Abstract
Single crystal XRD structure of the title compound reveals that the molecule adopt non-planar structure. The molecule is puckered with the total puckering amplitude of (Q) = 0.368(3)Å. Crystals of the title molecules are interconnected by intermolecular O–H⋯O and C–H⋯O interactions to develop 1D chains extending infinitely along the crystallographic a-axis. The intermolecular interactions were explored by Hirshfeld surfaces and their associated fingerprint graphs are obtained which revealed that the H⋯H and H⋯C pairs of inter atomic contacts were pre-dominant in the crystal packing of title compound. The energy of intermolecular interactions are computed using the accurate energy density model of B3LYP/6–31 G(d,p).
Keywords: Single crystal XRD, Boratanaphthalene, Intermolecular interactions, Hirshfeld surfaces, 3D-interaction energies
Graphical abstract
Single Crystal XRD; Boratanaphthalene; Intermolecular interactions; Hirshfeld surfaces; 3D-Interaction energies.
Specification Table
| Subject area | X-ray crystallography |
|---|---|
| Compound | 2,2-Diphenyl-1-oxa-3-oxonia-2-boratanaphthalene |
| Data category | Crystallographic data |
| Data acquisition format | CIF |
| Data type | Analysed |
| Procedure | Crystal structure determination, interpretation and computational analysis |
| Data accessibility | CCDC: 2064704 |
1. Introduction
Boron fused tri- and tetra-coordinated molecules have found great importance for multifunctional applications such as OLEDs, bio-imaging etc. due to their wide window of electronic properties. Some of the tetra-coordinated boron chelated N, O structures have found to be highly emissive in solid state with colour tunability due to their rigid π-conjugated structures [1, 2]. Crystal structure and molecular packing play major role in the light emissive devices wherein weak π···π interactions greatly helps for enhanced emission [3]. Hence understanding the structural dynamics of the molecule is of great significance in this perspective. The literature study strongly reveals that, the organic compounds bearing hetero atoms possess significant biological activities such as anti-viral, anti-fungal, anti-cancer, etc. [4, 5, 6] Further, presence of inter-molecular interactions in the architecture of crystal packing and their co-operation with hydrogen bonding guides in the synthetic and catalytic utility. Owing to this, the intermolecular interactions present in the crystal were investigated by three dimensional Hirshfeld surfaces (3D-HS) and also their energies were evaluated using accurate energy density wavefunction of B3LYP/6-31G(d,p). With respect to all the above perspectives and in continuation of our ongoing work on structural studies, we now report the structural and Hirshfeld surface studies of the compound 2,2-diphenyl-1-oxa-3-oxonia-2-boratanaphthalene.
2. Procedures
2.1. Experimental
2,2-Diphenyl-1-oxa-3-oxonia-2-boratanaphthalene was synthesized according to the reported procedure [7]. 2-(diphenylboryl)ethanamine (100 mg, 0.96 mmol) was refluxed with 2-hydroxybenzaldehyde (100μL, 0.96 mmol) in 5 mL methanol for 4 h to obtain cream colour solid with 90% yield (Figure 1). Single crystals were grown in ethanol using slow evaporation method.
Figure 1.
Schematic diagram of the compound 2,2-diphenyl-1-oxa-3-oxonia-2-boratanaphthalene.
2.2. Single crystal XRD
The structural and refinement details of the title compound are given in Table 1. A suitable size of block shaped single crystal was selected carefully for X-ray diffraction study. The diffraction data were collected on an Apex Bruker-II diffractometer equipped with MoKα radiation of wavelength 0.71073Å. Complete X-ray intensity data set was processed by SAINT software [8]. The crystal and molecular structure was solved by direct method using SHELXS and refined by full-matrix least squares based on F2 SHELXL [9, 10, 11]. All the non-hydrogen atoms were refined anisotropically while the hydrogen atoms were refined isotropically and placed at chemically ideal positions with isotropic displacement parameters as C–H = 0.93Å with Uiso(H) = 1.2Ueq(C) for aromatic rings and O–H = 0.82Å with Uiso(H) = 1.5Ueq(O). The molecular and packing diagram were generated using the MERCURY [12] while PLATON software was used to obtain geometric parameters viz., bond distances, inter-bond angles and torsional angles [13, 14]
Table 1.
