Abstract
The Nar two-component regulatory system, consisting of the dual sensor-transmitters NarX and NarQ and the dual response regulators NarL and NarP, controls the expression of various anaerobic respiratory pathway genes and fermentation pathway genes. Although both NarX and NarQ are known to detect the two environmental signals nitrate and nitrite, little is known regarding the sensitivity and selectivity of ligand for detection or activation of the sensor-transmitters. In this study, we have developed a sensitive anion-specific in vitro assay for NarX autophosphorylation by using Escherichia coli membranes highly enriched in the full-length NarX protein. In this ATP- and magnesium-dependent reaction, nitrate elicited a greater signal output (i.e., NarX autophosphorylation) than did nitrite. Nitrate stimulation occurred at concentrations as low as 5 μM, and the half-maximal level of NarX autophosphorylation occurred at approximately 35 μM nitrate. In contrast, nitrite-dependent stimulation was detected only at 500 μM, while 3.5 mM nitrite was needed to achieve half-maximal NarX autophosphorylation. Maximal nitrate- and nitrite-stimulated levels of NarX phosphorylation were five and two times, respectively, over the basal level of NarX autophosphorylation. The presence of Triton X-100 eliminated the nitrate-stimulated kinase activity and lowered the basal level of activity, suggesting that the membrane environment plays a crucial role in nitrate detection and/or regulation of kinase activity. These results provide in vitro evidence for the differential detection of dual signaling ligands by the NarX sensor-transmitter protein, which modulates the cytoplasmic NarX autokinase activity and phosphotransfer to NarL, the cognate response regulator.
Nitrate- as well as nitrite-dependent gene expression in Escherichia coli is accomplished by the Nar two-component regulatory system, composed of the NarX, NarQ, NarL, and NarP proteins (6, 8). The Nar system has many features in common with the superfamily of bacterial sensor-transmitter response regulators (for a review, see reference 15), which as a group function to detect a variety of external or internal signals and control a variety of cellular metabolic processes, ranging from gene expression to enzyme activity and cell motility. The Nar phosphorelay system is unusual in that it has two sensor-transmitter members, NarX and NarQ, that can independently detect nitrate or nitrite signals. It also has two response regulators, NarL and NarP, that interact with DNA. The reception of either the nitrate or nitrite signal by NarX or NarQ elicits a message transfer to NarL and NarP in the form of covalent protein phosphorylation (3, 19, 22) that activates the two DNA binding proteins so they can then modulate gene expression.
NarL-phosphate and NarP-phosphate control the transcription of many genes involved in anaerobic respiration and fermentation (1, 7, 8, 20). The respiratory pathway genes include those for two cellular nitrate reductases (narGHJI and napA), two nitrite reductases (nirBDC and nrfABCDEFG), a nitrite exporter (narK), a formate dehydrogenase (fdnGHI), a dimethyl sulfoxide/trimethylamine-N-oxide reductase (dmsABC), and a fumarate reductase (frdABCD). The fermentation pathway genes include those for alcohol dehydrogenase (adhE) and pyruvate formate lyase (pfl). Finally, NarX and NarQ also possess a cophosphatase activity that stimulates the rate of NarL-phosphate and NarP-phosphate dephosphorylation to recycle these DNA binding proteins to their inactive states (3, 19, 22).
The NarX and NarQ sensor-transmitter proteins are transmembrane receptors anchored in the cytoplasmic membrane. They show considerable amino acid sequence homology (i.e., 32% identity and 68% similarity) and contain a conserved 17-amino-acid region designated the P box (2, 4, 5) that is exposed to the periplasmic space of the cell. From in vivo studies, the reception of either the nitrate or nitrite signal in the periplasmic space of the cell is proposed to activate an ATP-dependent autokinase located in the cytoplasm-exposed domain of NarX and NarQ (2, 5). Recent in vitro studies have demonstrated that either nitrate or nitrite can stimulate NarX autokinase activity relative to that when no ligand is present (24). In that study, nitrate was only marginally more effective than nitrite in stimulation of NarX phosphorylation (a half-maximal level of 1 mM for nitrate versus 5 mM for nitrite). It was also not evident how the membrane environment or other biochemical parameters affected NarX phosphorylation.
In this study, we have examined the in vitro ligand response of NarX in membranes isolated from E. coli cells overproducing the sensor-transmitter protein. The addition of either nitrate or nitrite stimulated protein autophosphorylation above a basal level, i.e., five- and twofold, respectively. However, nitrate did so at a concentration that was 2 orders of magnitude lower than that for nitrite. The requirements for the membrane-bound kinase activity were examined, as were the effects of addition of detergent, other oxyanions, and various inhibitors of NarX autophosphorylation.
MATERIALS AND METHODS
Strains and plasmids.
The host strain used for all gene manipulations was E. coli XL-1 Blue (Stratagene). To construct the narX expression vector pKK1, a 3.6-kb DraI-HindIII fragment containing the narXL gene region from plasmid pIS19 (18) was excised and inserted into the SmaI- and HindIII-digested plasmid vector pKK223-3 (Pharmacia Biotech). PCR with the two oligonucleotides 5′GGCGGGAAGCTTTTACTTAGTTAAGATCTAACAGGATCAGA3′ and 5′GTAACGAACTGAATGCATCCTGGG3′ was used to introduce stop codons in each reading frame following narX and to introduce a HindIII site just prior to the first BglII site within narL (12). The resulting 650-bp DNA fragment was then inserted into plasmid pKK1 that had been digested with NsiI and HindIII to generate plasmid pKK2. This manipulation deleted the narL gene sequences following the BglII site and terminated NarL translation at codon 25. Oligonucleotides 5′TGTGAATTCCATATGCTTAAACGTTGTCTCTCTCCGCT3′ and 5′CGTTCTGCTGCTCGAGTCAA3′ were then used to generate a second PCR fragment that contained (i) an ATG start codon for narX within an NdeI site and (ii) an EcoRI site immediately upstream of the NdeI site. The resulting 260-bp fragment was inserted into plasmid pKK-2 between the EcoRI and XhoI sites to give plasmid pKK-4. The narX and associated narL sequences were then excised from pKK-4 by using EcoRI and HindIII and introduced between the corresponding sites in the vector pGem-7zf (Promega) to create plasmid pGem7-4. The intended DNA sequences of all new segments of pGem7-4 were confirmed by dideoxy sequencing (17). The narX-narL gene region was excised from pGem7-4 and inserted into the pET3a expression vector (Stratagene) between the NdeI and BamHI sites to give the narX overexpression plasmid pET3a-4.
