This prespecified analysis of the SURVAVI trial compares the clinical outcomes, health status, and bioprosthetic valve performance after self-expanding transcatheter aortic valve replacement or surgery at 5 years of follow-up.
Key Points
Question
What are the 5-year outcomes in randomized transcatheter aortic valve replacement (TAVR) vs surgery trials in intermediate-risk patients with severe aortic valve stenosis?
Findings
In this prespecified 5-year analysis of a randomized clinical trial, older patients with severe aortic stenosis at intermediate operative risk had no difference in the primary composite outcome of death or disabling stroke between TAVR and surgery at 5 years. Overall, there were more valve-related reinterventions in the TAVR group at 5 years, but after 2 years, there were no differences in rehospitalizations or reinterventions between groups.
Meaning
Among intermediate-risk patients with symptomatic severe aortic stenosis, clinical outcomes at 5 years were similar and bioprosthetic valve performance remained stable after TAVR and surgery.
Abstract
Importance
In patients with severe aortic valve stenosis at intermediate surgical risk, transcatheter aortic valve replacement (TAVR) with a self-expanding supra-annular valve was noninferior to surgery for all-cause mortality or disabling stroke at 2 years. Comparisons of longer-term clinical and hemodynamic outcomes in these patients are limited.
Objective
To report prespecified secondary 5-year outcomes from the Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement (SURTAVI) randomized clinical trial.
Design, Setting, and Participants
SURTAVI is a prospective randomized, unblinded clinical trial. Randomization was stratified by investigational site and need for revascularization determined by the local heart teams. Patients with severe aortic valve stenosis deemed to be at intermediate risk of 30-day surgical mortality were enrolled at 87 centers from June 19, 2012, to June 30, 2016, in Europe and North America. Analysis took place between August and October 2021.
Intervention
Patients were randomized to TAVR with a self-expanding, supra-annular transcatheter or a surgical bioprosthesis.
Main Outcomes and Measures
The prespecified secondary end points of death or disabling stroke and other adverse events and hemodynamic findings at 5 years. An independent clinical event committee adjudicated all serious adverse events and an independent echocardiographic core laboratory evaluated all echocardiograms at 5 years.
Results
A total of 1660 individuals underwent an attempted TAVR (n = 864) or surgical (n = 796) procedure. The mean (SD) age was 79.8 (6.2) years, 724 (43.6%) were female, and the mean (SD) Society of Thoracic Surgery Predicted Risk of Mortality score was 4.5% (1.6%). At 5 years, the rates of death or disabling stroke were similar (TAVR, 31.3% vs surgery, 30.8%; hazard ratio, 1.02 [95% CI, 0.85-1.22]; P = .85). Transprosthetic gradients remained lower (mean [SD], 8.6 [5.5] mm Hg vs 11.2 [6.0] mm Hg; P < .001) and aortic valve areas were higher (mean [SD], 2.2 [0.7] cm2 vs 1.8 [0.6] cm2; P < .001) with TAVR vs surgery. More patients had moderate/severe paravalvular leak with TAVR than surgery (11 [3.0%] vs 2 [0.7%]; risk difference, 2.37% [95% CI, 0.17%- 4.85%]; P = .05). New pacemaker implantation rates were higher for TAVR than surgery at 5 years (289 [39.1%] vs 94 [15.1%]; hazard ratio, 3.30 [95% CI, 2.61-4.17]; log-rank P < .001), as were valve reintervention rates (27 [3.5%] vs 11 [1.9%]; hazard ratio, 2.21 [95% CI, 1.10-4.45]; log-rank P = .02), although between 2 and 5 years only 6 patients who underwent TAVR and 7 who underwent surgery required a reintervention.
Conclusions and Relevance
Among intermediate-risk patients with symptomatic severe aortic stenosis, major clinical outcomes at 5 years were similar for TAVR and surgery. TAVR was associated with superior hemodynamic valve performance but also with more paravalvular leak and valve reinterventions.
Introduction
Guidelines recommend transfemoral transcatheter or surgical aortic valve replacement for elderly patients with symptomatic severe aortic stenosis.1,2 Pivotal randomized clinical trials in elderly patients across the operative risk spectrum have reported at least equipoise in terms of outcomes to 2 years for these 2 strategies.3,4,5,6,7 Longer follow-up to 5 and 10 years is important to detect differences in clinical outcomes and bioprosthetic valve performance for proper counseling of patients with a longer life expectancy. Initial high-risk trials enrolled patients with a reserved long-term prognosis and therefore provided limited long-term insights.3,4 A randomized clinical trial evaluating patients at intermediate operative risk reported no significant difference in the incidence of death or disabling stroke at 5 years following transcatheter aortic valve replacement (TAVR) with a second-generation balloon expandable transcatheter heart valve or surgery.8 However, the frequency of heart failure or valve-related hospitalizations and redo valve interventions was higher after TAVR than after surgery.8,9 A small randomized clinical trial reported similar clinical outcomes with self-expanding TAVR or surgery in 280 low-risk patients but less valve degeneration after TAVR at 8 years of follow-up.10 The Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI; NCT0158691) randomized clinical trial enrolled patients with severe aortic valve stenosis at intermediate operative risk and demonstrated clinical noninferiority between self-expanding TAVR and surgery at 2 years.7 This report from the SURTAVI trial compares the clinical outcomes, health status, and bioprosthetic valve performance after self-expanding TAVR or surgery at 5 years of follow-up.
