Abstract
The development of a one-step amination–cyclization cascade reaction for the synthesis of N-substituted iminosugars from iodo-pentoses and hexoses is reported. This novel methodology allows for the stereoselective conversion of easily accessible iodo-aldoses and iodo-ketoses into iminosugars in a single step, in highly efficient yields (63–95%), and in aqueous media. Furthermore, the use of functionalized amines allows for the synthesis of N-functionalized iminosugars without additional steps. To illustrate this methodology, a number of biologically important iminosugars were prepared, including 1-deoxynojirimycin, (3S,4R,5S,6R)-azepane-3,4,5,6-tetraol, and N-functionalized 1-deoxymannojirimycins.
Introduction
Iminosugars are naturally occurring monosaccharide analogues in which the ring-oxygen is replaced by nitrogen. As carbohydrate mimics, iminosugars have a variety of biological properties, which is predominantly due to their ability to interact with the active site of glycosidases, and to a lesser extent, glycosyltransferases.1 For example, the piperidine 1-deoxymannojirimycin (DMJ, 1, Figure 1) exhibits promising mammalian α-fucosidase activity,2 while the gluco-configured 1-deoxynojirimycin (DNJ, 2) is an α-glucosidase inhibitor, and the related N-alkylated derivatives Miglustat (3) and Miglitol (4) have found application in the treatment of Gaucher’s disease3,4 and type II diabetes,5 respectively. The seven-membered iminosugar (3S,4R,5S,6R)-azepane-3,4,5,6-tetraol (5) inhibits numerous glycosidases, including α- and β-galactosidase, β-glucosidase, and α-fucosidase.6 More recently, various N-functionalized piperidines have been found to inhibit α-glucosidases that are crucial to protein synthesis pathways exploited within virus-infected cells, thus allowing for the potential host-directed treatment of viral infections such as HIV,7,8 hepatitis C,9 and influenza.10
Figure 1.
Representative Iminosugars.
The low natural abundance and growing pharmacological application of iminosugars have led to this class of compounds remaining a relevant and interesting target for synthetic chemists. As such, there is a large body of work pertaining to their syntheses.11 In recent years, we have established efficient strategies for the protecting-group-free synthesis of pyrrolidines;12−14 however, applying this methodology to the synthesis of piperidines proved more challenging.15,16
To date, the most efficient routes for the synthesis of piperidines rely on either double reductive aminations17−24 or the incorporation of a nitrogen atom into a ketose sugar followed by intramolecular reductive amination using either catalytic hydrogenation or a borohydride reagent. For example, in 1965, Paulsen and co-workers reported the first synthesis of DNJ25 and demonstrated that intramolecular reductive amination of 6-amino-6-deoxy-l-sorbose (6a) proceeds to selectively provide DNJ (2, Scheme 1). DNJ (2) was isolated as the sole isomer, as the intermediate imine is selectively attacked from the α-face.17 Several years later, it was shown that 6-deoxy-6-benzyloxycarbonylamido-ketose 6b(26) or 6-deoxy-6-azido-ketose 6c, prepared either enzymatically27 or chemically,28 provided convenient access to DNJ (2) using the same hydrogenation strategy, again with exclusive formation of the gluco-isomer, and others have subsequently developed routes for the synthesis of polyhydroxypiperidines utilizing iminium ions as key reactive intermediates.29
Scheme 1. Strategies for the Synthesis of DNJ and Related Iminosugars.
Building on the aforementioned studies and our experience with iminosugar synthesis from ω-deoxy-ω-iodo-glycosides,30,31 we envisioned that iodo-glycosides would be ideal starting materials for a cascade reaction leading to the efficient syntheses of a variety of iminosugars with minimal use of protecting groups (Scheme 2). Key in our approach is the avoidance of elaborate protecting-group strategies and functional group interconversions to reach the imine intermediate. As such, imine formation between the ketose carbonyl of I and amine II could be followed by an intramolecular displacement of the iodide to give cyclic imine III, which could then be stereoselectively reduced to form the desired iminosugar(IV). This overall process not only reduces the number of steps required for iminosugar synthesis but also keeps the use of protecting groups to a minimum making the route highly atom-economic. In addition, this reaction would allow for the incorporation of N-substituents in the same step.
Scheme 2. Proposed Syntheses of N-Substituted Iminosugars.
Results and Discussion
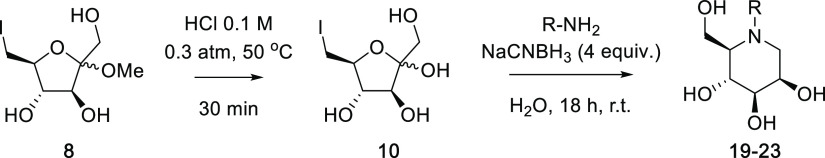
To explore the proposed synthetic route, we commenced our studies using d-fructose (7) as the starting sugar (Scheme 3). To this end, d-fructose (7) was converted into methyl glycoside 8 and subsequently iodinated to give methyl 6-deoxy-6-iodo-d-fructofuranoside (9) in two steps and 65% overall yield.23 Hydrolysis of methyl iodoglycoside 9 was then optimized, whereby exposure to 0.1 M HCl in H2O at 0.3 atm and 50 °C resulted in complete conversion into 6-deoxy-6-iodo-d-fructose (10), as evidenced by thin layer chromatography (TLC). While 6-deoxy-6-iodo-d-fructose (10) could be isolated, this compound was sensitive to pH and decomposed rapidly in vacuo under weakly acidic or basic conditions. Thus, a crude reaction mixture of 10 in 0.1 M aqueous HCl was treated with excess AcONH4 (130 equiv) and aq. NH3 (90 equiv) followed by NaCNBH3 (4 equiv), and the reaction mixture was stirred at 80 °C for 18 h. After purification by Dowex-H+ resin and silica gel flash column chromatography, DMJ (1) was then isolated in 88% yield as the sole stereoisomer.
Scheme 3. Synthesis of DMJ (1) from d-fructose (7).
Spurred on by these results, we then sought to optimize the synthesis of DMJ (1) by altering the reaction conditions for the reductive amination. A large excess of ammonium salt is a requirement for the effective reductive amination of aldehydes and aldoses;32 however, the reductive amination of ketoses does not suffer from the dimerization reactions observed in the synthesis of primary amines.24 Accordingly, the reductive amination reaction of ketose 10 was repeated with aq. NH3 in the absence of AcONH4, which led to DMJ (1) being formed in 89% yield and moreover, facilitated the purification process. Further optimization studies involving changes to reaction concentration, temperature, and time then followed, which ultimately allowed for DMJ (1) to be synthesized in 95% yield from 6-deoxy-6-iodo-d-fructose (9) via a two-step-one-pot reaction using aq. NH3 (90 equiv) and NaCNBH3 (4 equiv) and stirring the reaction mixture at r.t. for 18 h. Taken together, this resulted in a four-step (three-pot) synthesis of DMJ (1) in 62% overall yield from readily available d-fructose (6). Other notable syntheses of DMJ include those published by Furneaux et al. (five steps, 25% overall yield from d-fructose),33 by Maier et al. (seven steps, 35% overall yield from 1,5-anhydro-d-fructose),34 and several enzymatic and chemo/enzymatic syntheses (2–5 steps, 9–44% yield).35−37
To account for the stereoselectivity of the amination–cyclization cascade reaction en route to the synthesis of DMJ (1), it is postulated that exposure of iodide 10 to ammonia leads to ring opening, imine formation, and subsequent intramolecular iodide displacement at the 6-position to give cyclic iminium ion 11 (Scheme 3). Iminium ion 11 then undergoes a stereoselective reduction, whereby the lowest energy transition state for the reduction is suggested to occur when the maximum number of substituents is in a pseudoequatorial orientation,38−40 which can be achieved when conformer 11 undergoes Si face reduction to give DMJ (1), a selectivity previously observed in Paulsen’s first synthesis of DMJ through hydrogenation.17
To determine whether the amination–cyclization cascade reaction could be successfully applied to the synthesis of other piperidines, the synthesis of 1-deoxynojirimycin (DNJ, 2) from l-sorbose (12) was then attempted (Scheme 4). First, l-sorbose (12) was converted into methyl glycoside 13(41) and selectively iodinated at the 6-position to afford methyl 6-deoxy-6-iodo-l-sorbofuranose (14) in 43% yield (over two steps). Sorbofuranoside 14 was then subjected to the previously optimized two-step-one-pot amination–cyclization cascade reaction to yield DNJ (2) selectively and in 95% yield. Once again, the preferential formation of DNJ could be explained via the stereoselective reduction of the intermediate cyclic imine so as to generate a transition state that places the maximum number of substituents in pseudoequatorial orientations. Taken as a whole, the total synthesis of DNJ was thus completed in four steps (three-pot) and 41% overall yield from inexpensive and readily available l-sorbose. Other efficient strategies for the synthesis of DNJ include those involving the use of enzymes (three steps, 55–65% overall yield from glucose or protected derivatives thereof)42−44 and a nonenzymatic route developed by Demailly et al. (four steps, 54% overall yield from l-sorbose).20
Scheme 4. Synthesis of DNJ (2) from l-sorbose (12).
Next, we attempted to synthesize l-1-deoxygalactonojirimycin (l-DGJ) from d-tagatose using our amination–cyclization cascade strategy. First synthesized in 1990,45l-DGJ has since been identified as an inhibitor and molecular chaperone of galactosidases and galactosyl transferases and thus shows much promise for the treatment of lysosomal storage disorders and other protein deficiencies.46−49 While our syntheses of DMJ (1) and DNJ (2) involved Fischer glycosylation and the subsequent installation of an iodide at the 6-position of d-fructose and l-sorbose, respectively, we previously noted that Fischer glycosylation and iodination of d-tagatose led to a complex mixture of products.22 Accordingly, d-tagatose (15) was protected with isopropylidene groups to give 1,2:3,4-di-O-isopropylidene-d-tagatofuranose 16 in which the 6-position was subsequently iodinated to give 6-iodo-tagatoside 17 in 78% yield over two steps (Scheme 5).50 Acid-mediated deprotection of 17 under the agency of 0.15 M HCl at 50 °C and 0.3 atm for 18 h in MeOH, rather than H2O, led to complete conversion of 17 to 6-deoxy-6-iodo-d-tagatose, as evidenced by TLC. The crude reaction mixture was then subjected to NH3 (aq.) and NaCNBH3, and the solution was stirred at room temperature for a further 18 h. Workup and purification of the reaction mixture then provided l-DGJ (18) in 86% yield over the two steps. As with DMJ (1) and DNJ (2), formation of the major product l-DGJ (18) resulted from the stereoselective reduction of an intermediate imine to place the maximum number of substituents in the pseudoequatorial orientation. Thus, our total synthesis of l-DGJ was achieved in four steps (three-pot) and 67% overall yield from d-tagatose. The most comparable and efficient synthesis was reported by Jenkinson et al. in 2011,42 with l-DGJ being synthesized in four steps and in 66% overall yield from d-tagatose.
