Summary
Age is the strongest determinant of COVID-19 mortality, and over 2 billion people have received primary series vaccination with BNT162b2 (mRNA) or ChAdOx1 (adenoviral vector). However, the profile of sustained vaccine immunogenicity in older people is unknown. Here, we determine spike-specific humoral and cellular immunity to 8 months following BNT162b2 or ChAdOx1 in 245 people aged 80–98 years. Vaccines are strongly immunogenic, with antibodies retained in every donor, while titers fall to 23%–26% from peak. Peak immunity develops rapidly with standard interval BNT162b2, although antibody titers are enhanced 3.7-fold with extended interval. Neutralization of ancestral variants is superior following BNT162b2, while neutralization of Omicron is broadly negative. Conversely, cellular responses are stronger following ChAdOx1 and are retained to 33%–60% of peak with all vaccines. BNT162b2 and ChAdOx1 elicit strong, but differential, sustained immunogenicity in older people. These data provide a baseline to assess optimal booster regimen in this vulnerable age group.
Keywords: COVID-19, vaccines, elderly, primary series, BNT162b2, ChAdOx1
Graphical abstract

Highlights
-
•
Dual COVID-19 vaccination with BNT162b2 or ChAdOx1 is immunogenic in older people
-
•
Extending BNT162b2 dose interval increases antibody level but limits cellular response
-
•
BNT162b2 gives higher antibody level, and ChAdOx1 stimulates strong T cell response
-
•
Immune responses fall by 41%–76% at 8 months, less than reported in younger people
Dual “primary series” SARS-CoV-2 vaccination is the core of global vaccine protection. Parry et al. determine an 8-month profile of antibody and cellular immunity in people aged >80 years following mRNA or adenovirus vaccination. Both are immunogenic, but subtype and dose interval elicit differential immune platforms for booster vaccines.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is estimated to have led to the death of over 18 million people to date,1 and age is the strongest determinant of risk.2 Indeed, the median age of death within the first 6 months of the pandemic within the UK was 83 years.3 SARS-CoV-2 vaccines have transformed control of the COVID-19 pandemic and provide strong protection against severe disease and mortality.4, 5, 6 As such, there is considerable interest in the immunogenicity and clinical utility of vaccines in older population, and although older people were underrepresented within vaccine registration studies, a number of studies have demonstrated robust short term immunogenicity7 and are likely to underpin the strong clinical protection following vaccination observed in this group.8
Despite this, the longer-term immunogenicity of COVID-19 vaccines in this vulnerable population requires further investigation. In particular, there remains uncertainty regarding the potential importance of immune senescence on the magnitude and relative waning of vaccine responses in the very elderly.9 Initial reports indicate that spike-specific antibody titer can fall by over 18-fold from peak within 6 months,10 and such a rate of decline could be particularly important in those people with somewhat lower peak responses. Furthermore, clinical protection against vaccine breakthrough is likely to depend on the balance of humoral and cellular immunity, and while most studies have focused on antibody responses, it is now recognized that cellular immunity provides an essential component of immune protection.11 Vaccine subtypes employ differential mechanisms of delivery of the spike protein that are likely to influence the profile of spike-specific immune response, and previous studies early after the first12 and second dose7 have shown that mRNA vaccines elicit higher humoral immunity in older people, while adenoviral delivery can enhance cellular responses.13,14 A further influence on immunogenicity will relate to the time interval between the first and second doses of the dual vaccination. The BNT162b2 vaccine is generally delivered with a 3-week interval, but an extended 10- to 12-week interval has been used in some countries in order to maximize delivery of at least 1 vaccine across the population. This extended dose interval is also the standard approach for the ChAdOx1 primary series. Several reports have now shown that the extended interval regimen enhances mRNA-induced humoral immunity after the second dose, but this leaves a 10-week period of single-dose immunity during which low titer antibody responses are observed.15, 16, 17, 18 In contrast, the standard regimen induces a rapid immune response, although peak titers remain lower than observed following use of an extended interval regime.
Most COVID-19 vaccines comprise a two-dose primary series regimen, and although booster vaccines have been implemented in some settings, it remains critical to understand longer-term immunogenicity after the primary series, as this provides the platform on which boosters operate. Furthermore, booster vaccines have not been recommended in many countries, and compliance for acceptance of a third dose vaccine is far from complete. As such, primary series vaccination provides the platform for global immune protection. Here, we provide a detailed prospective assessment on spike-specific immune responses up to 8 months following 3 different COVID-19 vaccine regimens in older people and compare this profile with that seen at younger ages. Differential responses are seen in relation to vaccine subtype and dose interval, which provide insights that should help to guide future vaccine policy.
Results
Antibody responses are retained in all donors and titers increased 3.7-fold with extended interval mRNA vaccine
Blood samples were taken from three groups of older donors living independently in the UK who had undergone dual COVID-19 vaccination with the first vaccine given 8 months previously. All donors were seronegative at each sample time point for previous SARS-CoV-2 infection, as determined by anti-nucleocapsid response, and none had received third booster vaccines. There were no cases of breakthrough infection identified in the 8 months follow up following the first vaccine dose. Two cohorts had received homologous BNT162b2 mRNA vaccination with either a standard 3-week or extended 11-week interval between the two doses. The third had received homologous ChAdOx1 vaccination with an 11-week interval between doses (Figure 1A).
Figure 1.
Spike-specific antibody titers at 8 months are enhanced following extended interval BNT162b2 vaccine regimen
(A) Schematic representation of vaccine delivery within study groups. Three different cohorts of vaccinees were studied: 3-week, standard-interval BNT162b2 (sBNT162b2); 11-week, extended-interval BNT162b2 (eBNT162b2), or ChAdOx1 (11-week interval). Blood samples were taken at (1) 5 weeks post first vaccine (2 weeks after second dose for sBNT162b2 and 5 weeks after first dose for other two cohorts); (2) 13 weeks (10 weeks after second vaccine in sBNT162b2 cohort and 2 weeks after second dose for other two cohorts), and (3) 8 months.
(B) Dot plot of spike-specific antibody responses in three cohorts at 8 months after the first vaccination (sBNT162b2 [n = 66], eBNT162b2 [n = 62], and ChAdOx1 [n = 72]). Statistical analysis with Kruskal-Wallis test followed by multiple comparisons. (sBNT162b2 versus eBNT162b2 p < 0.0001 and eBNT162b2 versus ChAdOx1 p < 0.0001).
