Abstract
Introduction
Vitamin K (VK) as a nutrient, is a cofactor in the carboxylation of osteocalcin (OC), which can bind with hydroxyapatite to promote bone mineralization and increase bone strength. However, some studies have been inconsistent on whether vitamin K2 (VK2) can maintain or improve bone mineral density (BMD) and reduce the incidence of fractures in postmenopausal women. Therefore, the main objective of this meta-analysis was to determine the effect of VK2 as a nutritional supplement on BMD and fracture incidence in postmenopausal women.
Methods
We searched PubMed, EMBASE, and Cochrane Library databases (published before March 17, 2022) and then extracted and pooled data from all randomized controlled trials (RCTs) that met the inclusion criteria.
Results
Sixteen RCTs with a total of 6,425 subjects were included in this meta-analysis. The overall effect test of 10 studies showed a significant improvement in lumbar spine BMD (BMD LS) (P = 0.006) with VK2. The subgroup analysis of VK2 combination therapy showed that BMD LS was significantly maintained and improved with the administration of VK2 (P = 0.03). The overall effect test of the six RCTs showed no significant difference in fracture incidence between the two groups (RR=0.96, P=0.65). However, after excluding one heterogeneous study, the overall effect test showed a significant reduction in fracture incidence with VK2 (RR = 0.43, P = 0.01). In addition, this meta-analysis showed that VK2 reduced serum undercarboxylated osteocalcin (uc-OC) levels and the ratio of uc-OC to cOC in both subgroups of VK2 combined intervention and alone. However, for carboxylated osteocalcin (cOC), both subgroup analysis and overall effect test showed no significant effect of VK2 on it. And the pooled analysis of adverse reactions showed no significant difference between the VK2 and control groups (RR = 1.03, 95%CI 0.87 to 1.21, P = 0.76).
Conclusions
The results of this meta-analysis seem to indicate that VK2 supplementation has a positive effect on the maintenance and improvement of BMD LS in postmenopausal women, and it can also reduce the fracture incidence, serum uc-OC levels and the ratio of uc-OC to cOC. In conclusion, VK2 can indirectly promote bone mineralization and increase bone strength.
Keywords: vitamin K2, postmenopausal women, osteoporosis, fracture, meta-analysis, randomized controlled trial
Introduction
Osteoporosis (OP) has become a global public health problem, which is mainly characterized by the reduction of bone trabecular number, destruction of bone microstructure and the increase of bone fragility, which predisposes to fractures (1). OP and OP-related fractures are increasingly common in women over 55 years and men over 65 years, especially in older postmenopausal women (2). Hip fracture due to OP in particular can be devastating to the elderly, because it is expensive to treat and carries a high risk of death (3). Postmenopausal osteoporosis (PMOP) is the most common type in primary osteoporosis. When a woman goes through menopause, a sharp drop of estrogen levels will lead to more osteoclastogenesis than osteoblastogenesis, which can lead to the increase of bone resorption, the breakdown of bone metabolism balance, and eventual OP.
Osteocalcin (OC), as a biomarker of bone metabolism, is synthesized and secreted by osteoblasts. It plays an important role in regulating bone calcium metabolism. However, undercarboxylated osteocalcin (uc-OC) has no biological function, because it cannot bind to hydroxyapatite and does not facilitate the deposition of bone calcium. Only with the assistance of VK can uc-OC be converted to carboxylated osteocalcin (cOC), which has a high affinity for calcium and promotes hydroxyapatite formation and bone mineralization (4–7). VK is a fat-soluble vitamin that is a cofactor for the carboxylation of osteocalcin (8, 9). VK includes phylloquinone (VK1) and menaquinone (VK2) (10, 11). VK1 is mainly found in plants, while VK2 is mainly found in some fermented foods, most commonly natto, and VK2 can also be synthesized by intestinal bacteria (12). In addition, there are twelve MK subtypes in VK2, among which MK-4 and MK-7 mainly maintain bone health, as MK-4 and MK-7 can promote bone calcium deposition and increase bone strength (13).
But so far, the answer to the question of whether VK2 significantly maintains or improves bone mineral density (BMD) in various parts of the body and whether it significantly reduces the incidence of fracture is unclear. Some studies (14–20) suggest that VK2 can do this, but some suggest that it cannot (9, 11, 21). However, unlike VK2, the role of vitamin D and calcium in the prevention and treatment of OP has been confirmed by high-quality studies and has been included in guidelines for the prevention and treatment of OP (22, 23). Previous studies have shown that VK2 can promote the mineralization of 1,25 (OH)2D3-induced in human osteoblasts, and that the effect of VK2 in combination with vitamin D3 or calcium or other anti-osteoporosis drugs (such as alendronate) on bone mass is much greater than that of VK2 alone (24–27). Although previous meta-analyses have evaluated the effects of VK (28–34), to our knowledge, no meta-analysis has examined the effects of VK2 combination therapy and VK2 alone therapy on PMOP. Therefore, we consider it necessary to conduct this systematic review and meta-analysis to determine the role of VK2 in the prevention and treatment of PMOP.
Materials and methods
Search strategy
According to the PRISMA statement, we searched those authoritative literature databases (PubMed, EMBASE, and the Cochrane Library). The search terms used were vitamin K, menaquinone, menatetrenone, postmenopausal and osteoporosis. In order to retrieve these studies comprehensively, we adopted the search method of combining keywords and Boolean Operators. Medical Subject Heading (MeSH) phrases were also used appropriately, and we did not limit the year and language of publication during the search. We have stored the search strategy for the above database in Supplementary material.
Inclusion and exclusion criteria
Based on the principles of PICOS (Population/Intervention/ Comparison/Outcome/Study design), the following inclusion criteria were developed: 1. Study design was a randomized controlled trial; 2. Study population was postmenopausal women; 3. Intervention group was VK2 alone or in combination with other therapeutic measures; 4. Control group was given another drug or placebo; 5. Outcomes should include at least one of the following:(1) percentage change in BMD of lumbar spine, femoral neck, hip, or forearm; (2) Incidence of fractures; (3) percentage change in serum uc-OC; (4) percentage change in serum cOC; (5) percentage change in serum uc-OC to cOC ratio and (6) incidence of adverse reactions.
