This cohort study examines registry data for patients with type A aortic dissection to assess mortality rates in the first 48 hours after hospital arrival and reflect any updates due to the advances in diagnostic testing and treatment of the contemporary era.
Key Points
Question
What is the mortality for patients in the early hours after presentation with acute type A aortic dissection in the contemporary era?
Findings
In this cohort study, nonoperative patients presenting with acute type A aortic dissection had a mortality in the first 48 hours of 0.5% per hour. This rate decreased to 0.09% per hour over this period for those receiving surgery or intending to receive surgery.
Meaning
Acute type A aortic dissection still may carry a high mortality for patients not receiving surgery.
Abstract
Importance
Early data revealed a mortality rate of 1% to 2% per hour for type A acute aortic dissection (TAAAD) during the initial 48 hours. Despite advances in diagnostic testing and treatment, this mortality rate continues to be cited because of a lack of contemporary data characterizing early mortality and the effect of timely surgery.
Objective
To examine early mortality rates for patients with TAAAD in the contemporary era.
Design, Setting, and Participants
This cohort study examined data for patients with TAAAD in the International Registry of Acute Aortic Dissection between 1996 and 2018. Patients were grouped according to the mode of their intended treatment, surgical or medical.
Exposure
Surgical treatment.
Main Outcomes and Measures
Mortality was assessed in the initial 48 hours after hospital arrival using Kaplan-Meier curves. In-hospital complications were also evaluated.
Results
A total of 5611 patients with TAAAD were identified based on intended treatment: 5131 (91.4%) in the surgical group (3442 [67.1%] male; mean [SD] age, 60.4 [14.1] years) and 480 (8.6%) in the medical group (480 [52.5%] male; mean [SD] age, 70.9 [14.7] years). Reasons for medical management included advanced age (n = 141), comorbidities (n = 281), and patient preference (n = 81). Over the first 48 hours, the mortality for all patients in the study was 5.8%. Among patients who were medically managed, mortality was 0.5% per hour (23.7% at 48 hours). For those whose intended treatment was surgical, 48-hour mortality was 4.4%. In the surgical group, 51 patients (1%) died before the operation.
Conclusions and Relevance
In this study, the overall mortality rate for TAAAD was 5.8% at 48 hours. For patients in the medical group, TAAAD had a mortality rate of 0.5% per hour (23.7% at 48 hours). However, among those in the surgical group, 48-hour mortality decreased to 4.4%.
Introduction
The mortality rate for type A acute aortic dissection (TAAAD) during the initial 24 to 48 hours after symptom onset is commonly described as 1% to 2% per hour, based on data from the 1950s, before medical therapy improved and cardiovascular surgery became routine.1 In the contemporary era, consensus guidelines have emphasized the need for earlier recognition and triage of aortic dissection, facilitated by widely available computed tomographic imaging.2,3 Despite significant improvements in perioperative management as well as cardiovascular, endovascular, and hybrid surgical techniques,2,3 hospital mortality remains high.4,5,6,7,8
In the series by Hirst et al1 from the 1950s, mortality was estimated at 21% in the first 24 hours and 37% in the first 48 hours. A previous International Registry of Acute Aortic Dissection (IRAD) report evaluated the AD mortality risk as delineated by time from symptom onset and described a heightened risk in the first 24 hours, especially for TAAAD.4 Patients may not undergo timely surgery because of delays in recognition or treatment, patient preference, comorbidities, or advanced age. A subset of patients may be planned for surgery but experience preoperative resuscitation or hypotension/shock, important predictors of surgical mortality that may prevent or delay repair.5,6,7 Given the significant advancements in aortic care over the last 6 decades, we aimed to delineate TAAAD mortality risk in the first 48 hours and the clinical features associated with death before (or without) surgery.
Methods
IRAD has collected data for consecutive patients at 56 tertiary care centers in 14 countries since 1996.5,8,9,10 Patients with spontaneous or iatrogenic AD diagnosed within 2 weeks of symptom onset are enrolled at presentation or identified retrospectively via hospital discharge diagnosis or operative or imaging records. Diagnosis is based on clinical history, imaging results, surgical inspection, and/or postmortem evaluation.5 Institutional review board approval was obtained at each site and consent done in accordance with each site’s approval.
A 347-item form captures comprehensive data regarding demographics, medical history, clinical presentation, physical examination findings, imaging results, management, and outcomes, including complications and cause of death where available. Race was determined from the medical record to assess whether race influenced the likelihood a patient received nonoperative management or died before surgery.