Scheme 5. Synthesis of l-DGJ (18) from d-tagatose (15).
Having demonstrated the versatility of the amination–cyclization cascade reaction for the synthesis of a variety of piperidines, the applicability of the route for the preparation of N-functionalized piperidines was then investigated. To this end, methyl 6-deoxy-6-iodo-d-fructofuranoside (9) was treated with 0.1 M aqueous HCl, and the intermediate, 6-deoxy-6-iodo-fructose 10, was subjected to n-butylamine in the presence of NaCNBH3 (4 equiv) at room temperature for 18 h (Entry 1, Table 1). Following purification by Dowex-H+ ion exchange resin and silica gel flash column chromatography, N-butyl-DMJ (19) was isolated in 69% yield (over two steps). Here, optimization studies revealed that the use of 10 equivalents of n-butylamine gave the best yield of N-butyl-DMJ without unnecessarily complicating the purification process. Our four-step synthesis of N-butyl-DMJ (19) from d-fructose is the highest yielding to date (45% overall yield), with previous syntheses having been achieved via the alkylation of DMJ,51,52 the epimerization of N-butyl-DNJ,53 or through the synthesis and modification of d-mannolactam.54
Table 1. Synthesis of N-Functionalized DMJ Derivativesa,b.
All reaction mixtures used NaCNBH3 (4 equiv) and were stirred at room temperature for 18 h followed by concentration in vacuo and purification using Dowex-H+ exchange resin and silica gel flash column chromatography.
Isolated yield calculated over two steps.
Next, the synthesis of N-methyl-DMJ (20) was attempted (Entry 2, Table 1). Given the low boiling point of methylamine (−6 °C), a large excess of this reagent (26 equiv) was used as the excess reagent could be readily removed by evaporation following completion of the reaction. In this way, N-methyl-DMJ (20) was isolated in an excellent 87% yield following purification using Dowex-H+ exchange resin and silica gel flash column chromatography (56% overall yield from d-fructose). For the synthesis of N-benzyl-DMJ (21) (Entry 3), N-phenethyl-DMJ (22) (Entry 4), and N-(2-hydroxyethyl)-DMJ (23) (Entry 5), 10 equivalents of amine were required to prevent difficulties in separating the residual amine from the desired products; however in all instances, the reactions occurred smoothly to give 21, 22, and 23 in 63% yield, 64% yield, and 67% yield, respectively. As with N-butyl-DMJ (19), the total syntheses of N-methyl-DMJ (20),55−57N-benzyl-DMJ (21),58−60 and N-(2-hydroxyethyl)-DMJ (23)43,44,51 reported herein represent the shortest and highest yielding syntheses to date. The synthesis of N-phenethyl-DMJ (22) was hitherto unpublished.
Having demonstrated the versatility of our amination–cyclization cascade reaction when using ketose sugars as starting materials, we then sought to extend this strategy to include aldoses. To this end, d-xylose (24) was subjected to Fischer glycosylation and subsequent iodination using previously optimized conditions12 to give methyl 6-deoxy-6-iodo-d-xylofuranoside (25) in 60% yield over the two steps (Scheme 6). Next, iodoxyloside 25 was refluxed in a 0.3 M HCl solution for an hour, after which point the in situ generated 5-deoxy-5-iodo-d-xylose was exposed to NH3 (aq.) and NaCNBH3 at room temperature for 18 h. Following purification via Dowex-H+ resin and silica gel flash column chromatography, (3R,4r,5S)-piperidine-3,4,5-triol (26) was isolated in an excellent 88% yield (53% overall yield from d-xylose). Triol 26 belongs to a class of iminosugars first synthesized in the mid-1960s,61−64 with the shortest and highest yielding synthesis of 26 previously being achieved in five steps and 40% overall yield from methyl 6-deoxy-6-bromo-α-d-glucopyranoside.65
Scheme 6. Synthesis of (3R,4r,5S)-Piperidine-3,4,5-triol (26) from d-xylose (24).
Finally, we sought to extend our methodology to the synthesis of azepanes (Scheme 7). Because of the difficulty associated with the hydrolysis of methyl aldohexosides,66 our synthesis thus began with isopropylidene protection and subsequent iodination of d-galactose (27) to give protected iodogalactoside 28 in 78% yield (two steps).16 To avoid the formation of methyl 6-deoxy-6-iodo-d-galactopyranose in the subsequent deprotection step, isopropylidene deprotection was initially achieved using a 9:1 mixture of AcOH:H2O under reflux,67 whereby full conversion to 6-deoxy-6-iodo-d-galactose (29) was observed via TLC analysis after 2 days. Alternatively, treatment of protected iodogalactoside 28 with a 9:1 mixture of TFA/H2O at 50 °C and 0.3 atm led to the formation of 6-deoxy-6-iodo-d-galactose (29) within 30 min. Unlike the previously described iodo-sugars, iodide 29 was stable in the presence of acid in vacuo (pH = 1) and thus could be isolated following CH2Cl2:H2O extraction and subsequent concentration in vacuo. Next, 6-deoxy-6-iodo-d-galactose (29) was exposed to NH3 (aq.) and NaCNBH3 at room temperature for 18 h. Purification of the crude product by Dowex-H+ resin, requiring careful elution with 0.1% aq. NH3, then afforded (3S,4R,5S,6R)-azepane-3,4,5,6-tetraol (5) in 68% yield over two steps (55% overall yield from d-galactose). Comparably, Wong et al. achieved the most efficient synthesis of azepane 5 to date in four steps and 63% overall yield, also from d-galactose.6
Scheme 7. Synthesis of (3S,4R,5S,6R)-Azepane-3,4,5,6-tetraol (5) from d-Galactose (27).
Conclusions
In conclusion, we developed an amination–cyclization cascade reaction that has been successfully applied to a variety of readily available ketose and aldose carbohydrate starting materials. In this way, DMJ (1), DNJ (2), l-DGJ (18), (3R,4r,5S)-piperidine-3,4,5-triol (26), and (3S,4R,5S,6R)-azepane-3,4,5,6-tetraol (5) were all prepared in four steps and in overall yields that were comparable or higher than those previously reported. Key in these syntheses was the formation of an appropriate iodoglycoside intermediate, which was readily accessible in two steps from the corresponding monosaccharide. In addition, the scope of the amination–cyclization cascade reaction was further exemplified through the reaction of 6-deoxy-6-iodo-d-fructose with various amines thereby allowing for the first reported synthesis of N-(2-phenyl)ethyl-DMJ (22) and the shortest and highest yielding syntheses of N-butyl-DMJ (19), N-methyl-DMJ (20), N-benzyl-DMJ (21), and N-(2-hydoxyl)ethyl-DMJ (23) to date. Given the versatility of this synthetic methodology, it is thus anticipated that it can be readily adapted to the synthesis of other iminosugars, particularly other N-functionalized derivatives, without compromising overall yields.
Experimental Section
Unless otherwise stated, all reactions were performed under atmospheric air. THF (Lab-Scan) was distilled from activated zinc prior to use. MeOH (Pure Science), EtOH (absolute, Pure Science), AcOH (Lab Scam), CH2Cl2 (LabServ), 30% aqueous NH3 (Fisher Science), isopropanol (BDH), d-fructose (Carbosynth), d-xylose (Carbosynth), d-galactose (Carbosynth), NaCNBH3 (Aldrich), imidazole (Aldrich), I2 (BDH), triphenyl phosphine (Acros), AcONH4 (Aldrich), aqueous 35% HCl (Univar), and 98% H2SO4 (Panreac), d-tagatose (Carbosynth), NaOH (Pure Science), anhydrous CuIISO4 (Scientific & Chemical Supplies), TFA (Aldrich), aminodiphenylmethane (Aldrich), n-butylamine (Aldrich), ethanolamine (BDH), benzylamine (Aldrich), phenylethylamine (BDH), and aq. 40% methylamine (BDH) were used as received. Drum petroleum ether and ethyl acetate were distilled before use. Distilled H2O was generated using a Millipore RiOs 8 purifier. Zn dust was activated by the careful addition of conc. H2SO4 to Zn powder in the presence of ethanol, and the solid was decanted, washed with ethanol, diethyl ether, and then finally washed (and stored) in petroleum ether. All solvents were removed by evaporation under reduced pressure (in vacuo). Reactions were monitored by TLC analysis on Macherey-Nagel silica gel-coated plastic sheets (0.20 mm, with fluorescent indicator UV254) with detection by UV absorption (254 nm), by dipping in 10% H2SO4 in MeOH or 3% ninhydrin in EtOH followed by charring at ∼150 °C. Column chromatography was performed on Pure Science silica gel (40–63 μm). Dowex-H+ 50wx8-100 ion exchange resin was activated by 1 hour exposure to 1 M HCl. High-resolution mass spectra were recorded on a Waters Q-TOF Premier Tandem Mass Spectrometer using positive electrospray ionization. Optical rotations were recorded using a PerkinElmer 241 polarimeter at the sodium D-line. Infrared (IR) spectra were recorded as thin films using a Bruker Tensor 27 Fourier transform infrared spectrometer, equipped with an attenuated total reflectance sampling accessory, and are reported in wave numbers (cm–1). Nuclear magnetic resonance (NMR) spectra were recorded at 20 °C in CDCl3 or D2O using either a Varian Unity-INOVA operating at 300 MHz or a Varian Unity operating at 500 MHz. Chemical shifts are given in ppm (δ) and are relative to chloroform or water, and all given 13C spectra are proton decoupled. NMR peak assignments were made using correlation spectroscopy, heteronuclear single quantum coherence, and heteronuclear multiple bond correlation experiments, and carbohydrate numbering has been employed where possible.
Methyl d-Fructofuranoside (8)
d-Fructose (7, 3.6 g, 20 mmol) and H2SO4 (1.0 mL, 18 mmol) were added to 200 mL of MeOH. After the solution was stirred for 15 min, aq. NH3 (4 mL, 30%) was added, and the reaction mixture was concentrated to ca. 50 mL in vacuo, cooled over ice, filtered, and concentrated in vacuo. The remaining oil was purified by silica gel flash column chromatography (EtOAc/MeOH, 99/1 to 95/5, v/v) to afford 8 in an anomeric mixture (3.43 g, 87%). α-8, Rf = 0.57, β-8, Rf= 0.70 (EtOAc/iPrOH/H2O, 6/4/1, v/v/v). HRMS: m/z calcd for [C7H14O6 + Na]+: 217.0682, obsd.: 217.0690. IR and NMR spectral data matched those previously reported in ref (68).