Studies would be excluded if they fulfilled any of the following conditions: 1. Non-randomized controlled trial; 2. Study was not about VK2; 3. Subjects had renal or immune disease; 4. Subjects were taking anti-osteoporosis drugs or other drugs that affect bone metabolism (such as glucocorticoids or immunosuppressants); 5. Subjects were taking drugs that affect the absorption or metabolism of VK2, such as warfarin.
Study selection
In accordance with the inclusion and exclusion criteria, two reviewers (ML. MA, ZJ.MA) browsed through the titles and abstracts of all studies for initial screening. Then, the full text of the studies was read carefully to identify the final included studies. During the screening process, two reviewers documented the reasons for exclusion of some studies. When two reviewers disagreed on the inclusion or exclusion of studies, a third reviewer (H. DONG) would be invited to discuss and make a final decision.
Data extraction
First, three reviewers (ML. MA, ZJ.MA and H.SUN) extracted and collated the basic information of the final included studies including author, publication year, study type, country, number of study center, sample size, age, follow-up time, intervening measures, as well as other relevant data required for this meta-analysis. Then, three reviewers assessed the quality of the included studies. Some studies presented results in bar or line graphs, without giving specific data. In view of this, we used the online tool for graphical data extraction (Web Plot Digitizer, https://automeris.io/WebPlotDigitizer/). The extracted and collated data were finally rechecked by ML.MA.
Outcomes
The percentage change in BMD (lumbar spine, femoral neck, hip, and forearm) before and after treatment with VK2 or other measures and the incidence of fractures were taken as primary outcomes. Meanwhile, the percentage change of serum uc-OC, cOC, and uc-OC to cOC ratio and the incidence of adverse reactions were taken as secondary outcomes.
Assessment of bias risk for included studies
Two reviewers (YL.HE, SX.LI) independently assessed the risk of bias for all included RCTs using the Cochrane Collaboration's tool. This tool assessed the risk of bias for RCTs through the following seven lists: (1) Random sequence generation (selection bias); (2) Allocation concealment (selection bias); (3) Blinding of participants and personnel (performance bias); (4) Blinding of outcome assessment (detection bias); (5) Incomplete outcome data (attrition bias); (6) Selective reporting (reporting bias); (7) Other bias. Each list has three options: high risk, low risk and unclear risk.
Data synthesis and analysis
We used the STATA software (STATA Software Version 16.0) to assess publication bias and conduct sensitivity analysis, and used the Review Manager Software (RevMan5.3) to make analysis for other data. For continuous outcome variables, we extracted the mean and standard deviation from the included studies and then calculated the weighted mean difference (WMD) and 95% confidence interval (95% CI), and for binary outcome variables, we extracted the number of positive events in both groups then calculated the risk ratio (RR) and 95%CI. The heterogeneity of studies was assessed using I2 statistics and Cochran's Q Test (35–37). The random-effects model was used when the heterogeneity between studies was large (P ≤ 0.1, I2 > 50%) and the fixed-effects model was used when the heterogeneity between studies was small (P > 0.1, I2 ≤ 50%). Subgroup analysis was based on VK2 and the studies were then divided into two subgroups: VK2 combined intervention subgroup and VK2 alone intervention subgroup. Sensitivity analysis was performed by removing studies that caused heterogeneity. The continuous variables in this meta-analysis were the percentage change of outcomes before and after treatment, which contributed to the elimination of differences in baseline data.
Results
Search results
We searched a total of 694 studies in PubMed, EMBASE and Cochrane Library databases, and then we removed 213 duplicated studies and obtained 481 studies initially. Next, by reading the titles and abstracts of these studies, we excluded 432 studies again for the following reasons:(1) subjects were uncorrelated with ours; (2) review or meta-analysis; (3) animal studies; (4) outcomes of studies did not meet our needs; (5) study population included either men or non-menopausal women; (6) mechanism researches for VK2. Next, the full texts of the remaining 49 studies were read precisely and carefully, and 16 studies (9, 14, 15, 18, 21, 26, 27, 38–46) were finally eligible for the inclusion in this meta-analysis. Another 33 studies were excluded for the following reasons: (1) the intervention was VK1 or partially VK1; (2) non-randomized controlled trials; (3) no access to study data; (4) study data were absolute values, not percentage change. Additionally, the majority of the included studies were conducted in Japan (18, 26, 27, 39–41, 44–46), and others were conducted in Denmark, the Netherlands, Norway, China and Indonesia (9, 14, 15, 21, 38, 42, 43). The screening process of the studies is shown in Figure 1.
Figure 1.
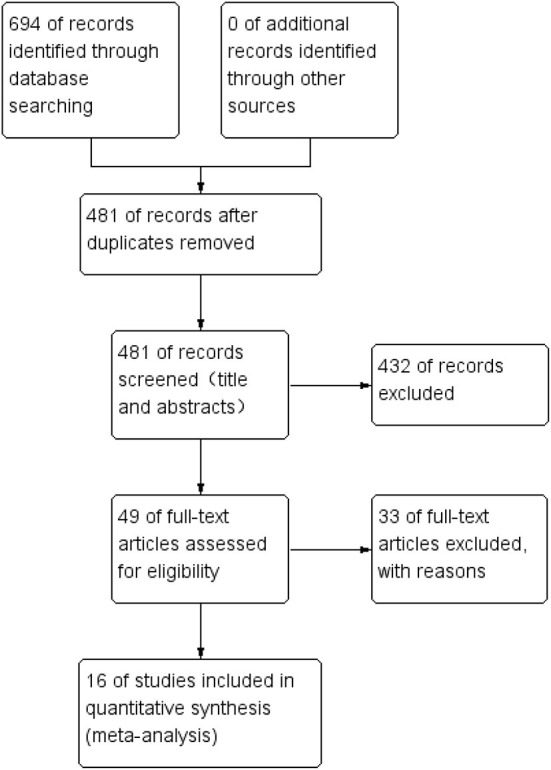
Flow diagram of studies selection.