Cell growth.
For NarX protein production, E. coli BL21(λDE3)/pLysE (Stratagene) was freshly transformed with plasmid pET3a-4, plated on L-agar plates containing 50 μg of ampicillin per ml, and incubated overnight at 37°C. Cells from one plate were resuspended in Luria-Bertani broth (13) and used to inoculate 100 ml of Luria-Bertani medium at an initial optical density at 600 nm of 0.4. IPTG (isopropyl-β-d-thiogalactopyranoside) and ampicillin were added to final concentrations of 0.5 mM and 100 μg/ml, respectively. The cultures were grown aerobically at 37°C for 3.5 h. Cells were then harvested by centrifugation for 10 min at 3,000 × g.
Preparation of E. coli membranes.
Cells were resuspended in breakage buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 5 mM 1,10-phenanthroline, 2 mM phenylmethylsulfonyl fluoride, 10% [vol/vol] glycerol). The cells were then sonicated for 20-s intervals for a total of 1 min. Following centrifugation at 20,000 × g for 10 min to remove cellular debris, membranes were isolated from the supernatant fraction by centrifugation at 100,000 × g for 60 min. The membrane pellet was resuspended in membrane storage buffer (cell breakage buffer plus 1 mM 1,2-dithiothreitol [DTT]), aliquoted, and stored at −80°C until needed. The cell breakage and storage buffers were prepared fresh and were chilled to 4°C prior to use. Since after two to three freeze-thaw cycles the membrane fractions exhibited reduced NarX kinase activity, the refreezing and rethawing of membrane samples were avoided.
Phosphorylation of NarX.
E. coli membrane preparations were diluted into phosphorylation buffer (HEPES [pH 8.0], 50 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, and 2 mM DTT) at 4°C to a final protein concentration of 0.1 to 0.4 μg/μl and then divided into the individual phosphorylation reaction mixtures. ATP-dependent phosphorylation of the NarX protein was then performed essentially as previously described (19). The phosphorylation reaction was initiated by addition of a 0.1 volume of a 10× reaction cocktail that contained 2.5 μM [γ-32P]ATP (3,000 Ci/mmol) (Andotek) and 247.5 μM ATP (Fisher Scientific). Reactions were stopped by addition of a gel loading buffer that contained 10 mM Tris-HCl (pH 8.0), 10% (wt/vol) sodium dodecyl sulfate (SDS), 20% (vol/vol) β-mercaptoethanol, 50% (vol/vol) glycerol, and 0.02% (wt/vol) bromophenol blue. Where indicated, valinomycin, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and 2,4-dinitrophenol (DNP) were added to the phosphorylation mix just before addition of the ATP. For the N-ethylmaleimide (NEM) studies, membranes were prepared as described above but resuspended in 100 mM KPO4 (pH 7.4)–10 mM MgSO4 in place of the usual storage buffer. Membranes were treated with NEM as described by Olami et al. (14). The NEM-treated membranes were pelleted and resuspended in phosphorylation assay buffer, and the reactions were performed as described above.
Phosphorylation of NarL by NarX in membranes.
For the studies of phosphorylation of NarL by NarX, the response regulator NarL was purified as described before (19). NarX was first autophosphorylated at room temperature as follows. Five microliters of 100 mM KNO3 was added to 5 μl of NarX membranes (5 μg of total protein per μl) and diluted into a phosphorylation mix containing 50 mM MOPS (morpholinepropanesulfonic acid) (pH 7.0), 50 mM KCl, 10 mM MgCl2, and 0.5 mM EDTA in a total volume of 50 μl. Phosphorylation was initiated by addition of [γ-32P]ATP (3,000 Ci/mmol) (NEN) and ATP (Fisher Scientific) to final concentrations of 0.25 and 24.75 μM, respectively, and the reaction mixture was incubated for 30 s. The phosphotransfer reaction was initiated by the addition of 1 μl of NarL (at a final concentration 0.5 μM) to the NarX autophosphorylation mix. The reaction mixture was incubated at room temperature for 1 min after the addition of NarL, and 10-μl samples were taken. Reactions were stopped by the addition of 4 μl of gel loading buffer. Phosphorylated proteins were separated by gel electrophoresis and visualized by using a PhosphorImager screen (Molecular Dynamics).
PAGE and Western blotting.
For Western blotting, proteins were separated by SDS–12.5% polyacrylamide gel electrophoresis (SDS–12.5% PAGE) by using the Phastsystem (Pharmacia Biotech). Protein transfer to nitrocellulose membranes (Micron Separations Inc.) was also performed with the Phastsystem. Western blot analysis was performed by the method of Towbin et al. (21) with a polyclonal antibody made against a NarX-TrpE fusion protein diluted 750-fold. For the NarX phosphorylation experiments, SDS-PAGE was carried out with 12% Ready Gels (Bio-Rad). Following electrophoresis, the gels were dried and placed on a PhosphorImager screen. Radioactivity was quantitated with the ImageQuant software package (Molecular Dynamics), where radioactivity is expressed as arbitrary units.
