Abstract
As a reference laboratory for measles and rubella surveillance in Lombardy, we evaluated the association between SARS-CoV-2 infection and measles-like syndromes, providing preliminary evidence for undetected early circulation of SARS-CoV-2. Overall, 435 samples from 156 cases were investigated. RNA from oropharyngeal swabs (N = 148) and urine (N = 141) was screened with four hemi-nested PCRs and molecular evidence for SARS-CoV-2 infection was found in 13 subjects. Two of the positive patients were from the pandemic period (2/12, 16.7%, March 2020–March 2021) and 11 were from the pre-pandemic period (11/44, 25%, August 2019–February 2020). Sera (N = 146) were tested for anti-SARS-CoV-2 IgG, IgM, and IgA antibodies. Five of the RNA-positive individuals also had detectable anti-SARS-CoV-2 antibodies. No strong evidence of infection was found in samples collected between August 2018 and July 2019 from 100 patients. The earliest sample with evidence of SARS-CoV-2 RNA was from September 12, 2019, and the positive patient was also positive for anti-SARS-CoV-2 antibodies (IgG and IgM). Mutations typical of B.1 strains previously reported to have emerged in January 2020 (C3037T, C14408T, and A23403G), were identified in samples collected as early as October 2019 in Lombardy. One of these mutations (C14408T) was also identified among sequences downloaded from public databases that were obtained by others from samples collected in Brazil in November 2019. We conclude that a SARS-CoV-2 progenitor capable of producing a measles-like syndrome may have emerged in late June-late July 2019 and that viruses with mutations characterizing B.1 strain may have been spreading globally before the first Wuhan outbreak. Our findings should be complemented by high-throughput sequencing to obtain additional sequence information. We highlight the importance of retrospective surveillance studies in understanding the early dynamics of COVID-19 spread and we encourage other groups to perform retrospective investigations to seek confirmatory proofs of early SARS-CoV-2 circulation.
Keywords: SARS-CoV-2, COVID-19, Pandemic, Emergence, Measles, Rash
1. Introduction
The betacoronavirus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) was first isolated in China in late December 2019 in samples collected from hospitalized patients with atypical pneumonia (Huang et al., 2020; Zhu et al., 2020; WHO, 2020; Gorbalenya et al., 2020; Singh et al., 2021). Molecular evolutionary analyses suggest that SARS-CoV-2 emerged as a capable human pathogen, likely from a bat reservoir, although the mechanism of the original spillover(s) is a subject of ongoing debate. Although the timeline of SARS-CoV-2 emergence has not yet been firmly established, evolutionary analyses predicted that the virus likely circulated in China for some time, even months, before the first recorded December outbreak in Wuhan (Andersen et al., 2020; Leitner and Kumar, 2020; MacLean et al., 2021; Kumar et al., 2021; Xia, 2021; Chiara et al., 2021).
By mid-January 2020, cases outside of Asia were recorded in North America and Europe (Worobey et al., 2020). Italy was the first European country reporting sustained community transmission of SARS-CoV-2, following the first identification of a non-travel related case in Codogno, Lombardy, on February 20, 2020. Italy quickly became the epicenter of the European epidemic, with Lombardy being the most affected region (Grasselli et al., 2020; Carletti and Pancrazi, 2021; Tosi and Chiappa, 2021; Odone et al., 2020; Onder et al., 2020; ISTAT ISS, 2021). The viral strain that dominated the Lombardy outbreak and that subsequently spread across Europe and beyond differs from the reference genome (Wuhan-Hu-1 strain) by the simultaneous presence of several mutations, including C3037T (synonymous), C14408T (RdRp P323L), and A23403G (Spike D614G) (Rambaut et al., 2020; Hadfield et al., 2018; Alteri et al., 2021; Hodcroft et al., 2021). This strain is now classified as 20 A in NextStrain (Hadfield et al., 2018) and B.1 in Pangolin (Rambaut et al., 2020), has an αβ mutational signature (Kumar et al., 2021), and is also referred to as DG1111 haplotype (Ruan et al., 2021).
Several lines of evidence suggest that SARS-CoV-2 had already been spreading unnoticed for some time prior to being recognized. In fact, SARS-CoV-2 RNA was detected in wastewaters in Northern Italy in late December 2019 (La Rosa et al., 2021) and in Brazil in November 2019 (Fongaro et al., 2021), and there is some indirect evidence of 2019 infections through antibody detection in France (Carrat et al., 2021), Italy (Apolone et al., 2020; Montomoli et al., 2022), UK (Ng et al., 2020), the USA (Basavaraju et al., 2021), and Angola (Paixao et al., 2022). In addition, SARS-CoV-2 RNA was detected as early as December 2019 in a respiratory sample from a French patient hospitalized for haemoptysis (Deslandes et al., 2020), in the oropharyngeal swab from a child from Milan (Lombardy) with suspected measles (Amendola et al., 2021), and in the blood and lungs of a patients that died in Milan because of acute circulatory insufficiency (Lai et al., 2022). Finally, viral antigens and RNA were detected in paraffin-preserved skin biopsies of a woman with dermatosis in November 2019 in Milan (Gianotti et al., 2021).
A variety of skin manifestations have been reported in patients with SARS-CoV-2 infection, as also frequently observed in Lombardy (Recalcati, 2020; Marzano et al., 2021; Colonna et al., 2020). These manifestations, which pose diagnostic difficulties in the context of COVID-19 (Visconti et al., 2021), may appear at any disease stage, although they are often characterized by late-onset, and have variable duration, severity, and prognosis (Freeman et al., 2021; Visconti et al., 2021; Giavedoni et al., 2020; Gisondi et al., 2020). Skin manifestations can also appear in the absence of respiratory symptoms (Visconti et al., 2021), posing a diagnostic challenge since respiratory swabs from affected patients can be RNA-negative when cutaneous manifestations appear (Giavedoni et al., 2020; Le Cleach et al., 2020; Frumholtz et al., 2021).
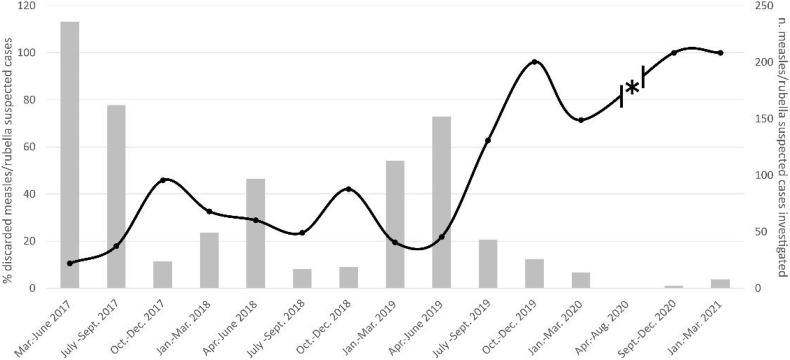
As a MoRoNet (Network of Italian Reference Laboratories for Measles and Rubella)-accredited and WHO-accredited Subnational Reference Laboratory for measles and rubella surveillance in Lombardy, we receive every year oropharyngeal swabs and/or urine from patients who present with morbilliform rash to perform molecular and serological diagnostics (Magurano et al., 2020; Bianchi et al., 2022). We monitor the metropolitan Milan area and its surroundings, a densely populated area of about 4 million inhabitants, as well as other Lombardy provinces, i.e., Brescia, Varese, Como, and Monza-Brianza. We also tabulate annual test performance indicators, including the rate of discarded non-measles and non-rubella cases, expressed as the number of non-measles/non-rubella cases in a year divided by the average population in the study area. In 2019, this rate was more than twice as high as the average of the previous two years (3.00 per 100,000 vs. 1.25 per 100,000) (Bianchi et al., 2019) and, since the summer of 2019, the percentage of suspected measles and rubella cases that tested negative for those diseases steadily increased from about 30% to 70% at the start of the first pandemic wave, and to 100% in 2021 (Fig. 1 ), suggesting a systematic etiological change for these manifestations.
