate of 0.1 °C /min from 45 °C to 20 °C. A blank experiment was conducted without the ultrasonic preprocessing at same conditions. During the cooling crystallization, the samples remained undisturbed.
2.4. INA-ethanol experiment
The concentration of INA in ethanol was 150. 92 mg ml − 1 which is saturated at 45 °C. The preparation process of the solution is the same as that of PZA.
The ultrasonic pretreatment experiment of INA is the same as PZA to pretreat the solution at 45 °C. Then, vails contained solution with ultrasound pretreatment were placed in a jacket crystallizer vessel connected with a thermostatic water bath with three different cooling rates of 0.1 °C /min, 5 °C /min and quench cooling from 45 °C to 20 °C. The continuous ultrasonic irradiation experiment of INA is to quench the solution from 45 °C to 20 °C firstly, then ultrasound is introduced and continue to work until nucleation at 20 °C. The crystallization experiments with and without continuous ultrasound were repeated for 80 times in order to determine the induction time of INA.
2.5. Characterizations
Powder X-ray diffraction (PXRD) (D8 Advance, Bruker, Germany) was adopted to determine the solid forms of the obtained crystals, the samples were scanned over a diffraction angle (2θ) angle of 5° to 40° at a scanning rate of 10° min−1. The 13C nuclear magnetic resonance (NMR) spectra were recorded on an 400 MHz Bruker Avance-III spectrometer equipped at 293.15 K.
3. Results and discussion
3.1. Polymorph control by ultrasonic pretreatment
Pyrazinamide is usually nucleated as a dimeric form in the cooling crystallization process of PZA-water solution. As the study shown [45], the nucleation outcomes of PZA in water is greatly affected and determined by the molecular self-assemblies in solution. Thus, PZA solution was pretreated by ultrasound to regulate the molecular self-associations in solution in order to achieve the selected nucleation of form γ.
The saturated solution was pretreated with ultrasonic power of 100 W, 160 W and 200 W, respectively. The obtained crystals from cooling crystallization of water were analyzed by PXRD. Comparing with simulated PXRD pattern (Fig. 1), crystals grown without ultrasonic pretreatment were all α form. As previous assumption, crystals obtained after ultrasonic pretreatment at 100 W and 160 W were all pure γ form. While, polymorphic mixture of α and γ were obtained at 200 W. The microscope images of obtained PZA in Fig. 2 illustrate distinct crystal morphologies of different polymorphs, the needle like α form (aspect ratio > 20) crystallized without ultrasonic pretreatment and the long flaky γ form (aspect ratio < 10) obtained with ultrasonic pretreatment.
Fig. 1.
PXRD patterns of the obtained PZA crystals a) without ultrasound, with ultrasound at the power of b) 200 W, c) 160 W and d) 100 W. Along with the simulated PXRD patterns of the α and γ-forms of PZA.
Fig. 2.
Microscope images of PZA crystals with water: A) Without ultrasound, B) ultrasound with 200 W, C) ultrasound with 160 W, D) ultrasound with 100 W.
The experimental results indicate that γ form prefer to crystallize by itself at ultrasonic power of 100 W and 160 W pretreatment, while the regulation of γ form nucleation with ultrasonic pretreatment seems to lose prominence at ultrasonic power of 200 W. The hypothesized mechanism of polymorph control by ultrasonic pretreatment is altering and regulating the molecular associations and hydrogen bonds by shear forces due to cavitation [47] as shown in Fig. 3. Based on that, form γ of PZA with chain structure could be obtained in water, when ultrasound was applied to break the original dimer structure in solution. Meanwhile, cavitation bubbles originated from ultrasound accelerate solute molecular movements, increasing the inter-molecular collisions in solution which would promote the solute–solute interactions [47], [48]. In this situation, higher ultrasonic power facilitates the nucleation of form α because of molecular collisions. Therefore, it is speculated that there is an optimum condition between the break of hydrogen bonds of dimer structure of PZA by shear forces and dimer re-formation by increased inter-molecular collisions to promote the nucleation of form γ.
Fig. 3.
Polymorph control mechanism of PZA by ultrasound pretreatment.
