Abstract
Nanotechnology advancements and applications have paved the way for new possibilities in regenerative medicine and tissue engineering. It is a relatively new field that has the potential to improve stem cell differentiation and therapy greatly. Numerous studies have demonstrated that nanomaterials can function as a physiological niche for the formation and differentiation of stem cells. However, quantum dots (QDs), such as carbon quantum dots (CQDs) and graphene quantum dots (GQDs), have shown considerable promise in the field of regenerative medicine. To date, most research has focused on stem cell tracking and imaging using CQDs. However, their interaction with stem cells and the associated possibility for differentiation by selectively focusing chemical signals to a particular lineage has received scant attention. In this mini-review, we attempt to categorize a few pathways linked with the role of CQDs in stem cell differentiation.
Introduction
In the domain of medicine, stem cell treatment has enabled us to understand a variety of problems that were before incomprehensible.1−3 Their limitless capacity for self-renewal and ability to differentiate into any cell type sets them apart from other cell types. Their classification is based on a hierarchical pattern. Totipotent stem cells, which are only found in the early embryonic stage, are the most primitive type of stem cells capable of forming a whole embryo. Subsequently, embryonic stem cells reach the pluripotent stage when they may develop entirely into the three germ layers: endoderm, mesoderm, and ectoderm. Subdivisions of these cells progressively destroy their pluripotency, leaving them stationary and restricted. When multipotent stem cells are supplemented with appropriate growth factors and inducers, they differentiate into a specific cell type. These cells are known as adult stem cells (ASCs), and practically, ASCs are found in all specialized tissues in a metabolically quiescent state.4 Embryos are destroyed during the isolation or extraction of embryonic stem cells (ESCs); hence, using ESCs for scientific purposes raises religious concerns and is deemed sometimes immoral. While induced pluripotent stem cells (iPSCs) are formed from somatic cells and may be employed for therapeutic purposes, many laboratories lack the necessary infrastructure and knowledge to manufacture iPSCs, making them an inconvenient source of stem cells. Since both ESCs and iPSCs have a limited number of drawbacks, researchers believe ASCs to be the first choice for stem cell-based therapeutics.5
Although stem cell treatment has a wide variety of applications and a promising future in the field of regenerative medicine, there are still several constraints connected with the use of stem cells.6−9 Long-term survival of engrafted cells, limited control over the fate of stem cells, and the irreversibility of the treatment are some of the potential risks associated with this procedure. Other potential risks include the type and proliferative status of the stem cells used in the procedure and the route of administration to deliver them to the intended region.10,11 Significant risks are the tumor formation ability of stem cells,12 immune response, and transmission of serendipitous agents.13 Due to the difficulty of controlling stem cell fate without physical or chemical cues, the existing stem cell therapies are ineffective at entirely replacing injured cells. Due to these limitations, the transplantation productivity and survival rate of ex vivo differentiated stem cells are extremely low.
Mesenchymal Stem Cells (MSCs)
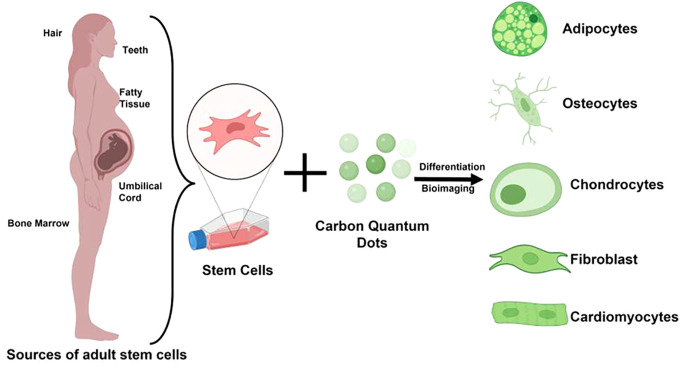
ASCs, often referred to as MSCs, have demonstrated tremendous potential for tissue engineering and regenerative medicine. Their limitless proliferation and differentiation capabilities have aided researchers in achieving new heights in the medical field. MSCs promote tissue regeneration and repair and are the ideal candidate for culture-based expansion due to their self-renewability, multipotency, ease of isolation and expansion, immunological privilege, genetic stability, and no ethical concerns.14 Friedenstein and Petrakova discovered MSCs in the stromal layer of bone marrow in 1968.15 MSCs, like other stem cells, are self-renewing and can differentiate into various cell types such as chondrocytes, neurons, adipocytes, cardiomyocytes, and osteocytes.16 MSCs can be isolated from various tissues and fluids, including the umbilical cord, adipose tissue, amniotic fluid, and dental pulp.17 According to the International Society for Cellular Therapy (ISCT) guidelines, MSCs should exhibit the following characteristics: (1) plastic adherence, (2) expression of mesodermal cell surface markers (CD73, CD90, and CD105) but not hematopoietic cell surface markers (CD11b, CD14, CD19, CD34, CD45, CD79, and HLA-DR), and (3) the ability to differentiate into mesodermal lineages such as chondrocytes, adipocytes, and osteocytes.18 However, MSCs have their own several limitations. Scientists are now searching for a noninvasive and effective method of monitoring MSCs in vivo. Additionally, the high cost associated with chemicals such as inducers to determine the fate of MSCs into appropriate lineage poses a significant financial burden. These impediments limit the utilization of MSCs in clinical settings. Therefore, the development of a cost-effective and reliable approach for steering stem cell differentiation. Significant progress has been made to overcome these obstacles, and a tremendous opportunity has been identified in nanotechnology. Researchers are exploring multidimensional nanomaterials to manipulate the fate and transport of stem cells.19−21 Due to the small size of nanomaterials, their surface can be modified to imitate the biophysical cues provided by extracellular matrix (ECM).22−24 Owing to their high surface area to volume ratio, they can be easily used for targeted drug delivery.25 Additionally, the nanoparticles can effectively direct the differentiation of stem cells into a particular lineage26−28 while also allowing for long-term monitoring following implantation.29,30 The physical and chemical properties of nanomaterials can influence the cell microenvironment and cell behavior, which, in turn, can modulate the cell response toward differentiation.31 Reportedly, using physical and chemical interactions, graphene-based nanomaterials can bind to biomolecules such as DNA, proteins, and other small molecules, thereby directing stem cell lineage.32
Major Issues Associated with MSCs Differentiation
