Abstract
Objectives:
Dengue and Zika infections cause illnesses with overlapping clinical manifestations. The aim of this study was to explore the association of each of these infections with single or grouped clinical and laboratory parameters.
Methods:
Clinical and laboratory data were collected prospectively from a cohort of patients seeking care for symptoms meeting the Pan American Health Organization’s modified case-definition criteria for probable Zika virus infection. Zika and dengue were diagnosed with RT-PCR. The relationship of clinical characteristics and laboratory data with Zika, dengue, and undefined acute illness (UAI) was examined.
Results:
In the univariate models, localized rash and maculopapular exanthema were associated with Zika infection. Generalized rash, petechiae, and petechial purpuric rash were associated with dengue. Cough and confusion/disorientation were associated with UAI. Platelets were significantly lower in the dengue group. A conditional inference tree model showed poor sensitivity and positive predictive value for individual viral diagnoses.
Conclusions:
Clusters of signs, symptoms, and laboratory values evaluated in this study could not consistently differentiate Zika or dengue cases from UAI in the clinical setting at the individual patient level. We identified symptoms that are important to Zika and dengue in the univariate analyses, but predictive models were unreliable. Low platelet count was a distinctive feature of dengue.
Keywords: Arbovirus infections, Signs, Symptoms, Laboratory values, Diagnosis
Introduction
Dengue and Zika viruses are both positive-sense, single-stranded RNA viruses belonging to the Flaviviridae family. They have similar genetic sequences, transmission cycles, and ecological niches, and overlapping clinical manifestations (Gould and Salomon, 2008). Dengue virus, the causative agent of dengue, was re-introduced to the Americas a few decades ago, established endemically, and currently circulates in annual peaks (Brathwaite et al., 2012). Zika virus, the causative agent of Zika, was recently introduced to the Americas, causing an outbreak of pandemic proportions (Nugent et al., 2017), and attracted special attention due to its association with Zika congenital syndrome and Guillain–Barré syndrome (Krauer et al., 2017).
Symptomatic Zika infection is characterized by rash, fever, conjunctivitis, malaise, myalgia, arthralgia, edema, headache, and retro-ocular pain (Brooks et al., 2017; Garza-González et al., 2017), which makes it difficult to differentiate clinically from dengue and chikungunya virus infections, which are both regionally endemic (Braga et al., 2017; Colombo et al., 2017). Moreover, concurrent outbreaks and even co-infections may occur (Braga et al., 2017; Mercado-Reyes et al., 2019), and the clinical presentation may vary geographically (Braga et al., 2017; Brenciaglia et al., 2018; Silva et al., 2019). Furthermore, dengue endemic regions where Zika virus now circulates, have other co-pathogens causing similar acute illnesses (Marcondes et al., 2017; Wilder-Smith et al., 2017).
Currently, there is no single clinical feature or group of clinical features that has been identified to distinguish Zika from dengue and other acute diseases (Silva et al., 2019; Waggoner et al., 2016). Research aiming to develop prediction models using signs and/or symptoms to diagnose Zika in the clinic have been unsuccessful. Previous studies have documented that a low platelet count, low white blood cell count, and myalgia are useful indicators of dengue infection compared to other undefined acute illnesses (UAI) (Potts and Rothman, 2008). Recent studies have attempted to differentiate Zika and dengue virus infections using the presence or absence of specific symptoms, with inconsistent results (Azeredo et al., 2018; Silva et al., 2019; Waggoner et al., 2016). Important limitations of these studies are that datasets included small numbers of Zika patients, data were collected retrospectively, and cohorts of patients were not assessed concurrently and were from different geographic regions. Finally, the studies did not include patients with other acute febrile illnesses that have similar manifestations as a third diagnostic group.
The aim of this analysis was to explore the association of patterns of aggregated symptoms, signs, and laboratory parameters (clinical features) with Zika and dengue infections in patients living in a dengue endemic area. Although a set of clinical features that could clinically diagnose Zika would be optimal, a less ambitious goal of identifying individual or groups of clinical features that are associated with Zika may be scientifically important. Identifying short-term characteristics of Zika could help clinicians include Zika in the differential diagnosis and conduct confirmatory testing. Clusters of clinical features may guide investigators as they search for long-term sequelae of Zika, with the specific clinical characteristics potentially giving insight into organ systems affected by the virus. This information could be used to help investigators design future studies that focus more systematically on the identified symptomatology, which may ultimately lead to clinical models for the diagnosis of Zika.
Methods
Study setting and design
This was a sub-analysis of the Zik01 cohort (Gouel-Cheron et al., 2019), a prospective cohort study that enrolled patients with possible Zika infections in four government-funded, clinical care centers in the city of Tapachula in the state of Chiapas in Mexico between June 2016 and July 2018 (ClinicalTrials.gov, NCT02831699). Tapachula is located 23 km west of the border with Guatemala along the Pacific coast. This area is considered hyper-endemic for dengue, with an estimated seroprevalence of 83% (95% confidence interval 73.8%–88.9%) in school children aged 13–17 years (Amaya-Larios et al., 2018).
Participants were locally enrolled by principal investigators at each center. There were two primary healthcare centers, a general hospital, and a third-level healthcare hospital. The primary healthcare centers provide preventive and ambulatory care services for employees of private companies (Instituto Mexicano del Seguro Social or IMSS) or federal government employees (Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado or ISSSTE) affiliated to public options for health insurance. The General Hospital of Tapachula City provides ambulatory and inpatient healthcare services for the uninsured and is funded and managed by the State of Chiapas Ministry of Health. Finally, the Hospital Regional de Alta Especialidad Ciudad Salud is a federal government-funded, regional referral center that provides care for uninsured patients with complex diseases.