To explore the rationality of previous hypothesis, the changes of molecular arrangements of PZA in solution with and without ultrasonic pretreatment were analyzed by 13C NMR. It was supposed that there should be 13C shifts for the PZA in solution when the intrinsic dimer structures of PZA were destructed and the hydrogen bonds between C O and NH2 groups were broken. As shown in Fig. 4, the change in 13C chemical shift of C O for PZA after ultrasound pretreatment is 0.0023 ppm, compared with that without ultrasound. The changes of 13C chemical shifts for C O were attributed to the rupture of the hydrogen bond dimers between C O and NH2 groups in the solution, which lead to the increase of the density of electron clouds around the carbon core. Thus, the 13C chemical shift at the carbonyl position after ultrasound pretreatment is partially reduced.
Fig. 4.
13C NMR spectra of PZA-water‑d2 solution a) without ultrasound, b) with ultrasound.
Here, we propose a new ultrasound petreatment strategy in regulating polymorph nucleation for the solution crystallization systems, where the synthons present in the crystal structures mirror the self-associates in solution. For such crystallization systems, the outcomes of nucleation are mostly controlled by the molecular self-assemblies in solution. After the pretreatment of ultrasonic irradiation, original intermolecular hydrogen bonds like dimer are broken by the shear forces generated by the collapse of cavitation bubbles and the modes of molecular self-assemblies change accordingly, leading to the nucleation of selected form. In order to further demonstrate the applicability of the ultrasound pretreatment strategy, DHB-toluene crystallization system, whose nucleation outcome was controlled by the molecular self-associations in solution, was selected [49]. DHB molecules were reported to prefer form dimer associates in toluene and nucleate as form I with dimer structure. The crystals obtained by ultrasound pre-processing were determined to be form II with chain synthons. (Fig. S1 and S2) Based on that, ultrasonic pretreatment was confirmed to be an effective method in polymorph control and regulation.
3.2. Polymorph control with continuous ultrasound
According to the reports of Kulkarni [39], [50], both dimer and chain self-associates are present for INA molecules in ethanol, while, only form I with dimer structure crystallizes. The crystallization outcomes indicated that the dimer structures of INA dominate in the competing processes of cluster formation with chain associates in ethanol. Thus, the polymorph nucleation of INA in ethanol is controlled by not only the molecular self-assemblies, but largely by kinetic factors.
According to that, ultrasound pretreatment strategy valid for PZA-water, which could only alter and disrupt the molecular self-associations before crystallization to control nucleation outcomes, seems not effective for INA crystallization in ethanol. The INA crystallization outcomes with ultrasonic pretreatment confirmed our assumption. The nucleation outcomes of INA remained unchanged even with various ultrasonic times (Fig. 5A) and the various ultrasonic powers (Fig. S3). It has been reported that the kinetic factors have a very significant effect on the nucleation outcomes of INA, so we also chose different cooling rates for INA crystallization in ethanol with 5℃/min and quenched after ultrasonic pretreatment shown in Fig. 5B and Fig. 5C. The experimental results indicate that larger cooling rate has a slight effect on the INA crystallization outcomes with little form IV nucleated regardless of ultrasonic pretreatment or not. Analysis of 13C NMR spectra of INA found that the change in 13C chemical shift of C O for INA after ultrasound pretreatment is 0.105 ppm as shown in Fig. 6, which indicates that the molecular arrangement of INA molecule changes after ultrasonic pretreatment. Both NMR experiments of PZA and INA after ultrasonic processing verify that the feasibility of ultrasound to regulate and disrupt the molecular self-associations and arrangements in solution.
Fig. 5.
PXRD patterns of obtained INA crystals at A: 0.1℃ / min, B: 5℃ / min cooling rate and C: crush cooling with a) no ultrasound, b) ultrasound for 5 min, c) ultrasound for 10 min, d) ultrasound for 30 min at 200 W of ultrasonic power, along with the simulated PXRD patterns of the Forms I, and IV of INA.
Fig. 6.
13C NMR spectra of INA-ethanol-d6 solution a) without ultrasound, b) with ultrasound.