Fig. 1.
Measles and rubella epidemiological trends observed in our laboratory from 2017 (start of surveillance activity) to 2021. The number of measles/rubella suspected cases investigated is indicated by grey bars (right axis), and the percentage of discarded measles/rubella suspected cases (suspected cases with negative diagnostic) is shown by the black line (left axis). The asterisk marks the period of interruption of surveillance activities due to lockdown measures.
These observations prompted us to investigate whether SARS-CoV-2 infection could be involved in morbilliform clinical manifestations, thus explaining an epidemiological trend that began several months before the first known COVID-19 cases observed in Italy. This study expands on our previous report (Amendola et al., 2021) and describes the results of the surveillance conducted on samples collected over two and a half years, beginning more than a year prior to the start of the COVID-19 pandemic. We simultaneously investigate the association between SARS-CoV-2 and measles-like syndromes and provide further evidence for undetected early circulation of SARS-CoV-2 in Lombardy.
2. Materials and methods
2.1. Specimens
All samples from measles/rubella negative patients (discarded cases) received within the measles and rubella surveillance framework since August 2018 were the focus of our investigation. Overall, 435 samples (148 oropharyngeal swabs, 141 urine samples, and 146 serum samples) collected from 156 cases were investigated for SARS-CoV-2 infection. These included samples from 44 cases collected between August 2019 (when the unexpected increase in discarded measles-rubella cases was first noted) and the beginning of the pandemic in late February 2020 (pre-pandemic cases), samples from 12 cases collected between March 2020 and March 2021 (pandemic cases), and samples from 100 cases collected between August 2018 and July 2019 (control cohort). The small number of samples received during the pandemic period was due to lockdown measures that impacted measles surveillance activities throughout Europe (Nicolay et al., 2020). Since all samples were received from hospitals, patients from the pandemic period had already tested negative for SARS-CoV-2 with standard diagnostic protocols (Real Time PCR on nasal swab samples) performed by the admitting hospitals. Samples included oropharyngeal swabs (Copan, Copan Italia SPA, Italy) submerged in 3 ml universal transport medium (UTM), urine, and sera, all stored at −80 °C in the biobank of the Laboratory at the University of Milan. These samples were collected when the first tentative diagnosis of measles or rubella had been made, which is when the skin manifestations became evident, even in absence of respiratory symptoms. Patient characteristics are summarized in Table 1 .
Table 1.
Demographic characteristics of the patients included in this study.
| Control | Pre-pandemic | Pandemic | |
|---|---|---|---|
| N. (M, F) | 100 (54, 46) | 44 (22, 22) | 12 (5, 7) |
| 0–1 years N (%) | 27 (27.0) | 7 (15.9) | 2 (16.7) |
| 2–4 years N (%) | 7 (7.0) | 4 (9.1) | 2 (16.7) |
| 5–14 years N (%) | 6 (6.0) | 5 (11.4) | 1 (8.3) |
| 15–39 years N (%) | 33 (33.0) | 19 (43.2) | 2 (16.7) |
| 40–64 years N (%) | 18 (18.0) | 8 (18.2) | 4 (33.3) |
| >65 years N (%) | 9 (9.0) | 1 (2.3) | 1 (8.3) |
| Average age | 24.6 | 23.8 | 29.4 |
| Median age | 19 | 23 | 32 |
| Vaccination status N. (yes, no)a | 81 (38, 43) | 34 (19, 15) | 9 (5, 4) |
This was known only for a subset of patients. Yes: 1 or 2 measle vaccination doses; No: no measles vaccination.
Ethical review and approval were waived, as the study was carried out as part of the Integrated Measles-Rubella Surveillance, performed by law in accordance with the Prime Minister's Decree of 3 March 2017 (https://www.gazzettaufficiale.it/eli/id/2017/05/12/17A03142/sg). The study was conducted in the absence of consent following the declaration of a state of emergency caused by the SARS-CoV-2 pandemic, in accordance with letter (i), article 9 of the GDPR (https://eurlex.europa.eu/legal-content/IT/TXT/HTML/?uri=OJ:L:2016:119:FULL&from=FI).
2.2. Molecular testing and sequence analysis
In total, 289 samples (all oropharyngeal swabs and urine samples) were tested for the presence of SARS-CoV-2 RNA. When not already available from previous surveillance activities, RNA was freshly isolated. RNA isolation was performed with the NucliSENS® easyMAG™ automated system (bioMérieux bv, Lyon, France) from 1 ml of UTM and/or using pellet obtained from 5 to 15 ml of urine as input, and cDNA was synthesized using SuperScript™ II Reverse Transcriptase (ThermoFisher, Waltham, Massachusetts) with the reverse primer (final concentration of 20 μM), according to the manufacturer's instructions. All 289 samples were screened with three different hemi-nested PCRs designed to amplify genomic fragments within the non-structural protein 3 (NsP3), the RNA-dependent RNA-polymerase (RdRp), and the spike protein (Spike A) spanning regions that are known to contain some of the key mutations and the furin cleavage site. Furthermore, 252 samples collected since January 2019 were screened with a fourth hemi-nested PCR targeting another fragment of the Spike gene (Spike B) (Table 2 ). PCRs were performed with the DreamTaq DNA polymerase (ThermoFisher, Waltham, Massachusetts) using 5 μl of cDNA. Amplicons from positive samples were purified with the NucleoSpin Gel and PCR Clean-Up kit (Macherey-Nagel GmbH & Co. KG, Germany) and outsourced for Sanger sequencing. Isolated RNA was also tested for SARS-CoV-2 by Real-Time PCR according to the diagnostic protocol of the CDC (CDC, 2020). Each step was performed in physically separated laboratories and a negative control (H2O) and a positive control (RNA isolated from a SARS-CoV-2-positive patient) were included in each amplification round to validate the tests.
Table 2.
Primers used for the molecular detection of SARS-CoV-2 RNA.
| Target Gene | Primer Name (orientation) | Nucleotide sequence 5′-3′ | Taa (°C) | Amplicon size | Positionb nucleotide (amino acid) | Mutation or Motifc | Reference |
|---|---|---|---|---|---|---|---|
| NsP3 | C3037T F1/2 (+) | TTGATTTAGATGAGTGGAGTATGGCTAC | 60 | 263 bp | 2949-3212 (76–164) | C3037T (F106F) | This study |
| C3037T R1 (−) | GTCTGAACAACTGGTGTAAGTTCC | ||||||
| C3037T R2 (−) | CATCATCTAACCAATCTTCTTCTTGCT | ||||||
| RdRp | C14408T F1/2 (+) | TTTGGATGACAGATGCATTCTGC | 58 | 335 bp | 14343-14678 (300–412) | C14408T (P323L) | This study |
| C14408T R1 (−) | GATAGTAGTCATAATCGCTGATAGCAG | ||||||
| C14408T R2 (−) | CCGGGTTTGACAGTTTGAAAAGC | ||||||
| Spike A | A23403G F1 (+) | TTCAACTTCAATGGTTTAACAGGCA | 60 | 421 bp | 23288-23709 (575–715) | A23403G (D614G) FCSd |
This study |
| A23403G F2 (+) | GTCCGTGATCCACAGACACTTG | ||||||
| A23403G R1/2 (−) | GTGGGTATGGCAATAGAGTTATTAGAGT | ||||||
| Spike B | OUT-F (+) | AGGCTGCGTTATAGCTTGGA | 55 | 470 bp | 22882-23352 (440–596) | T22917G (L452R), G23012A (E484K), G23012C (E484Q), A23063T (N501Y), C23271A (A570D) | Amendola et al. (2021) |
| MaSi_AR (−) | ACACTGACACCACCAAAAGAAC | ||||||
| SiMa_BF (+) | TCTTGATTCTAAGGTTGGTGGT |
Annealing temperature.