It has been a goal of scientists for a very long time to control the behavior and stem cell fate according to the need of intended therapeutic application. Several approaches exist to externally regulate stem cell fate. The long-term objective is to extract stem cells from patients and, using a variety of external stimuli, develop specialized cells that can be implanted back into patients. These cells will then be used to treat the patient condition. While stem cell-based therapies are novel and are reshaping the world, they have some drawbacks. Before using stem cells for clinical trials, several significant hurdles must be overcome, including their tumorigenicity, rapid proliferation, and differentiation into undesirable lineages.20 Thus, it is critical to regulate and perceive stem cell behavior in terms of cell attachment, differentiation, proliferation, and ECM secretion33 in response to a variety of chemical, mechanical, and environmental variables/inducers.31 Although these inducers direct stem cell differentiation into the desired lineage, a high cost is associated with them. Several kinase signal transduction pathways govern the many factors that play a role in keeping MSCs in their pluripotent state and determining their fate. Wingless-related integration site (Wnt) signaling, transforming growth factor (TGF-β) signaling, leukemia inhibitory factor (LIF) signaling, and fibroblast growth factor (FGF) signaling are a few of these pathways.34,35 The Wnt pathway is required to maintain MSC pluripotency, migration, and polarity, while TGF-β signaling is critical for stem cell self-renewal and differentiation.36−38 Numerous transcription factors are required for the proper regulation of these pathways, including the Yamanaka factors, Octamer-binding transcription factor 4 (Oct-4), sex-determining region Y-box 2 (Sox-2), Krüppel-like factor 4 (Klf4), cellular-Myc (c-Myc), and runt-related transcription factor 2 (RUNX2).39 More importantly, synchronization, balance, and systematic orchestration of these elements are necessary for stem cell self-renewal, differentiation, and proliferation to a specific cell linage.40 Physical cues such as surface stiffness, density, topology, and porosity can be used to influence stem cells extremely readily.41−43 Thus, it is anticipated that manipulating these external stimuli can be beneficial in directing the stem cell lineage according to our needs. Extensive research is being conducted to identify substitutes for the inducers. Additionally, this substitute should be inexpensive and capable of differentiating stem cells into the desired lineage. Herein, we delineate the role of nanoscale quantum dots in stem cell lineage modulation.
Quantum Dots
If only one dimension of a three-dimensional structure is nanoscale, the structure is referred to as a quantum well; if two dimensions are tiny, the structure is referred to as a quantum wire; and if all three dimensions are nanoscale, the structure is referred to as a quantum dot (QD). In 1998, quantum dots became the first carbon-based nanomaterial used in biological research.44 QDs can be synthesized in either a top-down or bottom-up fashion. Researchers are concentrating their efforts on the green synthesis of quantum dots, owing to their high quantum yield. The emission of QDs can be tuned in the near-infrared region (NIR), enabling deeper tissue imaging. QDs have a unique property of broad excitation range and narrow emission range, which allows multiplexed imaging with a single excitation source.
Additionally, QDs have a high quantum yield of photoluminescence that is proportional to the imaging contrast. Additionally, QDs are resistant to photobleaching and other environmental conditions, allowing for prolonged exposure and outperforming other organic fluorophores. Another distinguishing feature of QDs is their compatibility with biomolecular functionalization and the enhanced permeability and retention (EPR) phenomenon, enabling them to target tumor cells.45 These properties of QDs are gathering the attention of biologists as QDs hold the potential to start a new era of fluorescence imaging and can help in unravelling the secrets of biology at a molecular level.
Carbon Quantum Dots
CQDs are attracting considerable attention in the biological area due to their small size, stable photoluminescence, improved surface grafting, biocompatibility, and high-water solubility.46−49 Numerous nanoparticles such as magnetic nanoparticles and quantum dots are available for labeling stem cells. However, due to the difficulty of detecting magnetic signals in MRI scanners due to cell division and dilution of magnetic nanoparticles (MNPs), scientists are moving toward quantum dots that are fluorescence-based.50 CQDs have been observed to be ingested by cells via endocytosis processes.51,52 CQDs are also critical for stem cell differentiation into osteocytes, adipocytes, and neurocytes. For instance, CQDs have previously been shown to promote osteogenic differentiation via bone morphogenetic protein (BMP), TGF-β, and Wnt signaling pathways.
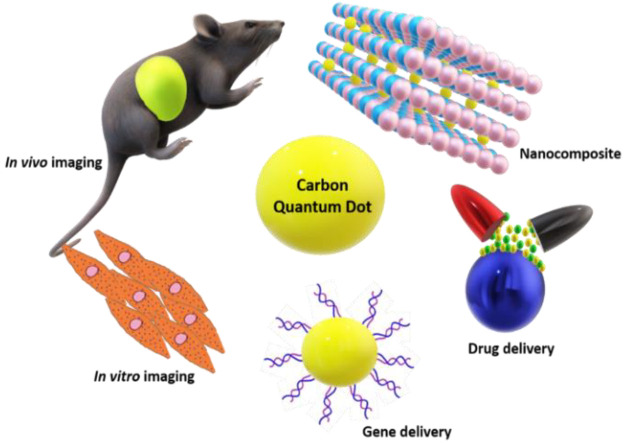
CQDs are a novel nanomaterial with numerous biomedical applications such as cell imaging due to their strong fluorescence. They are highly water-soluble, and their fabrication process is economical. They are safe for biological applications due to their greater biocompatibility and minimal cytotoxicity. The exceptional biological features of CQDs, such as hydrophilicity, low toxicity, chemical stability, and strong biocompatibility, ensure that their promised applications in biosensors, drug administration, gene delivery, bioimaging and the manufacture of nanocomposites, which can be employed for a variety of tissue engineering and regenerative medicine applications. CQDs have flourished in a variety of disciplines due to their superior chemical stability, optical characteristics, and renal clearance capabilities. Compared to organic dyes and conventional QDs, the improved photostability of CQDs may be attributed to their structure and composition.53,54 CQDs are core–shell composites consisting of a carbon core and surface passivation with various functional groups, such as hydroxyl, carboxyl, and amine, among others. This makes them hydrophilic and allows for a variety of surface functionalization and passivation processes to be carried out.55 Due to the π–π stacking, they are useful as carriers for various aromatic anticancer medicines. The cells take up these nanoscale quantum dots via energy-dependent endocytosis pathways.
