Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) has been linked to gallstone disease (GSD) in observational studies; however, the relationships between certain lipid profiles and GSD remain unclear.
Methods
We adopted a two-sample Mendelian randomization (MR) framework by applying different statistical methods to assess causalities between lipid profiles and GSD. We identified single-nucleotide polymorphisms (SNPs) for blood lipids and NAFLD from separate previous genome-wide association studies (GWASs).
Results
We retrieved GSD SNPs attributed to 10,520 cases and 361,194 controls and validated our estimates using GWAS summary data from UK Biobank. We also performed sex-stratified analyses. Based on the summary estimates of 41, 59, 35, and 2 SNPs for low-density lipoprotein cholesterol (LDLC), high-density lipoprotein cholesterol (HDLC), triglycerides (TGs), and NAFLD, respectively, we found no evidence of a causal relationship between genetically-predicted lipid profiles and GSD. The odds ratios were 0.995 for LDLC [95% confidence interval (CI): 0.994–0.998] per 0.98 mmol/L, 0.999 for HDLC (95% CI: 0.996–1.003) per 0.41 mmol/L, 0.997 for TGs (95% CI: 0.994–1.001) per 1 mmol/L, and 0.993 for NAFLD (95% CI: 0.984–1.003). No evidence of associations between lipid profile s and GSD in validation MR analyses or the sex-stratification analyses was noted.
Conclusions
Genetically predicted hyperlipidemia or NAFLD is not causally associated with GSD.
Keywords: Blood lipids, nonalcoholic fatty liver disease (NAFLD), gallstone disease (GSD), Mendelian randomization (MR)
Introduction
Gallstone disease (GSD), also known as cholelithiasis, is one of the most common and costly known gastrointestinal diseases (1-3) and affects 10.5–15% of the population in the developed world (4). The prevalence of GSD varies by race, with the highest (48%) seen among Native Americans and Hispanics, the lowest (5%) recorded in African populations, and midrange figures reported in Asian populations (5–20%) (5-10). GSD is the most common digestive disease leading to hospital admissions in Europe and the USA. An estimated 1.8 million ambulatory care visits result in diagnosis of GSD annually, with an associated treatment cost of $6.2 billion in the USA (11). Complications of GSD include cholecystitis, cholangitis, and pancreatitis. In addition, GSD is an important risk factor for gallbladder cancer (12) and is associated with significant complications and poor patient prognosis. Consequently, reducing the prevalence of GSD may also yield benefits in the clinical treatment of gallbladder cancer.
It is known that obesity is a risk factor for GSD (1,2), and obesity tends to be associated with unhealthily high levels of blood lipids and fatty liver disease (3,4). The association between hyperlipidemia and GSD is, however, controversial. Several clinical studies, mostly observational investigations and systematic reviews, have reported a positive correlation between hyperlipidemia and GSD (13,14). However, an epidemiological study found that blood lipid profiles did not differ in patients with and without GSD (5). Meanwhile, Ferkingstad et al. reported that blood lipid levels of low-density lipoprotein cholesterol (LDLC) are not causative factors in gallstone formation (15). Most obese individuals suffer from nonalcoholic fatty liver disease (NAFLD), and previous clinical retrospective observational studies have reported that NAFLD is an independent risk factor for GSD (16,17). Importantly, observational research can easily be influenced by confounding factors and sample size, and stronger evidence is needed to verify the relationship between lipid profiles in the blood or liver and GSD. Drugs to reduce lipid levels are widely used for asymptomatic GSD patients, but the use of these drugs is linked to many side effects, and further research is needed to guide treatment (18).
Mendelian randomization (MR) is a useful method by which causal associations may be inferred through the adoption of genetic information such as single-nucleotide polymorphisms (SNPs) or copy number variations as instrumental variables to test for causality (19-21). MR takes advantage of the random segregation of alleles inherited by offspring from their parents during meiosis. An MR study is analogous to random allocation of the treatment in a randomized controlled trial and can overcome both reverse causation and confounding (22). In this study, we sought to identify any causal relationships between lipid profiles in the blood or liver and GSD using the MR method in 2 steps. First, we used two-sample Mendelian randomization (TSMR) analysis to estimate the causal effect of lipid profiles on GSD. Second, we validated the estimates using one-sample MR analysis. We present the following article in accordance with the STREGA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4007/rc).
Methods
Genome-wide association studies (GWASs) data of blood lipids
We selected genetic variants that were associated with blood lipids, including LDLC, high-density lipoprotein cholesterol (HDLC), and triglycerides (TGs), at a genome-wide significance level in the Global Lipids Genetics Consortium (GLGC) (23) covering data from 60 studies. We selected summary estimates of 126 SNPs that (I) have been shown to be associated with blood lipids in the GLGC GWAS (P<5×10−8) and included 188,577 participants (90% European ancestry), and that (II) were independent variants, using data from the 1000 Genomes Project (linkage disequilibrium threshold of r2<0.001 and located 1 Mb apart from each other (Tables S1-S3). A detailed description of the statistical methods and quality-control efforts was provided in a previous publication by the GLGC (23). The effect sizes were calculated with respect to the effect allele per 1 standard deviation increase in the plasma lipid level (which was equal to 0.98 mmol/L for LDLC, 0.41 mmol/L for HDLC, and 1 mmol/L for TGs).