Methyl 6-Deoxy-6-iodo-d-fructofuranoside (9)
Methyl glycoside 8 (2.02 g, 10.5 mmol), PPh3 (4.12 g, 15.7 mmol), and imidazole (1.54 g, 20.9 mmol) were dissolved in dry THF (84 mL) and brought to reflux. A solution of I2 (3.99 g, 15.7 mmol) in THF (42 mL) was added dropwise to the refluxing solution. The resulting solution was refluxed for a further 10 mins, cooled to room temperature, filtered over celite (washing with THF), and concentrated in vacuo. The remaining orange oil was purified via silica gel flash column chromatography (Petroleum ether/EtOAc, 4/1 to 1/2, v/v) and reverse-phase column chromatography (H2O/MeOH, 100/0 to 9/1, v/v) to afford the desired product 9 (2.37 g, 75% yield) as a colorless oil. α-9, Rf = 0.34, 1H-NMR (500 MHz, D2O) δ 4.16 (d, J3,4 = 2 Hz, 1H, H-3), 3.93–3.89 (m, 2H, H-4, H-5), 3.79 (d, J1a,1b = 12.5 Hz, 1H, H-1a), 3.68 (d, J1b,1a = 12.5 Hz, 1H, H-1b), 3.49 (dd, J6a,6b = 4.5 Hz, J6b,5 = 10.5 Hz, 1H, H-6a), 3.41–3.41 (m, 1H, H–6b), 3.32 (s, 3H, OMe). 13C-NMR (125 MHz, D2O) δ 108.1 (C-2), 81.7 (C-5), 80.7 (C-4), 80.4 (C-3), 57.7 (C-1), 48.2 (OMe), 5.2 (C-6). β-9, Rf = 0.29 (DCM/MeOH, 5/1, v/v). 1H-NMR (500 MHz, D2O) δ 4.20 (d, J3,4 = 8 Hz, 1H, H-3), 4.06 (t, J4,3 = 8 Hz, 1H, H-4), 3.88–3.84 (m, 1H, H-5), 3.71 (d, J1a,1b = 12.5 Hz, H-1a), 3.66 (d, J1b,1a = 12.5 Hz, H-1b), 3.49 (dd, J6a,6b = 4.5 Hz, J6b,5 = 10.5 Hz, 1H, H-6a), 3.41–3.41 (m, 1H, H-6b), 3.36 (s, 3H, OMe). 13C-NMR (125 MHz, D2O) δ 103.7 (C-2), 80.1 (C-5), 78.6 (C-4), 77.0 (C-3), 59.4 (C-1), 49.3 (OMe), 6.9 (C-6). IR (film) 3350, 2895, 1462, 1039, 1031 cm–1. HRMS: m/z calcd. For [C7H13IO5 + Na]+: 326.9699, obsd.: 326.9704. Spectral data matched those previously reported in ref (69).
6-Deoxy-6-iodo-d-fructofuranose (10)
Methyl 6-deoxy-6-iodo-d-fructofuranoside (9, 0.25 g, 0.82 mmol) was dissolved in 8.2 mL of a 0.15 M HCl solution and stirred at room temperature until TLC confirmed full conversion to 6-deoxy-6-iodo-d-fructofuranoside (ca. 3 days). The resulting mixture was neutralized using NaHCO3, filtered over celite, and concentrated in vacuo to give the desired product (0.054 g, 0.18 mmol, 23%), Rf = 0.25 (DCM/MeOH, 5/1, v/v), 1H-NMR (500 MHz, D2O) δ 4.20 (d, J3,4 = 8.4 Hz, 1H, H-3), 4.13 (t, J4,3 = J4,5 = 7.7 Hz, 1H, H-4), 3.85 (m, 1H, H-5), 3.65 (d, J1a,1b = 12.3 Hz, 1H, H-1a), 3.60 (d, J1b,1a = 12.3 Hz, 1H, H-1b), 3.56 (dd, J6a,6b = 10.8 Hz, J6a,5 = 5.2 Hz, 1H, H-6a), 3.45 (dd, J6b,6a = 10.8 Hz, J6b,5 = 6.3 Hz, 1H, H-6b). 13C-NMR (125 MHz, D2O) δ 101.5 (C-2), 79.5 (C-5), 78.4 (C-4), 75.5 (C-3), 62.7 (C-1), 7.3 (C-6). HRMS: m/z calcd. For [C6H11IO5 + H]+: 290.9724, obsd.: 290.9728.
Methyl l-Sorbofuranoside (13)
To a flask containing H2SO4 in MeOH (0.03 M, 400 mL) was added l-sorbose (12, 2.00 g, 11.1 mmol), and the reaction mixture was stirred at room temperature for 2 h, before the addition of aq. 35% NH3 (3 mL). The reaction mixture was concentrated in vacuo to ca. 100 mL and filtered over celite (cold MeOH wash), and the mother liquor was collected and concentrated. The residue was purified using silica gel flash column chromatography (EtOAc to EtOAc/MeOH, 9/1) to give an α,β mixture of methyl-d-sorbofuranoside (1.19 g, 55%), which was used as is for subsequent reactions. α-13Rf = 0.32 (EtOAc/i-PrOH/H2O, 6/4/1, v/v/v). β-13Rf = 0.30 (EtOAc/i-PrOH/H2O 6/4/1, v/v/v). Spectral data matched those previously reported in ref (33).
Methyl 6-Deoxy-6-iodo-l-sorbofuranoside (14)
To an α,β-mixture of methyl l-sorbofuranoside (13, 0.55 g, 2.8 mmol) in THF (28 mL) were added PPh3 (1.85 g, 70 mmol) and imidazole (0.57 g, 84 mmol). The reaction mixture was heated to 70 °C, and a solution of I2 (1.43 g, 56 mmol) in THF (14 mL) was added portion wise over 5 min. The reaction mixture was stirred at 70 °C until TLC showed complete conversion of the starting material to the desired product (ca. 7 h), after which time MeOH (15 mL) was added and the reaction mixture was concentrated in vacuo. The resulting mixture was purified using silica gel flash column chromatography (Petroleum ether/EtOAc 4/1 to 1/1, v/v) and HP20 (H2O to H2O/MeOH, 9/1, v/v) to give an α,β-mixture (α:β = 1:5) of methyl 6-deoxy-6-iodo-l-sorbofuranoside (0.67 g 78%).: α-14Rf = β-14Rf = 0.35 (DCM/MeOH, 5/1, v/v). IR (film) 3401, 2980, 2880, 1462, 1039, 1031 cm–1. β-141H-NMR (500 MHz, D2O) δ 4.41 (m, 2H, H-4, H-5), 4.36 (m, 1H, H-3), 3.76 (d, J1a,1b = 12.2 Hz, 1H, H-1a), 3.66 (d, J1b,1a = 12.2 Hz, 1H, H-1b), 3.35 (dd, J6a,6b = 9.3 Hz, J6a,5 = 5.4 Hz, 1H, H-6a), 3.29–3.23 (m, 1H, H-6b), 3.31 (s, 3H, OMe).13C-NMR (125 MHz, D2O) δ 108.2 (C-2), 80.6 (C-4), 75.2 (C-3), 71.5 (C-5), 58.5 (C-1), 48.8 (OMe), −0.4 (C-6). HRMS: m/z calcd. For [C7H13IO5 + Na]+: 326.9699, obsd.: 326.9694.
1,2:3,4-Di-O-isopropylidene-d-tagatofuranose (16)
Anhydrous Cu(II)SO4 (4.17 g, 26 mmol) and d-tagatose (15, 1.17 g, 6.5 mmol) were added to a flask under an argon atmosphere. To this flask, H2SO4 (36 mM) in acetone (distilled and degassed, 22 mL) was added, and the resulting mixture was stirred at room temperature for 18 h. The reaction mixture was quenched with sodium carbonate, filtered over celite, concentrated, and purified via silica gel flash column chromatography (Petroleum ether/EtOAc, 100/0 to 4/1, v/v) to give 16 as a colorless oil (1.47 g, 87% yield), Rf = 0.3 (petroleum ether/EtOAc, 1/1, v/v). Spectral data matched those previously reported in ref (42).
1,2:3,4-Di-O-isopropylidene-6-deoxy-6-iodo-d-tagatofuranose (17)
Diisopropylidene-protected sugar 16 (2.31 g, 8.9 mmol), PPh3 (6.75 g, 25.8 mmol), and imidazole (1.81 g, 26.6 mmol) were added to freshly distilled THF (89 mL), and the solution was brought to reflux. To this, I2 (4.56 g, 18 mmol) in THF (44 mL) was added dropwise over 1.5 h. The resulting mixture was refluxed for a further 12 h, then quenched with methanol, and concentrated. The residue was subjected to silica gel flash column chromatography (Petroleum ether/EtOAc, 100/0 to 4/1, v/v) to give iodide 17 as a white crystalline solid (2.84 g, 87% yield), m.p. 41–42 °C, Rf = 0.8 (petroleum ether/EtOAc, 1/1, v/v). [α]D20 = + 46.1 (c = 1.1, CDCl3). IR (film) 2989, 2391, 1376, 1209, 1028, 851 cm–1. 1H-NMR (500 MHz, CDCl3) δ 4.82 (m, 1H, H-4), 4.63 (d, J3,4 = 5.5 Hz, 1H, H-3), 4.23 (dd, J1a,1b = 9.5 Hz, J1a,3 = 1 Hz, 1H, H-1a), 4.20 (dd, J1a,1b = 9.5 Hz, J1b,3 = 1 Hz, 1H, H-1b), 4.20 (m, 1H, H-5), 3.28 (m, 2H, H-6a,b), 1.41 (s, 3H, H-8), 1.32 (s, 3H, H-9), 1.47 (s, 3H, H-11), 1.39 (s, 3H, H-12); 13C-NMR (125 MHz, CDCl3) δ 112.9 (C-2), 111.8 (C-10), 111.8 (C-7), 85.4 (C-3), 79.8 (C-5), 79.7 (C-4), 69.3 (C-1), 26.4 (C-11), 26.4 (C-12), 26.0 (C-8), 25.0 (C-9), −0.9 (C-6). HRMS: m/z calcd. For [C12H20IO5 + H]+: 371.0350, obsd.: 371.0347. Spectral data matched those previously reported in ref (70).
