Abstract

Polyphosphazenes are an inorganic molecular hybrid family with multifunctional properties due to their wide range of organic substitutes. This review intends to propose the basics of the synthetic chemistry of polyphosphazene, describing for researchers outside the field the basic knowledge required to design and prepare polyphosphazenes with desired properties. A special emphasis is placed on recent advances in chemical synthesis, which allow not only the synthesis of polyphosphazenes with controlled molecular weights and polydispersities but also the synthesis of novel branched designs and block copolymers. We also investigated the synthesis of polyphosphazenes using various functional materials. This review aims to assist researchers in synthesizing their specific polyphosphazene material with unique property combinations, with the hope of stimulating further research and even more innovative applications for these highly interesting multifaceted materials.
1. Introduction
The word phospohazene refers to the vast variety of molecules containing nitrogen and phosphorus atoms attached via alternatingly arranged saturated and unsaturated bonds in their backbones, providing either cyclic rings or linear chains as shown in Figure 1 (structures 1.1–1.2). Here, R is an organic group, representing the wide variety of functional groups.1 Their unique backbone distinguishes them from the other wide varieties of polymers, which is due to the inorganic nature of the polymer while most other polymers have an organic nature. However, the attached side chains could be organic or inorganic in nature.2 To date, a lot of classes of organic–inorganic polymers have been known as polyphosphazenes.2 Although chemical engineers and synthetic chemists have very few options to bank on as far as the choice of backbone in a specific polymer of polyphosphazenes is related, extraordinary multiplicity in properties of these polyphosphazene materials has been attained due to the vast substitutional potential of chlorine atoms involved in their intrinsic structure by R side groups.3 A few examples out of a vast range of structural diversity in polyphophsazene material are shown in Figure 1 (structures 1.3–1.7).
Figure 1.
Structural representation of linear polyphosphazenes (1.1), cyclic polyphosphazenes (1.2), film and fiber precursors (1.3), elastors (1.4), bioerodible polymers (1.5), microencapsulationg polymers (1.6), and solid polymer electrolytes (1.7).
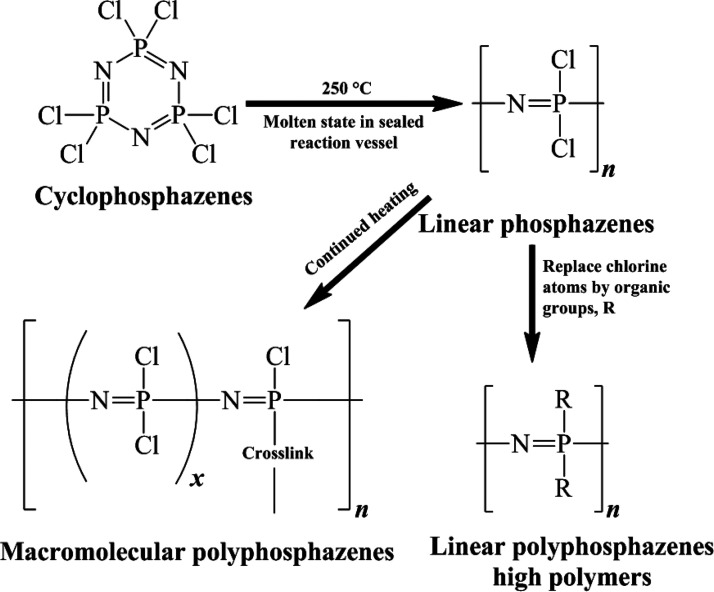
Polyphosphazenes contain a wide range of potential applications from fire retardants to fibers of optical materials,4,5 fuel cells,6 elastomers via films and coatings,7 composites and carbon materials,8 a variety of different membranes,9 electro-optical to biomedical materials,10 as well as solid battery electrolytes.11 In the context of this review, this is necessary to study the history of polyphosphazene development over the years before going on to the actual study of polyphosphazene related to our research interest. Back in the days of 1834, Wohler and Rose worked on the reaction of ammonia (NH3) and phosphorus pentachloride (PCl5), which produced the white crystalline product that could be collected without any decomposition as a pure compound.12 However, the empirical formula of this compound was proposed as NPCl2 in 1844. Further, Gladstone and Holmes verified this formula by using the vapor density method and suggested the formula (NPCl2)3.13
However, in the context of polyphosphazene chemistry, it was Stokes who first proposed the cyclic structure of polyphosphazene and reported “inorganic rubber” by heating the cholorophosphazenes.14 After the huge contribution of Stokes, there was less progress made in phosphazene polymer chemistry and material chemistry until the mid-1960s. Allcock, Valan, and Kugel reported a series of experiments over polyphosphazene and revealed the basic features of phosphazenes. Those findings paved the path of polyphosphazene chemistry to where it stands now.15 Their work can be summarized by the following points: (i) The excellent reactivity of a special P–-Cl bond results in the formation of a lot of hybrid polymers, both inorganic and organic in nature. (ii) Poly(dichlorophosphazene) (PDCP) can produce hexachlorocyclophosphazene (HCCP) by controlling the temperature, time, termination of the reaction, and purity and converting a cyclic trimer into a linear polymer. (iii) Hydrolytically stable compounds can be produced by the nucleophilic substitution of chlorine via PDCP treatment with alkali metal salts of alcohols and primary or secondary amines.
Further, Allcock also proposed the IUPAC name of hexachlorocyclotriphosphazene [NPCl2]3 as 2,2,4,4,6,6-hexachloro-1,3,5,2,4,6-triazotriphosphorine, which is commonly known as HCCP.16,17 This work provides the foundation of synthesis processes related to polyphosphazenes with unique, interesting, and innovative properties.
2. Types of Polyphosphazenes
The classification of polyphosphazene is mainly based on its structural diversity and the hybrid nature of the backbone comprised of alternatingly arranged phosphorus and nitrogen atoms in inherent structure along with two chlorine atoms attached to each phosphorus atom.18 Polyphosphazene chemistry can be modified and improved by chemical substitution of phosphorus attached to two chlorine atoms and various mono-, bi-, tri-, or multiple functional groups consisting of nucleophiles.19−21 Those nucleophiles could be organometallic, inorganic, or organic in nature due to the extensive variety of polyphosphazene polymers used in multiple applications.22
Hence, the main chemical shift in features of polyphosphazenes is brought via a nucleophilic substitution reaction between attacking nucleophiles on the chlorine atom attached to the phosphorus atom.23 Putting all the above facts into a list, polyphosphazene can be classified into four categories. The major highlights of these four types are shown in Figure 2.
Figure 2.
Types of polyphosphazenes based on their structural and functional group diversity.
2.1. Small-Molecule Cyclic Polyphosphazenes
The significant and characteristic properties of cyclic phosphazene chemistry depend on the existence of a succession of rings of large sizes with general structures exhibited in structures 1.8 and 1.9 in Figure 3, which can easily extend from tens to hundreds.24 The cyclophosphazene term is used for ring species. The middle word of cyclophosphazene denotes the actual number of repeating units in the cyclic structure. Structures 1.8 and 1.9 are named as cyclotriphosphazene and cyclotetraphosphazene, respectively. The vast variety of polymers can be produced by varying the stereochemical and structural positions in mixed substituent cyclophosphazenes.25 The position of various types and numbers of side groups on phosphorus atoms inside the cyclic structure is convenient in refereeing the skeletal name of a molecule by giving changed atoms a number or trans/cis arrangement or geminal and nongeminal stereoisomeric terms.26
Figure 3.

Structural representation of cyclic trimer (1.8), cyclic tetramer (1.9), and N-trimethylsilyl-P-trichlorophosphazane (1.10).
2.2. Small-Molecule Linear Polyphosphazenes
The organic reagents and linear polyphosphazene PDCP combined to produce the new class of “small molecule linear polyphosphazenes”. These small-molecule linear polyphosphazene polymers are vital for the synthesis of new polymers, and they are utilized as a structural and reaction model for the excellent performance of polymers.27 One of the typical examples of these polymers is shown in Figure 3 (structure 1.10). The monophosphazene or phosphirimine terminology is used for the monomeric phosphazene. However, recently these monomeric phosphazenes have been named phosphoronimines due to suggestions of modern nomenclature. As this class of phosphazene can be utilized as a pioneer material to synthesize polyphosphazene polymers, it is proposed as a monomer or cross-linker inside a large chain of polyphosphazene polymers.
2.3. Linear Polyphosphazene High Polymers
It is important to distinguish between various levels of polyphosphazenes. They are differentiated by the degree of polymerization, denoted as n, inside dissimilar polymeric classes based on the number of monomers tangled to form a specific one.28 The term oligomers is used for polymers consisting of 2–100 repeating units; however, low weight polymers contain 1000–15000 monomers, and polyphosphazene polymers are often comprised of more than 1000–15 000 or an even higher number of monomers in their structure. This differentiation is very significant to make depending on the monomer’s existence in chains in order to reveal the benefits of polymers containing stable chemical and physical properties, length of chains, solubility patterns, and arrangement of the side groups on small oligomers.29 In the context of high molecular weight polyphosphazenes, the most crucial factors involved to develop the features and application of polyphosphazenes are (1) the type of attached side groups and (2) the way monomers are organized inside the chain.29
The existence of two or more different classes of functional side groups on similar polymer chains may produce numerous classes of polyphosphazene designs.17 Two identical kinds of side groups existing on a phosphorus atom are known as geminal, but those with one of each dissimilar side group type on the phosphorus atom are called nongeminal, with the addition of cis and trans arrangements (Figure 4; structures 1.11–1.13). When a block of one type of functional side group coexists with the other type of block already present inside the same polyphosphazene polymer chain, they are termed as block copolymers, as shown in Figure 4 (structure 1.14). The term block polymer is used when a block of one class of functional side group coexists with another class of polymer in the same polyphosphazene chain (Figure 4; structure 1.14). The block copolymer of phosphazenes is linked with other organic polymers.14
Figure 4.