At the time of study planning, it was decided to enroll up to 600 participants across three different cohorts (symptomatic people seeking care for symptoms compatible with Zika, patients with Guillain–Barré syndrome, and asymptomatic household contacts of symptomatic participants). The sample size was based on convenience and feasibility given the uncertainty of the number of people that would be infected. The protocol specified that separate analyses would be performed for each cohort. This paper analyses data from the cohort of symptomatic participants. The symptomatic cohort included 467 participants, while the Guillain–Barré syndrome and asymptomatic household contact cohorts included 29 and 103, respectively.
Cohort criteria entry for the symptomatic cohort were based on the Pan American Health Organization (2016) definition of probable Zika infection, modified as follows: any two signs/symptoms (rash, elevated body temperature >37.2 °C, arthralgia, myalgia, non-purulent conjunctivitis, conjunctival hyperemia, headache, or malaise) with onset in the previous 7 days preceding the initial visit, not explained by other illness. A modified definition was used due to the uncertainty of the spectrum of the clinical manifestations of Zika at the emergence of the disease as a pandemic, to allow some flexibility and in an attempt to improve sensitivity of the definition. Symptoms, signs, blood and urine samples were collected at enrollment (visit 1) and 3, 7, 28, and 180 days later (referred to as visits 2, 3, 4, and 5). For each patient, visit 1 occurred anywhere between the first day of symptoms and 7 days later. Visit 2 was performed 3 days after visit 1. Therefore, these data represent a cross-section of values from 3 to 10 days after the initial symptoms occurred.
Procedures and definitions
Participants were classified as having confirmed Zika or dengue virus infection (chikungunya virus was also tested, but only one patient was chikungunya-positive, so this patient was excluded from the analysis) when viral RNA was detected in serum or urine samples at visit 1, 2, or 3. Patients with no detectable viral RNA were classified as having an undefined acute illness (UAI). Patients with no identifiable RNA in a urine or blood sample, but with missing data for at least two follow-up visits were excluded from this analysis. Viral RNA was identified in serum and urine with sequence amplification via PCR, as described previously (Gouel-Cheron et al., 2019).
All participants 12 years of age or older were included in this analysis. The characteristics that are examined in this paper are self-reported symptoms collected at visits 1 and 2, signs on physical examination, laboratory tests, neurocognitive score, and disability score (all collected at visit 1). Clinical features occurring before the first visit were not collected in a uniform way between the sites in this study, so these data was not used in the analysis. Neurocognitive impairment was assessed using the Montreal Cognitive Assessment (MoCA), a screening tool for mild neurocognitive dysfunction (Aguilar-Navarro et al., 2018). Disability was assessed using the 12-item World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) (Üstün et al., 2010).
Statistical analysis
Continuous variables were standardized for ease of comparison. A multinomial regression model (with the dependent variable being disease group) was used to examine the univariate relationship of each clinical feature to disease group. An overall test was performed to determine whether there was any relationship between the clinical feature and disease, and comparisons of the odds of the clinical features between disease groups were performed. 95% confidence intervals for the odds ratios were calculated.
A conditional tree model was used to explore groups of characteristics more likely to be associated with specific disease categories. This analysis was performed using the ctree function of the partykit package in R (Hothorn and Zeileis, 2015; Hothorn et al., 2006). This approach considers all possible dichotomizations of the covariates as potential predictors, while controlling for the multiple comparisons inherent in the procedure through a Bonferroni adjustment. Since the number of Zika and dengue cases was small compared to the number of clinical features, some of the signs/symptoms were combined into broader categories when there was strong overlap in the clinical features within an individual. The conditional tree model also included weights to account for differences in sample size between the groups (Zika n = 33, dengue n = 59, and UAI n = 276). The weights were selected so that the model would not over-emphasize the UAI patients, since we were more interested in characteristics associated with dengue and Zika. Weights of 3.70, 2.14, and 0.45 were given to each Zika, dengue, and UAI patient, respectively. The sum of the weights for all participants is equal to the overall sample size with each disease group receiving a third of the total weight. In order to evaluate the ability of the model to predict group membership, the sensitivity and positive predictive values (PPV) for the model were estimated using leave-one-out cross-validation.
Ethical considerations
This study adhered to the principles of the Declaration of Helsinki. The study protocol was evaluated and approved by an institutional review board in all Mexican participating institutions. Participation was voluntary, and participant agreements were documented through written informed consent procedures. Participants younger than 18 years gave their assent and their parents or legal representatives authorized their participation. All data were captured in de-identified datasets. The study protocol was reviewed and approved by the Comité de Ética en Investigación (Committee on Ethics in Research) and the Comité de Investigación (Research Committee) at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INF-2636-18-19-1).