Data for patients with TAAAD enrolled in IRAD between January 1996 and November 2018 were analyzed (N = 5780). Patients with an iatrogenic cause of dissection were excluded, leaving a cohort of 5611. Patients were subdivided into 2 groups by intended treatment: planned surgical treatment vs medical treatment. A subanalysis was conducted of patients who were candidates for surgical treatment according to IRAD investigators but died before repair. For medically managed cases, the reasons for medical therapy were delineated as patient preference, comorbid conditions, and advanced age.
Summary statistics for median (IQR) time are presented in hours. The Kruskal-Wallis test was used for data with skewed distributions. Analysis of variance for multiple groups was used to compare normally distributed continuous variables. Multiple χ2 tests were used to compare categorical data. Kaplan-Meier mortality curves were developed for all patients with TAAAD with defined times of hospital arrival and death based on the initial 48 hours after hospital arrival (n = 3503). Only those cases with available information were included in the denominator for each variable, as reflected in the tables. Hall and Wellner bands were added to the Kaplan-Meier curves to illustrate 95% CIs for each group.
To investigate which patients died awaiting surgery, a multivariable Cox proportional hazards model was generated for cumulative incidence of death in the first 48 hours, for only those patients who were deemed surgical candidates. A Kaplan-Meier curve was also generated for time to repair in those patients receiving surgery. Further, Kaplan-Meier analysis was used to compare 48-hour mortality between patients presenting in the first 11.5 years of IRAD vs those presenting in the later 11.5 years. Multivariable logistic regression was performed to assess the independent association of preoperative variables, including time to repair, with 48-hour mortality among only those patients receiving surgery.
Results
There were 5611 patients with TAAAD assessed, including 5131 (91.4%) with intended or actual surgery and 480 (8.6%) managed medically. Reasons for medical management included advanced age (45.5%), comorbid illness (72%), and patient preference (29%) (more than 1 option could be selected).
Patients in the medical group were more likely to be older (mean [SD] age, 70.9 [14.7] years vs 60.4 [14.1] years in the surgical group) and female (228/480 [47.5%] vs 1689/5131 [32.9%] in the surgical group). Patients managed medically were also more likely to have more comorbidities, including a history of AD and aortic surgery (Table 1). For surgical patients, the median (IQR) time to diagnosis was 2.5 hours (1.2-5.3), and the median (IQR) time from presentation to surgery was 6 hours (4.0-15.0).
Table 1. Demographics and History.
| No./total No. (%) | P value | ||
|---|---|---|---|
| Intended surgery | Medical management | ||
| No. of patients (%) | 5131 (91.4) | 480 (8.6) | |
| Age | <.001 | ||
| No. of patients | 5130 | 480 | |
| Mean (SD), y | 60.4 (14.1) | 70.9 (14.7) | |
| Gender | |||
| Male | 3442/5131 (67.1) | 252/480 (52.5) | <.001 |
| Female | 1689/5131 (32.9) | 228/480 (47.5) | |
| Race | |||
| Asian | 221/4667 (4.7) | 38/436 (8.7) | <.001 |
| Black | 507/4667 (10.9) | 30/436 (6.9) | .01 |
| White | 3735/4667 (80.0) | 355/436 (81.4) | .49 |
| BMIa | .20 | ||
| No. of patients | 3103 | 200 | |
| Median (IQR) | 27.7 (24.6-31.9) | 27.3 (23.5-32.2) | |
| Patient transferred | 3681/5131 (71.7) | 308/480 (64.2) | <.001 |
| Time from symptom onset to presentation | .85 | ||
| No. of patients | 2517 | 241 | |
| Median (IQR), h | 1.5 (0.8-3.3) | 1.5 (0.8-3.4) | |
| Time from presentation to diagnosis | .005 | ||
| No. of patients | 2697 | 238 | |