Crystal, structural and refinement statistics.
| CCDC | 2064704 |
|---|---|
| Chemical formula | C21H19BNO2 |
| Mr | 328.18 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 290 |
| a, b, c, β (Å,°) | 9.1024(6), 17.8682(15), 10.9790(9), 96.933(2) |
| V (Å−3), Z | 1772.6(2), 4 |
| Radiation type | MoKα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.40 × 0.36 × 0.31 |
| Diffractometer | APEX (Bruker, 2006) |
| No. of measured, independent and observed reflections | 22044, 3649, 1990 |
| Rint | 0.05 |
| (sin θ/λ)max (Å−1) | 0.627 |
| Goodness-of-Fit (S) | 1.048 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0632, 0.2065, 1.048 |
| No. of reflections | 3649 |
| No. of parameters | 226 |
| Data/Restraints/Parameters | 3649/0/226 |
| H-atom treatment | H-atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (eÅ−3) | 0.55, -0.34 |
| Index ranges | h = -11→10, k = -22→22, l = -13→13 |
| Theta range (°) | Θmax = 25.2, θmin = 2.2 |
2.3. Computational studies
Three dimensional Hirshfeld surfaces (3D-HS) mapped on both dnorm and electrostatic potentials (ESP), and their associated two dimensional fingerprint graphs are carried out making use of Crystal Explorer 17.5 software and to compute three dimensional interaction energies [15, 16, 17, 18, 19, 20]
3. Results and discussions
3.1. Structural commentary
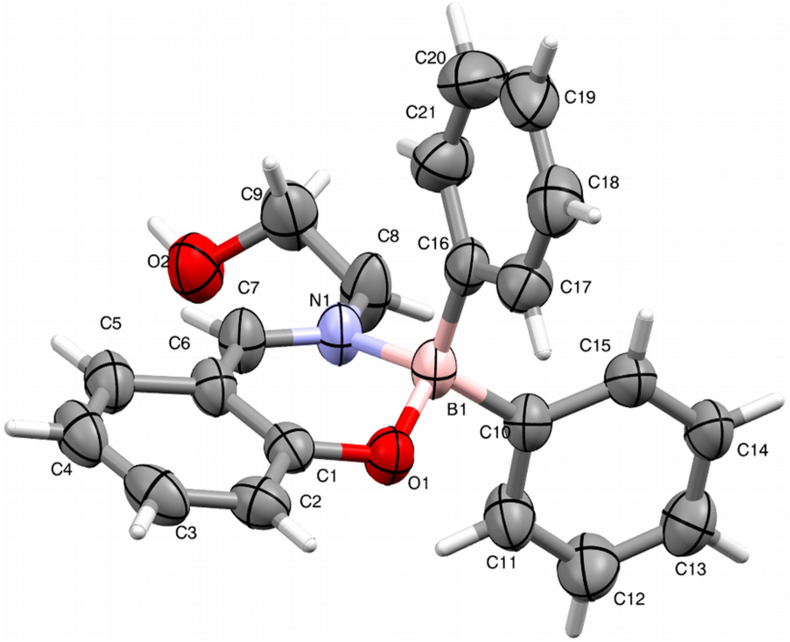
The molecular structure of the title compound consists of a ten-membered heterocyclic ring [O1–B1–N1–C1/C7] and two phenyl rings [C10/C15] and [C16/C21] which are interlinked such that to have a non-coplanar view, and an -ethoxy group as an extended conformation as given in thermal ellipsoids drawn at 50% probability (Figure 2). The ten-membered ring [O1–B1–N1–C1/C7] displayed a puckering environment at B1 with the total puckering amplitude (Q) of 0.368 (3)Å and displayed a half-chair conformation. The pseudorotation (θ) and the relative phase (φ) angles are 114.0(3)° and 136.6 (4)° respectively [21, 22]
Figure 2.
Thermal ellipsoids of a title molecule at 50% of probability.
