Abstract
A simple and efficient sample extraction and preconcentration method based on reversed-phase ionic liquid dispersive liquid-liquid microextraction (RP-IL-DLLME) had been developed and used to quantify the domoic acid in human urine samples. The analysis was performed by ultra-performance liquid chromatography and photodiode array detection. During the procedure, hydrophilic ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [C4mim] BF4 as dispersive solvent and NaOH solution was chosen as extraction solvent. Some important parameters in the method were investigated to get high enrichment factors. Under optimal conditions, the linearity of the method was in the range of 0.1–10 ng mL−1 and the correlation coefficient was above 0.9996. The relative standard deviations (RSDs) of the developed methods for intra-day (n = 5) and inter-day (n = 5) precision ranged from 1.9 to 3.9%. Meanwhile, limit of detection (LOD) was 0.03 ng mL−1(S/N = 3) and that of quantification (LOQ) was 0.1 ng mL−1(S/N = 10) with the enrichment factors (EF) being 230. Eventually, the proposed method was successfully applied to the determination of Dominic acid in human urine samples.
Keywords: Reversed-phase dispersive liquid-liquid microextraction, Ionic liquid, Domoic acid, Ultra-high-performance liquid chromatography
Reversed-phase dispersive liquid-liquid microextraction; Ionic liquid; Domoic acid; Ultra-high-performance liquid chromatography.
1. Introduction
Domoic acid (DA) as a natural non-protein neural amino acid is a strong neurotoxic substance produced by pseudo-rhomboid algae and rhomboid algae. Filter feeding on marine shellfish and fish can accumulate DA in the body which cause humans and other marine mammals poisoned. As a neurotransmitter, DA has the same function of stimulating nerve cells as glutamate. But its intensity is 100 times higher than that of glutamate. DA may damage the memory-related areas of the hippocampus and thalamus of the brain, which cause memory loss and even death. In 1987, shellfish poisoning occurred for the first time in Canada and killed three people [1, 2]. Subsequent studies showed that DA should be responsible for the accident. It’s the first time that pseudo-rhomboid algae and rhomboid algae toxin had been declared poisonous. These toxic algae were first discovered in North America and then later seen in Europe, New Zealand and other waters. So far, these toxic algae were almost distributed all the oceans in the world. the world. Meanwhile, the enrichment of DA in shellfish also happened in these areas [3, 4, 5]. This made accurate determination of trace DA in human body important. It’s not only for etiological diagnosis but also for metabolic kinetics study for patients with DA toxicosis.
At present, the main methods for the analysis of DA included high-performance liquid chromatography-ultraviolet detector (HPLC-UV) [6, 7, 8, 9, 10, 11, 12], liquid chromatography-tandem mass spectrometry (LC-MS/MS) [13, 14, 15, 16, 17], Capillary electrochromatography (CE) [18, 19, 20], Animal experiment [21, 22], Enzyme-linked immunoassay (ELISA) [23, 24] assay and so on. However, the low concentrations of DA and high matrix complexities made it more difficult for precise quantitative analysis in complex samples. Pretreatment becomes necessary because it not only increases the concentration of the analyte, but also cleans up the sample to minimize matrix effects. Currently, the main pretreatment methods for ultra-trace DA are liquid-liquid extraction (LLE) and solid-phase extraction (SPE). Although these two techniques are currently widely used in the separation and purification of complex samples, they still have problems to be solved, such as high solvent consumption, time-consuming, large analyte loss, and unsatisfactory selectivity or sensitivity etal [25]. Therefore, it’s necessary to develop a developing a low cost, easy operation, sensitive, and less hazardous sample pretreatment method.
Recently, dispersive liquid-liquid microextraction (DLLME) has emerged as one of the most interesting extraction methods for analyte enrichment, as it eliminates matrix effects and increases enrichment rates [26, 27]. In the traditional DLLME method, the analyte is transferred from the aqueous phase to the organic phase (as the extraction solvent) with the help of a dispersing solvent. Although this method can reduce the sample pretreatment time and greatly reduce the use of organic solvents, it still requires some toxic volatile aromatic organic solvents. In addition, DLLME also has some difficulties when applied to biological samples. To address these problems of the DLLME method, a reversed-phase dispersive liquid-liquid microextraction (RP-DLLME) method is proposed to pretreatment samples [28, 29, 30, 31]. In this method, a small volume (μL level) of aqueous solvent replaces the toxic organic solvent as the extractant phase. Dispersion of the aqueous extractant phase into the organic sample using a medium polarity solvent as dispersant. After mixing and centrifugation, the precipitated phase is an aqueous micro drop containing the target analyte that is directly acceptable for the detection step of the analytical instrument. Therefore, RP-DLLME has the advantages of simplicity, high efficiency, small sample volume, low solvent consumption, low cost, low waste generation, and high enrichment factor. However, it still relies on volatile organic extractants such as acetonitrile, benzene, chloroform, and carbon tetrachloride, which are quite harmful to humans and the environment. Hence new kind of alternative solvents should be explored in order to solve the limitations of traditional organic solvents in RP-DLLME.