Methods
Study Details
The SURTAVI trial details have been previously reported.7 The trial protocol and statistical analysis plan are available in Supplement 1. The SURTAVI trial followed the principles of the Declaration of Helsinki11 and good clinical practice. Each institutional review board or ethics committee approved the trial protocol, and all patients provided informed signed consent.
In brief, patients with severe symptomatic aortic stenosis deemed to be at intermediate operative risk by a multidisciplinary screening committee based on a Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) score and other coexisting comorbidities were randomized 1:1 to TAVR with a supra-annular self-expanding bioprosthesis or surgery from June 19, 2012, to June 30, 2016, at 87 centers in Canada, Europe, and the United States. Complete data on race and ethnicity were not collected. Randomization was stratified by clinical site and the need for revascularization determined by the multidisciplinary heart team. The investigators and research personnel participating and trial committees are provided in eTables 1 and 2 in Supplement 2.
Detailed inclusion and exclusion criteria are provided in eTable 3 in Supplement 2. Patients in the TAVR group underwent implant of a first-generation (CoreValve; Medtronic) or second-generation (Evolut R valve; Medtronic) TAVR device and surgical valve type was per operator’s choice, although mechanical valves were not allowed. Staged or concomitant percutaneous coronary intervention or coronary artery bypass grafting was performed when indicated.
Patient assessments were performed at baseline, hospital discharge, 30 days, 12 months, 18 months, and annually through years postprocedure. An independent clinical events committee adjudicated all adverse clinical events and an independent core laboratory evaluated available echocardiograms at baseline, discharge, 6 months, 12 months, 2 years, and 5 years.12 Follow-up is planned to 10 years.
Clinical End Points
The primary end point for this analysis was the composite of all-cause mortality or disabling stroke at 5 years. Prespecified secondary end points included all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, aortic valve–related reintervention and rehospitalizations, prosthetic valve endocarditis and clinical thrombosis, conduction disturbances requiring permanent pacemaker implantation, and echocardiographic assessment of valve performance (effective orifice area, mean gradient, and valve regurgitation) at 5 years. Detailed definitions are shown in eTable 4 in Supplement 2. Additional prespecified secondary end points included health status measured by the Kansas City Cardiomyopathy Questionnaire overall summary score and the New York Heart Association functional class. Clinical safety end points and prosthesis-patient mismatch (PPM) were defined per Valve Academic Research Consortium–2 definitions.13
Statistical Analysis
The primary analysis cohort is the modified intention-to-treat population (eTable 4 in Supplement 2) of patients who underwent an attempted TAVR or surgery. Echocardiographic outcomes are reported for the cohort of patients who underwent implantation. Categorical variables are reported as counts and frequencies and compared using the χ2 or Fisher exact test, where appropriate. Continuous variables are presented as mean (SD) and compared using the t test. For ordinal data, the Cochran-Mantel-Haenszel test was used. Clinical events are reported as Kaplan-Meier estimates and compared using the log-rank test. The mean (SD) of the change in Kansas City Cardiomyopathy Questionnaire score from baseline to each time point are compared using the t test. Post-hoc analyses included landmark analysis of the key clinical outcomes at 2 to 5 years and subgroup analysis performed using Cox proportional hazards. Kaplan-Meier estimates of all-cause mortality were compared for (1) patients with a pacemaker at baseline vs those with and those without a new permanent pacemaker implanted within 30 days of the index procedure, (2) patients with none or trace vs mild vs moderate or severe paravalvular leak (PVL) at discharge, and (3) patients with vs without PPM at discharge. No adjustments were made for multiple comparisons. All testing used a 2-sided α level of .05. All statistical analyses were performed using SAS software version 9.4 (SAS Institute). Analysis took place between August and October 2021.