Results
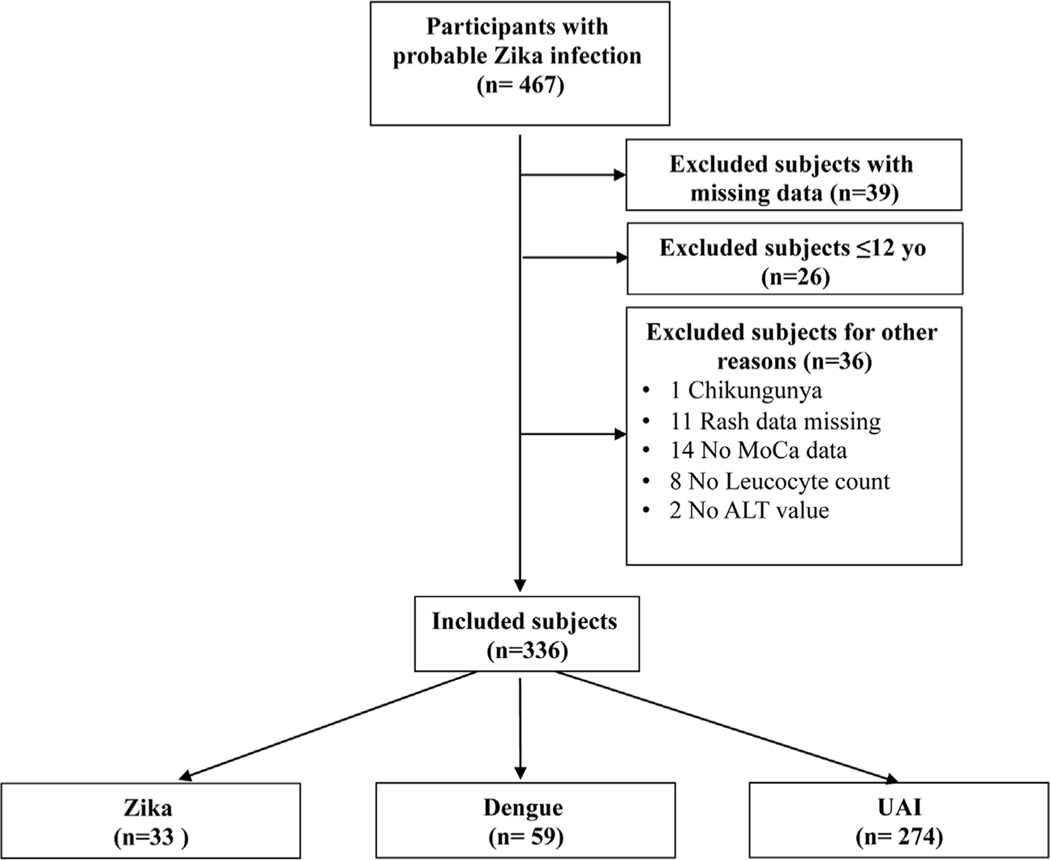
A total 467 symptomatic participants with possible Zika infection were enrolled. Thirty-nine patients with missing data on more than one follow-up visit, for whom Zika and dengue infections could not be confidently ruled out, and 26 patients younger than 12 years old were excluded; another 36 were excluded for other reasons (Figure 1). Thus, 366 patients were included in this analysis: 33 with confirmed Zika virus infection, 59 with confirmed dengue, and 274 with UAI. The sex distribution was similar across the groups. Patients with dengue were the youngest on average, while those with UAI were the oldest (Table 1). Patients with symptomatic Zika infections tended to present to care later during their acute illness than patients with dengue or UAI.
Figure 1. Study flow chart.
The flow chart describes the study population included in the analysis.
Table 1.
Demographic characteristics of 366 patients seeking care for an acute illness compatible with probable Zika infection in the city of Tapachula, state of Chiapas, Mexico (2016–2018) by diagnosis group.
| Zika (n = 33) | Dengue (n = 59) | Undefined acute illness (n = 274) | |
|---|---|---|---|
|
| |||
| Female, n (%) | 20 (61) | 31 (53) | 170 (62) |
| Age in years, mean ± SD | 33.7 ± 12.2 | 27.4 ± 11.4 | 35.2 ± 15.05 |
| Age in years, n (%) | |||
| 0 | 4 (12) | 0 (0) | 7 (2) |
| 1 | 1 (3) | 3 (5) | 32 (12) |
| 2 | 8 (24) | 3 (5) | 48 (18) |
| 3 | 8 (24) | 7 (12) | 45 (16) |
| 4 | 5 (15) | 15 (25) | 51 (19) |
| 5 | 4 (12) | 10 (17) | 40 (15) |
| 6 | 3 (9) | 13 (22) | 42 (15) |
| 7 | 0 (0) | 8 (14) | 9 (3) |
SD, standard deviation.
Table 2 gives the characteristics that were collected on each patient, along with the data collection time range and whether the characteristic was self-reported or from the physical examination. The table presents the characteristics that were highly correlated and grouped together for the tree analysis and the proportion of the overall sample that had each characteristic. Malaise was the most often observed eligibility criterion (98%). Conjunctivitis (48%) and rash (46%) were the least observed eligibility criteria. The other eligibility criteria were observed in 87–90% of participants. Myalgia (63%) and myoarthralgia, both collected at visit 2 (61%), were the most frequently observed symptoms of the non-eligibility characteristics. The mean MoCA score was at the population normalized value (MoCA score values <26 indicate lower than normal cognitive function). The WHODAS score does not have normative values and the scale was developed with 0 meaning no disability and 100 meaning total disability (Üstün et al., 2010). Twenty people in this study had scores of 35, a score that falls at the 90th percentile of the general population (Üstün et al., 2010). Overall, laboratory values included in the analysis were within normal limits except for the alanine aminotransferase (ALT) level, which was mildly elevated.
Table 2.
List of symptoms, signs, laboratory parameters, and neurological and disability tests in 366 patients with probable Zika virus infection in Tapachula, Chiapas, Mexico.
