Abstract
Anti-proliferative agents have been the primary therapeutic drug of choice to inhibit restenosis after endovascular treatment. However, recent safety and efficacy concerns for patients who underwent peripheral artery disease revascularization have demonstrated the need for alternative therapeutics. The aim of this investigation was to investigate the efficacy of a cell-specific RNA aptamer inhibiting vascular smooth muscle cell proliferation and migration. First, the impact of the RNA aptamer (Apt 14) on the wound healing of primary cultured porcine vascular smooth muscle cells (VSMCs) was examined in response to a scratch wound injury. We then evaluated the effect of local luminal delivery of Apt 14 on neointimal formation in a clinically relevant swine iliofemoral injury model. In contrast with a non-selected control aptamer (NSC) that had no impact on VSMC migration, Apt 14 attenuated the wound healing of primary cultured porcine VSMCs to platelet-derived growth factor-BB. Histological analysis of the Apt 14-treated arteries demonstrated a significant reduction in neointimal area percent diameter stenosis compared with arteries treated with saline and NSC controls. The findings of this study suggest that aptamers can function as selective inhibitors and thus provide more fine-tuning to inhibit selective pathways responsible for neointimal hyperplasia.
Key words: MT: oligonucleotides, therapies and applications; RNA; aptamer; cell-specific therapy; in vitro; cell migration; vascular smooth muscle cell; endovascular; perfusion catheter; neointimal hyperplasia; swine; injury model
Graphical abstract
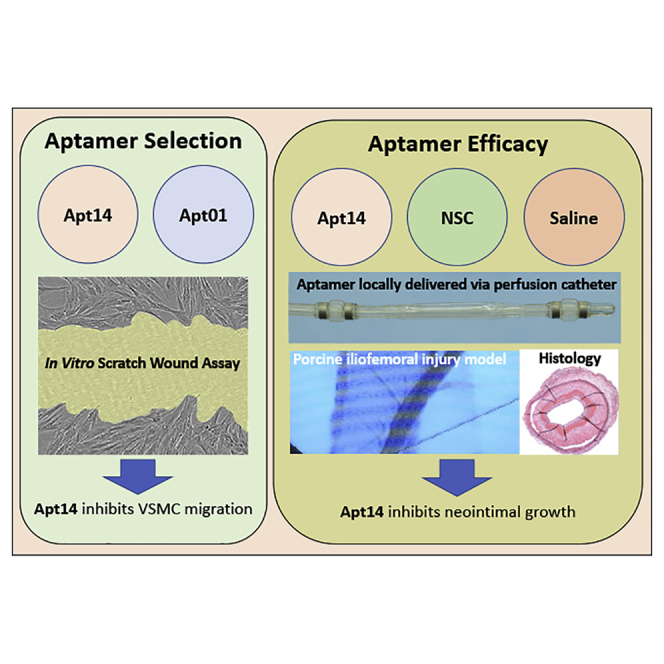
Apt 14 specifically selected to target VSMCs inhibited the wound healing of VSMCs in culture. Intravascular delivery of Apt 14 to the iliofemoral artery attenuated neointimal formation in a large animal model of peripheral vascular disease.
Introduction
Treatment of peripheral artery disease (PAD) affects more than 8 million Americans and costs approximately $21 billion annually.1 The high cost is mainly due to the large number of peripheral vascular operations and interventions that fail, often requiring repeat interventions.2, 3, 4, 5 The problem is re-stenosis, the re-occlusion of the artery where the original obstruction was treated. However, treatment results in injury to the vessel wall that activates vascular smooth muscle cells (VSMCs) to migrate from the tunica media to the subendothelial space and proliferate, resulting in intimal hyperplasia and severely restricted blood flow.