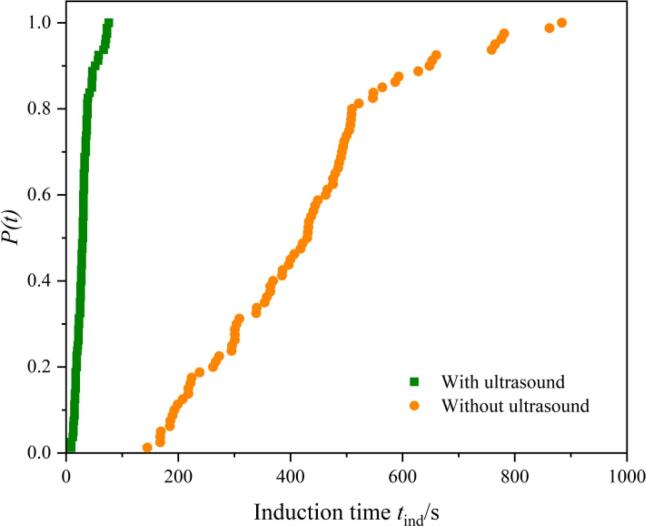
Thus, polymorph control of INA in ethanol by breaking the self-assemblies of molecules before crystallization didn’t work out, since the nucleation of INA in ethanol is determined by the competition of different self-associates during crystallization. Ultrasonic pretreatment can’t affect the competition of clusters during nucleation. According to the nucleation mechanism of INA in ethanol, the moment of ultrasound application was chosen in the nucleation stage. The saturated INA ethanol solution at 45℃ was quenched to 20℃, meanwhile ultrasound was induced and continued until nucleation. As shown in Fig. 7 and Fig. S4, the mixed crystals of forms II and IV both with chain structures are obtained with continuous ultrasound during nucleation process, the form I with dimer structures nucleated without ultrasound introduction. The mechanism of polymorph control by continuous ultrasound was supposed to divide into two steps. Firstly, the shear forces generated by ultrasound destroy a small amount of dimer structures in solution during the nucleation, and inhibit the generation of new hydrogen-bonded dimer clusters. Then, the collapse of ultrasonic cavitation bubbles produces localized high supersaturation in the solution. Under such highly supersaturated conditions, the molecular clusters with chain structure didn’t have sufficient time to reorient and rearrange. The solution system is likely to form less stable polymorphic forms. Therefore, the crystals with chain associates dominate during the competing of molecular clusters in solution with the aid of ultrasound. These two decisive factors together contributed to the crystallization of INA form II and IV under continuous ultrasound treatment. The 13C NMR spectra of INA confirmed the first step of our hypothesized mechanism of polymorph control by continuous ultrasonic irradiation during nucleation process, in which ultrasound could disrupt the self-associations and break the interactions between molecules. To further verify the second step of continuous ultrasound-assisted polymorph control mechanism, the experiments of induction times of INA in ethanol were conducted and the probability distribution was shown in Fig. 8. The results indicate that the induction time was decreased under ultrasonic irradiation from 514.5 s to 42.5 s, which demonstrates the powerful promotion effect on INA nucleation of continuous ultrasound. The drastically decreased induction time explain that chain clusters dominate during nucleation as less time for molecules to reorient and rearrange before nucleation and the structure of nucleated crystals (Forms II and IV) resembles the chain clusters in solution.
Fig. 7.
PXRD patterns of obtained INA crystals at quench crystallization a) without ultrasound, b) with continuous ultrasound at 200 W of ultrasonic power, along with the simulated PXRD patterns of the Forms I, II and IV of INA.
Fig. 8.
Induction time probability distributions of INA at different conditions.
As been reported [51], [52], there have some systems like INA-ethanol [53], whose molecular self-associate in solution are not directly related to the corresponding crystal structure. For this kind of crystallization systems, nucleation is controlled not only by the molecular self-assemblies in solution as a molecular rearrangement process may be involved before nucleation. Thus, aiming at the “no direct correspondence” crystallization systems, a different polymorph control strategy was put forward. Application of continuous ultrasonic irradiation during nucleation not only breaks the original molecular interactions but also interferes the molecular reorientation and rearrangement by accelerating nucleation rates, which ultimately facilitates the selected nucleation of target polymorph.
4. Conclusion
In this article, we put forward two different ultrasound application strategies to control polymorph nucleation for different crystallization systems. For systems such as PZA-water and DHB-toluene where a direct relationship exists between the molecular self-assembly in solution and the synthon in crystal structure, ultrasound pretreatment before crystallization was proposed. The shear forces generated by the collapse of cavitation bubbles broke the original molecular interactions like dimers in PZA-water and DHB-toluene systems. As a result, polymorph with chain structure crystallized after ultrasonic irradiation preprocessing instead of dimer form. Besides that, there seems exists an optimal ultrasonic power intensity in regulating polymorph nucleation by pretreatment strategy, as higher power may accelerate molecular collision probability and promote the molecular reorientation and rearrangement.