Characteristics of the included studies
Through searching and screening, 16 studies were included in this meta-analysis, all of which were RCTs with a total of 6,425 subjects. These studies were mainly distributed in Europe (9, 15, 21, 38, 42) and Asia (14, 18, 26, 27, 39–41, 43–46). Most of them were single-center studies, and only three were multicenter studies (14, 21, 41). The characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of included studies.
| Reference | Study type | Country | Research center | Number | Age | Years of menopause | Osteoporosis | Follow-up | Intervention | Comparison |
|---|---|---|---|---|---|---|---|---|---|---|
| Rønn et al. (9) | RCT | Denmark | SC | 142 | 67.3 ± 4.4 | >2 | No | 36 M | 375μg/day MK-7 + 38μg/day VD3 + 800 mg/day Ca | 38μg/day VD3 + 800 mg/day Ca |
| Rønn et al. (38) | RCT | Denmark | SC | 142 | 60–80 | >2 | No | 12 M | 375μg/day MK-7 + 38μg/day VD3 + 800 mg/day Ca | 38μg/day VD3 + 800 mg/day Ca |
| Kasukawa et al. (26) | RCT | Japan | SC | 101 | >60 | - | Yes | 12 M | 17.5 mg/week risedronate + 45 mg/day menatetrenone | 17.5 mg/week risedronate |
| Koitaya et al. (39) | RCT | Japan | SC | 48 | 58.4 ± 3.8 | 7.3 ± 3.8 | No | 12 M | 1.5 mg/day MK-4 | placebo |
| Jiang et al. (14) | RCT | China | MC | 213 | 45–75 | >5 | Yes | 12 M | 45 mg/day menatetrenone + 500 mg/day Ca | 0.5 μg/day alfacalcidol + 500 mg/day Ca |
| Knapen et al. (15) | RCT | Netherlands | SC | 244 | 55–65 | 9 ± 6 | No | 36 M | 180μg/day MK-7 | placebo |
| Emaus et al. (21) | RCT | Norway | MC | 334 | 50–60 | 1-5 | No | 12 M | 360 mg/day MK-7 | placebo |
| Shiraki et al. (40) | RCT | Japan | SC | 121 | 68.6 ± 7.6 | 19.6 ± 9.3 | Yes | 6 M | 45 mg/day menatetrenone | 133.8 mg/day Ca |
| Inoue et al. (41) | RCT | Japan | MC | 4378 | >50 | 20.55 ± 9.89 | Yes | 48 M | 45 mg/day menatetrenone + Ca | Ca |
| Hirao et al. (27) | RCT | Japan | SC | 48 | 68.5 ± 2.1 | - | Yes | 12 M | 45 mg/day VK2 + 5 mg/day alendronate | 5 mg/day alendronate |
| Knapen et al. (42) | RCT | Netherlands | SC | 325 | 55–75 | >2 | No | 36 M | 45 mg/day MK-4 | placebo |
| Purwosunu et al. (43) | RCT | Indonesia | SC | 63 | 60–75 | >2 | Yes | 12 M | 45 mg/day menatetrenone + 1500 mg/day Ca | placebo + 1500 mg/day Ca |
| Ushiroyama et al. (44) | RCT | Japan | SC | 172 | 53.41 ± 5.90 | 2.50 ± 4.16 | Yes | 24 M | 45 mg/day MK-4 + 1μg/day VD3 | 1μg/day VD3 |
| Iwamoto et al. (45) | RCT | Japan | SC | 72 | 53-78 | >5 | Yes | 24 M | 45 mg/day MK-4 | 2 g/day Ca |
| Iwamoto et al. (46) | RCT | Japan | SC | 92 | 55-81 | >5 | Yes | 24 M | 45 mg/day menatetrenone + 0.75 μg/day VD3 | 0.75 μg/day VD3 |
| Iwamoto et al. (18) | RCT | Japan | SC | 72 | 53.82 ± 5.24 | 5.21 ± 4.18 | No | 12 M | 45 mg/day MK-4 | 1.0 g/day VD3 |
RCT, Randomized Controlled Trial; SC, single-center; MC, multiple-center; M, month; VK2, vitamin K2; VD3, vitamin D3; Ca, calcium.
Quality assessment of the included studies
Two reviewers (YL. HE, SX.LI) independently assessed the quality of each included randomized controlled trial using the Cochrane Collaboration's tool. Although all studies included in this meta-analysis were randomized controlled trials, some studies did not clearly describe how populations were grouped, resulting in a high risk of selection bias (15, 18, 27, 40, 44–46). There were also some studies that did not implement the double-blind method well, leading to a high risk of performance bias (26, 40, 41, 45). The results of bias risk assessment for the included RCTs are shown in Figure 2.
Figure 2.