In the molecule, the mean planes of phenyl rings [(C10/C15) and (C16/C21)] are inclined with the dihedral angle of 77.05 (1)°. The central ten-membered ring (O1–B1–N1–C1/C7) makes the dihedral angle of 85.65(12)° and 87.55(1)° with the mean planes of phenyl rings (C10/C15) and (C16/C21) respectively, which reveals that the rings exhibits nearly an orthogonal relation to each other. The chain, N1–C8–C9–O2 adopts a -syn-clinal conformation with the torsional angle of -75.27°. The bond lengths and inter-bond angle values agree with the standard values [23, 24, 25, 26, 27, 28, 29].
3.1.1. Crystal packing
The crystal of the titled molecule are governed by an intermolecular O2–H2⋯O1 and C14–H14⋯O2 interactions (Table 2), to develop an independent 1D polymeric chains propagating indefinitely along crystallographic a-axis, as depicted in Figure 3. In addition to this, the titled molecule also comprise an intra-molecular C7–H7⋯O2 interaction, incorporating a self-assembly closed S(6) loop.
Table 2.
Hydrogen bonding geometry (Å,°).
| Interaction | D-H...A | d(H...A) | d(D...A) | Angle (D-H...A) | Symmetry code |
|---|---|---|---|---|---|
| Intra-molecular | C7–H7⋯O2 | 2.30 | 2.970(4) | 129 | |
| Intermolecular | O2–H2⋯O1 | 2.08 | 2.900(3) | 176 | -1/2+x,1/2-y,1/2+z |
| C14–H14⋯O2 | 2.52 | 3.363(4) | 152 | 1+x,y,z |
Figure 3.
Packing of the title molecules when it is viewed along crystallographic a-axis.
3.2. Computational analysis
The volume of 3D-HS mapped on dnorm (Figure 4) and electrostatic potential (Figure 5) are 369.88Å2 and 435.97Å3 respectively, with the colour scales of [−0.5324 a.u (red) and +1.4509 a.u (blue)], and [−0.1112 a.u (red) and +0.1478 a.u (blue)] respectively. The surfaces are given in transparent mode to understand the position of all the elements and the functional groups available in the molecule. The dnorm furnishes both the donor and acceptor regions of hydrogen bonding interactions by forming red colour circular spots on its surface with equal brightness.
Figure 4.
Front (a) and rear (b) views of Hirshfeld surface mapped on dnorm.
Figure 5.
Front (a) and rear (b) views of HS mapped on electrostatic potentials.
For instance, the donor and acceptor regions of intermolecular O2–H2⋯O1 interaction can be viewed on the front and rear views of dnorm surfaces, given in Figures 4 a and b respectively. In Figure 4a the red spot surrounding O2–H2 and at the vicinity of O1 in Figure 4b are the indicators of donor and acceptor regions of O2–H2⋯O1 interaction (Table 2) [30, 31, 32, 33]. This is also substantiated by electrostatic potentials (Fig. 5a and b are front and rear views) by reflecting intense blue coloured patch around O2–H2, while the red coloured patch around O1 represents the electrostatic positive donor and negative acceptor potentials respectively. These electrostatic interactions serve as an important tool in the binding of protein targets [34].
The two-dimensional fingerprint graphs were generated in an expanded mode for all the major contacts, and it is delineated in Figure 6. Total contribution from all the pairs of inter atomic contacts is given in Figure 6(a). The highest contribution is represented by a pair of H⋯H inter atomic contacts with 60.9%, reflected as a pair of blue coloured spoke like pattern, merged almost in the middle of the graph at de + di = 1.1Å Figure 6(b). The 29.9% of contribution was made to the Hirshfeld surface from H⋯C pair of inter atomic contacts producing a pair of unique blue coloured wings between the region of 1.12Å<(de + di) < 1.72Å Figure 6(c). The pair of H⋯O inter atomic contacts represents only 7.9% of total contribution which is emerged as a characteristic sharp spikes over the region of 0.79 Å<(de + di) < 1.18Å Figure 6(d). Presence of H⋯O and H⋯C contacts correlates with the intermolecular interactions given in Table 2. Lastly, the C⋯C inter atomic contacts comprise only 2.2% to the crystal packing which is observed as an arrowhead pattern in the region of de + di ≈ 1.70Å pointing diagonally left downwards Figure 6(e). Overall study of fingerprint graphs reveals that the pair of H⋯H and H⋯C inter atomic contacts are the predominant ones. While H⋯O and C⋯C contacts are less significant in the formation of a three dimensional Hirshfeld surface.