In crystallization system like INA-ethanol, the solute molecular self-associates can’t give sufficient information to predict the nucleated polymorph. The nucleation of this kind of systems was largely controlled by the solute molecular rearrangement before nucleation, which usually were affected by kinetic factors, like nucleation rates, supersaturation, desolvation and so on. Based on that, we choose the method of introducing continuous ultrasonic irradiation in the nucleation stage. It is found that this method can obtain crystals of form II and form IV of INA. The induction of ultrasound during nucleation process broke the interactions of dimers firstly by shear forces and accelerate the occurrence of nucleation to avoid the re-dimerization of solute molecules.
In conclusion, the introduction of ultrasound was demonstrated to have the abilities to alter and disrupt the molecular self-assembly of solute in solution. It’s the first time that different ultrasound application methods were proposed based on the nucleation mechanisms of various crystallization systems. These strategies were proved to be effective in polymorph control and have a degree of applicability. Moreover, ultrasound pretreatment method avoids the involvement of ultrasonic equipment during crystallization process. It may reduce the costs and difficulties of equipment build-up especially for industrial production and provide a new inspiration in polymorph control.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the financial support of Zhengzhou University, National Natural Science Foundation of China (NSFC 21908100) and Key R & D plan of Jiangsu Province (BE2019001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106118.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Shi Q., Chen H.B., Wang Y.A., Xu J., Liu Z.Y., Zhang C. Recent advances in drug polymorphs: aspects of pharmaceutical properties and selective crystallization. Int. J. Pharmaceutics. 2022;611 doi: 10.1016/j.ijpharm.2021.121320. [DOI] [PubMed] [Google Scholar]
- 2.Chistyakov D., Sergeev G. The polymorphism of drugs: new approaches to the synthesis of nanostructured polymorphs. Pharmaceutics. 2020;12(1) doi: 10.3390/pharmaceutics12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar J., Zahn D. Polymorphic phase transitions: macroscopic theory and molecular simulation. Adv. Drug Delivery Rev. 2017;117:47–70. doi: 10.1016/j.addr.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Thakur T.S., Thakuria R. Crystalline multicomponent solids: an alternative for addressing the hygroscopicity issue in pharmaceutical materials. Cryst. Growth Des. 2020;20(9):6245–6265. doi: 10.1021/acs.cgd.0c00654. [DOI] [Google Scholar]
- 5.Luo H.Y., Hao X., Gong Y.Q., Zhou J.H., He X., Li J.J. Rational crystal polymorph design of olanzapine. Cryst. Growth Des. 2019;19(4):2388–2395. doi: 10.1021/acs.cgd.9b00068. [DOI] [Google Scholar]
- 6.R. Hilfiker, Polymorphism - In the Pharmaceutical Industry, 2006. 10.1002/3527607889.
- 7.Zhou Y., Wang J., Xiao Y., Wang T., Huang X. The effects of polymorphism on physicochemical properties and pharmacodynamics of solid drugs. Curr. Pharm. Des. 2018;24(21):2375–2382. doi: 10.2174/1381612824666180515155425. [DOI] [PubMed] [Google Scholar]
- 8.Belenguer A.M., Lampronti G.I., De Mitri N., Driver M., Hunter C.A., Sanders J.K.M. Understanding the influence of surface solvation and structure on polymorph stability: a combined mechanochemical and theoretical approach. J. Am. Chem. Soc. 2018;140(49):17051–17059. doi: 10.1021/jacs.8b08549. [DOI] [PubMed] [Google Scholar]