GWAS data of NAFLD
NAFLD ranges from hepatic steatosis to steatohepatitis and, finally, to fibrosis. Computed tomography can be used to measure hepatic steatosis, while steatohepatitis or fibrosis must be assessed histologically. We selected the significant SNPs (P<5×10−8) associated with hepatic steatosis and histologic NAFLD from the largest-to-date GWAS study (24,25). Patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 and transmembrane 6 superfamily member 2 (TM6SF2) rs58542926, the 2 strongest genetic predictors of NAFLD, were used as proxies for hepatic steatosis and histologic NAFLD (25). Because rs58542926 was not genotyped in most of the GWAS summary data used in this investigation, rs2228603 at the NCAN gene locus, which exists in strong linkage disequilibrium with rs58542926 (pairwise R2=0.76 based on the phase III data of the 1000 Genomes Project in European individuals) and which is significantly associated with liver fat content (26), was used in place of TM6SF2 rs58542926.
GWAS data of LDLC, HDLC, TGs, and GSD in UK Biobank
We used data from UK Biobank, one of the largest available prospective cohort study databases, which includes more than 500,000 participants (aged 40–69 years) recruited between 2006 and 2010. The biochemical assays, genotyping, and follow-up of the study design have been published elsewhere (27). UK Biobank GWAS results are available for 371,714 unrelated individuals of European ancestry from Neale Lab (http://www.nealelab.is/uk-biobank/). Genetic associations of both sexes in combination and individually, together with LDLC, HDLC, and TGs, were obtained for validation analyses, where the associations (sex, age, age-squared, the interaction of sex and age, and the interaction of sex and age-squared) were discerned via multivariable linear regression adjusted for the first 20 principal components (28). The trait phenotypes for LDLC, HDLC, and TGs can be found on the UK Biobank showcase using codes 30780, 30706, and 30870, respectively. Unfortunately, the sample size for the NAFLD phenotype present in UK Biobank was insufficient for us to have any confidence in the results, so we did not make use of the NAFLD phenotype data from UK Biobank.
Genetic associations of both sexes in combination and individually with GSD were obtained from UK Biobank summary statistics provided by Neale Lab (Cambridge, MA, USA) as outcomes. The GSD phenotype could be found as part of the International Classification of Diseases, 10th revision code listings on the UK Biobank showcase using code 41202.
Statistical analysis
The instrument variables were first assessed to discern whether they were robustly associated with their lipid traits by computing the proportion of variance explained and the F score values. For MR estimation with LDLC, HDLC, TGs, and NAFLD as the exposure variables and GSD as the outcome variable, MR-pleiotropy residual sum and outlier (MR-PRESSO) was used to identify and remove outliers at a P value <0.05. After dropping the outliers, we harmonized the summary data from the exposure and outcome parameters to ensure that the effect of an SNP on the exposure and the effect of the same SNP on the outcome each corresponded to the same allele (29). We employed 4 different methods to estimate the causal association between the lipid profiles and GSD: inverse variance-weighting (IVW) (random-effects model), MR-Egger, weighted median, and simple median. We adopted Cochran’s Q test to assess the heterogeneity. In addition to the heterogeneity test, we used the MR-Egger regression method to test for horizontal pleiotropy (30). Heterogeneity can be revealed by a scatterplot, while horizontal pleiotropy can be represented by a forest plot and funnel plot. We considered the association as causal if the directions of the estimates were consistently determined by at least 3 methods. Furthermore, we performed one-sample MR analyses using the LDLC, HDLC, TGs, and GSD GWAS summary data of combined genders from UK Biobank as a validation data set. To conduct sex-stratified analyses, we performed one-sample MR analysis on female- or male-specific GWAS summary data of LDLC, HDLC, TGs, and GSD from UK Biobank with the same SNPs chosen as instrument variables as were used in the previous TSMR analysis.
In addition to the 4 different MR methods, leave-one-out sensitivity analysis was conducted to test the robustness of the MR estimation by excluding a single variant from the analysis at a time. The fluctuation of the estimates in response to this exclusion reflected the influence of the variant in the causal estimation.