Referred to Wuhan-Hu-1 (NC_045512.2).
Amino acid mutations are indicated in parenthesis.
FCS: furin cleavage site (amino acids 680–689).
Obtained sequences (GenBank accession numbers MZ223385 - MZ223398) were compared to the reference strain Wuhan-Hu-1 (NC_045512.2) and to sequences previously obtained from human sewage samples collected in Brazil in November 2019 (Fongaro et al., 2021). Illumina sequence reads (PRJNA679821) were downloaded from GenBank, adapters were removed with BBDuk, and remaining reads were mapped to Wuhan-Hu-1 using Geneious R11 (Biomatters). Mutations with respect to the reference strain were only recorded if simultaneously present in both the forward and reverse reads. Identified mutations were classified using a recently developed mutation order analysis approach (Kumar et al., 2021), and the time of emergence of the SARS-CoV-2 progenitor (proCoV2) was approximated using a previously calibrated molecular clock (Pekar et al., 2021).
2.3. Serological testing
For serological investigations, 146 sera (98 control cases, 38 pre-pandemic cases, and 10 pandemic cases) were tested for anti-SARS-CoV-2 IgG, IgM, and IgA using the semi-quantitative Anti-SARS-CoV-2 ELISA (Euroimmun, Lübeck, Germany) tests, according to manufacturer's instructions.
Positive sera were then sent to the Italian National Institute of Health (ISS) to confirm the results and quantify the titer of neutralizing antibodies by SARS-CoV-2 plaque reduction neutralization test (serum dilution causing 80% plaque reduction, PRNT80) conducted as previously described (Magurano et al., 2021).
3. Results
3.1. SARS-CoV-2 infection detection
Molecular evidence for SARS-CoV-2 infection was found in 13 subjects, with a positivity rate of 16.7% (2/12) for the pandemic cases and 25% (11/44) for the pre-pandemic cases (Table 3 ). For six of the 13 patients (46.2%) viral RNA was found in urine, while for the remaining seven patients (53.8%) the virus was found in respiratory material. The virus was found only in the urine of patients from the pandemic period (2021), consistent with the negativity of respiratory samples reported by the hospitals that performed the initial SARS-CoV-2 screening at the time of sample collection. Samples from nine of the 11 patients of the pre-pandemic period were collected in 2019. The first sample that tested positive for SARS-CoV-2 RNA was a urine sample collected as early as September 12th, 2019, from an 8-months old child whose serum was also IgG and IgM positive (Table 3). Samples became positive after nested PCRs, and none of the samples tested positive with the Real-Time PCR diagnostic protocol. This suggests a low viral load at the detection threshold. None of the 191 swab and urine samples collected before September 2019 (183 samples from the control cohort and 8 samples collected in August 2019) as well as none of the negative controls turned positive with our molecular methods. Additionally, it never occurred that two samples processed next to one-another, including those next to positive controls, tested positive.
Table 3.
Details of identified positive casesa.
| Patient ID | Area | Collection date | Delayb | Age (yr.) | Sex | RT PCR | NsP3c | RdRpc | Spike Ac | Spike Bc | IgMd | IgAd | IgGd | PRNT 80%e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pandemic cases | ||||||||||||||
| 1 | Milan | 12/09/2019 | 3 | 0.67 | M | – | – | – | – | + (U) | + | - | + | – |
| 2 | East Milan | 12/10/2019 | 0 | 18 | F | – | – | – | – | + (S) | – | – | – | – |
| 3 | Brescia | 17/10/2019 | 1 | 9 | F | – | – | + (U) | – | – | – | – | – | – |
| 4 | North-West Milan | 19/10/2019 | 1 | 1 | M | – | – | + (S) | – | – | – | + | + | – |
| 5 | Brescia | 22/10/2019 | 1 | 1 | F | – | + (S) | – | – | – | + | – | – | -f |
| 6 | East Milan | 23/10/2019 | NA | 1 | F | – | – | + (S) | – | – | + | – | – | – |
| 7 | North-West Milan | 22/11/2019 | 7 | 2 | M | – | + (S) | – | – | – | NA | NA | NA | NA |
| 8 | West Milan | 05/12/2019 | 4 | 4 | M | – | – | – | – | + (S) | NA | NA | NA | NA |
| 9 | South-East Milan | 15/12/2019 | 2 | 25 | F | - | - | + (U) | + (U) | + (U) | – | – | – | – |
| 10 | Milan | 09/01/2020 | 1 | 53 | M | – | – | – | – | + (U) | – | – | – | – |
| 11 | Brescia | 14/01/2020 | 4 | 40 | M | – | – | – | – | + (S) | – | – | – | NA |
| Pandemic cases | ||||||||||||||
| 12 | North-West Milan | 15/01/2021 | 1 | 65 | F | – | – | – | – | – | – | + | + | 1:60 |
| 13 | Lodi | 16/01/2021 | 9 | 32 | F | – | + (U) | – | + (U) | – | + | - | - | – |
| 14 | North Milan | 25/01/2021 | 4 | 1 | F | – | – | – | – | – | + | + | + | 1:160 |
| 15 | Milan | 26/02/2021 | 5 | 47 | M | – | – | – | – | + (U) | - | - | - | – |
Only patients that tested positive in PCR or that presented neutralizing antibodies are included.
Days from exanthema onset to sample collection.
U: urine sample was positive,S: oropharyngeal swab was positive.
NA: serum/data not available.
PRNT: plaque-reduction neutralization test.
This serum caused 62% plaque reduction at a 1:10 dilution.
Four of the eleven SARS-CoV-2 RNA-positive patients from the pre-pandemic period tested positive for anti-SARS-CoV-2 antibodies, with IgM being the most frequently detected antibody class. However, only one of these sera contained partially neutralizing antibodies (causing 62% plaque reduction at a dilution of 1:10). Additionally, neutralizing antibodies were detected in two subjects who had been sick during the pandemic period, and both were SARS-CoV-2 RNA-negative. Interestingly, independently from the date of sample collection, we detected antibodies in all five positive children aged one year or younger, while only two out of eight patients older than one year had positive ELISA results and they were both patients from the pandemic period (Table 3).
Twelve samples collected before the first sample in which we detected viral RNA (October 2018–September 11, 2019) showed IgM (N = 2), IgA (N = 8), or IgG (N = 2) positivity alone. Since no molecular evidence for SARS-CoV-2 was found and only partial neutralization was observed for four of these samples (50–59% plaque reduction at a dilution of 1:10), we concluded that the evidence of current infection was weak and considered the diagnosis for these patients inconclusive. A similar conclusion was made for two other patients who presented symptoms during the pre-pandemic period and for other two who were sick during the pandemic period (Supplementary Table S1). Results obtained for pre-pandemic and pandemic cases were comparable, since each patient presented a unique pattern of positivity, with none showing a positive result for every test performed (Table 3).
None of the positive patients reported any history of travel abroad in the two weeks prior to the onset of rash and there were no known epidemiological connections between patients. The first pre-pandemic cases were mainly localized east of Milan and Brescia (September–October 2019), while later cases were identified in north-western Milan (November–December 2019). No cases were reported from Como, Monza-Brianza, and Varese, cities not particularly affected by COVID-19 during the first epidemic wave (Carletti and Pancrazi, 2021; Tosi and Chiappa, 2021). Five out of 11 pre-pandemic cases occurred in the period October 12–23, 2019. Pandemic cases were mostly localized in the province of Milan and were all detected in the first months of 2021.