- 9.Salem A., Hagymasi A., Voros-Horvath B., Safarik T., Balic T., Szabo P., Gosi F., Nagy S., Pal S., Kunsagi-Mate S., Szechenyi A. Solvent dependent 4-aminosalicylic acid-sulfamethazine co-crystal polymorph control. Eur. J. Pharm. Sci. 2021;156 doi: 10.1016/j.ejps.2020.105599. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T., Szilágyi B., Gong J., Nagy Z.K. Thermodynamic polymorph selection in enantiotropic systems using supersaturation-controlled batch and semibatch cooling crystallization. Cryst. Growth Des. 2019;19(11):6715–6726. doi: 10.1021/acs.cgd.9b01076. [DOI] [Google Scholar]
- 11.Javid N., Kendall T., Burns I.S., Sefcik J. Filtration suppresses laser-induced nucleation of glycine in aqueous solutions. Cryst. Growth Des. 2016;16(8):4196–4202. doi: 10.1021/acs.cgd.6b00046. [DOI] [Google Scholar]
- 12.Ruggiero M.T., Zeitler J.A., Korter T.M. Concomitant polymorphism and the martensitic-like transformation of an organic crystal. Phys. Chem. Chem. Phys. 2017;19(42):28502–28506. doi: 10.1039/c7cp04666a. [DOI] [PubMed] [Google Scholar]
- 13.Farmer T.C., Carpenter C.L., Doherty M.F. Polymorph selection by continuous crystallization. AICHE J. 2016;62(9):3505–3514. doi: 10.1002/aic.15343. [DOI] [Google Scholar]
- 14.Nicoud L., Licordari F., Myerson A.S. Polymorph control in batch seeded crystallizers. A case study with paracetamol. CrystEngComm. 2019;21(13):2105–2118. doi: 10.1039/c8ce01428k. [DOI] [Google Scholar]
- 15.Liu Y., Xu S.J., Liu Y.M., Chen M.Y., Chen Y.F., Du S.C., Wang Y.P., Sun P.P., Sun M.M., Shi P., Gong J.B. Seed-assisted effects on solution-mediated phase transformation: a case study of L-Histidine in antisolvent crystallization. Ind. Eng. Chem. Res. 2018;57(2):784–793. doi: 10.1021/acs.iecr.7b04762. [DOI] [Google Scholar]
- 16.Zhang K., Xu S., Liu S., Tang W., Fu X., Gong J. Novel strategy to control polymorph nucleation of gamma pyrazinamide by preferred intermolecular interactions during heterogeneous nucleation. Cryst. Growth Des. 2018;18(9):4874–4879. doi: 10.1021/acs.cgd.8b00943. [DOI] [Google Scholar]
- 17.Capacci-Daniel C., Gaskell K.J., Swift J.A. Nucleation and growth of metastable polymorphs on siloxane monolayer templates. Cryst. Growth Des. 2010;10(2):952–962. doi: 10.1021/cg9012697. [DOI] [Google Scholar]
- 18.Zhao Y., Hou B., Liu C., Ji X., Huang Y., Sui J., Liu D., Wang N., Hao H. Mechanistic study on the effect of magnetic field on the crystallization of organic small molecules. Ind. Eng. Chem. Res. 2021;60(43):15741–15751. doi: 10.1021/acs.iecr.1c03393. [DOI] [Google Scholar]
- 19.Zeng Q.Y., Mukherjee A., Muller P., Rogers R.D., Myerson A.S. Exploring the role of ionic liquids to tune the polymorphic outcome of organic compounds. Chem. Sci. 2018;9(6):1510–1520. doi: 10.1039/c7sc04353h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polat S., Sayan P. Effect of ultrasonic irradiation on morphology and polymorphic transformation of glycine. Ultrason. Sonochem. 2018;47:17–28. doi: 10.1016/j.ultsonch.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Fang C., Tang W., Wu S., Wang J., Gao Z., Gong J. Ultrasound-assisted intensified crystallization of L-glutamic acid: crystal nucleation and polymorph transformation. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105227. [DOI] [PubMed] [Google Scholar]
- 22.Fang C., Yang P., Liu Y., Wang J., Gao Z., Gong J., Rohani S. Ultrasound-assisted theophylline polymorphic transformation: selective polymorph nucleation, molecular mechanism and kinetics analysis. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain M.N., Jordens J., Kuhn S., Braeken L., Van Gerven T. Ultrasound as a tool for polymorph control and high yield in flow crystallization. Chem. Eng. J. 2021;408 doi: 10.1016/j.cej.2020.127272. [DOI] [Google Scholar]