| Median (IQR), h | 2.5 (1.2-5.3) | 3.5 (1.4-7.3) | |
| Time from presentation to surgery | |||
| No. of patients | 2893 | ||
| Median (IQR), h | 6.0 (4.0-15.0) | ||
| Time from presentation to death | <.001 | ||
| No. of patients | 470 | 133 | |
| Median (IQR), h | 64.7 (16.2-231.4) | 27.7 (8.4-70.4) | |
| History of hypertension | 3603/4718 (76.4) | 349/446 (78.3) | .37 |
| Diabetes | 416/4342 (9.6) | 68/422 (16.1) | <.001 |
| Marfan syndrome | 166/4294 (3.9) | 17/407 (4.2) | .76 |
| Atherosclerosis | 748/4289 (17.4) | 135/416 (32.5) | <.001 |
| Known aortic aneurysm | 583/4336 (13.4) | 94/424 (22.2) | <.001 |
| Prior aortic dissection | 181/4293 (4.2) | 42/423 (9.9) | <.001 |
| Bicuspid aortic valve | 172/4072 (4.2) | 7/337 (2.1) | .06 |
| Aortic valve disease | 485/4297 (11.3) | 51/408 (12.5) | .46 |
| Other aortic disease | 91/4228 (2.2) | 11/402 (2.7) | .45 |
| Current smoker | 907/2777 (32.7) | 57/240 (23.8) | .005 |
| Family history of aortic disease | 246/2732 (9.0) | 21/209 (10.0) | .61 |
| Peripheral arterial disease | 112/2977 (3.8) | 17/223 (7.6) | .005 |
| COPD | 304/3057 (9.9) | 43/232 (18.5) | <.001 |
| Chronic kidney insufficiency | 218/3036 (7.2) | 29/229 (12.7) | .002 |
| Prior cardiac surgery | 516/4276 (12.1) | 114/424 (26.9) | <.001 |
| Aneurysm/AAD | 223/4226 (5.3) | 63/421 (15.0) | <.001 |
Abbreviations: AAD, acute aortic dissection; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Calculated as weight in kilograms divided by height in meters squared.
There was heterogeneity between continents in the initial strategy, with more patients managed medically in Asian centers compared with North American and European sites (44 [16.5%] vs 436 [8.2%], respectively; P < .001). The Asian centers were underrepresented in IRAD, composing only 4.8% of the entire group (267 patients).
Presenting signs, symptoms, and diagnostic testing are shown in eTables 1 and 2 in the Supplement. Most patients with TAAAD presented with abrupt chest pain, though these features were less prevalent in those managed medically. Aortic insufficiency was more common in the surgical group. Patients with intramural hematoma were more likely to be treated medically.
Complications
Complications in the 2 treatment groups are summarized in Table 2. Several complications were evident on presentation or shortly after admission in the patients managed medically; these differences likely contributed to the initial medical management strategy. Coma and stroke were more prevalent in the medically managed group as were mesenteric ischemia, kidney failure, and hypotension.
Table 2. Complications.
| No./total No. (%) | P value | ||
|---|---|---|---|
| Intended surgery | Medical management | ||
| Neurological deficit | |||
| Stroke | 188/4375 (4.3) | 49/402 (12.2) | <.001 |
| Coma | 80/4354 (1.8) | 36/402 (9.0) | <.001 |
| Spinal cord ischemia | 52/4344 (1.2) | 7/398 (1.8) | .33 |
| Myocardial ischemia | 239/2100 (11.4) | 29/270 (10.7) | .76 |
| Myocardial infarction | 143/2110 (6.8) | 23/268 (8.6) | .28 |
| Mesenteric ischemia/infarction | 119/4436 (2.7) | 29/408 (7.1) | <.001 |
| Acute kidney failure | 326/4438 (7.3) | 72/411 (17.5) | <.001 |
| Extension of dissection | 223/4399 (5.1) | 49/401 (12.2) | <.001 |
| Hypotension | 885/4466 (19.8) | 131/409 (32.0) | <.001 |
| Cardiac tamponade | 569/4484 (12.7) | 61/404 (15.1) | .17 |
| Limb ischemia | 457/4445 (10.3) | 37/404 (9.2) | .48 |
Mortality
Cause of death differed between groups (Table 3) with sudden and often unpredictable complications of aortic rupture and cardiac tamponade more common in patients managed medically. Conversely, surgical patients were more likely to die of multiorgan failure and bleeding complications. Cause of death was unknown in 313 patients (28.5%).