3.2. Sequence analysis
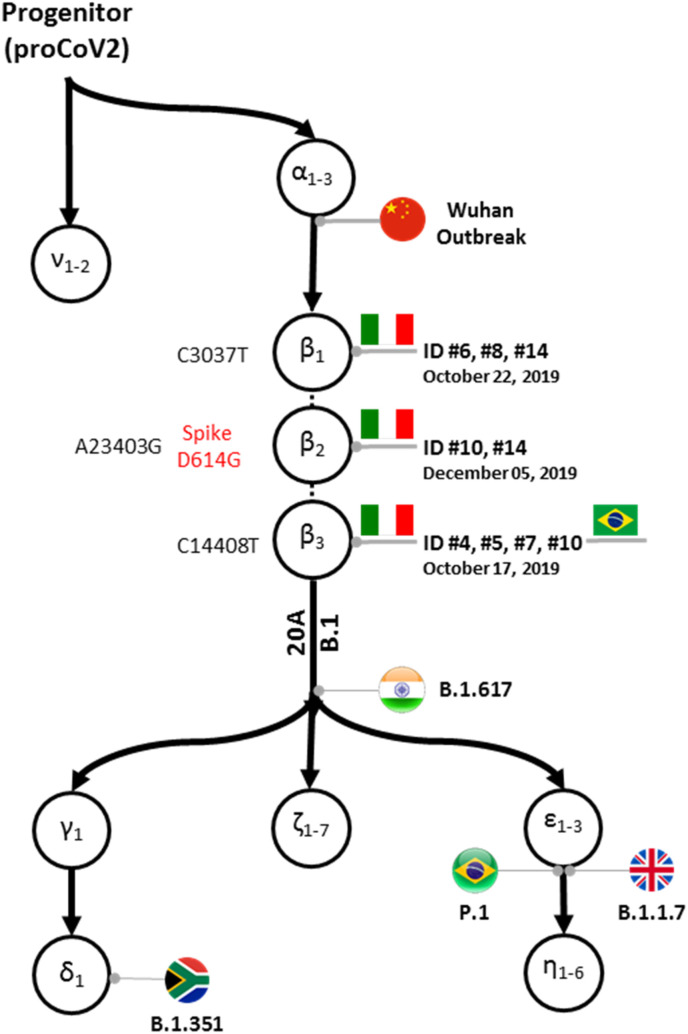
In total, we obtained 15 sequences, including 12 from pre-pandemic cases (Table 3). All three major mutations (C3037T, C14408T, and A23403G), which had first been detected weeks after the outbreak in China, were observed whenever these regions were sequenced, indicating that sequences from October 2019 already carried mutations that had been absent in the first sampled strains (e.g., Wuhan-Hu-1) reported from China (Supplementary Table S2, Fig. 2 ). Six out of the seven partial S sequences (fragment B) were 100% identical to the reference sequence Wuhan-Hu-1.
Fig. 2.
Schematic display of mutations identified in this study mapped on to the global mutational history of SARS-CoV-2. The backbone mutational tree was reconstructed from an analysis of >68,000 genomes estimate in which mutations are denoted by Greek letters (Kumar et al., 2021). Variants observed in the Italian samples analyzed in this study are marked by the patient identifiers (ID, see Table 3) along with the earliest sample acquisition date for each variant in our samples. Two patients (#9 and #13) appear twice because they contained two mutations each (β2 and β3, and β1 and β2, respectively). Nucleotide mutations are indicated in black while the amino acid mutation in the spike protein (β2) is indicated in red. Also shown are the mutation β3 found in Brazil in November 2019, indicated by a Brazilian flag, the points of attachment of 20 A and B.1 coronavirus lineages as well as some Variants of Concern (VOC): B.1.617 (delta) from India, B.1.351 (beta) from South Africa, P.1 (gamma) from Brazil, and B.1.1.7 (alpha) from the UK. A dotted line is connecting β mutations because their relative order in the cluster of three mutations could not be established with a high statistical confidence (Kumar et al., 2021). ProCoV2 is the most recent common ancestor (progenitor) of all known SARS-CoV-2 genomes sequenced to date. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We found no mutations within the furin cleavage site. One sequence (case ID #5) contained two additional non-synonymous mutations in NsP3, unique in our dataset, T2987C (F908L) and T3012C (L916S), whose significance is unclear. Interestingly, the simultaneous presence of multiple variants was noted in one patient (case ID #10) as two double peaks were observable in the electropherograms of the S sequences: 23000C/T and 23222G/A (Supplementary Fig. S1).
The three common mutations (C3037T, C14408T, and A23403G) belong to the β group of mutations (Kumar et al., 2021) previously not found in samples collected before late January 2020. All globally circulating genomes with β mutations also carry three ⍺ mutations (T18060C, T8782C, and C28144T), inferred to precede them temporally; however, these mutations lie outside the fragments sequenced in this study. Nonetheless, the mutation order analysis (Kumar et al., 2021) predicts that these mutations are likely to be present in the pre-pandemic strains, implying that these strains could be at least six mutations away from the inferred SARS-CoV-2 progenitor strain and belong to the αβ lineage, which has produced almost all the major offspring strains circulating today (Fig. 2). Finally, among the 125 sequence reads obtained by others from samples collected in Brazil in late 2019 (Fongaro et al., 2021) that could be mapped towards the reference strain we observed mutations β3 (C14408T), ⍺2 (T8782C), and ⍺3 (C28144T).
Since all three β mutations were present in samples collected on October 17, 2019, we estimate that the progenitor of SARS-CoV-2 could have already existed 11.6–16.2 weeks earlier than October 17, i.e., in late June 2019 to late July 2019, using a simple extrapolation assuming six mutations from the ancestral genome, constant substitution rate, and the published range of mutation rate estimates (Kumar et al., 2021; Pekar et al., 2021). Given that only short partial sequences are available and that the level of sequence divergence is low, more sophisticated molecular clock methods, including those which allow for variable clock rates (Miura et al., 2020; Fisher et al., 2021) are not directly applicable, and estimate precision is likely low.
4. Discussion
4.1. SARS-CoV-2 and measles-like rash
There is a clear association between morbilliform eruptions and SARS-CoV-2 infection (Freeman et al., 2021), although diagnosis remains challenging given the non-specific clinical spectrum, inconsistencies in laboratory results, and the lack of an optimal time for testing (Freeman et al., 2021). While antibody testing might not provide a definitive diagnosis, especially in cases of sustained viral spread, molecular detection of the virus is complicated by lower viral loads. In fact, all samples that we identified as SARS-CoV-2-positive (pre-pandemic and pandemic cases) were positive only after two rounds of amplification. Although the use of nested-PCRs could raise concerns of false positivity, due to the high sensitivity of this method, the totality of results show that this interpretation is unlikely. The lack of viral detection in all negative controls and in samples collected from the control cohort as well as the identification of unique mutations in two of the obtained sequences provide strong support for the biological plausibility of obtained results. Additionally, there was concordance between our test results and diagnostics performed at the hospital as none of the respiratory samples obtained during the pandemic period tested positive, while SARS-CoV-2 RNA was identified only in urine samples collected from two patients during this period. The heterogeneous pattern of positivity found for these samples, which is indicative of low viral load and potentially of RNA degradation after long-term storage, makes viral determination difficult and complicates diagnostics.