Ionic liquids (ILs) are organic molten salts with a melting temperature below 100 °C that exhibit a number of favorable physicochemical properties such as negligible vapor pressure, high thermal stability, high electrical conductivity, non-flammability, and the ability to achieve tunable properties through the selection of cationic or anionic components making them an attractive alternative to organic solvents and contributing to the accuracy and safety of analytical measurements. More than that, ILS’ physical and chemical properties can be fine-tuned by structural changes in their cationic and anionic fractions. So, the improvement of extraction yields and selectivity became easy. ILS are now widely employed in several extraction modes, including SPE, single drop microextraction (SDME) and DLLME etal [32, 33, 34]. These suggest that RP-IL-DLLME has great potential as an effective sample pretreatment method. To our knowledge, this is the first report of pretreatment of trace DA in urine samples using the RP-IL-DLLME method.
Although the HPLC-UV method is now widely used as the main detection method for the analysis of DA, it has the drawbacks of low method sensitivity and long analysis time. With the development of instrumentation and column technology, ultra-high performance liquid chromatography (UHPLC) has emerged. Compared with conventional HPLC, UHPLC can significantly improve chromatographic performance, analytical sensitivity and sample throughput, while minimizing solvent and sample consumption. Therefore, UHPLC-UV is ideal as a fast, economical, easy-to-operate and reliable method for DA quality evaluation [35, 36].
In this study, a rapid, simple and inexpensive reversed-phase ionic liquid dispersive liquid-liquid microextraction (RP-IL-DLLME) method was established for the pretreatment of DA from human urine. The extraction parameters, such as the type and volume of dispersive solvent, the type and volume of ionic liquid, the pH of the sample solution, ionic strength, centrifuging speed and time, were evaluated. Under optimal conditions, the established RP-IL-DLLME combined with UHPLC-UA method was successfully applied to the separation and determination of DA in human urine. Since this method needn’t some special or expensive equipment (e.g. LC-MS/MS, ELISA, etc.), it could be performed under routine testing conditions. Meanwhile, the method had a short analysis cycle, which improved the applicability and accuracy of DA analysis in qualitative and quantitative aspects and was important for routine DA quality control.
2. Experimental methods
2.1. Chemicals and solutions
DA (purity≥99%) was obtained from Sigma (St. Louis, MO, USA). 1-Butyl-3-methylimidazolium tetrafluoroborate [C4mim] BF4, 1-Hexyl-3-MethylImidazolium tetrafluoro-borate [C6mim] BF4, 1-octyl-3-methylimidazolium tetrafluoroborate [C8mim] BF4 (≥99%) were purchased from Monils Chemical (Shanghai) Co. Ltd., China. Other chemicals were of analytical grade and obtained from Merck (Darmstadt, Germany). HPLC-grade acetonitrile was got from Fisher (Pittsburgh, PA, USA). Ultrapure water was produced by a Milli-Q system (Millipore, MA, USA).
Stock solution of DA was prepared by dissolving accurately weighed DA in acetonitrile-water (10:90, v/v) to yield final concentrations of 1.0 mg mL−1. The stock solutions were stored at 4 °C (stable for no less than 6 months) in the dark and carried to room temperature before use. Calibration standard working solutions at five concentration levels, 0.1, 0.5, 1.0, 5.0 and 10.0 ng mL−1, were freshly prepared by appropriate dilution of the stock solution with ultrapure water.
2.2. Sample collection and preparation
The CDC Human Subjects Institutional Review Board (Shenzhen University Health Sciences Center Ethics and Institutional Review Board) approved anonymous urine collection for method development and validation. All participants received written informed consent and each subject received a gift card of RMB 10 for participation in the study. To confirm the validity of this approach, we analyzed six urine samples collected at the Shenzhen University Health Science Center from six healthy adult male and female volunteers who fasted in the morning and had no recorded exposure to the target neurotoxicant.
Before analysis, the urine samples were filtered through a 0.45 μm membrane filter. In order to get spiked samples, calculated volumes of working solutions of DA should be added into filtrated blank urine samples and the volume of resulting solutions was 5 mL. Then the samples were left to stand for 30 min so as to allow mix well between the analyte and the urine sample.
2.3. Instrumentation and apparatus
The chromatographic system consisted of Nexera UHPLC system with LC-30AD pump, SIL-30AC automatic sampler, DGU-20AR online degassing device and SPD-M30A photodiode array detector. Data were evaluated by LabSolutions software. Separations were accomplished by a Shim-pack XR-ODSIII (2.0 × 50 mm id, 1.6 μm) column kept at 30 °C with a flow rate of 0.6 mL min−1 and the detection at 245 nm. The mobile phase was acetonitrile-0.1% formic acid (35:65, v/v). 2 μL injection volume was applied.
A pH meter (Starter 3000 pH, Ohaus, Switzerland) with a resolution of ±0.1 pH unit served for pH measurements. A high-speed centrifuge (Mistral 2000, MSE, UK) was employed to centrifuge the sample solutions.
2.4. RP-IL-DLLME procedure
A 5 mL of sample solution spiked with DA was placed in a 10 mL centrifuge tube. A mixture of 300 μL [C4mim] BF4 (dispersive solvent), 30 μL 0.5 M NaOH solution (extraction solvent) and 0.5% w/v Na2SO4 was quickly introduced into the sample solution, which resulted in the formation of cloudy solution. Then The mixture was gently shaken by hand for 20 s and centrifuged at 8000 rpm for 6 min to achieve phase separation. The enriched aqueous phase containing the extracted analyte was obtained (20 μL) using a 50 μL micro-syringe while the sedimented phase containing [C4mim] BF4 was left in the tube. 1.0 mL acetonitrile with water (25:75, v/v) was added to lower the viscosity of the aqueous phase. After that 2 μL of solution was injected into the UHPLC system for analysis. In the process, each experiment was run three times to reduce the error.