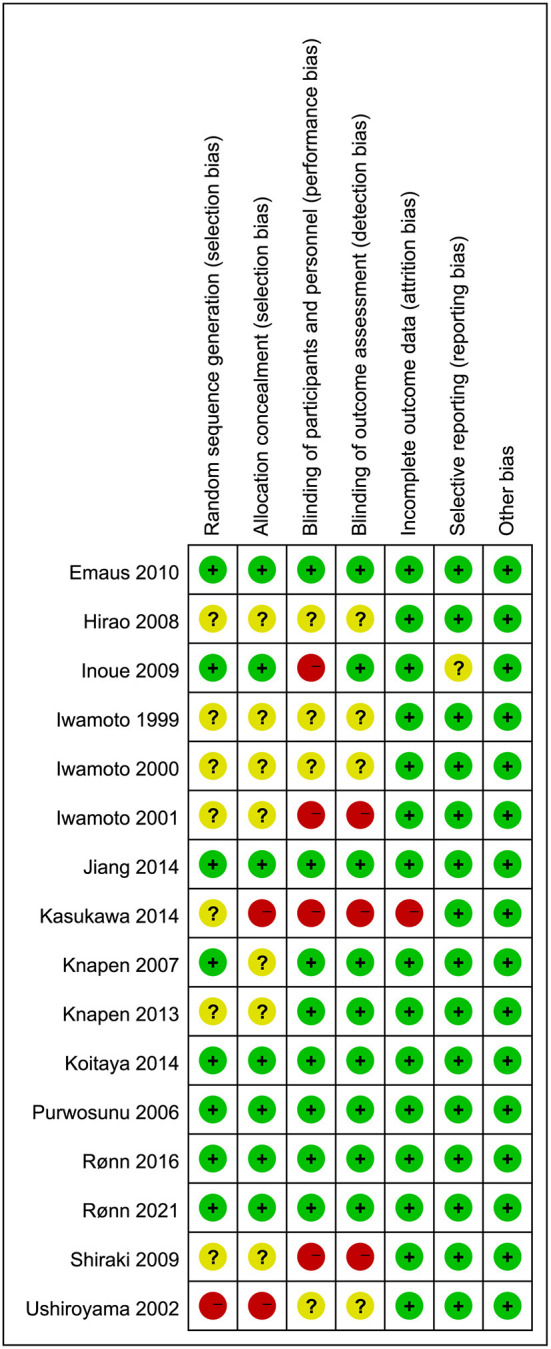
Risk of bias summary of included studies.
Primary outcomes
Pooled analysis for the changes in BMD
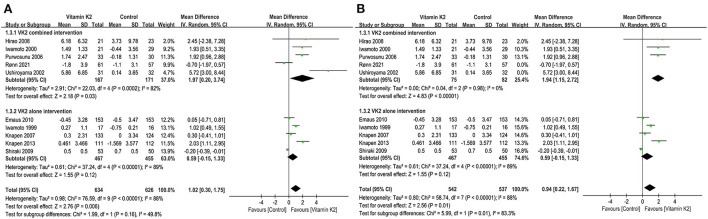
We first analyzed the change in BMD LS before and after treatment with VK2. A total of ten studies (9, 15, 18, 21, 27, 40, 42–44, 46) reported the changes in BMD LS, and the test for the overall effect of these ten studies showed that VK2 maintained and improved BMD LS (MD = 1.02, 95%CI 0.30 to 1.75, P=0.006) compared with the control group (Figure 3A). Subgroup analysis of VK2 combined intervention showed that the effect of VK2 on BMD LS was superior to that of the control group (MD = 1.97, 95% CI 0.20 to 3.74, P = 0.03) (Figure 3A). However, the subgroup analysis of VK2 alone intervention showed a similar effect of VK2 and control groups on change in BMD LS (P = 0.160) (Figure 3A). In the subgroup of VK2 combined intervention, heterogeneity between studies was eliminated after removing Rønn et al. (9) and Ushiroyama et al. (44) (Figure 3B). And we found that the Z value of the test for overall effect became larger (Z = 4.83), the P-value became smaller (P < 0.00001), and the 95% confidence interval was narrow down (1.15 to 2.72) (Figure 3B). These changes further suggest that VK2 combined intervention appears to be better at maintaining BMD LS.
Figure 3.
(A) Forest plot of the change in BMD LS. (B) Forest plot of the change in BMD LS after removed the studies by Rønn and Ushiroyama.
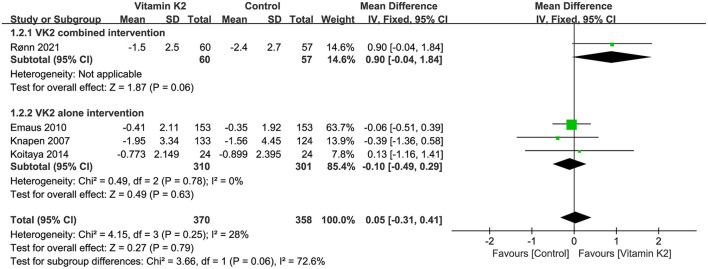
The changes in hip BMD were reported in four studies (9, 21, 39, 42), and the overall effect test showed no significant difference in hip BMD changes in the VK2 group compared with the control group (P = 0.79) (Figure 4).
Figure 4.
Forest plot of the change in hip BMD.
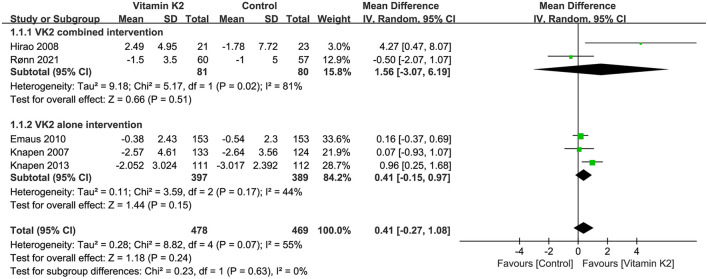
Five studies (9, 15, 21, 27, 42) reported changes in femoral neck BMD, and the overall effect test showed no significant difference in femoral neck BMD change between the VK2 and control groups (p = 0.24) (Figure 5).
Figure 5.
Forest plot of the change in femoral neck BMD.
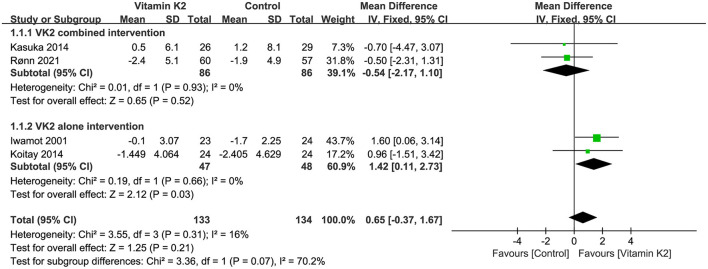
Four studies (9, 26, 39, 45) reported percentage changes in forearm BMD. Both the overall effect test and the subgroup analysis of VK2 combined intervention showed no significant difference in BMD change between the VK2 and control groups (P = 0.21, P = 0.52). However, the subgroup analysis of VK2 alone intervention showed that VK2 had a superior effect on forearm BMD than controls (MD = 1.42, 95%CI 0.11 to 2.73, P = 0.03) (Figure 6).