The identification of viral RNA in urine indicates that a systemic infection can occur concurrently with the development of the morbilliform skin rash and the low detectability in respiratory samples could reflect the frequently observed shifted (before or after) appearance of the skin rash with respect to respiratory symptoms. Additionally, it has been shown that SARS-CoV-2 persists in urine and can be detected in this sample type even in patients who already recovered or whose nasopharyngeal swab tests negative (Baj et al., 2020). Molecular testing of these patients while respiratory symptoms were present might have led to a higher detection rate in oropharyngeal samples. Furthermore, our analysis of the pandemic samples might be biased towards cases that are “difficult to detect” as in this period we only received samples from patients whose respiratory samples tested negative for SARS-CoV-2 RNA using RT-qPCR, which is the standard diagnostic method. Therefore, COVID-19 cases with rash that already tested positive in the hospital were not sent to us for measles identification. Finally, since some of the cases identified during the pandemic period declared a close contact with COVID-19 confirmed cases during the days and weeks preceding symptom onset, epidemiological investigations remain crucial in helping diagnose these cases. Future studies should therefore elucidate the relationship between SARS-CoV-2 and morbilliform skin rash.
4.2. Indication for SARS-CoV-2 circulation during the pre-pandemic period
Following the first demonstration of early SARS-CoV-2 circulation in Northern Italy (Amendola et al., 2021), we decided to screen all available urine and oropharyngeal swab samples collected from measles-negative patients that were submitted to our laboratory since August 2018. Our results provide preliminary evidence that SARS-CoV-2 was already circulating in Northern Italy by late 2019, with the first molecular evidence of infection dating to September 12th, 2019, and no PCR-positive result for any of the 191 samples collected before this date. The involvement of SARS-CoV-2 in morbilliform eruptions can, at least in part, explain the increase in the rate of discarded non-measles and non-rubella cases observed since late 2019. Importantly, none of the identified cases, including the nine cases from 2019, were related to travel abroad.
Sequence analysis showed that β mutations (diagnostic of lineage 20 A and B.1) were already present in strains found in samples from the last quarter of 2019, both in Italy and in Brazil. Although all three β mutations are always found together in almost all the genomes that have been sequenced (Kumar et al., 2021), because of partial sequencing, all three mutations were never detected together in our samples. Although complete genome sequencing would be critical to confirm our observations, these results imply that a lineage of the coronavirus substantially different from the putative progenitor was likely already circulating at that time, pushing back the predicted date of the progenitor SARS-CoV-2 to the period between late June 2019 and late July 2019. These date estimates are likely imprecise given limited sequence data, low genetic divergence, and strong assumptions on evolutionary rates, but the overall qualitative range of summer 2019 is nonetheless supported. This observation corroborates recent computational estimates (Kumar et al., 2021; Xia, 2021; Chiara et al., 2021), although molecular dating studies using SARS-CoV-2 genomes have sometimes yielded inconsistent estimates and revealed methodological limitations (Xia, 2021). Our findings might seem surprising as β mutations have so far only been identified in strains from 2020. However, the pool of available sequences from 2019 is very small, and genomes carrying these mutations may have simply gone undetected early on. A recent analysis of recovered sequences from thirteen early Chinese strains showed how the addition of even a few early strains can significantly shift the relative likelihoods for the most likely source of the viral outbreak (Bloom, 2021). Although it is possible that viruses carrying β mutations were imported into Europe and South America, it is also conceivable that these mutations evolved in parallel outside of China as the virus was circulating in other geographical areas, as previously hypothesized (Kumar et al., 2021; Ruan et al., 2021). Finally, we detected multiple variants in samples collected during the pre-pandemic period, supporting the hypothesis that several SARS-CoV-2 lineages were spreading worldwide for several weeks before the first reported COVID-19 cases (Kumar et al., 2021; Petti, 2022).
Two possible hypotheses can be formulated to explain an unnoticed circulation of the virus for a few months in 2019. It is possible that a somewhat genetically different virus with reduced transmissibility and/or virulence was circulating. Indeed, since the beginning of the pandemic, several variants have emerged that were more infectious than previously circulating ones (Tian et al., 2022). This could also explain why we observed a lower degree of neutralization for sera from 2019 than for those collected in 2021, possibly indicating a weaker virus-antibody bound (Chen et al., 2021). However, immunological data suggest that the adaptive immune response against SARS-CoV-2 varies among patients and that a significant proportion of patients do not develop neutralizing antibodies or that their titer decreases rapidly (Chia et al., 2021). Indeed, serological evidence for infection in pre-pandemic cases was inconclusive and immunological markers were used to integrate the molecular investigation that, combined with sequencing, allowed us to draw much stronger conclusions.
Since strains with an αβ mutational signature have produced all major lineages, an alternative hypothesis of a strain capable of efficient human-to-human transmission that was circulating undetected in northern Italy by September 2019 seems more likely, as recently highlighted (Miura et al., 2020). Identification delay, epidemiological stochasticity, and the non-linear relationship between incidence and mortality and the time of onset of community circulation (Ylli et al., 2020) could explain the long time-span between virus introduction in Lombardy (possibly no later than September 2019, according to our results) and the excess by over 60% in all-cause mortality, compared to the same period in the quinquennia 2015–2019, that was recorded in March–April 2020 (www.istat.it, report of the 5th of March 2021) (ISTAT ISS, 2021).
The Italian influenza surveillance network, which as of 2009 also monitors severe and complicated forms of respiratory diseases, was upgraded in January 2020 to also encompass the surveillance of SARS-CoV-2. Retrospective studies performed in Lombardy on samples collected within the framework of this network identified SARS-CoV-2 for the first time in March 2020 (Galli et al., 2021, Giardina et al., 2021), at least 10 days after the first identified clinical case, when the virus had already spread unnoticed in Lombardy (Valenti et al., 2021; Cereda et al., 2021). It is plausible that the surveillance of influenza and other respiratory diseases was not sensitive enough to detect the earliest positive patients, which may have gone unsampled among the high number of respiratory infections that commonly occur in autumn-winter (Percivalle et al., 2020; Canuti et al., 2022). In contrast, some of these patients could be picked up by more comprehensive surveillance systems, such as those monitoring measles and wastewaters (La Rosa et al., 2021; Amendola et al., 2021; Petti, 2022; Canuti et al., 2022). Indeed, a recent retrospective study evidenced a high viral load in Lombardy wastewaters in March–April 2020, in concomitance with the first pandemic wave. Viral load steadily decreased as the number of active cases declined as consequence of the lockdown (Castiglioni et al., 2021). These observations strengthen the hypothesis that a number of active cases must have been already present at the end of 2019 in Lombardy given the detection of the virus in the wastewater of Milan on December 18, 2019 reported by the Italian National Institute of Health (La Rosa et al., 2021).
5. Conclusions
Our study is the first to provide SARS-CoV-2 sequence information acquired from clinical samples collected prior to December 2019. Our results have potentially relevant implications for the global effort to clarify the chain of events that led to the emergence of SARS-CoV-2 in the human population and suggest that a wider geographical area and a broader timespan should be considered during virus origin investigations, as we recently highlighted (Canuti et al., 2022). Limitations of our study include the relatively low number of investigated patients, and the sub-optimal conditions for SARS-CoV-2 detection as samples were not collected within the framework of a respiratory disease surveillance and presented low viral load. Additionally, our preliminary data will have to be complemented by follow-up studies. A metagenomic approach is warranted to obtain more sequence information from the detected strains to investigate the virus evolutionary history and more accurately estimate the time of virus introduction. Nonetheless, our preliminary findings should encourage other groups to perform additional retrospective studies to seek confirmatory proof of early SARS-CoV-2 cases and more accurately identify the time and location of viral emergence (Canuti et al., 2022), especially considering that, among the high number of published papers about SARS-CoV-2 and COVID-19, only a few articles are dedicated to explore the origin of the virus (Domingo, 2021). The crucial need for further retrospective investigations in China and other areas where there is evidence for early SARS-CoV-2 circulation has also been indicated as one of the priority research areas by the WHO (Mallapaty, 2021a, 2021b) as well as by the recently established Scientific Advisory Group for the Origins of Novel Pathogens (SAGO) (SAGO, 2022). Finally, our study highlights the crucial role of sound surveillance systems in managing epidemics at their onset and as a tool to retrospectively investigate early stages of pathogen transmission.