Results
Study Population, Procedures, and Follow-up
A total of 1746 patients were randomized and 1660 (modified intention-to-treat cohort) underwent an attempted TAVR (n = 864) or surgical (n = 796) procedure (eFigure 1 in Supplement 2). The mean (SD) age was 79.8 (6.2) years, 724 (43.6%) were female, and the mean (SD) STS-PROM score was 4.5% (1.6%) (Table 1). In the TAVR cohort, an iliofemoral approach was applied in 808 individuals (93.6%). The first-generation bioprothesis was implanted in 724 patients (84%) and the second-generation device in 139 patients (16%). General anesthesia during TAVR was used in 653 patients (75.7%). Percutaneous coronary intervention was performed in 126 patients (13.1%) in the TAVR group (either staged [76 patients] or concomitantly [50 patients]). Concomitant coronary artery bypass grafting was performed in 176 patients (22.1%) in the surgery group. Clinical 5-year follow-up was available for 503 patients who underwent TAVR (93.7%) and 426 patients in the surgery group (95.5%). Echocardiographic 5-year follow-up was available for 390 patients undergoing TAVR (72.6%) and 312 undergoing surgery (70.0%). The baseline characteristics of the patients who were alive but without 5-year follow-up data are presented in eTables 5, 6, and 7 in Supplement 2. Compared with patients with follow-up data, these patients were older, had a higher STS-PROM score, and were more likely to be frail.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| TAVR (n = 864) | Surgery (n = 796) | |
| Age, mean (SD), y | 79.9 (6.2) | 79.7 (6.1) |
| Female | 366 (42.4) | 358 (45.0) |
| Male | 498 (57.6) | 438 (55.0) |
| STS-PROM score, mean (SD), %a | 4.4 (1.5) | 4.5 (1.6) |
| Body surface area, mean (SD), m2 | 1.9 (0.2) | 1.9 (0.2) |
| New York Heart Association class | ||
| I | 0 | 0 |
| II | 344 (39.8) | 333 (41.8) |
| III | 472 (54.6) | 411 (51.6) |
| IV | 48 (5.6) | 52 (6.5) |
| Coronary artery disease | 541 (62.6) | 511 (64.2) |
| Prior myocardial infarction | 125 (14.5) | 111 (13.9) |
| Prior coronary artery bypass grafting | 136 (15.7) | 137 (17.2) |
| Prior percutaneous coronary intervention | 184 (21.3) | 169 (21.2) |
| Cerebrovascular | 151 (17.5) | 130 (16.3) |
| Peripheral vascular disease | 266 (30.8) | 238 (29.9) |
| Diabetes | 296 (34.3) | 277 (34.8) |
| Chronic lung disease/COPD | 305 (35.4) | 267 (33.5) |
| Serum creatinine >2.0 mg/dL | 14 (1.6) | 17 (2.1) |
| Preexisting pacemaker | 84 (9.7) | 72 (9.0) |
| Prior atrial fibrillation/flutter | 243 (28.1) | 211 (26.5) |
| Falls in past 6 mo | 102 (11.8) | 102 (12.7) |
| 5-m Gait speed >6 s | 428 (51.8) | 403 (52.9) |
Abbreviations: COPD, chronic obstructive pulmonary disease; STS-PROM, Society of Thoracic Surgery Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
SI conversion factor: To convert creatinine to µmol/L, multiply by 88.4.
STS-PROM provides an estimate of the risk of death at 30 days among patients undergoing surgical aortic valve replacement based on several demographic and procedural variables.
Clinical Outcome at 5 Years
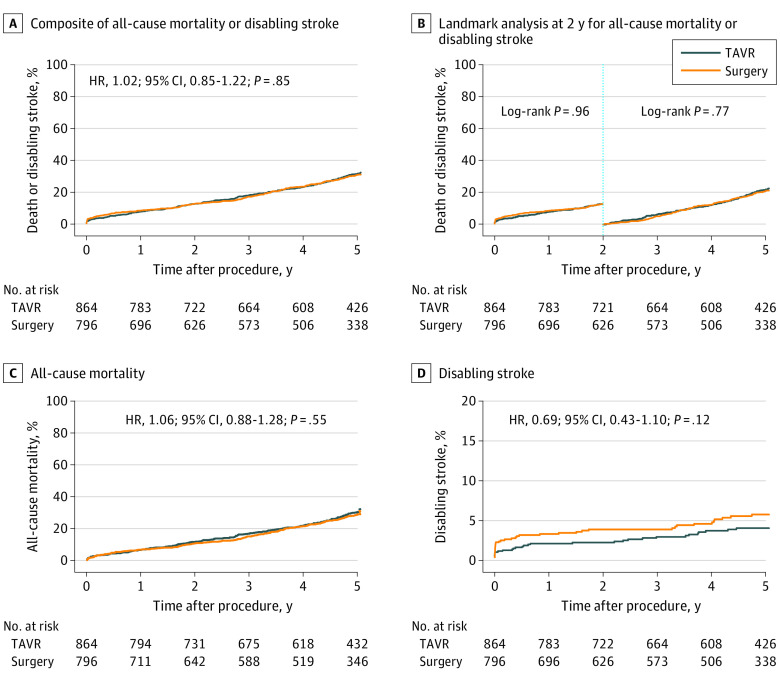
Clinical outcomes are shown in Table 2 and Figure 1. The primary composite end point of all-cause mortality or disabling stroke occurred in 255 patients undergoing TAVR (31.3%) and 217 undergoing surgery (30.8%) (hazard ratio [HR], 1.02 [95% CI, 0.85-1.22]; P = .85). In the TAVR cohort, 243 patients (30.0%) died compared with 200 (28.7%) in the surgery cohort (HR, 1.06 [95% CI, 0.88-1.28]; P = .55) and 31 patients undergoing TAVR (4.1%) vs 40 (5.8%) undergoing surgery experienced a disabling stroke (HR, 0.69 [95% CI, 0.43-1.10]; P = .12). There was no heterogeneity of treatment effect on mortality for all tested subgroups (eFigure 2 in Supplement 2). Among patients who were stratified to the need for revascularization prior to randomization, concomitant coronary artery bypass grafting was performed in 138 of 163 patients (84.7%) in the surgery cohort and concomitant or staged percutaneous coronary intervention was performed in 126 of 169 patients (74.6%) in the TAVR cohort. Baseline characteristics by stratification to need for revascularization are shown in eTable 8 in Supplement 2. Overall, there was no difference in the rates of death or disabling stroke at 5 years among patients (TAVR and surgery) who were stratified by need for revascularization vs patients who did not need revascularization (106 [34.3%] vs 366 [30.3%]; HR, 1.19 [95% CI, 0.96-1.48]; P = .11). There was also no difference between the patients in the TAVR vs surgery groups who were stratified by the need for revascularization (61 [37.5%] vs 45 [30.6%]; HR, 1.19 [95% CI, 0.81-1.76]; P = .37) or in those who did not need revascularization (194 [29.8%] vs 172 [30.9%]; HR, 0.97 [95% CI, 0.79-1.19]; P = .79).