Table 3. Cause of Death.
| No./total No. (%) | P value | ||
|---|---|---|---|
| Intended surgery | Medical management | ||
| Neurologic | 96/857 (11.2) | 18/241 (7.5) | .09 |
| Tamponade | 21/857 (2.5) | 31/241 (12.9) | <.001 |
| Visceral ischemia | 52/857 (6.1) | 15/241 (6.2) | .93 |
| Bleeding | 38/857 (4.4) | 3/241 (1.2) | .02 |
| Multiorgan failure | 111/857 (13.0) | 11/241 (4.6) | <.001 |
| Cardiac | 136/857 (15.9) | 27/241 (11.2) | .07 |
| Rupture | 115/857 (13.4) | 50/241 (20.7) | .005 |
| Unknown | 242/857 (28.2) | 71/241 (29.5) | .71 |
| Other | 46/857 (5.4) | 15/241 (6.2) | .61 |
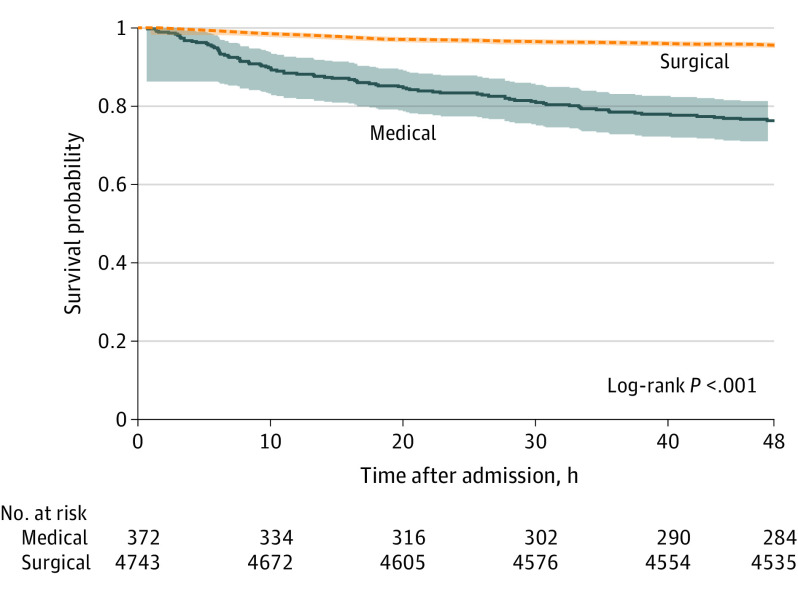
Among all patients with TAAAD in this study, 48-hour mortality was 5.8% (0.12%/hour). Overall mortality among those with planned or completed surgery was 4.4% at 48 hours (0.09%/hour) (Figure). In comparison, among the patients with medical management, mortality was 23.7% (0.5%/hour). Analysis of the early and later intervals of the IRAD (before and after July 2007), demonstrated no difference in 48-hour mortality in patients managed medically (eFigure 1 in the Supplement), but surgical mortality decreased in the more recent time period (from 5.5% to 3.9%; log-rank P = .01).
Figure. Survival in the Surgical and Medical Management Groups.
Kaplan-Meier analysis of survival at 48 hours in surgical patients (including patients with planned surgery) vs those receiving medical management alone.
Multivariable logistic regression was used to assess the independent association of various factors with 48-hour mortality among the patients managed surgically (Table 4). Time from initial hospital admission to surgery was forced into the model. As time to surgery increased, 48-hour mortality decreased. Advanced age; presenting hypotension, shock, or tamponade; prior cardiac surgery; preoperative malperfusion; and higher body mass index were conversely associated with worse postoperative mortality.
Table 4. Multivariable Logistic Regression for Predictors of In-Hospital Death Among Those Receiving Surgerya.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Time from initial hospital admission to surgery, h | 0.96 (0.94-0.99) | .02 |
| Age per 10 y | 1.51 (1.22-1.87) | <.001 |
| Presenting hypotension, shock, or tamponade | 2.58 (1.53-4.35) | <.001 |
| Prior cardiac surgery | 2.80 (1.31-5.99) | .008 |
| Preoperative malperfusion | 3.09 (1.83-5.23) | <.001 |
| Body mass index | 1.05 (1.02-1.08) | .002 |
C statistic: 0.787; Hosmer-Lemeshow P = .39.