(C) Dot plot to show percentage of spike RBD-specific B cells as a proportion of the memory B cell population at 13–14 weeks post first dose of vaccine. Statistical analysis by Kruskal-Wallis test followed by multiple comparisons (sBNT162b2 0.7%, n = 20; eBNT162b2 0.6%, n = 19, and ChAdOx1 0.5%, n = 21).
Initial studies were undertaken 8 months following the first dose to determine spike-specific antibody titers within the three study groups using the Roche ELECCYS quantitative assay. Median values were 290 AU/mL for donors following standard-interval BNT162b2 (n = 66), 1,069 AU/mL for those after extended-interval BNT162b2 (n = 62), and 329 AU/mL following ChAdOx1 (n = 72) (Figure 1B). As such, antibody levels after extended-interval BNT162b2 were 3.7- and 3.2-fold higher than standard-interval BNT162b2 or ChAdOx1, respectively (p ≤ 0.0001).
The relative proportion of SARS-CoV-2 spike-specific B cells recognizing the spike receptor-binding domain (RBD) was then determined using a spike-tetramer flow cytometric assay on a subgroup of participants (19 on extended-interval BNT162b2, 20 on standard-interval BNT162b2, and 21 who received ChAdOx1). No difference was observed in age or gender distribution within these subgroups (p = 0.2). Median values of spike tetramer-specific B cells were 0.7%, 0.6%, and 0.5% across the vaccine regimens, values comparable to those seen after natural infection at this time point,19,20 with no difference seen between groups (Figure 1C).
These data indicate that antibody titers following the standard BNT162b2 and ChAdOx1 regimes are equivalent at 8 months after primary vaccination in older people. However, values are 3.2- to 3.7-fold higher when an extended-interval BNT162b2 protocol is employed.
Spike-specific neutralizing antibody activity at 8 months is superior following BNT162b2 mRNA vaccination
We next went on to assess the functional activity of spike-specific antibodies post-vaccination through measurement of neutralizing activity against ancestral and Omicron SARS-CoV-2 variants using HIV(SARS-CoV-2) pseudotypes. Reciprocal of serum dilution mediating 50% neutralization (ND50) titers against ancestral virus were 143 and 237 respectively following standard (n = 62) or extended-interval (n = 60) BNT162b2 vaccination. These compared to a value of 53 following ChAdOx1 (n = 73), indicating that neutralizing activity is relatively enhanced following mRNA vaccination (Figure 2A). Neutralization of Omicron was markedly impaired, with ND50 values <50 in the majority of donors, with no difference between vaccine cohorts.
Figure 2.
Viral neutralization activity within serum at 8 months post-vaccine is enhanced following BNT162b2 vaccination
(A) Neutralization of HIV(SARS-CoV-2) pseudotypes bearing ancestral or Omicron spike glycoproteins by vaccine sera. ND50 calculated as reciprocal of dilution at which infectivity reduced to 50%. Dot plot shows ND50 from the three cohorts at 8 months post-vaccine. The lower limit of detection was >ND50, and values on the ND50 line are considered negative. Statistical difference analyzed by Kruskal-Wallis test followed by multiple comparisons. (sBNT162b2 versus ChAdOx1 p = 0.0006 and eBNT162b2 versus ChAdOx1 p < 0.0001).
(B) Correlation between the spike-specific antibody titer and the pseudotype-derived ND50 against ancestral (red dot) or Omicron (black dot) between the three cohorts (ancestral: sBNT162b2 R = 0.48, p < 0.0001; eBNT162b2 R = 0.64, p ≤ 0.0001; ChAdOx1 R = 0.69, p < 0.0001; Omicron: sBNT162b2 R = −0.12, p = 0.41; eBNT162b2 R = 0.27, p = 0.07; ChAdOx1 R = 0.15, p = 0.31), with linear regression shown.
We further determined the relationship between antibody titer and neutralizing activity to assess the relative functional activity of antibodies following each vaccination regimen. As expected, these values were correlated in all cases, although neutralization was somewhat suppressed compared with the antibody titer following ChAdOx1 vaccination (Figure 2B).
Spike-specific cellular immune responses are enhanced following ChAdOx1 vaccination
An assessment of spike-specific cellular response following stimulation of peripheral blood mononuclear cells (PBMCs) with peptide pools from the spike protein of SARS-CoV-2 was next performed. The major focus was on interferon gamma (IFN-γ) release following peptide stimulation, and this was assessed by two platforms, ELISpot assay and plasma QuantiFERON concentration.
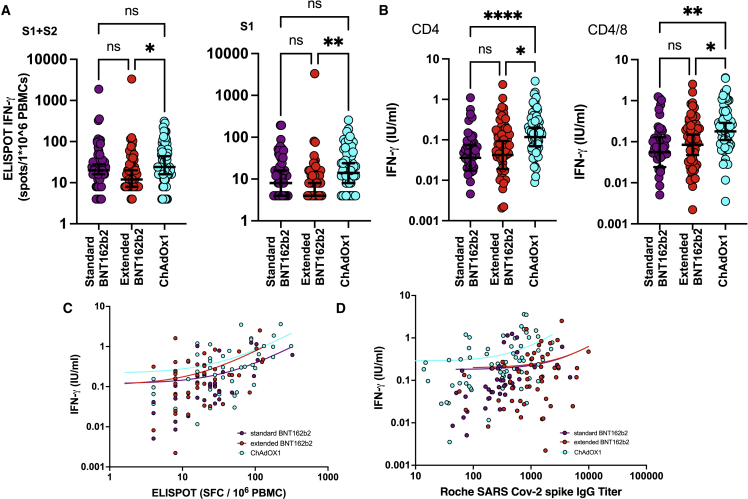
IFN-γ release was enhanced following ChAdOx1 vaccination in both assay systems. In particular, median ELISpot values following stimulation with peptides from the spike S1 domain were 14 spots/106 in ChAdOx1 vaccinees (n = 72) compared with 8/106 (n = 60) and 4/106 (n = 57), respectively, following standard-or extended-interval BNT162b2 vaccination (p = 0.6 and 0.0013, respectively) (Figure 3A). Comparable values following stimulation with combined S1 and S2 peptide pools were 24, 20, and 12 across these three cohorts, respectively (ChAdOx1 versus extended BNT162b2, p = 0.012) (Figures 3A and S1). These data show that ELISpot responses are comparable between ChAdOx1 and standard-interval BNT162b2 vaccines but that values after extended-interval BNT162b2 vaccination are lower than ChAdOx1.
Figure 3.
