This cohort study examines the risk and incidence of alcohol-related cancers and all cancers among adults in Korea who self-reported their drinking behavior.
Key Points
Question
How does the risk of developing cancer change after alcohol consumption is increased, stopped, or reduced?
Findings
In this cohort study of 4 513 746 insured adults in Korea, those who increased their alcohol consumption had a higher risk for alcohol-related cancers and all cancers compared with those who had sustained levels of drinking, whereas those who reduced their alcohol consumption had a lower risk. Although an increased risk was observed temporarily after quitting drinking, no increased risk was observed when quitting was sustained.
Meaning
Findings of this study suggest that drinking cessation and reduction should be reinforced for the prevention of cancer.
Abstract
Importance
Although numerous studies have shown an association between alcohol consumption and cancer, how changes in drinking behavior increase or decrease the incidence of cancer is not well understood.
Objective
To investigate the association between the reduction, cessation, or increase of alcohol consumption and the development of alcohol-related cancers and all cancers.
Design, Setting, and Participants
This population-based cohort study analyzed adult beneficiaries in the Korean National Health Insurance Service. Participants (aged ≥40 years) included those who underwent a national health screening in both 2009 and 2011 and had available data on their drinking status. Data were analyzed from April 16 to July 6, 2020.
Exposures
Alcohol consumption level, which was self-reported by participants in health screening questionnaires, was categorized into none (0 g/d), mild (<15 g/d), moderate (15-29.9 g/d), and heavy (≥30 g/d) drinking. Based on changes in alcohol consumption level from 2009 to 2011, participants were categorized into the following groups: nondrinker, sustainer, increaser, quitter, and reducer.
Main Outcomes and Measures
The primary outcome was newly diagnosed alcohol-related cancers (including cancers of the head and neck, esophagus, colorectum, liver, larynx, and female breast), and the secondary outcome was all newly diagnosed cancers (except for thyroid cancer).
Results
Among the 4 513 746 participants (mean [SD] age, 53.6 [9.6] years; 2 324 172 [51.5%] men), the incidence rate of cancer was 7.7 per 1000 person-years during a median (IQR) follow-up of 6.4 (6.1-6.6) years. Compared with the sustainer groups at each drinking level, the increaser groups had a higher risk of alcohol-related cancers and all cancers. The increased alcohol-related cancer incidence was associated with dose; those who changed from nondrinking to mild (adjusted hazard ratio [aHR], 1.03; 95% CI, 1.00-1.06), moderate (aHR, 1.10; 95% CI, 1.02-1.18), or heavy (aHR, 1.34; 95% CI, 1.23-1.45) drinking levels had an associated higher risk than those who did not drink. Those with mild drinking levels who quit drinking had a lower risk of alcohol-related cancer (aHR, 0.96; 95% CI, 0.92-0.99) than those who sustained their drinking levels. Those with moderate (aHR, 1.07; 95% CI, 1.03-1.12) or heavy (aHR, 1.07; 95% CI, 1.02-1.12) drinking levels who quit drinking had a higher all cancer incidence than those who sustained their levels, but when quitting was sustained, this increase in risk disappeared. Compared with sustained heavy drinking, reduced heavy drinking levels to moderate levels (alcohol-related cancer: aHR, 0.91 [95% CI, 0.86-0.97]; all cancers: aHR, 0.96 [95% CI, 0.92-0.99]) or mild levels (alcohol-related cancer: aHR, 0.92 [95% CI, 0.86-0.98]; all cancers: aHR, 0.92 [95% CI, 0.89-0.96]) were associated with decreased cancer risk.
Conclusions and Relevance
Results of this study showed that increased alcohol consumption was associated with higher risks for alcohol-related and all cancers, whereas sustained quitting and reduced drinking were associated with lower risks of alcohol-related and all cancers. Alcohol cessation and reduction should be reinforced for the prevention of cancer.
Introduction
Cancer is the second leading cause of death globally, accounting for an estimated 9.6 million deaths in 2018.1 Alcohol consumption is the third major, modifiable cancer risk factor after tobacco use and excess body weight,2 and it is an established cause of at least 7 types of cancer.3
Although numerous studies have found an association between alcohol consumption and cancer,4 there is paucity of research into how the incidence of cancer increases or decreases with changes in drinking habits. Some studies of the association between alcohol cessation and risk of several cancers, including laryngeal or pharyngeal,5 esophageal,6 and liver cancers,7 reported reduced incidence. We found only 1 cohort study that reported an association between reduction in alcohol consumption and risk of cancer. The study found a modest decrease in the risk of upper digestive tract (oral cavity, pharynx, larynx, and esophagus) cancers among individuals who reduced their alcohol intake and an elevated risk among those who increased their alcohol intake.8
We conducted a cohort study to investigate the association between the reduction, cessation, or increase of alcohol consumption and the development of alcohol-related cancers and all cancers. Specifically, we measured alcohol consumption in a large cohort of Korean adults at 2 time points and incidence of cancer.
Methods
This retrospective, population-based cohort study was approved by the Institutional Review Board of the Samsung Medical Center, which waived the informed consent requirement because the data were public and anonymized under confidentiality guidelines. This study was designed and conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.9
Study Setting and Population
Korea has a mandatory social insurance system with insurance premiums that are determined by income level and not by health status. The National Health Insurance Service (NHIS) is a single insurer in Korea that covers approximately 97% of the population except for 3% of beneficiaries of the Medical Aid Program. Data on the use of medical facilities and records of prescriptions with International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes are gathered by the NHIS. In addition, the NHIS provides free biennial health screening for all beneficiaries older than 40 years and all employees regardless of age. This screening includes a self-administered questionnaire on health behavior (eg, medical history, smoking status, and drinking status), anthropometric measurements (eg, body mass index [calculated as weight in kilograms divided by height in meters squared] and blood pressure), and laboratory tests (eg, fasting glucose and lipid levels).10 The NHIS also collects information on demographic factors (eg, age, sex, place of residence, and income level) and links the data to a death registry database to manage the qualification of the enrollees. The NHIS database has been used to establish the cohort data for various epidemiologic studies.11
Using the NHIS database, we initially included in the present cohort 4 961 441 individuals (aged ≥40 years) who had available data on their drinking status in 2 consecutive biennial health screenings (2009 and 2011). Individuals were excluded if (1) they had a history of any cancer (n = 141 566) or cardiovascular disease (n = 65 108) before the 2011 health screening date; (2) they had any cancer (n = 40 982), any cardiovascular disease (n = 14 081), or died (n = 7881) within 1 year after the 2011 health screening date; or (3) they had any missing information (n = 178 077). Ultimately, 4 513 746 individuals were included in the primary analysis. These participants were followed up from 1 year after the 2011 health screening date to the date of incident cancer, death, or end of study (December 31, 2018), whichever occurred first (eFigure 1 in the Supplement).