Structural representation of geminal side group arrangement (1.11), cis-nongeminal (1.12), trans-nongeminal (1.13), and block copolymers (1.14).
2.4. Macromolecular and Composite Polyphosphazene Materials
Nowadays, the most common species used is known as a composite, a mixture of polymer and nonpolymeric material.30 These composites are classified as interpenetrating polymers and polymer blends. Polymer blends are featured as physical mixtures of two other polymers, subdivided into distinct and homogeneous domains. An interpenetrating polymer is produced when both or only one of the polymer constituents are cross-linked. However, both of these classes can be found in polyphosphazene synthesis procedures and can be formed by two polyphosphazene precursors, or one of them could be an organic polymer. Some of the structural examples of IPN polymers are given below in Figure 5.
Figure 5.

Structural representation of organic polymer blocks linked to polyphosphazene blocks (1.15–1.18).
3. Geometric and Electronic Properties of Polyphosphazenes
It is important to better understand the intrinsic electronic and geometric study featured in the structural arrangement and morphological properties of polyphosphazene.22 To date, the researchers working on polyphosphazenes have faced difficulties in determining the unique arrangement of electrons in small molecules of polyphosphazene. The huge problem is the accountability of the four extra electrons per repeating unit when the outer shell electrons of phosphorus, nitrogen, and their side unit groups are coupled into two-electron system (Figure 5; structures 1.15–1.18).31 The outlook and reactivity trend of the four electrons per repeating unit has puzzled scientific researchers investigating polyphosphazenes in the last 50 years.
In this context, the following evidence needs to be taken into account while perusing experiments around polyphosphazenes: (1) Bond length of nitrogen–phosphorus atoms inside linear or cyclic polyphosphazenes is smaller when it is compared to normal covalent σ bond lengths in −N–P– due to electronegativity occurrence.32 (2) These bond lengths toward a short chain or inside a ring are mostly constant unless the impact of end groups or side groups influences a change.33 (3) However, some rings are planar, and others are nonplanar; however, the structural stability of six-membered, eight-membered, or even higher membered rings remains the same. This result concludes with the fact that there is no obvious spectral or electronic absorption from the skeletal appearance of polyphosphazenes throughout the visible region or at the ultraviolet region with similar spectra irrespective of the chain length and ring size.34 (4) The strongly basic skeletal nitrogen atoms have the capacity to create the coordination covalent bond with transition metals and protons if high electron-donating side groups exist in the backbone of polyphosphazenes.35 (5) The side groups do not contribute to binding energies of polyphosphazenes, and the ring size is shown via X-ray photoelectron spectroscopy (XPS). (6) According to the reported literature, phosphazene rings cannot be reduced by polarographic reduction into radical anions and electron spin resonance.36 (7) Restriction of rotation in the phosphorus–nitrogen bond in polyphosphazenes is less, which creates a phosphazene ring and makes the chain structure quite stable.37
The above-discussed facts all logically proved the uniqueness of the intrinsic structural and geometric properties of polyphosphazenes. This evidence obviously suggests the diversity in skeletal bonding structures of polyphosphazenes when compared to any other electron-rich organic species. These compounds exhibit a unique class of polymers with a very rare form of bonding that is neither completely unsaturated nor aromatic like benzene in nature.38 Further, it can be simply concluded that the contribution of two electrons to occupy a lone-pair orbital radially engaged outside the ring is due to the basic nature of nitrogen in the polyphosphazene skeleton. In fact, it is the intrinsic nature of two electrons per repeating unit, on each phosphorus and nitrogen atom, that can build ambiguity for arrangements of electrons.39
To overcome this difficulty, many researchers worked to predict the actual arrangement of polyphosphazenes bonds; e.g., Paddock, Craig, Mitchel, and Dewar put forward their theories.12 Their arguments mainly focused on the fact that the phosphorus atom belongs to the third row of the periodic table, so it holds 3d orbitals as well as 2p, 2s, and s–p hybrid orbitals.40 In a simple way, it can be stated that electrons inside polyphosphazenes do not take part in the group or skeletal lone pair basicity or σ bonds, but they are involved in stabilizing the excited state chemistry, which is different from the π-orbital bond symmetry of inorganic molecules. In the context of polyphosphazene bonding conception, Dewar contradicted the Craig and Paddock “dπ–pπ bonding model” which revealed that the wide electron delocalization in the π-system could be possible by exhibiting the island-bonding model, which can be explained by orbital symmetry considerations by indicating the possibility of delocalization in restricted islands from one phosphorus atom to another.41
There could be two possibilities for π-bonding arrangements in polyphosphazene ring chemistry.42 The first postulate is that the lone pair of nitrogen is radially oriented toward the phosphazene ring, so it can donate electrons to the neighboring 3dxy orbital of phosphorus that is oriented planar to the ring (Figure 6). At the end, it can be suggested that the nonplanar dxz orbitals of phosphorus can overlap to the adjacent 2pz orbitals of nitrogen to create a conjugation above and below the plane of the ring (Figure 6).26 However, the diffused symmetry of the d orbital will create a misalliance over each phosphorus atom, which further generates the nodes at these sites. This is the reason that stabilization of polyphosphazenes is independent of the size of their rings.43
Figure 6.

Graphical representation of the π-bonding pattern of the d-orbital and the lone pair of phosphorus and nitrogen, respectively.42
As further evidence, the complete planarity of the ring stabilization is not needed because the skeletal bond formation is twisted in various d-orbital swappings. This is the main difference when it is compared to other aromatic ring systems. In short-chain polyphosphazenes, this logic is valid to explain the much higher bond torsion. Therefore, it can be concluded that it is still very difficult to explain the bond arrangements through only the method, and it can be explained by applying the collective methods of all related spectroscopic techniques to reveal the structural mysteries, hidden in those compounds. To this end, computational chemistry studies and simulation would play a role in the future with much higher output.43
4. Syntheses Methods of Polyphosphazenes
4.1. Syntheses Methods of Linear Polyphosphazenes
A brief comprehensive effort has been shown in Figure 7 where we can locate the possible substituents for the reaction of polyphosphazenes. This procedure is derived from the HCCP ring-opening polymerization to synthesize linear polyphosphazenes, also known as PDCPs. This reaction follows the nucleophilic substitution of all chlorine atoms attached to phosphorus atoms and replaced by bifunctional organic or organometallics species all along the chain length.44,45 This synthesis pathway is controlled by the main component PDCP which is completely inorganic in nature and used by various kinds of polyphosphazene materials known in the literature, such as polymeric intermediates, macromolecular reactants, parent compounds, as well as polymeric sources.
Figure 7.
Broad stepwise reaction summary for the synthesis of the polyphosphazenes.44,45
Additionally, controlled polymerization of PDCP is also possible through a living cationic polymerization of trichlorophosphoranimine (Cl3PNSiMe3). This procedure can be carried out simply in solution at room temperature via reaction of Cl3PNSiMe3 with 2 equiv of PCl5, giving a cationic species [Cl3PNPCl3]+ with PCl6– as the counterion.46 This species can start the polymerization with the addition of further equivalents of Cl3PNSiMe3, leading to polymer chains with a “living” cationic end group.47 The formation of 1 equiv of ClSiMe3 as a side product with each monomer molecule added to the polymer makes this polymerization a rare example of a polycondensation reaction. The living chain growth mechanism, with one cationic initiator per propagating chain, not only permits the use of molecular weight via the feed monomer to the initiator ratio but also takes the lead for poly(dichloro)phosphazenes with narrow polydispersities. Reaction times may vary depending on the desired polymer chain length, the monomer concentration, and the nature of the counterion,47 but Cl3PNSiMe3 is noted to be consumed completely relatively quickly within a few hours in the preferred solvent dichloromethane.48
All of these key features indicate the excellent reactivity of chlorine atoms attached to skeletal phosphorus present in polyphosphazene chains.37,49 The key objective of this review is to highlight cyclophosphazene chemistry, specifically HCCP derivatives, so the maximum weight will be positioned on cyclophosphazene chemistry instead of linear polyphosphazenes.
4.2. Syntheses Methods of the Cyclomatrix Type of Polyphosphazenes
Cyclomatrix types of polyphosphazenes are cross-linked or self-assembled, less well-known compared to the other classes of polyphosphazenes, and are utilized in limited applications due to their difficult preparation methods.50,51 However, using HCCP as the monomer, where six chlorine atoms are easily replaceable, can be the most ideal candidate to start any sort of polyphosphazene reaction. Due to its extraordinary stereochemical structure and easily substituted chlorine atoms under an ultrasonication technique, a wide range of micro- and nano-shaped materials were fabricated utilizing a one-pot method.52−55 Tang and co-workers revealed the synthesis of these polyphosphazenes via an SN2 mechanism of nucleophilic substitution.56Figure 7 shows the model bidentate ligand for nucleophilic substitution. In the first step, activation of a nucleophilic ligand occurred in the presence of basic triethylamine (TEA). In the second step, the chlorine atom attached to phosphorus atoms of HCCP is replaced via attacking the activating ligand to form the nucleophilic phosphorus bond. In the third step, the removal of chlorine atoms from the HCCP structure is done by replacement of halogen.