Percutaneous intervention—balloon angioplasty and stenting—is preferred over bypass surgery in PAD because of lower morbidity and mortality rates and shorter in-hospital stays.6,7 However, 50%–85% of patients develop hemodynamically significant restenosis within 2 years of the procedure.5,8 Anti-proliferative drugs, used in combination with bare metal stents, termed drug-eluting stents (DES), were a significant breakthrough and highly successful in treating coronary artery disease,9,10 but stents have shown very poor clinical outcomes in treating PAD since they are prone to fracture in the periphery.11 Drug-coated balloons (DCBs) have emerged as a promising approach for treating PAD, but data supporting their long-term clinical efficacy have been inconsistent. Specifically, recent reports have shown an increased risk of death and amputations after local paclitaxel delivery to treat PAD.12
To date, all commercially available DES and DCBs have incorporated paclitaxel or a –limus (e.g., sirolimus, everolimus, zotarolimus) as an anti-proliferative agent to inhibit restenosis. In this study, we investigated an RNA aptamer as an alternative to current therapeutic agents to inhibit restenosis. RNA aptamers are single-stranded nucleic acids whose binding properties depend on their sequence and three-dimensional structure. We have previously identified a VSMC-targeted RNA aptamer (Apt 14) binds to the platelet-derived growth factor receptor (PDGFR)-β to inhibit its phosphorylation and prevent phosphatidylinositol 3-kinase/protein kinase-B (PI3K/Akt) activation.13 In doing so, Apt 14 selectively inhibited migration but not proliferation, of cultured VSMC while having no effect on the migration or proliferation of endothelial cells. Furthermore, Apt 14 attenuated neointimal formation in a murine model of carotid injury when applied to the peri-adventitial surface.13 In this study, we extend these findings toward the next step of potential application to human vascular disease by testing the efficacy of luminal delivery of Apt 14 on neointimal formation in a clinically relevant swine iliofemoral injury model.
Results
Apt 14 inhibits swine VSMC migration
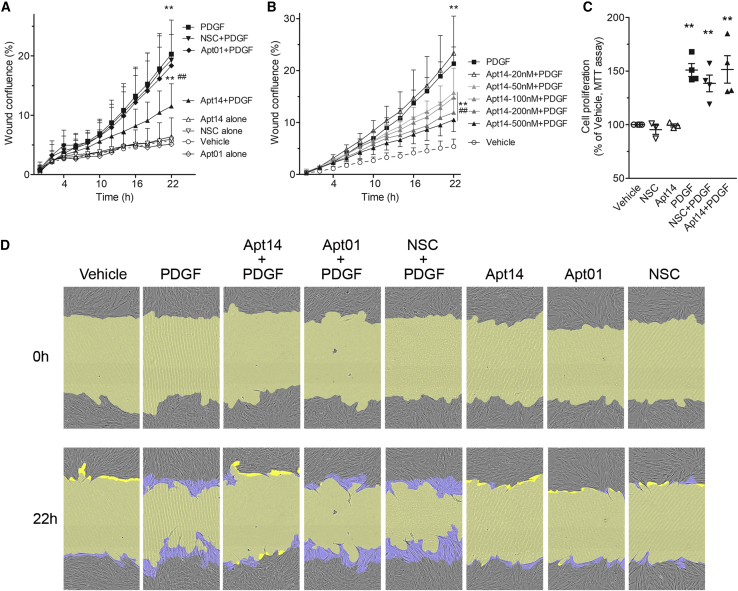
To assess the effect of the RNA aptamers on VSMC function, we first evaluated their effect in vitro on cell migration in response to PDGF-BB (PDGF). As shown in Figure 1A, Apt 14 (200 nM) decreased the migration of PDGF-stimulated swine VMSCs, whereas the non-specific control aptamer (NSC) (200 nM) or Apt 01 (200 nM) had no impact (wound confluence at 22 h: PDGF, 20.31 ± 5.72%; NSC-PDGF, 19.30 ± 6.72%; Apt 01-PDGF, 18.36 ± 5.26%; Apt 14-PDGF, 11.53 ± 3.76%; p < 0.001 vs. PDGF). Also, aptamer alone did not affect cell migration (Figure 1A). A dose dependence of Apt 14 was observed, ranging from 20 nM to 500 nM of Apt 14 (Figure 1B). Despite its ability to inhibit VSMC migration, Apt 14 did not affect PDGF-BB-mediated swine VMSC proliferation (Figure 1C).
Figure 1.
Effect of RNA aptamers on porcine VSMC migration
Cells were cultured to confluence followed by 2-day serum starvation then subject to scratch wound injury and treated with PDGF-BB (5 ng/mL) in the absence or presence of aptamers. Aptamers or vehicle were added to cells 30 min before PDGF. Cell images were captured every 2 h and wound confluence was calculated by the IncuCyte S3 system for more than 22 h. Migration was assessed by wound confluence. (A) Aptamer 14 (Apt 14) but not Apt 01 or NSC decreases PDGF-induced VSMC migration. Aptamers was added at 200 nM. n = 8–12. (B) Dose-response of Apt 14 in inhibition of PDGF-mediated cell migration. n = 3–4. (C) Effect of RNA aptamers (200 nM) on cell proliferation to PDGF-BB (2.5 ng/mL). Aptamers were added 30 min before PDGF. Cells were treated with PDGF for 24 h n = 3–4. (D) Representative cell phase images showing the wound confluence at 0 h and 22 h after PDGF exposure. The initial scratch wound and scratch wound area were masked in blue and yellow, respectively. Migration data were analyzed by two-way ANOVA, repeated measures, with Bonferroni post tests. Proliferation data were analyzed by one-way ANOVA with Tukey post hoc test. ∗p < 0.05, ∗∗p < 0.01 vs. vehicle, #p < 0.05, ##p < 0.01 vs. PDGF.