Exposure
Information on alcohol consumption was collected from the self-administered questionnaires at 2 separate health screenings in 2009 and 2011. The amount of pure alcohol intake per day was calculated from the drinking frequency per week and the typical amount consumed on each occasion. First, we grouped participants according to drinking levels based on their self-reported daily alcohol consumption: none (0 g/d), mild (<15 g/d), moderate (15-29.9 g/d), or heavy (≥30 g/d) drinking.12,13 Second, for the description of baseline characteristics and interpretation, we classified these participants into 5 groups based on their drinking levels between 2009 and 2011: (1) nondrinker, defined as sustained alcohol abstinence; (2) sustainer, defined as maintained baseline level of alcohol consumption; (3) increaser, defined as elevated level of alcohol consumption; (4) quitter, defined as stopping alcohol consumption from a baseline mild, moderate, or heavy level; or (5) reducer, defined as decreased level of alcohol consumption but not quitting.
Outcomes
The primary outcome was newly diagnosed alcohol-related cancers, and the secondary outcome was all newly diagnosed cancers (ICD-10 codes C00-C99) except for thyroid cancer (ICD-10 code C73). Alcohol-related cancers were defined as established cancers, including cancers of the head and neck (oral cavity and pharynx; ICD-10 codes C01-C10 and C12-14), esophagus (ICD-10 code C15), colorectum (ICD-10 codes C18-C20 but excluding appendix [ICD-10 code C18.1]), liver (ICD-10 code C22), larynx (ICD-10 code C32), and female breast (ICD-10 code C50) according to the list of cancers of the National Cancer Institute,14 World Cancer Research Fund and American Institute for Cancer Research,4 and International Agency for Research on Cancer.15 We excluded thyroid cancer from the definition of all cancers because it is a representative example of overdiagnosis by inadvertent thyroid cancer screening in Korea.16,17
To define cancer incidence, we used a special registration code in addition to the ICD-10 diagnosis code. The NHIS has established a special co-payment reduction program to enhance health coverage and relieve the financial burden of patients with cancer. For example, patients pay only 5% of the total medical bill incurred for cancer-related medical care. Because enrollment in this co-payment reduction program is indicated by a special co-payment reduction code for cancer (V193) and requires a medical certificate from a physician, the cancer diagnoses included in this study are considered to be sufficiently reliable, and this method has been used in previous studies.18,19
We considered socioeconomic position, including income level and place of residence (urban or rural), to be a potential covariate. Household income was categorized into quartiles according to insurance premium levels, and those covered by the Medical Aid Program (the poorest 3% of the Korean population) were merged into the lowest income quartile. Smoking status was classified into never, former (<20 pack-years or ≥20 pack-years), or current smoker (<20 pack-years or ≥20 pack-years). Participants were also categorized according to whether they engaged in regular exercise. Regular exercise was defined as more than 30 minutes of moderate physical activity at least 5 times per week or more than 20 minutes of strenuous physical activity at least 3 times per week. Comorbidities (hypertension, diabetes, chronic kidney disease, and chronic obstructive pulmonary disease) were based on claims data before the screening date and health screening test results. To assess the overall comorbidity load, we used the primary care equivalent of the Charlson Comorbidity Index.
Statistical Analysis
The association between changes in the drinking level and the incidence of cancer was estimated using a Cox proportional hazards regression model initially (crude model [model 1]) and then using a multivariable-adjusted model (model 2; adjusted for age, sex, socioeconomic position, smoking status, physical activity, comorbidities, and Charlson Comorbidity Index). To observe the associations between changes in drinking level and cancer incidence, we selected the sustainer group (no change from baseline) for each alcohol consumption level as the reference group. Stratified analysis was performed by age, sex, and smoking status in 2009.
For the secondary analysis, we explored the association of changes in the drinking level that occurred during the follow-up. We selected 3 542 927 participants (78.5%) whose health screening data were available for 2013. Using information on drinking levels at 3 consecutive health screenings, we repeated the same analysis of drinking level change from 2009 to 2011 to 2013.
All statistical analyses were performed using the SAS statistical package, version 9.4 (SAS Institute Inc). A 2-sided P < .05 was considered to be statistically significant. Data were analyzed from April 16 to July 6, 2020.
Results
The 4 513 746 participants in the cohort had a mean (SD) age of 53.6 (9.6) years and included 2 324 172 men (51.5%) and 2 189 574 women (48.5%). From 2009 to 2011, 26.6% of participants with mild drinking, 9.6% with moderate drinking, and 8.6% with heavy drinking levels quit drinking. Compared with the quitter group, the increaser group tended to be younger, be male, have higher incomes, be current smokers, not engaged in regular exercise, and have a lower Charlson Comorbidity Index (Table 1).
Table 1. Characteristics of Study Participants According to Changes in Drinking Level Between 2009 and 2011 .
| Characteristic | Participant drinking level, No. (%) | ||||
|---|---|---|---|---|---|
| Nondrinker group (n = 2 218 002) | Quitter group (n = 377 325) | Reducer group (n = 302 732) | Sustainer group (n = 990 873) | Increaser group (n = 624 814) | |
| Age, mean (SD), y | 55.9 (10.3) | 53.2 (9.9) | 51.2 (8.8) | 50.6 (8.7) | 51.4 (9.1) |
| Sex | |||||
| Female | 1 622 546 (73.2) | 161 250 (42.7) | 26 903 (8.9) | 202 492 (20.4) | 176 383 (28.2) |
| Male | 595 456 (26.9) | 216 075 (57.3) | 275 829 (91.1) | 788 381 (79.6) | 448 431 (71.8) |