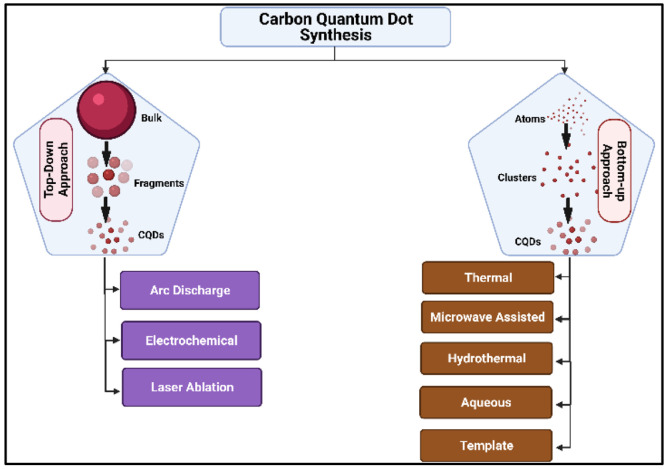
CQDs can be synthesized via various physical, chemical, and biological ways but top-down and bottom-up approaches are the two most widely used methods.56 While any method can be used to synthesize CQDs, researchers favor the biological approach since it is cost-effective, cleaner, greener, and environmentally friendly.
The top-down strategy involves the chemical or physical breakdown of bulk material into nanostructures. Although this technique is straightforward and easily adjustable to meet our requirements, there are disadvantages, such as imperfections on the surface of the nanostructures. On the other hand, the bottom-up method may result in less waste and, therefore, can be more cost-effective. Bottom-up approaches entail assembling material atom by atom, then molecule by molecule or cluster by cluster, ultimately producing nanostructures.55 Various techniques involved in the bottom-up and top-down approaches have been illustrated in Figure 1.
Figure 1.
Schematic representation of the techniques involved in the bottom-up and top-down approach in CQD synthesis.
CQDs: Role in Bioimaging
Numerous organic fluorophores are commercially available; however, QDs are favored due to their excellent resistance to photobleaching.57 CQDs made from natural products exhibit a greater luminescence, biocompatibility, and solubility than those synthesized from other sources.58 Compared to organic dyes and other cadmium-based QDs, CQDs feature emission peaks that are more clearly defined and broader excitation spectra. So, by varying the size of CQDs and the excitation wavelength, the same CQDs can give different fluorescence.54 CQDs made with citric acid monohydrate and diethylene glycol BIS ether was extremely fluorescent, and their synthesis was also straightforward, environmentally friendly, and scalable.59 Mukherjee et al. demonstrated the green production of sulfur-doped GQDs using sugar cane molasses. The GQDs were extremely fluorescent, biocompatible, and had a quantum yield of 47%.60 Shen et al. manufactured nitrogen-doped carbon quantum dots (N-CQDs) for bioimaging applications using glucose and m-phenylamine. The quenching of Fe3+ and CrO42– was found to be static.61 CQDs were produced for multicolor imaging utilizing citric acid and the doping ions YB3+ and ND3+. The synthesized CQDs also emit in the near-infrared range, indicating their suitability for visible and infrared bioimaging.62 CQDs have been shown to attach to DNA and RNA in Caenorhabditis elegans live cells and emit distinct fluorescence, allowing for detecting DNA and RNA localization.63 The quantum yield of CQDs produced with alanine as the carbon source and ethylenediamine as the passivation was 46.2%. CQDs tagged MCF-7 cells and were suitable for bioimaging. The addition of dihydronicotinamide adenine dinucleotide (NADH) into the as-synthesized CQDs quenched their fluorescence, resulting in the generation of a new protocol for NADH determination inside the cells.64 CQDs not only mark cells but also have the capacity to target other elements. CQDs were coupled with amino TPEA (N-(2-aminoethyl)-N,N′,N′-tris(pyridine-2-yl-methyl)ethane-1,2-diamine) to enable imaging of Cu2+ inside cells. The compound aided in the production of Cu2+ and exhibited extremely minimal cytotoxicity.65 Because autofluorescence might cause interference with CQDs, Wei et al. produced boron and nitrogen codoped CQDs (BN-CQDs) utilizing o-phenylenediamine as the carbon and nitrogen source and boric acid as the source of boron and used them for bioimaging of HeLa cells.66 In greater quantities, CQDs have been reported to be hazardous. Extended exposure to CQDs may be detrimental to certain cell types. Another group reported that CQDs synthesized from waste cotton linter were economical, easy to synthesize, and had high fluorescence. On the other hand, when these CQDs were incubated for a longer duration or at higher concentrations, they proved to be toxic for HUVEC and H2452 cells.67
CQDs: Effect on Cancer Cells
Cancer is one of the most lethal diseases, and there is ongoing research to unravel a potential cure for it. To this end, nanoparticles can be utilized in combating cancer. Nanoparticles internalize into cells via the endocytosis mechanism. Furthermore, CQDs are biocompatible and are employed exclusively for bioimaging; however, a few CQDs may also generate excessive reactive oxygen species (ROS) and can be toxic to cells. By inhibiting the expression of F-actin, VEGF, MMP-2, and MMP-9, CQDs/Cu2O produced by the Jiaqiang group preferentially destroyed SKOV3 ovarian cells. Additionally, these CQDs destroyed the cells via apoptosis by halting them in the S phase.68
CQDs synthesized with o-phenylenediamine were readily taken up by HeLa cells, and when activated with blue light, they generated a substantial amount of ROS, leading to cell death.69
Highly fluorescent quantum dots were synthesized from tea leaf extract and were apoptotic for lung cancer cells (A549).70 Cancer CSCs are a subtype of stem-like cells seen within malignant tumors. These cells exhibit characteristics of both stem cells and malignant cells.71,72 They are regarded as the architect of tumor spread and relapse. Their capacity for self-renewal and resistance to chemotherapeutic drugs jeopardizes cancer treatment.73,74 As a result, CSCs may be a therapeutic target for tumor metastasis and relapse prevention. Su et al. demonstrated that doxorubicin-loaded red emission CQDs enter the nucleus of CSCs, exhibiting anticancer activity, hence eradicating the possibility of metastasis.75 There are various ways via which CQDs could be used for the elimination of CSCs (Figure 2).
Figure 2.