General Amination–Cyclization Cascade Reaction Conditions
The methyl iodo-glycosides (1.0 mmol) were added to a solution of aq. HCl (0.15 M, 10 mL) and stirred under reduced pressure (0.3 atm) at 50 °C until TLC analysis showed full conversion to the corresponding iodo-ketofuranose. Following this, the appropriate amine and NaCNBH3 (4 mmol) were added sequentially, and the solution was stirred at room temperature for 18 h. The resulting mixture was concentrated in vacuo and purified using Dowex-H+ and silica gel flash column chromatography.
1-Deoxymannojirimycin (1)
Methyl 6-deoxy-6-iodo-d-fructofuranoside (9, 0.48 g, 1.56 mmol) was treated to the general amination–cyclization cascade reaction conditions using aq. 35% NH3 (8 mL, 144 mmol) and purified using Dowex-H+ (1% aq. NH3) and silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 20/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 96% yield (0.24 g, 1.50 mmol). Rf = 0.10 (DCM/EtOH/MeOH/aq. NH3, 5/2/2/1, v/v/v/v); [α]D20 -15.7 (c = 1.6, H2O); lit.25 [α]D -15.0 (c = 2, H2O). IR (film) 3420, 3305, 2992, 2883 cm–1; 1H-NMR (500 MHz, D2O): δ 4.26 (td, J2,1a = J2,3 = 3.1 Hz, J2,1b = 1.5 Hz, 1H, H-2), 4.00 (dd, J6a,6b = 12.6 Hz, J6a,5 = 3.3 Hz, 1H, H-6a), 3.87 (t, J3,4 = J4,5 = 10.1 Hz, 1H, H-4), 3.86 (dd, J6a,6b = 12.8 Hz, J6b,5 = 6.7 Hz, 1H, H-6b), 3.70 (dd, J3,4 = 9.5 Hz, J3,2 = 3.1 Hz, 1H, H-3), 3.42 (dd, J1a,1b = 13.6 Hz , J1a,2 = 3.1 Hz, 1H, H-1a), 3.25 (dd, J1b,1a = 13.6 Hz, J1b,2 = 1.5 Hz, 1H, H-1b), 3.15 (ddd, J5,4 = 10.3 Hz, J5,6a = 6.7 Hz, J5,6b = 3.3 Hz, 1H, H-5); 13C-NMR (125 MHz, D2O): δ 72.2 (C-3), 65.7 (C-2), 65.6 (C-4), 60.0 (C-5), 57.9 (C-6), 47.4 (C-1); HRMS: m/z calcd. For [C6H13NO4 + H]+: 164.0917, obsd.: 164.0915. Spectral data matched those previously reported in ref (71).
N-Butyl-1-deoxymannojirimycin (19)
Methyl-6-deoxy-6-iodo-d-fructofuranoside (9, 0.312 g, 1.0 mmol) was treated to the general amination–cyclization cascade reaction conditions using n-butylamine (1.0 mL, 10 mmol) and purified using silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 20/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 69% yield (0.156 g, 0.70 mmol). [α]D20 -42.6o (H2O, c = 0.96); IR (film, MeOH): 3277, 3197, 2920, 1656,1103, cm–1; 1H-NMR (500 MHz, D2O): δ 4.26–4.24 (m, 1H, H-2), 4.09 (dd, J6a,6b = 13.4 Hz, J6a,5 = 1.80 Hz, 1H, H-6a), 3.99 (dd, J6a,6b = 13.1 Hz, J6b,5 = 2.61 Hz, 1H, H-6b), 3.98 (t, J4,3 = J4,5 = 10.0 Hz, 1H, H-4), 3.69 (dd, J3,4= 9.7 Hz, J3,2= 3.3 Hz, 1H, H-3), 3.50 (dd, J1a,1b = 13.2 Hz, J1a,1b = 3.1 Hz, 1H, H-1a), 3.43 (d, J1a,1b = 13.3 Hz, 1H, H-1b), 3.29 (t, J7,8 = 8.6 Hz, 2H, H-7), 3.13 (app d, J4,5 = 10.4 Hz, 1H, H-5), 1.73–1.64 (m, 2H, H-8), 1.38 (qd, J9,10 = 7.45 Hz, J8,9 = 1.55 Hz, 2H, H-9), 0.93 (t, J9,10 = 7.4 Hz, 3H, H-10); 13C-NMR (125 MHz, D2O): δ 72.0 (C-3), 65.5 (C-5), 65.4 (C-2), 65.0 (C-4), 54.7 (C-1), 54.1 (C-6), 52.8 (C-7), 23.7 (C-8), 19.2 (C-9), 12.7 (C-10); HRMS: m/z calcd. For [C10H21NO4 + H]+: 220.1543, obsd.: 220.1548. Spectral data matched those previously reported in ref (43).
N-Methyl-1-deoxymannojirimycin (20)
Methyl-6-deoxy-6-iodo-d-fructofuranoside (9, 0.125 g, 0.41 mmol) was treated to the general amination–cyclization cascade reaction conditions using aq. 40% methylamine (0.82 mL, 10.6 mmol) and purified using silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 20/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 87% yield (0.063 g, 0.35 mmol). [α]D20 -58.9 (H2O, c = 0.8); IR (film, MeOH): 3285, 3258, 2838, 2804, 1399 cm–1; 1H-NMR (500 MHz D2O): δ 4.18–4.16 (m, 1H, H-2), 4.04 (d, J6a,6b = 12.9 Hz, 1H, H-6a), 3.99 (dd, J6a,6b = 13.2 Hz, J6b,5 = 2.5 Hz, 1H, H-6b), 3.93 (t, J4,3 = J4,5 = 10.0 Hz, 1H, H-4), 3.79 (dd, J3,4= 9.74 Hz, J3,2= 3.3 Hz, 1H, H-3), 3.41 (d, J1a,1b = 12.6 Hz, 1H, H-1a), 3.24 (d, J1a,1b = 12.6 Hz, 1H, H-1b), 3.18–3.14 (m, 1H, H-5), 2.84 (s, 3H, NMe); 13C-NMR (125 MHz, D2O): δ 72.8 (C-3), 67.8 (C-5), 66.0 (C-2), 65.1 (C-4), 58.65 (C-1), 54.8 (C-6), 40.0 (NMe); HRMS: m/z calcd. For [C7H15NO4 + H]+: 178.1074, obsd.: 178.1077. Spectral data matched those previously reported in ref (72).
N-Benzyl-1-deoxymannojirimycin (21)
Methyl-6-deoxy-6-iodo-d-fructofuranoside (9, 0.124 g, 0.40 mmol) was treated to the general amination–cyclization cascade reaction conditions using benzylamine (0.44 mL, 4.0 mmol) and purified using silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 25/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 63% yield (0.065 g, 0.256 mmol). [α]D20 -49.3o (MeOH, c = 0.6); IR (film, MeOH): 3254, 3222, 2888, 2173, 1457, 1078 cm–1; 1H NMR (500 MHz D2O): δ 7.50 (br s, 5H, Harom), 4.70 (d, J7a,7b = 13.3 Hz, 1H, H-7a), 4.32 (dd, J7a,7b = 13.3 Hz, 1H, H-7b), 4.30 (d, J6a,6b = 13.5 Hz, 1H, H-6a) 4.23 (dd, J6a, 6b = 13.5, 1H, H-6b), 4.14–4.12 (m, 1H, H-2), 4.01 (t, J4,3 = J4,5 = 10.0 Hz, 1H, H-4), 3.61 (dd, J3,4= 9.7, J3,2= 2.7 Hz, 1H, H-3), 3.35 (d, J1a,1b,= 13.2 Hz, 1H, H-1a), 3.22 (d, J1a,1b = 12.57 Hz, 1H, H-1b) 3.14 (app. d, J4,5 = 10.1 Hz, 1H, H-5); 13C NMR (125 MHz, D2O): δ 131.7 (C-9), 130.3 (C-11), 129.3 (C-10), 127.7 (C-8), 71.9 (C-3), 66.3 (C-2), 65.1 (C-4 and 5), 56.9 (C-7), 54.5 (C-1), 54.3 (C-6); HRMS: m/z calcd. For [C13H19NO4 + H]+: 254.1387, obsd.: 254.1393. Spectral data matched those previously reported in ref (52).
N-(2-Phenylethyl)-1-deoxymannojirimycin (22)
Methyl-6-deoxy-6-iodo-d-fructofuranoside (9, 0.125 g, 0.41 mmol) was treated to the general amination–cyclization cascade reaction conditions using 2-phenylethan-1-amine (0.516 mL, 4.11 mmol) and purified using silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 25/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 64% yield (0.070 g, 0.26 mmol). [α]D20 -35.6 (MeOH, c = 0.9); IR (film, MeOH): 3347, 3153, 3048, 2969, 1737, 1407, 1080 cm–1; 1H NMR (500 MHz D2O): δ 7.42–7.35 (m, 5H, Harom), 4.27 (br s, 1H, H-2), 4.10 (d, J6a,6b = 13.6 Hz, 1H, H-6a) 4.04 (d, J6a, 6b = 13.6, 1H, H-6b), 3.98 (t, J4,3 = J4,5 = 9.9 Hz, 1H, H-4), 3.73 (d, J3,4= 9.5 Hz, 1H, H-3), 3.65 (d, J1a,1b = 13.1 Hz, 1H, H-1a), 3.55–3.53 (m, 2H, CH2–7), 3.52 (d, J1a,1b = 12.9 Hz, 1H, H-1b), 3.25 (d, J4,5 = 10.5 Hz, 1H, H-5), 3.17–3.07 (m, 2H, CH2–8); 13C NMR (125 MHz, D2O): δ 136.9 (C-10), 129.1 (C-12), 129.0 (C-11), 128.8 (C-9), 72.01 (C-3), 65.7 (C-5), 65.4 (C-2), 65.1 (C-4), 55.0 (C-1), 54.2 (C-6) 53.7 (C-7), 28.3 (C-8); HRMS: m/z calcd. For [C14H21NO4 + H]+: 268.1543, obsd.: 268.1559.