| Income level by quartile | |||||
| Quartile 1 (lowest) | 529 519 (23.9) | 84 178 (22.3) | 52 584 (17.4) | 178 708 (18.0) | 125 231 (20.0) |
| Quartile 2 | 429 539 (19.4) | 72 359 (19.2) | 53 326 (17.6) | 168 921 (17.1) | 113 589 (18.2) |
| Quartile 3 | 528 717 (23.8) | 90 324 (23.9) | 79 150 (26.2) | 242 315 (24.5) | 152 820 (24.5) |
| Quartile 4 (highest) | 730 227 (32.9) | 130 464 (34.6) | 117 672 (38.9) | 400 929 (40.5) | 233 174 (37.3) |
| Urban place of residence | 978 883 (44.1) | 171 834 (45.5) | 139 161 (46.0) | 474 503 (47.9) | 284 642 (45.6) |
| Smoking status | |||||
| Never | 1 858 340 (83.8) | 218 936 (58.0) | 75 193 (24.8) | 370 041 (37.3) | 326 109 (52.2) |
| Former | |||||
| <20 Pack-years | 105 598 (4.8) | 46 639 (12.4) | 53 513 (17.7) | 181 246 (18.3) | 80 489 (12.9) |
| ≥20 Pack-years | 70 497 (3.2) | 26 806 (7.1) | 36 332 (12.0) | 93 257 (9.4) | 46 251 (7.4) |
| Current | |||||
| <20 Pack-years | 79 152 (3.6) | 40 049 (10.6) | 56 010 (18.5) | 162 259 (16.4) | 82 311 (13.2) |
| ≥20 Pack-years | 104 415 (4.7) | 44 895 (11.9) | 81 684 (27.0) | 184 070 (18.6) | 89 654 (14.4) |
| Regular exercise | 442 596 (20.0) | 88 107 (23.4) | 76 842 (25.4) | 241 280 (24.4) | 138 972 (22.2) |
| BMI, mean (SD) | 23.9 (3.1) | 24.0 (4.9) | 24.3 (6.0) | 24.0 (2.8) | 24.1 (2.9) |
| Comorbidities | |||||
| Hypertension | 236 908 (10.7) | 40 014 (10.6) | 36 792 (12.2) | 96 711 (9.8) | 64 681 (10.4) |
| Diabetes | 770 554 (34.8) | 123 141 (32.6) | 110 233 (36.4) | 309 989 (31.3) | 199 546 (32.0) |
| Dyslipidemia | 552 048 (24.9) | 78 497 (20.8) | 60 629 (20.0) | 186 462 (18.8) | 122 018 (19.5) |
| CKD | 290 413 (13.1) | 42 905 (11.4) | 27 260 (9.0) | 93 833 (9.5) | 59 704 (9.6) |
| COPD | 108 370 (4.9) | 15 511 (4.1) | 9629 (3.2) | 30 654 (3.1) | 22 245 (3.6) |
| CCI, mean (SD) | 1.2 (1.4) | 1.1 (1.3) | 0.9 (1.2) | 0.8 (1.2) | 0.9 (1.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
Alcohol Consumption Change and Cancer
We followed participants for a median (IQR) of 6.4 (6.1-6.6) years, yielding a total of 28 090 140 person-years. During this period, there were 215 676 cancer events (7.7 per 1000 person-years), 37.2% (80 263 cases) of which were alcohol-related cancers. Table 2 and eTable 1 in the Supplement show the associations between the change in alcohol consumption and risk of cancer, with the sustainer group at each drinking level as the reference group.
Table 2. Associations Between Changes in Drinking Level and Cancer.
| Drinking level | No. (%) | Person-years | Cancer incidence rate per 1000 person-years | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| 2009 | 2011 | Participants | Events | Model 1a | Model 2b | |||
| Alcohol-related cancers | None | None | 2 218 002 (49.1) | 39 765 (49.5) | 13 985 909.0 | 2.8 | 1 [Reference] | 1 [Reference] |
| None | Mild | 297 258 (6.6) | 4958 (6.2) | 1 873 661.5 | 2.6 | 0.93 (0.90-0.96) | 1.03 (1.00-1.06) | |
| None | Moderate | 43 165 (1.0) | 816 (1.0) | 270 934.5 | 3.0 | 1.06 (0.99-1.14) | 1.10 (1.02-1.18) | |
| None | Heavy | 24 761 (0.5) | 577 (0.7) | 154 205.1 | 3.7 | 1.32 (1.21-1.43) | 1.34 (1.23-1.45) | |
| Mild | None | 305 582 (6.8) | 5425 (6.8) | 1 923 062.0 | 2.8 | 1.15 (1.12-1.19) | 0.96 (0.93-0.99) | |
| Mild | Mild | 663 193 (14.7) | 10 214 (12.7) | 4 178 738.4 | 2.4 | 1 [Reference] | 1 [Reference] | |
| Mild | Moderate | 138 073 (3.1) | 2358 (2.9) | 867 957.0 | 2.7 | 1.11 (1.06-1.16) | 1.10 (1.05-1.15) | |
| Mild | Heavy | 41 864 (0.9) | 856 (1.1) | 261 888.9 | 3.3 | 1.34 (1.25-1.43) | 1.17 (1.09-1.25) | |
| Moderate | None | 44 370 (1.0) | 984 (1.2) | 276 633.2 | 3.6 | 1.35 (1.26-1.45) | 1.05 (0.98-1.14) | |
| Moderate | Mild | 160 850 (3.6) | 2822 (3.5) | 1 009 333.3 | 2.8 | 1.06 (1.01-1.12) | 0.98 (0.93-1.04) | |
| Moderate | Moderate | 177 446 (3.9) | 2938 (3.7) | 1 114 432.3 | 2.6 | 1 [Reference] | 1 [Reference] | |
| Moderate | Heavy | 79 693 (1.8) | 1495 (1.9) | 499 352.0 | 3.0 | 1.14 (1.07-1.21) | 1.04 (0.98-1.11) | |
| Heavy | None | 27 373 (0.6) | 778 (1.0) | 168 497.5 | 4.6 | 1.28 (1.18-1.38) | 1.04 (0.96-1.12) | |
| Heavy | Mild | 53 347 (1.2) | 1149 (1.4) | 332 919.1 | 3.5 | 0.95 (0.89-1.02) | 0.92 (0.86-0.98) | |
| Heavy | Moderate | 88 535 (2.0) | 1736 (2.2) | 553 395.5 | 3.1 | 0.87 (0.82-0.92) | 0.91 (0.86-0.97) | |
| Heavy | Heavy | 150 234 (3.3) | 3392 (4.2) | 937 483.6 | 3.6 | 1 [Reference] | 1 [Reference] | |
| All cancers (except thyroid) | None | None | 2 218 002 (49.1) | 104 645 (48.5) | 13 834 229.0 | 7.6 | 1 [Reference] | 1 [Reference] |
| None | Mild | 297 258 (6.6) | 12 916 (6.0) | 1 854 643.0 | 7.0 | 0.92 (0.90-0.94) | 0.98 (0.97-1.00) | |
| None | Moderate | 43 165 (1.0) | 2267 (1.1) | 267 413.2 | 8.5 | 1.12 (1.08-1.17) | 0.98 (0.94-1.03) | |
| None | Heavy | 24 761 (0.5) | 1600 (0.7) | 151 910.8 | 10.5 | 1.40 (1.33-1.47) | 1.12 (1.07-1.18) | |
| Mild | None | 305 582 (6.8) | 14 624 (6.8) | 1 901 404.6 | 7.7 | 1.11 (1.09-1.14) | 0.98 (0.96-1.00) | |
| Mild | Mild | 663 193 (14.7) | 28 515 (13.2) | 4 134 254.3 | 6.9 | 1 [Reference] | 1 [Reference] | |
| Mild | Moderate | 138 073 (3.1) | 6512 (3.0) | 858 167.6 | 7.6 | 1.10 (1.07-1.13) | 1.02 (0.99-1.05) | |
| Mild | Heavy | 41 864 (0.9) | 2383 (1.1) | 258 324.6 | 9.2 | 1.34 (1.28-1.40) | 1.09 (1.04-1.13) | |
| Moderate | None | 44 370 (1.0) | 2852 (1.3) | 272 309.1 | 10.5 | 1.41 (1.35-1.47) | 1.07 (1.03-1.12) | |
| Moderate | Mild | 160 850 (3.6) | 7930 (3.7) | 997 209.7 | 8.0 | 1.07 (1.04-1.10) | 0.98 (0.95-1.02) | |
| Moderate | Moderate | 177 446 (3.9) | 8213 (3.8) | 1 102 016.0 | 7.5 | 1 [Reference] | 1 [Reference] | |
| Moderate | Heavy | 79 693 (1.8) | 4097 (1.9) | 493 498.5 | 8.3 | 1.11 (1.07-1.16) | 1.01 (0.97-1.05) | |
| Heavy | None | 27 373 (0.6) | 2143 (1.0) | 165 607.5 | 12.9 | 1.32 (1.26-1.38) | 1.07 (1.02-1.12) | |
| Heavy | Mild | 53 347 (1.2) | 3111 (1.4) | 328 378.0 | 9.5 | 0.97 (0.93-1.01) | 0.92 (0.89-0.96) | |
| Heavy | Moderate | 88 535 (2.0) | 4789 (2.2) | 546 300.8 | 8.8 | 0.89 (0.86-0.93) | 0.96 (0.92-0.99) | |
| Heavy | Heavy | 150 234 (3.3) | 9079 (4.2) | 924 473.2 | 9.8 | 1 [Reference] | 1 [Reference] | |
Abbreviation: HR, hazard ratio.