Funding
This research was funded by Romeo and Enrica Invernizzi Pediatric Research Center, University of Milan, Milan, Italy; U.S. National Science Foundation to S.K. and S.M. (DEB‐2034228) and S.P. (DBI‐2027196); U.S. National Institutes of Health to S.K. (GM‐139504-03) and S.P. (AI‐134384).
Institutional review board statement
Ethical review and approval were waived for this study, as it was carried out as part of the Integrated Measles-Rubella Surveillance, performed by law in accordance with the Prime Minister's Decree of 3 March 2017 (https://www.gazzettaufficiale.it/eli/id/2017/05/12/17A03142/sg). The retrospective study was conducted in the absence of consent following the declaration of a state of emergency caused by the SARS-CoV-2 pandemic, in accordance with letter (i), article 9 of the GDPR (https://eurlex.europa.eu/legal-content/IT/TXT/HTML/?uri=OJ:L:2016:119:FULL&from=FI).
Informed consent statement
Patient consent was waived following the declaration of a state of emergency caused by the SARS-CoV-2 pandemic, in accordance with letter (i), article 9 of the GDPR (https://eurlex.europa.eu/legalcontent/IT/TXT/HTML/?uri=OJ:L:2016:119:FULL&from=FI).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Dr. Marino Faccini and the staff of the local health authorities of Milan and Brescia involved in the outbreak investigations and Mrs. Paola Bucci of the Istituto Superiore di Sanità (ISS) for technical support. We would also like to thank Dr. Otto Kolbl for insightful conversation. The authors acknowledge support from the Department of Health Science of University of Milan through the APC initiative.
This study is dedicated to the memory of Dr. Raffaele Gianotti, to whom we will be forever grateful for improving our knowledge of COVID-19-related skin manifestations, for appreciating our work, and for encouraging us to continue our research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.113979.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alteri C., Cento V., Piralla A., Costabile V., Tallarita M., Colagrossi L., Renica S., Giardina F., Novazzi F., Gaiarsa S., Matarazzo E., Antonello M., Vismara C., Fumagalli R., Epis O.M., Puoti M., Perno C.F., Baldanti F. Genomic epidemiology of SARS-CoV-2 reveals multiple lineages and early spread of SARS-CoV-2 infections in Lombardy, Italy. Nat. Commun. 2021;12(1):434. doi: 10.1038/s41467-020-20688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola A., Bianchi S., Gori M., Colzani D., Canuti M., Borghi E., Raviglione M.C., Zuccotti G.V., Tanzi E. Evidence of SARS-CoV-2 RNA in an oropharyngeal swab specimen, milan, Italy, early december 2019. Emerg. Infect. Dis. 2021;27(2):648–650. doi: 10.3201/eid2702.204632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolone G., Montomoli E., Manenti A., Boeri M., Sabia F., Hyseni I., Mazzini L., Martinuzzi D., Cantone L., Milanese G., Sestini S., Suatoni P., Marchianò A., Bollati V., Sozzi G., Pastorino U. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori. 2020;107(5):446–451. doi: 10.1177/0300891620974755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj A., Azzi L., Dalla Gasperina D., Genoni A., Tamborini A., Gambarini C., Carcano G., Grossi P., Sessa F. Pilot study: long-term shedding of SARS-CoV-2 in urine: a threat for dispersal in wastewater. Front. Public Health. 2020;8:569209. doi: 10.3389/fpubh.2020.569209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavaraju S.V., Patton M.E., Grimm K., Rasheed M.A.U., Lester S., Mills L., Stumpf M., Freeman B., Tamin A., Harcourt J., Schiffer J., Semenova V., Li H., Alston B., Ategbole M., Bolcen S., Boulay D., Browning P., Cronin L., David E., Desai R., Epperson M., Gorantla Y., Jia T., Maniatis P., Moss K., Ortiz K., Park S.H., Patel P., Qin Y., Steward-Clark E., Tatum H., Vogan A., Zellner B., Drobeniuc J., Sapiano M.R.P., Havers F., Reed C., Gerber S., Thornburg N.J., Stramer S.L. Serologic testing of US blood donations to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–Reactive antibodies: december 2019–january 2020. Clin. Infect. Dis. 2021;72(12):e1004–e1009. doi: 10.1093/cid/ciaa1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S., Faccini M., Lamberti A., Senatore S., Civeri G., Frati E.R., Colzani D., Gori M., Cereda D., Gramegna M., Auxilia F., Tanzi E., Amendola A. Measles surveillance activities in the metropolitan area of milan during 2017-2018. J. Prev. Med. Hyg. 2019;60(4):E286–E292. doi: 10.15167/2421-4248/jpmh2019.60.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S., Gori M., Fappani C., Ciceri G., Canuti M., Colzani D., Dura M., Terraneo M., Lamberti A., Baggieri M., Senatore S., Faccini M., Magurano F., Tanzi E., Amendola A. Characterization of vaccine breakthrough cases during measles outbreaks in milan and surrounding areas, Italy, 2017-2021. Viruses. 2022;14(5):1068. doi: 10.3390/v14051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J.D. Recovery of deleted deep sequencing data sheds more light on the early wuhan SARS-CoV-2 epidemic. Mol. Biol. Evol. 2021;38(12):5211–5224. doi: 10.1093/molbev/msab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuti M., Bianchi S., Kolbl O., Pond S.L.K., Kumar S., Gori M., Fappani C., Colzani D., Borghi E., Zuccotti G.V., Raviglione M.C., Tanzi E., Amendola A. Waiting for the truth: is reluctance in accepting an early origin hypothesis for SARS-CoV-2 delaying our understanding of viral emergence? BMJ Glob. Health. 2022;7(3):e008386. doi: 10.1136/bmjgh-2021-008386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti M., Pancrazi R. Geographic negative correlation of estimated incidence between first and second waves of coronavirus disease 2019 (COVID-19) in Italy. Mathematics. 2021;9(2) doi: 10.3390/math9020133. 133. [DOI] [Google Scholar]
- Carrat F., Figoni J., Henny J., Desenclos J.-C., Kab S., de Lamballerie X., Zins M. Evidence of early circulation of SARS-CoV-2 in France: findings from the population-based “CONSTANCES” cohort. Eur. J. Epidemiol. 2021;36(2):219–222. doi: 10.1007/s10654-020-00716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni S., Schiarea S., Pellegrinelli L., Primache V., Galli C., Bubba L., Mancinelli F., Marinelli M., Cereda D., Ammoni E., Pariani E., Zuccato E., Binda S. SARS-CoV-2 RNA in urban wastewater samples to monitor the COVID-19 pandemic in Lombardy, Italy (March–June 2020) Sci. Total Environ. 2021;806:150816. doi: 10.1016/j.scitotenv.2021.150816. 150816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. Research Use Only 2019-Novel Coronavirus (2019-NCoV) Real-Time RT-PCR Primers and Probes. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (accessed 2022 -03 -09).