N-Hydroxyethyl-1-deoxymannojirimycin (23)
Methyl-6-deoxy-6-iodo-d-fructofuranoside (9, 0.324 g, 1.0 mmol) was treated to the general amination–cyclization cascade reaction conditions using ethanolamine (0.64 mL, 10 mmol) and purified using silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 7/2/2/1, v/v/v/v) to give the title compound as a white amorphous solid in 67% yield (0.147 g, 0.71 mmol), [α]D20 -82.1 (H2O, c = 0.55); IR (film, MeOH): 3314, 2942, 2831, 1448, 1020 cm–1; 1H NMR (500 MHz D2O): δ 4.26 (br s, 1H, H-2), 4.10 (d, J6a,6b = 13.5 Hz, 1H, H-6a), 4.06 (d, J6a,6b = 13.5, 1H, H-6b), 4.01–4.00 (m, 1H, H-4), 3.99 (t, J7,8 = 6.5 Hz, 2H, CH2–8), 3.74 (dd, J3,4= 9.5, J3,2= 2.5 Hz, 1H, H-3), 3.66 (d, J1a,1b = 13.5 Hz, 1H, H-1a), 3.49 (d, J1a,1b = 13.5 Hz, 1H, H-1b), 3.61–3.58 (m, 1H, H-7a), 3.42–3.38 (m, 1H, H-7b), 3.26–3.25 (m, 1H, H-5); 13C NMR (125 MHz, D2O): δ 71.9 (C-3), 66.5 (C-5), 65.4 (C-2), 65.2 (C-4), 55.3 (C-1), 54.9, 54.2 (C-6, C-7 and C-8); HRMS: m/z calcd. For [C8H17NO5 + H]+: 208.1179, obsd.: 208.1184. Spectral data matched those previously reported in ref (43).
1-Deoxynojirimycin (2)
Methyl-6-deoxy-6-iodo-l-sorbofuranoside (14, 0.42 g, 1.3 mmol) was treated to the general amination–cyclization cascade reaction conditions using aq. 35% NH3 (6.5 mL, 120 mmol) and purified using Dowex-H+ (5% NH3) and silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 20/2/2/1, v/v/v/v) to give 1-deoxynojirimycin (0.21 g, 95%) as a white amorphous solid. Rf = 0.10 (DCM/EtOH/MeOH/aq. NH3, 5/2/2/1, v/v/v/v). [α]D20 46 (c = 0.8, H2O), lit.20 [α]D 44 [c = 0.2, H2O]; IR (film) 3420, 3305 cm–1; 1H-NMR (500 MHz, D2O): δ 3.93 (dd, J6a,6b = 12.8 Hz, J6a,5 = 3.1 Hz, 1H, H-6a), 3.86 (dd, J6b,6a = 12.7 Hz, J6b,5 = 5.3 Hz, 1H, H-6b), 3.77 (ddd, J2,1b = 11.6 Hz, J2,3 = 9.2 Hz, J2,1a = 5.1 Hz, 1H, H-2), 3.58 (dd, J4,5 = 10.5 Hz, J4,3 = 9.3 Hz, 1H, H-4), 3.52–3.44 (m, 2H, H-1a, H-3), 3.18 (ddd, J5,4 = 10.6 Hz, J5,6b = 5.3 Hz, J5,6a = 3.1 Hz, 1H, H-5), 2.95 (dd, J1b,1a = 12.5 Hz, J1b,2 = 11.6 Hz, 1H, H-1b); 13C-NMR (125 MHz, D2O): δ 76.0 (C-3), 67.6 (C-4), 66.8 (C-2), 59.8 (C-5), 57.5 (C-6), 45.7 (C-1); HRMS: m/z calcd. For [C6H13NO4 + H]+: 164.0917, obsd.: 164.0918. Spectral data matched those previously reported in ref (73).
l-1-Deoxygalactonojirimycin (18)
To a solution of HCl in MeOH (0.15 M, 12 mL) was added 1,2:3,4-di-O-isopropylidene-6-deoxy-6-iodo-d-tagatose (17, 0.45 g, 1.21 mmol), and the solution was put under reduced pressure (0.3 atm) and heated in a 50 °C water bath until the starting material had completely reacted (observed via TLC analysis, 2 h), following which H2O (12 mL) was added, the MeOH was removed in vacuo, and the remaining solution was stirred at room temperature for 18 h to give complete conversion to 6-deoxy-6-iodo-d-tagatoside (observed via TLC analysis). Following this, 35% aq. NH3 (6.0 mL, 111 mmol) and NaCNBH3 (0.30 g, 4.84 mmol) were added sequentially to the reaction mixture, which was stirred at room temperature for a further 18 h. The resulting mixture was concentrated in vacuo and purified using Dowex-H+ (5% aq. NH3) and silica gel flash column chromatography (DCM/EtOH/MeOH/ aq. NH3, 20/2/2/1, v/v/v/v) to give 1-deoxygalactojirimycin (0.17 g, 86%). Rf = 0.10 (DCM/EtOH/MeOH/ aq. NH3, 5/2/2/1, v/v/v/v). [α]D20 -10.1 (c = 0.96, H2O), lit.42 [α]D -9.2 (c = 0.425, H2O); IR (film) 3380 cm–1; 1H-NMR (500 MHz, D2O): δ 4.19 (dd, J4,3 = 3.0 Hz, J4,5 = 1.4 Hz, 1H, H-4), 4.09 (ddd, J2,1b = 11.4 Hz, J2,3 = 9.6 Hz, J2,1a = 5.3 Hz, 1H, H-2), 3.90 (dd, J6a,6b = 12.3 Hz, J6a,5 = 4.9 Hz, 1H, H-6a), 3.82 (dd, J6b,6a = 12.2 Hz, J6b,5 = 8.8 Hz, 1H, H-6b), 3.66 (dd, J3,2 = 9.7 Hz, J3,4 = 3.0 Hz, 1H, H-3), 3.53 (dd, J1a,1b = 12.5 Hz, J1a,2 = 5.4 Hz, 1H, H-1a), 3.44 (ddd, J5,6b = 8.9 Hz, J5,6a = 4.8 Hz, J5,4 = 1.4 Hz, 1H, H-5), 2.90 (dd, J1b,1a = 12.5 Hz, J1b,2 = 11.5 Hz, 1H, H-1b); 13C-NMR (125 MHz, D2O): δ 72.8 (C-3), 66.8 (C-4), 64.6 (C-2), 60.0 (C-5), 59.0 (C-6), 46.0 (C-1). HRMS: m/z calcd. For [C6H13NO4 + H]+: 164.0917, obsd.: 164.0912. Spectral data matched those previously reported in ref (42).
Methyl 5-Deoxy-5-iodo-α/β-d-xylofuranoside (25)
To a solution of d-xylose (24, 4.16 g, 27.7 mmol) in MeOH (138 mL), AcCl (0.42 mL) was added and the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was neutralized by the addition of Dowex-OH–, filtered, and concentrated. The resulting oil was purified by flash chromatography (MeOH/EtOAc, 1/9, v/v) to give the pure methyl xylofuranosides. To a solution of the methyl xylofuranosides (27.7 mmol) in dry THF (152 mL) under an argon atmosphere, PPh3 (10.9 g, 41.5 mmol) and imidazole (3.71 g, 55.4 mmol) were added. I2 (10.4 g, 41.5 mmol) in dry THF (42 mL) was cannulated into the reaction vessel. The reaction mixture was refluxed for 2 h, then cooled, filtered, and concentrated. The product was dissolved in petroleum ether/EtOAc (3/1, v/v), filtered, and then purified by reverse-phase HP20 (MeOH/H2O, 5/1, v/v) to give methyl 5-deoxy-5-iodo-α/β-d-xyloside (25) as a colorless oil (4.63 g, 61%). Rf = 0.65 (EtOAc/MeOH, 9/1, v/v); [α]D20 = −19.7 (c = 1.5, CHCl3); IR (film) 3446, 1216, 770 cm–1; α-25:1H-NMR (500 MHz, CDCl3): δ 5.06 (d, J1,2 = 4.4, 1H, H-1), 4.40 (m, 1H, H-4), 4.29 (dd, J2,3 = 3.3 Hz, J3,4 = 4.6 Hz, 1H, H-3), 4.17 (dd, J1,2 = 4.4 Hz, J2,3 = 3.3 Hz, 1H, H-2), 3.51 (s, 3H, OMe), 3.31 (dd, J4,5a = 7.6 Hz, J5a,5b = 9.8 Hz, 1H, H-5a), 3.25 (dd, J4,5b = 6.1 Hz, J5a,5b = 9.8 Hz, 1H, H-5b); 13C-NMR (125 MHz, CDCl3): δ 102.2 (C-1), 79.1 (C-4), 78.4 (C-2), 76.9 (C-3), 56.2 (OMe), 1.6 (C-5); β-25:1H-NMR (500 MHz, CDCl3): δ 4.93 (s, 1H, H-1), 4.60 (dt, J3,4 = 3.9 Hz, J4,5a = J4,5b = 7.7 Hz, 1H, H-4), 4.28 (s, 1H, H-2), 4.14 (d, J3,4 = 3.9 Hz, 1H, H-3), 3.40 (s, 3H, OMe), 3.32 (d, J4,5 = 7.7 Hz, 2H, H-5a,b); 13C-NMR (125 MHz, CDCl3): δ 109.0 (C-1), 83.7 (C-4), 79.7 (C-2), 76.0 (C-3), 55.5 (OMe), 1.9 (C-5); HRMS: m/z calcd. For [C6H11O4I + Na]+: 296.9594, obsd.: 296.9601.
(3R,4r,5S)-Piperidine-3,4,5-triol (26)
A solution of methyl 5-deoxy-5-iodo-α/β-d-xyloside (25, 0.39 g, 1.42 mmol) in aqueous HCl (0.3 M, 14 mL) was refluxed until full conversion of the starting material was observed via TLC (ca. 1 h). Following this, 35% aq. NH3 (7 mL, 130 mmol) and NaCNBH3 (0.36 g, 5.68 mmol) were added sequentially to the reaction mixture, which was stirred at room temperature for a further 18 h. The resulting mixture was concentrated in vacuo and purified using Dowex-H+ (5% aq. NH3) and silica gel flash column chromatography (DCM/EtOH/MeOH/aq. NH3, 20/2/2/1, v/v/v/v) to give (3R,4r,5S)-piperidine-3,4,5-triol (0.16 g, 88%). Rf = 0.10 (DCM/EtOH/MeOH/aq. NH3 5/2/2/1 v/v/v/v); IR (film) 3420, 3350, 2902, 2887 cm–1; 1H-NMR (500 MHz, D2O): δ 3.64 (ddd, J2,1b = 10.4 Hz, J2,3 = 8.7 Hz, J2,1a = 4.8 Hz, 2H, H-2), 3.40 (t, J3,2 = 8.7 Hz, 1H, H-3), 3.30 (dd, J1a,1b = 12.7 Hz, J1a,2 = 4.8 Hz, 2H, H-1a), 2.71 (dd, J1b,1a = 12.7 Hz, J1b,2 = 10.4 Hz, 2H, H-1b); 13C-NMR (125 MHz, D2O): δ 76.1 (C-3), 68.5 (C-2), 47.4 (C-1). HRMS: m/z calcd. For [C6H12NO3 + H]+: 135.0812, obsd.: 135.0817. Spectral data matched those previously reported in ref (74).