The actual polymerization starts when TEA plays the role of a base to accept all the chlorine from HCCP and synthesize its chlorine salt crystal, triethylammonium chloride (TEACl). This step facilitates the polymerization and allows the nucleophilic ligand to replace all the reactive chlorine attached to the phosphorus atoms of HCCP to synthesize the highly cross-linked cyclopolyphosphazenes. This preparation method has more benefits than many other time-consuming and complicated approaches: this method is a facile and green approach to form morphology-controlled polymers, and these products are functional in numerous types of present-day applications.57 Using the one-pot method of synthesis for cyclopolyphosphazenes, the four techniques are applied to get the desired product with controlled morphology by changing the various solvents, temperature, and ratio of monomers.
The template-assisted method is influenced by the synthesis of a byproduct during the reaction, such as TEACl crystal formation during the polymerization precipitation method by using any monomer in the presence of TEA. These TEACl crystals provide the adherence of primary nucleus particles over them because of high surface energy, and then TEACl crystals act as a template to synthesize the nanotubes or nanorods.58 This side product can be removed after complete polymerization by washing with water, alcohol, or acetone to get purified products. However, an ex situ template such as carbon nanotubes (CNTs) is also utilized to synthesize core–shell polyphosphazenes@CNTs and can be further modified to the desired morphology for advanced applications, e.g., energy storage.59 This ex situ technique is famous not only for using carbon templates such as graphene or CNTs but also because it can be used to wrap some inorganic ex situ templates such as ZIF-67.60 In summary, this method gives fruitful products in the absence as well as in the presence of the external template.
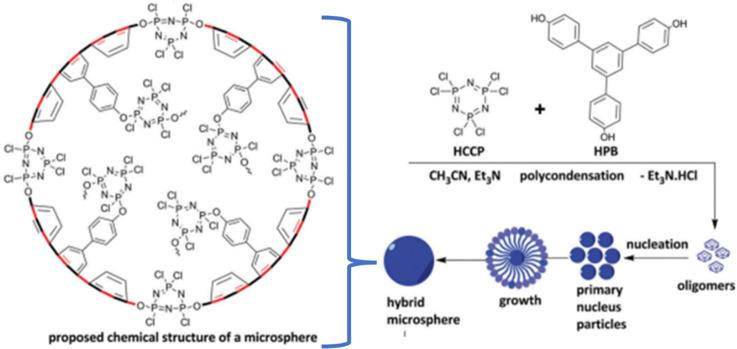
In contrast to the ex/in situ template method, which is mostly employed to obtain nanotubes or rod-shaped morphology, the primary particles self-assembled and grew the fibers, spheres, and hollow materials through the “self-assembly method”, as shown in Figure 8.61,62 The obtained morphologies are based upon the principle of oligomeric absorbing polyphosphazene mechanisms which can be developed by the facilitation of a specific solvent system to obtain the desired morphology by adjusting the monomer ratios, temperature, time, and power of ultrasonic irradiation. The nucleation process dominates after the initial formation of oligomers which leads to the creation of primary nucleus particles. Their self-assembly contributes to a specific homogeneous morphology at the final nano- or microlevel particles, and the growth of the monodispersed product is highly demanded for the consistency of any fruitful application.63 The absolute purity of monomers is required to get the monodispersion of a product; otherwise, target morphology is very difficult to achieve.
Figure 8.
Mechanistic and schematic illustration of the poly[cyclotriphosphazene-co-1,3,5-tri(4-hydroxyphenyl)benzene] microspheres with ideally proposed chemical structure.62 Adapted from ref (62) with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry.
The “skelton convert method” is more useful than above-mentioned two methods when it comes to usage of their end product in wide variety of applications. Cyclomatrix kind of polymers can easily be converted by treating them with high temperature 600–900 °C. Their structure and conventional - P=N - bond convert into - C - C - bond networks with minor amount of heterogeneous atoms trapped - C - C - structure such as nitrogen, phosphorus, sulfur depending upon different pyrolysis temperature.63 The doped heteroatoms such as phosphorus, nitrogen, boron, oxygen, sulfur either react with each other or transform into their reactive species during the pyrolysis treatment and finally these reactive species reformed into obtained carbon material. The precursor morphology and stability of pyrolysis polyphosphazenes is consistent even at too high temperature, which allows them to be functional in various of advanced applications e.g. supercapacitors and electrocatalysts.8 This method has literally widened the new horizons toward the growth of cyclopolyphosphazenes nano- and micromaterials or the transformation of carbon nano- and micromaterials into macromolecule structures to obtain entirely new polymer systems.
The modification or surface functionalization of the cyclopolyphosphazenes can be obtained naturally due to the micro- or nanomaterial’s high surface energy. This high surface energy is actually based on the presence of active groups on their surfaces such as −OH or −NH2 groups.61 The existence of these kinds of functional groups facilitates cyclopolyphosphazenes to form the advanced level of functional materials. This method is generally known as the “surface polymerization method”, and this method is functional to attain a class of core–shell designs where various metallic nanoparticles impregnate the cyclomatrix type of the polyphosphazene surface to form carbon nanotubes, silver nanowires, and magnetic nanotubes with the assistance of carbon, silver salts, and Fe2O3 precursors, respectively.64−66
These methods have been comprehensively studied during the past decade to reveal the extensive properties of cyclopolyphosphazenes. On the basis of the above-discussed variety of techniques, cyclopolyphosphazenes have been engaged in an enormous amount of modern-day applications. It is important to study briefly each of those advanced applications. Nowadays, cyclopolyphosphazenes are utilized in every field of life where polymers can act as materials ranging from flame retardants to biodegradable materials, electro-optical materials to army applications, and from porous materials to energy conversions. However, in this review the core topic is the applications of cyclopolyphosphazenes and their hybrids.
5. Applications of the Cyclomatrix Type of Polyphosphazenes
Their applications are mainly based on the molecular structure and arrangement and the way the materials interact with each other and liquid, solid, and gaseous phase media. Relying on their mode of interactions and their properties, various other applications are further classified. The molecular level of features is almost the same with two important features: (1) the existence of different types of functional groups and (2) the type of unique backbone structure polyphosphazenes have. These two features play an important role in cyclomatrix-type polyphosphazene chemistry, as the targeted number of special functional groups on the side and backbone structure of polyphosphazenes of phosphorus and nitrogen groups is alternatingly arranged to make them highly demanded polymeric species.67−69 Based on this discussion, a very wide classification of polyphosphazene applications due to their intrinsic structural and functional group tendencies is shown in Figure 9. It obviously highlights that novel polymeric design and fabrication of polyphosphazenes has applications in membrane and controlled surfaces, biomedical applications, semiconductors, energy conversion materials, storage materials, as well as high performance and fire-resistant elastomers.70−76 It is a difficult task to study the broad range of applications of polyphosphazenes. Therefore, here we will only discuss the modern-day properties of cyclopolyphosphazenes as well as applications, which are mostly dependent on their morphological properties.
Figure 9.
Diagram showing the six main application areas being developed for polyphosphazenes.
5.1. Biomedical Applications
The cyclopolyphosphazenes have been used in biomedical applications and play a role in human health betterment via a drug delivery technique.77,78 This drug delivery technique has many benefits over traditional and conventional types of drug dosage systems.79,80 The drug delivery system reduced the toxicity, improved the efficiency in the context of target tissues, and improved the health condition of the patient.81−83 Morphology-focused carriers with uniform hollow structures need an hour to efficiently run a drug delivery system, and these materials have the potential to deliver the guest molecules in systems with minimum damage to human body cells.84−86 Until now, polyphosphazene microspheres have been utilized for modern day drug delivery systems because they can provide the monodispersion in solutions carrying large amounts of guest molecules of the drugs.80,87−89 The biocompatibility and biodegrability of cyclopolyphosphazene materials are well-known, and many research groups87,90 have used these molecules as biomaterials. Liu and co-worker synthesized the highly cross-linked, mesoporous, and most importantly hollow core submicrospheres and used them as drug delivery carriers.91 These hollow submicrospheres exhibited a sustainability of 15 days, released drug in a controlled way, and stored the 380 mg doxorubicin hydrochloride drug with extraordinary biocompatibility and uniform dispersion in an organic medium.
