Abstract
Objective
Fibroblast growth factor 21 (FGF21) is a peripherally-derived endocrine hormone that acts on the central nervous system (CNS) to regulate whole body energy homeostasis. Pharmacological administration of FGF21 promotes weight loss in obese animal models and human subjects with obesity. However, the central targets mediating these effects are incompletely defined.
Methods
To explore the mechanism for FGF21's effects to lower body weight, we pharmacologically administer FGF21 to genetic animal models lacking the obligate FGF21 co-receptor, β-klotho (KLB), in either glutamatergic (Vglut2-Cre) or GABAergic (Vgat-Cre) neurons. In addition, we abolish FGF21 signaling to leptin receptor (LepR-Cre) positive cells. Finally, we examine the synergistic effects of FGF21 and leptin to lower body weight and explore the importance of physiological leptin levels in FGF21-mediated regulation of body weight.
Results
Here we show that FGF21 signaling to glutamatergic neurons is required for FGF21 to modulate energy expenditure and promote weight loss. In addition, we demonstrate that FGF21 signals to leptin receptor-expressing cells to regulate body weight, and that central leptin signaling is required for FGF21 to fully stimulate body weight loss during obesity. Interestingly, co-administration of FGF21 and leptin synergistically leads to robust weight loss.
Conclusions
These data reveal an important endocrine crosstalk between liver- and adipose-derived signals which integrate in the CNS to modulate energy homeostasis and body weight regulation.
Keywords: Neurons, FGF21, Betaklotho, Glutamatergic, Leptin, Adipose, Liver, Synergy
Highlights
-
•
Pharmacological FGF21 administration signals to glutamatergic, but not GABAergic, neurons to reduce body weight.
-
•
Central leptin signaling is required for FGF21 to fully stimulate weight loss during obesity.
-
•
Co-administration of leptin with FGF21 synergistically enhances weight loss.
1. Introduction
Obesity is a major health and economic burden [1,2] with significant contributions to the global epidemic of metabolic diseases including diabetes [3], cardiovascular disease [4] and non-alcoholic steatohepatitis [5]. While significant advancements have been made in understanding the mechanisms that modulate energy intake and expenditure to regulate body weight, the development of therapeutic compounds that effectively manage obesity remains extremely challenging. In particular, although many anti-obesity drugs are initially successful in reducing energy intake and subsequently body weight, a compensatory decrease in energy expenditure leads to weight regain [6,7]. Pharmaceutical agents that target energy expenditure are effective at ameliorating obesity, however, limiting off-target effects with these drugs remains a challenge.
Fibroblast growth factor 21 (FGF21) is a liver-derived hormone that regulates energy and nutrient homeostasis. FGF21 signals to specific tissues that express both the traditional FGF receptor (FGFR1c) and a co-receptor termed β-klotho (KLB) [8,9]. KLB confers specificity for FGF21 action and is absolutely required for FGF21 signaling [[10], [11], [12], [13]]. Pharmacological administration of FGF21 to obese animal models improves insulin sensitivity [14,15] and increases energy expenditure to promote weight loss [16,17]. Moreover, extended administration of FGF21 analogs to obese humans significantly lowers triglyceride levels and reduces body weight with minimal adverse events [18,19]. The mechanism for the effects of FGF21 to acutely regulate insulin sensitivity and chronically reduce body weight are dissociable with direct FGF21 signaling to adipose tissues being required for the acute insulin-sensitizing effects of FGF21 [20,21], while direct FGF21 signaling to the central nervous system (CNS) is required for the extended effects of FGF21 to lower body weight and secondarily improve insulin sensitivity [[21], [22], [23], [24]].
Although FGF21 signaling to the CNS regulates body weight and energy homeostasis, several lines of evidence suggest that adipose tissues are also critical for FGF21-mediated regulation of body weight, even if FGF21 signaling to adipose tissue is not required. For example, mice lacking mature adipocytes are refractory to the metabolic effects of FGF21 [25] or FGFR1-based agonists [26]. In addition, disruption of leptin signaling using ob/ob or db/db mice abrogates the beneficial effects if FGF21 to lower body weight but does not impair FGF21's acute glucose lowering effect [[27], [28], [29], [30], [31]]. Despite its potent metabolic effects, the mechanism of FGF21 action to promote body weight loss is still unknown and the specific population of KLB+ cells in the CNS that are necessary to control energy expenditure have remained elusive.
Here we show that FGF21 signaling to glutamatergic, but not GABAergic, neurons is necessary for FGF21 to promote weight loss. In addition, we demonstrate that FGF21 signals to leptin receptor (LepR)-expressing cells to regulate body weight, and that central leptin signaling is required for FGF21's full effects to reduce body weight. Finally, we show that administration of FGF21 and leptin in combination has potent weight-reducing effects through regulation of both caloric intake and energy expenditure. Together, this work elucidates the neuronal population through which FGF21 regulates energy expenditure and body weight, and demonstrates that, in part, FGF21 requires leptin signaling to promote weight loss.
2. Materials and methods
2.1. Animals
The following mice were utilized in these studies: KLB-Cre [32], KLBfl/fl [12], Vglut2-IRES-Cre (stock #028863) [33], Vgat-IRES-Cre (stock #028862) [33], Vglut2-IRES-Flp (stock #030212), Ai65 (RCFL-tdT)-D (stock #021875) [34] and LepR-Cre (stock #008320) [35]. All mice used in experiments were males on a C57BL/6 J genetic background. Mice were individually housed in a 12 h light/dark cycle at 22–23 °C or 30 °C as indicated. Mice were given ad libitum access to food, chow (Teklad; 2920X) or 60% HFD (Research Diets; D12492), except when pair fed. Health status was normal for all animals. All experiments presented in this study were conducted according to the animal research guidelines from NIH and were approved by the University of Iowa IACUC.
2.2. Administration of recombinant FGF21
KLBVglut2−KO mice: 16–18 week old DIO WT and KLBVglut2−KO mice on 60% HFD (Research Diets, D12492) for 8 weeks were randomized by body weight to receive either vehicle or FGF21 (1 mg/kg/day) via minipump (ALZET, 1002). Mice were individually housed for 2 weeks prior to surgery. Mice were then subcutaneously implanted with osmotic minipumps and body weight was recorded daily for 2 weeks.
KLBVgat−KO mice: 16–18 week old DIO WT and KLBVgat−KO mice on 60% HFD (Research Diets, D12492) for 8 weeks were randomized by body weight to receive either vehicle or FGF21 (1 mg/kg/day) via minipump (ALZET, 1002). Mice were individually housed for 2 weeks prior to surgery. Mice were then subcutaneously implanted with osmotic minipumps and body weight was recorded daily for 2 weeks.
KLBLepR−KO mice: 16–18 week old DIO WT and KLBLepR−KO mice on 60% HFD for 8 weeks were randomized by body weight to receive either vehicle or FGF21 (1 mg/kg/day) by i.p. administration. Mice were individually housed for 1 week prior to 5 days of acclimation vehicle injections. All mice received a single treatment injection daily for 3 weeks. Body weights were recorded daily.
Lean WT mice: 12 week-old WT mice on chow diet were randomized by body weight to receive either vehicle, FGF21 (1 mg/kg/day), leptin (250 ng/h; R&D) or FGF21 plus leptin via minipump (ALZET, 1002). Mice were individually housed for 2 weeks prior to surgery. Mice were then subcutaneously implanted with osmotic minipumps and body weight was recorded daily for 2 weeks.
DIO WT mice: 16–18-week-old WT mice on 60% HFD for 8 weeks were randomized by body weight to receive either vehicle, FGF21 (1 μg/day), leptin antagonist (LA; Protein Laboratories Rehovot Ltd; 8 μg/day) or FGF21 plus LA via ICV cannula (ALZET, Brain Infusion Kit III) connected to an osmotic minipump (ALZET, 1004). Mice were individually housed for 2 weeks prior to surgery. Under isoflurane anesthetic, mice were placed in a stereotaxic frame, the minipump was inserted under the skin, and the cannula was implanted using the following coordinates: 1 mm lateral, 0.34 mm caudal to bregma, and 2.5 mm ventral from the surface of the skull. The cannula was secured to the skull using Vetbond (3 M) and dental cement.
2.3. Pair feeding
Prior to any treatment, all mice were acclimated to pair feeding in home cages for 1 week. All food was removed from the hoppers and food was placed on the floor of the home cage twice daily. For ICV minipump experiments, one group of FGF21 plus leptin antagonist (LA) mice were pair fed to the FGF21-treated group. This FGF21 plus LA pair fed group was given the same amount of food consumed by the FGF21 group on the previous day with half of the food provided at the start of the light cycle and half provided at the end of the light cycle. A parallel group of FGF21 plus LA mice were fed ad libitum. For FGF21 and leptin infusion experiments, one group of FGF21-treated mice was pair fed to the FGF21 plus leptin group. The FGF21 pair fed animals were given the same amount of food consumed by the FGF21 plus leptin animals on the previous day with half of the food provided at the start of the light cycle and half provided at the end of the light cycle. A parallel group of FGF21-treated mice were fed ad libitum.