Figure 6.
Forest plot of change in forearm BMD.
Pooled analysis for fracture incidence
Six studies (14, 15, 21, 26, 41, 45) reported fracture incidence, and the overall effect test showed that VK2 did not reduce the incidence of fractures (RR = 0.56, 95% CI 0.28 to 1.11, P = 0.10) (Figure 7A). However, when the study by Inoue et al. (41) was removed, the subgroup analysis of VK2 combined intervention showed a significant difference in fracture incidence between the VK2 and control groups (RR = 0.25, 95% CI 0.07 to 0.87, P = 0.03, I2 = 0%) (Figure 7B). And the overall effect test also showed that VK2 was effective in reducing the incidence of fractures compared with the control group (RR = 0.38, 95% CI 0.20 to 0.76, P = 0.006, I2 = 0%) (Figure 7B).
Figure 7.
(A) Forest plot of the incidence of fractures. (B) Forest plot of the incidence of fractures after removed the study by Inoue.
Secondary outcomes
Pooled analysis for the change in serum uc-OC
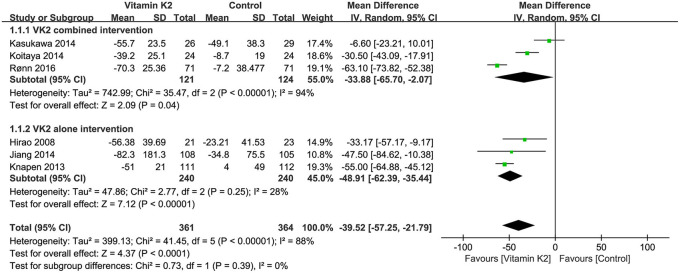
Six studies (14, 15, 26, 27, 38, 39) reported the percentage changes in serum uc-OC. The overall effect test showed a significant difference in serum uc-OC levels between the VK2 and control groups (MD = −39.52, 95%CI−57.25 to−21.79, P < 0.0001). Subgroup analyses of both VK2 combined and alone intervention showed that VK2 significantly reduced serum UC-OC levels compared with the control group (P < 0.05) (Figure 8).
Figure 8.
Forest plot of the change in serum uc-OC.
Pooled analysis for the change in serum cOC
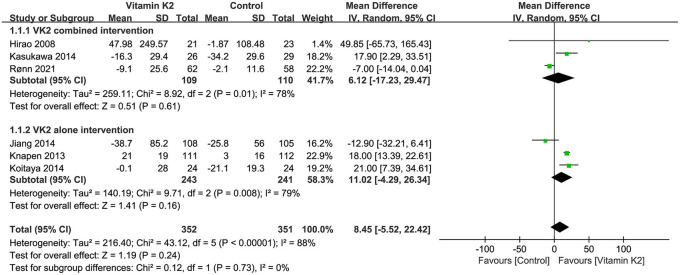
Six studies (9, 14, 15, 26, 27, 39) reported percentage changes in serum cOC that were not significantly different between the VK2 and control groups by overall effect test (MD = 8.45, 95% CI−5.52 to 22.42, p = 0.24) (Figure 9).
Figure 9.
Forest plot of the change in serum cOC.
Pooled analysis for the change in the ratio of serum uc-OC to cOC
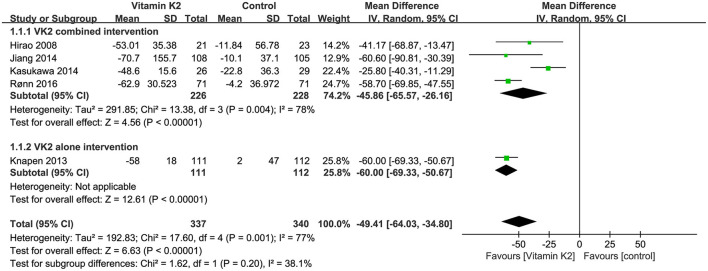
Five studies (14, 15, 26, 27, 38) reported the percentage changes in the ratio of serum uc-OC to cOC, and the overall effect test showed a significant difference between the VK2 and control groups (MD = −49.41, 95% CI 64.03 to−34.80, P < 0.00001). Similarly, the subgroup analyses of VK2 combined and alone intervention showed that VK2 significantly reduced the ratio of serum uc-OC to cOC (P < 0.00001) (Figure 10).
Figure 10.
Forest plot of the change in the ratio of serum uc-OC to cOC.
Pooled analysis for the incidence of adverse reactions
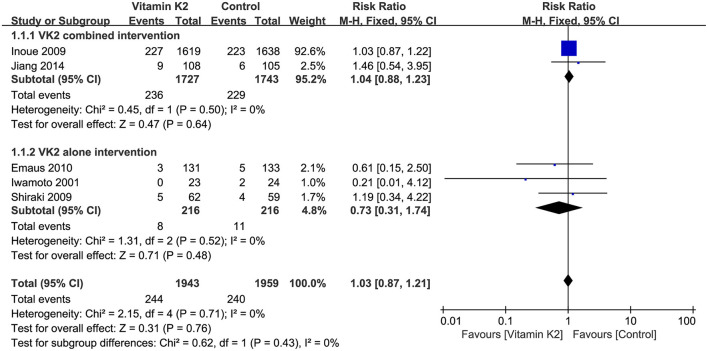
The incidence of adverse reactions was reported in five studies (14, 21, 40, 41, 45), and overall effect test showed no significant difference in the incidence of adverse reactions between the VK2 and control groups (RR = 1.03, 95% CI 0.87 to 1.21, P = 0.76) (Figure 11).
Figure 11.