The enrichment factors (EF) and the extraction recoveries (%ER) were calculated by means of Eqs:
| ER= (Vs/Vin) (EF) 100% | (1) |
| EF = Cs /Cin | (2) |
Where Vs was the volume of aqueous phase (20 μL) and Vin was the volume of initial solution (5 mL). Cs was the final concentration of analyte in the aqueous phase and Cin was the concentration of the initial concentration of analyte in the initial solution [37, 38].
2.5. Method validation
The analytical methods were validated according to international guidelines [39, 40] to check the limit of detection (LOD), limit of quantification (LOQ), linearity, intra- and inter-day precision, accuracy, recovery, selectivity and stability in urine samples.
3. Results and discussion
3.1. Evaluating of the RP-IL-DLLME procedure
Sample extraction in RP-IL-DLLME was investigated because several factors such as the type and volume of the dispersing solvent, the type and volume of the extracting solvent, the pH of the sample solution, the effect of salt, and the centrifugation speed and time affected the sample extraction in RP-IL-DLLME. 2 mL of aqueous solution containing 0.5 ng mL-1 DA was used as the sample for all evaluation experiments.
3.1.1. Selection of type and volume of dispersive solvent
In RP-IL-DLLME, a suitable dispersive solvent was the key to improve the extraction efficiency because of its great influence by changing the cloudy state. The suitable dispersive solvent should have both miscibility with the extraction solvent and no-volatility. Thus, three ionic liquids [C2mim] BF4 [C4mim] BF4 and [C8mim] BF4 were investigated as dispersive solvent. It’s noted that [C8mim] BF4 might be unable to form precipitation at the bottom of the centrifuge tube because of its higher solubility. So [C8mim] BF4 would not be further studied. With [C2mim] BF4 as dispersive solvent, although opacification system could be formed, the extraction recovery was pretty low. In comparison [C4mim] BF4 gave higher extraction recovery and better peak shape. Therefore [C4mim] BF4 was chosen as the dispersive solvent in subsequent experiments.
Except the type, the volume of the dispersive solvent also affected the extraction recovery by its dispersibility in aqueous phase. So, the selected [C4mim] BF4’s volume must be optimal. Different volumes (100–600 μL) of [C4mim] BF4 were screened for the experiments. Figure 1 shows the extraction recovery of DA increased rapidly with the increasing volume of [C4mim] BF4 up to 300 μL. But beyond this point, the DA’s extraction recovery slightly decreased. This may be due to the fact that when the volume of ILS was less than 100 μL, the volume of the ILS phase was too small to achieve phase separation, so the effective extraction of DA could not be obtained. With the increase of the volume of ILS, the volume of ILS phase increases, and the extraction recovery of DA increases gradually. When 300 μL ILS is added, the phase separation is the most obvious, and the extraction recovery of DA is the highest. However, when the volume of ILS exceeded 300 μL, the extraction rate decreased slightly because the hydrophobicity of ILS increased with its volume and the solubility of DA in water increased, which was not easy to be extracted. Therefore, the addition volume of [C4mim] BF4 was 300 μL in this experiment.
Figure 1.
Effect of volume of [C4mim] BF4.
3.1.2. Selection of type and volume of extraction solvent
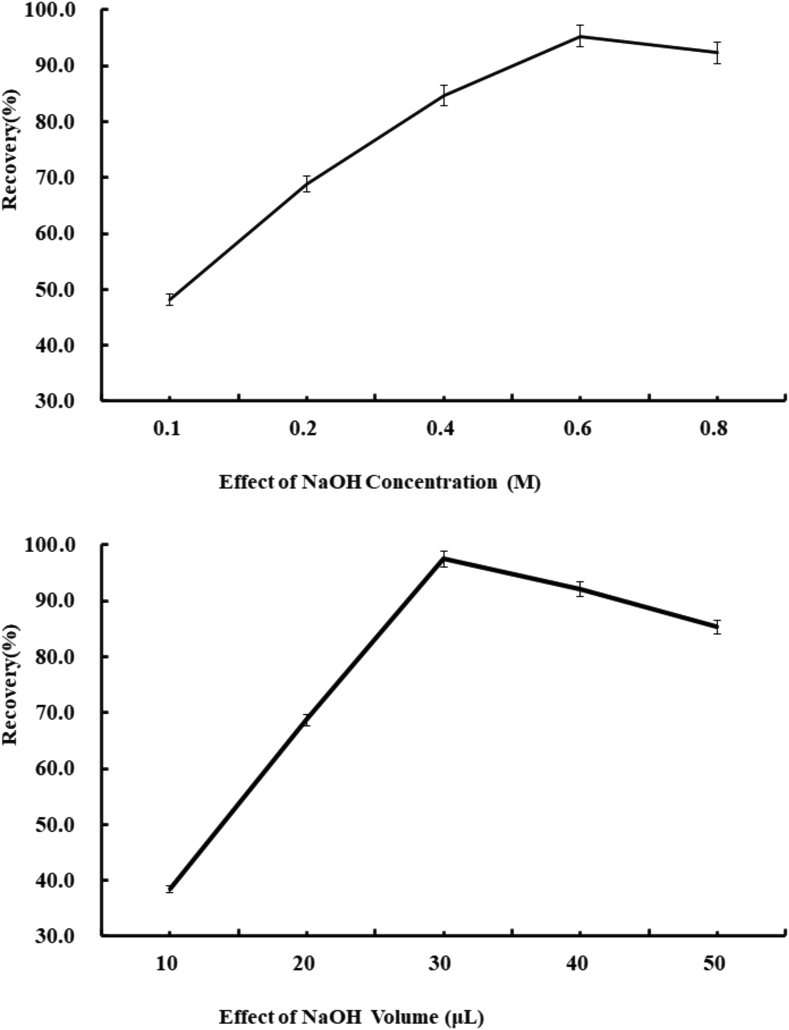
In view of the fact that DA is a weakly acidic compound with multiple carboxyl groups. DA’s existing form varied with the pH value of the environment (neutral molecular form or ionic form) changed. 50 μL of aqueous NaOH solutions with different concentrations (0.1–1.0 M) was used to extract the spiked blank sample and the corresponding results are shown in Figure 2A. The results indicated that 0.5M aqueous NaOH solution was the most efficient extraction solvent in the proposed method and therefore applied in the subsequent experiments.