2.4. Infrared imaging
Tail temperature was imaged in fully awake, unrestrained WT mice receiving vehicle, FGF21, leptin, or FGF21 plus leptin by minipump using a high-resolution infrared camera (A655sc Thermal Image; FLIR Systems, Inc.) as previously described [36]. Tail temperature values were taken from the base of the tail, as previously described [37].
2.5. PHP.eB infection
4–8 week old KLB-Cre mice were anesthetized with isoflurane and injected retro-orbitally with PHP.eB viruses: PHP.eB-hSyn-DIO-hM3Dq (3.2 × 1013 GC/mL; Addgene #: 44,361) or PHP.eB- FLEX-tdTomato (1.5 × 1013 GC/mL; Addgene #: 28,306). Following injection, mice were allowed to recover and were placed on 60% HFD to induce obesity.
2.6. Indirect calorimetry
DIO WT and KLBVglut2−KO mice, DIO KLB-Cre mice, and lean WT mice implanted with minipumps administering vehicle, FGF21, leptin or FGF21 plus leptin were individually housed in the Promethion Metabolic System (Sable Systems International) at the University of Iowa Metabolic Phenotyping Core on a 12 h light–dark cycle with ad libitum access to water and food. DIO WT and KLBVglut2−KO mice were injected daily with vehicle (i.p.) for 5 days followed by FGF21 (i.p., 1 mg/kg/day) for 7 days. KLB-Cre mice injected with PHP.eB-hSyn-DIO-hM3Dq or PHP.eB-FLEX-tdTomato were injected daily with vehicle for 2 days (i.p.) followed by 2 days of CNO treatment (i.p., 1 mg/kg). Cages were mounted inside thermally controlled cabinets maintained at 22 °C (KLB-Cre and WT mice) or 30 °C (DIO WT and KLBVglut2−KO mice). Each cage was connected to a flow regulator and gas analyzer to measure oxygen consumption and carbon dioxide production.
2.7. Insulin tolerance tests
DIO WT, KLBVglut2−KO and KLBLepR−KO mice were fasted for 5 h and then injected with 0.75U insulin/kg BW (i.p.) and tail blood was collected at 0, 15, 30, 60, 90, and 120 min. Lean WT and KLBVglut2−KO mice were fasted for 5 h and co-injected with insulin (0.25U/kg, i.p.) and vehicle. One week later, the same cohort of mice was co-injected with insulin (0.25U/kg, i.p.) and FGF21 (1 mg/kg, i.p.).
2.8. Immunohistochemistry
Following an overnight fast, KLB-Cre; Ai14-tdTomato mice were administered 100 μg leptin (i.p.). Mice were transcardially perfused with saline followed by 4% paraformaldehyde (PFA). Brain tissue was post-fixed in 4% PFA overnight followed by 30% sucrose for 48 h. Coronal brain sections (40 μm) were collected using a cryostat (Leica) and pSTAT3 staining was performed as previously described [38]. Briefly, free-floating sections were incubated in 1% H2O2 to quench endogenous peroxidases, permeabilized with 0.3% Triton X-100 and blocked in 3% goat serum, followed by incubation in primary antibody (Cell Signaling 9145) overnight at 4 °C. The following day, sections were incubated in secondary antibody (goat anti-rabbit 488; Life Technologies) for 1 h at room temperature and mounted onto slides. All sections were mounted with VECTASHIELD antifade mounting media with DAPI (Vector Laboratories) and imaged using an Olympus BX61 Light Microscope.
2.9. Gene expression
Gene expression analyses were performed as described previously [15]. Briefly, RNA was isolated from the indicated tissues following Trizol (Invitrogen) protocol. 2 μg RNA from each sample was used to generate cDNA (High Capacity cDNA Reverse Transcription Kit; Life Technologies), and QPCR was conducted using SYBR green (Invitrogen). QPCR primer sequences are as follows: Ucp1: 5′-AAGCTGTGCGATGTCCATGT-3′, 5′-AAGCCACAAACCCTTTGAAAA-3′, U36B4: 5′-CGTCCTCGTTGGAGTGACA-3′, 5′CGGTGCGTCAGGGATTG-3′ Bmp8b: 5′- TCAACACAACCCTCCACATCA-3′, 5′-AGATCGGAGCGTCTGAAGATC-3′; Elovl3: 5′-CTTCGAGACGTTTCAGGACTTAAG-3′, 5′-TCTGGCCAACAACGATGAG-3′.
2.10. Plasma analysis
Blood was collected into 300K2E microvettes (Sarstedt) and spun at 3000 rpm for 30 min at 4 °C to separate plasma. Human FGF21 (Biovendor), mouse insulin (Crystal Chem Inc) and mouse leptin levels (Millipore) were measured using commercially available ELISAs. Plasma triglycerides (Infinity, Thermo Scientific) and glucose (Wako Diagnostics) were measured using colorimetric assays. All measurements were performed according to the manufacturer's instructions.
2.11. Sympathetic nerve recordings
BAT sympathetic nerves were recorded under anesthesia as previously described [39]. Briefly, animals were anesthetized and instrumented with a colonic temperature probe. Cannulae were implanted into the common carotid artery and jugular vein. A sympathetic nerve subserving the BAT was isolated and suspended on 36-gauge platinum-iridium electrodes and secured with silicone gel. Electrodes were interfaced to a high-impedance probe (HIP-511; Grass Instruments), and the neural signal from BAT was amplified 100,000 times, filtered at low- and high-frequency cutoffs at 100 and 1,000 Hz, respectively, and routed to a resetting voltage integrator (B600c; University of Iowa Bioengineering). Data was recorded using an ADInstruments PowerLab with the associated Chart software.
2.12. Statistical analysis
Data were analyzed using GraphPad Prism and presented as mean ± SEM; p < 0.05 was considered significant. The statistical test used for each comparison is described in the corresponding figure legends.
3. Results
3.1. Activation of KLB+ neurons is sufficient to increase energy expenditure and decrease body weight
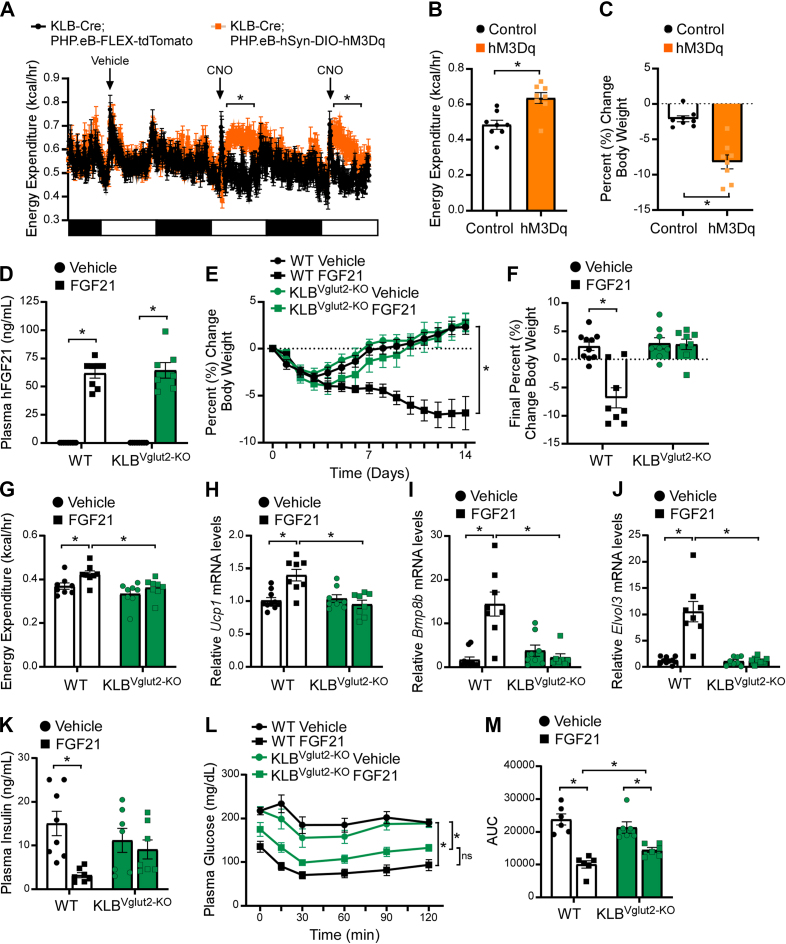
Numerous studies have implicated the CNS as the site of action for FGF21-mediated regulation of body weight [[21], [22], [23], [24]]. Despite this, it remains unclear whether these actions are mediated by neurons or other cell populations within the brain (i.e., glial, ependymal, pericytes, tanycytes). To determine whether activation of KLB+ neurons is sufficient to elevate energy expenditure and promote body weight loss, we infected KLB-Cre mice, which express Cre recombinase under the control of the endogenous Klb promoter [32], with a Cre-dependent viral construct that expresses an excitatory designer receptors exclusively activating designer drugs (DREADDs) (PHP.eB-hSyn-DIO-hM3D (Gq)-mCherry) or a control virus (PHP.eB-FLEX-tdTomato) specifically in neurons throughout the brain. Critically, intravenous injection of PHP.eB viruses has been shown to transduce a majority of neurons within the CNS [40]. Accordingly, we observed PHP.eB viral infection in regions of the brain consistent with KLB expression as previously reported in KLB-Cre; tdTomato mice [32]. Following PHP.eB infection, KLB-Cre mice were given 60% high fat diet (HFD) to induce obesity. Administration of the DREADD-specific ligand, clozapine-N-oxide (CNO), increased energy expenditure (Figure 1A,B) and decreased body weight (Figure 1C) in diet-induced obese (DIO) KLB-Cre mice infected with PHP.eB-hSyn-DIO-hM3Dq, but not in mice administered the control virus. Together, these data demonstrate that activation of KLB+ neurons is sufficient to increase energy expenditure and promote body weight loss.