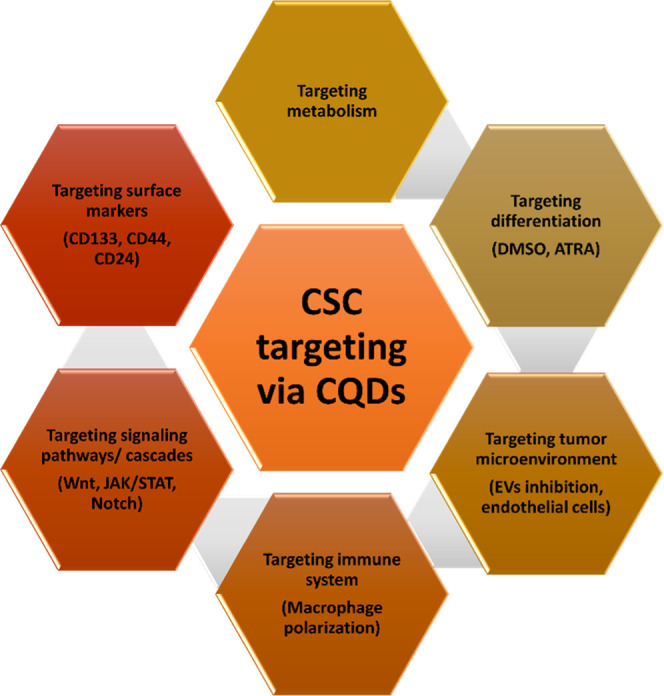
Potential role of CQDs in cancer stem cells targeting.
CQDs: Role in Stem Cell Differentiation
A range of diverse stem cell characteristics, such as pluripotency, differentiation, and reprogramming, may be regulated by several different small molecules. However, because most small molecules are hydrophobic, organic solvents like dimethyl sulfoxide (DMSO) are frequently used to dissolve the small molecules for cell culture supplementation. This practice can cause cytotoxicity and impair the differentiation of treated stem cells. The therapeutic use of small molecule medications is also hampered by their low water solubility. CQDs, on the other hand, are soluble in water, and the surface of these particles may be altered to facilitate targeted distribution.76 Even though positively charged CQDs are more cytotoxic and have lower photoluminescence (PL), in comparison to negatively charged CQDs, they nonetheless have a greater labeling efficiency due to their higher absorption capacity. Differentiation of stem cells into adipocytes and osteocytes was disturbed due to positively charged CQDs. Relatively weak positively charged CQDs could have high biocompatibility and labeling efficiency in human stem cells.77 CQDs, by their nanostructure, also play a part in the differentiation of stem cells. (Figure 3). The healing of bone is a complex physiological phenomenon;78 during this process, the stem cells replace the damaged bone tissues with new bones.79 CQDs have been successfully used to promote osteogenic differentiation of MSCs and their bioimaging. An increase in the expression of specific markers indicates the differentiation of MSCs into a distinct lineage.
Figure 3.
Differentiation of stem cells into multiple lineages using growth factors and quantum dots.
Han et al. reported that adenosine aspirin-based CQDs upregulated the osteogenic markers alkaline phosphatase (ALP), RUNX2, OCN, and BSP in the presence of osteogenic inductive factors such as dexamethasone, ascorbic acid, and β-glycerophosphate, indicating osteogenic differentiation of bone marrow mesenchymal stem cells.80 Bu et al. synthesized CQDs using ascorbic acid as a precursor and demonstrated bioimaging and osteogenic properties. The osteogenic impact was further boosted by miR-2861, a promoter of osteoblast differentiation.81,82 Wang et al. synthesized carbon dots with Zn2+ passivation (Zn-CDs). Zn2+ is included due to its osteogenic activity. Zn-CDs promote wound healing by generating ROS. The ROS generated triggered oxidative stress, which activated the MAPK signaling pathway, resulting in osteogenic differentiation. However, further research is needed to elucidate the precise mechanism behind the upregulation of these components ted, and the pathways involved (see Figure 4).83 Ghafary et al. successfully absorbed the covalently bound pDNA complexed quantum dots into the stem cell nucleus.84
Figure 4.
Applications of carbon quantum dots in stem cell therapy.
Stem cell gene therapy may be used to treat various inherited genetic disorders, including severe combined immunodeficiency caused by adenosine deaminase, adrenoleukodystrophy, and infantile blindness. Among others, there is Leber Congenital Amaurosis (LCA).85,86 The treatment entails genetic material transferred into stem cells to mend and eradicate genetic abnormalities. Thus, the simultaneous delivery of genes and tracking of CQDs boosts their utility in stem cell therapy.
Conclusion
CQDs are promising agents for stem cell bioimaging due to their unique physicochemical features and biocompatibility. Moreover, the green synthesis of CQDs is an eco-friendly method for their fabrication. Importantly, CQDs promote the expression of many regulatory factors involved in determining the fate of stem cells. They can also be employed as an alternative to expensive induction factors, owing to their ability to direct stem cell differentiation and tracking in vivo. Depending on the size of the cargo to be internalized, many energy-dependent mechanisms exist to allow the extracellular cargo inside the cell. Caveolae mechanisms usually internalize smaller cargo, intermediate cargo internalizes by the clathrin mechanism, and large cargos are internalized by macropinocytosis. Depending upon the size of CQD, the photoluminescence and light emission color vary. This property of CQD can be leveraged for drug delivery and bioimaging for patients on multidrug therapy. Bestowed with robust and multifaceted nature, functionalized CQDs can also act as a drug carrier for targeted stem cell therapy. The possibility for CQDs to build up in organs and cause unwanted side effects can be a concern that should be factored in for their clinical applications. The recent development of hybrid CQDs with other biomolecules that can synergistically uplift the drug delivery and bioimaging properties of CQDs holds promise for future development. To date, there are only a handful of reports that describe some signaling transduction pathways in the determination of stem cell fate. However, further research is needed to elucidate the precise mechanisms of how CQDs direct stem cell lineage. While the theranostic properties of CQDs have made them an ideal candidate in the biological sector, it is critical to understand the mechanisms of their action to minimize side effects. We envision that rigorous investigation into the molecular basis for CQDs in directing stem cell lineage and other biological applications will open new avenues for translational research.