| Symptoms, signs, laboratory parametersa | Combined variables for regression trees | Proportion or mean ± SD of | |||
|---|---|---|---|---|---|
|
|
|||||
| All groups | Zika | Dengue | UAI | ||
|
| |||||
| Malaise/fatigue (1) | Malaise/Myalgia | 0.98 | 0.91 | 0.98 | 0.99 |
| Myalgia/muscle pain (1) | 0.89 | 0.91 | 0.9 | 0.89 | |
| Arthralgia/joint pain (1) | 0.9 | 0.67 | 0.95 | 0.91 | |
| Headache (1) | 0.9 | 0.82 | 0.95 | 0.91 | |
| Fever (1) | 0.87 | 0.70 | 0.95 | 0.87 | |
| Conjunctivitis (1) | 0.48 | 0.61 | 0.39 | 0.48 | |
| Rash (1) | 0.46 | 0.67 | 0.59 | 0.41 | |
| Myalgia/muscle pain (2) | 0.63 | 0.58 | 0.51 | 0.67 | |
| Myoarthralgia/back pain (2) | Pain | 0.61 | 0.58 | 0.54 | 0.63 |
| Arthralgia/joint pain (2) | 0.46 | 0.42 | 0.42 | 0.47 | |
| Malaise/fatigue (2) | 0.57 | 0.48 | 0.59 | 0.57 | |
| Photophobia (2) | 0.49 | 0.64 | 0.39 | 0.49 | |
| Sore throat (2) | 0.46 | 0.39 | 0.29 | 0.5 | |
| Cough (2) | 0.45 | 0.27 | 0.32 | 0.5 | |
| Periorbital pain (2) | 0.43 | 0.36 | 0.31 | 0.47 | |
| Difficulty standing (2) | Lack of mobility | 0.41 | 0.36 | 0.49 | 0.4 |
| Difficulty walking (2) | 0.34 | 0.3 | 0.34 | 0.35 | |
| Muscle weakness (2) | 0.33 | 0.27 | 0.34 | 0.34 | |
| Itch (2) | 0.38 | 0.52 | 0.53 | 0.33 | |
| Nausea (2) | Nausea/vomiting | 0.33 | 0.27 | 0.4 | 0.35 |
| Vomiting (2) | 0.09 | 0.06 | 0.1 | 0.09 | |
| Erythematous rash (3) | 0.32 | 0.27 | 0.54 | 0.28 | |
| Localized rash (3) | 0.32 | 0.58 | 0.19 | 0.31 | |
| Generalized rash (3) | 0.16 | 0.09 | 0.47 | 0.1 | |
| Maculopapular rash (3) | 0.13 | 0.36 | 0.08 | 0.11 | |
| Petechiae (3) | 0.07 | 0 | 0.24 | 0.05 | |
| Petechial or purpuric rash (3) | 0.05 | 0.03 | 0.14 | 0.03 | |
| Confusion/disorientation (2) | 0.22 | 0.09 | 0.1 | 0.26 | |
| Behavior alterations (2) | 0.21 | 0.15 | 0.15 | 0.23 | |
| Diarrhea (2) | 0.19 | 0.06 | 0.25 | 0.2 | |
| Stiff neck (2) | 0.18 | 0.12 | 0.2 | 0.19 | |
| Mouth ulcers (2) | 0.15 | 0.09 | 0.14 | 0.16 | |
| Bleeding (2) | 0.05 | 0.03 | 0.05 | 0.05 | |
| Ecchymosis or hematomas (3) | 0.02 | 0.03 | 0.03 | 0.01 | |
| MoCA score (3) | 26.28 (4.01) | 25.30 (3.31) | 26.64 (4.08) | 26.31 (4.07) | |
| WHODAS score (3) | 36.91 (14.65) | 33.13 (15.88) | 42.71 (15.80) | 36.12 (13.96) | |
| ALT IU/μl (3) | 63.77 (63.26) | 47.61 (31.71) | 114.25 (107.33) | 54.85 (46.08) | |
| Leukocyte count cell/ml (3) | 7.14 (3.62) | 6.06 (2.82) | 4.38 (2.31) | 7.86 (3.63) | |
| Neutrophil % (3) | 55.27 (17.13) | 56.76 (12.76) | 40.51 (17.73) | 58.27 (15.82) | |
| Platelet count IU/μl (3) | 220.55 (97.87) | 259.64 (96.22) | 96.36 (61.59) | 242.59 (83.12) | |
ALT, alanine aminotransferase; MoCA, Montreal Cognitive Assessment; SD, standard deviation; UAI, undefined acute illness; WHODAS, World Health Organization Disability Assessment Schedule.
(1) Indicates self-reported eligibility symptoms collected 0–7 days after symptom onset. (2) Indicates self-reported symptoms collected 3–10 days after symptom onset. (3) Indicates data collected at physical examination 0–7 days after symptom onset.
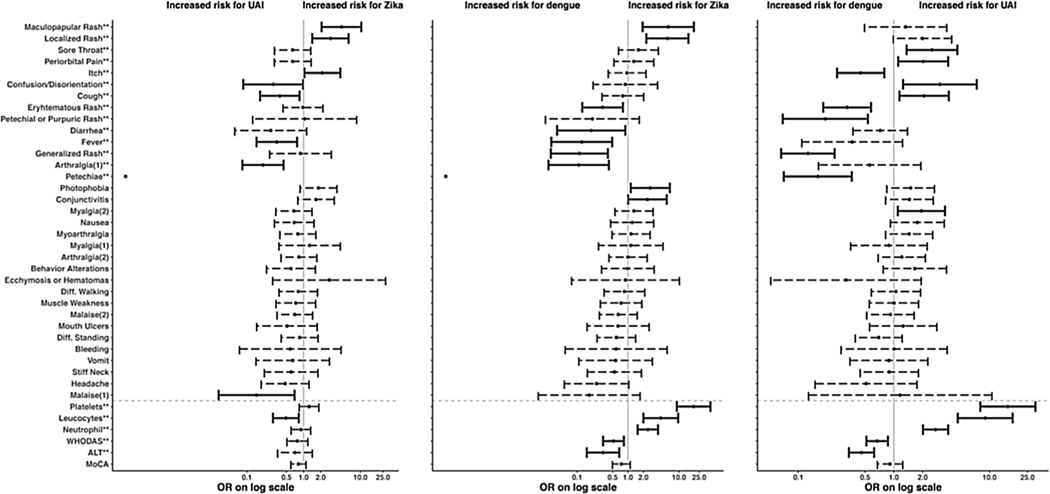
Figure 2 gives the odds ratios and 95% confidence intervals for the associations of each clinical feature and the disease categories. For continuous variables (lower section of the figure below the gray dashed line), the odds ratio reflects the effect of an increase of 1 standard deviation. The figure shows that the odds of being in the Zika group are higher if localized rash or maculopapular exanthema is present, and lower if arthralgia or fever is present. The odds of being in the dengue group are increased with the presence of generalized rash, erythematous rash, or petechiae (only present in dengue patients); with decreasing platelets, neutrophils, or leukocytes; or with increasing WHODAS or ALT. The odds of being in the UAI group are increased if confusion, cough, or high leukocytes are present.