RESULTS
Expression and phosphorylation of NarX in membrane fractions.
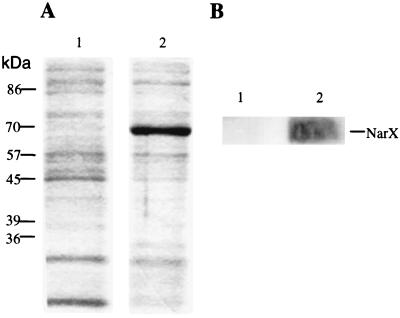
To prepare E. coli membranes enriched in the NarX sensor-transmitter protein, an inducible narX plasmid expression system was devised (see Materials and Methods). Following addition of IPTG to induce narX gene expression and subsequent preparation of the membrane fraction, a significant amount of a 70-kDa protein was accumulated in the membrane fraction (Fig. 1A, lane 2). This protein was not seen in membranes prepared from cells lacking the narX overexpression plasmid (Fig. 1A, lane 1). Western blotting with antibodies to NarX confirmed that the predominant 70-kDa protein was NarX (Fig. 1B, lane 2). No NarX-cross-reacting material was detected in control membranes (Fig. 1B, lane 1). Finally, no NarX protein was detected in the soluble cytoplasmic fraction of the IPTG-induced cells (data not shown).
FIG. 1.
Overexpression of NarX. (A) SDS-PAGE of membrane fractions prepared from strains lacking the narX overexpression plasmid pET3a-4 (lane 1) or containing pET3a-4 induced with IPTG (lane 2). Equal amounts of total membrane proteins were loaded in lanes 1 and 2. The host strain was BL21(λDE3)/pLysE that was induced with IPTG as indicated in Materials and Methods. (B) Western blot analysis of the corresponding membrane fractions shown in panel A.
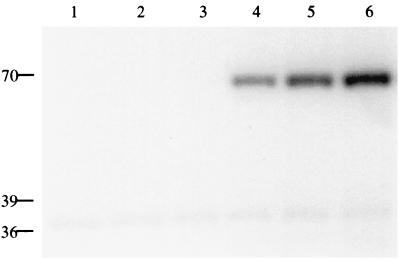
To determine whether the membrane-bound NarX protein was competent for autophosphorylation, we employed a modified protocol of Schroeder et al. (19), which was previously used to examine the ATP-dependent covalent phosphorylation of N-terminally truncated forms of NarX and NarQ proteins (see Materials and Methods). When the NarX-containing membrane fraction was tested with [γ-32P]ATP as the phosphodonor, a 70-kDa 32P-labeled protein was observed (Fig. 2, lane 4). However, when membranes derived from the strain lacking the narX overexpression plasmid were tested, no 32P-labeled NarX protein was seen (Fig. 2, lanes 1 to 3). These findings are consistent with prior reports that NarX autophosphorylates even in the absence of ligand (19, 22, 24), although it cannot be ruled out that this basal level of activity is the result of NarX overexpression. The membranes from both the control and overexpression strains all contained a small amount of a 37-kDa 32P-labeled protein (Fig. 2). This material did not cross-react with NarX antibodies (data not shown). A similar 37-kDa 32P-labeled protein was also observed by Walker and DeMoss (22) and appears to be independent of the strain or plasmid employed.
FIG. 2.
NarX-phosphate accumulation is stimulated by either nitrate or nitrite. Autophosphorylation of membrane proteins from cells lacking the narX expression plasmid (lanes 1 to 3) or harboring the narX expression plasmid and IPTG induced (lanes 4 to 6) is shown. Additions to the phosphorylation assay were as follows: lanes 1 and 4, 10 mM KCl; lanes 2 and 5, 10 mM KNO2; lanes 3 and 6, 10 mM KNO3. Reactions were performed at 20°C for 30 s prior to the addition of gel loading buffer. Numbers on the left are molecular masses in kilodaltons.
Stimulation of NarX autokinase activity by addition of nitrite and nitrate.
To establish whether the membrane-associated NarX autophosphorylation activity was responsive to either of the environmental signals, nitrite or nitrate, each anion was added to the reaction mixture at a final concentration of 10 mM. The intensity of the 70-kDa 32P-labeled protein was increased in each case (Fig. 2, compare lane 4 to lanes 5 and 6). These findings indicate that the NarX-containing membrane preparations are responsive to each anion, in accordance with prior in vivo (2, 5) and in vitro (24) studies. The observed nitrate effect cannot be accounted for by the presence of contaminating nitrite, since nitrite was present at less than 0.001% (wt/wt). Addition of 25 mM EDTA, a chelating agent of divalent cations, completely inhibited NarX autophosphorylation (data not shown), consistent with the requirement of Mg2+ for NarX autokinase (19).
Effect of membrane concentration on NarX phosphorylation.
NarX autophosphorylation was also examined as a function of total membrane protein concentrations. The amount of radiolabeled NarX increased proportionally to the amount of membrane protein used in the range from 0.028 to 0.61 μg/μl (data not shown). This indicated that at the membrane concentrations typically used in the assay (0.1 to 0.4 μg of total membrane protein per μl), NarX protein was not in excess.
Time course of NarX autophosphorylation.