Notably, some of the instrument variables used in the previous MR analyses were associated with more than one lipid profile. Meanwhile, multivariable MR has an advantage over univariate MR in that it accounts for potential pleiotropic influence. We conducted multivariable MR using the IVW method to estimate the direct causal effect of LDLC, HDLC, and TGs on the outcomes by applying the method to the complete set of 126 lipid-associated SNPs. All MR analyses were performed using the “MendelianRandomization”, “TwoSampleMR”, and “MRPRESSO” packages in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
The GWAS summary data used for MR analyses in this investigation are publicly available (23,24). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
No causal effect of hyperlipidemia on GSD
The characteristics of the populations included in the GLGC and UK Biobank are shown in Table 1. We first selected SNPs that could serve as valid instrumental variables for each blood lipid (LDL, HDL, and TGs) in the European population based on association summary statistics from the GLGC study. From the GLGC study, following MR-PRESSO and harmonization correction, we obtained a total of 41, 59, and 35 index SNPs to serve as instrumental variables for LDL, HDL, and TGs, respectively (Tables S1,S2,S4). The selected SNPs in total explained 6.90%, 3.67%, or 4.27% of the observed phenotypic variance for LDL, HDL, or TGs, respectively. Importantly, the F score values for these SNPs were 150.3, 59.9, and 116.7, respectively, all of which were larger than 10, suggesting that the selected SNPs had a sufficiently strong effect to serve as valid instruments and that weak instrument bias was unlikely to occur.
Table 1. Characteristics of Global Lipids Genetics Consortium and UK Biobank datasets.
| Exposure/outcome | Datasets | No. SNPs | Sample size (No. of cases) | Population |
|---|---|---|---|---|
| LDL-cholesterol | GLGC | 41 | 83,198 | 90% European |
| HDL-cholesterol | GLGC | 59 | 92,860 | 90% European |
| Triglycerides | GLGC | 35 | 91,598 | 90% European |
| Hepatic steatosis | GOLD | 2 | 7,176 | 100% European |
| Histological NAFLD | AGES | 2 | 2,868 | 100% European |
| LDL-cholesterol | UK Biobank | 41 | 343,621 | 100% European |
| HDL-cholesterol | UK Biobank | 59 | 315,133 | 100% European |
| Triglycerides | UK Biobank | 35 | 343,992 | 100% European |
| Main outcome | ||||
| Gallstone disease | UK Biobank | 371,714 (10,520) | 100% European |
LDL, low-density lipoprotein; HDL, high-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; GLGC, Global Lipids Genetics Consortium; GOLD, Genetics of Obesity-related Liver Disease; AGES, Age, Gene/Environment Susceptibility-Reykjavik Study; SNPs, single-nucleotide polymorphisms.
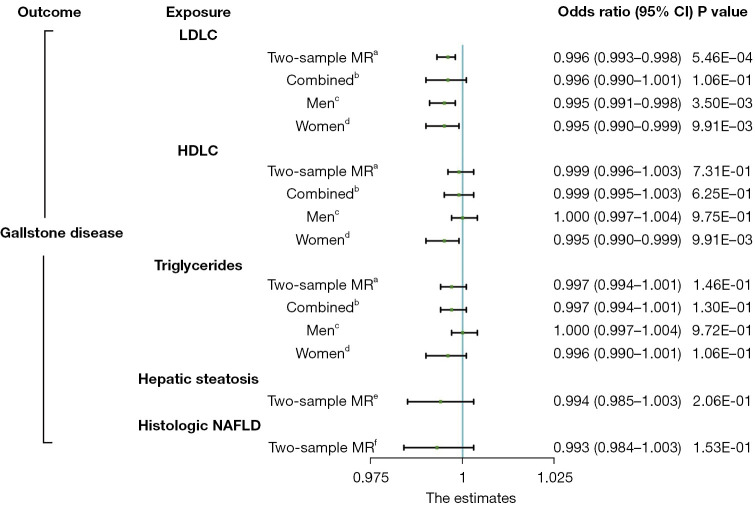
In UK Biobank, we identified 10,520 participants with GSD and subsequently obtained association summary statistics of GSD from UK Biobank for the selected instrumental variables of the blood lipids. To investigate the potential association between blood lipids and GSD, we applied 4 different methods to complete TSMR analyses (Table 2, Figures S1-S4). The IVW analysis indicated a marginal negative association between the LDLC level and GSD [odds ratio (OR) 0.995; 95% confidence interval (CI): 0.994–0.998; P<0.001]. Meanwhile, no evidence was found for a causal relationship between the HDLC level and GSD (OR 0.999, 95% CI: 0.996–1.003; P=0.731) or the TGs level and GSD (OR 0.997, 95% CI: 0.994–1.001; P=0.146). These results suggested that genetically predicted blood lipid levels were not associated with GSD. The results of the TSMR analyses were consistent in the four methods.