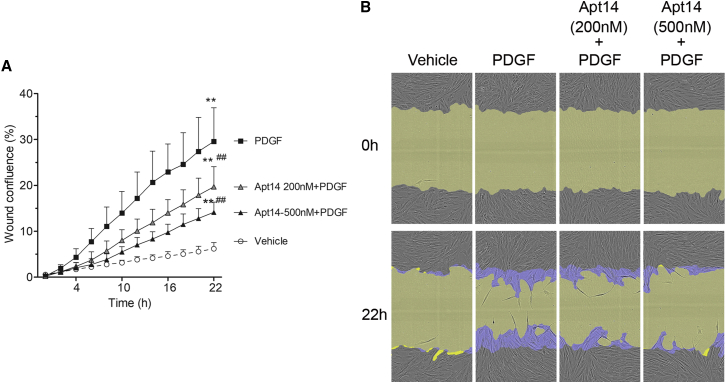
Contrast agent Omnipaque (iohexol) injection 300 (G.E. Healthcare) was used to deliver the aptamers to the vessel. The contrast agent (osmolality at 672 mOsm/kg vs. 285 for average human plasma level) was first diluted with distilled H2O (1mL of 300 + 1.3 mL of H2O) to be isotonic and then diluted two- or four-fold with serum-free DMEM. To ensure that the contrast agent does not affect the ability of Apt 14 to inhibit swine VSMC migration to PDGF, Apt 14 was added to a diluted isotonic contrast agent at both 200 nM and 500 nM concentrations. Results demonstrated that the effectiveness of Apt 14 was not altered in the presence of contrast. Additionally, the higher concentration of Apt 14 resulted in a better inhibition of the VSMC migration to PDGF (Figure 2).
Figure 2.
RNA aptamer 14 (Apt 14) inhibits porcine VSMC migration in the presence of contrast agent
Cells were cultured to confluence then subject to scratch wound injury and treated with PDGF-BB (5 ng/mL). Apt 14 was diluted in isotonic contrast agent at a concentration of 200 nM or 500 nM. (A) Wound confluence was measured by the Incucyte S3 system for more than 22 h. (B) Representative cell phase images were shown at 0 h and 22 h after PDGF exposure. The initial scratch wound and scratch wound area were masked in blue and yellow, respectively. Data were analyzed by two-way ANOVA, repeated measures, with Bonferroni post tests. ∗p < 0.05, ∗∗p < 0.01 vs. vehicle, #p < 0.05, ##p < 0.01 vs. PDGF. n = 4.
Apt 14 inhibits neointimal formation in vivo
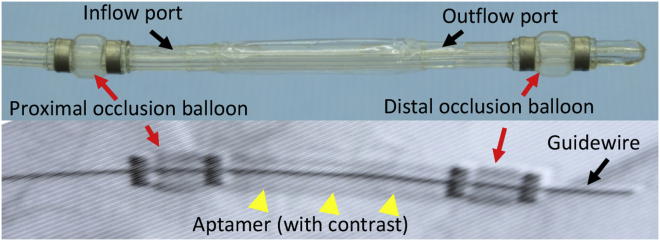
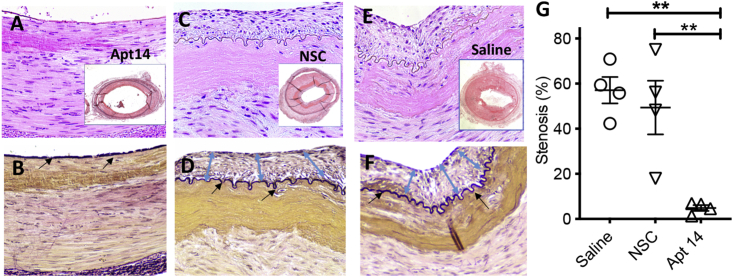
To evaluate the effect on neointimal formation, we delivered Apt 14 directly into the medial vessel wall swine femoral arteries after balloon injury using an endovascular perfusion catheter (Figure 3). We have previously demonstrated that our endovascular approach effectively delivers Apt 14 to the vessel wall.14 The arteries were harvested 14 days after treatment with Apt 14. No evidence of dissection or thrombosis was observed at the treatment site. Histological analysis of the treatment groups showed no differences in internal elastic lamina injury (p = 0.45) and no differences in the measured external elastic lamina (p = 0.30), internal elastic lamina (p = 0.32), and medial area (p = 0.32). The Apt 14 treated group had a larger lumen area (saline 1.86 ± 0.63 mm2 vs. NSC 1.27 ± 0.80 mm2 vs. Apt 14 4.10 ± 2.28 mm2; p = 0.0488) and a smaller neointimal area (saline 2.61 ± 1.08 mm2 vs. NSC 1.50 ± 1.27 mm2 vs. Apt 14 0.244 ± 0.22 mm2; p = 0.023) and percent diameter stenosis (saline 57.13 ± 11.78% vs. NSC 49.38 ± 23.78% vs. Apt 14 4.85 ± 2.54%; p = 0.0140) as compared with controls (Figure 4).