Figure 2.
(a). Effect of concentration of NaOH solutions (M). (b). Effect of volume of NaOH solutions.
Moreover, the volume of extraction solvent also affected the extraction efficiency directly. So, series volume of 0.5M NaOH solutions ranging from 10 μL to 100 μL were studied. The results show (Figure 2B.) that the extraction recovery increased significantly with the volume of extraction solvent raised from 10 μL to 50 μL. However, when the volume exceeds 50 μL, the extraction recovery has a decline. This may be due to the dilution effect caused by the increased extraction solution volume. So, the volume of 50 μL was selected in follow up experiments.
3.1.3. Salt effect
In the RP-IL-DLLME process, the presence of salt ions not only changes the solubility of the target compound in the sample solution, but also changes the distribution behavior of the extractant in the sample solution. On the one hand, the presence of salt ions will lead to salting-out effect and improve the distribution coefficient of DA in the ionic liquid phase; on the other hand, the addition of salt ions will increase the density and viscosity of the sample solution and increase the mass transfer resistance. Therefore, we can investigate the type and concentration of salt to help us to obtain higher extraction efficiency. We compared the effects of three common salts (NaCl, Na2SO4 and Na2CO3) on the extraction recovery. The results showed that after adding Na2SO4, not only the solution phases were separated quickly, the interface was clear, no turbidity and no flocculation, but also the extraction recovery was the highest. This may be due to the fact that when NaCl exists in the sample, part of the anion BF4- in [C4mim] BF4 is exchanged with Cl−, resulting in the conversion of part of [C4mim] BF4 into a more hydrophilic ionic liquid [C4mim] Cl, so the extraction recovery may be too low when NaCl is used. When adding Na2CO3 to the solution, the viscosity of the sample solution changes accordingly, which is not conducive to the diffusion of ionic liquids under the condition of high viscosity, resulting in poor extraction recovery. So Na2SO4 was selected for the subsequent experimental parameters.
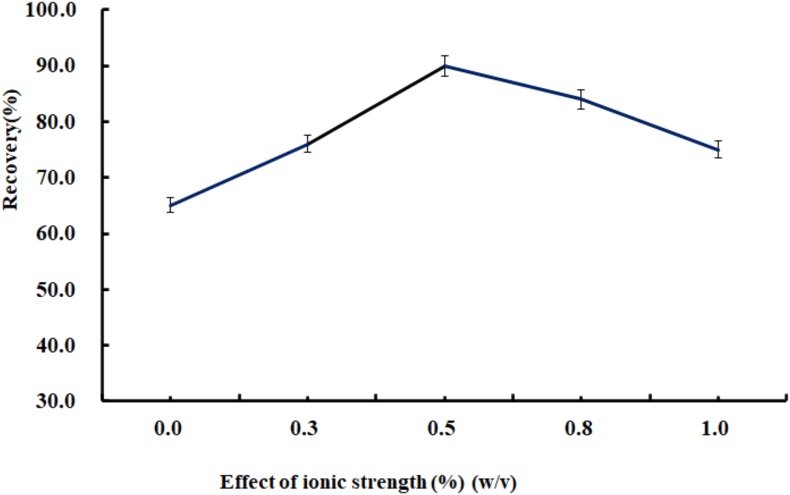
After determining Na2SO4 as the type of salt, the next step was to study the effect of 0%–1.0% (w/v) Na2SO4 solution on the extraction recovery. The experimental results are shown in Figure 3. The extraction efficiency increases at first and then decreases with the increase of Na2SO4 concentration. The highest extraction efficiency can be obtained when the concentration of Na2SO4 is 0.5% (w/v). This phenomenon can be explained as follows: ① when the concentration of Na2SO4 is low, the salting-out effect is dominant, there is a strong hydration between salt and water molecules, and free water molecules tend to combine with salt molecules, resulting in a relative increase in the concentration of target compounds in aqueous solution and more distribution to the ionic liquid phase. ② when the salt concentration increases gradually, there is a strong electrostatic interaction between the salt molecule and the target compound molecule, which reduces the target and mass transfer process, resulting in the decrease of extraction efficiency. Therefore, the concentration of Na2SO4 0.5% (w/v) was selected in the follow up experiment.
Figure 3.
Effect of the ionic strength.