Table 2. Two-sample Mendelian randomization estimations showing the effect of lipids on GSD.
| Exposure | Methods | Odds ratioa | 95% CI | P value | Ph | Q-statistics | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| LDLC | IVW | 0.996 | 0.993 | 0.998 | 5.46E−04 | 9.06E−03 | 64.1 |
| MR-Egger | 0.995 | 0.992 | 0.999 | 7.97E−03 | 7.33E−03 | 63.8 | |
| Weighted median | 0.997 | 0.994 | 1.000 | 4.25E−02 | – | – | |
| Simple median | 0.997 | 0.993 | 1.001 | 1.06E−01 | – | – | |
| MR-Egger interceptb | 0.0001 | −0.0002 | 0.0003 | 6.57E−01 | – | – | |
| HDLC | IVW | 0.999 | 0.996 | 1.003 | 7.31E−01 | 2.76E−04 | 102.6 |
| MR-Egger | 0.997 | 0.989 | 1.004 | 3.51E−01 | 2.82E−04 | 101.2 | |
| Weighted median | 0.997 | 0.993 | 1.002 | 2.48E−01 | – | – | |
| Simple median | 0.998 | 0.993 | 1.003 | 4.06E−01 | – | – | |
| MR-Egger interceptb | 0.0002 | −0.0002 | 0.0005 | 3.77E−01 | – | – | |
| Triglycerides | IVW | 0.997 | 0.994 | 1.001 | 1.46E−01 | 3.79E−01 | 35.9 |
| MR-Egger | 0.993 | 0.987 | 0.999 | 2.98E−02 | 4.70E−01 | 32.9 | |
| Weighted median | 0.998 | 0.993 | 1.003 | 4.17E−01 | – | – | |
| Simple median | 1.003 | 0.996 | 1.009 | 4.39E−01 | – | – | |
| MR-Egger interceptb | 0.0001 | 0.0000 | 0.0005 | 9.45E−02 | – | – | |
| Hepatic steatosis | IVW | 0.994 | 0.985 | 1.003 | 2.06E−01 | 4.63E−03 | 8.0 |
| Histologic NAFLD | IVW | 0.993 | 0.984 | 1.003 | 1.53E−01 | 8.88E−03 | 6.8 |
a, odds ratio per 1 SD increase; b, regression coefficient (95% CI). GSD, gallstone disease; CI, confidence interval; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; Ph, P value for heterogeneity; SD, standard deviation; IVW, inverse variance-weighting; MR, Mendelian randomization.
Cochran’s Q test indicated that there was significant heterogeneity for LDLC and HDLC (Table 2). However, the leave-one-out analyses did not materially change the results of the TSMR estimate. The funnel and forest plots showed an absence of directional pleiotropy, with a symmetrical distribution of variant effects (Figures S4-S12). To validate the estimate, we performed one-sample MR analyses with the identified SNPs using the GWAS summary data of LDLC, HDLC, TGs, and GSD from UK Biobank. The resultant findings were similar to those of the TSMR analyses (Figure 1, Table S5).
Figure 1.
Comparison of the total causal estimations with heterogeneity and pleiotropic effect between lipid profiles and gallstone disease risk being considered via Mendelian randomization. a, two-sample MR analysis of the Global Lipids Genetics Consortium study and the UK Biobank cohort; b, one-sample MR analysis of all participants in the UK Biobank cohort; c, one-sample MR analysis of male participants in the UK Biobank cohort; d, one-sample MR analysis of female participants in the UK Biobank cohort; e, two-sample MR analysis of the Genetics of Obesity-Related Liver Disease study and the UK Biobank cohort; f, two-sample MR analysis of the Age, Gene/Environment Susceptibility-Reykjavik study and the UK Biobank cohort. CI, confidence interval; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; MR, Mendelian randomization.
Female sex has been identified as a risk factor for GSD (31). To investigate whether any of the 3 blood lipids showed evidence of sex-specific effects, we performed a sex-stratified MR analysis on sex-specific GWAS data from UK Biobank. No evidence was found to support an association between blood lipids and GSD in either men or women (Figure 1, Tables 3,4). No heterogeneity or pleiotropy was apparent between blood lipids and GSD in either sex (Figures S1-S3). In summary, our MR study did not support a causal association between hyperlipidemia and GSD.
Table 3. Mendelian randomization estimations showing the effect of lipid profiles on GSD in male.
| Exposure | Methods | Odds ratioa | 95% CI | P value | Ph | Q-statistics | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| LDLC | IVW | 0.995 | 0.991 | 0.998 | 3.50E−03 | 8.25E−02 | 52.9 |
| MR-Egger | 0.994 | 0.990 | 0.999 | 1.82E−02 | 6.90E−02 | 52.8 | |
| Weighted median | 0.993 | 0.989 | 0.998 | 6.69E−03 | – | – | |
| Simple median | 0.991 | 0.985 | 0.998 | 6.91E−03 | – | – | |
| MR-Egger interceptb | 0.0001 | −0.0002 | 0.0003 | 7.51E−01 | – | – | |
| HDLC | IVW | 1.000 | 0.997 | 1.004 | 9.75E−01 | 4.05E−02 | 78.1 |
| MR-Egger | 0.998 | 0.992 | 1.004 | 4.53E−01 | 4.14E−02 | 76.8 | |
| Weighted median | 1.000 | 0.995 | 1.005 | 9.16E−01 | – | – | |
| Simple median | 1.000 | 0.995 | 1.006 | 9.62E−01 | – | – | |
| MR-Egger interceptb | 0.0001 | −0.0001 | 0.0004 | 3.34E−01 | – | – | |
| Triglycerides | IVW | 1.000 | 0.997 | 1.004 | 9.72E−01 | 4.47E−01 | 34.4 |
| MR-Egger | 0.998 | 0.993 | 1.003 | 4.67E−01 | 4.48E−01 | 33.4 | |
| Weighted median | 1.000 | 0.995 | 1.006 | 8.63E−01 | – | – | |
| Simple median | 1.003 | 0.996 | 1.010 | 3.60E−01 | – | – | |
| MR-Egger interceptb | 0.0001 | −0.0001 | 0.0004 | 3.18E−01 | – | – | |
a, odds ratio per 1 SD increase; b, regression coefficient (95% CI). GSD, gallstone disease; CI, confidence interval; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; Ph, P value for heterogeneity; SD, standard deviation; IVW, inverse variance-weighting; MR, Mendelian randomization.