Figure 3.
Angiography showing aptamer being delivered into the arterial wall of a pig iliofemoral artery
Figure 4.
Verhoeff-Van Gieson- and hematoxylin and eosin-stained images of iliofemoral arteries from a pig injury model
The pig arteries were subject to endothelial denudation using a angioplasty balloon catheter, treated with Apt 14 (500 nM), NSC (500 nM) or saline via a perfusion catheter and harvested at 14 days after treatment. (A–F) Neointimal growth is marked by blue double-headed arrows. The internal elastic lamina is marked by black arrows. (G) Percent stenosis graph based on morphometric analysis of the Apt 14-, NSC-. and saline (control)-treated arteries. Data were analyzed by one-way ANOVA with Tukey post tests. ∗p < 0.05, ∗∗p < 0.01. n = 4.
Discussion
This report provides the first demonstration of cell-targeted delivery of a synthetic RNA ligand to inhibit neointimal growth in a preclinical, large animal model of vascular disease. The study confirmed that (i) the RNA aptamer Apt 14 is a potent inhibitor of PDGF-simulated swine VSMC, (ii) the contrast agent had no adverse impact on Apt 14 efficacy, (iii) Apt 14 can be locally delivered in vivo using an endovascular approach, and (iv) Apt 14 attenuates neointimal formation in a clinically relevant preclinical model.
To date, the current endovascular approach to inhibit restenosis after revascularization uses DES or DCBs. These devices deliver anti-proliferative agents such as paclitaxel or –limus drugs onto the luminal surface of the target lesion. For the treatment of PAD, paclitaxel has been the drug of choice, which is not specific to VSMCs and inhibits proliferation in all cells, including endothelial cells. Two recent meta-analyses suggest an increased risk of death after applications of paclitaxel-coated DCBs in the femoropopliteal and infrapopliteal regions.12,15 These studies evaluated multiple randomized trials with long-term follow-up (≤5 years). Although the exact mechanism is unknown, it was postulated that drug (paclitaxel) mobility (with unknown consequences) may have contributed to these findings. Bio-drugs, such as aptamers, can thus play a significant role in developing safer targeted therapeutic options.
For this study, two aptamers (Apt 01 and Apt 14) were selected that demonstrated high selective VSMC internalization and a NSC. Previously, we have shown that both Apt 01 and Apt 14 aptamers were distinct in sequence similarity and structure similarity.16 Apt 14 functions as a smart drug by inhibiting activation of PI3K/Akt signaling in response to multiple agonists by a mechanism that involves inhibition of PDGFR-β phosphorylation.13 Our results confirmed previous observations that Apt 14 inhibits migration of PDGF-stimulated swine VMSCs, whereas the NSC had no impact. Additionally, a dose-dependent response of Apt 14 was observed. For translational purposes, we also confirmed that a radiopaque contrast agent did not affect Apt 14 function.