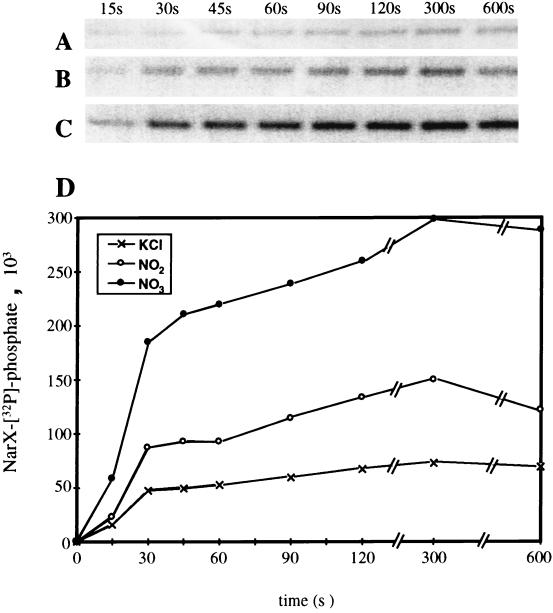
When the phosphorylation reaction was performed at 20°C, the phosphorylation of NarX was rapid and reached the maximal level within 30 s. Over the following 2 to 5 min, a significant fraction of the NarX-phosphate was dephosphorylated (data not shown). When the assay temperature was lowered to 4°C, NarX phosphorylation proceeded at a noticeably lower rate for the first 1 to 2 min and then remained relatively constant over the following 10 min (Fig. 3A). When nitrate (10 mM) was present, NarX autophosphorylation proceeded at a higher initial rate relative to that with no nitrate addition (compare Fig. 3A to C) and achieved a fivefold-higher final level of [32P]phosphate incorporation (Fig. 3D). When nitrite (10 mM) was used in place of nitrate, the rate of NarX phosphorylation was also higher than that when no nitrite was added (compare Fig. 3A to B), but it was lower than that when nitrate was present (compare Fig. 3B to C). The final level of [32P]phosphate incorporated by 5 min in the presence of nitrite was only 2.2-fold higher than that when no anion was present (Fig. 3D). Thus, the addition of either nitrate or nitrite to a final concentration of 10 mM significantly enhanced the rate of NarX phosphorylation. The presence of ligand clearly stimulates NarX kinase activity so that subsequent transfer of the phosphate group to NarL or NarP can occur (5, 19).
FIG. 3.
Time course of NarX autophosphorylation in the presence or absence of added nitrate or nitrite. (A to C) To initiate each set of NarX autophosphorylation reactions, 7 μl of a 10× ATP cocktail was added to 63 μl of diluted membranes which contained 10 mM (final concentration) KCl (A), 10 mM KNO2 (B), or 1 mM KNO3 (C). At the indicated time points, 9.5-μl aliquots were removed and mixed with 4 μl of gel loading buffer. The amount of protein applied per lane was 0.40 μg. All phosphorylation reactions were performed at 4°C. (D) Plot of NarX protein phosphorylation with time. Units of NarX-[32P]phosphate are arbitrary units.
Effect of anion ligand concentration on NarX autophosphorylation.
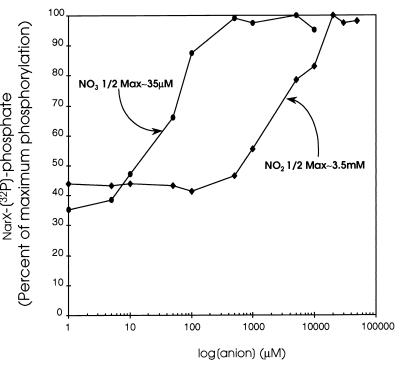
Since both nitrate and nitrite elicited different NarX phosphorylation kinetics relative to those when no ligand was added (Fig. 3D), we next examined how variations in each oxyanion concentration affect NarX autophosphorylation. To test this, the amount of NarX-phosphate produced in 30 s at 20°C was measured with different levels of nitrate or nitrite, ranging from 1 to 50,000 μM (Fig. 4). The threshold for stimulation of NarX-phosphate formation was observed at 5 μM nitrate. Half-maximal amounts of NarX-phosphate were observed at approximately 35 μM relative to the amount seen when saturating amounts of nitrate ligand were present. In contrast, the threshold of the nitrite response was at approximately 500 μM. Half-maximal phosphorylation was not seen until about 3.5 mM nitrite. This represents a 100-fold-less-sensitive half-maximal response for nitrite stimulation than for nitrate stimulation. Correspondingly, optimal NarX-phosphate formation was seen when nitrate was present at 500 μM, whereas optimal NarX-phosphate formation in the presence of nitrite occurred only at concentrations above 20 mM nitrite. Ligand-dependent NarX phosphorylation clearly is more responsive to nitrate than to nitrite as a signal (i.e., in both the threshold and sensitivity of the signal response).
FIG. 4.
NarX phosphorylation as a function of nitrate and nitrite concentrations. The reaction mixture contained 1 μl of 10× KNO2 or KNO3 stock solution that was added to 8 μl of diluted membranes. One microliter of 10× ATP cocktail was then added to initiate the phosphorylation reaction. Phosphorylation was allowed to proceed for 30 s at room temperature before addition of 4 μl of gel loading buffer. [32P]phosphate incorporation into NarX in the presence of nitrite (⧫) or nitrate (●) is expressed as the percentage of maximum phosphorylation. 1/2 Max, half-maximal.
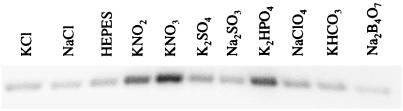
Effect of other oxyanions on NarX phosphorylation.
Anions structurally related to nitrate and nitrite were also tested for their ability to stimulate ATP-dependent NarX autophosphorylation (Fig. 5). When tested at final concentrations of up to 10 mM, sulfate, sulfite, carbonate, chlorate, and borate neither enhanced nor inhibited the amount of 32P-NarX formed. Interestingly, addition of 10 mM phosphate stimulated the reaction above background (approximately 30%). Because changes in pH can affect CheA autophosphorylation (11), we also tested the addition of phosphate solutions adjusted to pH 5.8, 7.6, and 10. NarX autokinase activity was above background at all pH levels and therefore was not due to changes in the pH of the phosphorylation reaction mixture upon addition of phosphate (data not shown).
FIG. 5.