Figure 1.
FGF21 signaling to glutamatergic neurons is required to promote weight loss, but not improve insulin sensitivity, in DIO mice. (A–C) Energy expenditure (A–B) and percent change in body weight (C) in diet-induced obese (DIO) KLB-Cre mice infected with PHP.eB-hSyn-DIO-hM3D (Gq)-mCherry (hM3Dq) or PHP.eB-FLEX-tdTomato (Control) and subsequently treated with vehicle for 2 days followed by CNO (i.p., 1 mg/kg) for 2 days (n = 8/group). (D–F) 16–18 week old DIO WT and KLBVglut2−KO mice were administered vehicle or FGF21 (1 mg/kg/day) by osmotic minipump for 2 weeks (n = 7–10/group). (D) Plasma FGF21 levels, (E) daily percent change in body weight and (F) final percent change in body weight. (G) Energy expenditure in 16–18 week old DIO WT and KLBVglut2−KO mice after 5 days of daily vehicle and 7 days of daily FGF21 injections (i.p., 1 mg/kg) (n = 8/group). (H–K) 16–18 week old DIO WT and KLBVglut2−KO mice were administered vehicle or FGF21 (1 mg/kg/day) by osmotic minipump for 2 weeks (n = 8–10/group). (H–J) Brown adipose tissue mRNA expression of (H) Ucp1, (I) Bmp8b and (J) Elovl3, and (K) plasma insulin levels (n = 7–8/group). (L–M) Plasma glucose levels during an insulin tolerance test (ITT) in DIO WT and KLBVglut2−KO mice at baseline and after 3 weeks of daily FGF21 injections (i.p, 1 mg/kg) (n = 6/group). (M) Quantification of the area under the curve for ITT in (L). Values are mean ± SEM. ∗p < 0.05. Statistical analyses were conducted using either one-way ANOVA or two-way ANOVA with Holms-Sidak's multiple comparisons test.
3.2. FGF21 signaling to glutamatergic neurons is required for its effects to reduce body weight
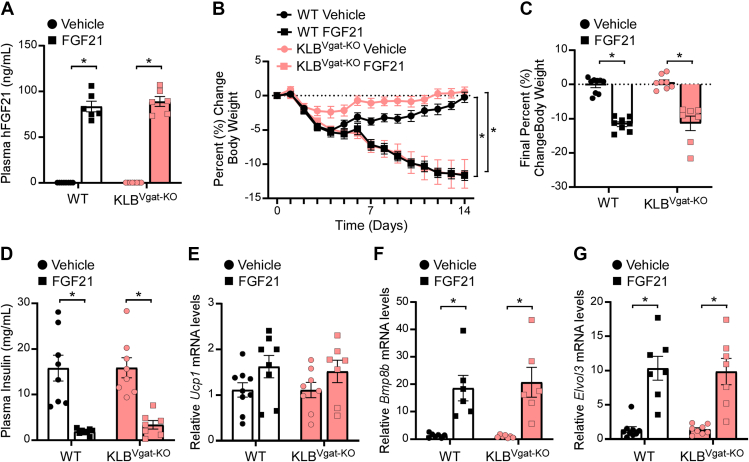
Previous work from our lab and others has demonstrated that KLB is expressed in both excitatory (glutamatergic, Vglut2+) and inhibitory (GABAergic, Vgat+) neurons [32,41]. To begin to explore the molecular identity of neurons mediating FGF21's effects on energy expenditure, we generated mice that lack KLB in glutamatergic (KLBVglut2−KO) or GABAergic (KLBVgat−KO) neurons by crossing KLBfl/fl mice with Vglut2-Cre or Vgat-Cre mice [33], respectively. Following consumption of 60% HFD, weight matched DIO KLBVglut2−KO, KLBVgat−KO and control wild-type (WT) littermates (Supplemental Figure 1a and Supplemental Figure 3a) were administered FGF21 by osmotic minipump for 2 weeks. As expected, FGF21 treatment significantly elevated plasma FGF21 levels in WT littermates, as well as both KLBVglut2−KO and KLBVgat−KO mice (Figure 1, Figure 2A). Consistent with previous studies, continuous FGF21 administration to DIO WT mice resulted in significant weight loss (Figure 1E,F and Figure 2B,C). However, while DIO KLBVgat−KO responded similarly to WT mice with significant weight loss in response to FGF21 (Figure 2B,C and Supplemental Figure 3b), DIO KLBVglut2−KO mice, in contrast, were completely resistant to FGF21-mediated decreases in body weight (Figure 1E,F).
Figure 2.
FGF21 signaling to GABAergic neurons is not required to promote weight loss in DIO mice. (A–G) 16–18 week old diet-induced obese (DIO) WT and KLBVgat−KO mice were administered vehicle or FGF21 (1 mg/kg/day) by osmotic minipump for 2 weeks (n = 6–9/group). (A) Plasma FGF21 levels, (B) daily percent change in body weight, (C) final percent change in body weight, (D) plasma insulin levels, and (E–G) brown adipose tissue mRNA expression of (E) Ucp1, (F) Bmp8b, and (G) Elovl3. Values are mean ± SEM. ∗p < 0.05. Statistical analyses were conducted using two-way ANOVA with Holms-Sidak's multiple comparisons test.
To determine whether FGF21-mediated activation of energy expenditure was attenuated in KLBVglut2−KO mice, DIO WT and KLBVglut2−KO mice were housed in metabolic cages (Promethion) and administered vehicle daily via intraperitoneal (i.p.) injection for 5 days followed by 7 days of daily FGF21 (1 mg/kg, i.p.). Consistent with no differences in baseline body weight (Supplemental Figure 1a), WT and KLBVglut2−KO mice exhibited similar oxygen consumption (VO2) (Supplemental Figure 1i, j) and energy expenditure (Figure 1G and Supplemental Figure 1f-h) during both the light and dark cycle following vehicle treatment. As expected, FGF21 treatment significantly increased VO2 (Supplemental Figure 1i) and energy expenditure (Figure 1G) during the dark cycle in WT mice; however, KLBVglut2−KO mice were resistant to FGF21-mediated increases in VO2 (Supplemental Figure 1i, j) and energy expenditure (Figure 1G and Supplemental Figure 1g). Consistent with the effects on energy expenditure, FGF21 treatment significantly increased water intake in WT mice, but not KLBVglut2−KO mice (Supplemental Figure 1k, l), likely due to an increase in postprandial thirst as elevations in energy expenditure lead to increases in food consumption. Importantly, administration of FGF21 did not alter physical activity in WT mice (Supplemental Figure 1m, n), although there was a trend toward increased activity following FGF21 treatment in KLBVglut2−KO mice during the dark cycle (Supplemental Figure 1m), suggesting that FGF21 may be able to regulate other aspects of energy homeostasis when circuits controlling energy expenditure are impaired. Consistent with the metabolic cage data, DIO WT and KLBVgat−KO mice administered FGF21 exhibited significantly increased thermogenic gene expression in brown adipose tissue (BAT) compared to vehicle treated controls (Figure 1H–J and Figure 2E–G), and this induction was completely prevented in the BAT of DIO KLBVglut2−KO mice treated with FGF21 (Figure 1H–J). Together, these data indicate that KLB expression in glutamatergic, but not GABAergic, neurons is required for FGF21 to increase energy expenditure and promote body weight loss in DIO mice.