IFN-γ release following peptide stimulation is enhanced at 8 months following ChAdOx1 vaccination
(A) Dot plot of spike-specific cellular responses measured by IFN-γ ELISpot within the three cohorts (spot-forming units [SFUs] per 106 PBMCs). The left panel shows response following stimulation with total spike peptide pool (sBNT162b2 versus eBNT162b2 p = 0.12 and eBNT162b2 versus ChAdOx1 p = 0.01) and right panel shows response using spike S1 peptide pool (eBNT162b2 versus ChAdOx1 p = 0.001). Statistical analysis by Kruskal-Wallis test followed by multiple comparisons.
(B) Dot plot of spike-specific cellular responses measured by QuantiFERON within the three cohorts. Data are shown as plasma concentration of IFN-γ (IU/mL) following peptide stimulation. The left panel shows the response following stimulation with HLA class II-binding peptides from the RBD region (sBNT162b2 versus ChAdOx1 p < 0.0001 and eBNT162b2 versus ChAdOx1 p = 0.01) and right panel shows response following stimulation of HLA class I- and class II-binding peptides from whole spike protein (sBNT162b2 versus ChAdOx1 p = 0.003 and eBNT162b2 versus ChAdOx1 p = 0.03). Statistical difference analyzed with Kruskal-Wallis test followed by multiple comparisons.
(C) Correlation between ELISpot and QuantiFERON analysis following stimulation with using total spike peptide pool (sBNT162b2 R = 0.55, p = 0.0004; eBNT162b2 R = 0.53, p < 0.0001; ChAdOx1 R = 0.47, p = 0.0002) with linear regression line.
(D) Correlation between spike-specific antibody titer and IFN-γ concentration from CD4/8 QuantiFERON assay (sBNT162b2 R = 0.68, p < 0.0001; eBNT162b2 R = 0.31, p = 0.02; ChAdOx1 R = 0.46, p < 0.0001) with linear regression line.
A more marked influence of vaccine platform was seen with the QuantiFERON assay where IFN-γ plasma concentration following whole-blood stimulation with HLA class II-binding peptides from RBD was 0.16 IU/mL following ChAdOx1 vaccination (n = 69), a value 3.2- and 2-fold higher than following standard- or extended-interval BNT162b2 vaccination, respectively (0.05 [n = 43] and 0.08 IU/mL [n = 55]) (Figure 3B). Comparable values following stimulation with combined CD4+ and CD8+-stimulatory peptides across spike were 0.20, 0.09, and 0.12 IU/mL, respectively, indicating a 2.2- and 1.7-fold increase within the ChAdOx1 group (Figure 3B).
ELISpot values and QuantiFERON IFN-γ concentration were correlated with a trend toward higher relative IFN-γ production following QuantiFERON stimulation in recipients of ChAdOx1 vaccine (Figure 3C). Assessment of QuantiFERON IFN-γ production in relation to spike-specific antibody titer also revealed a correlation within each cohort (Figure 3D).
These observations indicate that the adenovirus-based spike vaccine delivery elicits stronger IFN-γ response following peptide stimulation in older people at 8 months following dual vaccination.
IL-2 release following spike peptide stimulation is increased following ChAdOx1 vaccination
SARS-CoV-2-spike-specific T cells are characterized by significant interleukin-2 (IL-2) production,21 with tumor necrosis factor (TNF) also produced early after natural infection.22 As such, we next went on to measure the concentration of IL-2 and TNF within QuantiFERON plasma samples following peptide stimulation. A LEGENDplex assay was used to measure IFN-γ, IL-2, and TNF concentrations within plasma samples, in the absence or presence of SARS-CoV-2 peptide stimulation, and these values were assessed as a ratio of increase following stimulation.
IFN-γ ratios correlated strongly with the absolute plasma concentrations and, again, were higher in samples from ChAdOx1 vaccinees. In particular, values were 3.1 and 4.3 following stimulation with CD4 (RBD only) or CD4/8 peptides (whole spike), respectively, compared with 1.5 and 1.6 or 1.3 and 2.5 in recipients of BNT162b2 with either a standard- or extended-interval regime, respectively (CD4: ChAdOx1 versus s-BNT162b2, p = 0.003; ChAdOx1 versus e-BNT162b2, p = 0.025; CD4/8: ChAdOx1 versus s-BNT162b2, p = 0.0055; ChAdOx1 versus e-BNT162b2, p = 0.4) (Figures 4A and 4B).
Figure 4.
IL-2 production from spike-stimulated cells is relatively enhanced at 8 months following ChAdOx1 compared with BNT162b2 vaccination
Legendplex technology was used to determine cytokine concentrations in plasma from QuantiFERON tubes following incubation in the absence or presence of spike peptides. The ratio of [cytokine] +peptide/−peptide is expressed on the y axis.
(A) The top panels show the assay using CD4 T cell-specific peptides from the RBD region (IFN-γ: sBNT162b2 vs ChAdOx1 p=0.003 and eBNT162b2 vs ChAdOx1 p=0.03; IL-2: sBNT162b2 vs ChAdOx1 p=0.0007 and eBNT162b2 vs ChAdOx1 p=0.03).
(B) Bottom panels show the assay using both CD4 T cell- and CD8 T cell-specific peptides from whole spike protein. The statistical difference was analyzed with Kruskal-Wallis test followed by multiple comparisons. (IFN-γ: sBNT162b2 versus ChAdOx1 p = 0.006; IL-2: sBNT162b2 versus ChAdOx1 p = 0.008 and eBNT162b2 versus ChAdOx1 p = 0.02).
IL-2 ratios were also markedly increased in recipients of the ChAdOx1 vaccine, with values of 6 and 6.8 following stimulation with CD4 or CD4/8 peptides, respectively, compared with 2.4 and 2.9 or 2 and 2.9 in recipients of BNT162b2 with either a standard- or extended-interval regime (CD4: ChAdOx1 versus s-BNT162b2, p = 0.0007; ChAdOx1 versus e-BNT162b2, p = 0.0026; CD4/8: ChAdOx1 versus s-BNT162b2, p = 0.0077; ChAdOx1 versus e-BNT162b2, p = 0.012) (Figures 4A and 4B). TNF values showed little increment following peptide stimulation and no variation in relation to the different vaccine regimens.
As such, the cellular response to spike peptide stimulation includes substantial IL-2 secretion at 8 months following each vaccine regimen, although this is enhanced following ChAdOX1.
Antibody responses are comparable to those seen in adults aged between 59 and 80, while cellular responses are lower following BNT162b2
The relative importance of immune senescence in relation to SARS-CoV-2-specific vaccine responses is critical for assessment of future vaccine policy. We next compared vaccine responses with two additional cohorts of 26 individuals aged between 70 and 73 years old for ChAdOx1 vaccine and 48 individuals aged between 59 and 75 years old for BNT162b2 vaccine (Table 1). Of note, donors aged <80 years who had received BNT162b2 with a standard 3-week interval were not available for study as this regimen had been discontinued in favor of an extended-interval regime for these younger age groups (Figure 5A).