6-Deoxy-6-iodo-1,2:3,4-di-O-isopropylidene-α-d-galactopyranose (28)
To a mixture of 1,2:3,4-di-O-isopropylidene-α-d-galactopyranose16 (2.6 g, 10 mmol), PPh3 (3.93 g, 15 mmol), and imidazole (1.36 g, 20 mmol) in dry THF (100 mL) was added I2 (3.81 g, 15 mmol) in small portions. After refluxing for 1 h, the reaction mixture was cooled to room temperature and quenched by the addition of 10% aq. Na2S2O4. The product was extracted with EtOAc, and the combined organic layers were washed with brine, dried (MgSO4), filtered, and concentrated. Distillation of the residue gave 6-deoxy-6-iodo-1,2:3,4-O-di-isopropyl-α-d-galactopyranose as a yellow oil (3.14 g, 85%). Rf = 0.55 (Petroleum ether/EtOAc, 4/1, v/v); [α]D20 -52.1 (c = 1, DCM), lit.75 [α]D -57.3 (c = 1, DCM); 1H-NMR (500 MHz, CDCl3): δ 5.52 (d, J1,2 = 5.0 Hz, 1H, H-1), 4.59 (dd, J3,4 = 7.9 Hz, J3,2 = 2.5 Hz, 1H, H-3), 4.38 (dd, J4,3 = 7.9 Hz, J4,5 = 1.9 Hz, 1H, H-4), 4.28 (dd, J2,1 = 5.0 Hz, J2,3 = 2.5 Hz, 1H, H-2), 3.92 (ddd, J5,6b = 7.2 Hz, J5,6a = 6.8 Hz, J5,4 = 1.9 Hz, 1H, H-5), 3.29 (dd, J6a,b = 10 Hz, J6a,5 = 6.8 Hz, 1H, H-6a), 3.19 (dd, J6b,a = 10 Hz, J6b,5 = 7.2 Hz, 1H, H-6b), 1.52 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.33 (s, 3H, CH3), 1.31 (s, 3H, CH3); 13C-NMR (125 MHz, CDCl3): δ 109.7 (C-7), 109.0 (C-10), 96.8 (C-1), 71.5 (C-4), 71.1 (C-3), 70.5 (C-2), 68.9 (C-5), 26.2 (C-8), 26.1 (C-11), 25.0 (C-9), 24.6 (C-12), 2.5 (C-6); HRMS: m/z calcd. For [C12H20IO5 + H]+: 371.0350, obsd.: 371.0355.
(3S,4R,5S,6R)-Azepane-3,4,5,6-tetraol (5)
A 9:1 mixture of TFA:H2O (4.3 mL) and 6-deoxy-6-iodo-1,2:3,4-di-O-isopropylidene-α-d-galactopyranose (28, 0.32 g, 0.86 mmol) was put under reduced pressure (0.3 atm) and heated in a 50 °C water bath for 30 min, when complete consumption of the starting material was observed by TLC. The resulting mixture was concentrated in vacuo and redissolved in a mixture of DCM and H2O, from which the product was extracted with H2O and concentrated to give 6-deoxy-6-iodo-d-galactose (29) as a brown oil that was used without further purification. [Rf = 0.35 (DCM/MeOH, 5/1, v/v); α-29: 1H-NMR (500 MHz, D2O): δ 5.19 (d, J1,2 = 3.8 Hz, 1H, H-1), 4.20–4.14 (m, 1H, H-5), 4.10 (dd, J4,3 = 3.3 Hz, J4,5 = 1.1 Hz, 1H, H-4), 3.81 (m, 1H, H-3), 3.79 (dd, J2,3 = 10.3 Hz, J2,1 = 3.9 Hz, 1H, H-2), 3.35–3.16 (m, 2H, CH2–6); 13C NMR (125 MHz, D2O) δ 92.2 (C-1), 70.7 (C-5), 69.9 (C-4), 69.0 (C-3), 67.8 (C-5), 2.3 (C-6); β-29: 1H NMR (500 MHz, D2O) δ 4.54 (d, J1,2 = 7.9 Hz, 1H, H-1), 4.06 (dd, J4,3 = 3.5 Hz, J4,5 = 1.0 Hz, 1H, H-4), 3.81 (m, 1H, H-5), 3.59 (dd, J3,2 = 10 Hz, J3,4 = 3.5 Hz, 1H, H-3), 3.43 (dd, J2,3 = 10 Hz, J2,1 = 7.9 Hz, 1H, H-2), 3.35–3.16 (m, 2H, CH2–6); 13C NMR (125 MHz, D2O) δ 92.2 (C-1), 70.7 (C-5), 69.9 (C-4), 69.0 (C-3), 67.8 (C-5), 2.3 (C-6); HRMS: m/z calcd. For [C6H11IO5 + H]+: 290.9724, obsd.: 290.9730]. Next, 6-deoxy-6-iodo-d-galactopyranose (29) was dissolved in H2O (8.6 mL), after which aq. NH3 (4.3 mL, 80 mmol) and NaCNBH3 (0.22 g, 3.45 mmol) were added, and the reaction mixture was stirred at room temperature for 2 days. The resulting mixture was concentrated in vacuo and purified using Dowex-H+ exchange resin (0.1% NH3) to give the title compound as a white solid (95.4 mg, 68% two steps). Rf = 0.10 (DCM/EtOH/MeOH/aq. NH3, 5/2/2/1, v/v/v/v); IR (film) 3401, 2988, 2896 cm–1; 1H-NMR (500 MHz, D2O): δ 4.00 (d, J3,2 = 6.5 Hz, 2H, H-3), 3.87 (ddd, J2,3 = 6.5 Hz, J2,1b = 4.8 Hz, J2,1a = 4.2 Hz, 2H, H-2), 3.08 (dd, J1a,1b = 14.7 Hz, J1a,2 = 4.2 Hz, 2H, H-1a), 2.90 (dd, J1b,1a = 14.7 Hz, J1b,2 = 4.8 Hz, 2H, H-1b). 13C-NMR (125 MHz, D2O): δ 73.9 (C-3), 70.0 (C-2), 51.0 (C-1). HRMS: m/z calcd. For [C6H13NO4 + H]+: 164.0917, obsd.: 164.0923. Spectral data matched those previously reported in ref (6).
Acknowledgments
The authors would like to thank the Royal Society of New Zealand, Marsden Fund, for financial support (11-VUW-057).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01646.
Copies of 1H- and 13C-NMR spectra of all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Borges de Melo E.; da Silveira Gomes A.; Carvalho I. α- and β-Glucosidase Inhibitors: Chemical Structure and Biological Activity. Tetrahedron 2006, 62, 10277–10302. 10.1016/j.tet.2006.08.055. [DOI] [Google Scholar]
- Kato A.; Kato N.; Kano E.; Adachi I.; Ikeda K.; Yu L.; Okamoto T.; Banba Y.; Ouchi H.; Takahata H.; Asano N. Biological Properties of d- and l-1-Deoxyazasugars. J. Med. Chem. 2005, 48, 2036–2044. 10.1021/jm0495881. [DOI] [PubMed] [Google Scholar]
- Nicholls K.; Germain D. P.; Feliciani C.; Shankar S.; Ezgu F.; Janmohamed S. G.; Laing S. M.; Schroyer R. O.; Bragat A. C.; Sitaraman S.; Boudes P. F. Phase 3 study of migalastat HCl for Fabry disease: Stage 1 results. Mol. Genet. Metab. 2013, 108, S70. 10.1016/j.ymgme.2012.11.182. [DOI] [Google Scholar]
- Schulze H.; Sandhoff K. Lysosomal Lipid Storage Diseases. Cold Spring Harb. Perspect. Biol. 2011, 3, a004804 10.1101/cshperspect.a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L. J.; Spencer C. M. Miglitol: A Review of its Therapeutic Potential in Type 2 Diabetes Mellitus. Drugs 2000, 59, 521–549. 10.2165/00003495-200059030-00012. [DOI] [PubMed] [Google Scholar]
- Morís-Varas F.; Qian X. H.; Wong C. H. Enzymatic/Chemical Synthesis and Biological Evaluation of Seven-Membered Iminocyclitols. J. Am. Chem. Soc. 1996, 118, 7647–7652. 10.1021/ja960975c. [DOI] [Google Scholar]
- Fischer P. B.; Karlsson G. B.; Butters T. D.; Dwek R. A.; Platt F. M. N-butyldeoxynojirimycin-Mediated Inhibition of Human Immunodeficiency Virus Entry Correlates With Changes in Antibody Recognition of the V1/V2 Region of gp120. J. Virol. 1996, 70, 7143–7152. 10.1128/jvi.70.10.7143-7152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P. B.; Karlsson G. B.; Dwek R. A.; Platt F. M. N-butyldeoxynojirimycin-Mediated Inhibition of Human Immunodeficiency Virus Entry Correlates with Impaired gp120 Shedding and gp41 Exposure. J. Virol. 1996, 70, 7153–7160. 10.1128/jvi.70.10.7153-7160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel C.; Garcia C.; Roingeard P.; Zitzmann N.; Dubuisson J.; Dwek R. A.; Trépo C.; Zoulim F.; Durantel D. Antiviral Effect of α-glucosidase Inhibitors on Viral Morphogenesis and Binding Properties of Hepatitis C Virus-Like Particles. J. Gen. Virol. 2006, 87, 861–871. 10.1099/vir.0.81503-0. [DOI] [PubMed] [Google Scholar]
- Tyrrell B. E.; Sayce A. C.; Warfield K. L.; Miller J. L.; Zitzmann N. Iminosugars: Promising Therapeutics for Influenza Infection. Crit. Rev. Microbiol. 2017, 43, 521–545. 10.1080/1040841X.2016.1242868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For some relevant reviews, see; a Compain P.; Chagnault V.; Martin O. R. Tactics and strategies for the synthesis of iminosugar C-glycosides: a review. Tetrahedron 2009, 20, 672–711. 10.1016/j.tetasy.2009.03.031. [DOI] [Google Scholar]; b Stocker B. L.; Dangerfield E. M.; Win-Mason A. L.; Haslett G. W.; Timmer M. S. M. Recent developments in the synthesis of pyrrolidine-containing iminosugars. Eur. J. Org. Chem. 2010, 2010, 1615. 10.1002/ejoc.200901320. [DOI] [Google Scholar]; c Natori Y.; Imahori T.; Yoshimura Y. Development of Stereoselective Synthesis of Biologically Active Nitrogen-heterocyclic Compounds: Applications for Syntheses of Natural Product and Organocatalyst. J. Syn. Org. Chem. Jpn. 2016, 74, 335–349. 10.5059/yukigoseikyokaishi.74.335. [DOI] [Google Scholar]; d Nicolas C.; Martin O. R. Glycoside Mimics from Glycosylamines: Recent Progress. Molecules 2018, 23, 1612. 10.3390/molecules23071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangerfield E. M.; Timmer M. S. M.; Stocker B. L. Total Synthesis Without Protecting Groups: Pyrrolidines and Cyclic Carbamates. Org. Lett. 2009, 11, 535–538. 10.1021/ol802484y. [DOI] [PubMed] [Google Scholar]