3.1.4. Effect of centrifuging speed and time
The rapid separation of extractant and sample solution can be completed by the help of centrifugation. Centrifugation time would affect the volume of the ionic liquid phase directly. A reasonable time not only contributed to gain high extraction recovery but also reflected the superiority of the method. To probe the effect of this feature, the centrifugation time and speed were studied in the range of 2–10 min and 5000–10000 rpm respectively. The results showed that centrifugation at 8000 rpm for 6 min was sufficient to achieve phase separation and clarity of the enriched phase, and even higher speeds and times did not significantly affect the results. Since the enriched phase was completely separated out, increasing the speed and time did not have any significant effect on the results. In order to make the experimental operation easier and shorten the experimental time, 8000 rpm and 6min were selected as the speed and time for further experiments.
3.2. Analytical performance of the proposed method
The method was validated in terms of linearity, precision, accuracy and recovery. Calibration curves were constructed using weighted (1/x2) linear least-squares regression analysis of the observed peak area of DA against concentration. During the experiments, at least five described concentrations of the solution were analyzed in triplicate. In this proposed method, the calibration curve was linear in the ranges of 0.1–10.0 ng mL−1 with the R2 value of 0.9996 for DA. Limits of detection (LOD) for DA was found to be 0.03 ng mL−1 (S/N = 3), and limits of quantification (LOQ) were 0.1 ng mL−1 (S/N = 10).
Three concentrations of quality control (QC) analytes were analyzed to assess the precision and accuracy of the proposed method. The intra-day accuracy, inter-day accuracy and precision values for the assay are shown in Table 1. All intra-day RSDs (%) for DA were less than 3.3%. All inter-day RSDs (%) were less than 3.9%. Accuracy was determined by comparing the average calculated concentration with the spiked target concentration of the quality control samples. Intraday and inter-day accuracies for DA were found to be within 94.8% and 97.6%. To determine the recovery of DA in human urine samples, DA was added to blank human urine samples to achieve final concentrations of 0.1, 1.0, and 10 ng m L−1. The urine samples were subjected to RP-IL-DLLME procedure and injected into UHPLC. The average recoveries of DA in urine samples at concentrations of 0.1, 1.0, and 10 ng m L−1 were 94.6%, 95.9%, and 97.3%, respectively.
Table 1.
Quantitative characteristics of the proposed RP-IL-DLLME method for the analysis of DA in the urine sample (n = 5).
| Concentration (ng mL −1) | Precision (RSD%) |
Accuracy (%) |
Recovery (%) | ||
|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | ||
| 0.1 | 3.3 | 3.9 | 94.8 | 95.6 | 94.6 |
| 1.0 | 2.9 | 3.5 | 95.9 | 96.4 | 95.9 |
| 10.0 | 1.9 | 2.6 | 96.3 | 97.6 | 97.3 |
Selectivity was assessed by comparing the chromatograms of blank urine samples and spiked urine samples that were subjected to the RP-IL-DLLME procedure and injected into UHPLC. DA in urine samples was stable when stored at -20 °C for at least 15 days. The relative error (RE) % between the initial concentration of DA in the urine samples and the concentrations of the three subsequent freeze-thaw cycles ranged from 3.4% to 4.6%, indicating that DA was stable during the three freeze-thaw cycles. Treated samples were also found to be stable for at least 4 h at room temperature. The above stability data are summarized in Table 2. The results indicate that no significant deterioration of the analyte was observed under any of these conditions.
Table 2.
Stability f or the assay of DA in urine sample (n = 5).
| Concentration found (ng mL−1) (mean ± SD) | Concentration added (ng mL−1) (mean ± SD) |
||
|---|---|---|---|
| 0.1 | 1.0 | 10.0 | |
| Freeze and thaw stability | |||
| At the beginning | 0.0946 ± 0.0061 | 0.959 ± 0.047 | 9.68 ± 0.35 |
| After three freeze-thaw cycle | 0.0990 ± 0.0065 | 0.995 ± 0.052 | 10.01 ± 0.57 |
| Bias (RE%) | 4.6 | 3.8 | 3.4 |
| Short-term room temperature stability | |||
| At the beginning | 0.0946 ± 0.0061 | 0.959 ± 0.047 | 9.68 ± 0.35 |
| After 4 h at room temperature | 0.0983 ± 0.0042 | 0.991 ± 0.042 | 9.90 ± 0.46 |
| Bias (RE%) | 3.9 | 3.4 | 2.3 |
| Long-term cold storage stability | |||
| At the beginning | 0.0946 ± 0.0061 | 0.959 ± 0.047 | 9.68 ± 0.35 |
| After 15 days at -20 °C | 0.1004 ± 0.0039 | 1.012 ± 0.038 | 10.12 ± 0.42 |
| Bias (RE%) | 6.1 | 5.5 | 4.5 |
a Bias (RE%)= (Cactual −Ccalculated)/Cactual (%).
3.3. Comparison of RP-IL-DLLME with other pretreatment methods
To investigate the novelty and effectiveness of RP-IL-DLLME for the quantitative analysis of DA in urine samples, the performance of RP-IL-DLLME was compared with that of traditional RP-DLLME (without ILs). Spiked sample (1 ng mL−1 DA standard solution) was applied for comparative extraction. In the traditional RP-DLLME process, toluene was chosen as the dispersant. Other conditions were the same as the RP-IL-DLLME procedure. Based on the results expressed in Table 3, this method demonstrates a high enrichment factor, a desirable linear range and a low LOD value for quantitative DA analysis.
Table 3.