Table 4. Mendelian randomization estimations showing the effect of lipid profiles on GSD in female.
| Exposure | Methods | Odds ratioa | 95% CI | P value | Ph | Q-statistics | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| LDLC | IVW | 0.995 | 0.990 | 0.999 | 9.91E−03 | 6.95E−02 | 53.9 |
| MR-Egger | 0.995 | 0.989 | 1.000 | 5.66E−02 | 5.63E−02 | 53.9 | |
| Weighted median | 0.996 | 0.991 | 1.001 | 9.88E−02 | – | – | |
| Simple median | 0.992 | 0.985 | 0.999 | 1.72E−02 | – | – | |
| MR-Egger interceptb | 0.0002 | −0.0004 | 0.0004 | 9.79E−01 | – | – | |
| HDLC | IVW | 0.999 | 0.994 | 1.003 | 5.57E−01 | 2.48E−02 | 81.0 |
| MR-Egger | 0.996 | 0.988 | 1.005 | 4.26E−01 | 2.19E−02 | 80.5 | |
| Weighted median | 0.996 | 0.99 | 1.003 | 2.81E−01 | – | – | |
| Simple median | 0.997 | 0.99 | 1.004 | 4.67E−01 | – | – | |
| MR-Egger interceptb | 0.0002 | −0.0003 | 0.0006 | 5.67E−01 | – | – | |
| Triglycerides | IVW | 0.996 | 0.990 | 1.001 | 1.06E−01 | 2.56E−01 | 39.0 |
| MR-Egger | 0.992 | 0.983 | 1.000 | 7.30E−02 | 2.66E−01 | 37.6 | |
| Weighted median | 0.993 | 0.984 | 1.001 | 8.80E−02 | – | – | |
| Simple median | 0.998 | 0.989 | 1.008 | 7.45E−01 | – | – | |
| MR-Egger interceptb | 0.0002 | −0.0002 | 0.0007 | 2.84E−01 | – | – | |
a, odds ratio per 1 SD increase; b, regression coefficient (95% CI). GSD, gallstone disease; CI, confidence interval; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; Ph, P value for heterogeneity; SD, standard deviation; IVW, inverse variance-weighting; MR, Mendelian randomization.
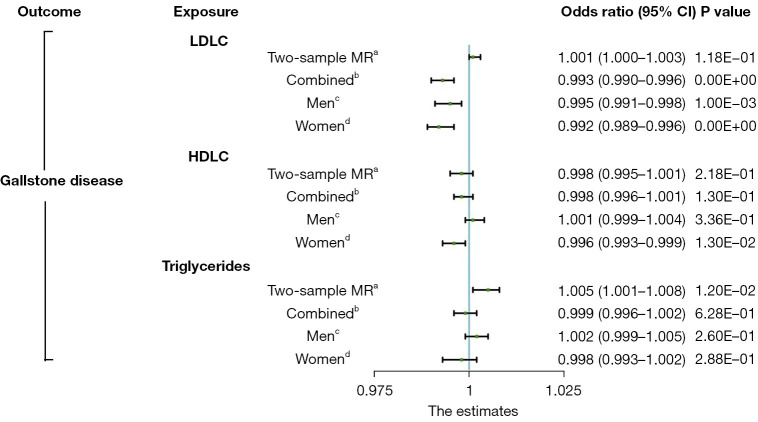
In the leave-one-out analysis, we confirmed that no single genetic variant was strongly driving the overall effect of each lipid profile on GSD (Figures S13-S15). In the multivariable MR analysis that adjusted for the effect of each blood lipid, the results remained unchanged (Figure 2, Table S6). The multivariable-adjusted β values were 0.002 (95% CI: –0.001 to 0.005; P=0.261) for LDLC, 0.000 (95% CI: –0.006 to 0.006; P=0.983) for HDLC, and 0.005 (95% CI: –0.002 to 0.013; P=0.148) for TGs (Table S2).
Figure 2.