Model 1: crude model.
Model 2: adjusted for age, sex, socioeconomic position (income level and place of residence), smoking status, physical activity, comorbidities (hypertension, diabetes, dyslipidemia, chronic kidney disease, and chronic obstructive pulmonary disease), and Charlson Comorbidity Index.
Compared with the sustainer group, the increaser group had a higher risk of alcohol-related cancers, showing a dose-response association: nondrinking status was associated with increased risk of alcohol-related cancers as the status changed to mild drinking (adjusted hazard ratio [aHR], 1.03; 95% CI, 1.00-1.06), moderate drinking (aHR, 1.10; 95% CI, 1.02-1.18), or heavy drinking (aHR, 1.34; 95% CI, 1.23-1.45) (Table 2; Figure 1). Similarly, higher rates of alcohol-related cancers were found among those with a mild drinking level who changed to moderate (aHR, 1.10; 95% CI, 1.05-1.15) or heavy (aHR, 1.17; 95% CI, 1.09-1.25) drinking levels compared with those who sustained mild drinking levels. This pattern was also present for all cancers: increased risk was associated not only with nondrinking that became heavy drinking (aHR, 1.12; 95% CI, 1.07-1.18) but also mild drinking that became heavy drinking (aHR, 1.09; 95% CI, 1.04-1.13). When we examined specific cancer sites, we found that the increaser (from nondrinking) group had a high incidence of stomach, liver, gallbladder, and lung cancer; multiple myeloma; and leukemia (eTable 2 and eFigure 2 in the Supplement).
Figure 1. Risk of All Cancers and Alcohol-Related Cancers by Changes in Drinking Level Between 2009 and 2011.
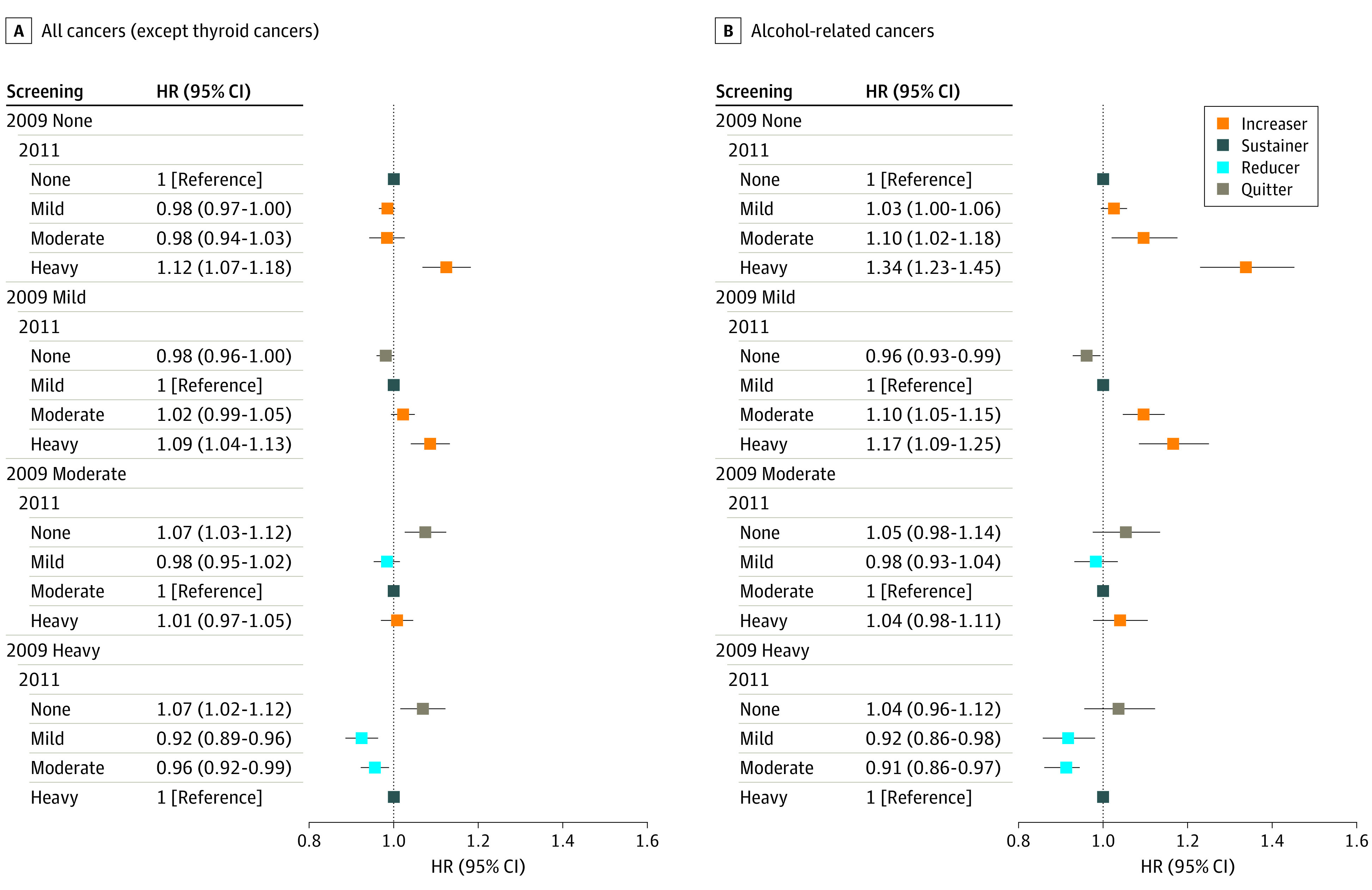
Hazard ratios (HRs) were adjusted for age, sex, socioeconomic position (income level and place of residence), smoking status, physical activity, comorbidities (hypertension, diabetes, dyslipidemia, chronic kidney disease, chronic obstructive pulmonary disease), and Charlson Comorbidity Index. Error bars indicate the 95% CIs.