Comparison of the present study with traditional RP-DLLME methods for the determination of DA.
| Sample preparation | LOD (ng mL-1) | LR (ng mL-1) | RSD % | EF |
|---|---|---|---|---|
| RP-IL-DLLME | 0.03 | 0.1–10 | ≤3.3 | 230 |
| RP-DLLME | 0.31 | 1–100 | ≤9.7 | 86 |
EF, enrichment factor; LR, linear range.
The proposed method was compared with the pretreatment and determination of DA in different matrices reported in the literature and the results are presented in Table 4. RP-IL-DLLME has higher extraction efficiency, shorter extraction time and extraction temperature. In addition, compared with LLE, SPE and traditional RP-DLLME, this method does not require the addition of any organic dispersive solvent, which has a significant advantage in terms of environmental impact.
Table 4.
Comparison of the present study with some recent methods selected from the literature for the determination of DA.
| Samples | Methods | Sample preparation | LOD | LR | Time (min) | RSD % | Refs |
|---|---|---|---|---|---|---|---|
| Shellfish | ELISA | LLE | ≥25 ng g−1 | 25–500 ng g−1 | >120 | ≤4.12 | [19] |
| mussels | HPLC/PCD | SPE | ≥25 ng mL−1 | 50–1500 ng mL−1 | >25 | ≤1.9 | [23] |
| seawater | RRLC-MS/MS | SPE | ≥0.02 ng mL−1 | 0.1–10.0 ng mL−1 | >10 | ≤19.0 | [24] |
| seawater | HPLC/DAD | MIPs-SPE | ≥0.01 mg mL−1 | 0–50 mg mL−1 | >15 | ≤5.0 | [25] |
| Plasma | HPLC-MS/MS | LLE | ≥0.16 ng mL−1 | 0.16–16 ng mL−1 | >> 60 | ≤7.3 | [17] |
| Urine | HPLC-MS/MS | LLE | ≥7.8 ng mL−1 | 7.8–1000 ng mL−1 | >> 15 | ≤11.4 | [17] |
| urine | UHPLC/DAD | RP-IL-DLLME | ≥0.03 ng mL−1 | 0.1–10.0 ng mL−1 | <10 | ≤6.4 | Present method |
ELISA: enzyme-linked immunosorbent assa.
HPLC-PCD: high-performance liquid chromatography/post-column derivatization.
RRLC-MS/MS: rapid resolution liquid chromatography-tandem mass spectrometry.
HPLC-DAD: high-performance liquid chromatography/diode-array detector.
MIPs: Molecularly imprinted polymers.
3.4. Analysis of real samples
The applicability of the proposed RP-IL-DLLME method to real samples was assessed under the optimal conditions. Six urine samples (5 mL) were used without dilution. Each experiment was carried out three times under the same conditions. The results are reported in Table 5. DA was detected in two of the six urine samples, which may be related to the volunteers’ consumption of DA contaminated fish and shellfish. Figure 4 shows typical chromatograms of a blank urine sample spiked with DA (1.0 ng mL−1) and a real urine sample extracted with RP-IL-DLLME. It shows no significant interference from endogenous substances observed in the place of the analytes. As can be seen from this preliminary investigation, the method has proved to have good selectivity and sensitivity to determine and quantify DA in the urine of human not specifically exposed to the target neurotoxicants.
Table 5.
Quantification of DA in six human urine samples by RP-IL-DLLME (n = 3).
| Sample | DA (mean ± SD; ng mL−1) |
|---|---|
| 1 | ND |
| 2 | 1.78 ± 0.043 |
| 3 | ND |
| 4 | ND |
| 5 | ND |
| 6 | 0.15 ± 0.028 |
Figure 4.
UHPLC-UV chromatogram of (a) human blank urine and (b) spiked urine sample (1.0 ng mL−1) of DA extracted by RP-IL-DLLME.
4. Conclusions
Extraction of trace compounds from biological substrates is a hot topic in the field of analysis. In this work, a convenient, economical and effective ultra-performance liquid chromatography method based on RP-IL-DLLME for preconcentration and determination of DA in human urine samples was developed. NaOH solution was used as extraction solvent and ionic liquid [C4mim] BF4 was used as dispersion solvent. As a result, potentially hazardous substances, such as volatile organic solvents, are avoided, thus reducing the risk of operation.
Under optimal conditions, enrichment factors of up to 230-fold and effective sample clean-up can be achieved. More importantly, the high linearity, reproducibility, intra- and inter-day precision and accuracy suggest that the developed method is a promising approach for the analysis of low levels of DA in biological samples.
Declarations
Author contribution statement
Qiao feng Wang: Performed the experiments.
Li Jun Liang: Analyzed and interpreted the data.
Jiang Bing Sun: Contributed reagents, materials, analysis tools or data.