Orum et al. synthesized the novel cyclomatrix polyphosphazene nanospheres that contain curcumin and quercetin, which were prepared by one-pot drug self-framed precipitation polymerization. Curcumin and quercetin were used both as monomers in the synthesis of nanospheres and as anticancer released drugs.80
Further, microspheres of trimethoprime (TMP) and HCCP can be prepared by a one-pot method, which can be utilized as an antibiotic drug carrier. The TMP microspheres attained a 92% degradation ratio after 50 days when an in vitro biodegradation test was performed in acidic medium. These TMP microspheres have been used in model drug-controlled delivery systems such as Rhodamine 6G and vitamin B12. A synthetic kind of polyphosphazene-derived macromolecule such as PCPP has the potential to play a role as a microfabricating agent and is a potential candidate for an intradermal immune adjuvant in the fabrication of coated microneedles by using an intradermal vaccination method.92,93
5.2. Fluorescent Active Materials
Two steps are involved in the synthesis of fluorescent active molecules. In the first step, polymeric material is synthesized via emulsion polymerization, the sol–gel method, or the precipitation polymerization technique. In the second step the compounds such as fluorophores, quantum dots, or dye molecules are bonded by either van der Waals forces or chemical interactions.55,94 The existence of heavy metals of quantum dots and nonspecific tagging of dye molecules make them bioincompatible for many fluorescent and bio applications. Opposite to this, fluorescent active species can be used and easily prepared by the self-assembly method. These active fluorescent methods possesses the intrinsic optical features due to their backbone structural stability and π–π conjugation.55,95 In this context, various polyphosphazene polymers which possess the suitable morphology and exhibit the fluorescent properties are chosen to be used as a biosensor, detector, and biomaterial.96−98 The poly(cyclotriphosphazene-co-phloroglucinol) (PCTP) fluorescent microspheres,99 poly(cyclotriphosphazene-co-fluorescein) (PCTPF) nanoshells,99 intrinsic poly(cyclotriphosphazene-co-resveratrol) (PRS) hollow spheres,100 and poly[cyclotriphosphazene-co-bis(aminomethyl)ferrocene] (PCPF) microspheres,101 and the hybrid poly[cyclotriphosphazene-co-1,3,5-tri(4-hydroxyphenyl)benzene] (PCTHB) microsphere62 has exhibited great results as a fluorescent active and drug carrier biomaterial because of its porous nature and high surface area.
5.3. Carbon Material Derivatives
In recent days, carbon materials have attracted the attention of researchers. In particular, porous carbon materials possess a good pore volume with high surface area, extraordinary mechanical stability with less density, and excellent surface permeability with chemical inertness.102−105 These highly noteworthy morphological and structural properties make porous materials very suitable candidates for adsorbents, storage materials, catalyst supports, supercapacitors, and sensors and in solar cells and fuel cells to act as an electrocatalyst for the oxygen evolution reaction (OER), hydrogen evolution reaction (HER), and oxygen reduction reaction (ORR).6,106−109 The cyclopolyphosphazene material pyrolysis has improved their morphological properties from being even on the surface to porous, rigid solid, to hollow and from traditional P=N -bond to̵ C–C -graphitic bond networks in nature.110 Dufek and co-workers synthesized the PZS and carbonized them at 1000 °C to form porous carbon materials and used lithium ion electrodes which exhibited the stable capacity of 1200 mAh g–1, with almost 95% retention after 40 cycles.111
Sekar et al. have prepared an electrocatalyst by the immobilization of NiCo2O4 on a phosphazene-based covalent organic polymer (P-COP) through a facile hydrothermal method. The NiCo2O4-P-COP-based electrode was simultaneously used for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), and it displayed a minimum overpotential of 270 and 130 mV (V vs RHE), respectively, at a current density of 10 mA cm–2. In addition, it acted as an oxygen reduction catalyst with a half-wave potential of 0.83 V (V vs RHE) and a maximum current density of 4.5 mA cm–2 (Figure 10).107
Figure 10.
Schematic illustration of the synthesis of the P-COP and NiCo2O4-P-COP (a), TEM images of NiCo2O4-P-COP (b), and OER, HER, and ORR mechanisms for NiCo2O4-P-COP (c).107 Reprinted (adapted) with permission from ref (107). Copyright (2021) American Chemical Society
5.4. Adsorbent of Toxic Compounds
Organic dyes have been used as dye compounds in the industry since ancient times. In modern days, these organic dyes are a source of pigmentation and are utilized in a wide range of industries such as food, paper, cosmetics, textile industries, and so on.51,112 However, disposal of their waste is a huge problem for many industries as they not only can be very dangerous for humans but also interrupt the marine ecosystem due to their mutagenic and carcinogenic nature.113,114 To this day, various industries are trying to solve this problem by using different methods. Use of adsorbent is one of the easy, cost-economic, and effective ways to remove these dyes from the system.72,115,116 Nowadays, different types of adsorbents are commercially available for their adsorbance, such as zeolite, silica, natural polymers, and activated carbons. However, the area of consumption of these adsorbents is so large that these adsorbents could not fulfill the needs. Their porous morphology, large surface area hollow in structure, abundant hydroxyl groups, presence of heteroatoms, active −NH2 groups, and π–π stacking make polyphosphazenes excellent candidates as adsorbents (shown in Figure 11).101,117,118
Figure 11.
Proposed mechanism of tetracycline decontamination through PZS@rGO involving π–π stacking, electrostatic interactions, H-bonding, and Lewis acid–base interactions.118 Reprinted with permission from ref (118).
Wu et al. selected porous carbon nanosheets (PCNs) with a specific area of 1032.1 m2 g–1 as a carrier to in situ load aminated cyclomatrix polyphosphazene to generate PCNs@PCP composites. For that, low molecular weight polyethylenimine (PEI) and HCCP were utilized as ideal comonomers to conduct polymerization on PCNs. Subsequently, a mass of La3+ ions was anchored onto the PCNs@PCP framework via an impregnation process to obtain PCNs@PCP-La for phosphate removal. The investigation demonstrated that the strong coordination can be formed between electron-deficient La3+ and electron-rich N atoms in PEI units and phosphazene rings. Compared to unmodified PCNs@PCP, the phosphate adsorption capacity of PCNs@PCP-La increased remarkably (80.1 mg P g–1 vs 121.2 mg P g–1). Moreover, the adsorption capacity of PCNs@PCP-La stayed at a high level (more than 100 mg P g–1) over a wide pH region of 3.0–9.0, exhibiting high pH adaptability and thereby offering great potential for cleaning real phosphate-containing wastewater.102
Chen and coworkers have synthesized the poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes and used them for adsorption of methylene blue. They exhibited excellent adsorption capacity of 69.16 mg/g in 15 min, which is measured by the various parameters such as pH range, equilibrium at 25 °C, and initial concentration of the adsorbate. Kinetic studies and thermodynamic data revealed that absorption of methylene blue by polyphosphazene materials is a spontaneous and endothermic process, which followed the Langmuir isotherm.119 In the end, it can be concluded that the π–π stacking and high negatively charged surface properties of polyphosphazene materials along with high electrostatic attractions have increased the absorption capacity of the polyphosphazene materials.
5.5. Support materials for Nanoparticles
In recent days, nanoparticle-based hybrid robotics have been hot topics in research. Transition metals and the noble metals both can be used to carve the nanoparticles and can be used in every field of life such as catalysis, microelectronics, sensors, data storage, etc. due to their unparalleled properties.120 With increasing research on nanoparticles, it has been revealed that these nanoparticles tends to aggregate with each other when no support is involved, and this aggregation restricts performance in various applications.121 To sort out this problem, various types of support materials, specifically polymers, have been used to stabilize and control their uniform nanosize so they can retain their optimum potential. It is investigated that a number of polymeric species work as a support for nanoparticles, and in this way they can act with optimum potential by residing on a polymeric support.76,122,123 Polymers, CNTs, ionic liquids, and ligands were our choice, but their synthesis and morphology control after nanoparticle deposition is not an easy task. Cyclotriphosphazene is the best alternative for stabilizing nanoparticles.124 It consists of a 6-membered ring with alternative single and double bonds between phosphorus and nitrogen.
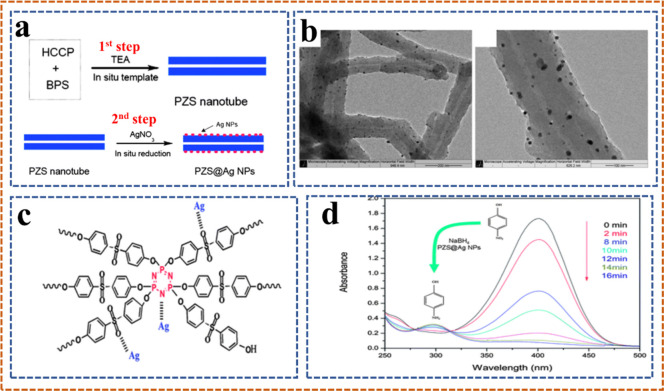
Recently, we prepared Ag-Cu NPs at polyphopshazene nanotubes, and these catalysts were efficiently utilized for a prototype 4-nitrophenol reduction reaction: the schematic representation of Ag-Cu NPs@PZS is shown in Figure 12.125 It is proved from studies that deposition of silver nanoparticles was done on the poly[cyclotriphosphazene-co-(4,4′-sulfonyl diphenol] material on its surface via an inorganic reaction, while reduction of 4-nitrophenol (4-NP) was employed as a precursor of AgNO3, as shown in Figure 13.126 By this reaction, homogeneous nanoparticles of uniform size are obtained. Moreover, this process is less costly and totally based on green synthesis.
Figure 12.
Schematic representation of Ag-Cu NP@PZS nanotubes.125 Reprinted (adapted) with permission from ref (125). Copyright (2022) American Chemical Society.
Figure 13.
Two-step illustration of the procedure for preparing the PZS@Ag NP composites (a), TEM images of PZS@Ag NP composites (b), the schematic of the coordination behavior between Ag NPs and the PZS nanotubes (c), and successive UV–vis absorbance spectra for the reduction of 4-NP by NaBH4 in the presence of the PZS@Ag NP composites (d).126 Reproduced from ref (126) with permission from the Royal Society of Chemistry.
6. Conclusion
We have presented a detailed description of features and applications of cyclomatrix phosphazene compounds. We predict that this study on hybrid polyphosphazenes will provide a foundation for guiding and understanding the robotic chemistry of polyphosphazene-derived hybrids with excellent quality for industrial research and universities. It has potential to revolutionize the green chemistry and energy industries.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Project No. 51773010). M.I. and M.A.A. express appreciation to the Research Center for Advanced Materials Science (RCAMS) at King Khalid University Abha, Saudi Arabia, through grant KKU/ RCAMS/22.