Figure 6.
Two dimensional fingerprints graphs for the title compound. The di and de along x and y represents the nearest adjacent nuclei internal and external from the three dimensional Hirshfeld surface. (a) Total contribution, (b) H⋯H (c) H⋯C (d) H⋯O and (e) C⋯C inter atomic contacts.
Three-dimensional interaction energies were computed for the title compound using the CrystalExplorer17.5 software [35, 36]. Interaction energy was calculated using an accurate energy density model of B3LYP/6-31G(d,p), during the calculation a cluster of seventeen molecules (comprising a total of 748 atoms) of various colour coding scheme from different Cartesian co-ordinates were surrounding the original one (showed by black coloured ball and stick model) within the default radius of 3.8Å. The total interaction energy (Etot) in the energy framework have been classified into classic electrostatic (Eele), polarization (Epol), dispersion (Edis) and exchange repulsion (Erep) energies [37].
Further, in the generation of electrostatic, dispersion and total energy frameworks, the tube size of 50 and threshold cut-off energy level of 10 kJ/mol were maintained. The Coloumbic (Eele) energy terms are represented by red coloured solid cylinders (Figure 7), and green coloured solid cylinders in the construction of dispersion energy (Etot) frameworks given in Figure 8. The total energy (Etot) terms are constructed by using the blue coloured solid cylinders (Figure 9). In each of the energy profile the difference in thickness of solid cylinders reveals the relative strength of interaction with the constituent members of a cluster [38, 39], and plays an important role in the integrity of a crystal packing. Also, the red- and orange-coloured dashed lines in all the energy profiles reveal the molecules involved in the hydrogen bonding interactions given in Table 2. The total interacting energies in the cluster of title compound are given in component form (Table 3). Accordingly, the highest total interaction energy (Etot = -67.6 kJ/mol from all the components) was noticed with a pair of symmetric pale-green coloured molecules interacting at the centroid distance of R = 7.76Å which correlates with the intermolecular O2–H2⋯O1 interaction. The total interaction energy (Etot = -18.2 kJ/mol) associated with an intermolecular C14–H14⋯O2 interaction confirms with the red coloured molecules at the centroid distance of R = 9.10Å. The least total interaction energy was observed as Etot = -1.8 kJ/mol associated with a couple of pink coloured molecules having the longest molecular centroid distance of R = 13.14Å. Further, the total energy from all the components in a cluster, the dispersion energy (Edis) terms were dominated over the other terms as observed in the reported compounds [40, 41, 42]. Further, the total interaction energies are varying with the molecular centroid distances obeying the laws of electrostatics.
Figure 7.
Three-dimensional interaction energy profile for Coloumbic (Eele) terms generated with the tube size of 50 and cut-off energy of 10 kJ/mol for clarity purpose.
Figure 8.
Three-dimensional interaction energy profile for dispersion (Edis) terms generated with the tube size of 50 and cut-off energy of 10 kJ/mol for clarity purpose.
Figure 9.
Three-dimensional interaction energy profile for total energy (Etot) terms generated with the tube size of 50 and cut-off energy of 10 kJ/mol for clarity purpose.
Table 3.
Three-dimensional interaction energies (kJ/mol) in component form for the title compound for electron density B3LYP/6-31G(d,p). Colour code: Colour coding scheme of molecules, N: Number of interacting molecules with the reference one, Symop: Rotational symmetry operator, R: Molecular centroid distance. Scale factor: Etot= kelex Eele+ kpolx Epol+ kdisx Edis+ krepx Erep Mackenzie et al. [43].
4. Conclusion
The title compound is crystallized in a monoclinic system with P21/n space group. The molecular structure of the compound was puckered at B1 and adopts half-chair conformation. The packing of molecules were governed by intra- and intermolecular C–H⋯O and O–H⋯O interactions and, they are explored by three dimensional Hirshfeld surfaces and fingerprint calculations, revealing that the H⋯H and H⋯C pairs of inter atomic contacts have made the significant contribution towards the formation of three dimensional Hirshfeld surfaces. The interaction energy calculations reveal -67.6 kJ/mol is the highest total interaction energy (from all the components) associated with an intermolecular O2–H2⋯O1 interaction. Further, in the energy frameworks, in each case the dispersion energy Edis term dominated over other components obeying laws of electrostatics.