Table 2. Clinical Outcomes at 2 and 5 Years for Patients in the Modified Intention-to-Treat Population.
| Outcome | No. (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 y | 5 y | Events between 2 and 5 yb | |||||||
| TAVR | Surgery | P value | TAVR | Surgery | P value | TAVR | Surgery | P value | |
| All-cause mortality or disabling stroke | 108 (12.7) | 97 (12.7) | .96 | 255 (31.3) | 217 (30.8) | .85 | 147 (21.3) | 120 (20.7) | .77 |
| All-cause mortality | 98 (11.5) | 80 (10.5) | .53 | 243 (30.0) | 200 (28.7) | .55 | 145 (20.9) | 120 (20.3) | .79 |
| Cardiovascular | 65 (7.8) | 54 (7.1) | .66 | 136 (17.8) | 116 (17.4) | .84 | 71 (10.9) | 62 (11.1) | .89 |
| Noncardiovascular | 33 (4.1) | 26 (3.7) | .64 | 107 (14.8) | 84 (13.7) | .50 | 74 (11.2) | 58 (10.4) | .61 |
| Aortic valve reintervention | 21 (2.5) | 4. (0.5) | .002 | 27 (3.5) | 11 (1.9) | .02 | 6 (1.0) | 7 (1.3) | .60 |
| Stroke or transient ischemic attack | 79 (9.5) | 85 (11.2) | .26 | 129 (17.2) | 123 (18.0) | .53 | 50 (8.5) | 38 (7.6) | .64 |
| Strokec | 50 (6.0) | 65 (8.5) | .05 | 86 (11.6) | 93 (13.6) | .16 | 36 (6.0) | 28 (5.5) | .76 |
| Disabling | 19 (2.3) | 30 (3.9) | .05 | 31 (4.1) | 40 (5.8) | .11 | 12 (1.9) | 10 (2.0) | .97 |
| Nondisabling | 32 (3.9) | 35 (4.6) | .44 | 60 (8.3) | 54 (8.1) | .92 | 28 (4.6) | 19 (3.6) | .44 |
| Transient ischemia attack | 33 (4.0) | 23 (3.2) | .32 | 48 (6.3) | 36 (5.4) | .42 | 15 (2.4) | 13 (2.3) | .99 |
| Myocardial infarction | 22 (2.7) | 16 (2.1) | .51 | 45 (6.2) | 30 (4.7) | .23 | 23 (3.7) | 14 (2.6) | .30 |
| Permanent pacemaker implantationd | 262 (30.9) | 75 (9.8) | <.001 | 293 (35.8) | 101 (14.6) | <.001 | 31 (7.1) | 26 (5.3) | .27 |
| Permanent pacemaker implantatione | 259 (33.9) | 69 (10.0) | <.001 | 289 (39.1) | 94 (15.1) | <.001 | 30 (7.8) | 25 (5.7) | .20 |
| Valve endocarditis | 3 (0.4) | 6 (0.8) | .25 | 7 (1.0) | 12 (1.8) | .15 | 4 (0.6) | 6 (1.0) | .39 |
| Clinical valve thrombosis | 3 (0.4) | 0 (0.0) | .10 | 4 (0.5) | 2 (0.4) | .51 | 1 (0.2) | 2 (0.4) | .47 |
| Aortic valve– and heart failure–related rehospitalization | 105 (12.8) | 71 (9.5) | .06 | 180 (23.9) | 136 (20.8) | .13 | 75 (12.7) | 65 (12.5) | .89 |
Data presented as the number of patients with an event (Kaplan-Meier rates as percentage).
Landmark analysis.
In 1 patient, the type of stroke was undetermined.
Patients with a pacemaker or implanted cardioverter-defibrillator at baseline are included.
Patients with a pacemaker or implanted cardioverter-defibrillator at baseline are excluded.
Figure 1. Kaplan-Meier Time to Event Analyses.
Cox proportional hazard ratio (HR) and 95% CIs are reported. TAVR indicates transcatheter aortic valve replacement.
Need for new permanent pacemaker (eFigure 3 in Supplement 2) and the occurrence of more than trace PVL (eFigure 4 in Supplement 2) after TAVR did not affect mortality rates at 5 years. Patients with a baseline pacemaker had a numerically higher 5-year mortality rate than patients with or without a new pacemaker implanted within 30 days post-TAVR. Hospitalization for heart failure– or valve-related reasons was similar between groups (TAVR vs surgery: 180 [23.9%] vs 136 [20.8%]; HR, 1.19 [95% CI, 0.95-1.49]; P = .13). The incidence of clinical valve thrombosis and endocarditis out to 5 years was equally rare for TAVR and surgery. At 5-year follow-up, more patients required a valve reintervention in the TAVR cohort compared with the surgery cohort (27 [3.5%] vs 11 [1.9%]; HR, 2.21 [95% CI, 1.10-4.45]; log-rank P = .02). This difference was driven by more reinterventions after TAVR than surgery in the first 2 years after the index procedure (21 [2.5%] vs 4 [0.5%]; HR, 4.81 [95% CI, 1.65-14.0]; log-rank P = .002) and driven mainly by percutaneous reintervention in the TAVR arm.7 After 2 years, 6 patients who underwent TAVR and 7 who underwent surgery needed valve reintervention including a similar proportion of surgical and transcatheter techniques. Details about need for valve reinterventions between 2 and 5 years are shown in eTable 9 in Supplement 2.