Figure 2.
Odds ratios and 95% confidence intervals for the comparisons of disease groups for each characteristic in a univariate model.
The darker lines indicate the odds ratios that are significantly different from 1 at the 0.05 level. Characteristics marked with ** represent the significance from a likelihood ratio test comparing a model with the characteristic versus intercept alone. Characteristics below the gray dashed line are continuous and standardized. Note that there is no interval associated with petechiae for comparisons with Zika; there were no Zika participants with this characteristic and therefore the interval was not accurately estimable. (1) Indicates self-reported eligibility symptoms collected 0–7 days after symptom onset; (2) indicates self-reported symptoms collected 3–10 days after symptom onset.
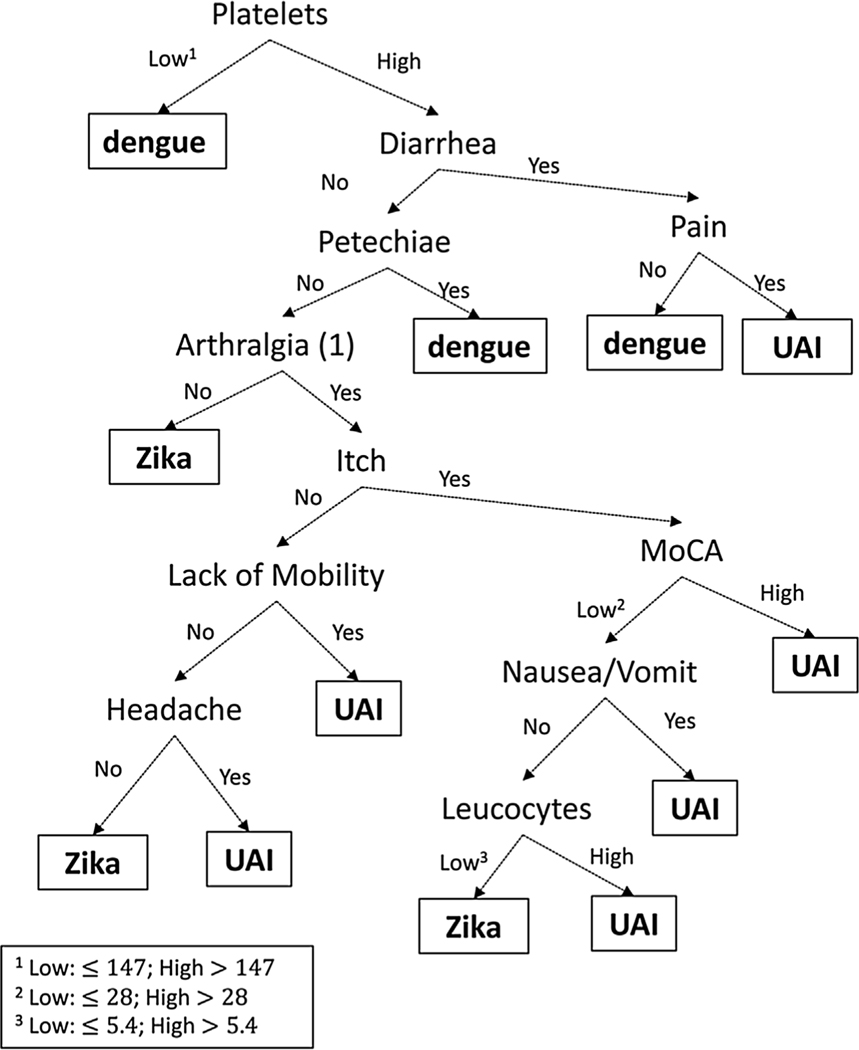
In the classification tree models, low platelets (≤147 × 109 cells/l), diarrhea, petechiae, and any myoarticular pain are the most important clinical features that discriminate dengue from the other two disease groups (Figure 3). The diagnostic values of the decision tree model are presented in Tables 3 (sensitivity) and 4 (positive predictive value). The leave-one-out cross-validation method was used to estimate sensitivity and positive predictive value, with the Bonferroni-adjusted conditional classification tree regrown each time, in order to obtain estimates of how well the model would fit if an independent set of data was used to estimate sensitivity and positive predictive value, and thus make the results more generalizable.
Figure 3. Results from the tree model.
Clinical features that split categories early are more important than later splits. The arrows show paths with the presence or absence of clinical features that predict the different disease groups. The (1) for arthralgia indicates when the data were collected, as described in Table 1.
Table 3.