Table 1.
Patient demographics
| BNT162b2, 3 week, >80 years | BNT162b2, 11 week, >80 years | ChAdOx1, >80 years | BNT162b2, 11 week, <80 years | ChAdOx1, <80 years | |
|---|---|---|---|---|---|
| Number | 87 | 73 | 85 | 33 | 53 |
| Median age | 83 | 84 | 83 | 63 | 72 |
| Age range | 80–96 | 80–97 | 80–98 | 42–78 | 56–79 |
| Age IQR | 81–87 | 82–88 | 81–86 | 55–75 | 70–73 |
| Male | 37 | 33 | 34 | 14 | 20 |
| Female | 50 | 40 | 51 | 19 | 33 |
Figure 5.
SARS-CoV-2-specific antibody responses in older donors are equivalent to younger people, but cellular responses are impaired following BNT162b2 vaccination
Dot plots of spike-specific antibody and cellular responses at 8 months post-vaccination in donors <80 years (blue) and >80 years (green).
(A) Spike-specific antibody titers (<80: n = 26 for BNT162, n = 48 for ChAdOx1; >80: n = 62 for BNT162b2, n = 72 for ChAdOx1) (for under 80, BNT162b2 versus ChAdOx1 p = 0.0006; for over 80, BNT162b2 versus ChAdOx1 p < 0.0001).
(B) Spike-specific cellular response by IFN-γ ELISpot (SFUs/106 PBMCs) (<80: n = 25 for BNT162, n = 40 for ChAdOx1; >80: n = 64 for BNT162b2, n = 72 for ChAdOx1) (for over 80, BNT162b2 versus ChAdOx1 p = 0.004; for BNT162b2, under 80 versus over 80 p = 0.013).
(C) Spike-specific cellular response by plasma IFN-γ concentration following peptide stimulation in QuantiFERON assay (<80: n = 8 for BNT162, n = 30 for ChAdOx1; >80: n = 54 for BNT162b2, n = 68 for ChAdOx1) (for over 80, BNT162b2 versus ChAdOx1 p = 0.03). Median age of cohorts (years): 69 (interquartile rate [IQR] 59–75) and 83 (IQR 82–88) for BNT162b2; 72 (IQR 70–73) and 83 (IQR 81–86) for ChAdOx1. Statistical difference was analyzed with Kruskal-Wallis test followed by multiple comparisons.
SARS-CoV-2-specific antibody titers were broadly equivalent in both cohorts at 8 months following extended-interval BNT162b2 vaccination. Values were also comparable following ChAdOx1 vaccination, although, as observed within donors aged over 80 years, antibody titers were lower than those seen after mRNA vaccination within both age groups.
Spike-specific cellular immune responses were next compared across the age groups using IFN-γ ELISpot and Quantiferon assays. ELISpot responses against spike S1 peptides pool following BNT162b2 vaccination were higher in donors aged <80 years compared with those >80 years (20 versus 4 spots/106, p = 0.01) (Figures 5B and 5C). A trend was also observed toward a lower Quantiferon response in the older donors, but as only 8 donors were available for analysis in the <80 years cohort, this was not conclusive. In contrast, cellular responses after ChAdOx1 were comparable within the two age groups.
These data indicate that there is no evidence for an impact of extreme immune senescence on antibody responses following SARS-CoV-2 vaccination within this cohort, although reduced levels of cellular response are observed following BNT162b2 vaccination.
A differential profile of humoral and cellular immunity is observed in relation to vaccine subtype and dose interval
In previous studies, we have reported on early antibody and cellular immunity following the first and second vaccine doses for participants in this study.23 As such, we combined these data with results at 8 months to determine the prospective profile of immune response following each vaccine platform (Figure 6).
Figure 6.
Profile of humoral and cellular immunity for 8 months following dual BNT162b2 or ChAdOx1 vaccination in donors aged >80 years
(A) Spike-specific antibody titer at three time points serially collected among participants. Median titer with 95% confidence intervals (s-BNT162b2: 1,195, 450, and 290; e-BNT162b2: 16, 4,170, and 1,069; ChAdOx1: 19, 1,410, and 329).
(B) Spike-specific cellular response at three time points. Median ELISPOT SFUs/106 PBMCs with 95% confidence intervals (s-BNT162b2: 70, 24, and 20; e-BNT162b2: 8, 20, and 12; ChAdOx1: 20, 44, and 24). Number of samples, antibody: standard BNT162 n = 85, 74, and 62 at respective time points, extended BNT162b2 n = 69, 55, and 62, ChAdOx1 n = 84, 74, and 72; cellular: standard BNT162b2 n = 87, 77, and 60, extended BNT162b2 n = 72, 64, and 57, ChAdOx1 n = 85, 74, and 72.
Standard 3-week-interval BNT162b2 vaccination produced an early rapid antibody rise, which then fell by 62% over 2 months before settling to a decline of 7.2% per month over the subsequent 5 months. As previously described, peak antibody responses are enhanced following extended interval BNT162b2 vaccination, and this increment was maintained over the following 5 months, with a decline of 15% per month. A comparable decline of 15% per month was also seen following ChAdOx1 vaccination.
A different profile was seen in relation to cellular responses where ELISpot analysis was available at each time point. Standard interval BNT162b2 vaccination induced a strong response at 70 spot-forming units (SFUs)/106, which then demonstrated a biphasic fall of 33% and 3.4% per month over the subsequent 2 and 5 months, respectively. Extended-interval BNT162b2 vaccination elicited a weaker peak cellular response after the second vaccine, with a subsequent decline of 8% per month, and a similar kinetic profile was observed after ChAdOx1 vaccination, with a decline of 9% per month, although median values were higher both at the early and late time points after the second vaccine.
These data indicate that the immunogenicity of COVID-19 vaccines in older people is influenced substantially by both vaccine subtype and the time interval between doses.
Discussion
Age is a strong determinant of clinical severity following SARS-CoV-2 infection,24 but this risk has been markedly reduced through the introduction of COVID-19 vaccines.25 Here, we undertook a detailed analysis of the vaccine immunogenicity in older people living in the community. This is arguably the most important demographic to study, as most COVID-19 deaths occur in elderly people and the great majority continue to live at home rather than in residential care. Furthermore, we studied BNT162b2 and ChAdOx1, two of the most widely utilized vaccines globally, which have been delivered to over 2 billion people to date. The results reveal a range of insights with importance for future global vaccine strategy.