Author Contributions
¶ M.A. and T.N. contributed equally to this work.
The authors declare no competing financial interest.
References
- Singler R. E.; Hagnauer G. L.; Sicka R. W.. Phosphazene elastomers: synthesis, properties, and applications; ACS Publications, 1984. [Google Scholar]
- Arbuckle-Keil G. In Polymers in inorganic chemistry: A first-hand account; Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, 2015. [Google Scholar]
- Allcock H. R.Chemistry and applications of polyphosphazenes; Wiley-Intersci. Ser., 2003. [Google Scholar]
- Qiu S.; Wang X.; Yu B.; Feng X.; Mu X.; Yuen R. K.; Hu Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017, 325, 327–339. 10.1016/j.jhazmat.2016.11.057. [DOI] [PubMed] [Google Scholar]
- Jia Y.; Li J.; Wang Y.; Jin J.; Liu H. Preparation and properties of optical acrylate modified with sulfur-containing cyclophosphazene polymer. Prog. Org. Coat. 2021, 156, 106249. 10.1016/j.porgcoat.2021.106249. [DOI] [Google Scholar]
- Ali Z.; Basharat M.; Wu Z. A Review on the Morphologically Controlled Synthesis of Polyphosphazenes for Electrochemical Applications. ChemElectroChem. 2021, 8 (5), 759–782. 10.1002/celc.202001352. [DOI] [Google Scholar]
- Allcock H. R.; McDonnell G. S.; Desorcie J. L. Synthesis of new polyphosphazene elastomers. Macromolecules 1990, 23 (17), 3873–3877. 10.1021/ma00219a001. [DOI] [Google Scholar]
- Liu W.; Zhang S.; Dar S. U.; Zhao Y.; Akram R.; Zhang X.; Jin S.; Wu Z.; Wu D. Polyphosphazene-derived heteroatoms-doped carbon materials for supercapacitor electrodes. Carbon 2018, 129, 420–427. 10.1016/j.carbon.2017.12.016. [DOI] [Google Scholar]
- Veronese F. M.; Marsilio F.; Lora S.; Caliceti P.; Passi P.; Orsolini P. Polyphosphazene membranes and microspheres in periodontal diseases and implant surgery. Biomaterials 1999, 20 (1), 91–98. 10.1016/S0142-9612(97)00104-X. [DOI] [PubMed] [Google Scholar]
- Schacht E.; Vandorpe J.; Crommen J.; Seymour L.. Biodegradable Polyphosphazenes for Biomedical Applications. In Advanced biomaterials in biomedical engineering and drug delivery systems; Springer, 1996; pp 81–85. [Google Scholar]
- Zhang J.; Huang X.; Fu J.; Huang Y.; Liu W.; Tang X. Novel PEO-based composite solid polymer electrolytes incorporated with active inorganic–organic hybrid polyphosphazene microspheres. Mater. Chem. Phys. 2010, 121 (3), 511–518. 10.1016/j.matchemphys.2010.02.016. [DOI] [Google Scholar]
- De Jaeger R.; Gleria M. Poly (organophosphazene) s and related compounds: synthesis, properties and applications. Prog. Polym. Sci. 1998, 23 (2), 179–276. 10.1016/S0079-6700(97)00027-0. [DOI] [Google Scholar]
- Allcock H. R. Phosphonitrilic chemistry. Chem. Eng. News 1968, 46 (18), 68–81. 10.1021/cen-v046n018.p068. [DOI] [Google Scholar]
- Allcock H. R. A perspective of polyphosphazene research. J. Inorg. Organomet. Polym. Mater. 2007, 16 (4), 277. 10.1007/s10904-006-9052-9. [DOI] [Google Scholar]
- Allcock H. R.; Kugel R. L. Phosphonitrilic compounds. VIII. Reaction of o-aminophenol with phosphazenes. J. Am. Chem. Soc. 1969, 91 (20), 5452–5456. 10.1021/ja01048a009. [DOI] [Google Scholar]
- Allcock H. R. Recent advances in phosphazene (phosphonitrilic) chemistry. chemical Reviews 1972, 72 (4), 315–356. 10.1021/cr60278a002. [DOI] [Google Scholar]
- Bowers D. J.; Wright B. D.; Scionti V.; Schultz A.; Panzner M. J.; Twum E. B.; Li L.-L.; Katzenmeyer B. C.; Thome B. S.; Rinaldi P. L.; et al. Structure and Conformation of the Medium-Sized Chlorophosphazene Rings. Inorg. Chem. 2014, 53 (17), 8874–8886. 10.1021/ic500272b. [DOI] [PubMed] [Google Scholar]
- Bîrcă A.; Gherasim O.; Grumezescu V.; Grumezescu A. M., Introduction in thermoplastic and thermosetting polymers. In Materials for Biomedical Engineering,; Elsevier, 2019; pp 1–28. [Google Scholar]
- Amin A. M.; Wang L.; Wang J.; Yu H.; Gao J.; Li C.; Huo J.; Amer W. A.; Yan G.; Ma L. Recent research progress in the synthesis of polyphosphazene elastomers and their applications. Polym. Plast Technol. Eng. 2010, 49 (14), 1399–1405. 10.1080/03602559.2010.496387. [DOI] [Google Scholar]
- Işıklan M.; Asmafiliz N.; Özalp E. E.; Ilter E. E.; Kılıç Z.; Çoşut B. n.; Yeşilot S.; Kılıç A.; Öztürk A.; Hökelek T.; et al. Phosphorus– nitrogen compounds. 21. syntheses, structural investigations, biological activities, and DNA interactions of new N/O spirocyclic phosphazene derivatives. The NMR behaviors of chiral phosphazenes with stereogenic centers upon the addition of chiral solvating agents. Inorg. Chem. 2010, 49 (15), 7057–7071. 10.1021/ic100781v. [DOI] [PubMed] [Google Scholar]
- Elmas G.; Okumuş A.; Koç L. Y.; Soltanzade H.; Kılıç Z.; Hökelek T.; Dal H.; Açık L.; Üstündağ Z.; Dündar D.; et al. Phosphorus–nitrogen compounds. Part 29. Syntheses, crystal structures, spectroscopic and stereogenic properties, electrochemical investigations, antituberculosis, antimicrobial and cytotoxic activities and DNA interactions of ansa-spiro-ansa cyclotetraphosphazenes. Eur. J. Med. Chem. 2014, 87, 662–676. 10.1016/j.ejmech.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zhiping X. T. M. Q. M.Research and application of polyphosphazenes. New Chem. Mater. 2010, 3. [Google Scholar]
- Allcock H. R. The expanding field of polyphosphazene high polymers. Dalton Trans. 2016, 45 (5), 1856–1862. 10.1039/C5DT03887A. [DOI] [PubMed] [Google Scholar]
- Amin A. M.; Wang L.; Wang J.; Yu H.; Huo J.; Gao J.; Xiao A. Recent research progress in the synthesis of polyphosphazene and their applications. Des. Monomers Polym. 2009, 12 (5), 357–375. 10.1163/138577209X12486896623373. [DOI] [Google Scholar]
- Lee D. K.; Jackson A.-M. S.; Fushimi T.; Yennawar H.; Allcock H. R. Synthesis and inclusion behavior of cyclotriphosphazene molecules with asymmetric spiro rings. Dalton Trans. 2010, 39 (22), 5341–5348. 10.1039/b925734a. [DOI] [PubMed] [Google Scholar]
- İlter E. E.; Asmafiliz N.; Kılıç Z.; Işıklan M.; Hökelek T.; Çaylak N.; Şahin E. Phosphorus– nitrogen compounds. 14. synthesis, stereogenism, and structural investigations of novel N/O spirocyclic phosphazene derivatives. Inorg. Chem. 2007, 46 (23), 9931–9944. 10.1021/ic701216f. [DOI] [PubMed] [Google Scholar]
- Honeyman C. H.; Manners I.; Morrissey C. T.; Allcock H. R. Ambient temperature synthesis of poly (dichlorophosphazene) with molecular weight control. J. Am. Chem. Soc. 1995, 117 (26), 7035–7036. 10.1021/ja00131a040. [DOI] [Google Scholar]
- Allen C. W. Linear, cyclic and polymeric phosphazenes. Coord. Chem. Rev. 1994, 130 (1–2), 137–173. 10.1016/0010-8545(94)80004-9. [DOI] [Google Scholar]
- Allcock H. R. Generation of structural diversity in polyphosphazenes. Appl. Organomet. Chem. 2013, 27 (11), 620–629. 10.1002/aoc.2981. [DOI] [Google Scholar]
- Allcock H. R. Recent developments in polyphosphazene materials science. Curr. Opin. Solid State Mater. Sci. 2006, 10 (5–6), 231–240. 10.1016/j.cossms.2007.06.001. [DOI] [Google Scholar]
- Shaw R. The phosphazenes--a family of compounds containing an alternating phosphorus-nitrogen skeleton. Endeavour 1968, 27 (101), 74–80. 10.1016/0160-9327(68)90098-7. [DOI] [PubMed] [Google Scholar]
- Uslu A.; Yeşilot S. Chiral configurations in cyclophosphazene chemistry. Coord. Chem. Rev. 2015, 291, 28–67. 10.1016/j.ccr.2015.01.012. [DOI] [Google Scholar]
- Allcock H. R.Mechanisms and catalysis in cyclophosphazene polymerization; ACS Publications, 1992. [Google Scholar]
- Allcock H. R.; Kugel R. Degradation of halogenophosphazenes to a new phosphorane system. ChemComm (London) 1968, 24, 1606–1607. 10.1039/c19680001606. [DOI] [Google Scholar]