Acknowledgments
The authors would like to express their gratitude to the thousands of reviewers and experts who contributed to this unprecedented scientific and clinical crisis. We would also like to express our credit to BIORENDER, for the figures. R.C. and M.M. acknowledge the Department of Science and Technology Women Scientist Scheme—A, New Delhi, Govt. of India for funding the project (DST/WOSA/CS-106/2021).
Glossary
ABBREVIATIONS
- QDs
quantum dots
- CQDs
carbon quantum dots
- MSCs
mesenchymal stem cells
- CSC
cancer stem cells
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03285.
Author biographies (PDF)
Author Contributions
The manuscript was written with the contributions of all authors. All authors have given approval to the final version of the manuscript.
Author Contributions
§ M.M. and P.G. have contributed equally as first author.
Author Contributions
∥ R.C. and A.A. contributed equally as second authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Gama K. B.; Santos D. S.; Evangelista A. F.; Silva D. N.; de Alcântara A. C.; dos Santos R. R.; Soares M. B. P.; Villarreal C. F. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem Cells International 2018, 2018, 1. 10.1155/2018/8179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A.; Coelho P.; Soares R.; Costa R. Human Umbilical Cord Mesenchymal Stem Cells in Type 2 Diabetes Mellitus: The Emerging Therapeutic Approach. Cell and Tissue Research 2021 385:3 2021, 385 (3), 497–518. 10.1007/s00441-021-03461-4. [DOI] [PubMed] [Google Scholar]
- Pokrovskaya L. A.; Zubareva E. v.; Nadezhdin S. v.; Lysenko A. S.; Litovkina T. L. Biological Activity of Mesenchymal Stem Cells Secretome as a Basis for Cell-Free Therapeutic Approach. Research Results in Pharmacology 2020, 6 (1), 57–68. 10.3897/rrpharmacology.6.49413. [DOI] [Google Scholar]
- Clevers H.; Watt F. M. Defining Adult Stem Cells by Function, Not by Phenotype. Annu. Rev. Biochem. 2018, 1015–1027. 10.1146/annurev-biochem-062917-012341. [DOI] [PubMed] [Google Scholar]
- Lukomska B.; Stanaszek L.; Zuba-Surma E.; Legosz P.; Sarzynska S.; Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells International. 2019, 2019, 1. 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. T. J.; Gronthos S.; Shi S. Critical Reviews in Oral Biology & Medicine: Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. Journal of Dental Research. 2009, 792–806. 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M. Z.; Kucia M.; Jadczyk T.; Greco N. J.; Wojakowski W.; Tendera M.; Ratajczak J. Pivotal Role of Paracrine Effects in Stem Cell Therapies in Regenerative Medicine: Can We Translate Stem Cell-Secreted Paracrine Factors and Microvesicles into Better Therapeutic Strategies. Leukemia. 2012, 1166–1173. 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- Nelson T. J.; Behfar A.; Yamada S.; Martinez-Fernandez A.; Terzic A. Stem Cell Platforms for Regenerative Medicine. Clinical and Translational Science. 2009, 222–227. 10.1111/j.1752-8062.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang N. S.; Zhang C.; Hwang Y. S.; Varghese S. Mesenchymal Stem Cell Differentiation and Roles in Regenerative Medicine. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 2009, 1 (1), 97–106. 10.1002/wsbm.26. [DOI] [PubMed] [Google Scholar]
- Polak D. J.Regenerative Medicine. Opportunities and Challenges: A Brief Overview. Journal of the Royal Society Interface.; Royal Society December 6, 2010. 10.1098/rsif.2010.0362.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M.; Mantalaris S. Stem Cells Bioprocessing: An Important Milestone to Move Regenerative Medicine Research into the Clinical Arena. Pediatr. Res. 2008, 461–466. 10.1203/PDR.0b013e31816a8c1c. [DOI] [PubMed] [Google Scholar]
- Knoepfler P. S. Deconstructing Stem Cell Tumorigenicity: A Roadmap to Safe Regenerative Medicine. Stem Cells 2009, 27 (5), 1050–1056. 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S.; Ding S.; Rando T. A.; Trounson A. Translational Strategies and Challenges in Regenerative Medicine. Nature Medicine. 2014, 814–821. 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- Wei X.; Yang X.; Han Z. P.; Qu F. F.; Shao L.; Shi Y. F. Mesenchymal Stem Cells: A New Trend for Cell Therapy. Acta Pharmacologica Sinica. 2013, 747–754. 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porada C.; Zanjani E.; Almeida-Porada G. Adult Mesenchymal Stem Cells: A Pluripotent Population with Multiple Applications. Current Stem Cell Research & Therapy 2006, 1 (3), 365–369. 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- Wang J.; Chen Z.; Sun M.; Xu H.; Gao Y.; Liu J.; Li M. Characterization and Therapeutic Applications of Mesenchymal Stem Cells for Regenerative Medicine. Tissue and Cell 2020, 64, 101330. 10.1016/j.tice.2020.101330. [DOI] [PubMed] [Google Scholar]
- Sheng G. The Developmental Basis of Mesenchymal Stem/Stromal Cells (MSCs). BMC Developmental Biology 2015 15:1 2015, 15 (1), 1–8. 10.1186/S12861-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M.; le Blanc K.; Mueller I.; Slaper-Cortenbach I.; Marini F. C.; Krause D. S.; Deans R. J.; Keating A.; Prockop D. J.; Horwitz E. M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8 (4), 315–317. 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Arzaghi H.; Rahimi B.; Adel B.; Rahimi G.; Taherian Z.; Sanati A. L.; Shiralizadeh Dezfuli A. Nanomaterials Modulating Stem Cell Behavior towards Cardiovascular Cell Lineage. Materials Advances. 2021, 2231–2262. 10.1039/d0ma00957a. [DOI] [Google Scholar]
- Zhou X.; Yuan L.; Wu C.; Chen C.; Luo G.; Deng J.; Mao Z. Recent Review of the Effect of Nanomaterials on Stem Cells. RSC Advances. 2018, 17656–17676. 10.1039/c8ra02424c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki A.; Kim J. D.; Lee K. B. Nanotechnology for Regenerative Medicine: Nanomaterials for Stem Cell Imaging. Nanomedicine 2008, 3 (4), 567–578. 10.2217/17435889.3.4.567. [DOI] [PubMed] [Google Scholar]