Subjects were studied at 8 months after the first vaccine as this was the latest time point to assess responses following primary series vaccination, as a booster vaccine was recommended for older people at this point. Importantly, our analysis excluded people who had serological evidence of prior natural infection in order to assess true vaccine efficacy within a naive population. Interestingly, no subjects in any of the cohorts had evidence of breakthrough infection in the 8-month period following the first vaccine dose. This is likely to reflect vaccine efficacy and the “shielding” policy adopted by older people and recommended by the Department of Health in the UK during most of the study period. A striking feature was the impressive immunogenicity of both COVID-19 vaccines with SARS-CoV-2-specific antibody responses detected in every participant. Antibody titers were consistently higher following BNT162b2 vaccination compared with ChAdOx1,26,27 and mRNA delivery is known to generate intense humoral immune responses with prolonged local lymphadenopathy and germinal center formation.28 In addition, the BNT162b2 construct incorporates two proline residues that stabilize the spike protein in the pre-fusion conformation and may impact on the quality of immune response.29 Viral neutralization was also superior following mRNA vaccination. Spike-specific antibody levels and viral neutralization are emerging as correlates of immune protection,30,31 and these features are likely to underpin the impressive clinical efficacy of BNT162b2 in prevention of SARS-CoV-2 infection.
A further feature was that use of an extended time interval of 10–12 weeks between the two doses of BNT162b2 led to a sustained increase in the SARS-CoV-2-specific antibody titer, which was 3.7-fold higher at 8 months following vaccination. A comparable increase of 3.5-fold was observed at 2 weeks following the second dose,18 and as such, this enhancement is retained over the subsequent 5 months. Similar findings have been reported in younger adults at 4–8 weeks following the second vaccine,16,17 although the relative increment seems marked in older people. The immunological basis for this association is uncertain, but the sustained difference suggests that enhancement of spike-specific plasma cell generation is likely. Extended interval delivery might represent a viable option for delivery of the BNT162b2 vaccine,32 and an 8-week interval has recently been considered as potentially optimal for donors up to age 39 years in the United States.33 However, clinical efficacy has been excellent with the standard 3-week interval between doses,34 and T cell responses continued to be somewhat lower following an extended-interval regime, as seen at earlier time points.16,18 Furthermore, the standard interval also provides earlier peak antibody protection. Further research is now needed to assess how the extended-interval regime impacts on long-term humoral and cellular responses, and this work should extend beyond peripheral blood to study immunity at sites of viral replication such as mucosal surfaces within the respiratory tract.
A different pattern of immunogenicity was apparent in relation to spike-specific cellular responses, which showed relative enhancement following delivery of ChAdOx1 by several methods. Stronger cellular responses were seen previously at early time points following this vaccine,12 and here we show this is maintained up to 8 months. Median ELISpot responses were twice as high compared with the extended interval BNT162b2, although they were not significantly increased above standard BNT162b2 delivery. A similar finding was revealed in a review of vaccine immunogenicity,13 and the Ad26.COV2.S adenovirus-based vaccine also elicits particularly strong cellular responses.14 The Quantiferon SARS-CoV-2 assay35 was used for the first time in this cohort at the 8-month time point and also showed markedly increased IFN-γ release after peptide stimulation within ChAdOx1 vaccinees compared with both mRNA regimens.
QuantiFERON stimulation allows assessment of additional cytokine concentrations following peptide stimulation, and robust IL-2 responses were observed with each of the vaccine regimens, which augurs well for the generation and maintenance of spike-specific T cell memory. However, IL-2 production was >2-fold higher following ChAdOx1, indicating that production of both IFN-γ and IL-2 is enhanced following adenoviral-based vaccine delivery. The basis for this is uncertain, but the use of a viral vector may potentially enhance antigen presentation of spike peptides for cellular responses through infection of a fibroblast niche.36 In contrast to antibody neutralization, vaccine-induced cellular immunity is relatively preserved against SARS-CoV-2 variants,37 and recent data show excellent protection from severe Omicron infection in older donors who were primed with ChAdOx1 prior to an mRNA booster.38 Enhanced cellular responses following ChAdOx1 might be expected to translate into superior maintenance or affinity maturation of humoral responses,39 and a somewhat lower rate of spike-specific antibody decline following ChAdOx1 vaccination has been reported.26 However, this pattern was not observed within this cohort, and antibody neutralization activity was somewhat lower following this regimen.
The availability of samples at earlier time points from each participant allowed comparison of the kinetics of antibody and cellular responses between the three vaccine regimens. In recipients of standard-interval BNT162b2, we had access to a blood sample between the peak response and the 8-month assessment. This revealed an early rapid decline in antibody and cellular response over the first 2 months after the second vaccine, as seen in younger donors,10 before immune waning stabilized over the subsequent 5 months, with reductions of 7.2% and 3.4% per month in antibody titer and cellular response, respectively. The median antibody titer at 8 months was 24% of that seen at peak, a less marked reduction than reported in younger people,10,40,41 while cellular responses fell to 33% of peak value.
A notable feature was that the kinetics of antibody and cellular responses were almost identical following BNT162b2 or ChAdOx1 when an extended dose interval of 11 weeks was employed. The rates of antibody and cellular decline after peak response, at 15% and 8%–9% each month, respectively, were very similar, although differences were seen in absolute values. The lack of sampling in the 5-month period between peak response and 8-month assessment did not permit intermediate time point analysis of immune waning. However, median antibody titers at 8 months remained at 26% and 23% of those seen at peak for the extended BNT162b2 and ChAdOx1 regimes, respectively, similar to the standard BNT162b2 regime. Importantly, this decline over 8 months is substantially less marked than the 17- to 29-fold decline reported for donors aged 30–42 years after mRNA vaccination.14 Cellular responses were retained at 60% and 55% of peak value, respectively, and in keeping with increased stability of long-term T cell memory.
Participants had a median age of 85 years, and while vaccine immunogenicity was impressive, we were interested in comparing responses with those seen in adults aged <80 years. Antibody responses were entirely comparable with people of a median age of 67 years, showing no impact of extreme aging on the magnitude of the humoral response. However, we did not compare these values with younger adults, where several reports have observed a difference in antibody responses compared with older people.10,26,40
A somewhat different profile was seen in relation to cellular immunity where reduced IFN-γ responses were seen in older people who had received the BNT162b2 vaccine.42 In contrast, no age-related decline was observed following ChAdOx1, and this is noteworthy in relation to previous reports that antibody responses following adenoviral-based vaccines are also less influenced by factors such as age and underlying health conditions compared with mRNA regimens.26 It is important to note that the age distributions of these control cohorts varied (59–75 for BNT162b2 and 70–73 years for ChAdOx1) and as such may present potential bias in the interpretation of the vaccine responses observed.