Comparison of the direct causal estimates between plasma lipids and gallstone disease risk via multivariable Mendelian randomization. a, two-sample MR analysis of the Global Lipids Genetics Consortium study and the UK Biobank cohort; b, one-sample MR analysis of all participants in the UK Biobank cohort; c, one-sample MR analysis of male participants in the UK Biobank cohort; d, one-sample MR analysis of female participants in the UK Biobank cohort. CI, confidence interval; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; MR, Mendelian randomization.
No causal effect of NAFLD on GSD
We used 2 well-established hepatic steatosis-associated variants as genetic instruments to test the causal effect of hepatic steatosis on GSD (Table S4). The 2 SNPs explained 3.2% of the variance in hepatic steatosis and the mean F sore value was 118.56. With only 2 SNPs used as instrument variables, we performed a conventional MR analysis using the IVW method on GSD (Figures S4,S8). As listed in Table 2, we observed no significant association between genetically instrumented hepatic steatosis and GSD (OR 0.994, 95% CI: 0.985–1.003; P=0.206).
We further tested whether a genetically increased risk for histologic NAFLD has a different effect on GSD as compared to that of hepatic steatosis. Consistent with the results of hepatic steatosis, however, no significant causal relationship was found between genetically driven histologic NAFLD and GSD (Table 2). Taken together, the results of our MR study did not support a causal association between NAFLD and GSD.
Discussion
To our knowledge this was the first large-scale study to assess the causal relationship between lipid profiles in the blood or liver and GSD, and our results suggest that hyperlipidemia and NAFLD are not causally associated with the risk of GSD. This finding was robust and consistent in the various sensitivity analyses including 4 different MR methods, the validation dataset, sex-stratified assessment, and multivariable MR analysis.
Cholesterol, phospholipid, and bile salts are three major lipid components of bile, and cholesterol supersaturation leads to the precipitation of cholesterol monohydrate crystals followed by agglomeration of the crystals into macroscopic stones (32-36). Results from previous observational studies and reviews showed that hyperlipidemia is a risk factor for GSD (37-40), but the association between each blood lipid and GSD is still controversial. Atamanalp et al. found that high LDLC levels were associated with high GSD rates but that low HDLC levels were not (39). However, Andreotti et al. reported that high levels of TGs and low levels of HDLC were significantly associated with an increased risk of GSD, while LDLC levels were inversely associated with risk of GSD (40). To date, the conclusions of the relevant research have been inconsistent. Given the limitations of these observational studies, these results might have been driven by biases such as unmeasured confounders or reverse causation (21).
Contrary to previous observational studies, Ferkingstad et al. used binomial testing and found that lipid serum levels were not in themselves causative factors in gallstone formation (15). Supporting this finding, Stender et al. reported that elevated levels of LDLC were not causally associated with an increased risk of GSD in a one-sample MR study that included 3,323 cases of GSD (41). In our study, each type of blood lipid was considered separately, and hence our MR analysis had a higher power to confirm that there was no causal association between hyperlipidemia and GSD.
Hepatic steatosis and GSD are commonly found to coexist (19,42-44), and NAFLD and its severity have been independently associated with an increase in GSD (45). However, previous studies were observational investigations, and it has been difficult to perform randomized controlled trials for NAFLD and GSD. It therefore remains unclear whether there is a causal association between NAFLD and GSD. Aside from this, our MR study detected no causal association between genetically driven hepatic steatosis or histologic NAFLD and GSD.
One of the key strengths of our study is that it included 2 very large GWASs with more than 700,000 participants, helping to overcome the power limitations of MR analysis and facilitate the application of several analytical approaches. MR studies are also more robust against confounding than are traditional observational studies because an individual’s genetically determined risk for a given condition is fixed throughout their lifetime. Since MR analysis has a high assumption level (46,47), we performed sensitivity analyses, heterogeneity testing, and pleiotropy testing, all of which supported the main findings. To avoid weak instrument bias, we only selected SNPs strongly associated with exposure, and the F score values were all larger than 10 for each instrument variable.
In conclusion, this MR study indicates that genetically predicted lipid profiles are not causally associated with GSD in and of themselves. However, like many other MR analyses, this study has several limitations. First, although our findings with respect to the effect of hyperlipidemia and NAFLD on GSD are consistent in TSMR and one-sample MR analyses, the instrument variables only explain approximately 3–8% of the variance of exposure, and thus this study might have been underpowered to detect medium to small effects. Second, with the use of publicly available summary-level GWAS data, we only stratified analyses by sex and were unable to stratify analyses by other covariates of interest such as age, body mass index, and sex hormones. Finally, by using the GLGC study and UK Biobank cohort the majority of participants in our research were of European ancestry, and we were therefore unable to investigate the relationship between lipid profiles and GSD in Asian and African populations.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the participants in the Global Lipids Genetics Consortium study, UK Biobank cohort, the Genetics of Obesity-related Liver Disease study, and the Age, Gene/Environment Susceptibility-Reykjavik study. The authors acknowledge high-quality GWAS resources provided by consortium for researchers. Summary statistic data are available from the corresponding GWAS consortium. The authors are also grateful to Prof. Jinxin Bei for assistance.