For alcohol-related cancers, moderate or heavy drinking that changed to nondrinking was not significantly associated with the increased rate (Table 2; Figure 1). Although not to a great magnitude, an association was found between quitting from moderate drinking (aHR, 1.07; 95% CI, 1.03-1.12) or heavy drinking levels (aHR, 1.07; 95% CI, 1.02-1.12) and increased risk of all cancers. The quitter group was associated with higher incidences of head and neck, esophagus, stomach, colorectum, liver, gallbladder, larynx, cervix uteri, and pancreas cancers compared with sustained nondrinking (eTable 2 and eFigure 2 in the Supplement). Such associations were most pronounced for esophageal cancers, with an aHR of 3.66 (95% CI, 2.77-4.83) for those with a heavy drinking level who stopped drinking.
The risk reduction for alcohol-related cancer was greater for those with a heavy drinking level than for those with a moderate or mild drinking level who decreased their alcohol consumption. Reduced drinking among participants with formerly heavy drinking levels was associated with lower rates of alcohol-related cancers, regardless of whether it became moderate drinking (aHR, 0.91; 95% CI, 0.86-0.97) or mild drinking (aHR, 0.92; 95% CI, 0.86-0.98) (Figure 1; Table 2). This association was consistent for all cancers. Heavy drinking that changed to moderate drinking (aHR, 0.96; 95% CI, 0.92-0.99) or mild (aHR, 0.92; 95% CI, 0.89-0.96) was associated with lower risks of alcohol-related cancers compared with sustained heavy drinking. For cancer site, participants who changed from heavy to moderate drinking levels had a lower incidence of breast, kidney, and gallbladder cancer than those who sustained a heavy drinking level (eTable 2 and eFigure 2 in the Supplement).
Secondary Analysis
We analyzed alcohol behavior from 3 consecutive health screenings for participants whose data were available (eTables 3 and 4 in the Supplement). Those who quit drinking at the 2011 screening and remained nondrinking at the 2013 screening no longer showed an increased incidence of alcohol-related cancers compared with those who sustained the same level of drinking from baseline: mild drinking (aHR, 0.92; 95% CI, 0.87-0.98), moderate drinking (aHR, 1.01; 95% CI, 0.86-1.19), and heavy drinking (aHR, 0.91; 95% CI, 0.76-1.10) (Figure 2). A similar pattern was observed for all cancers. We also found that those with heavy drinking levels who greatly reduced their alcohol intake to mild drinking levels at the 2011 screening and maintained mild drinking levels were found to have an even lower rate of alcohol-related cancers (aHR, 0.85; 95% CI, 0.73-0.99) (Figure 2). Those with heavy drinking levels who greatly decreased their alcohol consumption to mild drinking levels at the 2011 screening and maintained mild (aHR, 0.89; 95% CI, 0.81-0.98) to moderate (aHR, 0.88; 95% CI, 0.80-0.98) drinking levels were found to have an even lower rate of all cancers (Figure 3).
Figure 2. Risk of Alcohol-Related Cancers by Changes in Drinking Level From 2009 to 2013.
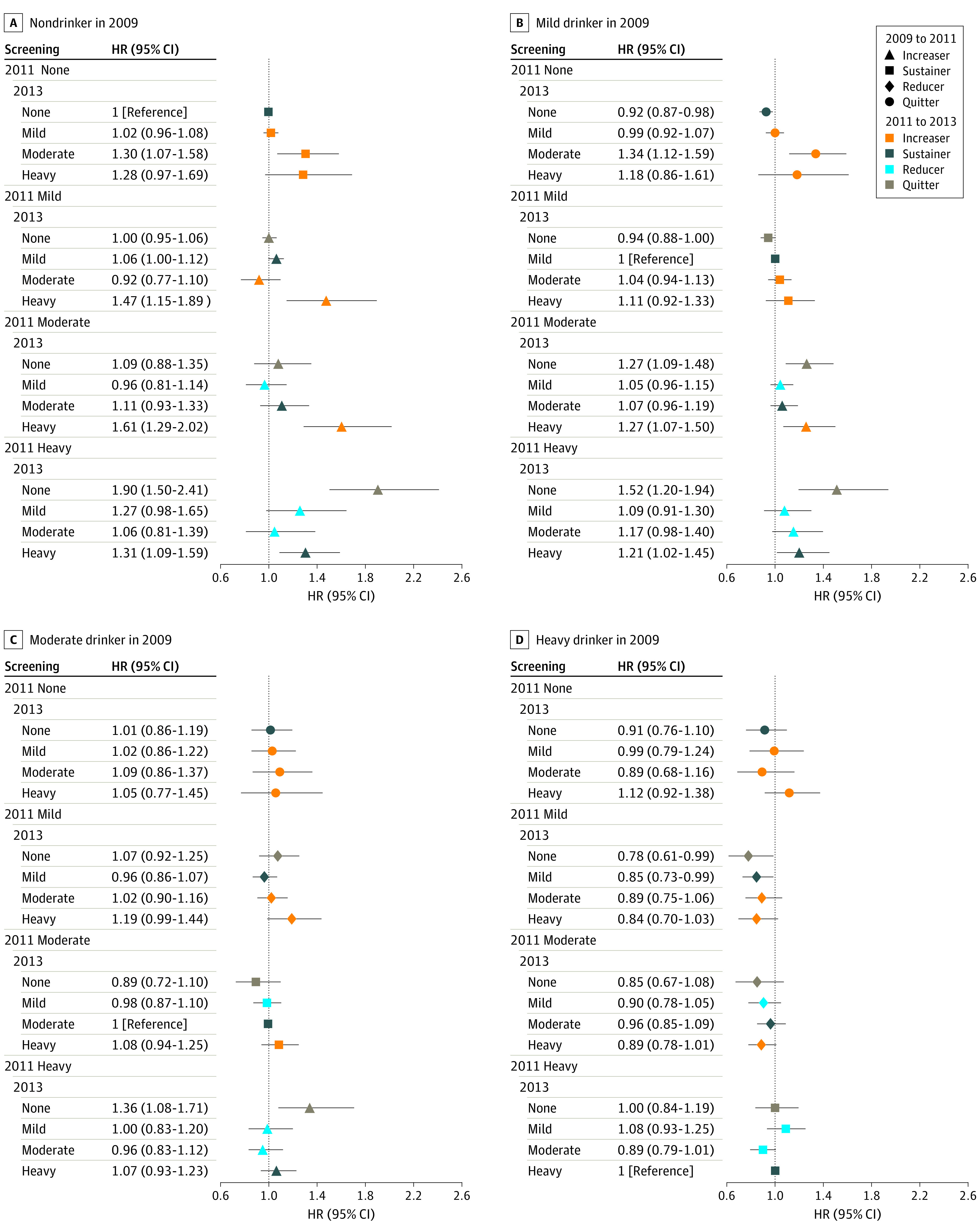
Sustainers from 2009 to 2013 were the reference. Hazard ratios (HRs) were adjusted for age, sex, socioeconomic position (income level and place of residence), smoking status, physical activity, comorbidities, and Charlson Comorbidity Index. Error bars indicate the 95% CIs.