- Boileau S.; Illy N. Activation in anionic polymerization: Why phosphazene bases are very exciting promoters. Prog. Polym. Sci. 2011, 36 (9), 1132–1151. 10.1016/j.progpolymsci.2011.05.005. [DOI] [Google Scholar]
- Birdsall W. J. Electroreduction and electron spin resonance studies with phosphazenes and related systems. Thesis; 1971; p 7209440. [Google Scholar]
- Allcock H. R. General introduction to phosphazenes. Phosphazenes: A Worldwide Insight 2004, 485. [Google Scholar]
- Marre M.; Boisdon M.; Sanchez M.. Spirophosphazenes from Dicoordinated Phosphorus Derivatives. Chem. Inf.-Dienst. 1982. [Google Scholar]
- Allcock H. R.; Birdsall W. Phosphonitrilic compounds. XI. Electroreduction and electron spin resonance spectra of phosphazenes. Inorg. Chem. 1971, 10 (11), 2495–2499. 10.1021/ic50105a025. [DOI] [Google Scholar]
- Sun H.; You X.; Deng J.; Chen X.; Yang Z.; Ren J.; Peng H. Novel graphene/carbon nanotube composite fibers for efficient wire-shaped miniature energy devices. Adv. Mater. 2014, 26 (18), 2868–2873. 10.1002/adma.201305188. [DOI] [PubMed] [Google Scholar]
- Stone D. A.; Allcock H. R. A New Polymeric Intermediate for the Synthesis of Hybrid Inorganic– Organic Polymers. Macromolecules 2006, 39 (15), 4935–4937. 10.1021/ma061079g. [DOI] [Google Scholar]
- Sun J.; Wang X.; Wu D. Novel spirocyclic phosphazene-based epoxy resin for halogen-free fire resistance: synthesis, curing behaviors, and flammability characteristics. ACS Appl. Mater. Interfaces 2012, 4 (8), 4047–4061. 10.1021/am300843c. [DOI] [PubMed] [Google Scholar]
- Liu J.; Tang J.; Wang X.; Wu D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2 (13), 5789–5799. 10.1039/c2ra20739g. [DOI] [Google Scholar]
- Gleria M.; De Jaeger R. Aspects of phosphazene research. J. Inorg. Organomet. Polym. Mater. 2001, 11 (1), 1–45. 10.1023/A:1013276518701. [DOI] [Google Scholar]
- Neilson R. H.; Wisian-Neilson P.. Synthesis and Characterization of New Phosphazene Polymers; Texas Christian Univ Fort Worth Dept of Chemistry, 1988. [Google Scholar]
- Blackstone V.; Soto A. P.; Manners I. Polymeric materials based on main group elements: the recent development of ambient temperature and controlled routes to polyphosphazenes. Dalton Trans. 2008, 33, 4363–4371. 10.1039/b719361k. [DOI] [PubMed] [Google Scholar]
- Blackstone V.; Pfirrmann S.; Helten H.; Staubitz A.; Presa Soto A.; Whittell G. R.; Manners I. A Cooperative Role for the Counteranion in the PCl5-Initiated Living, Cationic Chain Growth Polycondensation of the Phosphoranimine Cl3P= NSiMe3. J. Am. Chem. Soc. 2012, 134 (37), 15293–15296. 10.1021/ja307703h. [DOI] [PubMed] [Google Scholar]
- Wilfert S.; Henke H.; Schoefberger W.; Brüggemann O.; Teasdale I. Chain-End-Functionalized Polyphosphazenes via a One-Pot Phosphine-Mediated Living Polymerization. Macromol. Rapid Commun. 2014, 35 (12), 1135–1141. 10.1002/marc.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Xu Q.. Green Manufacturing and the Application of High-Temperature Polymer-Polyphosphazenes. In Green and Sustainable Manufacturing of Advanced Material; Elsevier: 2016; pp 603–646. [Google Scholar]
- Allcock H. R. Generation of structural diversity in polyphosphazenes. Appl. Organomet. Chem. 2013, 27 (11), 620–629. 10.1002/aoc.2981. [DOI] [Google Scholar]
- Tang X.-Z.; Huang X.-B., Synthesis and Assembly Chemistry of Inorganic Polymers. In Modern Inorganic Synthetic Chemistry; Elsevier: 2017; pp 279–306. [Google Scholar]
- Hu Y.; Meng L.; Lu Q. Fastening” porphyrin in highly cross-linked polyphosphazene hybrid nanoparticles: powerful red fluorescent probe for detecting mercury ion. Langmuir 2014, 30 (15), 4458–4464. 10.1021/la500270t. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Huang X.; Fu J.; Huang Y.; Zhu Y.; Tang X. A one-pot approach to novel cross-linked polyphosphazene microspheres with active amino groups. Macromol. Chem. Phys. 2009, 210 (9), 792–798. 10.1002/macp.200800597. [DOI] [Google Scholar]
- Huang Z.; Zheng F.; Chen S.; Lu X.; van Sittert C. G. C. E.; Lu Q. A strategy for the synthesis of cyclomatrix-polyphosphazene nanoparticles from non-aromatic monomers. RSC Adv. 2016, 6 (79), 75552–75561. 10.1039/C6RA13486F. [DOI] [Google Scholar]
- Wan C.; Huang X. Cyclomatrix polyphosphazenes frameworks (Cyclo-POPs) and the related nanomaterials: Synthesis, assembly and functionalisation. Mater. Today Commun. 2017, 11, 38–60. 10.1016/j.mtcomm.2017.02.001. [DOI] [Google Scholar]
- Zhu L.; Huang X.; Tang X. One-Pot Synthesis of Novel Poly (cyclotriphosphazene-co-sulfonyldiphenol) Microtubes without External Templates. Macromol. Mater. Eng. 2006, 291 (6), 714–719. 10.1002/mame.200600015. [DOI] [Google Scholar]
- Potin P.; De Jaeger R. Polyphosphazenes: Synthesis, structures, properties, applications. Eur. Polym. J. 1991, 27 (4–5), 341–348. 10.1016/0014-3057(91)90185-Q. [DOI] [Google Scholar]
- Zhu L.; Xu Y.; Yuan W.; Xi J.; Huang X.; Tang X.; Zheng S. One-Pot Synthesis of Poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) Nanotubes via an In Situ Template Approach. Adv. Mater. 2006, 18 (22), 2997–3000. 10.1002/adma.200600562. [DOI] [Google Scholar]
- Dar S. U.; Din M. A. U.; Hameed M. U.; Ali S.; Akram R.; Wu Z.; Wu D. Oxygen reduction reaction of (C-PCTNB@ CNTs): A nitrogen and phosphorus dual-doped carbon electro-catalyst derived from polyphosphazenes. J. Power Sources 2018, 373, 61–69. 10.1016/j.jpowsour.2017.11.006. [DOI] [Google Scholar]
- Yang S.; Peng L.; Huang P.; Wang X.; Sun Y.; Cao C.; Song W. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew. Chem., Int. Ed. 2016, 55 (12), 4016–4020. 10.1002/anie.201600455. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Huang X.; Li W.; Fu J.; Tang X. Preparation of novel hybrid inorganic–organic microspheres with active hydroxyl groups using ultrasonic irradiation via one-step precipitation polymerization. Mater. Lett. 2008, 62 (8–9), 1389–1392. 10.1016/j.matlet.2007.08.062. [DOI] [Google Scholar]
- Dar S. U.; Ali S.; Hameed M. U.; Zuhra Z.; Wu Z. A facile synthesis, structural morphology and fluorescent properties of cross-linked poly (cyclotriphosphazene-co-1, 3, 5-tri (4-hydroxyphenyl) benzene) hybrid copolymer microspheres. New J. Chem. 2016, 40 (10), 8418–8423. 10.1039/C6NJ01578F. [DOI] [Google Scholar]
- Zhu Y.; Huang X.; Fu J.; Wang G.; Tang X. Morphology control between microspheres and nanofibers by solvent-induced approach based on crosslinked phosphazene-containing materials. J. mater. sci. eng., B 2008, 153 (1–3), 62–65. 10.1016/j.mseb.2008.10.027. [DOI] [Google Scholar]
- Fu J.; Huang X.; Huang Y.; Pan Y.; Zhu Y.; Tang X. Preparation of silver nanocables wrapped with highly cross-linked organic– inorganic hybrid polyphosphazenes via a hard-template approach. J. Phys. Chem. C 2008, 112 (43), 16840–16844. 10.1021/jp8063855. [DOI] [Google Scholar]
- Xu H.; Zhang X.; Liu D.; Yan C.; Chen X.; Hui D.; Zhu Y. Cyclomatrix-type polyphosphazene coating: Improving interfacial property of carbon fiber/epoxy composites and preserving fiber tensile strength. Compos. B. Eng. 2016, 93, 244–251. 10.1016/j.compositesb.2016.03.033. [DOI] [Google Scholar]
- Hu Y.; Meng L.; Niu L.; Lu Q. Facile synthesis of superparamagnetic Fe3O4@ polyphosphazene@ Au shells for magnetic resonance imaging and photothermal therapy. ACS Appl. Mater. Interfaces. 2013, 5 (11), 4586–4591. 10.1021/am400843d. [DOI] [PubMed] [Google Scholar]
- Baranwal B.; Das S.; Farva U. Structural characterization of new fungicidal material; complexes of copper (II) with cyclic phosphazenes. Prog. Cryst. Growth Charact. Mater. 2002, 45 (1–2), 43–49. 10.1016/S0960-8974(02)00026-8. [DOI] [Google Scholar]
- van de Grampel C. In Phosphazenes: A Worldwide Insight; Gleria M., De Jaeger R., Eds.; Nova Science Publishers, Inc., 2004, p 143. [Google Scholar]