Forest plot of the incidence of adverse reactions.
Publication bias and sensitive analysis
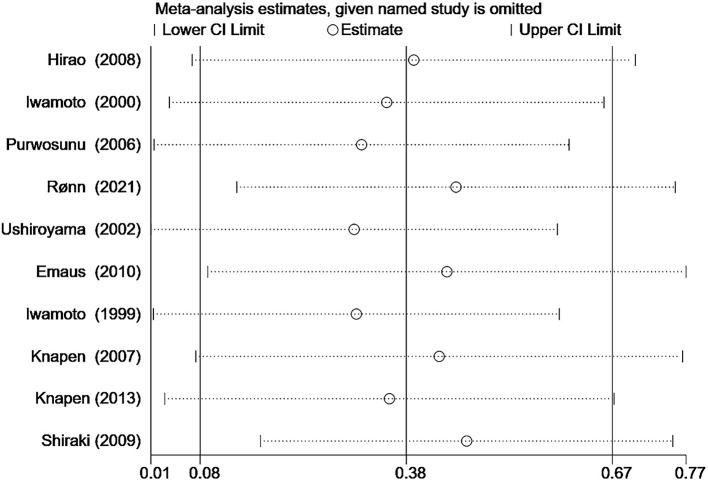
We imported the data from 10 studies (9, 15, 18, 21, 27, 40, 42–44, 46) that reported the percentage changes in BMD LS into STATA 16 software and then assessed publication bias by Egger's quantitative test. The results showed no publication bias for the ten studies (P = 0.134, 95%CI−1.19 to 7.41), and each study was roughly symmetrically distributed on both sides of the regression line (Figure 12). In addition, we also performed sensitivity analyses for the 10 studies using STATA 16 software to assess the stability of this meta-analysis model, and the results showed that when any of the 10 studies were removed, the dots representing statistical effect of the remaining studies were distributed around the vertical line of the overall statistical effect and within 95% confidence interval (Figure 13).
Figure 12.
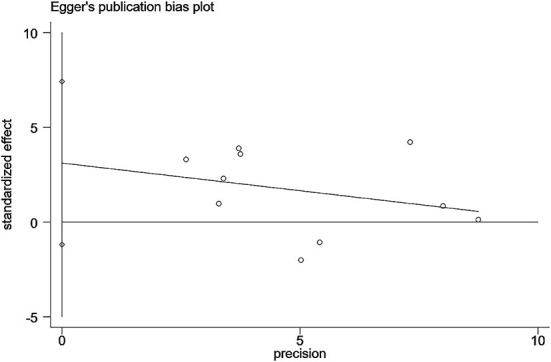
Publication bias test for the overall effect test of BMD LS change.
Figure 13.
Sensitive analysis for the overall effect test of BMD LS change.
Discussion
We searched for studies according to the PRISMA statement and screened studies according to the inclusion and exclusion criteria, and ultimately 16 RCTs were included in this meta-analysis. By pooled analysis, we found that VK2 maintained and improved the BMD LS, and the subgroup analysis of VK2 combined intervention yielded the same conclusion, but the subgroup analysis of VK2 intervention alone did not show the difference compared with the control group. In addition, the results of this meta-analysis showed no significant differences between the VK2 and control groups in terms of BMD changes in the hip, femoral neck and forearm. The overall effect test for fracture incidence showed that VK2 reduced fracture incidence after removing study with large heterogeneity (RR = 0.38,95% CI 0.20 to 0.76, P = 0.006, I2 = 0%) (41).
It is well known that the main aim of prevention and treatment of OP is to prevent fractures, so we took the BMD changes and fracture incidence as the primary outcome indicators in this meta-analysis. However, of all RCTs included in this meta-analysis, only 6 studies (14, 15, 21, 26, 41, 45) reported the incidence of fractures. Therefore, the effect of VK2 on fracture incidence cannot be assessed very accurately in this meta-analysis. The pooled analysis of the 6 studies showed that VK2 cannot reduce the fracture incidence, and subgroup analyses of VK2 combined and alone intervention also showed the similar results. However, when we performed sensitivity analyses on these six studies, we found that the study by Inoue et al. (41) was relatively heterogeneous in comparison with other studies. When the study by Inoue et al. was removed, the result of this meta-analysis showed that VK2 reduced the incidence of fractures. But in fact, the exclusion of the study by Inoue et al. may cause selection bias, because the sample size of this study was very large and the follow-up period was longer (48 months) than other studies. In the study by Inoue et al. there was no significant difference in the incidence of fractures between the VK2 and control groups. However, this study showed a significant reduction in the incidence of new vertebral fractures in patients treated with VK2 who had already had vertebral fractures, and it also showed that VK2 had a positive effect on the elderly population suffering from severe bone loss. Therefore, although the heterogeneity of this study is relatively large, it is undeniably a very valuable study. In view of this, we need to be cautious in excluding this study and also cautious in drawing conclusion that VK2 can reduce the incidence of fractures.
Osteocalcin (OC), also known as a gamma-carboxyl bone glaprotein (BGP), is a vitamin K-dependent protein that is synthesized and secreted by osteoblasts (47). OC has biological effects in regulating bone metabolism after OC is γ -carboxylated, but the process requires sufficient VK. Depending on the degree of carboxylation of osteocalcin, osteocalcin includes fully carboxylated osteocalcin (cOC) and undercarboxylated osteocalcin (uc-OC), while cOC can bind to hydroxyapatite and thus promote calcium deposition in bones. When serum VK is deficient, the carboxylation ability of osteocalcin will be weakened, serum uc-OC levels will be increased, and cOC levels will be decreased (48, 49). And low serum VK levels and high uc-OC levels are considered as risk factors for hip fracture in older women (50). In this meta-analysis, the overall effect test showed that VK2 significantly reduced serum uc-OC levels and the ratio of serum uc-OC to cOC, while subgroup analyses of the VK2 combined intervention and alone intervention also yielded similar conclusions. However, there was no significant difference in serum cOC levels between the VK2 group and the control group. Theoretically, when VK2 is maintained at a relatively high levels in serum, the γ-carboxylation of OC would be enhanced, thus contributing to the increase in serum cOC levels and the decrease in uc-OC levels. It should not be forgotten, however, that with increasing serum VK2 levels, more and more OC can become carboxylated and functional. And more of the functional OC can be bound to the bones rather than circulating in the blood, so serum cOC levels may not increase significantly.