Consistent with body weight loss, chronic FGF21 administration significantly decreased plasma insulin and glucose levels in DIO WT and KLBVgat−KO mice, but not DIO KLBVglut2−KO mice, compared to vehicle treated mice (Figure 1K, Figure 2D, and Supplemental Figure 1b). Concomitantly, FGF21 treatment reduced lipid content in the BAT and liver of WT, but not KLBVglut2−KO mice (Supplemental Figure 1c and Supplemental Figure 2a, b). To determine whether FGF21 administration improves insulin sensitivity in DIO KLBVglut2−KO mice, despite no effects on body weight, animals were subjected to insulin tolerance tests in response to vehicle treatment and after 3 weeks of daily FGF21 treatment. Interestingly, FGF21 administration effectively improved insulin sensitivity in both DIO WT and KLBVglut2−KO mice (Figure 1L,M). Importantly, similar improvements in insulin sensitivity were also observed in lean WT and KLBVglut2−KO mice acutely administered insulin plus FGF21 in combination (Supplemental Figure 1d, e). These data are consistent with previous research showing that the acute insulin sensitizing effects of FGF21 occur through action on adipose tissue and not the brain [15,21]. Together, these data demonstrate that FGF21 signaling to glutamatergic neurons is critical for the chronic pharmacological effects of FGF21 on body weight reduction, but not the acute effects of FGF21 to increase insulin sensitivity.
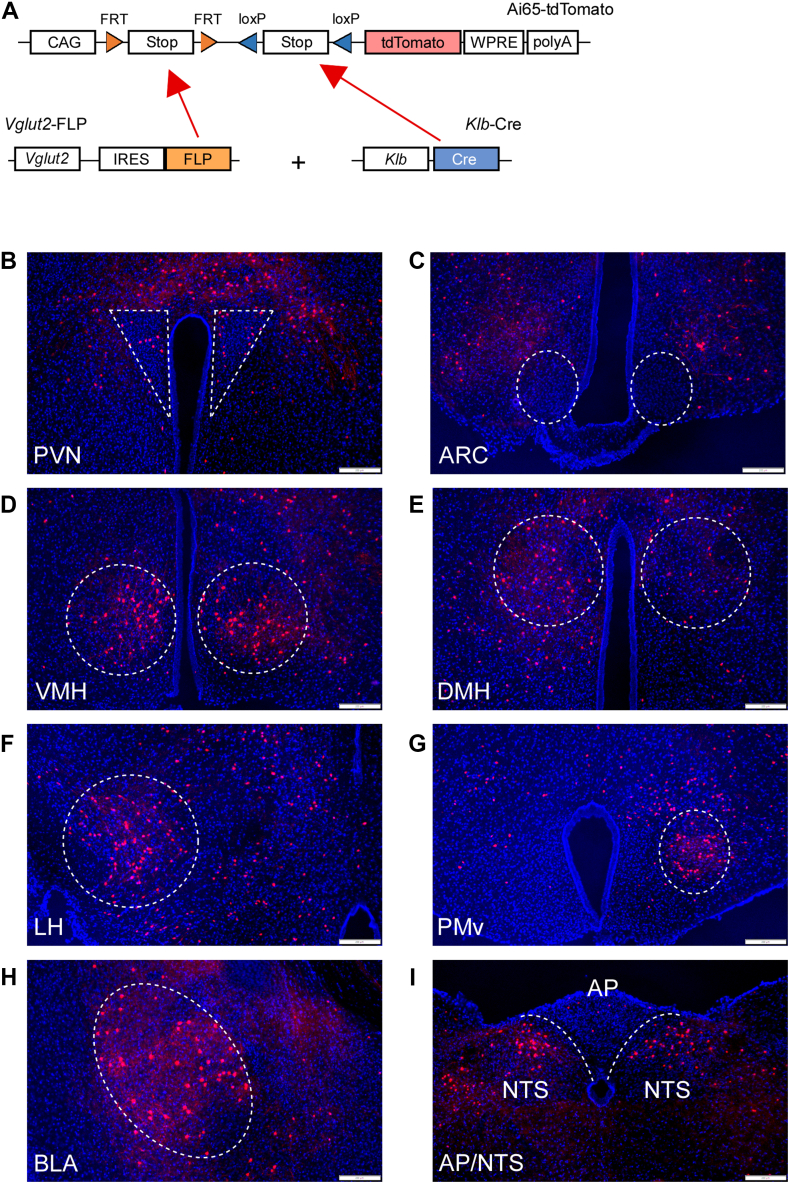
To investigate which brain region(s) contain Vglut2+/KLB+ neurons, we utilized reporter mice that provide tdTomato expression in a Flp- and Cre-dependent fashion (Ai65 reporter mice) [34]. Ai65 reporter mice were crossed to mice which express Flp recombinase under the control of the endogenous Vglut2 promoter (Vglut2-Flp) and KLB-Cre mice to generate KLB-Cre; Vglut2-Flp; Ai65 triple knock-in mice (Figure 3A). KLB-Cre; Vglut2-Flp; Ai65 triple knock-in mice exhibited tdTomato expression in multiple brain regions which have previously been demonstrated to express either Vglut2 [33] or KLB [32], including the paraventricular nucleus (PVN), ventromedial hypothalamus (VMH), lateral hypothalamus (LH), dorsomedial hypothalamus (DMH), basolateral amygdala (BLA), ventral premammillary nucleus (PMv) and nucleus of the solitary tract (NTS) (Figure 3B, D-I). However, little to no KLB+/Vglut2+ cells were observed in the arcuate nucleus of the hypothalamus (ARC) (Figure 3C). Comprehensive imaging of Vglut2+/KLB+ neurons using brain clearing and lightsheet microscopy revealed a significant number of Vglut2+/KLB+ neurons in the VMH, BLA, and hindbrain (Video 1 and Supplemental Videos 1-2).
Figure 3.
Distribution of KLB+/Vglut2+ neurons in the CNS. (A) Schematic representation of the generation of KLB-Cre; Vglut-FLP; Ai65-tdTomato triple-knockin mice. CAG, CAG promoter coding sequence; FRT, FRT sequence; LoxP, LoxP sequence; STOP, stop codons; tdtomato, tdtomato reporter sequence; WPRE, gene enhancer sequence; polyA, polyadenylation. (B–H) Fluorescent images of brain regions in KLB-Cre; Vglut-FLP; Ai65-tdTomato triple-knockin mice including the (B) paraventricular nucleus (PVN), (C) arcuate nucleus (ARC), (D) ventromedial hypothalamus (VMH), (E) dorsomedial hypothalamus (DMH), (F) lateral hypothalamus (LH), (G) ventral premammillary nucleus (PMv), (H) basolateral amygdala (BLA), and (I) area postrema (AP) and nucleus of the solitary tract (NTS).
The following is/are the supplementary data related to this article:
Video 1. Potential glutamatergic targets of direct FGF21 signaling. Light sheet imaging of a brain from KLB-Cre; Vglut2-FLP; Ai65-tdTomato mice processed by DISCO clearing. Video focused on the ventromedial hypothalamus (VMH).