Cross-validated summary of performance of the tree model with all variables (row proportions). The diagonal values are the sensitivity. The cells provide the number of subjects with each true diagnosis and predicted group.
| Cross-validated summary of performance of the tree model with all variables | |||||
|---|---|---|---|---|---|
|
| |||||
| Row proportions | |||||
|
| |||||
| Diagonal values are sensitivity | |||||
|
| |||||
| Predicted categories | Total | ||||
|
|
|||||
| Zika | Dengue | UAI | |||
|
| |||||
| True categories | Zika | 15 (0.45) | 2 (0.06) | 15 (0.48) | 33 (1.00) |
| Dengue | 2 (0.03) | 51 (0.86) | 6 (0.10) | 59 (1.00) | |
| UAI | 16 (0.06) | 63 (0.23) | 195 (0.71) | 274 (1.00) | |
| Total | 33 | 116 | 217 | ||
UAI, undefined acute illness.
Table 4.
Cross-validated summary of performance of the tree model with all variables (column proportions). The diagonal values are the positive predictive values. The cells provide the number of subjects with each true diagnosis and predicted group.
| Cross-validated summary of performance of the tree model with all variables | |||||
|---|---|---|---|---|---|
|
| |||||
| Column proportions | |||||
|
| |||||
| Diagonal values are positive predictive value | |||||
|
| |||||
| Predicted categories | Total | ||||
|
|
|||||
| Zika | Dengue | UAI | |||
|
| |||||
| True categories | Zika | 15 (0.45) | 2 (0.02) | 15 (0.07) | 33 |
| Dengue | 2 (0.06) | 51 (0.44) | 6 (0.03) | 59 | |
| UAI | 16 (0.48) | 63 (0.54) | 195 (0.90) | 274 | |
| Total | 33 (1.00) | 116 (1.00) | 217 (1.00) | ||
UAI, undefined acute illness.
True symptomatic dengue infections can be identified with good sensitivity (86%). We do not do as well with true UAI: 71% are predicted to be UAI, and only 45% of true symptomatic Zika infections are predicted to be such by the model. The model tends to over-predict dengue symptomatic infections, with 32% of all people predicted to have dengue when in truth only 16% have symptomatic dengue infections.
Discussion
In recent years, Zika and chikungunya were introduced into the Americas and now co-circulate in dengue endemic and hyper-endemic regions. The three arboviruses have overlapping presenting signs, symptoms, and laboratory abnormalities, which make clinical differentiation difficult for rapid decision-making in the clinical setting. In this analysis, the associations between Zika, dengue, and UAI with single and grouped clinical characteristics were examined. The purpose was to identify possible clinical patterns that could be associated with Zika or dengue symptomatic infections. Zika and dengue were confirmed in less than half of the participants and most patients had UAI. Several clinical features were associated with different disease groups: localized rash and maculopapular exanthema were associated with Zika, generalized rash, petechiae, and petechial purpuric rash were associated with dengue, while cough and confusion/disorientation were associated with UAI. Platelets were significantly lower in the dengue group and leukocytes were significantly different among all groups. Despite these findings, when evaluating all characteristics simultaneously, a decision tree model did not provide good prediction of group membership.
Previously, researchers in Singapore identified the concomitant presence of conjunctivitis and normal platelet and monocyte counts as a good predictor of Zika (88% sensitivity and 93% specificity) in a case–control study comparing Zika with dengue. A weakness of the study was that cases and controls were not collected over the same time period and the researchers did not include patients with other febrile illnesses, which occur concomitantly and increase the complexity of a differential diagnosis (Yan et al., 2018). The presence of rash, pruritus, and non-purulent conjunctivitis in the absence of fever were consistently associated with an increased probability of Zika in patients from Nicaragua and different regions in Brazil where Zika and dengue co-circulate (Braga et al., 2017; Silva et al., 2019; Waggoner et al., 2016), while arthralgia and vomiting were associated with dengue. Nonetheless, considerable overlap in the frequency of fever, arthralgia, and headache led to the conclusion in all studies that none of these signs or symptoms in isolation or as a group could be relied upon to establish a specific viral diagnosis, which is consistent with the present study results. These studies were either performed before the emergence of chikungunya and Zika pandemics, in different geographical areas, or developed to distinguish exclusively between dengue and Zika symptomatic infections without taking into account other acute illnesses that could be attributed to different pathogens co-circulating in dengue endemic, tropical and sub-tropical areas (Braga et al., 2017; Silva et al., 2019; Waggoner et al., 2016).
In a systematic review intending to identify signs, symptoms, and laboratory features that might help distinguish dengue from other febrile illnesses, the authors recognized heterogeneity of study populations, methodological short-comings of reviewed studies, lack of specific definitions and inclusion criteria, in addition to overlapping signs and symptoms between arboviral and other infections as probable reasons for the inability to clinically distinguish between dengue and other febrile illnesses (Potts and Rothman, 2008). In the present study, the disease characteristics were collected prospectively in a systematic way by trained staff in a cohort of patients seeking care for an acute illness compatible with the probable Zika case-definition and with no other obvious attributable disease, overcoming most limitations of previous studies. The data collection included a detailed description of symptoms and a physical examination including neurological evaluation and evaluation of neurocognitive involvement using a simple, brief, and widely available screening tool for mild neurocognitive impairment (MoCA), along with a disability tool (WHODAS). In addition, we accrued both dengue and Zika symptomatic patients over the same time frame and included participants with undefined illness who sought medical care for similar symptoms. Thus, the study adds reliable and robust information on the presenting clinical characteristics of Zika and dengue in the background presence of other undefined acute illnesses. The study population, which was enrolled in a diverse group of clinical care centers including primary care, hospital-based, and referral centers, strengthens the generalizability of the results.