Fifty-one patients (1%) were deemed operative candidates but died while awaiting surgery. Compared with those who had surgery, these patients were older and more likely to present with pulse deficits and coma or altered consciousness (eTable 3 in the Supplement). This cohort was more likely to develop preprocedure stroke, myocardial infarction, mesenteric ischemia/infarction, acute kidney failure, hypotension, cardiac tamponade, and limb ischemia when compared with those who ultimately received surgery. Critical hypotension and cardiac tamponade developed preoperatively but after hospital admission in 14 patients (28%) and 10 patients (21%), respectively. Of the patients who died awaiting surgery, 5 (9.8%) died of tamponade and 13 (25.5%) of rupture. Cox proportional hazard analysis demonstrated that older age, presenting hypotension or shock, and preoperative myocardial infarction were independently associated with dying before surgery (eTable 4 in the Supplement). Notably, the time to death in those who died awaiting surgery was 8.9 hours, which exceeded the median time to surgery (6 hours) (eFigure 2 in the Supplement).
Discussion
Since the early era of AD care, there have been important improvements in both diagnosis via imaging modalities and medical treatment, including β-blockers, as well as the advent of complex cardiovascular, vascular, and hybrid surgical techniques.2,3,8,11 Our data indicate that for patients with nonoperative TAAAD, the mortality is 23.7% within the first 48 hours, corresponding to approximately 0.5% per hour over this critical time period, which is lower than initial estimates from the 1950s but still substantial.1 A surgical strategy for acute AD leads to a mortality of 0.09% per hour, inclusive of patients who died before operative repair, and is lower in the recent years of this study.
The first 48 hours after onset of dissection identifies a particularly vulnerable time frame for patients with TAAAD. Because patients with aortic dissection are prone to aortic rupture, a subset of patients likely die before presenting to a medical facility or before AD is recognized.1,3,11,12 For this analysis, we identified the time of initial hospital admission to establish a group in whom mortality could be evaluated on an hourly basis. For these patients, the mortality rate in the first 48 hours among all patients deemed to be surgical candidates (with or without actually receiving surgery) was 0.09% per hour, compared with 0.5% per hour among those managed medically, who were older and had more comorbid conditions. Furthermore, the highest risk remains in the initial hours after symptom onset.1,3,13,14,15 Cause of death in these patients often is related to aortic rupture and cardiac tamponade, which can lead to sudden unexpected demise in a seemingly stable patient. As shown here, presenting hypotension and shock, potentially reflective of underlying cardiac tamponade, were important predictors of death in the surgical group. While surgical mortality has decreased significantly in recent years and even over the course of this study interval, in-hospital mortality for medical treatment alone has remained constant, at greater than 50%.10 It should be emphasized that the hospital mortality identified here does not capture those patients who die before arrival at the tertiary care center, including those at or transferred from referral hospitals.8 Population-based mortality of TAAAD is considered to be substantially higher.16
Medical Management Subgroup
There is a group of patients judged to have prohibitively high risk for surgery by various stakeholders (including physicians/surgeons, patient, and family). This heterogenous group includes patients with extreme age, those with severe comorbidities, and those who prefer to avoid surgery. Rates of surgical management in IRAD have increased over time, with 90% of patients with TAAAD undergoing surgery currently.10,17 In this analysis, we identified a subset of patients who at presentation appear to have evidence of cerebrovascular, mesenteric, coronary, and peripheral ischemia. Along with advanced age and prior cardiac surgery, findings of malperfusion are well-known markers of increased operative risk and may have influenced the decision-making process toward avoidance of surgery.17,18 Thus, once preoperative risks have been established, careful clinical evaluation and surgical center expertise is especially paramount in the highest-risk cases.3 Surgery in high-risk groups such as octogenarians has been shown to be a reasonable option, but the decision to offer surgery to octogenarian patients must balance the increased operative risks against the high mortality with medical therapy and mandates expeditious, informed decision-making with patients and their families.3,19
Complications in Patients Who Die Without Surgery
Cerebrovascular events and coma were more common in patients treated medically. Brain injury, including coma and stroke, may complicate AD in 10% of cases and increases the mortality risk across all treatment strategies. For patients who are surgical candidates and survive to surgery, operative repair offers lower mortality compared with medical management, and brain injury improves in the majority of patients.20 Time to surgery from symptom onset is critical in this respect.3,11 Visceral and peripheral malperfusion were also seen in a large percentage of surgical candidates who died awaiting surgery. Malperfusion of coronary, mesenteric, renal, and other vascular beds may occur in isolation or with other vascular territories and represents a particularly hazardous subset of patients with AD.21 Because patients with malperfusion syndromes have a high surgical mortality, surgeons may avoid operating in this high-risk group; thus, patients are much more likely to be treated with medical management alone, a perception that should be challenged.21 Although controversy exists over the exact timing of hybrid endovascular techniques for malperfusion, surgical/hybrid techniques have been reported to be superior to medical management alone as well as primary endovascular approaches.3,21 While some preoperative complications such as cerebrovascular or mesenteric ischemia may have influenced the initial treatment strategy, these complications also frequently developed while patients were awaiting surgery.