- Elsabahy M.; Wooley K. L. Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 2012, 41 (7), 2545–2561. 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q.; He X.; Li C.; He Y.; Peng Y.; Zhang Y.; Lu Y.; Chen X.; Zhang Y.; Chen Q.; Sun T.; Jiang C. Dandelion-Like Tailorable Nanoparticles for Tumor Microenvironment Modulation. Advanced Science 2019, 6 (21), 1901430. 10.1002/advs.201901430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekaran A.; Garcia A. J. Nanoscale Engineering of Extracellular Matrix-Mimetic Bioadhesive Surfaces and Implants for Tissue Engineering. Biochimica et Biophysica Acta (BBA) - General Subjects 2011, 1810 (3), 350–360. 10.1016/j.bbagen.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia M. A.; McNeil S. E. Immunological Properties of Engineered Nanomaterials. Nanoscience and Technology: A Collection of Reviews from Nature Journals 2009, 278–287. 10.1142/9789814287005_0029. [DOI] [Google Scholar]
- Ferreira L. Nanoparticles as Tools to Study and Control Stem Cells. Journal of Cellular Biochemistry 2009, 108 (4), 746–752. 10.1002/jcb.22303. [DOI] [PubMed] [Google Scholar]
- Dayem A. A.; Lee S. bin; Cho S. G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, Vol. 8, Page 761 2018, 8 (10), 761. 10.3390/NANO8100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayem A. A.; Choi H. Y.; Yang G. M.; Kim K.; Saha S. K.; Kim J. H.; Cho S. G. The Potential of Nanoparticles in Stem Cell Differentiation and Further Therapeutic Applications. Biotechnology Journal 2016, 11 (12), 1550–1560. 10.1002/biot.201600453. [DOI] [PubMed] [Google Scholar]
- Sun S. K.; Wang H. F.; Yan X. P. Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics. Acc. Chem. Res. 2018, 51 (5), 1131–1143. 10.1021/acs.accounts.7b00619. [DOI] [PubMed] [Google Scholar]
- González-Béjar M.; Francés-Soriano L.; Pérez-Prieto J. Upconversion Nanoparticles for Bioimaging and Regenerative Medicine. Frontiers in Bioengineering and Biotechnology 2016, 4 (JUN), 47. 10.3389/FBIOE.2016.00047/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong-Van E. K.; Madanagopal T. T.; Rosa V. Mechanisms of Graphene Influence on Cell Differentiation. Materials Today Chemistry 2020, 16, 100250. 10.1016/j.mtchem.2020.100250. [DOI] [Google Scholar]
- Kang H. K.; Kim D. J.; Kim M. S.; Kim D.-H.; Lee J. Y.; Sung E.-A.; Sarsenova M.; Chae Park S.; Hong B. H.; Kang K.-S. Improved Hepatoblast Differentiation of Human Pluripotent Stem Cells by Coffee Bean Derived Graphene Quantum Dots. 2D Materials 2022, 9 (3), 035012. 10.1088/2053-1583/ac6ba8. [DOI] [Google Scholar]
- Kenry; Lee W. C.; Loh K. P.; Lim C. T. When Stem Cells Meet Graphene: Opportunities and Challenges in Regenerative Medicine. Biomaterials 2018, 155, 236–250. 10.1016/j.biomaterials.2017.10.004. [DOI] [PubMed] [Google Scholar]
- NUSSE R. Wnt Signaling in Disease and in Development. Cell Research 2005 15:1 2005, 15 (1), 28–32. 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGFβ Signalling in Context. Nature Reviews Molecular Cell Biology 2012 13:10 2012, 13 (10), 616–630. 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D.; Massagué J. Roles of TGFβ in Metastasis. Cell Research 2009 19:1 2008, 19 (1), 89–102. 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- Croce J. C.; McClay D. R. Evolution of the Wnt Pathways. Methods Mol. Biol. 2008, 469, 3–18. 10.1007/978-1-60327-469-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R. The Wnts. Genome Biology 2001 3:1 2001, 3 (1), 1–15. 10.1186/GB-2001-3-1-REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Huang J.; Chen T.; Wang Y.; Xin S.; Li J.; Pei G.; Kang J. Yamanaka Factors Critically Regulate the Developmental Signaling Network in Mouse Embryonic Stem Cells. Cell Research 2008 18:12 2008, 18 (12), 1177–1189. 10.1038/cr.2008.309. [DOI] [PubMed] [Google Scholar]
- Cruciani S.; Santaniello S.; Montella A.; Ventura C.; Maioli M. Orchestrating Stem Cell Fate: Novel Tools for Regenerative Medicine. World Journal of Stem Cells 2019, 11 (8), 464. 10.4252/wjsc.v11.i8.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian K. A.; Bugarija B.; Lahn B. T.; Mrksich M. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (11), 4872–4877. 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam M. M.; Bonakdar S.; Shariatpanahi M. R.; Shokrgozar M. A.; Faghihi S. The Effect of Physical Cues on the Stem Cell Differentiation. Curr. Stem Cell Res. Ther 2019, 14 (3), 268–277. 10.2174/1574888X14666181227120706. [DOI] [PubMed] [Google Scholar]
- Higuchi A.; Ling Q. D.; Chang Y.; Hsu S. T.; Umezawa A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem. Rev. 2013, 113 (5), 3297–3328. 10.1021/cr300426x. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. J.; Chang J. C.; Kovtun O.; McBride J. R.; Tomlinson I. D. Biocompatible Quantum Dots for Biological Applications. Chemistry and Biology. 2011, 10–24. 10.1016/j.chembiol.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K. J.; Jing L.; Behrens A. M.; Jayawardena S.; Tang W.; Gao M.; Langer R.; Jaklenec A. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Adv. Mater. 2018, 30 (18), 1706356. 10.1002/adma.201706356. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Hu A. Carbon Quantum Dots: Synthesis, Properties and Applications. Journal of Materials Chemistry C 2014, 2 (34), 6921–6939. 10.1039/C4TC00988F. [DOI] [Google Scholar]
- Lin L.; Luo Y.; Tsai P.; Wang J.; Chen X. Metal Ions Doped Carbon Quantum Dots: Synthesis, Physicochemical Properties, and Their Applications. TrAC - Trends in Analytical Chemistry. 2018, 87–101. 10.1016/j.trac.2018.03.015. [DOI] [Google Scholar]
- Teradal N. L.; Jelinek R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthcare Mater. 2017, 6 (17), 1700574. 10.1002/adhm.201700574. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Zhang M.; Bhandari B.; Yang C.. Recent Development of Carbon Quantum Dots: Biological Toxicity, Antibacterial Properties and Application in Foods. Food Reviews International.; 2020. 10.1080/87559129.2020.1818255. [DOI] [Google Scholar]
- Edmundson M.; Thanh N. T. K.; Song B. Nanoparticles Based Stem Cell Tracking in Regenerative Medicine. Theranostics. 2013, 573–582. 10.7150/thno.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. W.; Bäumer W.; Monteiro-Riviere N. A. Cellular Uptake Mechanisms and Toxicity of Quantum Dots in Dendritic Cells. Nanomedicine 2011, 6 (5), 777–791. 10.2217/nnm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W.; Zhang X.; Zhang M.; Fan Z.; Sun Y.; Han M.; Fan L. The Uptake Mechanism and Biocompatibility of Graphene Quantum Dots with Human Neural Stem Cells. Nanoscale 2014, 6 (11), 5799–5806. 10.1039/C3NR06433F. [DOI] [PubMed] [Google Scholar]
- Tope S.; Saudagar S.; Kale N.; Khambayat S.; Bhise K.. Review: Therapeutic Application of Quantum Dots (QD). THE PHARMA INNOVATION-JOURNAL 2014.