- 24.Ike Y., Hirasawa I. Polymorph control of L-ArgHCl on antisolvent crystallization by ultrasonic irradiation. Chem. Eng. Technol. 2017;40(7):1318–1322. doi: 10.1002/ceat.201600678. [DOI] [Google Scholar]
- 25.Kaur Bhangu S., Ashokkumar M., Lee J. Ultrasound assisted crystallization of paracetamol: crystal size distribution and polymorph control. Cryst. Growth Des. 2016;16(4):1934–1941. doi: 10.1021/acs.cgd.5b01470. [DOI] [Google Scholar]
- 26.Seibel J., Amabilino D.B., De Feyter S. Preferred formation of minority concomitant polymorphs in 2D self-assembly under lateral nanoconfinement. Angew. Chem.-Int. Ed. 2019;58(37):12964–12968. doi: 10.1002/anie.201908552. [DOI] [PubMed] [Google Scholar]
- 27.Cheng S.X., McKenna G.B. Nanoconfinement effects on the glass transition and crystallization behaviors of nifedipine. Mol. Pharm. 2019;16(2):856–866. doi: 10.1021/acs.molpharmaceut.8b01172. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi M., Azimi N., Parvizian F. Using microparticles to enhance micromixing in a high frequency continuous flow sonoreactor. Chem. Eng. Process.: Process Intensification. 2013;70:250–258. doi: 10.1016/j.cep.2013.03.013. [DOI] [Google Scholar]
- 29.Renuka Devi K., Raja A., Srinivasan K. Ultrasound assisted nucleation and growth characteristics of glycine polymorphs–a combined experimental and analytical approach. Ultrason. Sonochem. 2015;24:107–113. doi: 10.1016/j.ultsonch.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Dalvi S.V., Yadav M.D. Effect of ultrasound and stabilizers on nucleation kinetics of curcumin during liquid antisolvent precipitation. Ultrason. Sonochem. 2015;24:114–122. doi: 10.1016/j.ultsonch.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Hazi Mastan T., Lenka M., Sarkar D. Nucleation kinetics from metastable zone widths for sonocrystallization of l-phenylalanine. Ultrason. Sonochem. 2017;36:497–506. doi: 10.1016/j.ultsonch.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez Vera H.U., Baillon F., Espitalier F., Accart P., Louisnard O. Crystallization of α-glycine by anti-solvent assisted by ultrasound. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104671. [DOI] [PubMed] [Google Scholar]
- 33.Xia D., Quan P., Piao H., Piao H., Sun S., Yin Y., Cui F. Preparation of stable nitrendipine nanosuspensions using the precipitation–ultrasonication method for enhancement of dissolution and oral bioavailability. Eur. J. Pharm. Sci. 2010;40(4):325–334. doi: 10.1016/j.ejps.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Thorat A.A., Dalvi S.V. Ultrasound-assisted modulation of concomitant polymorphism of curcumin during liquid antisolvent precipitation. Ultrason. Sonochem. 2016;30:35–43. doi: 10.1016/j.ultsonch.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Kurotani M., Hirasawa I. Effect of ultrasonic irradiation on the selective polymorph control in sulfamerazine. Chem. Eng. Res. Des. 2010;88(9):1272–1278. doi: 10.1016/j.cherd.2010.02.014. [DOI] [Google Scholar]
- 36.Belkacem N., Sheikh Salem M.A., AlKhatib H.S. Effect of ultrasound on the physico-chemical properties of poorly soluble drugs: Antisolvent sonocrystallization of ketoprofen. Powder Technol. 2015;285:16–24. doi: 10.1016/j.powtec.2015.06.058. [DOI] [Google Scholar]
- 37.Prasad R., Dalvi S.V. Understanding morphological evolution of griseofulvin particles into hierarchical microstructures during liquid antisolvent precipitation. Cryst. Growth Des. 2019;19(10):5836–5849. doi: 10.1021/acs.cgd.9b00859. [DOI] [Google Scholar]
- 38.Santos R.M., Ceulemans P., Van Gerven T. Synthesis of pure aragonite by sonochemical mineral carbonation. Chem. Eng. Res. Des. 2012;90(6):715–725. doi: 10.1016/j.cherd.2011.11.022. [DOI] [Google Scholar]