Geographic Heterogeneity
In the small population studied with medical management, an overall higher percentage of patients were managed medically in Asian sites. Prior analysis in AD subsets suggest distinct differences in an Asian population, where patients in general were felt to be of lower risk.17,22 Given the relatively few patients enrolled in Asian countries, we do not believe we have sufficient data to elucidate the differences in management between regions.
Time to Repair
Among surgical patients, increased time from initial admission to surgery was associated with improved in-hospital survival after adjusting for other factors. While it is not surprising that surviving to surgery ameliorates the effect of delayed repair, we are unable to study the associations with time delays in those who died while awaiting surgery. However, with a nonoperative mortality approaching 0.5% per hour, it is reasonable to assume that timely intervention in patients deemed operative candidates will improve outcomes, as the hourly mortality rate for surgical patients is more than 5-fold lower. With preoperative timing dependent on a variety of factors, including a surgeon’s assessment of risk, the association of time to repair and outcome in this cohort is likely a reflection of increased risk at presentation among those receiving early surgery.
Transformation of a Stable Subset
The numerical power of IRAD facilitated identification of crucial subsets of patients who died while awaiting surgery or on entry to the operating room. While some of the patients dying before surgery presented with complications, many others at presentation did not significantly differ from those undergoing surgery. Complications such as hypotension, tamponade, and peripheral ischemia may develop and transform seemingly stable patients into a potentially unstable and high-risk subgroup.8 Thus, improved processes and rapid access to care leading to earlier surgery could be associated with mortality in some patients, given the unpredictability of imminent complications.
Processes of Care and Aortic Centers
The critical group of patients who die before surgery highlights the importance of processes to rapidly identify AD and move these patients to surgery without delay.3 There are inherent delays in AD recognition and treatment, with times from presentation to diagnosis of 2.5 hours and from diagnosis to surgery of 3.5 hours, totaling 6 hours from emergency department arrival to surgery in IRAD centers.9 In the group of patients who died awaiting surgery, the median (IQR) time from presentation to death was 8.9 hours (4-32). Interhospital transfer is needed in more than 70% of cases, leading to inherent treatment delays, and several centers have developed regional transfer processes.3,23,24,25,26,27,28,29,30,31 A regional care model with emphasis on diagnosis and treatment protocols has been shown to reduce times to diagnosis and treatment.23
There is evidence that both center and surgeon volumes contribute to improved AD survival.32 Therefore, rapid transfer to aortic centers with cardiovascular and vascular specialists and anesthesiologists adept at complex aortic surgery, cerebral protection, and techniques to address malperfusion is critical in these patients.3 Transfer to regional high-volume aortic centers has been shown to decrease mortality across regions.24,25,28,29 In addition to addressing the hospital system components of timely diagnosis and treatment, it is important to address the time between the patient’s symptom onset and presentation (median [IQR], 1.5 hours [0.8-3.3]). Thus, patient education is critical such that high-risk patients recognize AD symptoms, present to the emergency department expeditiously, and advocate for aorta-specific testing when necessary.23,33
Limitations
Although it is the largest and most contemporary study to date evaluating initial mortality in TAAAD, the present study is limited by the retrospective nature and incomplete or missing events. As such, the reported mortality rates define those patients who survive to hospital stay and are recognized with AD, often after transfer to a tertiary IRAD center. As shown in the Oxford Vascular Study,16 a significant percentage of patients with acute AD do not survive to hospital admission. Additionally, because most IRAD centers are tertiary aortic referral centers, the mortality rates of medically treated cases in other centers with potentially less streamlined processes of care is not known. Therefore, while the number of patients who died awaiting surgery is relatively small, they are representative of a larger group who likely die en route to the tertiary care IRAD centers and are not captured in the registry. Given the retrospective nature of the analysis, it is difficult to determine if the delays in surgery led to the complications observed in surgical candidates who died awaiting surgery or if the complications themselves delayed or prohibited surgery. Further, the cause of death was not specified in 28.5% of the overall population. This likely is a reflection of the significant decline in autopsy rates in recent decades and in other cases may be due to difficulty in discerning the precise cause of death in complex patients, especially those with postsurgical, multiorgan failure. The IRAD comprises primarily European and North American centers, and thus Asian centers are underrepresented.