NarX phosphorylation in the presence of various anions. Phosphorylation procedures were by the protocol described in the legend to Fig. 4. Where indicated, a 10× stock of KCl, NaCl, K2SO4, Na2SO3, K2HPO4, NaClO4, KHCO3, or Na2B4O7 was used in place of 10× KNO2 or KNO3. Final concentrations were 10 mM for each anion and 50 mM for HEPES buffer.
The effect of phosphate on NarX signaling was also examined in vivo. When 10 mM phosphate was added to an anaerobically growing culture of cells carrying a NarX-dependent narG-lacZ reporter fusion, no induction of gene expression was seen, whereas addition of an equimolar amount of nitrate caused a 35-fold elevation in gene expression (data not shown). Thus, NarX is clearly able to discriminate between the two related anions in vivo.
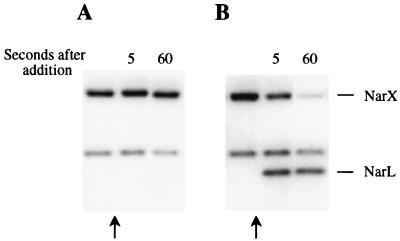
Phosphorylation of NarL by NarX in membranes.
We studied the transfer of phosphate from NarX to NarL by using the above-described NarX membrane preparations. NarX was first phosphorylated in the presence of 10 mM nitrate for 30 s. NarL was then added to the phosphorylated membrane-bound sensor and incubated for an additional minute. The results are summarized in Fig. 6. A control reaction in which no NarL was added was carried out to verify that NarX-phosphate was stable during the reaction period (Fig. 6A). The addition of NarL to the NarX-phosphate preparation resulted in a rapid dephosphorylation of NarX and transfer of phosphate to NarL (Fig. 6B). Transfer of phosphate continued during the next minute until almost no NarX-phosphate remained. Phosphotransfer from NarX-phosphate to NarL was also detected in the absence of added nitrate, although at a considerably reduced level (data not shown). Thus, in the presence of nitrate, a larger amount of NarL-phosphate was detected due to higher levels of nitrate-stimulated NarX-phosphate formation.
FIG. 6.
Phosphorylation of NarL by membrane-associated NarX-phosphate. Membrane-bound NarX was first phosphorylated as described in Materials and Methods. To monitor NarX autophosphorylation prior to the addition of NarL, 10-μl samples were taken at 30 s after the addition of ATP to the reaction mixture and transferred to tubes containing 4 μl of gel loading buffer. At 35 s after the addition of ATP (arrows), 1 μl of NarL phosphorylation buffer (A) or 1 μl of NarL protein (B) was added to the reaction mixture.
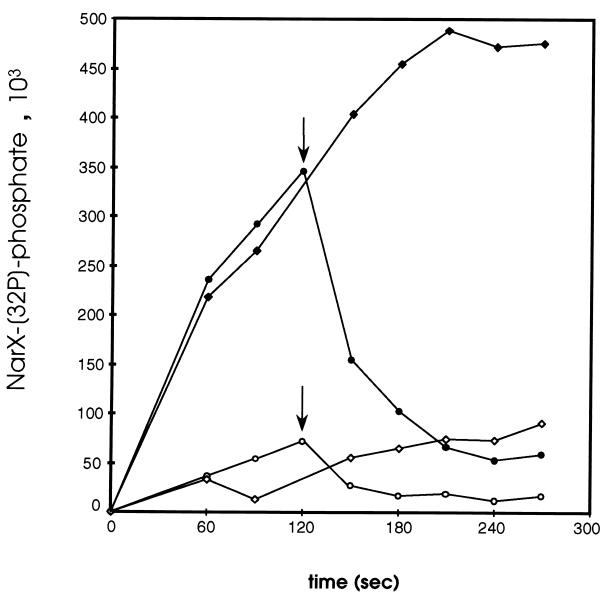
Stability of NarX-phosphate.
In order to examine the stability of NarX-phosphate in the presence of nitrate, NarX was phosphorylated for 120 s at 4°C with radiolabeled ATP, after which time excess unlabeled ATP was added (Fig. 7). The loss of NarX-phosphate was extremely rapid, with a half-life of approximately 20 s in the presence of 10 mM KNO3 or about 24 s when nitrate was absent. Thus, NO3 does not appear to significantly regulate the stability of phosphorylated NarX.
FIG. 7.
Pulse-chase of phosphorylated NarX. NarX, in the presence or absence of 10 mM KNO3, was phosphorylated with radiolabeled ATP for 120 s, at which time (arrows) HEPES buffer (to a final concentration of 50 mM) or nonradiolabeled ATP (to a final concentration of 1.03 mM) was added. Aliquots were collected at the indicated time points and mixed with gel loading buffer to stop the reaction. The amount of NarX-phosphate at 120 s (when cold ATP or 50 mM HEPES was added) was estimated by extrapolating from the previous data point. All reactions were carried out at 4°C. Symbols: ⧫, with KNO3, chased with HEPES; ●, with KNO3, chased with cold ATP; ◊, without KNO3, chased with HEPES; ○, without KNO3, chased with cold ATP.
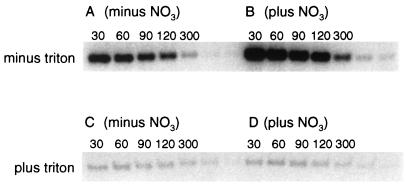
Effect of detergent addition on NarX autophosphorylation.
To determine if an intact membrane environment is required for NarX autophosphorylation, NarX-containing membranes were pretreated with 2% Triton X-100 for 60 min at 4°C prior to assay for nitrate-dependent NarX autophosphorylation at 20°C. Relative to that for the unsolubilized membrane controls, NarX autophosphorylation occurred at a much reduced level (ca. 40-fold) in the presence of Triton (data not shown and Fig. 8A and C). Strikingly, autophosphorylation of NarX in the detergent-solubilized preparation was also no longer stimulated by nitrate (compare Fig. 8A and B to C and D). Since the same amount of NarX protein was present in each lane, as determined by Coomassie blue staining, the difference could not be attributed to an unequal loading of NarX protein (data not shown).