- 39.Kulkarni S.A., Weber C.C., Myerson A.S., ter Horst J.H. Self-association during heterogeneous nucleation onto well-defined templates. Langmuir. 2014;30(41):12368–12375. doi: 10.1021/la5024828. [DOI] [PubMed] [Google Scholar]
- 40.Zhang K., Xu S., Gong J., Tang W. Revealing the critical role of template functional group ordering in the template-directed crystallization of pyrazinamide. CrystEngComm. 2019;21(42):6382–6389. doi: 10.1039/c9ce01236b. [DOI] [Google Scholar]
- 41.Parambil J.V., Poornachary S.K., Hinder S.J., Tan R.B.H., Heng J.Y.Y. Establishing template-induced polymorphic domains for API crystallisation: the case of carbamazepine. CrystEngComm. 2015;17(33):6384–6392. doi: 10.1039/c5ce01080b. [DOI] [Google Scholar]
- 42.Lai T.-T.-C., Cornevin J., Ferguson S., Li N., Trout B.L., Myerson A.S. Control of polymorphism in continuous crystallization via mixed suspension mixed product removal systems cascade design. Cryst. Growth Des. 2015;15(7):3374–3382. doi: 10.1021/acs.cgd.5b00466. [DOI] [Google Scholar]
- 43.Dai J., Jia L., Yang W., Zhu D., Xie C., Bao Y., Zhou L., Yin Q. Solid Forms selection of spironolactone: ternary phase diagram and nucleation process. Ind. Eng. Chem. Res. 2020;59(3):1350–1361. doi: 10.1021/acs.iecr.9b06153. [DOI] [Google Scholar]
- 44.Castro R.A.E., Maria T.M.R., Évora A.O.L., Feiteira J.C., Silva M.R., Beja A.M., Canotilho J., Eusébio M.E.S. A new insight into pyrazinamide polymorphic forms and their thermodynamic relationships. Cryst. Growth Des. 2009;10(1):274–282. doi: 10.1021/cg900890n. [DOI] [Google Scholar]
- 45.Song Y., Wu Y., Jiang Y., Yang H., Jiang Y. Polymers and solvent-induced polymorphic selection and preferential orientation of pyrazinamide crystal. Cryst. Growth Des. 2019;20(1):352–361. doi: 10.1021/acs.cgd.9b01287. [DOI] [Google Scholar]
- 46.Kulkarni S.A., Kadam S.S., Meekes H., Stankiewicz A.I., ter Horst J.H. Crystal nucleation kinetics from induction times and metastable zone widths. Cryst. Growth Des. 2013;13(6):2435–2440. doi: 10.1021/cg400139t. [DOI] [Google Scholar]
- 47.Prasad R., Dalvi S.V. Sonocrystallization: monitoring and controlling crystallization using ultrasound. Chem. Eng. Sci. 2020;226 doi: 10.1016/j.ces.2020.115911. [DOI] [Google Scholar]
- 48.Yao M., Wang L., Feng S., Li J., Fang C., Zhang S., Jin M., Tong L., Gao Z., Chen M., Gong J. Improving separation efficiency of crystallization by ultrasound-accelerated nucleation: the role of solute diffusion and solvation effect. Sep. Purif. Technol. 2022;294 doi: 10.1016/j.seppur.2022.121143. [DOI] [Google Scholar]
- 49.Davey R.J., Blagden N., Righini S., Alison H., Quayle M.J., Fuller S. Crystal polymorphism as a probe for molecular self-assembly during nucleation from solutions: the case of 2,6-dihydroxybenzoic acid. Cryst. Growth Des. 2001;1(1):59–65. doi: 10.1021/cg000009c. [DOI] [Google Scholar]
- 50.Kulkarni S., McGarrity E., Meekes H., Ter Horst J.H. Isonicotinamide self-association: the link between solvent and polymorph nucleation. Chem. Commun. (Cambridge, England) 2012;48:4983–4985. doi: 10.1039/c2cc18025a. [DOI] [PubMed] [Google Scholar]
- 51.Davey R.J., Schroeder S.L., ter Horst J.H. Nucleation of organic crystals–a molecular perspective. Angew. Chem. Int. Ed. Engl. 2013;52(8):2166–2179. doi: 10.1002/anie.201204824. [DOI] [PubMed] [Google Scholar]
- 52.Davey R.J., Back K.R., Sullivan R.A. Crystal nucleation from solutions–transition states, rate determining steps and complexity. Faraday Discuss. 2015;179:9–26. doi: 10.1039/c5fd00037h. [DOI] [PubMed] [Google Scholar]
- 53.Maggioni G.M., Bezinge L., Mazzotti M. Stochastic nucleation of polymorphs: experimental evidence and mathematical modeling. Cryst. Growth Des. 2017;17(12):6703–6711. doi: 10.1021/acs.cgd.7b01313. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.