Five studies (14, 21, 40, 41, 45) reported the incidence of adverse reactions in evaluating the safety of VK2 in the prevention and treatment of PMOP. The pooled analysis of the five studies showed that VK2 did not increase the incidence of adverse reactions compared to the controls (RR = 1.03, 95% CI 0.87 to 1.21, P = 0.76). It suggests to us that the use of VK2 for the prevention and treatment of PMOP is safe in the present situation.
In conclusion, we may conclude that VK2 is effective in the prevention and treatment of PMOP, because VK2 appears to maintain and improve the BMD LS. And VK2 may be particularly effective when used in combination with other preventive or therapeutic measures, such as vitamin D, calcium, or alendronate. The results of this meta-analysis gave us the greatest confidence that VK2 can significantly reduce serum levels of uc-OC. In the effect of VK2, more OC undergoes γ-carboxylation and are converted to cOC, which can promote bone mineralization and increase bone strength. Meanwhile this meta-analysis also suggests that VK2 may reduce the incidence of fractures, but this conclusion needs to be further verified by more multi-center, large sample size and prospective studies.
Although the 16 studies included in this meta-analysis were all randomized controlled trials, the following problems still remained in our meta-analysis: (1) the quality of the included studies was uneven, and there were various biases (selection bias, performance bias, detection bias, etc.); (2) the sample sizes of some RCTs were too small, which might lead to the conclusions that were accidental; (3) the follow-up time of the included studies was different, and we did not perform more detailed groupings; (4) the majority of the included studies were conducted in Japan, and these studies that concluded that VK2 had a positive effect on prevention and treatment of PMOP were also primarily conducted in Japan. Therefore, it remains to be discussed whether the positive effect of VK2 on prevention and treatment of PMOP can be extended to other countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
M-lM, Z-jM, and HS carried out the study design, study selection, data extraction, statistical analysis, and drafted the manuscript. BY, B-jR, and W-dZ participated in the study selection, data extraction, and drafted the manuscript. Y-lH and S-xL evaluated the quality of included studies. HD and Y-xW participated in the discussion for any discrepancies and supervised the study. All authors read and approved the final manuscript.
Funding
This study was supported by grants from TCM Science and Technology Development Program Project of Jiangsu Province (ZT202117) and Yangzhou Key Laboratory Cultivation Project (YZ2021143).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge all colleagues who participated in this study.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.979649/full#supplementary-material
References
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. 10.1016/S0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black DM, Rosen CJ. Clinical practice. postmenopausal osteoporosis. N Engl J Med. (2016) 374:254–62. 10.1056/NEJMcp1513724 [DOI] [PubMed] [Google Scholar]
- 3.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. (2019) 393:364–76. 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 4.Willems BA, Vermeer C, Reutelingsperger CP, Schurgers LJ. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. (2014) 58:1620–35. 10.1002/mnfr.201300743 [DOI] [PubMed] [Google Scholar]
- 5.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. (2009) 29:89–110. 10.1146/annurev-nutr-080508-141217 [DOI] [PubMed] [Google Scholar]
- 6.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. (2009) 297:C1358–1367. 10.1152/ajpcell.00216.2009 [DOI] [PubMed] [Google Scholar]
- 7.Nakao M, Nishiuchi Y, Nakata M, Kimura T, Sakakibara S. Synthesis of human osteocalcins: gamma-carboxyglutamic acid at position 17 is essential for a calcium-dependent conformational transition. Pept Res. (1994) 7:171–4. [PubMed] [Google Scholar]
- 8.Bügel S. Vitamin K and bone health in adult humans. Vitam Horm. (2008) 78:393–416. 10.1016/S0083-6729(07)00016-7 [DOI] [PubMed] [Google Scholar]
- 9.Rønn SH, Harsløf T, Oei L, Pedersen SB, Langdahl BL. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int. (2021) 32:185–91. 10.1007/s00198-020-05638-z [DOI] [PubMed] [Google Scholar]
- 10.Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. (2007) 109:3279–83. 10.1182/blood-2006-08-040709 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Liu Z, Duan L, Ji Y, Yang S, Zhang Y, et al. Effect of low-dose vitamin k2 supplementation on bone mineral density in middle-aged and elderly Chinese: a randomized controlled study. Calcif Tissue Int. (2020) 106:476–85. 10.1007/s00223-020-00669-4 [DOI] [PubMed] [Google Scholar]
- 12.Shearer MJ, Bach A, Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr. (1996) 126:1181s−6s. 10.1093/jn/126.suppl_4.1181S [DOI] [PubMed] [Google Scholar]
- 13.Wu WJ, Gao H, Jin JS, Ahn BY. A comparatively study of menaquinone-7 isolated from Cheonggukjang with vitamin K(1) and menaquinone-4 on osteoblastic cells differentiation and mineralization. Food Chem Toxicol. (2019) 131:110540. 10.1016/j.fct.2019.05.048 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Zhang ZL, Zhang ZL, Zhu HM, Wu YY, Cheng Q, et al. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: a multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin Interv Aging. (2014) 9:121–7. 10.2147/CIA.S54107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. (2013) 24:2499–507. 10.1007/s00198-013-2325-6 [DOI] [PubMed] [Google Scholar]
- 16.Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, et al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition. (2001) 17:315–21. 10.1016/S0899-9007(00)00554-2 [DOI] [PubMed] [Google Scholar]