FIG. 8.
Effect of Triton X-100 on NarX autophosphorylation. The indicated NarX-containing membranes were solubilized in 2% Triton X-100, kept on ice for 1 h, and then centrifuged. The supernatants, containing NarX detergent micelles, were then used in a phosphorylation reaction. Non-detergent-solubilized membranes were used directly in the phosphorylation assay. Phosphorylation reactions were performed as described in the legend to Fig. 3. (A and B) Phosphorylation time course in the absence of Triton X-100 with the addition of either 50 mM HEPES (A) or 10 mM KNO3 (B). (C and D) Phosphorylation time course of NarX in Triton X-100 micelles with the addition of either 50 mM HEPES (C) or 10 mM KNO3 (D). All times are in seconds.
Effects of DTT, NEM, and DTNB on NarX kinase activity.
During these experiments, we observed that membranes stored at −80°C eventually lost NarX kinase activity (after about 1 month). Addition of DTT to the inactive membrane fractions restored kinase activity (data not shown). We hypothesize that one or more cysteine residues are essential for NarX autokinase activity. To test this, we assayed NarX kinase activity in the presence of the cysteine-modifying agents NEM and DTNB (9, 16). If cysteine residues somehow play a role in NarX kinase activity, then modification of these residues would result in loss of NarX kinase. Indeed, increasing concentrations of NEM or DTNB inhibited NarX autophosphorylation. At 100 μM NEM, there was almost no NarX-phosphate formed (Table 1). At 500 μM DTNB, phosphorylation was inhibited by 49% compared to when no DTNB was added, while 5 mM DTNB inhibited phosphorylation completely (Table 1).
TABLE 1.
Effects of NEM, DTNB, DNP, and valinomycin on NarX autophosphorylationa
| Addition | Concn | % Inhibition of NarX phosphorylation |
|---|---|---|
| None | 0 | |
| NEM | 10 μM | 40 |
| 50 μM | 81 | |
| 100 μM | 98 | |
| DTNB | 500 μM | 49 |
| 5 mM | 100 | |
| Valinomycin | 100 μM | 2 |
| DNP | 1 mM | 28 |
| 3.33 mM | 32 |
All phosphorylation reactions were carried out (as described in Materials and Methods) at 20°C for 30 s in the absence of nitrate or nitrite.
Effects of ionophores on NarX kinase.
We next assayed whether a membrane potential was necessary for NarX phosphorylation by adding either valinomycin, a K+ ionophore, or DNP, a protonophore. Addition of valinomycin to concentrations of as high as 100 μM had no effect on NarX kinase activity (Table 1), while 1.0 to 3.33 mM DNP decreased NarX kinase activity by approximately 30% (Table 1). A proton motive force is not essential for NarX kinase activity.
DISCUSSION
Development of a ligand-responsive phosphorylation assay for NarX.
The NarX sensor-transmitter protein of the Nar two-component regulatory system, comprised of the NarX, NarQ, NarL, and NarP proteins, has allowed us to further examine the biochemical properties of ligand-regulated NarX signaling. While the two oxyanion ligands, nitrate and nitrite, were each able to enhance the rate of in vitro NarX autophosphorylation, nitrate was the more potent oxyanion signal (Fig. 4). The threshold for nitrate detection by NarX was about 2 orders of magnitude lower than that for nitrite. The nitrate-responsive NarX kinase activity occurred with concentrations as low as 5 μM nitrate, compared to 500 μM for nitrite. Half-maximal NarX phosphorylation was achieved at around 35 μM nitrate versus 3.5 mM nitrite. Nitrate also elicited a higher maximal level of NarX-phosphate formation than did nitrite; the ratio of maximum nitrate-induced to nitrite-induced to basal levels of NarX phosphorylation was 5:2:1 (compare maximum NarX-phosphate levels to basal levels with the addition of nitrate and nitrite [Fig. 3D]). Finally, the level of nitrate needed to achieve maximal NarX phosphorylation was about 500 μM, while at least 20 mM nitrite was required to give a maximal level of NarX phosphorylation (Fig. 4). Nitrate is approximately 100 times more potent as a signal than is nitrite.
The above-described findings are in contrast to those from the in vitro study by Williams and Stewart (24), who also used a membrane-bound NarX fraction to detect NarX-phosphate accumulation. However, the prior work did not reveal a significantly different ability of NarX to sense nitrate versus nitrite (half-maximal nitrate- and nitrite-stimulated phosphorylations occurred at 1 and 5 mM, respectively [24]). It is possible that variations in membrane preparations and phosphorylation conditions may account for differences between the two studies. The experimental parameters of the study by Williams and Stewart were not described in sufficient detail to allow a direct comparison with this work, although it is noted that a mol strain was used by Williams and Stewart. However, the ratios of maximum nitrate-stimulated to nitrite-stimulated to basal levels of phosphorylation that we observed (i.e., 5:2:1 [see above and Fig. 3]) are similar to the ratios of 6:3:1 reported by Williams and Stewart (24).
Discrimination between nitrate and nitrite by NarX.