Funding: This work was supported by grants from the National Key Research and Development Program (Nos. 2017YFA0104304 and 2017ZX10203205), National Natural Science Foundation of China (Nos. 81770648, 81770651, 81802402, 81901943, 81900597, and 81972286), Guangdong Basic and Applied Basic Research Foundation (Nos. 2015A030312013, 2017A030311034, and 2018A030310323), Sci-Tech Research Development Program of Guangdong Province (Nos. 2017B020209004, 20169013, 2017B030314027, 2017A020215023, 2018A030313705, and 2019A1515011698), Medical Scientific Research Foundation of Guangdong Province (No. A2018130), Sci-Tech Research Development Program of Guangzhou city (Nos. 2014Y2-00200, 201604020001, 201508020262, and 201400000001-3), Young Teacher Development Program of Sun Yat-sen University (Nos. 17ykpy57, 19ykpy35, 20ykpy41, and 20ykpy33), and the China Postdoctoral Science Foundation (No. 2019M653199).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4007/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4007/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4007/coif). The authors have no conflicts of interest to declare.
(English Language Editors: E. Davies and J. Gray)
References
- 1.Caballería L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol 2010;22:24-32. 10.1097/MEG.0b013e32832fcdf0 [DOI] [PubMed] [Google Scholar]
- 2.van den Berg EH, Amini M, Schreuder TC, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS One 2017;12:e0171502. 10.1371/journal.pone.0171502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55. 10.1053/j.gastro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 4.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006;20:981-96. 10.1016/j.bpg.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Méndez-Sánchez N, King-Martínez AC, Ramos MH, et al. The Amerindian's genes in the Mexican population are associated with development of gallstone disease. Am J Gastroenterol 2004;99:2166-70. 10.1111/j.1572-0241.2004.40159.x [DOI] [PubMed] [Google Scholar]
- 6.Everhart JE, Khare M, Hill M, et al. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999;117:632-9. 10.1016/S0016-5085(99)70456-7 [DOI] [PubMed] [Google Scholar]
- 7.Everhart JE, Yeh F, Lee ET, et al. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology 2002;35:1507-12. 10.1053/jhep.2002.33336 [DOI] [PubMed] [Google Scholar]
- 8.Maurer KR, Everhart JE, Ezzati TM, et al. Prevalence of gallstone disease in Hispanic populations in the United States. Gastroenterology 1989;96:487-92. 10.1016/0016-5085(89)91575-8 [DOI] [PubMed] [Google Scholar]
- 9.Everhart JE. Gallstones and ethnicity in the Americas. J Assoc Acad Minor Phys 2001;12:137-43. [PubMed] [Google Scholar]
- 10.Diehl AK, Stern MP, Ostrower VS, et al. Prevalence of clinical gallbladder disease in Mexican-American, Anglo, and black women. South Med J 1980;73:438-41, 443. 10.1097/00007611-198004000-00012 [DOI] [PubMed] [Google Scholar]
- 11.Biétry FA, Reich O, Schwenkglenks M, et al. Statin use and risk of cholecystectomy - A case-control analysis using Swiss claims data. Expert Opin Drug Saf 2016;15:1577-82. 10.1080/14740338.2016.1240782 [DOI] [PubMed] [Google Scholar]
- 12.Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349-64. 10.3322/canjclin.51.6.349 [DOI] [PubMed] [Google Scholar]
- 13.Zanlungo S, Rigotti A. Determinants of transhepatic cholesterol flux and their relevance for gallstone formation. Liver Int 2009;29:323-30. 10.1111/j.1478-3231.2009.01972.x [DOI] [PubMed] [Google Scholar]
- 14.Grigor'eva IN, Maliutina SK, Voevoda MI. Role of hyperlipidemia in cholelithiasis. Eksp Klin Gastroenterol 2010;(4):64-8. [PubMed] [Google Scholar]
- 15.Ferkingstad E, Oddsson A, Gretarsdottir S, et al. Genome-wide association meta-analysis yields 20 loci associated with gallstone disease. Nat Commun 2018;9:5101. 10.1038/s41467-018-07460-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung SC, Liao KF, Lai SW, et al. Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol 2011;11:111. 10.1186/1471-230X-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller T, Kollerova J, Hlavaty T, et al. Cholelithiasis and markers of nonalcoholic fatty liver disease in patients with metabolic risk factors. Scand J Gastroenterol 2012;47:197-203. 10.3109/00365521.2011.643481 [DOI] [PubMed] [Google Scholar]
- 18.Bodmer M, Brauchli YB, Krähenbühl S, et al. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA 2009;302:2001-7. 10.1001/jama.2009.1601 [DOI] [PubMed] [Google Scholar]
- 19.Loria P, Lonardo A, Lombardini S, et al. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol 2005;20:1176-84. 10.1111/j.1440-1746.2005.03924.x [DOI] [PubMed] [Google Scholar]