- Wang L.; Su X.; Xie J.-H.; Ming L.-J. Specific recognitions of multivalent cyclotriphosphazene derivatives in sensing, imaging, theranostics, and biomimetic catalysis. Coord. Chem. Rev. 2022, 454, 214326. 10.1016/j.ccr.2021.214326. [DOI] [Google Scholar]
- Chen F.; Teniola O. R.; Laurencin C. T. Biodegradable polyphosphazenes for regenerative engineering. J. Mater. Res. 2022, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand D. J.; Magiri R. B.; Wilson H. L.; Mutwiri G. K. Polyphosphazenes as Adjuvants for Animal Vaccines and Other Medical Applications. Front. Bioeng. Biotechnol. 2021, 9, 78. 10.3389/fbioe.2021.625482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.; Shi W.; Zhang K.; Xie Z. Bifunctional cyclomatrix polyphosphazene-based hybrid with abundant decorating groups: Synthesis and application as efficient electrochemical Pb (II) probe and methylene blue absorbent. J. Colloid Interface Sci. 2021, 587, 683–692. 10.1016/j.jcis.2020.11.028. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Qiu S.; He L.; Cai W.; Chu F.; Zhu Y.; Jiang X.; Song L.; Hu Y. Bifunctional linear polyphosphazene decorated by allyl groups: Synthesis and application as efficient flame-retardant and toughening agent of bismaleimide. Composites Part B: Engineering 2022, 233, 109653. 10.1016/j.compositesb.2022.109653. [DOI] [Google Scholar]
- Andrianov A. K.; Langer R. Polyphosphazene immunoadjuvants: Historical perspective and recent advances. J. Controlled Release 2021, 329, 299–315. 10.1016/j.jconrel.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Wang G.; Li F.; He Z.; Zhang J.; Chen J.; Wang R. High conductivity membrane containing polyphosphazene derivatives for vanadium redox flow battery. J. Membr. Sci. 2021, 630, 119322. 10.1016/j.memsci.2021.119322. [DOI] [Google Scholar]
- Sui Y.; Sima H.; Shao W.; Zhang C. Novel bioderived cross-linked polyphosphazene microspheres decorated with FeCo-layered double hydroxide as an all-in-one intumescent flame retardant for epoxy resin. Compos. B. Eng. 2022, 229, 109463. 10.1016/j.compositesb.2021.109463. [DOI] [Google Scholar]
- Ogueri K. S.; Ogueri K. S.; Allcock H. R.; Laurencin C. T. Polyphosphazene polymers: The next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vac. Sci. Technol. 2020, 38 (3), 030801. 10.1116/6.0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas Y.; Kneidinger M.; Fornaguera C.; Borrós S.; Brüggemann O.; Teasdale I. Dual stimuli-responsive polyphosphazene-based molecular gates for controlled drug delivery in lung cancer cells. RSC Adv. 2020, 10 (46), 27305–27314. 10.1039/D0RA03210G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood S.; Wang L.; Yu H.; Haq F.; Fahad S.; Alim Uddin M.; Haroon M. Recent progress on the preparation of cyclomatrix-polyphosphazene based micro/nanospheres and their application for drug release. ChemistrySelect 2020, 5 (20), 5939–5958. 10.1002/slct.201904844. [DOI] [Google Scholar]
- Örüm S. M. Novel cyclomatrix polyphosphazene nanospheres: Preparation, characterization and dual anticancer drug release application. Polym. Bull. 2022, 79 (5), 2851–2869. 10.1007/s00289-021-03654-5. [DOI] [Google Scholar]
- Zheng C.; Qiu L.; Yao X.; Zhu K. Novel micelles from graft polyphosphazenes as potential anti-cancer drug delivery systems: drug encapsulation and in vitro evaluation. Int. J. Pharm. 2009, 373 (1–2), 133–140. 10.1016/j.ijpharm.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Sharma B.; Bhardwaj T. R.; Singh R. K. Design, synthesis and studies on novel polymeric prodrugs of erlotinib for colon drug delivery. Curr. Med. Chem. Anticancer Agents) 2021, 21 (3), 383–392. 10.2174/1871520620666200811124013. [DOI] [PubMed] [Google Scholar]
- de Castilla P. E. M.; Tong L.; Huang C.; Sofias A. M.; Pastorin G.; Chen X.; Storm G.; Schiffelers R. M.; Wang J.-W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Delivery Rev. 2021, 175, 113801. 10.1016/j.addr.2021.05.011. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Zhai Y.; Xu J.; Zhou T.; Cen L. Microfluidic preparation of PLGA composite microspheres with mesoporous silica nanoparticles for finely manipulated drug release. International Int. J. Pharm. 2021, 593, 120173. 10.1016/j.ijpharm.2020.120173. [DOI] [PubMed] [Google Scholar]
- Aboelela S. S.; Ibrahim M.; Badruddoza A. Z. M.; Tran V.; Ferri J. K.; Roper T. D. Encapsulation of a highly hydrophilic drug in polymeric particles: A comparative study of batch and microfluidic processes. Int. J. Pharm. 2021, 606, 120906. 10.1016/j.ijpharm.2021.120906. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Benassi E.; Shi Y.; Yue X.; Cui L.; Yang S.; Liu Z.; Guo X. Modified biomimetic core–shell nanostructures enable long circulation and targeted delivery for cancer therapy. New J. Chem. 2021, 45 (45), 21359–21368. 10.1039/D1NJ04407A. [DOI] [Google Scholar]
- Allcock H. R.; Morozowich N. L. Bioerodible polyphosphazenes and their medical potential. Polym. Chem. 2012, 3 (3), 578–590. 10.1039/C1PY00468A. [DOI] [Google Scholar]
- Onder A.; Ozay H. Synthesis and characterization of biodegradable and antioxidant phosphazene-tannic acid nanospheres and their utilization as drug carrier material. Mater. Sci. Eng., C 2021, 120, 111723. 10.1016/j.msec.2020.111723. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhou N.; Zhang N.; Zhi Z.; Shao Y.; Meng L.; Yu D. Facile preparation of pH/redox dual-responsive biodegradable polyphosphazene prodrugs for effective cancer chemotherapy. Colloids Surf., B 2021, 200, 111573. 10.1016/j.colsurfb.2021.111573. [DOI] [PubMed] [Google Scholar]
- Lakshmi S.; Katti D.; Laurencin C. Biodegradable polyphosphazenes for drug delivery applications. Adv. Drug Delivery Rev. 2003, 55 (4), 467–482. 10.1016/S0169-409X(03)00039-5. [DOI] [PubMed] [Google Scholar]
- Liu W.; Huang X.; Wei H.; Chen K.; Gao J.; Tang X. Facile preparation of hollow crosslinked polyphosphazene submicrospheres with mesoporous shells. J. Mater. Chem. 2011, 21 (34), 12964–12968. 10.1039/c1jm11802a. [DOI] [Google Scholar]
- Ozay H.; Ozay O. Synthesis and characterization of drug microspheres containing phosphazene for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 99–105. 10.1016/j.colsurfa.2014.03.022. [DOI] [Google Scholar]
- Andrianov A. K.; Mutwiri G. Intradermal immunization using coated microneedles containing an immunoadjuvant. Vaccine 2012, 30 (29), 4355–4360. 10.1016/j.vaccine.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Stone D. A.; Chang Y.; Allcock H. R. Control of the conjugation length and solubility in electroluminescent polymers. J. Polym. Sci. A Polym. Chem. 2006, 44 (1), 69–76. 10.1002/pola.21140. [DOI] [Google Scholar]
- Çiftçi G. Y.; Eker Y.; Şenkuytu E.; Yuksel F. Structural and fluorescence properties of the 2, 2′-methylenediphenoxy and 1, 1′-methylenedi-2-naphthoxy cyclotriphosphazene derivatives. J. Mol. Struct. 2016, 1117, 164–172. 10.1016/j.molstruc.2016.03.030. [DOI] [Google Scholar]
- Metinoğlu Örüm S. A one pot synthesis and characterization of intrinsically fluorescent cross-linked polyphosphazene nanospheres. Phosphorus Sulfur Silicon Relat. Elem. 2022, 1–9. 10.1080/10426507.2022.2071897. [DOI] [Google Scholar]
- Long H.; Kuang W.-C.; Wang S.-L.; Zhang J.-X.; Huang L.-H.; Xiong Y.-Q.; Qing P.; Cai X.; Tan S.-Z. Preparation and Antimicrobial Activity of Antibacterial Silver-Loaded Polyphosphazene Microspheres. J. Nanosci. Nanotechnol. 2021, 21 (10), 5120–5130. 10.1166/jnn.2021.19335. [DOI] [PubMed] [Google Scholar]