A primary goal of this study was to demonstrate that intraluminal delivery of a selected RNA aptamer can inhibit injury-induced neointimal growth in a large animal model. Previously, we showed that the aptamer could be locally delivered using a perfusion catheter in an ex vivo porcine artery model.14 At 24 h after delivery, the results demonstrated that Apt 14 maintained and showed greater tissue levels as compared with Apt 01. Furthermore, the study confirmed that the aptamer was successfully delivered into the medial wall of the vessel, targeting the VSMCs. In this study, we evaluated the ability of the aptamer to inhibit neointimal formation in a clinically relevant swine iliofemoral injury model. While it is well recognized that vascular remodeling and repair occurs faster in animals than in humans after balloon injury, animal models can still provide a predictive value for observing biological effects that may be associated with drug delivery.17 Histopathologic evaluation of Apt 14, along with the NSC and saline only, were performed in a swine iliofemoral injury model, which is an appropriate model for evaluating endovascular devices.17 Histological evaluation demonstrated the effectiveness of Apt 14, based on vascular remodeling and healing. Specifically, neointimal area and percent diameter stenosis were significantly reduced in the Apt 14 group compared with controls.
While it is well known that neointimal formation induced by balloon injury depends on VSMC proliferation and migration from the media to the intima, most current therapeutic strategies primarily target cellular proliferation. In this study, we demonstrated that neointimal growth can be decreased by an Aptamer that specifically targets and inhibits VSMC migration, which has not been demonstrated previously in a large clinically relevant animal model. These results could be attributed to two main factors. First, the aptamer specifically target VSMCs, maintaining endothelial cell function and, therefore, natural healing (i.e., re-endothelialization) of the artery. Commonly used anti-proliferative drugs, such as paclitaxel, have shown to be cytotoxic, triggering secondary inflammatory reactions and altering the natural healing process.18 Second, the intraluminal approach delivers the liquid therapy directly to the medial wall, enabling the instantaneous uptake of the aptamer by the VSMCs. This approach differs from traditional endovascular techniques (stents and balloons) in that a high concentration of anti-proliferative therapy is deposited on the luminal surface. In this scenario, the anti-proliferative drugs may not be available or solubilized for uptake by the VSMCs for days, weeks, or months, in addition to inhibiting the natural re-endothelialization process.
The results from this study confirm our previous findings that Apt 14 inhibits neointimal formation in a murine carotid injury model.13 However, in the murine study, aptamer was delivered to the artery via a peri-adventitial application of a pluronic gel. This study implemented a minimally invasive endovascular strategy to deliver the aptamer to the target vessel, providing a more suitable clinical approach. Furthermore, the local liquid delivery approach allows for the potential delivery of multiple aptamers with complementary functions to inhibit VSMC migration, proliferation, and vascular inflammation.
Our results support the concept of the local delivery of Apt 14 to arterial segments to inhibit neointimal growth, however, the study was limited to a healthy animal model and thus did not consider diseased arteries. We also recognize that human lesions are more complex and often include fibrosis, calcification, hemorrhage, and, in most cases, require de-bulking using balloons and atherectomy devices, which may alter aptamer delivery and retention. Last, longer studies (>14 days) are warranted to determine aptamer efficacy on vascular remodeling.
In conclusion, these results provide the first evidence of using a perfusion catheter to directly deliver a VSMC-specific RNA aptamer into the medial wall, suppressing neointimal growth. The cell-specific targeted approach demonstrated in this study may be more effective and have better safety profiles than conventional nontargeted drugs. Additionally, our liquid therapeutic delivery achieves the preferred leave nothing behind strategy in the treatment of occlusive arterial disease.
Materials and Methods
Reagents
High glucose DMEM (11965), phenol red-free DMEM (31053), l-glutamine (25030-081), Minimum Essential Medium Non-Essential Amino Acids (MEM-NEAA) (Gibco, 11140), and penicillin/streptomycin (15140-122) were purchased from Gibco (Grand Island, NY). Fetal bovine serum (FBS, F2442), mouse PDGF-BB (SRP3229), and thiazolyl blue tetrazolium bromide (MTT, M2128) were purchased from Sigma (St. Louis, MO). DMSO (0231) was from VWR (Solon, Ohio). RNA aptamers were chemically synthesized by TriLink Biotechnologies (San Diego, CA) or Integrated DNA Technologies (Coralville, IA) with 2′ fluoro pyrimidines.