This study confirmed that even in the presence of other non-arboviral, acute illnesses with overlapping clinical manifestations, low platelets (and petechiae in this study) are more associated with symptomatic dengue virus infection than with the other disease categories. These observations might provide insight into fundamental differences in pathophysiological mechanisms between Zika and dengue viruses, considering that thrombocytopenia is a hallmark clinical manifestation of dengue but has consistently been shown to be an extremely rare finding in Zika virus infection (Van Dyne et al., 2018). The expression of nonstructural protein 1 (NS1), which is highly conserved across the Flavivirus genus and is vital for viral replication (Rastogi et al., 2016), has recently been implicated in the induction of thrombocytopenia in patients with dengue infection through different possible mechanisms: direct binding and infection of megakaryocytes and platelets (Simon et al., 2015), induction of platelet activation of an inflammatory profile via Toll-like receptor 4 (Chao et al., 2019), and enhancement of platelet phagocytosis through macrophages (Wan et al., 2016). Despite sequence and protein fold NS1 homology across the genus, crucial differences in surface electrostatic charge distribution and protein loop flexibility between Zika, dengue, and other flaviviruses might explain the pathophysiological differences explaining its diverse clinical manifestations (Poonsiri et al., 2018; Song et al., 2016). Further analyses detailing differences between Zika and dengue NS1 and their effects on megakaryocyte and platelet infection and activation are warranted (Guo and Rondina, 2019).
The conditional tree model presented in this paper had poor sensitivity and positive predictive value, and thus is not a candidate for use as a clinical tool to distinguish between the three categories of disease groups studied. Classification tree models, including the conditional trees used here, require large datasets when there are complicated relationships between prediction variables and the outcome of interest. The study dataset with only 33 Zika cases and 59 dengue cases was likely too small to be able to identify subtle groupings of symptoms to predict disease groups. This problem is common to the studies discussed above. When investigating small datasets, only very strong predictors of disease will be observed. Furthermore, with 34predictorvariables, the Bonferroni multiplicity adjustment would make it difficult to find any but the strongest associations. In addition, the imbalance in group sizes (33, 59, and 274) made an unweighted tree model overfit for UAI patients. The model was corrected by weighting the Zika and dengue patients more heavily, but this is not as optimal as having equal and large numbers of patients in each group, since important characteristics that occur at low percentages in the groups with few observations may not be observed. Other limitations of the study are that the patients came for their initial visit any time between 0 and 7 days after the onset of symptoms. Therefore, any early symptoms that were particular to a specific disease may have disappeared by the time some patients arrived at the clinic. We might also not have included unknown manifestations at the moment of designing the data collection forms, particularly if mild or inconspicuous. Also, the data collection forms were designed by infectious disease specialists, neurologists, and epidemiologists, which might have biased the instruments towards certain features and might have overlooked other important characteristics that other specialists (e.g., a dermatologist) may have been able to better distinguish.
In conclusion, this paper reports on the clinical features associated with Zika, dengue, and UAI patients seeking care for possible Zika virus infection. Clinical features that increase the odds of inclusion in each disease group were identified in the univariate analyses. A conditional tree model was used to explore more complex interactions between predictors, but did not have sufficient diagnostic ability for clinical usage, most likely due to the small number of Zika and dengue cases. A low platelet count was identified as a distinguishing feature of dengue, useful for discriminating dengue from Zika and other UAI. The results are consistent with previous findings suggesting that differences in the NS1 protein between Zika and dengue might explain the association between thrombocytopenia and dengue, but not in Zika (Chao et al., 2019). Further research with larger numbers of Zika and dengue cases is needed to understand the clinical characteristics of Zika and dengue in the context of other unidentified acute illnesses.
Acknowledgements
The Zik01 study team for the Mexican Emerging Infectious Diseases Clinical Research Network (LaRed) are Héctor Armando Rincón-León and Karla R. Navarro-Fuentes (Unidad de Medicina Familiar No.11, Instituto Mexicano del Seguro Social, Tapachula, Chiapas, Mexico), Sandra Caballero-Sosa, Francisco Camas-Durán, and Zoyla Priego-Smith (Clínica Hospital Dr. Roberto Nettel Flores, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado, Tapachula, Chiapas, Mexico), Emilia Ruiz (Hospital General de Tapachula, Tapachula, Chiapas, Mexico), José Gabriel Nájera-Cancino, Paul Rodriguez de La Rosa, Jesús Sepúlveda-Delgado, Alfredo Vera Maloof, Karina Trujillo, and Alexander López-Roblero (Hospital Regional de Alta Especialidad Ciudad Salud, Tapachula, Chiapas, Mexico), Raydel Valdés-Salgado, Yolanda Bertucci, Isabel Trejos, and Luis Diego Villalobos (Westat, Inc., Rockville, MD, USA), Pablo F. Belaunzarán-Zamudio, Pilar Ramos, Fernando J. Arteaga-Cabello, Lourdes Guerrero, and Guillermo Ruiz-Palacios (Departamento de Infectología, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico), Luis Mendoza-Garcés, Hugo Arroyo-Figueroa, Peter Quidgley, and Eli Becerril (LaRed Coordinating Center, Mexico City), John H. Powers III (Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD, USA), John H. Beigel and Sally Hunsberger (National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA).
This study has been registered at ClinicalTrials.gov (NCT02831699).
Role of funding source
LaRed is funded by the Mexico Ministry of Health and the US National Institute of Allergy and Infectious Diseases. This study was supported in part by Consejo Nacional de Ciencia y Tecnología (FONSEC SSA/IMSS/ISSSTE Projects No. 71260 and No. 127088); National Institute of Allergy and Infectious Diseases, National Institutes of Health, through its Intramural Research Programs and a contract with Westat, Inc. (Contract Number: HHSN2722009000031, Task Order Number: HHSN27200002); and in part with federal funds from the National Cancer Institute, National Institutes of Health (under Contract No. HHSN261200800001E and Contract No. 75N91019D00024, Task Order No. 75N91019F00130). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, or Westat, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Investigators in the LaRed Zik01 Study Team are listed in the Acknowledgments.