Recent in vivo findings by Wang et al. (23) have addressed the ability of the Nar regulatory system to discriminate between nitrate and nitrite. The in vivo data indicate that the Nar two-component regulatory system is much more responsive to nitrate (i.e., in the micromolar range) than to nitrite (i.e., in the millimolar range), in agreement with the results of this in vitro study. With a narG-lacZ reporter fusion in steady-state chemostat cultures, half-maximal narG-lacZ expression occurred with 3.0 μM nitrate present in the culture medium. In contrast, the half-maximal stimulation of narG-lacZ expression by nitrite occurred at 3.0 mM nitrite. Thus, two different experimental approaches indicate that nitrate stimulation of NarX activity occurs in the micromolar range, while nitrite stimulation occurs at concentrations several orders of magnitude higher. The physiological relevance of nitrite as an environmental signal in the Nar two-component system must now be reevaluated, since prior studies have not considered the regulatory implications of the differential ligand response by the NarX autokinase (discussed in reference 23).
The regulatory and catalytic domains of NarX.
Phosphorylation of the full-length membrane-bound NarX protein differs dramatically from phosphorylation of a soluble truncated version of NarX that contains only the cytoplasmic domain (19). The truncated NarX protein exhibits in vitro constitutive kinase activity regardless of whether ligand is present. This difference suggests that the periplasmic portion of NarX modulates the kinase activity contained in the cytoplasmic domain. This conclusion is supported by prior in vivo studies where expression of lacZ fusions with narG and frdA, two genes regulated by NarX, was shown to be nitrate independent when under the control of the truncated NarX lacking the periplasmic and putative transmembrane domains (2). Thus, NarX may be viewed as consisting of a regulatory domain (i.e., the periplasmic and transmembrane regions of NarX) involved in signal recognition and a catalytic domain (i.e., the cytoplasmic region of NarX) containing the ATP-dependent autokinase activity. Removal of the regulatory domain results in a constitutive or “locked-on” kinase (19). Additional support for this model is provided by recent genetic studies that revealed single amino acid changes in the periplasmic P-box region which caused loss-of-function (i.e., “locked-off”) control of NarX-dependent gene expression or gain-of-function (i.e., locked-on or ligand-independent regulation) for the NarX-controlled genes (2, 5). Similar observations have also been made for NarQ (5).
Phosphotransfer from NarX to NarL.
We also formally demonstrate that phosphate can be transferred from membrane-bound NarX-phosphate to NarL, its cognate response regulator. This phosphotransfer occurs either in the presence or in the absence of nitrate (data not shown), thus indicating that nitrate is not required for phosphorylation of NarL by NarX. However, since autophosphorylation of NarX in membranes is increased by the addition of nitrate, higher levels of NarL-phosphate result compared to when nitrate is absent.
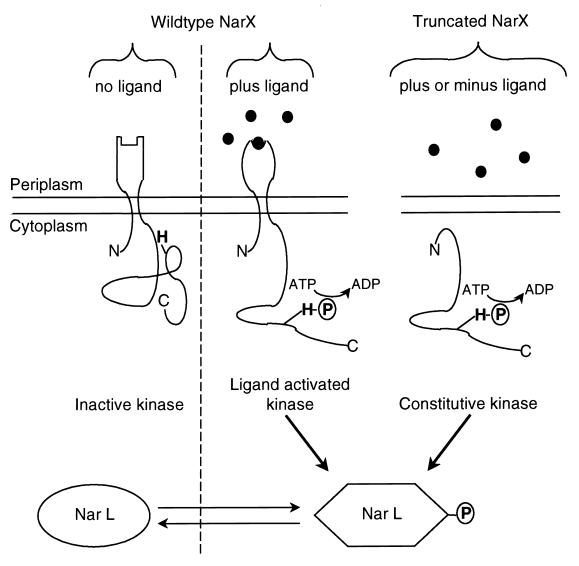
Working model.
The current working model for the operation of the NarX sensor-transmitter is shown in Fig. 9. The detection of either oxyanion signal by NarX occurs at the P-box region which is exposed to the periplasmic space (2, 5). Reception of the nitrate or nitrite signal somehow elicits an allosteric change in NarX to activate the NarX cytoplasmic autokinase (references 5, 12, and 24 and this work). The signal recognition process requires both the NarX periplasmic domain and an appropriate lipid environment (Fig. 8). Triton-treated NarX membranes exhibited diminished autophosphorylation and, most significantly, lost the ability to respond to the nitrate ligand. These results also explain why earlier experiments on NarX autophosphorylation by Triton-extracted preparations resulted in no nitrate sensitivity (22). Upon ligand recognition (Fig. 9), NarX-phosphate is formed in elevated amounts such that the protein can better activate either of the response regulator proteins, NarL and NarP, to give NarL-phosphate and NarP-phosphate (19). These phosphoproteins then bind at their DNA sites to activate or repress gene expression. Since the NarX kinase activity is not abolished in the absence of either ligand, NarX-phosphate can still form and thus transfer phosphate to NarL and NarP to provide a low basal level of activated response regulator. This is consistent with prior in vivo observations (5). However, the cophosphatase activity of NarX apparently prevents accumulation of elevated amounts of either NarL-phosphate or NarP-phosphate (3, 19, 22). We cannot fully specify how the two- to fivefold change in NarX-phosphate levels observed in this study affects the final level of transcriptional activation and repression, since the in vivo gene expression assays reflect multiple steps of the signal transduction process that includes the effect of nitrate on NarX phosphorylation, the subsequent phosphotransfer to NarL, and the binding of NarL-phosphate to DNA which then either activates or represses transcription. However, the availability of an in vitro ligand-responsive assay should allow us to further dissect the steps of the Nar signaling system. Furthermore, it will be interesting to compare mechanisms of nitrate regulation in E. coli to nitrate regulation in other organisms where NarX homologs have been identified, e.g., Pseudomonas stutzeri (10), Yersinia pestis (GenBank accession no. CAA21343), and Pseudomonas aeruginosa (GenBank accession no. CAA75537).
FIG. 9.