Figure 3. Risk of All Cancers by Changes in Drinking Level From 2009 to 2013.
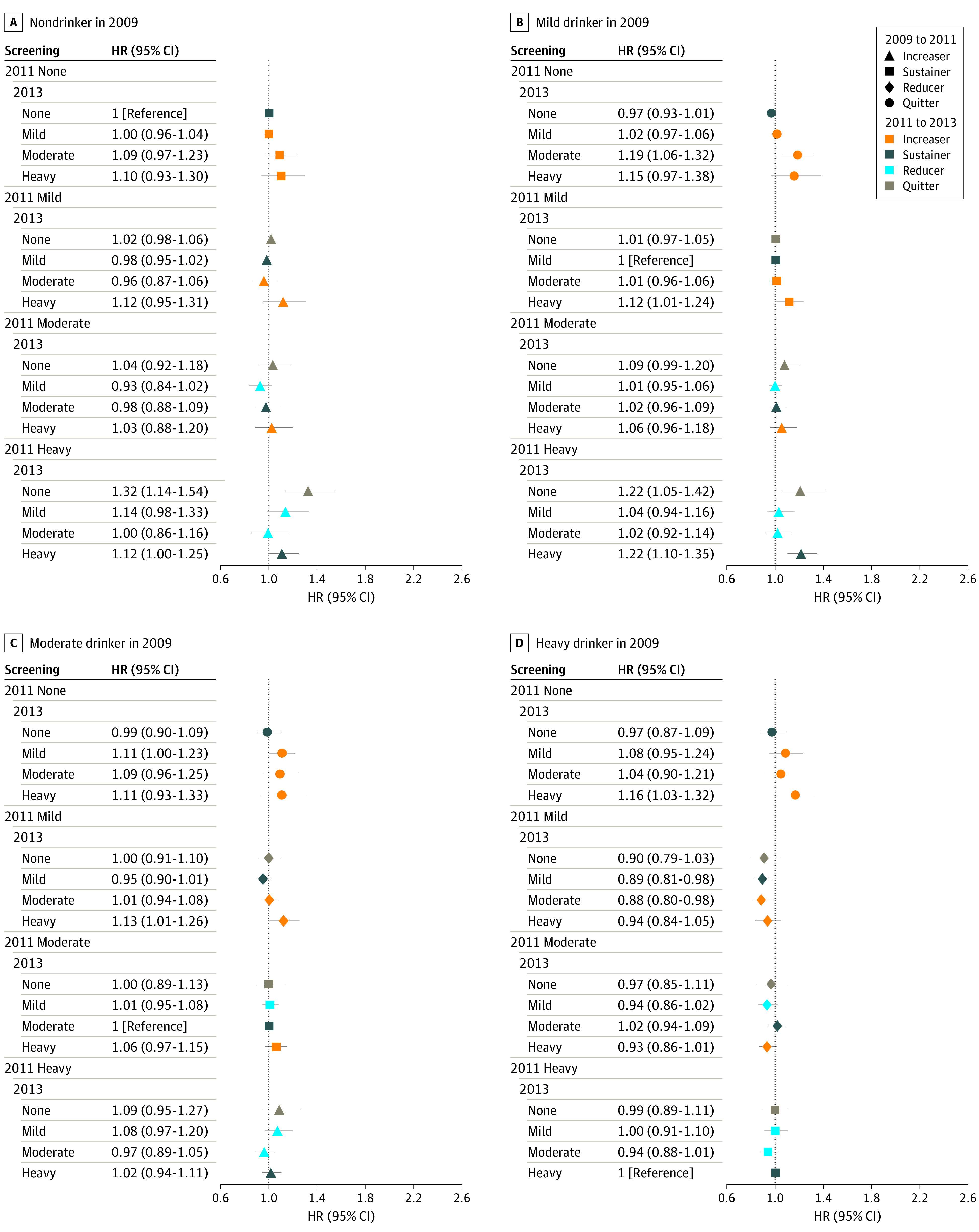
Sustainers from 2009 to 2013 were the reference. Hazard ratios (HRs) were adjusted for age, sex, socioeconomic position (income level and place of residence), smoking status, physical activity, comorbidities, and Charlson Comorbidity Index. Error bars indicate the 95% CIs.
Stratified Analysis
In stratified analyses according to age, sex, and smoking status, increased alcohol consumption was generally associated with an increased incidence of alcohol-related cancers and all cancers compared with sustained drinking, whereas a reduction in heavy drinking was generally associated with lower cancer risk, which was consistent with the main findings (eTables 5 to 8 in the Supplement). Associations between the change in alcohol consumption level and alcohol-related and all cancers were more prominent in participants who were older (eg, from nondrinking to heavy drinking in men aged ≥65 vs <65 years for alcohol-related cancers: aHR, 1.70 [95% CI, 1.48-1.94] vs 1.34 [95% CI, 1.20-1.50]), were male (eg, from nondrinking to heavy drinking in men vs women for alcohol-related cancers: aHR, 1.47 [95% CI, 1.35-1.60] vs 0.83 [95% CI, 0.60-1.16]), and had never smoker status (eg, from nondrinking to heavy drinking in never smoker vs current smoker with ≥20 pack-years for alcohol-related cancers: aHR, 1.77 [95% CI, 1.51-2.08] vs 1.49 [95% CI, 1.27-1.75]).
Discussion
In this large cohort study that used repeated measurements of alcohol consumption, we found that individuals who increased their alcohol consumption, regardless of their baseline drinking level, had an increased incidence of alcohol-related and all cancers compared with those who sustained their current level of drinking. Quitting was not associated with a lower incidence of alcohol-related cancer, but if abstinence was maintained over time, the incidence of alcohol-related and all cancers tended to decrease. Reducing drinking from heavy to moderate or mild levels was associated with a decreased risk of alcohol-related and all cancers.
Consumption of alcoholic drinks is an established risk for so-called alcohol-related cancers, such as mouth, pharynx and larynx, esophagus, liver, colorectum, and breast cancers.15,20 In addition, those who increased their level of drinking had a higher risk of dose-associated alcohol-related cancers than those who sustained their level of drinking. Although we did not find this association consistently for all alcohol-related cancers except liver cancer (eTable 2 and eFigure 2 in the Supplement), the findings may support a potential causal association between alcohol consumption and cancers.