- Singh I.; Arora R.; Dhiman H.; Pahwa R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turkish Journal of Pharmaceutical Sciences 2018, 15 (2), 219. 10.4274/tjps.63497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S.; Varma R. S. Green Synthesis, Biomedical and Biotechnological Applications of Carbon and Graphene Quantum Dots. A Review. Environmental Chemistry Letters 2020, 18 (3), 703–727. 10.1007/S10311-020-00984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G.; Wang H.; Yan L.; Wang X.; Pei R.; Yan T.; Zhao Y.; Guo X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39 (5), 1378–1383. 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Huang C.; Dong H.; Su Y.; Wu Y.; Narron R.; Yong Q. Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and in Vivo Bioimaging. Nanomaterials 2019, 9 (3), 387. 10.3390/nano9030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Zhang M.; Bhandari B.; Yang C. Recent Development of Carbon Quantum Dots: Biological Toxicity, Antibacterial Properties and Application in Foods. Food Reveuws INternations 2022, 28, 1513. 10.1080/87559129.2020.1818255. [DOI] [Google Scholar]
- Wang J.; Li Q.; Zhou J. E.; Wang Y.; Yu L.; Peng H.; Zhu J. Synthesis, Characterization and Cells and Tissues Imaging of Carbon Quantum Dots. Opt Mater. (Amst) 2017, 72, 15–19. 10.1016/j.optmat.2017.05.047. [DOI] [Google Scholar]
- Sangam S.; Gupta A.; Shakeel A.; Bhattacharya R.; Sharma A. K.; Suhag D.; Chakrabarti S.; Garg S. K.; Chattopadhyay S.; Basu B.; Kumar V.; Rajput S. K.; Dutta M. K.; Mukherjee M. Sustainable Synthesis of Single Crystalline Sulphur-Doped Graphene Quantum Dots for Bioimaging and Beyond. Green Chem. 2018, 20 (18), 4245–4259. 10.1039/C8GC01638K. [DOI] [Google Scholar]
- Shen T. Y.; Jia P. Y.; Chen D. S.; Wang L. N. Hydrothermal Synthesis of N-Doped Carbon Quantum Dots and Their Application in Ion-Detection and Cell-Imaging. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2021, 248, 119282. 10.1016/j.saa.2020.119282. [DOI] [PubMed] [Google Scholar]
- Wu F.; Su H.; Zhu X.; Wang K.; Zhang Z.; Wong W. K. Near-Infrared Emissive Lanthanide Hybridized Carbon Quantum Dots for Bioimaging Applications. J. Mater. Chem. B 2016, 4 (38), 6366–6372. 10.1039/C6TB01646D. [DOI] [PubMed] [Google Scholar]
- Han G.; Zhao J.; Zhang R.; Tian X.; Liu Z.; Wang A.; Liu R.; Liu B.; Han M. Y.; Gao X.; Zhang Z. Membrane-Penetrating Carbon Quantum Dots for Imaging Nucleic Acid Structures in Live Organisms. Angewandte Chemie - International Edition 2019, 58 (21), 7087–7091. 10.1002/anie.201903005. [DOI] [PubMed] [Google Scholar]
- Niu W. J.; Li Y.; Zhu R. H.; Shan D.; Fan Y. R.; Zhang X. J. Ethylenediamine-Assisted Hydrothermal Synthesis of Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probes for Sensitive Biosensing and Bioimaging. Sensors and Actuators, B: Chemical 2015, 218, 229–236. 10.1016/j.snb.2015.05.006. [DOI] [Google Scholar]
- Qu Q.; Zhu A.; Shao X.; Shi G.; Tian Y. Development of a Carbon Quantum Dots-Based Fluorescent Cu2+ Probe Suitable for Living Cell Imaging. Chem. Commun. 2012, 48 (44), 5473–5475. 10.1039/c2cc31000g. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Chen L.; Wang J.; Liu X.; Yang Y.; Yu S. Rapid Synthesis of B-N Co-Doped Yellow Emissive Carbon Quantum Dots for Cellular Imaging. Opt Mater. (Amst) 2020, 100, 109647. 10.1016/j.optmat.2019.109647. [DOI] [Google Scholar]
- Eskalen H.; Uruş S.; Cömertpay S.; Kurt A. H.; Özgan Ş. Microwave-Assisted Ultra-Fast Synthesis of Carbon Quantum Dots from Linter: Fluorescence Cancer Imaging and Human Cell Growth Inhibition Properties. Industrial Crops and Products 2020, 147, 112209. 10.1016/j.indcrop.2020.112209. [DOI] [Google Scholar]
- Chen D.; Li B.; Lei T.; Na D.; Nie M.; Yang Y.; Congjia; Xie; He Z.; Wang J. Selective Mediation of Ovarian Cancer SKOV3 Cells Death by Pristine Carbon Quantum Dots/Cu2O Composite through Targeting Matrix Metalloproteinases, Angiogenic Cytokines and Cytoskeleton. J. Nanobiotechnol. 2021, 19 (1), 1–17. 10.1186/s12951-021-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X.; Liu J.; Zhang Q.; Chen W.; Zhong X.; He J. Synthesis of Yellow-Fluorescent Carbon Nano-Dots by Microplasma for Imaging and Photocatalytic Inactivation of Cancer Cells. Nanoscale Res. Lett. 2021, 16 (1), 1–9. 10.1186/S11671-021-03478-2/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaji K.; Mani S.; Ponmurugan P.; de Castro C. S.; Lloyd Davies M.; Balasubramanian M. G.; Pitchaimuthu S. Green-Synthesis-Derived CdS Quantum Dots Using Tea Leaf Extract: Antimicrobial, Bioimaging, and Therapeutic Applications in Lung Cancer Cells. ACS Applied Nano Materials 2018, 1 (4), 1683–1693. 10.1021/acsanm.8b00147. [DOI] [Google Scholar]