Conclusions
In patients recognized with TAAAD in the contemporary era, the mortality rate in the first 48 hours is 0.12% per hour. This rate is lower than that reported in the 1950s and may in part be related to advances in surgery, medical treatment, and imaging over the past 60 years. Medically managed patients in this study have a 48-hour mortality of 23.7%, or 0.5% per hour. Patients who are deemed eligible for repair, most of whom ultimately receive surgery, have a lower mortality risk, with 4.4% mortality after 2 days. Importantly, a subgroup of initially stable patients experienced catastrophic and often unforeseen complications, including cardiac tamponade and aortic rupture, and died before surgery. These data serve as a sobering reminder that TAAAD merits efforts to improve recognition, as well as rapid transfer and surgical treatment protocols.
eTable 1. Presenting signs and symptoms
eTable 2. Imaging studies
eTable 3. Key differences between surgically managed patients and those who died waiting
eTable 4. Cox proportional hazard analysis for patients who died awaiting surgery
eFigure 1. Survival between early and later years separated by management type
eFigure 2. Time to surgery
References
- 1.Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore). 1958;37(3):217-279. doi: 10.1097/00005792-195809000-00003 [DOI] [PubMed] [Google Scholar]
- 2.Hiratzka LF, Bakris GL, Beckman JA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine . 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55(14):e27-e129. doi: 10.1016/j.jacc.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 3.Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58(24):2455-2474. doi: 10.1016/j.jacc.2011.06.067 [DOI] [PubMed] [Google Scholar]
- 4.Booher AM, Isselbacher EM, Nienaber CA, et al. ; IRAD Investigators . The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126(8):730.e19-730.e24. doi: 10.1016/j.amjmed.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 5.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897-903. doi: 10.1001/jama.283.7.897 [DOI] [PubMed] [Google Scholar]
- 6.Trimarchi S, Nienaber CA, Rampoldi V, et al. ; International Registry of Acute Aortic Dissection Investigators . Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129(1):112-122. doi: 10.1016/j.jtcvs.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 7.Chiappini B, Schepens M, Tan E, et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J. 2005;26(2):180-186. doi: 10.1093/eurheartj/ehi024 [DOI] [PubMed] [Google Scholar]
- 8.Evangelista A, Isselbacher EM, Bossone E, et al. ; IRAD Investigators . Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846-1860. doi: 10.1161/CIRCULATIONAHA.117.031264 [DOI] [PubMed] [Google Scholar]
- 9.Harris KM, Strauss CE, Eagle KA, et al. ; International Registry of Acute Aortic Dissection (IRAD) Investigators . Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124(18):1911-1918. doi: 10.1161/CIRCULATIONAHA.110.006320 [DOI] [PubMed] [Google Scholar]
- 10.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350-358. doi: 10.1016/j.jacc.2015.05.029 [DOI] [PubMed] [Google Scholar]
- 11.Jassar AS, Sundt TM III. How should we manage type A aortic dissection? Gen Thorac Cardiovasc Surg. 2019;67(1):137-145. doi: 10.1007/s11748-018-0957-3 [DOI] [PubMed] [Google Scholar]
- 12.Huynh N, Thordsen S, Thomas T, et al. Clinical and pathologic findings of aortic dissection at autopsy: review of 336 cases over nearly 6 decades. Am Heart J. 2019;209:108-115. doi: 10.1016/j.ahj.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 13.Perko MJ, Nørgaard M, Herzog TM, Olsen PS, Schroeder TV, Pettersson G. Unoperated aortic aneurysm: a survey of 170 patients. Ann Thorac Surg. 1995;59(5):1204-1209. doi: 10.1016/0003-4975(95)00132-5 [DOI] [PubMed] [Google Scholar]
- 14.Kuipers FM, Schatz IJ. Prognosis in dissecting aneurysm of the aorta. Circulation. 1963;27:658-661. doi: 10.1161/01.CIR.27.4.658 [DOI] [Google Scholar]
- 15.Lindsay J Jr, Hurst JW. Clinical features and prognosis in dissecting aneurysm of the aorta: a re-appraisal. Circulation. 1967;35(5):880-888. doi: 10.1161/01.CIR.35.5.880 [DOI] [PubMed] [Google Scholar]