- Dangerfield E. M.; Gulab S. A.; Plunkett C. H.; Timmer M. S. M.; Stocker B. L. A Fast, Efficient and Stereoselective Synthesis of Hydroxy-Pyrrolidines. Carbohydr. Res. 2010, 345, 1360–1365. 10.1016/j.carres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Dangerfield E. M.; Plunkett C. H.; Stocker B. L.; Timmer M. S. M. Protecting-Group-Free Synthesis of 2-Deoxy-Aza-Sugars. Molecules 2009, 14, 5298–5307. 10.3390/molecules14125298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkran H. M.; Munneke S.; Dangerfield E. M.; Stocker B. L.; Timmer M. S. M. Applications and Limitations of the I2-Mediated Carbamate Annulation for the Synthesis of Piperidines: Five- versus Six-Membered Ring Formation. J. Org. Chem. 2013, 78, 9791–9802. 10.1021/jo401512h. [DOI] [PubMed] [Google Scholar]
- Timmer M. S. M.; Dangerfield E. M.; Cheng J. M. H.; Gulab S. A.; Stocker B. L. Rapid Synthesis of 1-Deoxygalactonojirimycin Using a Carbamate Annulation. Tetrahedron Lett. 2011, 52, 4803–4805. 10.1016/j.tetlet.2011.07.044. [DOI] [Google Scholar]
- Reitz A. B.; Baxter E. W. Pyrrolidine and piperidine aminosugars from dicarbonyl sugars in one step. Concise synthesis of 1-deoxynojirimycin. Tetrahedron Lett. 1990, 31, 6777–6780. 10.1016/S0040-4039(00)97169-7. [DOI] [Google Scholar]
- Baxter E. W.; Reitz A. B. Concise synthesis of 1-deoxymannojirimycin. Bioorg. Med. Chem. Lett. 1992, 2, 1419–1422. 10.1016/S0960-894X(00)80524-7. [DOI] [Google Scholar]
- Martin O. R.; Saavedra O. M. Concise chemical synthesis of β-homonojirimycin and related compounds. Tetrahedron Lett. 1995, 36, 799–802. 10.1016/0040-4039(94)02389-S. [DOI] [Google Scholar]
- Steiner A. J.; Stütz A. E.; Tarling C. A.; Withers S. G.; Wrodnigg T. M. Iminoalditol-amino acid hybrids: synthesis and evaluation as glycosidase inhibitors. Carbohydr. Res. 2007, 342, 1850–1858. 10.1016/j.carres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Matassini C.; Mirabella S.; Goti A.; Cardona F. Double Reductive Amination and Selective Strecker Reaction of a D-Lyxaric Aldehyde: Synthesis of Diversely Functionalized 3,4,5-Trihydroxypiperidines. Eur. J. Org. Chem. 2012, 2012, 3920–3924. 10.1002/ejoc.201200587. [DOI] [Google Scholar]
- Liu B.; Van Mechelen J.; Van den Berg R. J. B. H. N.; Van den Nieuwendijk A. M. C. H.; Aerts J. M. F. G.; Van der Marel G. A.; Codée J. D. C.; Overkleeft H. S. Synthesis of Glycosylated 1-Deoxynojirimycins Starting from Natural and Synthetic Disaccharides. Eur. J. Org. Chem. 2019, 2019, 118–129. 10.1002/ejoc.201801461. [DOI] [Google Scholar]
- Clemente F.; Matassini C.; Cardona F. Reductive Amination Routes in the Synthesis of Piperidine IminoSugars. Eur. J. Org. Chem. 2020, 2020, 4447–4462. 10.1002/ejoc.201901840. [DOI] [Google Scholar]
- Iminosugars: From Synthesis to Therapeutic Applications; Compain P.; Martin O. R., (Eds.); Wiley: Berlin, 2007. [Google Scholar]
- Paulsen H.; Sangster I.; Heyns K. Monosaccharide mit Stickstoffhaltigem Ring, XIII. Synthese und Reaktionen von Keto-piperidinosen. Chem. Ber. 1967, 100, 802–815. 10.1002/cber.19671000314. [DOI] [Google Scholar]
- Kinast G.; Schedel M. A Four-Step Synthesis of 1-Deoxynojirimycin with a Biotransformation as Cardinal Reaction Step. Angew. Chem., Int. Ed. Engl. 1981, 20, 805–806. 10.1002/anie.198108051. [DOI] [Google Scholar]
- a Ziegler T.; Straub A.; Effenberger F. Enzyme-Catalyzed Synthesis of 1-Deoxymannojirimycin, 1-Deoxynojirimycin, and 1,4-Dideoxy-1,4-imino-d-arabinitol. Angew. Chem., Int. Ed. 1988, 27, 716–717. 10.1002/anie.198807161. [DOI] [Google Scholar]; b Durrwachter J. R.; Wong C. H. Fructose 1,6-Diphosphate Aldolase-Catalyzed Stereoselective Synthesis of C-Alkyl and N-Containing Sugars: Thermodynamically Controlled C-C Bond Formations. J. Org. Chem. 1988, 53, 4175–4181. 10.1021/jo00253a004. [DOI] [Google Scholar]
- Beaupere D.; Stasik B.; Uzan R.; Demailly G. Azidation Sélective du l-Sorbose. Application à la Synthése Rapide de la 1-Désoxynojirimycine. Carbohydr. Res. 1989, 191, 163–166. 10.1016/0008-6215(89)85058-X. [DOI] [Google Scholar]
- a Verhelst S. H. L.; Paez Martinez B.; Timmer M. S. M.; Lodder G.; van der Marel G. A.; Overkleeft H. S.; van Boom J. H. A Short Route toward Chiral, Polyhydroxylated Indolizidines and Quinolizidines. J. Org. Chem. 2003, 68, 9598–9603. 10.1021/jo0350662. [DOI] [PubMed] [Google Scholar]; b Senthilkumar S.; Prasad S. S.; Kumar P. S.; Baskaran S. A diversity oriented one-pot synthesis of novel iminosugar C-glycosides. Chem. Commun. 2014, 50, 1549–1551. 10.1039/c3cc48370c. [DOI] [PubMed] [Google Scholar]
- Hunt-Painter A. A.; Stocker B. L.; Timmer M. S. M. The Synthesis of the Molecular Chaperone 2,5-dideoxy-2,5-imino-d-altritol via Diastereoselective Reductive Amination and Carbamate Annulation. Tetrahedron 2018, 74, 1307–1312. 10.1016/j.tet.2018.01.011. [DOI] [Google Scholar]
- Hunt-Painter A. A.; Moggré G.-J.; Tyler P. C.; Stocker B. L.; Timmer M. S. M. Diastereoselective Carbamate Annulation for the Synthesis of 2,5-Dideoxy-2,5-iminoglycitols. ChemistrySelect 2017, 2, 8028–8032. 10.1002/slct.201701818. [DOI] [Google Scholar]
- Dangerfield E. M.; Plunkett C. H.; Win-Mason A. L.; Stocker B. L.; Timmer M. S. M. Protecting-Group-Free Synthesis of Amines: Synthesis of Primary Amines from Aldehydes via Reductive Amination. J. Org. Chem. 2010, 75, 5470–5477. 10.1021/jo100004c. [DOI] [PubMed] [Google Scholar]
- Furneaux R. H.; Tyler P. C.; Whitehouse L. A. A Short Practical Synthesis of Deoxymannojirimycin from d-Fructose. Tetrahedron Lett. 1993, 34, 3613–3616. 10.1016/S0040-4039(00)73650-1. [DOI] [Google Scholar]
- Maier P.; Andersen S. M.; Lundt I. 1,5-Anhydro-d-fructose as Chiral Building Block: A Novel Approach to 1-Deoxymannojirimycin. Synthesis 2006, 2006, 827–830. 10.1055/s-2006-926343. [DOI] [Google Scholar]
- Clapes Saborit P.; Joglar Tamargo J.; Castillo Exposito J. A.; Lozano Perez C.. Chemoenzymatic Process for the Preparation of Iminocyclitols. WO2008025826A1, March 6, 2008.
- Wei M.; Li Z.; Li T.; Wu B.; Liu Y.; Qu J.; Li X.; Li L.; Cai L.; Wang P. G. Transforming Flask Reaction into Cell-Based Synthesis: Production of Polyhydroxylated Molecules via Engineered Escherichia coli. ACS Catal. 2015, 5, 4060–4065. 10.1021/acscatal.5b00953. [DOI] [Google Scholar]
- de Raadt A.; Stütz A. E. A Simple Convergent Synthesis of the Mannosidase Inhibitor 1-Deoxymannonojirimycin from Sucrose. Tetrahedron Lett. 1992, 33, 189–192. 10.1016/0040-4039(92)88046-8. [DOI] [Google Scholar]
- Baxter E. W.; Reitz A. B. Expeditious Synthesis of Aza sugars by the Double Reductive Amination of Dicarbonyl Sugars. J. Org. Chem. 1994, 59, 3175–3185. 10.1021/jo00090a040. [DOI] [Google Scholar]
- Dhavale D. D.; Saha N. N.; Desai V. N. A Stereoselective Synthesis of 1,6-Dideoxynojirimycin by Double-Reductive Amination of Dicarbonyl Sugar. J. Org. Chem. 1997, 62, 7482–7484. 10.1021/jo970826s. [DOI] [PubMed] [Google Scholar]
- Look G. C.; Fotsch C. H.; Wong C. H. Enzyme-Catalyzed Organic Synthesis: Practical Routes to Aza Sugars and their Analogs for use as Glycoprocessing Inhibitors. Acc. Chem. Res. 1993, 26, 182–190. 10.1021/ar00028a008. [DOI] [Google Scholar]
- Bethell G. S.; Ferrier R. J. Studies with radioactive sugars: Part IV. The Methanolysis of d-Fructose and l-Sorbose. Carbohydr. Res. 1973, 31, 69–80. 10.1016/S0008-6215(00)82318-6. [DOI] [Google Scholar]
- Sethi M. K.; Mahajan S.; Bhandya S. R.; Anish K.. An Improved Process for the Preparation of 1-Deoxynojirimycin, an Intermediate of Miglitol. Indian Patent IN 2013CH03275, August 31, 2016.