Bioprosthetic Valve Performance
TAVR resulted in significantly larger effective orifice areas and lower mean gradients than surgery at all time points (all P < .001) (Figure 2). Total aortic regurgitation is shown in Figure 2B; PVL is shown in eFigure 5 in Supplement 2. Mild PVL was present in 98 patients in the TAVR cohort (27.1%) and 8 (2.7%) in the surgery cohort. More than mild PVL was noted in 11 patients undergoing TAVR (3.0%) and 2 (0.7%) undergoing surgery (risk difference, 2.37% [95% CI, 0.17%-4.85%]; P = .05). Severe PPM per Valve Academic Research Consortium–213 occurred in 12 patients in the TAVR cohort (3.8%) and 36 (14.0%) in the surgery group at 5 years (risk difference, −10.26% [95% CI, −14.98% to −5.53%]; P < .001). Severe PPM at discharge did not affect 5-year mortality after TAVR or surgery (eFigures 6 and 7 in Supplement 2).
Figure 2. Hemodynamics.
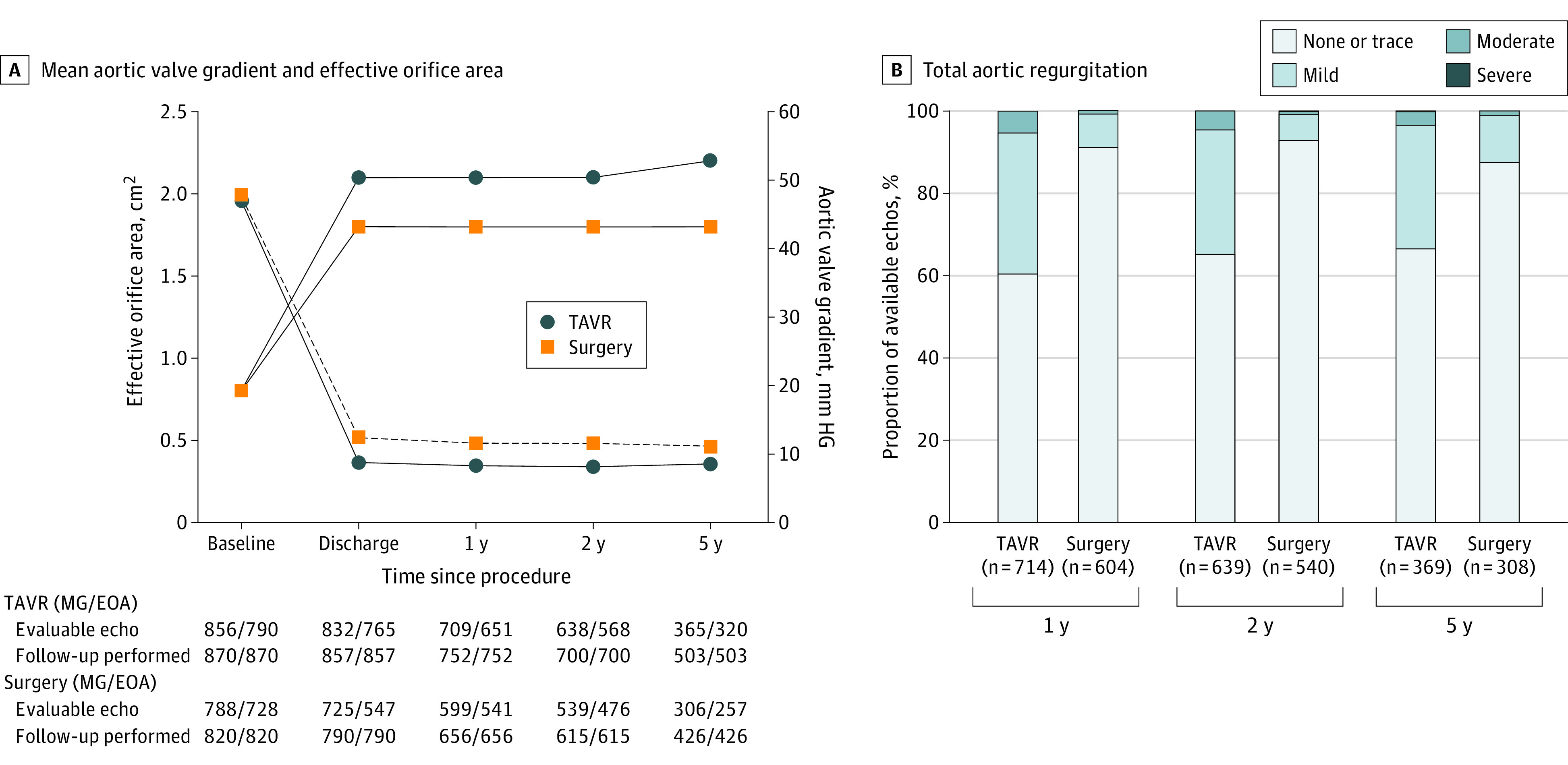
Patients in the transcatheter aortic valve replacement (TAVR) group had significantly larger effective orifice area and significantly lower mean gradient than the patients in the surgery group at all time points (all P < .001). ECHO indicates echocardiogram; MG/EAO, mean aortic valve gradient/effective orifice area.