Jun Zhou: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the Natural Science Basic Research Plan in Shaanxi Province of China (2020JZ-26).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Wright J.L.C., Falk M., Mcinnes A.G., Walter J.A. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from Eastern Prince Edward Island. Can. J. Chem. 1989;67:481–490. [Google Scholar]
- 2.Schmidt S. Developmental neurotoxicity of domoic acid: evidence for a critical window of exposure. Environ. Health Perspect. 2020;128:1240021–1240023. doi: 10.1289/EHP8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wekell J.C., Gauglitz E.J., Bamett H.J., Hatfield C.L., Simons D., Ayres D. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991-1993. Nat. Toxins. 1994;2:197–205. doi: 10.1002/nt.2620020408. [DOI] [PubMed] [Google Scholar]
- 4.Stobo L.A., Lacaze J.P.C.L., Scott A.C., Petrie J., Turrell E.A. Surveillance of algal toxins in shellfish from Scottish waters. Toxicon. 2008;51:635–648. doi: 10.1016/j.toxicon.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Beltrán A.S., Palafox-Uribe M., Grajales-Montiel J., Cruz-Villacorta A., Ochoa J.L. Sea bird mortality at Cabo Sea Lueas, Mexico: evidence that toxic diatom blooms are spreading. Toxcon. 1997;35:447–453. doi: 10.1016/s0041-0101(96)00140-7. [DOI] [PubMed] [Google Scholar]
- 6.James K.J., Gillman M., Lehane M., Martinez A.G. New fluorimetric method of liquid chromatography for the determination of the neurotoxin domoic acid in seafood and marine phytoplankton. J. Chromatogr. A. 2000;871(1):1–6. doi: 10.1016/s0021-9673(99)00917-6. [DOI] [PubMed] [Google Scholar]
- 7.Chan I.O.M., Tsang V.W.H., Chu K.K., Leung S.K., Lam M.H.W., Lau T.C., Lam P.K.S., Wu R.S.S. Solid-phase extraction-fluorimetric high performance liquid chromatographic determination of domoic acid in natural seawater mediated by an amorphous titania sorbent. Anal. chimica acta. 2007;583(1):111–117. doi: 10.1016/j.aca.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Maroulis M., Monemvasios I., Vardaka E., Rigas P. Determination of domoic acid in mussels by HPLC with post-column derivatization using 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) and fluorescence detection. J. Chromatogr. B. 2008;876(2):245–251. doi: 10.1016/j.jchromb.2008.10.053. 11. [DOI] [PubMed] [Google Scholar]
- 9.Mafra L.L., Léger C., Bates S.S., Quilliam M.A. Analysis of trace levels of domoic acid in seawater and plankton by liquid chromatography without derivatization, using UV or mass spectrometry detection. J. Chromatogr. A. 2009;1216(32):6003–6011. doi: 10.1016/j.chroma.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Botana L.M., Hess P., Munday R., Nathalie A., Degrasse S.L., Feeley M., Suzuki T., Berg M.V.D., Fattori V., Gamarro E.G. Derivation of toxicity equivalency factors for marine biotoxins associated with Bivalve Molluscs. Trends Food Sci. Technol. 2017;59:15–24. [Google Scholar]
- 11.He X., Chen J., Wang J., Tan L. Multipoint recognition of domoic acid from seawater by dummy template molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography. J. Chromatogr. A. 2017;1500(2):61–68. doi: 10.1016/j.chroma.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Witczak A., Sikorski Z.E. CRC Press; 2017. Toxins and Other Harmful Compounds in Foods. [Google Scholar]
- 13.Lawrence J.F., Lau P.Y., Leroux C.C., Lewis D. Comparison of UV absorption and electrospray mass spectrometry for the high-performance liquid chromatographic determination of domoic acid in shellfish and biological samples. J. Chromatogr. A. 1994;659(1):119–126. doi: 10.1016/0021-9673(94)85013-5. [DOI] [PubMed] [Google Scholar]
- 14.Blay P., Hui J.P.M., Chang J., Melanson J.E. Screening for multiple classes of marine biotoxins by liquid chromatography-high-resolution mass spectrometry. Anal. Bioanal. Chem. 2011;400(2):577–585. doi: 10.1007/s00216-011-4772-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Maucher-Fuquay J., Fire S.E., Mikulski C.M., Haynes B., Doucette G.J., Ramsdell J.S. Optimization of solid-phase extraction and liquid chromatography - tandem mass spectrometry for the determination of domoic acid in seawater, phytoplankton, and mammalian fluids and tissues. Anal. chimica acta. 2012;715:71–79. doi: 10.1016/j.aca.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Beach D.G., Liu H., Quilliam M.A. Sensitive determination of domoic acid in mussel tissue using dansyl chloride derivatization and liquid chromatography-mass spectrometry. Anal. Methods-UK. 2015;7(3):1000–1007. [Google Scholar]
- 17.Shum S., Kirkwood J.S., Jing J., Petroff R., Crouthamel B., Grant K.S., Burbacher T.M., Nelson W.L., Isoherranen N. Validated HPLC-MS/MS method to quantify low levels of domoic acid in plasma and urine after subacute exposure. ACS Omega. 2018;3:12079–12088. doi: 10.1021/acsomega.8b02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu W., Wu X., Lin X., Xie Z., Giesy J.P. Quantification of domoic acid in shellfish tissues by pressurized capillary electrochromatography. J. Sep.Sci. 2009;32(12):2117–2122. doi: 10.1002/jssc.200900017. [DOI] [PubMed] [Google Scholar]