- Liu P.; Wang L.; Yang Y.; Qu Y.; Ming L.-J. Recent advances of cyclotriphosphazene derivatives as fluorescent dyes. Dyes Pigm. 2021, 188, 109214. 10.1016/j.dyepig.2021.109214. [DOI] [Google Scholar]
- Pan T.; Huang X.; Wei H.; Wei W.; Tang X. Intrinsically Fluorescent Microspheres with Superior Thermal Stability and Broad Ultraviolet-Visible Absorption Based on Hybrid Polyphosphazene Material. Macromol. Chem. Phys. 2012, 213 (15), 1590–1595. 10.1002/macp.201200099. [DOI] [Google Scholar]
- Chang F.; Huang X.; Wei H.; Chen K.; Shan C.; Tang X. Intrinsically fluorescent hollow spheres based on organic–inorganic hybrid polyphosphazene material: Synthesis and application in drug release. Mater. Lett. 2014, 125, 128–131. 10.1016/j.matlet.2014.03.137. [DOI] [Google Scholar]
- Ali S.; Zuhra Z.; Butler I. S.; Dar S. U.; Hameed M. U.; Wu D.; Zhang L.; Wu Z. High-throughput synthesis of cross-linked poly (cyclotriphosphazene-co-bis (aminomethyl) ferrocene) microspheres and their performance as a superparamagnetic, electrochemical, fluorescent and adsorbent material. Chem. Eng. J. 2017, 315, 448–458. 10.1016/j.cej.2017.01.049. [DOI] [Google Scholar]
- Wu P.; Yu S.; Liu H.; Zhang X.; Hou L.; Liu S.; Fu J. Lanthanum ion modification of aminated cyclomatrix polyphosphazene-coated porous carbon nanosheets for rapid, efficient and selective removal of phosphate. Appl. Surf. Sci. 2022, 593, 153359. 10.1016/j.apsusc.2022.153359. [DOI] [Google Scholar]
- Wang Y.; Yang N.; Soldatov M.; Liu H. A novel phosphazene-based amine-functionalized porous polymer with high adsorption ability for I2, dyes and heavy metal ions. React. Funct. Polym. 2022, 173, 105235. 10.1016/j.reactfunctpolym.2022.105235. [DOI] [Google Scholar]
- Sun X.; Li L.; Yang Y.; Jia C.; Zhang X.; Wu J.; Zhu Z.; Wang J.; Yang J. Flame-retardant effect of hyperbranched phosphazene-based microspheres in poly (L-lactic acid). J. Mater. Sci. 2022, 1–20. 10.1007/s10853-021-06719-y. [DOI] [Google Scholar]
- Jia Y.; Jin J.; Meng H. Zirconium dioxide@ phosphazene for enhancing mechanical property, flame retardancy, and thermal property of polythiourethane composites. J. Mater. Sci. 2022, 139 (22), 52230. 10.1002/app.52230. [DOI] [Google Scholar]
- Abbas M. A.; Bang J. H. Rising again: opportunities and challenges for platinum-free electrocatalysts. Chem. Mater. 2015, 27 (21), 7218–7235. 10.1021/acs.chemmater.5b03331. [DOI] [Google Scholar]
- Sekar P.; Murugesh N.; Shanmugam R.; Senthil Kumar S.; Agnoli S.; Chandran N.; Vedachalam S.; Karvembu R. Phosphazene-Based Covalent Organic Polymer Decorated with NiCo2O4 Nanocuboids as a Trifunctional Electrocatalyst: A Unique Replacement for the Conventional Electrocatalysts. ACS Appl. Energy Mater. 2021, 4 (9), 9341–9352. 10.1021/acsaem.1c01550. [DOI] [Google Scholar]
- Dhiman N.; Pradhan D.; Mohanty P. Heteroatom (N and P) enriched nanoporous carbon as an efficient electrocatalyst for hydrazine oxidation reaction. Fuel 2022, 314, 122722. 10.1016/j.fuel.2021.122722. [DOI] [Google Scholar]
- Mondol M. M. H.; Bhadra B. N.; Jhung S. H. Molybdenum nitride@ porous carbon, derived from phosphomolybdic acid loaded metal-azolate framework-6: A highly effective catalyst for oxidative desulfurization. Appl. Catal., B 2021, 288, 119988. 10.1016/j.apcatb.2021.119988. [DOI] [Google Scholar]
- Fu J.; Chen Z.; Xu Q.; Chen J.; Huang X.; Tang X. The production of porous carbon nanofibers from cross-linked polyphosphazene nanofibers. Carbon 2011, 49 (3), 1037–1039. 10.1016/j.carbon.2010.10.050. [DOI] [Google Scholar]
- Dufek E. J.; Stone M. L.; Jamison D. K.; Stewart F. F.; Gering K. L.; Petkovic L. M.; Wilson A. D.; Harrup M. K.; Rollins H. W. Hybrid phosphazene anodes for energy storage applications. J.Power Sources 2014, 267, 347–355. 10.1016/j.jpowsour.2014.05.105. [DOI] [Google Scholar]
- Yao Y.; Xu F.; Chen M.; Xu Z.; Zhu Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101 (9), 3040–3046. 10.1016/j.biortech.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Lima E. C.; Royer B.; Vaghetti J. C.; Simon N. M.; da Cunha B. M.; Pavan F. A.; Benvenutti E. V.; Cataluña-Veses R.; Airoldi C. Application of Brazilian pine-fruit shell as a biosorbent to removal of reactive red 194 textile dye from aqueous solution: kinetics and equilibrium study. J. Hazard. Mater. 2008, 155 (3), 536–550. 10.1016/j.jhazmat.2007.11.101. [DOI] [PubMed] [Google Scholar]
- Yu S.; Feng M.; Wu P.; Liu H.; Liu S.; Fu J. Polyethyleneimine-grafted polyphosphazene microspheres for rapid and efficient capture of anionic dyes from wastewater. Mater. Today Chem. 2021, 21, 100525. 10.1016/j.mtchem.2021.100525. [DOI] [Google Scholar]
- Thakur S.; Pandey S.; Arotiba O. A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. 10.1016/j.carbpol.2016.06.104. [DOI] [PubMed] [Google Scholar]
- Hızal J.; Kanmaz N.; Yılmazoğlu M. Adsorption efficiency of sulfonated poly (ether ether ketone)(sPEEK) as a novel low-cost polymeric adsorbent for cationic organic dyes removal from aqueous solution. J. Mol. Liq. 2021, 322, 114761. 10.1016/j.molliq.2020.114761. [DOI] [Google Scholar]
- Fu J.; Chen Z.; Wu X.; Wang M.; Wang X.; Zhang J.; Zhang J.; Xu Q. Hollow poly (cyclotriphosphazene-co-phloroglucinol) microspheres: An effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem. Eng. J. 2015, 281, 42–52. 10.1016/j.cej.2015.06.088. [DOI] [Google Scholar]
- Ahmad M.; Nawaz T.; Alam M. M.; Abbas Y.; Ali S.; Imran M.; Zhang S.; Wu Z. Effective Poly (Cyclotriphosphazene-Co-4, 4′-Sulfonyldiphenol)@ rGO Sheets for Tetracycline Adsorption: Fabrication, Characterization, Adsorption Kinetics and Thermodynamics. Nanomaterials 2021, 11 (6), 1540. 10.3390/nano11061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Zhang J.; Fu J.; Wang M.; Wang X.; Han R.; Xu Q. Adsorption of methylene blue onto poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) nanotubes: kinetics, isotherm and thermodynamics analysis. J. Hazard. Mater. 2014, 273, 263–271. 10.1016/j.jhazmat.2014.03.053. [DOI] [PubMed] [Google Scholar]
- Daniel M.-C.; Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104 (1), 293–346. 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Vasilyeva S. V.; Vorotyntsev M. A.; Bezverkhyy I.; Lesniewska E.; Heintz O.; Chassagnon R. Synthesis and characterization of palladium nanoparticle/polypyrrole composites. J. Phys. Chem. C 2008, 112 (50), 19878–19885. 10.1021/jp805423t. [DOI] [Google Scholar]
- Manocchi A. K.; Horelik N. E.; Lee B.; Yi H. Simple, readily controllable palladium nanoparticle formation on surface-assembled viral nanotemplates. Langmuir 2010, 26 (5), 3670–3677. 10.1021/la9031514. [DOI] [PubMed] [Google Scholar]
- Richards P. I.; Steiner A. Cyclophosphazenes as nodal ligands in coordination polymers. Inorg. Chem. 2004, 43 (9), 2810–2817. 10.1021/ic035455e. [DOI] [PubMed] [Google Scholar]
- Hu X.; Yu S.; Yang G.; Long W.; Guo T.; Tian J.; Liu M.; Li X.; Zhang X.; Wei Y. Facile synthesis of inorganic–organic hybrid fluorescent nanoparticles with AIE feature using hexachlorocyclotriphosphazene as the bridge. J. Mol. Liq. 2022, 345, 117693. 10.1016/j.molliq.2021.117693. [DOI] [Google Scholar]
- Ahmad M.; Nawaz T.; Assiri M. A.; Hussain R.; Hussain I.; Imran M.; Ali S.; Wu Z. Fabrication of Bimetallic Cu–Ag Nanoparticle-Decorated Poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) and Its Enhanced Catalytic Activity for the Reduction of 4-Nitrophenol. ACS Omega 2022, 7 (8), 7096–7102. 10.1021/acsomega.1c06786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Fu J.; Huang D.; Zhang C.; Xu Q. Silver nanoparticles-decorated polyphosphazene nanotubes: synthesis and applications. Nanoscale 2013, 5 (17), 7913–7919. 10.1039/c3nr00010a. [DOI] [PubMed] [Google Scholar]