3.3. FGF21 signaling to leptin receptor-expressing cells is critical for its full effects to reduce body weight
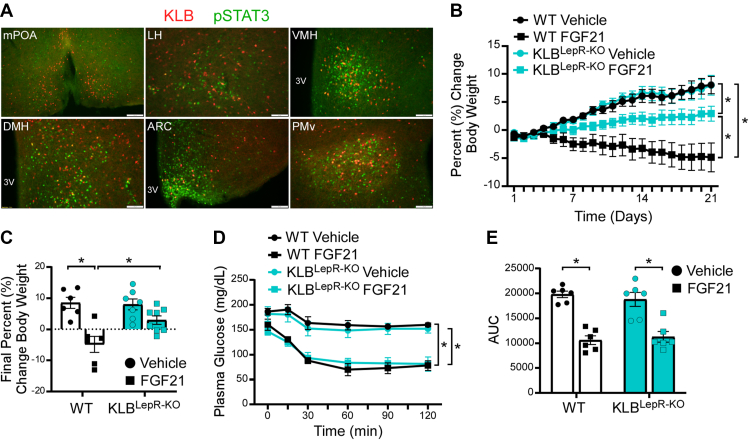
To further elucidate the molecular identity of KLB-expressing neurons we explored previously published datasets for neuronal markers within the hypothalamus [42] and in other brain regions [43]. In silico analysis of SMART-seq of cells from the hypothalamus [42] revealed that 95% of KLB+ cells express Vglut2 (521 of 546). Interestingly, 29% of these Vglut2+/KLB+ neurons also express the leptin receptor (LepR) (151 of 521). Importantly, previous research utilizing ob/ob mice [11,27], Zucker fatty rats [44], or mice lacking adipose tissue [25], suggests that signaling from the adipose-derived hormone, leptin, may be critical for FGF21 to reduce body weight [27,[29], [30], [31]]. Thus, to investigate whether FGF21 and leptin can signal to the same cells within the hypothalamus, we treated KLB-Cre; tdTomato mice with leptin and immunostained for leptin-induced phosphorylation of STAT3 (pSTAT3). Co-localization of tdTomato and pSTAT3 was observed in numerous regions of the hypothalamus, including the LH, VMH, DMH, ARC, and PMv (Figure 4A and Supplemental Figure 4), suggesting that FGF21 could act in these KLB+ leptin-sensitive cells. Interestingly, all of these regions, except the ARC, also contain neuronal populations which have also expressed both Vglut2 and KLB (Figure 3B–I). To next determine whether FGF21 action is required in leptin-sensitive cells to regulate body weight, we generated mice that lack KLB from leptin receptor-expressing cells (KLBLepR−KO) by crossing KLBfl/fl mice with LepR-Cre mice. Following consumption of 60% HFD, DIO KLBLepR−KO mice and WT littermates were administered FGF21 for 3 weeks. As expected, WT mice receiving FGF21 lost a significant amount of weight (Figure 4B,C). In contrast, KLBLepR−KO mice did not lose weight in response to FGF21 treatment; however, they also did not gain as much weight as vehicle treated KLBLepR−KO mice (Figure 4B,C) suggesting that FGF21 did retain some of its ability to regulate body weight. To determine how loss of FGF21 signaling to leptin-sensitive cells impacts FGF21-mediated improvements in insulin sensitivity, we conducted insulin tolerance tests in DIO KLBLepR−KO mice and WT littermates at baseline and following 3 weeks of daily FGF21 treatment. Similar to KLBVglut2−KO mice, FGF21 treatment significantly improved insulin sensitivity in KLBLepR−KO mice (Figure 4D,E) suggesting that FGF21 does not signal to LepR-expressing cells to acutely regulate insulin sensitivity. Together, these results support that FGF21 signaling to leptin-sensitive cells is required for FGF21's full effects to reduce body weight, but not for its role in the regulation of insulin sensitivity.
Figure 4.
FGF21 signals to leptin receptor-expressing cells to promote weight loss. (A) Representative immunofluorescence imaging for pSTAT3 in the medial preoptic area (mPOA), lateral hypothalamus (LH), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), arcuate nucleus (ARC) and ventral premammillary nucleus (PMv) of KLB-Cre; tdTomato mice administered leptin (i.p., 100 μg) following an overnight fast. 3 V = 3rd ventricle. (B–C) 16–18 week old diet-induced obese (DIO) WT and KLBLepR−KO mice were administered vehicle or FGF21 daily (i.p., 1 mg/kg) for 3 weeks (n = 6–8/group). (B) Daily percent change in body weight and (c) final percent chance in body weight. (D–E) Plasma glucose levels during an insulin tolerance test (ITT) in DIO WT and KLBLepR−KO mice at baseline and after 3 weeks of daily FGF21 injections (i.p, 1 mg/kg) (n = 6/group). (E) Quantification of the area under the curve for ITT in (D).
3.4. FGF21 and leptin co-treatment has potent weight-reducing effects
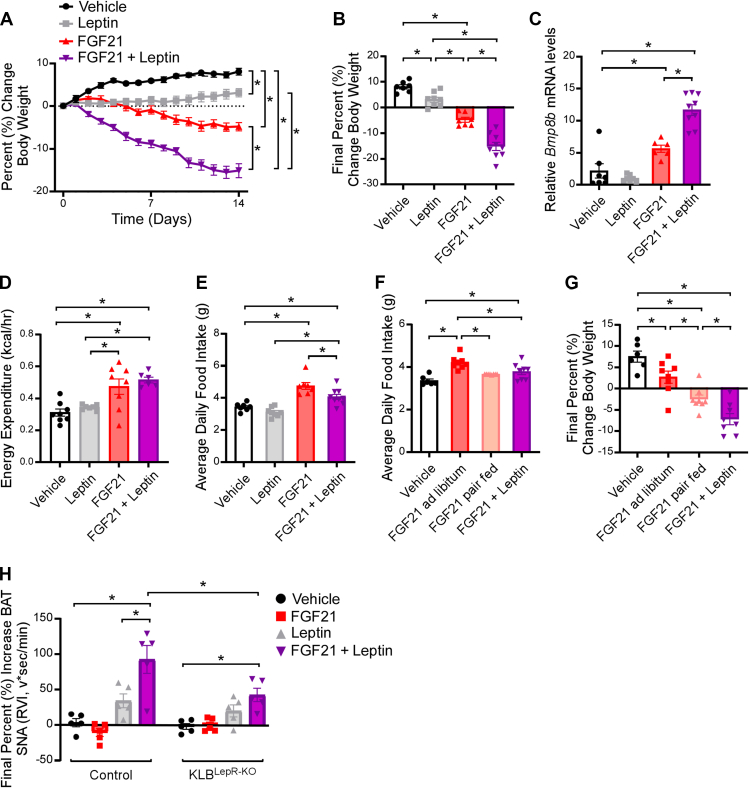
To investigate whether FGF21 has any functional effects on leptin signaling, we treated weight-matched lean WT mice with vehicle, FGF21 (1 mg/kg/day), a low dose of leptin (250 ng/h) [45], or FGF21 in combination with leptin for 14 days utilizing osmotic minipumps (Supplemental Figure 5a). As expected, mice receiving leptin exhibited elevated plasma leptin levels (Supplemental Figure 5c), and administration of FGF21 significantly increased plasma FGF21 (Supplemental Figure 5b). Co-administration of leptin with FGF21 resulted in significant reductions in body weight compared to leptin or FGF21 treatment alone (Figure 5A,B). Moreover, these mice had increased BAT thermogenic gene expression (Figure 5C and Supplemental Figure 5d) and elevated energy expenditure (Figure 5D) with no change in tail temperature (Supplemental Figure 5e, f). Consistent with body weight loss, FGF21 and leptin treatment significantly decreased plasma glucose and insulin levels compared to vehicle treated animals (Supplemental Figure 5g, h). As observed previously [17,46], FGF21 administration alone or in combination with leptin, significantly increased food intake compared to vehicle treated mice (Figure 5E), likely to support FGF21-mediated increases in energy expenditure. Notably, however, co-administration of FGF21 with leptin significantly attenuated the FGF21-mediated increase in food intake compared to FGF21 treatment alone (Figure 5E).
Figure 5.
FGF21 enhances leptin function to reduce body weight. (A–E) 10–12 week old, chow-fed WT mice were administered vehicle, leptin (250 ng/h), FGF21 (1 mg/kg/day) or leptin plus FGF21 by osmotic minipump for 2 weeks (n = 7–9/group). (A) Daily percent change in body weight, (B) final percent change in body weight, (C) Bmp8b mRNA in brown adipose tissue (n = 6–9/group), (D) energy expenditure, and (E) average daily food intake. (F–G) 10–12 week old, chow-fed WT mice were administered vehicle, FGF21 (1 mg/kg/day) or leptin plus FGF21 by osmotic minipumps for 2 weeks (n = 6–8/group). One group of FGF21 treated mice was pair fed to the FGF21 + leptin group. (F) Average daily food intake and (G) final percent change in body weight. (H) Brown adipose tissue sympathetic nerve activity recordings in 11–13 week old, chow-fed WT control or KLBLepR−KO mice administered a single IV injection of vehicle, FGF21 (1 mg/kg), leptin (0.5 μg/g), or FGF21+leptin (n = 5/group). Values are mean ± SEM. ∗p < 0.05. Statistical analyses were conducted using either one-way ANOVA or two-way ANOVA with Holms-Sidak's multiple comparisons test.
To determine the contribution of food consumption to the body weight loss by co-administration of FGF21 and leptin, we pair fed a group of weight-matched FGF21-treated mice to mice treated with FGF21 and leptin (Supplemental Figure 5i). To do so, FGF21 pair fed mice were given the amount of food eaten by the FGF21 and leptin group the previous day, with half provided at the start of the light cycle and half provided before the start of the dark cycle to limit food seeking behavior. As expected, pair fed FGF21-treated mice ate less (Figure 5F) and lost more weight than ad libitum fed FGF21-treated mice (Figure 5G). However, FGF21 pair fed mice did not lose as much weight as mice treated with FGF21 and leptin in combination (Figure 5G) suggesting that decreased food intake does not completely account for the profound weight loss observed in mice co-administered FGF21 and leptin. Administration of FGF21 plus leptin significantly increased thermogenic gene expression in the BAT compared to vehicle treated mice, although this induction was not significantly higher than FGF21 treatment alone (Supplemental Figure 5J-L).