We found an elevated risk for all cancers among participants who recently quit drinking compared with those who sustained their level of drinking. An explanation for this phenomenon may be the sick quitter phenomenon,21 the idea that individuals could have stopped consuming alcohol after feeling symptoms and/or other adverse health effects. Although we conducted a primary analysis with 2 assessments and a 1-year lag, it was not enough to address the sick quitter bias.22 However, in subgroup analyses with people undertaking 3 measurements, participants who quit drinking by the 2011 screening and remained nondrinking at the 2013 screening showed similar or even decreased risk compared with those who sustained the same level of drinking.
Although the potential mechanism underlying alcohol-induced carcinogenesis is not fully understood, cases of alcohol-induced carcinogenesis might be reversible because of physiologic homeostasis after quitting drinking. For example, 4-fold to 10-fold increases in cytochrome P450 2E1 (CYP2E1) have been found to be associated with alcohol consumption,23 which result in the production of acetaldehyde, reactive oxygen species, and other carcinogenic substrates, such as nitrosamines,24 whereas a rapid decline in CYP2E1 expression has been reported 3 days after the cessation of alcohol consumption.23 In addition, numerous reports have observed an association between drinking alcohol and suppression of natural killer (NK) cell function.25 Alcohol consumption is a factor in inhibiting the effector function of NK cells, suppressing its cytolytic activity, blocking NK cell release, and inducing NK cell apoptosis.25 However, after 3 months of alcohol withdrawal, the number of NK cells continue to increase.26 Thereafter, the increased cancer risk from alcohol consumption can be reversible by the cessation of drinking, which is consistent with results of previous studies.5,6,7,22,27,28
In addition, we observed that the risks of alcohol-related and all cancers tended to decrease slightly for those with mild drinking levels who quit drinking. Previous studies have raised concerns that drinking even a small amount of alcohol increases the risk of cancer,29 including most upper aerodigestive tract cancers and gastrointestinal cancers.30,31 The present study highlights that there is no safe level of alcohol consumption in terms of cancer risk.
Although participants with a heavy drinking level—even those who reduced their alcohol intake later—still had a higher cancer risk than those with sustained nondrinking (eTable 9 in the Supplement), we observed that those who reduced their heavy drinking to a mild or moderate level had a decreased risk of cancer compared with those with sustained heavy drinking levels. There may be several explanations for this finding. First, alcohol consumed per se could have directly contributed to the decreased risk of cancer among the reducer group. Given the well-established dose-response association between the amount of alcohol consumption and several cancer risks,32 it is likely that reduction in alcohol consumption is a factor in lower risk of cancers. Second, reducing alcohol intake could act as a temporary step toward permanent quitting, and decreased cancer risk among the reducer group could be associated with their increased probability of complete abstinence.
When we analyzed the risk by sex, we found that the association between changes in drinking level and cancer risks were not significant for women. The lack of an observed association may be attributable to sample size constraints. Throughout the study period, 73.1% of female participants had a nondrinking level, and not enough women changed their drinking levels (eg, from heavy to mild or none) to show statistical significance. In addition, men generally engaged in binge drinking more than women. An association between alcohol consumption and cancer may be mediated by dose, duration, and pattern of alcohol intake, including binge drinking.25 In vivo studies in mice showed that binge alcohol exposure was associated with inhibited activity of NK cells and reduced number and lytic activity of NK cells.25 Because of these sex-related differences in drinking patterns, it is possible that the association of alcohol consumption with cancer risk was less prominent in women than in men. In line with the results of this study, the American Cancer Society33 and World Cancer Research Fund and American Institute for Cancer Research4 recommend not drinking alcohol at all given that the less alcohol consumed, the lower the risk of cancer.
Limitations
This study has several limitations. First, we obtained lifestyle data, including level of alcohol consumption, from self-administered questionnaires, and it is probable that participants, especially women, underreported their alcohol consumption.34,35 Second, although examining long-term alcohol intake data before 2009 would be informative, information about long-term habits was not available from the NHIS database. The data covered a relatively short time span, preventing trajectory analyses or investigations of the impact of early-adulthood alcohol consumption.36,37 Third, because this study used data that were not originally designed for studying alcohol consumption, we were not able to assess aldehyde dehydrogenase gene status among participants, and we did not have pertinent information, such as reasons for reducing or stopping drinking and duration of drinking. Fourth, the results showing sex-based differences require cautious interpretation, which we attempted to address by performing sex-stratified analyses, because of the small number of women who reported drinking alcohol and even smaller number of women who reported heavy drinking levels. In addition, given the biological differences in alcohol metabolism between sexes, further studies that define drinking levels separately by sex may provide more insight into these associations among women.
Fifth, there might be unmeasured confounders, particularly those that would not be identified through routine health screening, such as stress or other mental health factors. Sixth, we did not consider concomitant health behavior changes in the analyses. It is possible that participants whose alcohol consumption behaviors changed over time may also experience changes in smoking status or physical activity. However, the sensitivity analysis among nonsmokers showed consistent results, suggesting that confounding from concomitant health behavior changes was not significant.
Conclusions
This large, population-based cohort study found that an increased level of alcohol consumption was associated with higher incidence of alcohol-related cancers and all cancers compared with sustained drinking levels, whereas reduced drinking (particularly heavy drinking) was associated with a lower cancer risk. Quitting drinking was associated with increased risk, but this risk increase disappeared when quitting was sustained. Alcohol cessation and reduction should be reinforced for the prevention of cancer.