- Xu Z.; Chen K.; Li G.; Yang J.. Novel Methods for Preparing 1-DNJ (1-Deoxynojirinmycin) and Precursor of 1-Deoxynojirinmycin. Chinese Patent CN 102702079 A, 3 September, 2012.
- Wang Y.; Tao Z.. Method for Preparing Miglitol Midbody N-Substituted-1-Deoxidization Nojirimycin Derivative. Chinese Patent CN101270378A, 24 September, 2008.
- Paulsen H.; Matzke M.; Orthen B.; Nuck R.; Reutter W. Monosaccharide mit Stickstoff im Ring, XXXIX. Synthese von modifizierten α-L-Fucosidase-Inhibitoren, die 1,5-Didesoxy-1,5-imino-L-fucit als Basisstruktur enthalten. Liebigs Ann. Chem. 1990, 1990, 953–963. 10.1002/jlac.1990199001176. [DOI] [Google Scholar]
- Wilson F. X.; Nash R. J.; Horne G.; Storer R.. Antiinfective Compounds. WO 2010029313 A1, 18 March, 2010.
- Wilson F. X.; Nash R. J.; Horne G.; Storer R.; Tinsley M.; Roach A. G.. Treatment of Energy Utilization Diseases. WO 2010049678 A2, 26 August, 2010.
- Wilson F. X.; Nash R. J.; Horne G.; Storer R.; Tinsley M.; Roach A. G.. Compounds for the Treatment of Flaviviral Infections. WO 2010015815 A2, 26 August, 2010.
- Wilson F. X.; Nash R. J.; Horne G.; Storer R.; Tinsley M.; Roach A. G.. Treatment of Lysosomal Storage Disorders and Other Proteostatic Diseases. WO 2010015816 A2, 26 August, 2010.
- Jenkinson S. F.; Fleet G. W. J.; Nash R. J.; Koike Y.; Adachi I.; Yoshihara A.; Morimoto K.; Izumori K.; Kato A. Looking-Glass Synergistic Pharmacological Chaperones: DGJ and l-DGJ from the Enantiomers of Tagatose. Org. Lett. 2011, 13, 4064–4067. 10.1021/ol201552q. [DOI] [PubMed] [Google Scholar]
- Concia A. L.; Lozano C.; Castillo J. A.; Parella T.; Joglar J.; Clapés P. d-Fructose-6-Phosphate Aldolase in Organic Synthesis: Cascade Chemical-Enzymatic Preparation of Sugar-Related Polyhydroxylated Compounds. Chem. – Eur. J. 2009, 15, 3808–3816. 10.1002/chem.200802532. [DOI] [PubMed] [Google Scholar]
- Lahav D.; Liu B.; van den Berg R. J. B. H. N.; van den Nieuwendijk A. M. C. H.; Wennekes T.; Ghisaidoobe A. T.; Breen I.; Ferraz M. J.; Kuo C.-L.; Wu L.; Geurink P. P.; Ovaa H.; van der Marel G. A.; van der Stelt M.; Boot R. G.; Davies G. J.; Aerts J. M. F. G.; Overkleeft H. S. A Fluorescence Polarization Activity-Based Protein Profiling Assay in the Discovery of Potent, Selective Inhibitors for Human Nonlysosomal Glucosylceramidase. J. Am. Chem. Soc. 2017, 139, 14192–14197. 10.1021/jacs.7b07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheron C.; Compain P.; Martin O. R. A stereodivergent approach to 1-Deoxynojirimycin, 1-Deoxygalactonojirimycin and 1-Deoxymannojirimycin Derivatives. Tetrahedron Lett. 2006, 47, 3081–3084. 10.1016/j.tetlet.2006.02.157. [DOI] [Google Scholar]
- Bhattacharya A. K.; Chand H. R.. Preparation of Glycolactam Compounds, Process for Preparing Them and Uses Thereof as Intermediates for Piperidine Alkaloids. Indian Patent IN 201611015813 A, 9 February, 2018.
- Kinast G.3,4,5-Trihydroxypiperidine, Process for their Preparation and their Use. German Patent DE 3507019 A1, 28 August, 1986.
- Ishiwata K.; Seki H.; Sasaki T.; Ishii S. I.; Nozaki T.; Senda M. Synthesis and Characteristics in Tumor-Bearing Mice of N-[11C]Methyl-1-Deoxynojirimycin and N-[11C]Methyl-1-Deoxymannojirimycin. Nucl. Med. Biol. 1993, 20, 843–847. 10.1016/0969-8051(93)90150-S. [DOI] [PubMed] [Google Scholar]
- Lopes C. C.; Lopes R. S. C.; Matos C. R. R.. Process for the Synthesis of Aza Sugars Having Biological Activity. Brazilian Patent BR 9902585 A, 26 September, 2000.
- Grabner R. W.; Landis B. H.; Rutter R. J.; Scaros M. G.. Production of N- substituted Polyhydroxy Nitrogen-Containing Heterocycles by Bioconversion. Canadian Patent CA 2091668 A1, 17 September, 1993.
- van den Broek L. A. G. M.; Vermaas D. J.; Heskamp B. M.; van Boeckel C. A. A.; Tan M. C. A. A.; Bolscher J. G. M.; Ploegh H. L.; van Kemenade F. J.; de Goede R. E. Y.; Miedema F. Chemical Modification of Azasugars, Inhibitors of N-Glycoprotein-Processing Glycosidases and of HIV-I infection: Review and Structure-Activity Relationships. Recl. Trav. Chim. Pays-Bas 1993, 112, 82–94. 10.1002/recl.19931120204. [DOI] [Google Scholar]
- Johnson C. R.; Golebiowski A.; Schoffers E.; Sundram H.; Braun M. P. Chemoenzymatic Synthesis of Azasugars: d-Talo- and d-Manno-1-Deoxynojirimycin. Synlett 1995, 1995, 313–314. 10.1055/s-1995-4967. [DOI] [Google Scholar]
- Jones J. K. N.; Turner J. C. 5-Acetamido-5-Deoxy-l-Arabinose: A Sugar Derivative Containing Nitrogen as the Hetero-Atom in the Ring. J. Chem. Soc. 1962, 4699–4703. 10.1039/jr9620004699. [DOI] [Google Scholar]
- Paulsen H. The Preparation of 5-Amino Sugars and their Transformation into Pyridine Derivatives. Angew. Chem. Int. Ed. 1962, 1, 454–454. 10.1002/anie.196204542. [DOI] [Google Scholar]
- Hanessian S.; Haskell T. H. Synthesis of 5-Acetamido-5-Deoxypentoses. Sugar Derivatives Containing Nitrogen in the Ring. J. Org. Chem. 1963, 28, 2604–2610. 10.1021/jo01045a029. [DOI] [Google Scholar]
- Jones J. K. N.; Szarek W. A. Synthesis of a Sugar Derivative with Nitrogen in the Ring. Can. J. Chem. 1963, 41, 636–640. 10.1139/v63-090. [DOI] [Google Scholar]
- Bernotas R. C.; Papandreou G.; Urbach J.; Ganem B. A New Family of Five-Carbon Iminoalditols which are Potent Glycosidase Inhibitors. Tetrahedron Lett. 1990, 31, 3393–3396. 10.1016/S0040-4039(00)97405-7. [DOI] [Google Scholar]
- Lindhorst T. K.Essentials of carbohydrate chemistry and biochemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Mravljak J.; Obreza A. The Efficient Synthesis of Amphiphilic Oximes of Galactose and Glucosamine. Tetrahedron Lett. 2012, 53, 2234–2235. 10.1016/j.tetlet.2012.02.086. [DOI] [Google Scholar]
- Angyal S. J.; Bethell G. S. Conformational Analysis in Carbohydrate Chemistry. III. The 13C N.M.R. Spectra of the Hexuloses. Aus. J. Chem. 1976, 29, 1249–1265. 10.1071/CH9761249. [DOI] [Google Scholar]
- Lauritsen A.; Madsen R. Synthesis of Naturally Occurring Iminosugars from d-Fructose by the Use of a Zinc-Mediated Fragmentation Reaction. Org. Biomol. Chem. 2006, 4, 2898–2905. 10.1039/b605818c. [DOI] [PubMed] [Google Scholar]
- Bechor Y.; Albeck A. Exocyclic Vinyl Ethers of Ketofuranosides. Tetrahedron 2008, 64, 2080–2089. 10.1016/j.tet.2007.12.049. [DOI] [Google Scholar]
- Singh A.; Kim B.; Lee W. K.; Ha H.-J. Asymmetric Synthesis of 1-Deoxyazasugars from Chiral Aziridines. Org. Biomol. Chem. 2011, 9, 1372–1380. 10.1039/c0ob00730g. [DOI] [PubMed] [Google Scholar]
- Asano N.; Kizu H.; Oseki K.; Tomioka E.; Matsui K.; Okamoto M.; Baba M. N-Alkylated Nitrogen-in-the-Ring Sugars: Conformational Basis of Inhibition of Glycosidases and HIV-1 Replication. J. Med. Chem. 1995, 38, 2349–2356. 10.1021/jm00013a012. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-X.; Wu B.; Wang B.; Li T.-H.; Zhang P.-F.; Guo L.-N.; Wang W.-J.; Zhao W.; Wang P. G. Facile and stereo-controlled synthesis of 2-deoxynojirimycin, Miglustat and Miglitol. Tetrahedron. Lett. 2011, 52, 3802–3804. 10.1016/j.tetlet.2011.05.063. [DOI] [Google Scholar]
- Ouchi H.; Mihara Y.; Takahata H. A New Route to Diverse 1-Azasugars from N-Boc-5-hydroxy-3-piperidene as a Common Building Block. J. Org. Chem. 2005, 70, 5207–5214. 10.1021/jo050519j. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Lu Y.-P.; Zhu L. Synthesis of Per-acetyl d-fucopyranosyl Bromide and Its Use in Preparation of Diphyllin d-fucopyranosyl Glycoside. J. Carbohydr. Chem. 2008, 27, 113–119. 10.1080/07328300802030860. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.