To begin to investigate how FGF21 may alter the function of leptin, we interrogated whether acute administration of a low dose of leptin and FGF21 in combination exhibits any effects on sympathetic drive to the BAT in comparison to leptin or FGF21 alone. Similar to the effects observed on body weight (Figure 5A,B), administration of leptin with FGF21 significantly increased leptin-mediated sympathetic nerve activity to the BAT compared to leptin or FGF21 treatment alone (Figure 5H). Importantly, and in contrast to previously published data in which FGF21 was administered directly into the brain [22] or chronically elevated throughout the course of the recording [22,47], acute IV administration of FGF21 alone did not activate BAT sympathetic nerve activity (SNA) (Figure 5H). Interestingly, the activation of BAT SNA by FGF21 and leptin co-administration was attenuated in KLBLepR−KO mice (Figure 5H), consistent with an attenuation of FGF21-mediated weight loss in these animals (Figure 4B,C). Together, these data support that co-administration of FGF21 and leptin synergistically promotes body weight loss.
3.5. FGF21 requires functional leptin signaling
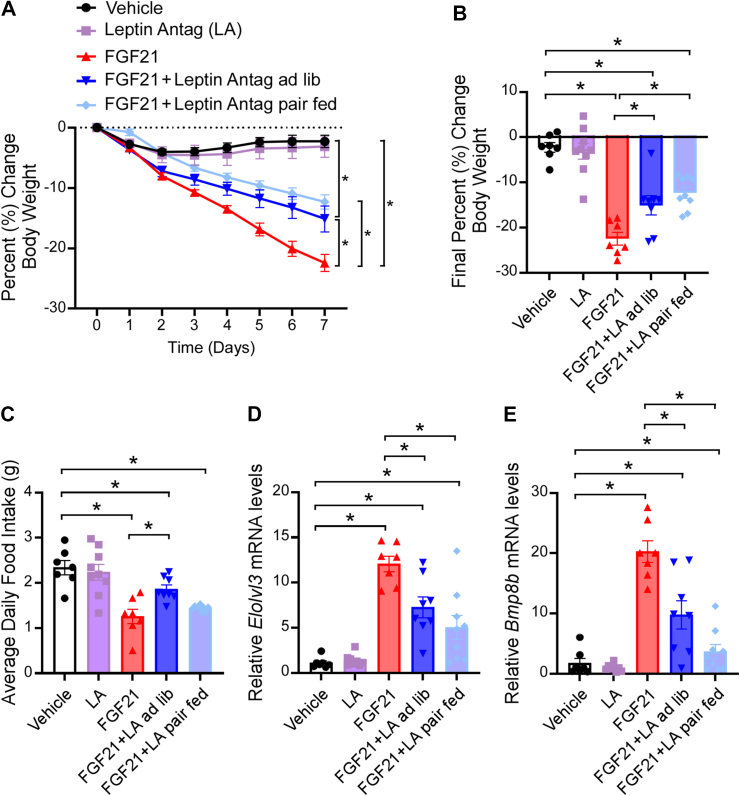
To determine whether functional leptin signaling is required for FGF21's metabolic effects, we acutely inhibited central leptin signaling by treating weight-matched DIO WT mice with intracerebroventricular (ICV) vehicle, FGF21, leptin antagonist (LA) or FGF21 plus LA via osmotic minipump for 7 days (Supplemental Figure 6a). To control for any alterations in food intake, one group of FGF21 plus LA treated mice was pair fed to mice receiving only FGF21. FGF21 plus LA pair fed mice were given the amount of food eaten by the FGF21-treated mice the previous day, with half provided at the start of the light cycle and half provided before the start of the dark cycle to limit food seeking behavior. As expected, central FGF21 administration significantly reduced body weight (Figure 6A,B). Interestingly, FGF21-mediated weight loss was abrogated in both ad libitum and pair fed mice treated with FGF21 and LA in combination (Figure 6A,B). Despite no differences in food intake (Figure 6C), FGF21 LA pair fed mice did not lose as much weight as mice treated with FGF21 alone suggesting that FGF21 requires leptin signaling to fully stimulate body weight loss. Consistent with this idea, both ad libitum and pair fed FGF21 LA treated groups exhibited attenuated induction of thermogenic gene expression in BAT compared to mice treated with FGF21 alone (Figure 6D,E). Moreover, plasma leptin levels corresponded to weight loss and importantly were not altered by ICV LA administration (Supplemental Figure 6b). While plasma leptin levels corresponded to body weight in these experiments, administration of FGF21 to WT DIO mice also acutely reduced plasma leptin levels suggesting FGF21 enhances leptin sensitivity (Supplemental Figure 6c). Despite significant weight loss, 7 days of ICV FGF21 treatment did not significantly reduce plasma glucose or insulin levels (Supplemental Figure 6d, e), however, plasma triglyceride levels were significantly decreased following FGF21 treatment compared to vehicle or pair fed LA FGF21 mice (Supplemental Figure 6f). Taken together, these data reveal that FGF21 requires, at least in part, leptin signaling to fully stimulate thermogenic gene expression and reduce body weight.
Figure 6.
FGF21 requires central leptin signaling to fully promote body weight loss. (A–E) 16–18 week old diet-induced obese (DIO) WT mice were administered vehicle, leptin antagonist (LA; 8 μg/day), FGF21 (1 μg/day), or LA and FGF21 by ICV osmotic minipump for 7 days (n = 7–9/group). (A) Daily percent change in body weight, (B) final percent change in body weight, (C) average daily food intake, and (D–E) brown adipose tissue mRNA expression of (D) Elovl3 and (E). Bmp8b Values are mean ± SEM. ∗p < 0.05. Statistical analyses were conducted using one-way ANOVA.
4. Discussion
This work provides important new insights into how FGF21 signals to the CNS to regulate energy homeostasis. Utilizing KLB-Cre mice, we demonstrate that activation of neurons expressing KLB is sufficient to elevate energy expenditure and decrease body weight. Moreover, the KLB-expressing neurons that are required for FGF21's ability to promote weight loss are glutamatergic, but not GABAergic. This is consistent with our recent studies showing that FGF21 signals to glutamatergic neurons to suppress sucrose intake [32] and promote weight loss following consumption of a low protein diet [23]. Finally, we also demonstrate that FGF21 requires, at least in part, central leptin signaling to decrease body weight and that administration of FGF21 and leptin together leads to potent weight loss through effects on both caloric intake and energy expenditure.
While FGF21 clearly acts on glutamatergic neurons to regulate body weight, it remains unclear whether these neurons are in a distinct brain region or if KLB+ glutamatergic neurons capable of regulating energy homeostasis in response to FGF21 are distributed in multiple brain regions. We demonstrate here that Vglut2+/KLB+ cells in the CNS can be found in several brain regions important for the control of energy homeostasis including the ventromedial hypothalamus, lateral hypothalamus, dorsomedial hypothalamus, paraventricular nucleus, ventral premammillary nucleus and nucleus of the solitary tract. Moreover, a subpopulation of these cells also expresses the leptin receptor (LepR) and leptin is involved in FGF21-mediated regulation of energy homeostasis. However, FGF21 still retains some of its ability to regulate body weight when KLB is ablated from LepR-expressing cells and when central leptin signaling is inhibited. Future work is needed to determine whether leptin signaling is also important for other aspects of FGF21 function including its role to regulate macronutrient preference. One component of FGF21's effect to increase energy expenditure involves FGF21-mediated enhancement of leptin-dependent increases in sympathetic nerve activity. Previous studies reported the ability of FGF21 to increase sympathetic nerve activity [22,47]. Our results provide further insight into these observations and reveal that FGF21 functions to acutely and significantly enhance leptin-mediated increases in sympathetic nerve activity to the brown adipose tissue, but FGF21 does not acutely increase sympathetic nerve activity on its own.
Both KLB and the LepR are expressed in regions of the hypothalamus including the VMH, DMH, and LH, as well as regions outside of the hypothalamus including the NTS. Previous research utilizing mice with both genetic ablation of KLB from the hindbrain and transgenic overexpression of FGF21 suggests that FGF21 may not require signaling to the NTS to regulate energy expenditure or body weight [22]. However, it is unclear if KLB is deleted from all cells within the NTS in this model or whether FGF21 action here is sufficient to promote body weight loss. In addition, although we have previously shown that FGF21 signaling to the VMH is not required for FGF21 to promote body weight loss [32], it remains possible that FGF21 signaling there is sufficient to regulate body weight, but that redundant neural circuits exist for FGF21-mediated regulation of energy homeostasis. Alternatively, chronic versus acute deletion of KLB from the VMH may produce different results similar to chronic versus acute deletion of the leptin receptor from AgRP neurons in the hypothalamus [48,49]. Finally, it is possible that FGF21 may act on different brain regions or neural circuits to promote body weight loss through context-dependent mechanisms to modulate not only energy expenditure, but also through the regulation of food intake, physical activity, or digestive efficiency [50].