Cell culture
Primary porcine VSMCs were isolated from the aorta by enzymatic digestion and cultured in DMEM containing 10% FBS, 1× MEM-NEAA, and 1× penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Briefly, after removal of the adventitia and scraping off of the luminal endothelium, the aorta was cut into very small pieces and digested at 37°C in an enzyme digestion medium containing type 2 collagenase (3 mg/mL), soybean trypsin inhibitor (0.375 mg/mL), elastase (0.5 mg/mL), and BSA (1 mg/mL). After sufficient dissociation (approximately 3 h), a growth medium was added to stop the protease digestion. The isolated cells were collected by centrifugation at 800×g for 5 min then resuspended in a growth medium and cultured in a dish for 1 h to allow the fibroblasts to attach. The medium containing unattached SMCs was transferred to a new dish for culture until cells reached confluence. The characteristic of VSMCs was confirmed by immunostaining for smooth muscle α-actin, a VSMC-specific marker. In the protocols described below, cells were synchronized for 1–2 days in phenol red-free DMEM containing 0.5% FBS or 0% FBS, 1 × l-glutamine and 1 × MEM-NEAA, and then aptamers were added 30 min before mouse PGDF-BB.
RNA aptamers
RNA aptamers were chemically synthesized by TriLink Biotechnologies or Integrated DNA Technologies with 5′ C12-NH2 and 2′ fluoro pyrimidines. The sequences of the aptamers are as follows:
Aptamer 14 5′-GGGAGGACGAUGCGGGCCUUUCGUUCUGACCUCCCCAGACGACUCGCCCGA -3′,
Aptamer 01 5′-GGGAGGACGAUGCGGUCCUGUCGUCUGUUCGUCCCCAGACGACUCGCCCGA-3′
Aptamer NSC 5′-GGGAGGACGAUGCGGAUUACGAGCUUGUUGCCUCGCAGACGACUCGCCCGA -3′.
RNA aptamers were folded at 5 μM in 1× Binding buffer (1×BB, 20 mM HEPES, 150 mM NaCl, 2 mM CaCl2) at 95°C for 5 min, 65°C for 15 min, and then 37°C for 20 min; or at 37°C for 20–30 min.
Migration assays
The migration of VSMCs was determined by scratch wound assay utilizing the WoundMaker-IncuCyte ZOOM-ImageLock plate system (Sartorius AG, Göttingen, Germany). Cells were seeded in 96-well plates at 20,000 cells/well in growth medium overnight, then in 0% FBS-DMEM for 2 days. After a scratch wound was made by the WoundMaker according to the manufacturer’s instructions, cells were treated in 0% FBS-DMEM with NSC, Apt 14, Apt 01, or vehicle (1×BB) for 30 min, and then 10 μL mouse PDGF-BB or vehicle (0% FBS-DMEM). The plates were placed in the IncuCyte, and the cells were imaged every 2 h. The phase images were processed, and wound confluence (%) within the wound region over time was determined by the Incucyte Analysis Software (2019B Rev3). All experiments were conducted in duplicate or triplicate.
Proliferation assays
Cell proliferation was determined by MTT assay. Cells were seeded in 96-well plates at 5000 cells/well in 0.5% FBS-DMEM for 1 day and then treated in 0% FBS-DMEM. Aptamers were added 30 min before PDGF-BB administration. At the indicated times, cell media was replaced with 0.5 mg/mL MTT in serum-free and phenol-free DMEM. After 4 h of incubation, media were removed, cells were dissolved in 100 μL DMSO for 5 min at 37°C, and absorbance was measured at 560 nm using a plate reader (Turner Biosystems, Sunnyvale, CA). Each treatment was conducted in 3–6 replicates.
Pig injury model
This study was approved by the Institutional Animal Care and Use Committee, conformed to the current Guide for the Care and Use of Laboratory Animals, and complies with the ARRIVE guidelines. The experimental preparation of the animal model has been previously reported.19, 20, 21 Six female pigs (12.3–14.1 kg) were pre-medicated with ketamine and midazolam, intubated, and placed on a ventilator. While the animals were ventilated on isoflurane gas anesthesia, the right carotid artery was exposed under a sterile field. The caudal end of the right carotid artery was tied off. Using micro scissors, a small incision was made to the right carotid artery, and a 6F guide sheath was inserted. A NITREX 0.014 guidewire (ev3 Inc., Plymouth, MN) was inserted and, under fluoroscopic guidance, endothelial denudation using a 4 × 12 mm angioplasty balloon catheter (Abbot Vascular, Abbott Park, IL) was performed on the left and right iliac arteries. After denudation, Apt 14, NSC, or saline was delivered via a perfusion catheter (3.0 mm × 30 mm, Advanced Catheter Therapies, Chattanooga, TN) at a luminal delivery pressure of 0.15 atm for 2 min. The perfusion catheter locally delivered 2.5 mL of the aptamer at a concentration of 500 nM. The perfusion catheter has two compliant occlusion balloons (one proximal and one distal), which define the treatment chamber. Luminal pressure, or treatment chamber pressure, was measured in real time via a sensor located within the treatment chamber, which was connected to an external pressure monitor to monitor the pressure during infusion of the liquid drug. For each group, two animals and four arteries were treated with either Apt 14, NSC or saline. Antiplatelet therapy consisted of aspirin (40 mg/day) given orally 24 h before catheterization, while single-dose intra-arterial heparin (150 IU/kg) and lidocaine were administered at the time of catheterization. Fourteen days after treatment, anesthetized animals were euthanized and the treated segments removed based on landmarks identified by angiography. The arteries were perfused with saline and formalin fixed under physiological pressure before removal. The segments were stored in 10% formalin at room temperature and then processed to paraffin blocks, sectioned, and stained with hematoxylin and eosin and Verhoeff-Van Gieson.