Health Status
Among patients who survived to 5 years, the functional improvement after the procedure was maintained and similar for TAVR and surgery (New York Heart Association class I or II with TAVR vs surgery: 393 [94.0%] vs 293 [91.3%]). The increase in Kansas City Cardiomyopathy Questionnaire overall summary score from baseline to 5 years was similar for TAVR (mean [SD], 15.3 [24.9]) and surgery (mean [SD], 14.3 [24.2]) (eFigure 8 in Supplement 2).
Discussion
This 5-year follow-up report of the SURTAVI trial demonstrated no difference in all-cause mortality or disabling stroke between supra-annular self-expanding TAVR and surgery in patients with symptomatic severe aortic stenosis at intermediate surgical risk. Clinical valve thrombosis and endocarditis were infrequent through 5 years with both TAVR and surgery. Rates of heart failure– or valve-related rehospitalization were similar for both strategies. Need for reintervention at 5 years was higher for TAVR than surgery and was driven by more early reinterventions after TAVR. Reintervention rates between 2 and 5 years were equally low for TAVR and surgery. Bioprosthetic valve performance was consistent through 5 years. TAVR demonstrated a better hemodynamic profile but also more mild or moderate PVL than surgery. Health status improved similarly after TAVR or surgery and was maintained at 5 years. Need for revascularization had no impact on clinical outcomes.
The Placement of Aortic Transcatheter Valves (PARTNER 2A) trial compared balloon expandable TAVR and surgery in patients with severe aortic stenosis at intermediate surgical risk and reported a 46% and 42% mortality with TAVR and surgery, respectively.8 Landmark analyses of clinical events between 2 and 5 years demonstrated more all-cause mortality, myocardial infarction, need for aortic valve reinterventions, and heart failure– or aortic valve–related hospitalizations in the TAVR group. This compares with the 30% and 28.7% all-cause mortality for TAVR and surgery in SURTAVI and no difference in clinical events between 2 and 5 years. Essential differences in study population and TAVR procedures preclude detailed comparison of patient end points between the PARTNER 2A and SURTAVI trials. Patients in PARTNER 2A were older and had a higher STS-PROM risk score. Transthoracic access was used in approximately a quarter of patients in the TAVR cohort and was associated with worse outcome. The second-generation balloon expandable transcatheter valve also showed more structural valve degeneration than the surgical valve.9 The third-generation balloon expandable transcatheter heart valve (SAPIEN 3; Edwards Lifesciences) appeared to have a more favorable profile in terms of clinical outcome and valve performance.14 We found no difference in the rate of heart failure– and aortic valve–related hospitalizations between TAVR and surgery in SURTAVI, which may attest to the maintained superior hemodynamic valve performance after supra-annular TAVR despite a higher incidence of PVL. Like the PARTNER 2A trial,8 the present study showed overall more valve reinterventions after TAVR than after surgery at 5 years. However, in SURTAVI, this was driven by more early reinterventions because of significant residual PVL with the early-generation nonrepositionable bioprosthesis design (CoreValve). Between 2 and 5 years, there was no difference in valve reintervention rate between TAVR and surgery: 6 patients after TAVR and 7 patients after surgery required a reintervention that equally consisted of transcatheter and surgical techniques and was predominantly for bioprosthetic valve degeneration. Conversely, in PARTNER 2A, there was no difference in valve reinterventions between TAVR and surgery within the first 2 years but significantly more valve reinterventions after TAVR between 2 and 5 years. Further research should determine whether long-term bioprosthetic valve performance of balloon expandable and self-expanding platforms is different relative to surgical bioprostheses and how that affects valve durability over time.
Consistent with other studies that compared supra-annular self-expanding TAVR with surgery, TAVR was associated with persistently larger aortic valve areas and lower transprosthetic gradients; however, TAVR had more mild or moderate PVL than surgery.3,10 The transcatheter valve design that was implanted in SURTAVI was mainly the first-generation bioprosthesis (CoreValve) (84%) and the remaining received the second-generation TAVR device (Evolut valve). The contemporary Evolut PRO valve (Medtronic) with an external pericardial wrap has demonstrated lower rates of significant PVL in a report from the Society of Thoracic Surgery/American College of Cardiology/Transcatheter Valve Therapy Registry but was not available for this trial.15 Severe PPM in SURTAVI occurred more often with surgery compared with TAVR (14.0% vs 3.8%), but severe PPM was not associated with clinical outcome at 5 years. This effect should be interpreted with caution given the relatively small sample size. Previous studies suggested a correlation between survival and severe PPM after surgery but not after TAVR.16,17 The need for new pacemakers in SURTAVI is consistent with previous literature on TAVR with a supra-annular self-expanding valve3,18,19 but was not associated with increased mortality at 5 years. Also in the ADVANCE trial, new pacemakers after TAVR with a CoreValve bioprosthesis were not associated with incremental mortality at 5 years of follow-up.20 The clinical impact of new pacemakers after TAVR is controversial and probably affected by the degree of pacemaker dependency as determined by sporadic or intermittent vs continuous right ventricular pacing. Modified implantation techniques that aim at higher valve implant depth and advanced computed tomography planning that considers the membranous septum length and location of the atrioventricular conduction bundle may mitigate new pacemaker rates.21,22
One-fifth of the study population was stratified by need of coronary revascularization prior to study randomization. Of these, more patients in the surgery arm than in the TAVR arm received coronary revascularization. Nevertheless, there was no difference in clinical event rates out to 5 years in patients who were stratified for coronary revascularization. Importantly, complex coronary artery disease was an exclusion criterion in SURTAVI, and the mean age of the patients was approximately 80 years. Complete revascularization may not be a prerequisite for good clinical outcome in elderly patients with severe aortic stenosis.23 The ACTIVATION trial (Assessing the Effects of Stenting in Significant Coronary Artery Disease Prior to Transcatheter Aortic Valve Implantation)24 randomized elderly patients with severe aortic stenosis and significant coronary artery disease to TAVR with vs without percutaneous coronary intervention and found no difference in the primary clinical end point but more bleeding in the PCI arm.