Previous studies showed that FGF21 can decrease body weight in obese pigs [51], which lack UCP1, as well as mice with acute or genetic ablation of UCP1-expressing cells [[52], [53], [54]]. Interestingly, instead of increasing energy expenditure, FGF21 increased physical activity and/or decreased food intake to promote negative energy balance. In addition, in non-human primates and humans, who have brown adipose tissue but do not depend as heavily on brown adipose tissue thermogenesis to regulate whole body energy homeostasis, FGF21 may promote weight loss, at least in part, by regulating food intake [18,55,56]. Thus, FGF21 could act through different brain regions or circuits to modulate discrete aspects of energy balance to ultimately promote body weight loss. Importantly, we found that FGF21 signaling to glutamatergic neurons is required for the body weight lowering effects of FGF21. In addition, we showed that functional leptin signaling is important for maximal FGF21-mediated weight loss. While loss of leptin signaling in GABAergic neurons [33,48] is responsible for weight gain, leptin action in glutamatergic SF1 neurons is critical for leptin's role in reducing body weight [57]. In addition, while leptin action in ARC AgRP neurons is critical for leptin's regulation of body weight [48,49], we did not observe KLB+/Vglut2+ neurons in the ARC. Since leptin regulates energy homeostasis through signaling to both glutamatergic [[57], [58], [59]] and GABAergic neurons [33,48], and because obesity represents a leptin resistant state [60], it is interesting to speculate that FGF21 may selectively improve leptin sensitivity in a subset of leptin receptor-containing neurons, particularly during obesity, when leptin levels are high. Importantly, unlike leptin resistance, obese rodents, primates, and humans with obesity respond to pharmacological levels of exogenous FGF21 or FGF21 analogues to lower body weight [50] even in a potentially FGF21 resistant state [28,61,62]. It will be interesting to explore whether FGF21 signaling to glutamatergic neurons is also required for FGF21-mediated weight loss in the models described above (i.e., UCP1 KO), in which thermogenesis is impaired, or whether alternative circuits are employed.
While the physiological significance of KLB in glutamatergic neurons is clear, the role of KLB in GABAergic neurons remains unknown since FGF21 does not act in this cell population to regulate either body weight (Figure 2 and [23]) or sucrose consumption [32]. Notably, KLB is also required for the action of another endocrine FGF, fibroblast growth factor 15/19 (FGF15/19), which may also act within the CNS [63]. Previous work suggests FGF19 can regulate the activation of GABAergic AgRP neurons [64], but more work is necessary to determine if KLB is required in these cells for FGF19 to regulate energy balance.
Both obese animals and human subjects with obesity exhibit significantly elevated plasma leptin levels because of leptin resistance [60,65]. Our data suggests that FGF21 requires leptin signaling to fully stimulate weight loss in obese animals. Therefore, it is interesting to speculate that plasma leptin levels may be a contributing factor to the beneficial effects of FGF21 analogues on weight loss in human patients. It is possible that patients with higher leptin levels will respond to FGF21 with a greater enhancement in leptin signaling leading to more significant weight loss. Importantly, our data also shows that FGF21 can acutely lower leptin levels following a single injection of FGF21 in DIO mice, likely due to an improvement in leptin sensitivity before any changes in body weight are observed. In contrast, human subjects with congenital leptin deficiency may be refractory to some of the beneficial metabolic effects of FGF21 administration, as is observed in mice deficient in leptin signaling [11,27]. Future studies are warranted to determine how baseline plasma leptin levels in human patients may correlate with weight loss in response to FGF21 analogues, and if changes in plasma leptin levels are observed before any significant changes in body weight occur. In addition to leptin, FGF21 is also a well-established insulin sensitizer [66]. A single injection of FGF21 to obese mice is sufficient to robustly improve insulin sensitivity and decrease plasma glucose levels [12,14,15]. FGF21's acute insulin-sensitizing effects occur through direct action on adipose tissues [15,21,67]. In contrast, we and others have shown that FGF21's effects on body weight occur through direct action on the CNS [[21], [22], [23], [24]]. Consistent with this, we show in this study that the acute, insulin-sensitizing effects of FGF21 are retained in mice lacking FGF21 signaling to glutamatergic neurons, but the secondary effects of FGF21 on improved glucose homeostasis and insulin sensitivity due to weight loss are lost in these mice. Recent work also suggests that FGF21 may potentiate the action of glucagon-like peptide-1 (GLP-1) as administration of a GLP-1/FGF21 dual agonist leads to robust weight loss, at least in part through reduced food intake, compared to FGF21 or GLP-1 alone [68,69]. Thus, FGF21 may function physiologically and pharmacologically on distinct tissues to promote systemic energy homeostasis by acting as a master sensitizer of other endocrine signals. Future work is needed to explore the central and peripheral signaling pathways by which FGF21 action sensitizes different endocrine hormones to regulate whole-body energy homeostasis. Together, our data demonstrate a previously unknown endocrine crosstalk between liver-derived and adipose-derived hormones that coordinately signal to the CNS to regulate body weight homeostasis.
Author contributions
K.E.C designed and performed experiments, interpreted data, and wrote the paper. M.C.N, A.I.S, T.J.N., K.H.F., D.A.M., S.O.J, L.V.Z, K.R. and Z.Z. performed experiments and interpreted data. M.J.P. conceived of the project, designed experiments, interpreted data, wrote the paper, and is responsible for the integrity of its content.
Acknowledgments
We thank Dr. Birgitte Andersen (Novo Nordisk) for providing FGF21 protein, Dr. Justin Grobe (Medical College of Wisconsin) for analytical expertise, and Dr. Lucas BonDurant for data collection and project support. This work was funded by the National Institutes of Health R01DK106104 (M.J.P.), R01AA027654 (M.J.P.), T32 HL007121 (K.E.C.), F32 DK117510 (K.E.C.), T32 DK112751 (K.H.F.), F31 DK117515 (S.O.J.), Veterans Affairs Merit Review Program I01BX004634 (M.J.P.), and the University of Iowa Carver College of Medicine (M.J.P). The authors would like to acknowledge use of the University of Iowa Central Microscopy Research Facility, the University of Iowa Metabolic Phenotyping Core and the Genomics Division of the Iowa Institute of Human Genetics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101564.
Conflict of interest
The authors have no conflicts to declare.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Afshin A., Reitsma M.B., Murray C.J.L. Health effects of overweight and obesity in 195 countries. New England Journal of Medicine. 2017;377(15):1496–1497. doi: 10.1056/NEJMc1710026. [DOI] [PubMed] [Google Scholar]
- 2.Bauer U.E., Briss P.A., Goodman R.A., Bowman B.A. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 3.Bjerregaard L.G., Baker J.L. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. New England Journal of Medicine. 2018;378(26):2537–2538. doi: 10.1056/NEJMc1805984. [DOI] [PubMed] [Google Scholar]
- 4.Twig G., Kark J.D. Body-mass index in adolescence and cardiovascular death in adulthood. New England Journal of Medicine. 2016;375(13):1300–1301. doi: 10.1056/NEJMc1609415. [DOI] [PubMed] [Google Scholar]
- 5.Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R., et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity. 2016;24(8):1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibel R.L., Seeley R.J., Darsow T., Berg E.G., Smith S.R., Ratner R. Biologic responses to weight loss and weight regain: report from an American diabetes association research symposium. Diabetes. 2015;64(7):2299–2309. doi: 10.2337/db15-0004. [DOI] [PubMed] [Google Scholar]
- 8.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V., et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki M., Uehara Y., Motomura-Matsuzaka K., Oki J., Koyama Y., Kimura M., et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Molecular Endocrinology. 2008;22(4):1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa Y., Kurosu H., Yamamoto M., Nandi A., Rosenblatt K.P., Goetz R., et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams A.C., Cheng C.C., Coskun T., Kharitonenkov A. FGF21 requires betaklotho to act in vivo. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding X., Boney-Montoya J., Owen B.M., Bookout A.L., Coate K.C., Mangelsdorf D.J., et al. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metabolism. 2012;16(3):387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S., Choi J., Mohanty J., Sousa L.P., Tome F., Pardon E., et al. Structures of beta-klotho reveal a 'zip code'-like mechanism for endocrine FGF signalling. Nature. 2018;553(7689):501–505. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J., Stanislaus S., Chinookoswong N., Lau Y.Y., Hager T., Patel J., et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. American Journal of Physiology. Endocrinology and Metabolism. 2009;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 15.BonDurant L.D., Ameka M., Naber M.C., Markan K.R., Idiga S.O., Acevedo M.R., et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metabolism. 2017;25(4):935–944. doi: 10.1016/j.cmet.2017.03.005. e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G., et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metabolism. 2016;23(3):427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L., et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism. 2013;18(3):333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 20.BonDurant L.D., Ameka M., Naber M.C., Markan K.R., Idiga S.O., Acevedo M.R., et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metabolism. 