- 16.Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM; Oxford Vascular Study . Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031-2037. doi: 10.1161/CIRCULATIONAHA.112.000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Montgomery D, Brinster DR, et al. Predicting in-hospital survival in acute type A aortic dissection medically treated. J Am Coll Cardiol. 2020;75(11):1360-1361. doi: 10.1016/j.jacc.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 18.Rampoldi V, Trimarchi S, Eagle KA, et al. ; International Registry of Acute Aortic Dissection (IRAD) Investigators . Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83(1):55-61. doi: 10.1016/j.athoracsur.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 19.Trimarchi S, Eagle KA, Nienaber CA, et al. ; International Registry of Acute Aortic Dissection Investigators . Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140(4):784-789. doi: 10.1016/j.jtcvs.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 20.Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145(3)(suppl):S213-21.e1. doi: 10.1016/j.jtcvs.2012.11.054 [DOI] [PubMed] [Google Scholar]
- 21.Di Eusanio M, Trimarchi S, Patel HJ, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145(2):385-390.e1. doi: 10.1016/j.jtcvs.2012.01.042 [DOI] [PubMed] [Google Scholar]
- 22.Pelzel JM, Braverman AC, Hirsch AT, Harris KM. International heterogeneity in diagnostic frequency and clinical outcomes of ascending aortic intramural hematoma. J Am Soc Echocardiogr. 2007;20(11):1260-1268. doi: 10.1016/j.echo.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 23.Harris KM, Strauss CE, Duval S, et al. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes. 2010;3(4):424-430. doi: 10.1161/CIRCOUTCOMES.109.920140 [DOI] [PubMed] [Google Scholar]
- 24.Andersen ND, Ganapathi AM, Hanna JM, Williams JB, Gaca JG, Hughes GC. Outcomes of acute type A dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63(17):1796-1803. doi: 10.1016/j.jacc.2013.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen ND, Benrashid E, Ross AK, et al. The utility of the aortic dissection team: outcomes and insights after a decade of experience. Ann Cardiothorac Surg. 2016;5(3):194-201. doi: 10.21037/acs.2016.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavaria JE, Brinster DR, Gorman RC, Woo YJ, Gleason T, Pochettino A. Advances in the treatment of acute type A dissection: an integrated approach. Ann Thorac Surg. 2002;74(5):S1848-S1863. doi: 10.1016/s0003-4975(02)04128-0 [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal B, Raymond CE, Randhawa MS, et al. Transfer metrics in patients with suspected acute aortic syndrome. Circ Cardiovasc Qual Outcomes. 2014;7(5):780-782. doi: 10.1161/CIRCOUTCOMES.114.000988 [DOI] [PubMed] [Google Scholar]
- 28.Vaja R, Talukder S, Norkunas M, et al. Impact of a streamlined rotational system for the management of acute aortic syndrome: sharing is caring. Eur J Cardiothorac Surg. 2019;55(5):984-989. doi: 10.1093/ejcts/ezy386 [DOI] [PubMed] [Google Scholar]
- 29.Goldstone AB, Chiu P, Baiocchi M, et al. Interfacility transfer of Medicare beneficiaries with acute type A aortic dissection and regionalization of care in the United States. Circulation. 2019;140(15):1239-1250. doi: 10.1161/CIRCULATIONAHA.118.038867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Froehlich W, Tolenaar JL, Harris KM, et al. Delay from diagnosis to surgery in transferred type A aortic dissection. Am J Med. 2018;131(3):300-306. doi: 10.1016/j.amjmed.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 31.Nienaber CA, Ince H. Surprising facts in acute aortic syndromes: the more we see, the less we save! Rev Esp Cardiol. 2009;62(3):239-241. doi: 10.1016/S0300-8932(09)70365-3 [DOI] [PubMed] [Google Scholar]
- 32.Chikwe J, Cavallaro P, Itagaki S, Seigerman M, Diluozzo G, Adams DH. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg. 2013;95(5):1563-1569. doi: 10.1016/j.athoracsur.2013.02.039 [DOI] [PubMed] [Google Scholar]
- 33.Helliker K, Burton T. Medical ignorance contributes to toll from aortic illness. The Wall Street Journal. November 4, 2003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Presenting signs and symptoms
eTable 2. Imaging studies
eTable 3. Key differences between surgically managed patients and those who died waiting
eTable 4. Cox proportional hazard analysis for patients who died awaiting surgery
eFigure 1. Survival between early and later years separated by management type
eFigure 2. Time to surgery