- Cereda D., Manica M., Tirani M., Rovida F., Demicheli V., Ajelli M., Poletti P., Trentini F., Guzzetta G., Marziano V., Piccarreta R., Barone A., Magoni M., Deandrea S., Diurno G., Lombardo M., Faccini M., Pan A., Bruno R., Pariani E., Grasselli G., Piatti A., Gramegna M., Baldanti F., Melegaro A., Merler S. The early phase of the COVID-19 epidemic in Lombardy, Italy. Epidemics. 2021;37 doi: 10.1016/j.epidem.2021.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., Liu J., Errico J.M., Xie X., Suryadevara N., Gilchuk P., Zost S.J., Tahan S., Droit L., Turner J.S., Kim W., Schmitz A.J., Thapa M., Wang D., Boon A.C.M., Presti R.M., O'Halloran J.A., Kim A.H.J., Deepak P., Pinto D., Fremont D.H., Crowe J.E., Corti D., Virgin H.W., Ellebedy A.H., Shi P.-Y., Diamond M.S. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021;27(4):717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.-W., Le Bert N., Tan C.W., Tiu C., Zhang J., Tan S.Y., Pada S., Chan Y.-H., Tham C.Y.L., Kunasegaran K., Chen M.I.-C., Low J.G.H., Leo Y.-S., Renia L., Bertoletti A., Ng L.F.P., Lye D.C., Wang L.-F. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara M., Horner D.S., Gissi C., Pesole G. Comparative genomics reveals early emergence and biased spatiotemporal distribution of SARS-CoV-2. Mol. Biol. Evol. 2021;38(6):2547–2565. doi: 10.1093/molbev/msab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna C., Genovese G., Monzani N.A., Picca M., Boggio F., Gianotti R., Marzano A.V. Outbreak of chilblain-like acral lesions in children in the metropolitan area of milan, Italy, during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;83(3):965–969. doi: 10.1016/j.jaad.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes A., Berti V., Tandjaoui-Lambotte Y., Alloui C., Carbonnelle E., Zahar J.R., Brichler S., Cohen Y. SARS-CoV-2 was already spreading in France in late december 2019. Int. J. Antimicrob. Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. Scientific evidence on the origin of SARS-CoV-2. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A.A., Ji X., Nishimura A., Lemey P., Suchard M.A. 2021. Shrinkage-based random local clocks with scalable inference arXiv:2105.07119. [DOI]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., Schörner M.A., Barazzetti F.H., Christoff A.P., de Oliveira L.F.V., Bazzo M.L., Wagner G., Hernández M., Rodríguez-Lázaro D. The presence of SARS-CoV-2 RNA in human sewage in santa catarina, Brazil, november 2019. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.E., McMahon D.E., Hruza G.J., Lipoff J.B., French L.E., Fox L.P., Fassett M.S. Timing of PCR and antibody testing in patients with COVID-19-associated dermatologic manifestations. J. Am. Acad. Dermatol. 2021;84(2):505–507. doi: 10.1016/j.jaad.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumholtz L., Bouaziz J.-D., Battistella M., Hadjadj J., Chocron R., Bengoufa D., Le Buanec H., Barnabei L., Meynier S., Schwartz O., Grzelak L., Smith N., Charbit B., Duffy D., Yatim N., Calugareanu A., Philippe A., Guerin C.l., Joly B., Siguret V., Jaume L., Bachelez H., Bagot M., Rieux-Laucat F., Maylin S., Legoff J., Delaugerre C., Gendron N., Smadja D.m., Cassius C., Saint-Louis CORE (COvid REsearch) Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br. J. Dermatol. 2021;185(6):1176–1185. doi: 10.1111/bjd.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C., Pellegrinelli L., Bubba L., Primache V., Anselmi G., Delbue S., Signorini L., Binda S., Cereda D., Gramegna M., Pariani E. The ili sentinel physicians group, null. When the COVID-19 pandemic surges during influenza season: lessons learnt from the sentinel laboratory-based surveillance of influenza-like illness in Lombardy during the 2019-2020 season. Viruses. 2021;13(4):695. doi: 10.3390/v13040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti R., Barberis M., Fellegara G., Galván‐Casas C., Gianotti E. COVID-19-Related dermatosis in november 2019: could this case be Italy’s patient zero? Br. J. Dermatol. 2021;184(5):970–971. doi: 10.1111/bjd.19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina F., Galli C., Pellegrinelli L., Paolucci S., Tallarita M., Pariani E., Piralla A., Baldanti F. No evidence of SARS-CoV-2 circulation in the framework of influenza surveillance between october 2019 and february 2020 in Lombardy, Italy. Trav. Med. Infect. Dis. 2021;40 doi: 10.1016/j.tmaid.2021.102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni P., Podlipnik S., Pericàs J.M., Fuertes de Vega I., García-Herrera A., Alós L., Carrera C., Andreu-Febrer C., Sanz-Beltran J., Riquelme-Mc Loughlin C., Riera-Monroig J., Combalia A., Bosch-Amate X., Morgado-Carrasco D., Pigem R., Toll-Abelló A., Martí-Martí I., Rizo-Potau D., Serra-García L., Alamon-Reig F., Iranzo P., Almuedo-Riera A., Muñoz J., Puig S., Mascaró J.M. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J. Clin. Med. 2020;9(10):3261. doi: 10.3390/jcm9103261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi P., PIaserico S., Bordin C., Alaibac M., Girolomoni G., Naldi L. Cutaneous manifestations of SARS-CoV-2 infection: a clinical update. J. Eur. Acad. Dermatol. Venereol. 2020;34(11):2499–2504. doi: 10.1111/jdv.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. Coronaviridae study group of the international committee on taxonomy of viruses. The species severe acute respiratory syndrome-related coronavirus : classifying 2019-NCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., Sagulenko P., Bedford T., Neher R.A. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E.B., Hadfield J., Neher R.A., Bedford T. Year-letter genetic clade naming for SARS-CoV-2 on Nextstrain.org - SARS-CoV-2 coronavirus. Software and Tools. 2021 https://virological.org/t/year-letter-genetic-clade-naming-for-sars-cov-2-on-nextstrain-org/498 (accessed 2021 -03 -10) [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 Novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT. ISS . 2021. Impatto Dell’epidemia COVID-19 Sulla Mortalita’ Totale Della Popolazione Residente Anno 2020. March 5. [Google Scholar]
- Kumar S., Tao Q., Weaver S., Sanderford M., Caraballo-Ortiz M.A., Sharma S., Pond S.L.K., Miura S. An evolutionary portrait of the progenitor SARS-CoV-2 and its dominant offshoots in COVID-19 pandemic. Mol. Biol. Evol. 2021;38(8):3046–3059. doi: 10.1093/molbev/msab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since december 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A., Tambuzzi S., Bergna A., Battistini A., Della Ventura C., Galli M., Zoja R., Zehender G., Cattaneo C. Evidence of SARS-CoV-2 antibodies and RNA on autopsy cases in the pre-pandemic period in milan (Italy) Front. Microbiol. 2022;13:886317. doi: 10.3389/fmicb.2022.886317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cleach L., Dousset L., Assier H., Fourati S., Barbarot S., Boulard C., Bourseau Quetier C., Cambon L., Cazanave C., Colin A., Kostrzewa E., Lesort C., Levy Roy A., Lombart F., Marco-Bonnet J., Monfort J.-B., Samimi M., Tardieu M., Wolkenstein P., Sbidian E., Beylot-Barry M., French Society of Dermatology Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br. J. Dermatol. 2020;183(5):866–874. doi: 10.1111/bjd.19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner T., Kumar S. Where did SARS-CoV-2 come from? Mol. Biol. Evol. 2020;37(9):2463–2464. doi: 10.1093/molbev/msaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean O.A., Lytras S., Weaver S., Singer J.B., Boni M.F., Lemey P., Pond S.L.K., Robertson D.L. Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen. PLoS Biol. 2021;19(3) doi: 10.1371/journal.pbio.3001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurano F., Baggieri M., Bucci P., D’Ugo E., Sabbatucci M., Maraglino F., Iannazzo S., Marchi A., Nicoletti L. MoRoNet a network to strengthen the quality of measles and rubella surveillance in Italy. Eur. J. Publ. Health. 2020;30(5) doi: 10.1093/eurpub/ckaa166.1336. ckaa166.1336. [DOI] [Google Scholar]