- 20.Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 2018;33:947-52. 10.1007/s10654-018-0424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133-63. 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 22.Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: Conceptual and methodological challenges. PLoS Genet 2017;13:e1006944. 10.1371/journal.pgen.1006944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274-83. 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speliotes EK, Yerges-Armstrong LM, Wu J, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 2011;7:e1001324. 10.1371/journal.pgen.1001324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Zhang Y, Graham S, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol 2020;73:263-76. 10.1016/j.jhep.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorden A, Yang R, Yerges-Armstrong LM, et al. Genetic variation at NCAN locus is associated with inflammation and fibrosis in non-alcoholic fatty liver disease in morbid obesity. Hum Hered 2013;75:34-43. 10.1159/000346195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Details and Considerations of The UK Biobank GWAS: The Neale Lab 2019. Available online: https://nealelab.github.io/UKBB_ldsc/h2_browser.html
- 29.Hartwig FP, Davies NM, Hemani G, et al. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol 2016;45:1717-26. 10.1093/ije/dyx028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512-25. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novacek G. Gender and gallstone disease. Wien Med Wochenschr 2006;156:527-33. 10.1007/s10354-006-0346-x [DOI] [PubMed] [Google Scholar]
- 32.Holan KR, Holzbach RT, Hermann RE, et al. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology 1979;77:611-7. 10.1016/0016-5085(79)90209-9 [DOI] [PubMed] [Google Scholar]
- 33.Sedaghat A, Grundy SM. Cholesterol crystals and the formation of cholesterol gallstones. N Engl J Med 1980;302:1274-7. 10.1056/NEJM198006053022302 [DOI] [PubMed] [Google Scholar]
- 34.Konikoff FM, Chung DS, Donovan JM, et al. Filamentous, helical, and tubular microstructures during cholesterol crystallization from bile. Evidence that cholesterol does not nucleate classic monohydrate plates. J Clin Invest 1992;90:1155-60. 10.1172/JCI115935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gantz DL, Wang DQ, Carey MC, et al. Cryoelectron microscopy of a nucleating model bile in vitreous ice: formation of primordial vesicles. Biophys J 1999;76:1436-51. 10.1016/S0006-3495(99)77304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res 1996;37:606-30. 10.1016/S0022-2275(20)37603-3 [DOI] [PubMed] [Google Scholar]
- 37.Chen LY, Qiao QH, Zhang SC, et al. Metabolic syndrome and gallstone disease. World J Gastroenterol 2012;18:4215-20. 10.3748/wjg.v18.i31.4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y, Li Z, Zhao E, et al. Risk factors in patients with hereditary gallstones in Chinese pedigrees. Med Princ Pract 2012;21:467-71. 10.1159/000337437 [DOI] [PubMed] [Google Scholar]
- 39.Atamanalp SS, Keles MS, Atamanalp RS, et al. The effects of serum cholesterol, LDL, and HDL levels on gallstone cholesterol concentration. Pak J Med Sci 2013;29:187-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreotti G, Chen J, Gao YT, et al. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. Int J Cancer 2008;122:2322-9. 10.1002/ijc.23307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stender S, Frikke-Schmidt R, Benn M, et al. Low-density lipoprotein cholesterol and risk of gallstone disease: a Mendelian randomization study and meta-analyses. J Hepatol 2013;58:126-33. 10.1016/j.jhep.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 42.Ramos-De la Medina A, Remes-Troche JM, Roesch-Dietlen FB, et al. Routine liver biopsy to screen for nonalcoholic fatty liver disease (NAFLD) during cholecystectomy for gallstone disease: is it justified? J Gastrointest Surg 2008;12:2097-102; discussion 2102. 10.1007/s11605-008-0704-7 [DOI] [PubMed] [Google Scholar]
- 43.Shen SS, Gong JJ, Wang XW, et al. Promotional effect of nonalcoholic fatty liver disease on Gallstone disease: A systematic review and meta-analysis. Turk J Gastroenterol 2017;28:31-9. [DOI] [PubMed] [Google Scholar]
- 44.Jaruvongvanich V, Sanguankeo A, Upala S. Significant Association Between Gallstone Disease and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 2016;61:2389-96. 10.1007/s10620-016-4125-2 [DOI] [PubMed] [Google Scholar]
- 45.Chang Y, Noh YH, Suh BS, et al. Bidirectional Association between Nonalcoholic Fatty Liver Disease and Gallstone Disease: A Cohort Study. J Clin Med 2018;7:458. 10.3390/jcm7110458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2018;47:358. 10.1093/ije/dyx275 [DOI] [PubMed] [Google Scholar]
- 47.Martens EP, Pestman WR, de Boer A, et al. Instrumental variables: application and limitations. Epidemiology 2006;17:260-7. 10.1097/01.ede.0000215160.88317.cb [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as