In conclusion, we show that BNT162b2 and ChAdOx1 vaccines are strongly immunogenic in older people and show substantial retention of antibody and cellular responses over at least 8 months. However, differential features of humoral and cellular immune responses are observed, and it will be important to assess how these will translate into long-term protection or modulate immunogenicity following booster vaccination.43
Limitations of the study
Limitations include the fact that this was not a clinical trial but a real-world analysis of vaccine responses. Indeed, these donors were some of the first people in the world to receive vaccination as part of a national vaccine campaign. Demographic information on participants is limited but all are independently living. In addition, while antibody and ELISpot were recorded at three time points, the QuantiFERON assay was assessed only at the 8-month time point.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| FITC conjugated anti-IgD | Biolegend | Cat # 348206; RRID:AB_10612567 |
| PerCP5.5 conjugated anti-CD20 | Biolegend | Cat # 302326; RRID:AB_893283 |
| Pe-Cy7 conjugated anti-CD38 | Biolegend | Cat # 303516;RRID:AB_2072782 |
| AF700 conjugated anti-IgM | Biolegend | Cat # 314538;RRID:AB_2566615 |
| APC-Cy7 conjugated anti-CD27 | Biolegend | Cat # 356424;RRID:AB_2566773 |
| BC510 conjugated anti-CD19 | Biolegend | Cat # 302242;RRID:AB_2561668 |
| Live dye | Thermo Fisher | Cat # L34955 |
| PE Streptavidin | Biolegend | Cat # 405245 |
| APC Streptavidin | Biolegend | Cat # 405207 |
| Recombinant DNA | ||
| SARS-CoV-2 spike gene expression vectors | Willett et al., 2022 | N/A |
| lentiviral vectors p8.91 | Davis et al., 2021 | N/A |
| pCSFLW | Davis et al., 2021 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Biotinylated spike RBD protein | Biolegend | Cat # 793906 |
| Dulbecco’s Phosphate Buffered Saline | Sigma | Cat # D8537 |
| Fetal Bovine Serum | Sigma | Cat # F9665 |
| GlutaMax Supplement | Sigma | Cat # G7513 |
| RPMI | Sigma | Cat # R8758 |
| DMEM | Sigma | Cat # D5796 |
| Penicillin-Streptomycin | Sigma | Cat # P4333 |
| Spike peptide pool | JPT Peptide Technologies | Cat # PM-WCPV-S-2 |
| Critical commercial assays | ||
| Immunotec T-SPOT® SARS-CoV-2 assay | Oxford Immunotec Ltd | Cat # COV.435/300 |
| QuantiFERON SARS-CoV-2 interferon-γ release assay | Qiagen | Cat # 626715 |
| LEGENDplex™ COVID-19 Cytokine Storm Panel 1 | Biolegend | Cat # 741090 |
| Experimental models: Cell lines | ||
| HEK293 | ATCC | Cat # CRL-1573 |
| HEK293T | ATCC | Cat # CRL-3216 |
| 293-ACE2 | Willett et al., 2022 | N/A |
| Software and algorithms | ||
| GraphPad Prism | GraphPad | V9 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Professor Paul Moss (P.moss@bham.ac.uk).
Materials availability
All requests for resources and reagents should be directed to the lead contact author. This includes viruses. All reagents will be made available on request after completion of a Materials Transfer Agreement.
Experimental model and subject detail
Patients and samples
Participants aged 80 years and older, and who were living independently, were invited to participate in the study and recruited between 29th December 2020 and 4th March 2021. Co-morbidities were permitted. Local primary care networks identified vaccinees aged 80 years and older who had received either the BNT162b2 or ChAdOx1 vaccines and who were not living in a residential or care home or requiring assisted living. Participants were either invited to participate on attending the local vaccination centres or sent invitation letters to take part. Following initial contact with the study team, participants were then given the participant information sheet and consented verbally over the phone. This was substantiated with written consent obtained at the first phlebotomy time point. Ethical approval was obtained from North West Preston Research Ethics Committee with favourable outcome (REC 20\NW\0240) and work was performed under the CIA UPH and conducted according to the Declaration of Helsinki and good clinical practice.
Home phlebotomy was organised for all participants and sample collection determined by participant availability at each time point. Donors were tested after each of the time points for evidence of SARS-CoV-2 infection, as determined by anti-nucleocapsid response and excluded from analysis if positive. None had received 3rd booster vaccines.
After excluding donors with natural infection, 87 donors received BNT162b2 on a 3 week dosing interval (median age 83 years, range 80–96; IQR 81–87; 37 male ((43%)); 73 donors received BNT162b2 on an extended interval (median age 84, range 80–97; IQR 82–88; 33 male (45%)) and 85 received ChAdOx1 (median age 83 years, range 80–98, IQR 81–86; 34 were male (40%)). Controls aged <80 were also recruited following identification from primary care records and a letter invitation (33 donors received the BNT162b2 on an extended interval (median age 63, range 42–78; IQR 55–75. 14 male (42%)) and 53 received the ChAdOx1 (median age 72 range 56–79 IQR 70–73. 20 male donors (38%)) (Demographics in Table 1).
Cells and viruses
For pseudoneutralisation assays HEK293, HEK293T and 293-ACE2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 200 mM L-glutamine, 100 μg/mL streptomycin and 100 IU/mL penicillin. 293-ACE2 target cells were maintained in complete DMEM supplemented with 2 μg/mL puromycin. HEK293T cells were transfected with the appropriate SARS-CoV-2 spike gene expression vector in conjunction with lentiviral vectors p8.9144 and pCSFLW (Davis C et al., 2021) using polyethylenimine (PEI, Polysciences, Warrington, USA). HIV (SARS-CoV-2) pseudotype-containing supernatants were harvested 48 h post-transfection, aliquoted and frozen at −80°C prior to use. The delta construct bore the following mutations relative to the ancestral Hu-1 sequence (GenBank: MN908947): T19R, G142D, E156del, F157del, R158G, L452R, T478K, D614G, P681R, D950N.