- Magurano F., Baggieri M., Marchi A., Rezza G., Nicoletti L., COVID-19 Study Group SARS-CoV-2 infection: the environmental endurance of the virus can Be influenced by the increase of temperature. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021;27(2):289.e5–289.e7. doi: 10.1016/j.cmi.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. Where did COVID come from? Five mysteries that remain. Nature. 2021;591:188–189. doi: 10.1038/d41586-021-00502-4. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. After the WHO report: what's next in the search for COVID's origins. Nature. 2021;592:337–338. doi: 10.1038/d41586-021-00877-4. [DOI] [PubMed] [Google Scholar]
- Marzano A.V., Genovese G., Moltrasio C., Gaspari V., Vezzoli P., Maione V., Misciali C., Sena P., Patrizi A., Offidani A., Quaglino P., Arco R., Caproni M., Rovesti M., Bordin G., Recalcati S., Potenza C., Guarneri C., Fabbrocini G., Tomasini C., Sorci M., Lombardo M., Gisondi P., Conti A., Casazza G., Peris K., Calzavara-Pinton P., Berti E. Italian skin COVID-19 network of the Italian society of dermatology and sexually transmitted diseases. The clinical spectrum of COVID-19-associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J. Am. Acad. Dermatol. 2021;84(5):1356–1363. doi: 10.1016/j.jaad.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Tamura K., Tao Q., Huuki L.A., Pond S.L.K., Priest J., Deng J., Kumar S. A new method for inferring timetrees from temporally sampled molecular sequences. PLoS Comput. Biol. 2020;16(1) doi: 10.1371/journal.pcbi.1007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montomoli E., Apolone G., Manenti A., Boeri M., Suatoni P., Sabia F., Marchianò A., Bollati V., Pastorino U., Sozzi G. Timeline of SARS-CoV2 Spread in Italy: Results from an Independent Serological Retesting. Viruses. 2022;14(1):61. doi: 10.3390/v14010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.W., Faulkner N., Cornish G.H., Rosa A., Harvey R., Hussain S., Ulferts R., Earl C., Wrobel A.G., Benton D.J., Roustan C., Bolland W., Thompson R., Agua-Doce A., Hobson P., Heaney J., Rickman H., Paraskevopoulou S., Houlihan C.F., Thomson K., Sanchez E., Shin G.Y., Spyer M.J., Joshi D., O'Reilly N., Walker P.A., Kjaer S., Riddell A., Moore C., Jebson B.R., Wilkinson M., Marshall L.R., Rosser E.C., Radziszewska A., Peckham H., Ciurtin C., Wedderburn L.R., Beale R., Swanton C., Gandhi S., Stockinger B., McCauley J., Gamblin S.J., McCoy L.E., Cherepanov P., Nastouli E., Kassiotis G. Preexisting and de Novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay N., Mirinaviciute G., Mollet T., Celentano L.P., Bacci S. Epidemiology of measles during the COVID-19 pandemic, a description of the surveillance data, 29 EU/EEA countries and the United Kingdom, january to may 2020. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2020;25(31):2001390. doi: 10.2807/1560-7917.ES.2020.25.31.2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A., Delmonte D., Scognamiglio T., Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Health. 2020;5(6) doi: 10.1016/S2468-2667(20)30099-2. e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Paixao J., Galangue M., Gaston C., Carralero R., Lino C., Júlio G., David Z., Francisco M., Sebastião C.S., Sacomboio E.N., Morais J., Francisco N.M. Early evidence of circulating SARS-CoV-2 in unvaccinated and vaccinated measles patients, september 2019–february 2020. Infect. Drug Resist. 2022;15:533–544. doi: 10.2147/IDR.S344437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekar J., Worobey M., Moshiri N., Scheffler K., Wertheim J.O. Timing the SARS-CoV-2 index case in hubei province. Science. 2021;372(6540):412–417. doi: 10.1126/science.abf8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E., Cambiè G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., Martino R.D., Isernia P., Mojoli F., Bruno R., Tirani M., Cereda D., Nicora C., Lombardo M., Baldanti F. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the lodi red zone in Lombardy, Italy, as at 06 april 2020. Euro Surveill. 2020;25(24) doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti S. Undetected and relatively sustained severe acute respiratory syndrome coronavirus 2 circulation worldwide during 2019. Clin. Infect. Dis. 2022;74(7):1313–1314. doi: 10.1093/cid/ciab727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O'Toole Á., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. JEADV. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- Ruan Y., Wen H., Hou M., He Z., Lu X., Xue Y., He X., Zhang Y.P., Wu C.I. The twin-beginnings of COVID-19 in Asia and Europe-one prevails quickly. Natl. Sci. Rev. 2021;9 doi: 10.1093/nsr/nwab223. nwab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGO . WHO Scientific Advisory Group for the Origins of Novel Pathogens (SAGO): Preliminary Report, 9 June 2022. World Health Organization; Geneva: 2022. https://www.who.int/publications/m/item/scientific-advisory-group-on-the-origins-of-novel-pathogens-report (accessed 2022 -06 -21) [Google Scholar]
- Singh S., McNab C., Olson R.M., Bristol N., Nolan C., Bergstrøm E., Bartos M., Mabuchi S., Panjabi R., Karan A., Abdalla S.M., Bonk M., Jamieson M., Werner G.K., Nordström A., Legido-Quigley H., Phelan A. How an outbreak became a pandemic: a chronological analysis of crucial junctures and international obligations in the early months of the COVID-19 pandemic. Lancet. 2021;398(10316):2109–2124. doi: 10.1016/S0140-6736(21)01897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Sun Y., Zhou J., Ye Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J. Med. Virol. 2022;94(3):847–857. doi: 10.1002/jmv.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi D., Chiappa M. Understanding the geographical spread of COVID-19 in relation with goods regional routes and governmental decrees: the Lombardy region case study. SN Comput. Sci. 2021;2(3) doi: 10.1007/s42979-021-00597-6. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti L., Bergna A., Pelusi S., Facciotti F., Lai A., Tarkowski M., Lombardi A., Berzuini A., Caprioli F., Santoro L., Baselli G., Ventura C.D., Erba E., Bosari S., Galli M., Zehender G., Prati D. Covid-19 donors study (CoDS) network (appendix 1). SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 outbreak in milan. Blood Transfus. Trasfus. Sangue. 2021;19(3):181–189. doi: 10.2450/2021.0324-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti A., Bataille V., Rossi N., Kluk J., Murphy R., Puig S., Nambi R., Bowyer R.C.E., Murray B., Bournot A., Wolf J., Ourselin S., Steves C.J., Spector T.D., Falchi M. Diagnostic value of cutaneous manifestation of SARS-CoV-2 infection. Br. J. Dermatol. 2021;184(5):880–887. doi: 10.1111/bjd.19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- Worobey M., Pekar J., Larsen B.B., Nelson M.I., Hill V., Joy J.B., Rambaut A., Suchard M.A., Wertheim J.O., Lemey P. The emergence of SARS-CoV-2 in Europe and north America. Science. 2020;370(6516):564–570. doi: 10.1126/science.abc8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. Dating the common ancestor from an NCBI tree of 83688 high-quality and full-length SARS-CoV-2 genomes. Viruses. 2021;13(9) doi: 10.3390/v13091790. 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylli A., Wu Y.Y., Burazeri G., Pirkle C., Sentell T. The lower COVID-19 related mortality and incidence rates in eastern European countries are associated with delayed start of community circulation. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.