2017;25(4):935–944. doi: 10.1016/j.cmet.2017.03.005. e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan T., Morgan D.A., Rahmouni K., Sonoda J., Fu X., Burgess S.C., et al. FGF19, FGF21, and an FGFR1/beta-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metabolism. 2017;26(5):709–718. doi: 10.1016/j.cmet.2017.09.005. e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen B.M., Ding X., Morgan D.A., Coate K.C., Bookout A.L., Rahmouni K., et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism. 2014;20(4):670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flippo K.H., Jensen-Cody S.O., Claflin K.E., Potthoff M.J. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-76593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douris N., Stevanovic D.M., Fisher F.M., Cisu T.I., Chee M.J., Nguyen N.L., et al. Central fibroblast growth factor 21 Browns white fat via sympathetic action in male mice. Endocrinology. 2015;156(7):2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veniant M.M., Hale C., Helmering J., Chen M.M., Stanislaus S., Busby J., et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foltz I.N., Hu S., King C., Wu X., Yang C., Wang W., et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Science Translational Medicine. 2012;4(162):162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 27.Adams A.C., Coskun T., Rovira A.R., Schneider M.A., Raches D.W., Micanovic R., et al. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale C., Chen M.M., Stanislaus S., Chinookoswong N., Hager T., Wang M., et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153(1):69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 29.Huang J., Ishino T., Chen G., Rolzin P., Osothprarop T.F., Retting K., et al. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. Journal of Pharmacology and Experimental Therapeutics. 2013;346(2):270–280. doi: 10.1124/jpet.113.204420. [DOI] [PubMed] [Google Scholar]
- 30.Kim H.W., Lee J.E., Cha J.J., Hyun Y.Y., Kim J.E., Lee M.H., et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology. 2013;154(9):3366–3376. doi: 10.1210/en.2012-2276. [DOI] [PubMed] [Google Scholar]
- 31.Wu A.L., Coulter S., Liddle C., Wong A., Eastham-Anderson J., French D.M., et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen-Cody S.O., Flippo K.H., Claflin K.E., Yavuz Y., Sapouckey S.A., Walters G.C., et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metabolism. 2020;32(2):273–286. doi: 10.1016/j.cmet.2020.06.008. e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vong L., Ye C., Yang Z., Choi B., Chua S., Jr., Lowell B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madisen L., Garner A.R., Shimaoka D., Chuong A.S., Klapoetke N.C., Li L., et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J.D., et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Z., Sierra A., Burnett C.M., Chen B., Subbotina E., Koganti S.R., et al. Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. The Journal of General Physiology. 2014;143(1):119–134. doi: 10.1085/jgp.201311063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A.W., Hoefig C.S., Abreu-Vieira G., de Jong J.M.A., Petrovic N., Mittag J., et al. Leptin raises defended body temperature without activating thermogenesis. Cell Reports. 2016;14(7):1621–1631. doi: 10.1016/j.celrep.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Cui H., Sohn J.W., Gautron L., Funahashi H., Williams K.W., Elmquist J.K., et al. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. Journal of Comparative Neurology. 2012;520(18):4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claflin K.E., Sandgren J.A., Lambertz A.M., Weidemann B.J., Littlejohn N.K., Burnett C.M., et al. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. Journal of Clinical Investigation. 2017;127(4):1414–1424. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan K.Y., Jang M.J., Yoo B.B., Greenbaum A., Ravi N., Wu W.L., et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nature Neuroscience. 2017;20(8):1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanov R.A., Zeisel A., Bakker J., Girach F., Hellysaz A., Tomer R., et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nature Neuroscience. 2017;20(2):176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D.W., Yao Z., Graybuck L.T., Kim T.K., Nguyen T.N., Smith K.A., et al. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell. 2019;179(3):713–728. doi: 10.1016/j.cell.2019.09.020. e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flippo K.H., Trammell S.A.J., Gillum M.P., Aklan I., Perez M.B., Yavuz Y., et al. FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit. Cell Metabolism. 2022;34(2):317–328. doi: 10.1016/j.cmet.2021.12.024. e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardo B., Lu M., Bandyopadhyay G., Li P., Zhou Y., Huang J., et al. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Scientific Reports. 2015;5 doi: 10.1038/srep11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halaas J.L., Boozer C., Blair-West J., Fidahusein N., Denton D.A., Friedman J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(16):8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., et al. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ameka M., Markan K.R., Morgan D.A., BonDurant L.D., Idiga S.O., Naber M.C., et al. Liver derived FGF21 maintains Core body temperature during acute cold exposure. Scientific Reports. 2019;9(1):630. doi: 10.1038/s41598-018-37198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J., Bartolome C.L., Low C.S., Yi X., Chien C.H., Wang P., et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556(7702):505–509. doi: 10.1038/s41586-018-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Wall E., Leshan R., Xu A.W., Balthasar N., Coppari R., Liu S.M., et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flippo K.H., Potthoff M.J. Metabolic messengers: FGF21. Nature Metabolismj. 2021;3(3):309–317. doi: 10.1038/s42255-021-00354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christoffersen B., Straarup E.M., Lykkegaard K., Fels J.J., Sass-Orum K., Zhang X., et al. FGF21 decreases food intake and body weight in obese Gottingen minipigs. Diabetes, Obesity and Metabolism. 2019;21(3):592–600. doi: 10.1111/dom.13560. [DOI] [PubMed] [Google Scholar]
- 52.Samms R.J., Smith D.P., Cheng C.C., Antonellis P.P., Perfield J.W., 2nd, Kharitonenkov A., et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Reports. 2015;11(7):991–999. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 53.Challa T.D., Dapito D.H., Kulenkampff E., Kiehlmann E., Moser C., Straub L., et al. A genetic model to study the contribution of Brown and brite adipocytes to metabolism. Cell Reports. 2020;30(10):3424–3433. doi: 10.1016/j.celrep.2020.02.055. e3424. [DOI] [PubMed] [Google Scholar]
- 54.Veniant M.M., Sivits G., Helmering J., Komorowski R., Lee J., Fan W., et al. Pharmacologic effects of FGF21 are independent of the "browning" of white adipose tissue. Cell Metabolism. 2015;21(5):731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Adams A.C., Halstead C.A., Hansen B.C., Irizarry A.R., Martin J.A., Myers S.R., et al. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson W.C., Zhou Y., Talukdar S., Musante C.J. PF-05231023, a long-acting FGF21 analogue, decreases body weight by reduction of food intake in non-human primates. Journal of Pharmacokinetics and Pharmacodynamics. 2016;43(4):411–425. doi: 10.1007/s10928-016-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V., et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 58.Bingham N.C., Anderson K.K., Reuter A.L., Stallings N.R., Parker K.L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149(5):2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berglund E.D., Vianna C.R., Donato J., Jr., Kim M.H., Chuang J.C., Lee C.E., et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. Journal of Clinical Investigation. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myers M.G., Jr., Heymsfield S.B., Haft C., Kahn B.B., Laughlin M., Leibel R.L., et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metabolism. 2012;15(2):150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S., et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markan K.R., Naber M.C., Small S.M., Peltekian L., Kessler R.L., Potthoff M.J. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Molecular Metabolism. 2017;6(6):602–610. doi: 10.1016/j.molmet.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markan K.R., Potthoff M.J. Metabolic fibroblast growth factors (FGFs): mediators of energy homeostasis. Seminars in Cell & Developmental Biology. 2016;53:85–93. doi: 10.1016/j.semcdb.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcelin G., Jo Y.H., Li X., Schwartz G.J., Zhang Y., Dun N.J., et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Molecular Metabolism. 2014;3(1):19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montez J.M., Soukas A., Asilmaz E., Fayzikhodjaeva G., Fantuzzi G., Friedman J.M. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2537–2542. doi: 10.1073/pnas.0409530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.BonDurant L.D., Potthoff M.J. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annual Review of Nutrition. 2018 doi: 10.1146/annurev-nutr-071816-064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M.Z., Chang J.C., Zavala-Solorio J., Kates L., Thai M., Ogasawara A., et al. FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/betaKlotho complex in non-adipocytes. Molecular Metabolism. 2017;6(11):1454–1467. doi: 10.1016/j.molmet.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilroy C.A., Capozzi M.E., Varanko A.K., Tong J., D'Alessio D.A., Campbell J.E., et al. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Science Advances. 2020;6(35) doi: 10.1126/sciadv.aaz9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muller T.D., Sullivan L.M., Habegger K., Yi C.X., Kabra D., Grant E., et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of Peptide Science. 2012;18(6):383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Potential glutamatergic targets of direct FGF21 signaling. Light sheet imaging of a brain from KLB-Cre; Vglut2-FLP; Ai65-tdTomato mice processed by DISCO clearing. Video focused on the ventromedial hypothalamus (VMH).
Data Availability Statement
Data will be made available on request.