Method details
Roche Elecsys® electrochemiluminescence immunoassay (ECLIA)
Total antibodies specific to SARS-CoV-2 were detected using electrochemiluminescence assays on the automated Roche cobas e801 analysers based at Public Health England (PHE) Porton. Calibration and quality control were performed as recommended by the manufacturer. Anti-nucleocapsid protein (NP) antibodies were detected using the qualitative Roche Elecsys® AntiSARS-CoV-2 ECLIA (COV2, Product code: 09203079190), whilst anti-spike (S) antibodies were detected using the quantitative Roche Elecsys® Anti-SARS-CoV-2 S ECLIA (COV2 S, Product code 09289275190. Anti-nucleocapsid results are expressed as cut-off index (COI) value, with a COI value of ≥1.0 considered positive for anti-nucleocapsid antibodies. Anti-spike results are expressed as units per mL (AU/mL), with samples with a result of ≥0.8 AU/mL considered positive for anti-spike antibodies within the fully quantitative range of the assay: 0.4–2,500 AU/mL. Samples >2,500 AU/mL were diluted further (1:100) to within the quantitative range.
Cellular assays
Peripheral blood mononuclear cells (PBMCs) were isolated from a whole blood sample using ‘T-Cell Xtend’ (Oxford Immunotec) and Ficoll. After quantification and dilution of recovered cells, 250,000 PBMC were plated into each well of a ‘T-SPOT Discovery SARS-CoV-2’ kit (Oxford Immunotec). This is designed to measure responses to overlapping peptides pools covering protein sequences of SARS-CoV-2 spike antigen. Negative control and PHA-stimulated cells as a positive control were included. Peptide sequences that showed high homology to endemic coronaviruses were removed from the sequences, but sequences that may have homology to SARS-CoV-1 were retained. Cells were incubated and interferon-γ secreting T cells were counted. The data was shown as spot forming units (SFU) per million PBMC.
Quantiferon assay
The T cell responses was also measured by quantiferon assay was carried out using the QuantiFERON SARS-CoV-2 assay (Qiagen). 1mL of whole blood was added to the test tubes, including QuantiFERON Nil, QFN SARS CoV-2 Ag1 and QFN SARS CoV-2 Ag2 tube. The first tube serves as negative control. The QFN SARS CoV-2 Ag1 and Ag2 tubes contain mixed epitopes either from Spike RBD region that are restricted through MHC-II or from whole spike that can stimulate both CD4 + and CD8 + T cells responses. After 18h incubation, plasma from all three test tubes are tested for IFN-γ using an enzyme-linked immunosorbent assay (ELISA)-based platform. The data was shown as concentration of IFN-γ in IU/mL after the deduction from the QFN-SARS-CoV-2 Nil tube.
Legendplex assay
The plasma sample from the Quantiferin Assay were assessed using a LEGENDplex™ COVID-19 Cytokine Storm Panel 1 (14-plex) (BioLegend) according to the manufacturer’s instructions. Briefly, the panel of capture beads are cocultured with the plasma samples before the biotinylated detection antibodies are added. At last the Streptavidin-phycoerythrin (SA-PE) is used to bind to the biotinylated detection antibodies. The samples were analysed flow cytometer and the concentration of the cytokine is determined according to the standard curve. Data were analysed with the LEGENDplex v.8.0 software. The data was shown as ratio of concentration of IFN-γ compare with that from the QFN-SARS-CoV-2 Nil tube.
B cell tetramer staining
To detect SARS-CoV-2 specific B cells, biotinylated spike RBD protein antigen (Biolegend) was multimerized with PE and APC labeled streptavidin. PBMC samples were stained with these two Spike tetramers before the surface antibodies were added. The data were acquired using Beckman Coulter Gallios Flow Cytometer and analysed with kaluza software.
The frequency of spike-specific memory B cells was expressed as a percentage of total memory B cells population.
Pseudotype-based neutralization assays
HEK293T cells were transfected with the SARS-CoV-2 S gene expression vector in conjunction with packaging lentiviral vectors to generate Human immunodeficiency virus (HIV) (SARS-CoV-2) pseudotype. Neutralizing activity in each sample was measured using serial dilution in triplicate from 1:50 to 1:36,450. After the incubation of HIV (SARS-CoV-2) pseudotypes with serum sample, they were plated onto 239-ACE2 target cells. After 48–72 h, luciferase activity was quantified on a PerkinElmer EnSight multimode plate reader. Antibody titer was then calculated by interpolating the point at which infectivity had been reduced to 90% of the value for the no serum control samples.
Quantification and statistical analysis
Data were tested for normality using Kolmogorov-Smirnov analysis. For comparative analysis of antibody titres and cellular responses between the cohorts, Kruskal-wallis with post hoc Dunn analysis was performed. The correlation was carried out using nonparametric Spearman’s rank-order correlation test. Antibody titres and T cell responses are presented as the median and IQR. 1. Statistical significance shown as: p < 0.05(∗), p < 0.01(∗∗), p < 0.001(∗∗∗) and p < 0.0001(∗∗∗∗). All analysis was performed using Graphpad prism v9.1.0 for Mac (San Diego, California USA).
Acknowledgments
We thank Dr. Rory Meade, Dr. Philip Saunders, Dr. Rosie Laugharne, Dr. Tom Groves, Dr. Parjit Dhillon, Dr. Eleanor Brodie, and the patients and staff at Harborne Medical Practice, Ridgacre House Surgery, Northumberland House Surgery, New Road Surgery, Lapal Medical Practice, and Wychbury Medical Group. We also thank Millie Manning, Danielle Sutherland, Tamsin Drury, and Alex Bray for their help in recruitment. We are grateful for support from Rajinder Jeet and Shahada Rahman Joli with phlebotomy services. The graphical abstract was created on BioRender. The work was funded by the National Core Studies Immunity award from UKRI and also the UK Coronavirus Immunology Consortium (UK-CIC). Additional support was from BBSRC (BB/R004250/1; BB/R019843/1) and MRC (MC_UU_12014/1).
Author contributions
H.P., R.B., and P.M. designed the study. P.M., H.P., and J.Z. wrote the manuscript, and H.P. and R.B. recruited participants. J.Z., C.S., R.A., P.S., N.L., S.S., S.N., K.V., and H.P. performed the experiments. H.P., B.W., G.M., J.Z., and P.M. verified and analyzed the data. All authors had full access to the data and approved submission of this report.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure that the study questionnaires were prepared in an inclusive way.
Published: August 25, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100739.
Supplemental information
Data and code availability
-
•
Data. All data (including raw data used to generate neutralizing and binding curves) reported in this paper will be shared by the lead contact upon request.
-
•
Code. This paper does not report original code.