eTable 1. Associations Between Changes in Drinking Level and Cancer Including Participants With Previous Cardiovascular History
eTable 2. The Risk of Site-Specific Cancer According to Changes in Drinking Level Between 2009 and 2011
eTable 3. Associations Between Changes in Drinking Level From 2009 to 2013 and Alcohol-Related Cancers
eTable 4. Associations Between Changes in Drinking Level From 2009 to 2013 and All Cancers
eTable 5. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Age
eTable 6. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Sex
eTable 7. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Smoking Status in 2009 (Male)
eTable 8. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Smoking Status in 2009 (Female)
eTable 9. Associations Between Changes in Drinking Level and Cancer (Sustained Non-Drinker as a Referent)
eFigure 1. Flow Chart of the Study Population
eFigure 2. The Risk of Site-Specific Cancer According to Changes in Drinking Level Between 2009 and 2011
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 3.Connor J. Alcohol consumption as a cause of cancer. Addiction. 2017;112(2):222-228. doi: 10.1111/add.13477 [DOI] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project expert report 2018: alcoholic drinks and the risk of cancer. Accessed May 30, 2022. http://www.dietandcancerreport.org/
- 5.Ahmad Kiadaliri A, Jarl J, Gavriilidis G, Gerdtham UG. Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: a systematic review and meta-analysis. PLoS One. 2013;8(3):e58158. doi: 10.1371/journal.pone.0058158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarl J, Gerdtham UG. Time pattern of reduction in risk of oesophageal cancer following alcohol cessation: a meta-analysis. Addiction. 2012;107(7):1234-1243. doi: 10.1111/j.1360-0443.2011.03772.x [DOI] [PubMed] [Google Scholar]
- 7.Heckley GA, Jarl J, Asamoah BO, G-Gerdtham U. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11:446. doi: 10.1186/1471-2407-11-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen LC, Keiding N, Johansen C, Grønbaek M. Changes in alcohol intake and risk of upper digestive tract cancer. Acta Oncol. 2007;46(8):1085-1089. doi: 10.1080/02841860701441806 [DOI] [PubMed] [Google Scholar]
- 9.Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis. 2017;38(9):859-872. doi: 10.1093/carcin/bgx067 [DOI] [PubMed] [Google Scholar]
- 10.Seong SC, Kim YY, Park SK, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7(9):e016640. doi: 10.1136/bmjopen-2017-016640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin DW, Cho B, Guallar E. Korean National Health Insurance database. JAMA Intern Med. 2016;176(1):138. doi: 10.1001/jamainternmed.2015.7110 [DOI] [PubMed] [Google Scholar]
- 12.Shin C, Baik I. Associations between alcohol consumption and leukocyte telomere length modified by a common polymorphism of ALDH2. Alcohol Clin Exp Res. 2016;40(4):765-771. doi: 10.1111/acer.13005 [DOI] [PubMed] [Google Scholar]
- 13.Lee YJ, Cho S, Kim SR. Effect of alcohol consumption on kidney function: population-based cohort study. Sci Rep. 2021;11(1):2381. doi: 10.1038/s41598-021-81777-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Alcohol and cancer risk. Accessed May 30, 2022. https://www.cancer.gov/about-cancer/causes-prevention/risk/alcohol/alcohol-fact-sheet
- 15.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Personal habits and indoor combustions. Volume 100 E: a review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt E):1-538. [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765-1767. doi: 10.1056/NEJMp1409841 [DOI] [PubMed] [Google Scholar]
- 17.Park S, Oh CM, Cho H, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016;355:i5745. doi: 10.1136/bmj.i5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo JE, Shin DW, Han K, et al. Association of the frequency and quantity of alcohol consumption with gastrointestinal cancer. JAMA Netw Open. 2021;4(8):e2120382. doi: 10.1001/jamanetworkopen.2021.20382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo Y, Shin DW, Han K, et al. Smoking, alcohol consumption, and the risk of thyroid cancer: a population-based Korean cohort study of 10 million people. Thyroid. 2022;32(4):440-448. doi: 10.1089/thy.2021.0675 [DOI] [PubMed] [Google Scholar]
- 20.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253-256. doi: 10.1017/S002966510800712X [DOI] [PubMed] [Google Scholar]
- 21.Park JE, Ryu Y, Cho SI. The association between health changes and cessation of alcohol consumption. Alcohol Alcohol. 2017;52(3):344-350. doi: 10.1093/alcalc/agw089m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehm J, Patra J, Popova S. Alcohol drinking cessation and its effect on esophageal and head and neck cancers: a pooled analysis. Int J Cancer. 2007;121(5):1132-1137. doi: 10.1002/ijc.22798 [DOI] [PubMed] [Google Scholar]
- 23.Oneta CM, Lieber CS, Li J, et al. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36(1):47-52. doi: 10.1016/S0168-8278(01)00223-9 [DOI] [PubMed] [Google Scholar]
- 24.McKillop IH, Schrum LW, Thompson KJ. Role of alcohol in the development and progression of hepatocellular carcinoma. Hepat Oncol. 2016;3(1):29-43. doi: 10.2217/hep.15.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratna A, Mandrekar P. Alcohol and cancer: mechanisms and therapies. Biomolecules. 2017;7(3):E61. doi: 10.3390/biom7030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laso FJ, Madruga JI, Girón JA, et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25(5):1096-1100. doi: 10.1002/hep.510250508 [DOI] [PubMed] [Google Scholar]
- 27.Altieri A, Bosetti C, Talamini R, et al. Cessation of smoking and drinking and the risk of laryngeal cancer. Br J Cancer. 2002;87(11):1227-1229. doi: 10.1038/sj.bjc.6600638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39(1):182-196. doi: 10.1093/ije/dyp291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. doi: 10.1136/bmj.h4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaitsu M, Takeuchi T, Kobayashi Y, Kawachi I. Light to moderate amount of lifetime alcohol consumption and risk of cancer in Japan. Cancer. 2020;126(5):1031-1040. doi: 10.1002/cncr.32590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YJ, Lee DH, Han KD, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population-based cohort study of South Korea. PLoS One. 2017;12(10):e0185778. doi: 10.1371/journal.pone.0185778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580-593. doi: 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. doi: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 34.Stockwell T, Zhao J, Macdonald S. Who under-reports their alcohol consumption in telephone surveys and by how much? an application of the ‘yesterday method’ in a national Canadian substance use survey. Addiction. 2014;109(10):1657-1666. doi: 10.1111/add.12609 [DOI] [PubMed] [Google Scholar]
- 35.Livingston M, Callinan S. Underreporting in alcohol surveys: whose drinking is underestimated? J Stud Alcohol Drugs. 2015;76(1):158-164. doi: 10.15288/jsad.2015.76.158 [DOI] [PubMed] [Google Scholar]
- 36.Hur J, Smith-Warner SA, Rimm EB, et al. Alcohol intake in early adulthood and risk of colorectal cancer: three large prospective cohort studies of men and women in the United States. Eur J Epidemiol. 2021;36(3):325-333. doi: 10.1007/s10654-021-00723-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. doi: 10.1136/bmj.k2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Associations Between Changes in Drinking Level and Cancer Including Participants With Previous Cardiovascular History
eTable 2. The Risk of Site-Specific Cancer According to Changes in Drinking Level Between 2009 and 2011
eTable 3. Associations Between Changes in Drinking Level From 2009 to 2013 and Alcohol-Related Cancers
eTable 4. Associations Between Changes in Drinking Level From 2009 to 2013 and All Cancers
eTable 5. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Age
eTable 6. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Sex
eTable 7. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Smoking Status in 2009 (Male)
eTable 8. Adjusted Hazard Ratios and 95% Confidence Intervals for Cancer According to Changes in Drinking Level by Smoking Status in 2009 (Female)
eTable 9. Associations Between Changes in Drinking Level and Cancer (Sustained Non-Drinker as a Referent)
eFigure 1. Flow Chart of the Study Population
eFigure 2. The Risk of Site-Specific Cancer According to Changes in Drinking Level Between 2009 and 2011