Limitations
Our study has several notable limitations. SURTAVI included elderly patients, and concomitant revascularization was limited to a fraction of the overall study population. Therefore, our results may not apply to younger patients or in the context of advanced coronary artery disease. Additionally, all patients underwent TAVR with a self-expanding valve or surgery at experienced TAVR centers, and the results may not be generalized outside of these parameters. As previously reported, there was a higher frequency of unplanned withdrawal in the surgery group. The severe COVID-19 pandemic appeared to have limited impact on clinical follow-up compliance but resulted in reduced scheduled in-person echocardiographic follow-up at 5 years. Missing echocardiography data at 5 years may affect our findings in terms of hemodynamic valve performance, and missing clinical data may include uncounted clinical end points. It is possible that missing data biased our findings. Overall, missing data were present in 19% of the total population, which is in line with other long-term follow-up studies in an intermediate risk population.8 These patients were older, with higher STS-PROM score and New York Heart Association symptoms, also in line with similar long-term follow-up studies of intermediate risk trials in this field. Other than missing patients because of random events such as withdrawal, missed visits, or loss to follow-up, most missing data are because of death. As such, and because of the 5-year mortality rates of 30.0% (TAVR) and 28.7% (surgery), we performed a joint model to examine the association between dropout (due to death) and effective orifice area/mean aortic valve gradient longitudinal trend (eMethods in Supplement 2).25 The results showed no evidence of an association between effective orifice area/mean aortic valve gradient and death (P = .44 and .79) (eTable 10 in Supplement 2).
The transcatheter heart valve that was predominantly used in the TAVR group is no longer clinically available. Contemporary device iterations have a smaller delivery profile, repositioning features, and a sealing wrap without compromising the hemodynamic valve performance, which may further improve device success and clinical outcome over time.15
Conclusions
Among patients with symptomatic severe aortic stenosis at intermediate surgical risk, clinical outcomes at 5 years were similar and bioprosthetic valve performance remained stable after TAVR and surgery.
Trial protocol and statistical analysis plan
eMethods.
eTable 1. Study Sites, Investigators and Key Personnel
eTable 2. SURTAVI Trial Committees
eTable 3. Inclusion and Exclusion Criteria
eTable 4. Major Clinical Event and Study Definitions
eTable 5. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (Overall Population)
eTable 6. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (TAVR)
eTable 7. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (Surgery)
eTable 8. Baseline Characteristics by Stratification to Revascularization
eTable 9. Reintervention Details From 2 to 5 Years
eTable 10. Joint Model Results: Association Between Effective Orifice Area/Mean Aortic Valve Gradient Longitudinal Trend and Time to Event (Death)
eFigure 1. Patient Flow
eFigure 2. Subgroup Analysis of All-Cause Mortality or Disabling Stroke at 5 Years
eFigure 3. 5-Year All-Cause Mortality in the TAVI Cohort by Permanent Pacemaker
eFigure 4. 5-Year All-Cause Mortality in the TAVR Cohort by Severity of Paravalvular Leak at Discharge
eFigure 5. Paravalvular Leak
eFigure 6. Prosthesis-Patient Mismatch
eFigure 7. 5-Year Mortality by Severity of PPM at Discharge
eFigure 8. Quality of Life
eReferences
Nonauthor collaborators. SURTAVI Trial Investigators
Data Sharing Statement
References
- 1.Vahanian A, Beyersdorf F, Praz F, et al. ; ESC/EACTS Scientific Document Group . 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 2.Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450-500. doi: 10.1016/j.jacc.2020.11.035 [DOI] [PubMed] [Google Scholar]
- 3.Gleason TG, Reardon MJ, Popma JJ, et al. 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72(22):2687-2696. doi: 10.1016/j.jacc.2018.08.2146 [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Leon MB, Smith CR, et al. 5-Year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. doi: 10.1016/S0140-6736(15)60308-7 [DOI] [PubMed] [Google Scholar]
- 5.Mack MJ, Leon MB, Thourani VH, et al. ; PARTNER 3 Investigators . Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 6.Popma JJ, Deeb GM, Yakubov SJ, et al. ; Evolut Low Risk Trial Investigators . Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706-1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 7.Reardon MJ, Van Mieghem NM, Popma JJ, et al. ; SURTAVI Investigators . Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 8.Makkar RR, Thourani VH, Mack MJ, et al. ; PARTNER 2 Investigators . Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382(9):799-809. doi: 10.1056/NEJMoa1910555 [DOI] [PubMed] [Google Scholar]