References
- Aguilar-Navarro SG, Mimenza-Alvarado AJ, Palacios-Garcia AA, Samudio-Cruz A, Gutierrez-Gutierrez LA, Avila-Funes JA. Validity and reliability of the Spanish version of the Montreal Cognitive Assessment (MoCA) for the detection of cognitive impairment in Mexico. Rev Colomb Psiquiat 2018;47(October–December (4)):237–43. [DOI] [PubMed] [Google Scholar]
- Amaya-Larios IY, Rojas-Russell M, López-Cervantes M, et al. Seroprevalence of dengue in school children in Mexico ages 6–17 years, 2016. Trans R Soc Trop Med Hyg 2018;112(May (5)):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo EL, dos Santos FB, Barbosa LS, Souza TMA, Badolato-Corrêa J, Sánchez-Arcila JC, et al. Clinical and laboratory profile of Zika and dengue infected patients: lessons learned from the co-circulation of dengue, Zika and chikungunya in Brazil. PLoS Curr 2018;(February):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga JU, Bressan C, Dalvi APR, et al. Accuracy of Zika virus disease case definition during simultaneous dengue and chikungunya epidemics. PLoS One 2017;12 (June (6))e0179725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite DO, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012;87 (October):584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenciaglia M, Noel T, Fields P, et al. Clinical, serological, and molecular observations from a case series study during the Asian lineage Zika virus outbreak in Grenada during 2016. Can J Infect Dis Med Microbiol 2018;2018(February):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks T, Roy-Burman A, Tuholske C, et al. Real-time evolution of Zika virus disease outbreak, Roatán, Honduras. Emerg Infect Dis 2017;23(August (8)):1360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo TE, Estofolete CF, Reis AFN, et al. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol 2017;96 (November):20–5. [DOI] [PubMed] [Google Scholar]
- Chao CH, Wu WC, Lai YC, et al. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog 2019;15(April (4))e1007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-González E, Mendoza-Olazarán S, Roman-Campos R, et al. Rapid spread of an ongoing outbreak of Zika virus disease in pregnant women in a Mexican hospital. Braz J Infect Dis 2017;21(June (5)):554–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouel-Cheron A, Lumbard K, Hunsberger S, Arteaga-Cabello FJ, Beigel J, Belaunzaran-Zamudio PF, et al. Serial real-time RT-PCR and serology measurements substantially improve Zika and dengue virus infection classification in a co-circulation area. Antivir Res 2019;172(December)104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, Salomon T. Pathogenic flaviviruses. Lancet 2008;371(February):500–9. [DOI] [PubMed] [Google Scholar]
- Guo L, Rondina MT. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol 2019;10(September):2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Hornik K, Zeleis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 2006;15(3):651–74. [Google Scholar]
- Hothorn T, Zeileis A. partykit: a modular toolkit for recursive partitioning in R. J Mach Learn Res 2015;16(December):3905–9. [Google Scholar]
- Krauer F, Riesen M, Reveiz L, Oladapo O, Martínez-Vega R, Porgo T, et al. Zika Virus infection as a cause of congenital brain abnormalities and guillain-barre syndrome: systematic review. PLoS Med 2017;14(January (1))e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes CB, Contigiani M, Gleiser RM. Emergent and reemergent arboviruses in South America and the Caribbean: why so many and why now?. J Med Entomol 2017;54(May (3)):509–32. [DOI] [PubMed] [Google Scholar]
- Mercado-Reyes M, Acosta-Reyes J, Navarro-Lechuga E, et al. Dengue, chikungunya and Zika virus coinfection: results of the national surveillance during the Zika epidemic in Colombia. Epidemiol Infect 2019;147(January):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent EK, Nugent AK, Nugent R, Nugent EK. Zika virus: epidemiology, pathogenesis and human disease. Am J Med Sci 2017;353(January):466–73. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization. Zika resources: case definitions [Internet]. Washington: PAHO; 2016. Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=11117:2015-zika-case-definitions-&Itemid=41532&lang=en [Accessed on 21 December 2019]. [Google Scholar]
- Poonsiri T, Wright GSA, Diamond MS, Turtle L, Solomon T, Antonyuk SV. Structural study of the C-terminal domain of nonstructural protein 1 from Japanese encephalitis virus. J Virol 2018;92(April (7)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 2008;13 (November (11)):1328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M, Sharma N, Singh SK. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J 2016;13(July):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MM, Tauro LB, Kikuti M, Anjos RO, Santos VC, Gonçalves TSF, et al. Concomitant transmission of dengue, chikungunya and Zika viruses in Brazil: clinical and epidemiological findings from surveillance for acute febrile illness. Clin Infect Dis 2019;69(October (8)):1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood 2015;126(July (3)):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Qi J, Haywood J, Shi Y, Gao GF. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat Struct Mol Biol 2016;23(May (5)):456–8. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. Developing the World Health Organization disability assessment schedule 2.0. Bull World Health Organ 2010;88(November (11)):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyne EA, Neaterour P, Rivera A, et al. Incidence and outcome of severe and nonsevere thrombocytopenia associated with Zika virus infection—Puerto Rico, 2016. Open Forum Infect Dis 2018;6(1) ofy325-ofy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JJ, Gresh L, Vargas MJ, Ballesteros G, Tellez Y, Soda KJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016;63(December (12)):1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan SW, Yang YW, Chu YT, et al. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb Haemost 2016;115(March (3)):646–56. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 2017;17(March (3)):e101–6. [DOI] [PubMed] [Google Scholar]
- Yan G, Pang L, Cook AR, et al. Distinguishing Zika and dengue viruses through simple clinical assessment, Singapore. Emerg Infect Dis 2018;24 (August):1565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]