- Yang L.; Shi P.; Zhao G.; Xu J.; Peng W.; Zhang J.; Zhang G.; Wang X.; Dong Z.; Chen F.; Cui H. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduction and Targeted Therapy 2020, 5 (1), 1–35. 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M.; Jordan C. T. The Increasing Complexity of the Cancer Stem Cell Paradigm. Science (1979) 2009, 324 (5935), 1670–1673. 10.1126/SCIENCE.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N.; Harris P. J.; Warren R. Q.; Ivy S. P. Targeting Cancer Stem Cells by Inhibiting Wnt, Notch, and Hedgehog Pathways. Nature Reviews Clinical Oncology 2011 8:2 2011, 8 (2), 97–106. 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E.; Ginestier C.; Iovino F.; Tarpin C.; Diebel M.; Esterni B.; Houvenaeghel G.; Extra J.-M.; Bertucci F.; Jacquemier J.; Xerri L.; Dontu G.; Stassi G.; Xiao Y.; Barsky S. H.; Birnbaum D.; Viens P.; Wicha M. S. Aldehyde Dehydrogenase 1-Positive Cancer Stem Cells Mediate Metastasis and Poor Clinical Outcome in Inflammatory Breast Cancer. Clin. Cancer Res. 2010, 16 (1), 45–55. 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W.; Guo R.; Yuan F.; Li Y.; Li X.; Zhang Y.; Zhou S.; Fan L. Red-Emissive Carbon Quantum Dots for Nuclear Drug Delivery in Cancer Stem Cells. J. Phys. Chem. Lett. 2020, 11 (4), 1357–1363. 10.1021/acs.jpclett.9b03891. [DOI] [PubMed] [Google Scholar]
- Li J.; Lee W. Y. W.; Wu T.; Xu J.; Zhang K.; Li G.; Xia J.; Bian L. Multifunctional Quantum Dot Nanoparticles for Effective Differentiation and Long-Term Tracking of Human Mesenchymal Stem Cells In Vitro and In Vivo. Adv. Healthcare Mater. 2016, 5 (9), 1049–1057. 10.1002/adhm.201500879. [DOI] [PubMed] [Google Scholar]
- Yan J.; Hou S.; Yu Y.; Qiao Y.; Xiao T.; Mei Y.; Zhang Z.; Wang B.; Huang C. C.; Lin C. H.; Suo G. The Effect of Surface Charge on the Cytotoxicity and Uptake of Carbon Quantum Dots in Human Umbilical Cord Derived Mesenchymal Stem Cells. Colloids Surf., B 2018, 171, 241–249. 10.1016/j.colsurfb.2018.07.034. [DOI] [PubMed] [Google Scholar]
- Borgiani E.; Duda G. N.; Checa S. Multiscale Modeling of Bone Healing: Toward a Systems Biology Approach. Frontiers in Physiology 2017, 8 (MAY), 287. 10.3389/fphys.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaquinta M. R.; Mazzoni E.; Bononi I.; Rotondo J. C.; Mazziotta C.; Montesi M.; Sprio S.; Tampieri A.; Tognon M.; Martini F. Adult Stem Cells for Bone Regeneration and Repair. Frontiers in Cell and Developmental Biology 2019, 7, 268. 10.3389/fcell.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.; Zhang F.; Zhang J.; Shao D.; Wang Y.; Li S.; Lv S.; Chi G.; Zhang M.; Chen L.; Liu J. Bioactive Carbon Dots Direct the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Colloids Surf., B 2019, 179, 1–8. 10.1016/j.colsurfb.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Bu W.; Xu X.; Wang Z.; Jin N.; Liu L.; Liu J.; Zhu S.; Zhang K.; Jelinek R.; Zhou D.; Sun H.; Yang B. Ascorbic Acid-PEI Carbon Dots with Osteogenic Effects as MiR-2861 Carriers to Effectively Enhance Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12 (45), 50287–50302. 10.1021/acsami.0c15425. [DOI] [PubMed] [Google Scholar]
- Li H.; Xie H.; Liu W.; Hu R.; Huang B.; Tan Y.-F.; Liao E.-Y.; Xu K.; Sheng Z.-F.; Zhou H.-D.; Wu X.-P.; Luo X.-H. A Novel MicroRNA Targeting HDAC5 Regulates Osteoblast Differentiation in Mice and Contributes to Primary Osteoporosis in Humans. J. Clin. Invest. 2009, 119 (12), 3666–3677. 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Yang M.; Liu L.; Yan G.; Yan H.; Feng J.; Li Z.; Li D.; Sun H.; Yang B. Osteogenic Potential of Zn2+-Passivated Carbon Dots for Bone Regeneration in Vivo. Biomaterials Science 2019, 7 (12), 5414–5423. 10.1039/C9BM01181A. [DOI] [PubMed] [Google Scholar]
- Ghafary S. M.; Nikkhah M.; Hatamie S.; Hosseinkhani S. Simultaneous Gene Delivery and Tracking through Preparation of Photo-Luminescent Nanoparticles Based on Graphene Quantum Dots and Chimeric Peptides. Scientific Reports 2017, 7 (1), 1–14. 10.1038/s41598-017-09890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnight E. R.; Wiley L. A.; Mullins R. F.; Stone E. M.; Tucker B. A. Gene Therapy Using Stem Cells. Cold Spring Harbor Perspectives in Medicine 2015, 5 (4), a017434. 10.1101/cshperspect.a017434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogrady B. Stem-Cell and Genetic Therapies Make a Healthy Marriage. Nature 2019, 569 (7756), S23–S23. 10.1038/d41586-019-01442-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.