Morphometric analysis
Histological sections were digitized, and measurements were performed using ImageJ software (NIH). Cross-sectional area measurements included the external elastic lamina, internal elastic lamina, and lumen area of each section. Using these measurements, the medial area, neointimal area, and percent area stenosis was calculated as previously described.22,23
Statistical analysis
All values were expressed as mean ± standard deviation. Continuous variables were compared between groups using one- or two-way analysis of variance (ANOVA) using GraphPad Prism 9 (GraphPad Software, La Jolla, CA). A value of p of 0.05 or less was considered statistically significant. If statistical significance was shown, a comparison of quantitative data of multiple groups was performed by Tukey’s multiple comparisons post hoc test or Bonferroni post hoc test.
Data and materials availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
F.J.M. is supported by the Office of Research and Development, Department of Veterans Affairs [I01BX001729], and the National Institutes of Health [1R01EB028798]. S.K.Y. is supported by the National Institute of Health [1R01EB028798]. The content of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
Author contributions
Writing – original conceptualization: S.K.Y., P.H.G., and F.J.M.; Methodology: S.K.Y., B.L., and F.J.M.; Data acquisition, curation, and analysis: S.K.Y., C.H., B.L., and F.J.M.; Resources: S.K.Y. and F.J.M.; Draft: S.K.Y., B.L., and F.J.M.; Writing – review and editing: S.K.Y., C.V.C., K.C., C.H., B.L., P.H.G., and F.J.M.; Supervision: S.K.Y. and F.J.M.
Declaration of interests
S.K.Y. serves on the Scientific Advisory Board of Advanced Catheter and has received grant support from Advanced Catheter Therapies, Alucent Biomedical, Toray Industries, Biosensors International, Advanced NanoTherapies, OrbusNeich Medical, and BD. The other co-authors have no conflict of interest to report.
References
- 1.Mahoney E.M., Wang K., Keo H.H., Duval S., Smolderen K.G., Cohen D.J., Steg G., Bhatt D.L., Hirsch A.T., Reduction of Atherothrombosis for Continued Health REACH Registry Investigators Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ. Cardiovasc. Qual. Outcomes. 2010;3:642–651. doi: 10.1161/circoutcomes.109.930735. [DOI] [PubMed] [Google Scholar]
- 2.Adam D.J., Beard J.D., Cleveland T., Bell J., Bradbury A.W., Forbes J.F., Fowkes F.G.R., Gillepsie I., Ruckley C.V., Raab G., Storkey H., BASIL trial participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/s0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 3.Conte M.S., Bandyk D.F., Clowes A.W., Moneta G.L., Seely L., Lorenz T.J., Namini H., Hamdan A.D., Roddy S.P., Belkin M., et al. PREVENT III Investigators Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 4.Schillinger M., Sabeti S., Loewe C., Dick P., Amighi J., Mlekusch W., Schlager O., Cejna M., Lammer J., Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 5.Schillinger M., Sabeti S., Dick P., Amighi J., Mlekusch W., Schlager O., Loewe C., Cejna M., Lammer J., Minar E. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/circulationaha.107.688341. [DOI] [PubMed] [Google Scholar]
- 6.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G.R., TASC II Working Group. Caporusso J., Durand-Zaleski I., Komori K., Lammer J., et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur. J. Vasc. Endovasc. Surg. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Tsetis D., Belli A.M. Guidelines for stenting in infrainguinal arterial disease. Cardiovasc. Intervent. Radiol. 2004;27:198–203. doi: 10.1007/s00270-004-0029-1. [DOI] [PubMed] [Google Scholar]