- 19.He Y., Fekete A., Chen G., Harir M., Zhang L., Tong P., Schmitt-Kopplin P. Analytical approaches for an important shellfish poisoning agent: domoic acid. J. Agric. Food Chem. 2010;58(22):11525–11533. doi: 10.1021/jf1031789. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X.W., Zhang Z.X. Quantification of domoic acid in shellfish samples by capillary electrophoresis-based enzyme immunoassay with electrochemical detection. Toxicon. 2012;59(6):626–632. doi: 10.1016/j.toxicon.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Wells M.L., Trick C.G., Cochlan W.P., Hughes M.P., Trainer V.L. Domoic acid: the synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 2005;50(6):1908–1917. [Google Scholar]
- 22.Saeed A.F., Awan S.A., Ling S., Wang R., Wang S. Domoic acid: attributes, exposure risks, innovative detection techniques and therapeutics. Algal Res. 2017;24:97–110. [Google Scholar]
- 23.Yu F.Y., Liu B.H., Wu T.S., Chi T.F., Su M.C. Development of a sensitive enzyme-linked immunosorbent assay for the determination of domoic acid in shellfish. J. Agric. Food Chem. 2004;52(17):5334–5339. doi: 10.1021/jf049303t. [DOI] [PubMed] [Google Scholar]
- 24.Tsao Z.J., Liao Y.C., Liu B.H., Su C.C., Yu F.Y. Development of a monoclonal antibody against domoic acid and its application in enzyme-linked immunosorbent assay and colloidal gold immunostrip. J. Agric. Food Chem. 2007;55(13):4921–4927. doi: 10.1021/jf0708140. [DOI] [PubMed] [Google Scholar]
- 25.Kanu A.B. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: a critical review. J. Chromatogr. A. 2021;1654 doi: 10.1016/j.chroma.2021.462444. [DOI] [PubMed] [Google Scholar]
- 26.Sorouraddin S.M., Farajzadeh M.A., Okhravi T. Development of a new method for extraction and preconcentration of cadmium and zinc ions in edible oils based on heat-induced homogeneous liquid–liquid microextraction. J. Iran. Chem. Soc. 2019;16:1537–1543. [Google Scholar]
- 27.Sorouraddin S.M., Farajzadeh M.A., Okhravi T. Application of deep eutectic solvent as a disperser in reversed-phase dispersive liquid-liquid microextraction for the extraction of Cd (II) and Zn (II) ions from oil samples. J. Food Compos. Anal. 2020;93 [Google Scholar]
- 28.Hashemi P., Raeisi F., Ghiasvand A.R., Rahimi A. Reversed-phase dispersive liquid-liquid microextraction with central composite design optimization for preconcentration and HPLC determination of oleuropein. Talanta. 2010;80(5):1926–1931. doi: 10.1016/j.talanta.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 29.Chisvert A., Benedé J.L., Peiró M., Pedrón I., Salvador A. Determination of N-nitroso-iethanolamine in cosmetic products by reversed-phase dispersive liquid-liquid microextraction followed by liquid chromatography. Talanta. 2017;166(1):81–86. doi: 10.1016/j.talanta.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Özzeybek G., Şahin İ., Erarpat S., Bakirdere S. Reverse phase dispersive liquid–liquid microextraction coupled to slotted quartz tube flame atomic absorption spectrometry as a new analytical strategy for trace determination of cadmium in fish and olive oil samples. J. Food Compos. Anal. 2020;90 [Google Scholar]
- 31.Shishov A., Volodina N., Semenova E., Navolotskaya D., Ermakov S., Bulatov A. Reversed-phase dispersive liquid-liquid microextraction based on decomposition of deep eutectic solvent for the determination of lead and cadmium in vegetable oil. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131456. [DOI] [PubMed] [Google Scholar]
- 32.Liu H., Jin P., Zhu F., Nie L., Qiu H. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. TrAC, Trends Anal. Chem. 2021;134 [Google Scholar]
- 33.Pena-Pereira F., Lavilla I., Bendicho C., Vidal L., Canals A. Speciation of mercury by ionic liquid-based single-drop microextraction combined with high-performance liquid chromatography-photodiode array detection. Talanta. 2009;78(2):537–541. doi: 10.1016/j.talanta.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Rykowska I., Ziemblińska J., Nowak I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: a review. J. Mol. Liq. 2018;259:319–339. [Google Scholar]
- 35.Baranowskaa I., Magiera S., Baranowski J. UHPLC method for the simultaneous determination of β-blockers, isoflavones and their metabolites in human urine. J. Chromatogr. B. 2011;879:615–626. doi: 10.1016/j.jchromb.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt A., Fiechter G., Fritz E.M., Maye H.K. Quantitation of capsaicinoids in different chilies from Austria by a novel UHPLC method. J. Food Compos. Anal. 2017;60:32–37. [Google Scholar]
- 37.Xu X.W., Huang L.Y., Wu Y.J., Yang L.J., Huang L.Y. Synergic cloud-point extraction using [C4mim] [PF6] and Triton X-114 as extractant combined with HPLC for the determination of rutin and narcissoside in Anoectochilus roxburghii (Wall.) Lindl. and its compound oral liquid. J. Chromatogr. B. 2021;1168 doi: 10.1016/j.jchromb.2021.122589. [DOI] [PubMed] [Google Scholar]
- 38.Chandrasekaram K., Alias Y., Mohamad S. Sporopollenin supported methylimidazolium ionic liquids based mixed matrix membrane for dispersive membrane micro-extraction of nitro and chloro-substituted phenols from various matrices. Microchem. J. 2022;172 [Google Scholar]
- 39.CDER e C.V.M. Food and Drug Administration; 2018. Bioanalytical Method Validation-Guidance for Industry. [Google Scholar]
- 40.International conference on harmonization of technical requirements forRegistration of pharmaceuticals for human use, ICH harmonised tripartite guideline (2005) Validation of Analytical Procedures: Text and MethodologyQ2. R1; Geneva: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.