-
•
Additional information. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. S0140-6736(21)02796–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Average age of death (median and mean) of persons whose death was due to COVID-19 or involved COVID-19, by sex, deaths registered up to week ending 2 October 2020, England and Wales - Office for National Statistics. (2020). https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/12376averageageofdeathmedianandmeanofpersonswhosedeathwasduetocovid19orinvolvedcovid19bysexdeathsregistereduptoweekending2october2020englandandwales.
- 4.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Otter A., Hallis B., Zuo J., Moss P. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun. Ageing. 2021;18:34. doi: 10.1186/s12979-021-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O’Doherty M., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann P., Curtis N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00084-18. e00084-18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 12.Parry H., Bruton R., Tut G., Ali M., Stephens C., Greenwood D., Faustini S., Hughes S., Huissoon A., Meade R., et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5-6 weeks post vaccine in participants aged 80 years or older: an exploratory analysis. Lancet. Healthy Longev. 2021;2:e554–e560. doi: 10.1016/S2666-7568(21)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier A.R.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N. Engl. J. Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tauzin A., Gong S.Y., Beaudoin-Bussières G., Vézina D., Gasser R., Nault L., Marchitto L., Benlarbi M., Chatterjee D., Nayrac M., et al. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30:97–109.e5. doi: 10.1016/j.chom.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne R.P., Longet S., Austin J.A., Skelly D.T., Dejnirattisai W., Adele S., Meardon N., Faustini S., Al-Taei S., Moore S.C., et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunau B., Goldfarb D.M., Asamoah-Boaheng M., Golding L., Kirkham T.L., Demers P.A., Lavoie P.M. Immunogenicity of extended mRNA SARS-CoV-2 vaccine dosing intervals. JAMA. 2022;327:279–281. doi: 10.1001/jama.2021.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Hallis B., Otter A., Zuo J., Moss P. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. npj Vaccines. 2021;7:1–5. doi: 10.1038/s41541-022-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan J.M., Mateus J., Kato Y., Hastie K.M., Faliti C.E., Ramirez S.I., Frazier A., Yu E.D., Grifoni A., Rawlings S.A., et al. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley G.E., Edwards E.S.J., Aui P.M., Varese N., Stojanovic S., McMahon J., Peleg A.Y., Boo I., Drummer H.E., Hogarth P.M., et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020;5:eabf8891. doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo J., Dowell A.C., Pearce H., Verma K., Long H.M., Begum J., Aiano F., Amin-Chowdhury Z., Hoschler K., Brooks T., et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4 + and CD8 + T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. Immunology. 2020 doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry H., Tut G., Bruton R., Faustini S., Stephens C., Saunders P., Bentley C., Hilyard K., Brown K., Amirthalingam G., et al. mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant. Elife. 2021;10:e69375. doi: 10.7554/eLife.69375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Driscoll M., Ribeiro Dos Santos G., Wang L., Cummings D.A.T., Azman A.S., Paireau J., Fontanet A., Cauchemez S., Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 25.Pastorino R., Pezzullo A.M., Villani L., Causio F.A., Axfors C., Contopoulos-Ioannidis D.G., Boccia S., Ioannidis J.P.A. Change in age distribution of COVID-19 deaths with the introduction of COVID-19 vaccination. Environ. Res. 2022;204:112342. doi: 10.1016/j.envres.2021.112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J., Pouwels K.B., Stoesser N., Matthews P.C., Diamond I., Studley R., Rourke E., Cook D., Bell J.I., Newton J.N., et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022;28:1072–1082. doi: 10.1038/s41591-022-01721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GeurtsvanKessel C.H., Geers D., Schmitz K.S., Mykytyn A.Z., Lamers M.M., Bogers S., Scherbeijn S., Gommers L., Sablerolles R.S.G., Nieuwkoop N.N., et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022;7:eabo2202. doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 30.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection (Infectious Diseases (except HIV/AIDS)) Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amirthalingam G., Bernal J.L., Andrews N.J., Whitaker H., Gower C., Stowe J., Tessier E., Subbarao V., Ireland G., Baawuah F., et al. Higher serological responses and increased vaccine effectiveness demonstrate the value of extended vaccine schedules in combatting COVID-19 in England. medRxiv. 2021 doi: 10.1101/2021.07.26.21261140. Preprint at. [DOI] [Google Scholar]
- 33.Interim clinical considerations for use of COVID-19 vaccines | CDC (2022). https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html.
- 34.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaganathan S., Stieber F., Rao S.N., Nikolayevskyy V., Manissero D., Allen N., Boyle J., Howard J. Preliminary evaluation of QuantiFERON SARS-CoV-2 and QIAreach anti-SARS-CoV-2 total test in recently vaccinated individuals. Infect. Dis. Ther. 2021;10:2765–2776. doi: 10.1007/s40121-021-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cupovic J., Ring S.S., Onder L., Colston J.M., Lütge M., Cheng H.-W., De Martin A., Provine N.M., Flatz L., Oxenius A., et al. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8+ T cells. Nat. Immunol. 2021;22:1042–1051. doi: 10.1038/s41590-021-00969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Chandrashekar A., Sellers D., Barrett J., Jacob-Dolan C., Lifton M., McMahan K., Sciacca M., VanWyk H., Wu C., et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022;603:493–496. doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stowe J., Andrews N., Kirsebom F., Mbbs M.R., Bernal J.L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation: test negative case-control study. medRxiv. 2022 doi: 10.1101/2022.04.01.22273281. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hipp N., Symington H., Pastoret C., Caron G., Monvoisin C., Tarte K., Fest T., Delaloy C. IL-2 imprints human naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. Nat. Commun. 2017;8:1443. doi: 10.1038/s41467-017-01475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., Haljasmägi L., Rumm A.P., Maruste R., Kärner J., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg. Health. Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., et al. Antibody persistence through 6 Months after the second dose of mRNA-1273 vaccine for covid-19. N. Engl. J. Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demaret J., Corroyer-Simovic B., Alidjinou E.K., Goffard A., Trauet J., Miczek S., Vuotto F., Dendooven A., Huvent-Grelle D., Podvin J., et al. Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front. Immunol. 2021;12:778679. doi: 10.3389/fimmu.2021.778679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., Bula M., Cathie K., Chatterjee K., Dodd K., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis C., Logan N., Tyson G., Orton R., Harvey W.T., Perkins J.S., Mollett G., Blacow R.M., Barclay W.S., et al. COVID-19 Genomics UK COG-UK Consortium Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;17:e1010022. doi: 10.1371/journal.ppat.1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data. All data (including raw data used to generate neutralizing and binding curves) reported in this paper will be shared by the lead contact upon request.
-
•
Code. This paper does not report original code.
-
•
Additional information. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.