- 8.Tosaka A., Soga Y., Iida O., Ishihara T., Hirano K., Suzuki K., Yokoi H., Nanto S., Nobuyoshi M. Classification and clinical impact of restenosis after femoropopliteal stenting. J. Am. Coll. Cardiol. 2012;59:16–23. doi: 10.1016/j.jacc.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Stone G.W., Ellis S.G., Cox D.A., Hermiller J., O'Shaughnessy C., Mann J.T., Turco M., Caputo R., Bergin P., Greenberg J., et al. TAXUS-IV Investigators A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 10.Morice M.C., Serruys P.W., Sousa J.E., Fajadet J., Ban Hayashi E., Perin M., Colombo A., Schuler G., Barragan P., Guagliumi G., et al. RAVEL Study Group. Randomized Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 11.Scheinert D., Scheinert S., Sax J., Piorkowski C., Bräunlich S., Ulrich M., Biamino G., Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Katsanos K., Spiliopoulos S., Kitrou P., Krokidis M., Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic Review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018;7:e011245. doi: 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiel W.H., Esposito C.L., Dickey D.D., Dassie J.P., Long M.E., Adam J., Streeter J., Schickling B., Takapoo M., Flenker K.S., et al. Smooth muscle cell-targeted RNA aptamer inhibits neointimal formation. Mol. Ther. 2016;24:779–787. doi: 10.1038/mt.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udofot O., Lin L.H., Thiel W.H., Erwin M., Turner E., Miller F.J., Jr., Giangrande P.H., Yazdani S.K. Delivery of cell-specific aptamers to the arterial wall with an occlusion perfusion catheter. Mol. Ther. Nucleic Acids. 2019;16:360–366. doi: 10.1016/j.omtn.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsanos K., Spiliopoulos S., Kitrou P., Krokidis M., Paraskevopoulos I., Karnabatidis D. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J. Vasc. Interv. Radiol. 2020;31:202–212. doi: 10.1016/j.jvir.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Thiel W.H., Bair T., Peek A.S., Liu X., Dassie J., Stockdale K.R., Behlke M.A., Miller F.J., Jr., Giangrande P.H. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS One. 2012;7:e43836. doi: 10.1371/journal.pone.0043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz R.S., Chronos N.A., Virmani R. Preclinical restenosis models and drug-eluting stents: still important, still much to learn. J. Am. Coll. Cardiol. 2004;44:1373–1385. doi: 10.1016/j.jacc.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Stampfl U., Radeleff B., Sommer C., Stampfl S., Lopez-Benitez R., Thierjung H., Kurz P., Berger I., Richter G.M. Paclitaxel-induced arterial wall toxicity and inflammation: part 2--long-term tissue response in a minipig model. J. Vasc. Interv. Radiol. 2009;20:1608–1616. doi: 10.1016/j.jvir.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Yazdani S.K., Sheehy A., Nakano M., Nakazawa G., Vorpahl M., Otsuka F., Donn R.S., Perkins L.E., Simonton C.A., Kolodgie F.D., Virmani R. Preclinical evaluation of second-generation everolimus- and zotarolimus-eluting coronary stents. J. Invasive Cardiol. 2013;25:383–390. [PubMed] [Google Scholar]
- 20.Turner E., Erwin M., Atigh M., Christians U., Saul J.M., Yazdani S.K. In vitro and in vivo assessment of Keratose as a novel excipient of paclitaxel coated balloons. Front. Pharmacol. 2018;9:808. doi: 10.3389/fphar.2018.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atigh M.K., Goel E., Erwin M., Greer R., 2nd, Ohayon J., Pettigrew R.I., Yazdani S.K. Precision delivery of liquid therapy into the arterial wall for the treatment of peripheral arterial disease. Sci. Rep. 2021;11:18676. doi: 10.1038/s41598-021-98063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel E., Erwin M., Cawthon C.V., Schaff C., Fedor N., Rayl T., Wilson O., Christians U., Register T.C., Geary R.L., et al. Pre-clinical investigation of Keratose as an excipient of drug coated balloons. Molecules. 2020;25 doi: 10.3390/molecules25071596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yazdani S.K., Pacheco E., Nakano M., Otsuka F., Naisbitt S., Kolodgie F.D., Ladich E., Rousselle S., Virmani R. Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug-coated balloon in a swine femoral artery model. Catheter. Cardiovasc. Interv. 2014;83:132–140. doi: 10.1002/ccd.24995. [DOI] [PubMed] [Google Scholar]