- 9.Pibarot P, Ternacle J, Jaber WA, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 Trial. J Am Coll Cardiol. 2020;76(16):1830-1843. doi: 10.1016/j.jacc.2020.08.049 [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen TH, Thyregod HGH, Ihlemann N, et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. 2021;42(30):2912-2919. doi: 10.1093/eurheartj/ehab375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Oh JK, Little SH, Abdelmoneim SS, et al. ; CoreValve U.S. Pivotal Trial Clinical Investigators . Regression of paravalvular aortic regurgitation and remodeling of self-expanding transcatheter aortic valve: an observation from the CoreValve U.S. Pivotal Trial. JACC Cardiovasc Imaging. 2015;8(12):1364-1375. doi: 10.1016/j.jcmg.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 13.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403-2418. doi: 10.1093/eurheartj/ehs255 [DOI] [PubMed] [Google Scholar]
- 14.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218-2225. doi: 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 15.Forrest JK, Kaple RK, Tang GHL, et al. Three generations of self-expanding transcatheter aortic valves: a report from the STS/ACC TVT Registry™. JACC Cardiovasc Interv. 2020;13(2):170-179. doi: 10.1016/j.jcin.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 16.Head SJ, Mokhles MM, Osnabrugge RL, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33(12):1518-1529. doi: 10.1093/eurheartj/ehs003 [DOI] [PubMed] [Google Scholar]
- 17.Ternacle J, Abbas AE, Pibarot P. Prosthesis-patient mismatch after transcatheter aortic valve replacement: has it become obsolete? JACC Cardiovasc Interv. 2021;14(9):977-980. doi: 10.1016/j.jcin.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 18.Grube E, Van Mieghem NM, Bleiziffer S, et al. ; FORWARD Study Investigators . Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis The International FORWARD Study. J Am Coll Cardiol. 2017;70(7):845-853. doi: 10.1016/j.jacc.2017.06.045 [DOI] [PubMed] [Google Scholar]
- 19.Manoharan G, Grube E, Van Mieghem NM, et al. Thirty-day clinical outcomes of the Evolut PRO self-expanding transcatheter aortic valve: the international FORWARD PRO study. EuroIntervention. 2020;16(10):850-857. doi: 10.4244/EIJ-D-20-00279 [DOI] [PubMed] [Google Scholar]
- 20.Gerckens U, Tamburino C, Bleiziffer S, et al. Final 5-year clinical and echocardiographic results for treatment of severe aortic stenosis with a self-expanding bioprosthesis from the ADVANCE Study. Eur Heart J. 2017;38(36):2729-2738. doi: 10.1093/eurheartj/ehx295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jilaihawi H, Zhao Z, Du R, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(18):1796-1807. doi: 10.1016/j.jcin.2019.05.056 [DOI] [PubMed] [Google Scholar]
- 22.Tang GHL, Zaid S, Michev I, et al. “Cusp-overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11(16):1663-1665. doi: 10.1016/j.jcin.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 23.Van Mieghem NM, van der Boon RM, Faqiri E, et al. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc Interv. 2013;6(8):867-875. doi: 10.1016/j.jcin.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 24.Patterson T, Clayton T, Dodd M, et al. ; ACTIVATION Trial Investigators . ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): a randomized clinical trial. JACC Cardiovasc Interv. 2021;14(18):1965-1974. doi: 10.1016/j.jcin.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Hernandez A, Rizopoulos D. %JM: a SAS macro to fit jointly generalized mixed models for longitudinal data and time-to-event responses. J Stat Softw. 2018;84. doi: 10.18637/jss.v084.i12 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods.
eTable 1. Study Sites, Investigators and Key Personnel
eTable 2. SURTAVI Trial Committees
eTable 3. Inclusion and Exclusion Criteria
eTable 4. Major Clinical Event and Study Definitions
eTable 5. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (Overall Population)
eTable 6. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (TAVR)
eTable 7. Baseline Characteristics for Patients With and Without Complete 5-Year Follow Up (Surgery)
eTable 8. Baseline Characteristics by Stratification to Revascularization
eTable 9. Reintervention Details From 2 to 5 Years
eTable 10. Joint Model Results: Association Between Effective Orifice Area/Mean Aortic Valve Gradient Longitudinal Trend and Time to Event (Death)
eFigure 1. Patient Flow
eFigure 2. Subgroup Analysis of All-Cause Mortality or Disabling Stroke at 5 Years
eFigure 3. 5-Year All-Cause Mortality in the TAVI Cohort by Permanent Pacemaker
eFigure 4. 5-Year All-Cause Mortality in the TAVR Cohort by Severity of Paravalvular Leak at Discharge
eFigure 5. Paravalvular Leak
eFigure 6. Prosthesis-Patient Mismatch
eFigure 7. 5-Year Mortality by Severity of PPM at Discharge
eFigure 8. Quality of Life
eReferences
Nonauthor collaborators. SURTAVI Trial Investigators
Data Sharing Statement