- 17.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. (2000) 15:515–21. 10.1359/jbmr.2000.15.3.515 [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, et al. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas. (1999) 31:161–4. 10.1016/S0378-5122(98)00114-5 [DOI] [PubMed] [Google Scholar]
- 19.Tasci AG, Bilgili H, Altunay H, Gecit MR, Keskin D. Prospective evaluation of Vitamin K2, Raloxifene and their co-administration in osteoporotic rats. Eur J Pharm Sci. (2011) 43:270–7. 10.1016/j.ejps.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 20.Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res. (2009) 24:983–91. 10.1359/jbmr.081254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. (2010) 21:1731–40. 10.1007/s00198-009-1126-4 [DOI] [PubMed] [Google Scholar]
- 22.Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe . BMJ. (2010) 340:b5463. 10.1136/bmj.b5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. (2012) 367:40–9. 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 24.Koshihara Y, Hoshi K, Ishibashi H, Shiraki M. Vitamin K2 promotes 1alpha,25(OH)2 vitamin D3-induced mineralization in human periosteal osteoblasts. Calcif Tissue Int. (1996) 59:466–73. 10.1007/BF00369212 [DOI] [PubMed] [Google Scholar]
- 25.Yasui T, Miyatani Y, Tomita J, Yamada M, Uemura H, Miura M, et al. Effect of vitamin K2 treatment on carboxylation of osteocalcin in early postmenopausal women. Gynecol Endocrinol. (2006) 22:455–9. 10.1080/09513590600900402 [DOI] [PubMed] [Google Scholar]
- 26.Kasukawa Y, Miyakoshi N, Ebina T, Aizawa T, Hongo M, Nozaka K, et al. Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated osteocalcin and osteocalcin levels in postmenopausal osteoporosis. J Bone Miner Metab. (2014) 32:290–7. 10.1007/s00774-013-0490-5 [DOI] [PubMed] [Google Scholar]
- 27.Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab. (2008) 26:260–4. 10.1007/s00774-007-0823-3 [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. (2012) 30:60–8. 10.1007/s00774-011-0287-3 [DOI] [PubMed] [Google Scholar]
- 29.Kuang X, Liu C, Guo X, Li K, Deng Q, Li D. The combination effect of vitamin K and vitamin D on human bone quality: a meta-analysis of randomized controlled trials. Food Funct. (2020) 11:3280–97. 10.1039/C9FO03063H [DOI] [PubMed] [Google Scholar]
- 30.Su S, He N, Men P, Song C, Zhai S. The efficacy and safety of menatetrenone in the management of osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. (2019) 30:1175–86. 10.1007/s00198-019-04853-7 [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Ji J, Li D, Meng J, Yu B. The combined effect of vitamin K and calcium on bone mineral density in humans: a meta-analysis of randomized controlled trials. J Orthop Surg Res. (2021) 16:592. 10.1186/s13018-021-02728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZB, Wan SL, Lu YJ, Ning L, Liu C, Fan SW. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos Int. (2015) 26:1175–86. 10.1007/s00198-014-2989-6 [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto J, Matsumoto H, Takeda T. Efficacy of menatetrenone (vitamin K2) against non-vertebral and hip fractures in patients with neurological diseases: meta-analysis of three randomized, controlled trials. Clin Drug Investig. (2009) 29:471–9. 10.2165/00044011-200929070-00005 [DOI] [PubMed] [Google Scholar]
- 34.Stevenson M, Lloyd-Jones M, Papaioannou D. Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health Technol Assess. (2009) 13:iii-xi1–134. 10.3310/hta13450 [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 36.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rønn SH, Harsløf T, Pedersen SB, Langdahl BL. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol. (2016) 175:541–9. 10.1530/EJE-16-0498 [DOI] [PubMed] [Google Scholar]
- 39.Koitaya N, Sekiguchi M, Tousen Y, Nishide Y, Morita A, Yamauchi J, et al. Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J Bone Miner Metab. (2014) 32:142–50. 10.1007/s00774-013-0472-7 [DOI] [PubMed] [Google Scholar]
- 40.Shiraki M, Itabashi A. Short-term menatetrenone therapy increases gamma-carboxylation of osteocalcin with a moderate increase of bone turnover in postmenopausal osteoporosis: a randomized prospective study. J Bone Miner Metab. (2009) 27:333–40. 10.1007/s00774-008-0034-6 [DOI] [PubMed] [Google Scholar]
- 41.Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Nakamura T, et al. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab. (2009) 27:66–75. 10.1007/s00774-008-0008-8 [DOI] [PubMed] [Google Scholar]
- 42.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. (2007) 18:963–72. 10.1007/s00198-007-0337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purwosunu Y. Muharram, Rachman IA, Reksoprodjo S, Sekizawa A. Vitamin K2 treatment for postmenopausal osteoporosis in Indonesia. J Obstet Gynaecol Res. (2006) 32:230–4. 10.1111/j.1447-0756.2006.00386.x [DOI] [PubMed] [Google Scholar]
- 44.Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas. (2002) 41:211–21. 10.1016/S0378-5122(01)00275-4 [DOI] [PubMed] [Google Scholar]
- 45.Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci. (2001) 6:487–92. 10.1007/s007760100002 [DOI] [PubMed] [Google Scholar]
- 46.Iwamoto J, Takeda T, Ichimura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci. (2000) 5:546–51. 10.1007/s007760070003 [DOI] [PubMed] [Google Scholar]
- 47.Kâ K, Rousseau MC, Tran SD, Kaartinen MT, Myneni VD, Henderson M, et al. Circulating undercarboxylated osteocalcin and gingival crevicular fluid tumour necrosis factor-α in children. J Clin Periodontol. (2014) 41:467–72. 10.1111/jcpe.12232 [DOI] [PubMed] [Google Scholar]
- 48.Jagannath VA, Thaker V, Chang AB, Price AI. Vitamin K supplementation for cystic fibrosis. Cochrane Database Syst Rev. (2020) 6:Cd008482. 10.1002/14651858.CD008482.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. (1993) 91:1769–74. 10.1172/JCI116387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. (2010) 29:761–5. 10.1016/j.clnu.2010.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.