Model for nitrate- and nitrite-dependent activation of NarX kinase. When neither anion signal is present, the majority of NarX is in the unphosphorylated state. The presence of either nitrate or nitrite induces a conformational change in NarX such that the autokinase activity is stimulated. Phosphate is subsequently transferred to NarL. Phosphorylated NarL in turn regulates expression from the various promoters under its control. A truncated (i.e., cytoplasmic) form of NarX, which lacks the regulatory ligand recognition domain, exhibits constitutive autokinase activity (2, 19). Phosphotransfer to NarL occurs in a ligand-independent fashion.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant AI21678 from the National Institutes of Health and by fellowships to A. Delgado from the Spanish Ministerio de Educación y Ciencia and the International Human Frontier Science Program.
We thank Mike Jarvis for providing the NarL protein used in this study.
REFERENCES
- 1.Bock A, Sawers G. Fermentation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Riley W S, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 262–282. [Google Scholar]
- 2.Cavicchioli R, Chiang R C, Kalman L V, Gunsalus R P. Role of the periplasmic domain of the Escherichia coli NarX sensor-transmitter protein in nitrate-dependent signal transduction and gene regulation. Mol Microbiol. 1996;21:901–911. doi: 10.1046/j.1365-2958.1996.491422.x. [DOI] [PubMed] [Google Scholar]
- 3.Cavicchioli R, Schroder I, Constanti M, Gunsalus R P. The NarX and NarQ sensor-transmitter proteins of Escherichia coli each require two conserved histidines for nitrate-dependent signal transduction to NarL. J Bacteriol. 1995;177:2416–2424. doi: 10.1128/jb.177.9.2416-2424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang R C, Cavicchioli R, Gunsalus R P. Identification and characterization of narQ, a second nitrate sensor for nitrate-dependent gene regulation in Escherichia coli. Mol Microbiol. 1992;6:1913–1923. doi: 10.1111/j.1365-2958.1992.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiang R C, Cavicchioli R, Gunsalus R P. ’Locked-on’ and ’locked-off’ signal transduction mutations in the periplasmic domain of the Escherichia coli NarQ and NarX sensors affect nitrate- and nitrite-dependent regulation by NarL and NarP. Mol Microbiol. 1997;24:1049–1060. doi: 10.1046/j.1365-2958.1997.4131779.x. [DOI] [PubMed] [Google Scholar]
- 6.Darwin A J, Stewart V. The Nar modulon systems: nitrate and nitrite regulation of anaerobic gene expression. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in E. coli. R. G. Austin, Tex: Landes Company; 1996. pp. 333–359. [Google Scholar]
- 7.Gennis R, Stewart V. Respiration. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Riley W S, Schaechter M, Umbarger M E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 217–261. [Google Scholar]
- 8.Gunsalus R P. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol. 1992;174:7069–7074. doi: 10.1128/jb.174.22.7069-7074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habeeb A F S A. Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol. 1972;25:457–464. doi: 10.1016/S0076-6879(72)25041-8. [DOI] [PubMed] [Google Scholar]
- 10.Hartig E, Zumft W G. Kinetics of nirS expression (cytochrome cd1 nitrite reductase) in Pseudomonas stutzeri during the transition from aerobic respiration to denitrification: evidence for a denitrification-specific nitrate- and nitrite-responsive regulatory system. J Bacteriol. 1999;181:161–166. doi: 10.1128/jb.181.1.161-166.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess J F, Bourret R B, Simon M I. Phosphorylation assays for proteins of the two-component regulatory system controlling chemotaxis in Escherichia coli. Methods Enzymol. 1991;200:188–204. doi: 10.1016/0076-6879(91)00139-n. [DOI] [PubMed] [Google Scholar]
- 12.Kalman L V, Gunsalus R P. Nitrate- and molybdenum-independent signal transduction mutations in narX that alter regulation of anaerobic respiratory genes in Escherichia coli. J Bacteriol. 1990;172:7049–7056. doi: 10.1128/jb.172.12.7049-7056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 14.Olami Y, Rimon A, Gerchman Y, Rothman A, Padan E. Histidine 225, a residue of the NhaA-Na+/H+ antiporter of Escherichia coli, is exposed and faces the cell exterior. J Biol Chem. 1997;272:1761–1768. doi: 10.1074/jbc.272.3.1761. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 16.Riordan J F, Vallee B L. Reactions with N-ethylmaleimide and p-mercuribenzoate. Methods Enzymol. 1972;25:449–455. doi: 10.1016/S0076-6879(72)25040-6. [DOI] [PubMed] [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder I, Darie S, Gunsalus R P. Activation of the Escherichia coli nitrate reductase (narGHJI) operon by NarL and Fnr requires integration host factor. J Biol Chem. 1993;268:771–774. [PubMed] [Google Scholar]
- 19.Schroeder I, Wolin C D, Cavicchioli R, Gunsalus R P. Phosphorylation and dephosphorylation of the NarQ, NarX, and NarL proteins of the nitrate-dependent two-component regulatory system of Escherichia coli. J Bacteriol. 1994;176:4985–4992. doi: 10.1128/jb.176.16.4985-4992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart V. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol Microbiol. 1993;9:425–434. doi: 10.1111/j.1365-2958.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 21.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide genes to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker M S, DeMoss J A. Phosphorylation and dephosphorylation catalyzed in vitro by purified components of the nitrate sensing system, NarX and NarL. J Biol Chem. 1993;268:8391–8393. [PubMed] [Google Scholar]
- 23.Wang H, Tseng C-P, Gunsalus R P. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J Bacteriol. 1999;181:5303–5308. doi: 10.1128/jb.181.17.5303-5308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams S B, Stewart V. Discrimination between structurally related ligands nitrate and nitrite controls autokinase activity of the NarX transmembrane signal transducer of Escherichia coli K-12. Mol Microbiol. 1997;26:911–925. doi: 10.1046/j.1365-2958.1997.6262002.x. [DOI] [PubMed] [Google Scholar]