Declarations
Author contribution statement
N. R. Sreenatha: Analyzed and interpreted the data; Wrote the paper.
D. P. Ganesha: Contributed reagents, materials, analysis tools or data.
A. S. Jeevan Chakravarthy, B. N. Lakshminarayana: Conceived and designed the experiments.
B Suchithra: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data associated with this study has been deposited at CCDC under the accession number 2064704.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are thankful to Dr. (Mrs) R. Chandramani, Professor (Rtd), Department of Physics, Bangalore University, Bengaluru (India) for her support, and Prof. M Jayashankar, St. Joseph’s Autonomous College, Bengaluru, Karnataka, India. Dedicating to our Respected Professor (Rtd), Dr. (Mrs.) R. Chandramani, Ph.D, D.Sc., Bangalore University, and beloved N. S. Vedha Karthik, Hassan and A. J. Siddhanth Chakravarthy, Bangalore.
References
- 1.Li D., Zhang H., Wang C., Huang S., Guo J., Wang Y. Construction of full-color-tunable and strongly emissive materials by functionalizing a boron-chelate four-ring-fused π-conjugated core. J. Mater. Chem. 2012;22:4319–4328. [Google Scholar]
- 2.Kubota Y., Kasatani K., Takai H., Funabiki K., Matsui M. Strategy to enhance solid-state fluorescence and aggregation-induced emission enhancement effect in pyrimidine boron complexes. Dalton Trans. 2015;44:3326–3341. doi: 10.1039/c4dt03318c. [DOI] [PubMed] [Google Scholar]
- 3.Feng S., Gong S., Feng G. Aggregation-induced emission and solid fluorescence of fluorescein derivatives. Chem. Commun. 2020;56:2511–2513. doi: 10.1039/c9cc09784h. [DOI] [PubMed] [Google Scholar]
- 4.Patel S.A., Rajale T., O’Brien E., Burkhart D.J., Nelson J.K., Twamley B., Blumenfeld A., Szabon-Watola M.I., Gerdes J.M., Bridges R.J., Natale N.R. Isoxazole analogues bind the system Xc-transporter: structure–activity relationship and pharmacophore model. Bioorg. Med. Chem. 2010;18:202–213. doi: 10.1016/j.bmc.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva N.M., Tributino J.L.M., Miranda A.L.P., Barrerio Carlos E.J., Fraga F.M. New isoxazole derivatives designed as nicotinic acetylcholine receptor ligand candidates. Eur. J. Med. Chem. 2002;37:163–170. doi: 10.1016/s0223-5234(01)01327-7. [DOI] [PubMed] [Google Scholar]
- 6.Kaffy J., Pontikis R., Carrez D., Croisy A., Monneret C., Florent J.-C. Isoxazole-type derivatives related to combretastatin A-4, synthesis and biological evaluation. Bioorg. Med. Chem. 2006;14:4067–4077. doi: 10.1016/j.bmc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Claas K.E., Hohaus E. Pre-column fluorescent derivatization for high-performance liquid chromatography with 2,2-diphenyl-1-oxa-3-oxonia-2-boratanaphthalene (DOOB): determination of aminoalkanols. Z. für Anal. Chem. 1985;322:343–347. [Google Scholar]
- 8.Bruker . Bruker AXS Inc.; Madison, Wisconsin, USA: 2006. APEX2 and SAINT. [Google Scholar]
- 9.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 10.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015;C71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheldrick G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015;A71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macrae C.F., Sovago I., Cottrell S.J., Galek P.T.A., McCabe P., Pidcock E., Platings M., Shields G.P., Stevens J.S., Towler M., Wood P.A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020;53:226–235. doi: 10.1107/S1600576719014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spek A.L. What makes a crystal structure report valid? Inorg. Chim. Acta. 2018;470:232–237. [Google Scholar]
- 14.Spek A.L. checkCIF validation ALERTS: what they mean and how to respond. Acta Crystallogr. 2020;E76:1–11. doi: 10.1107/S2056989019016244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spackman M.A., Jayatilaka D. Hirshfeld surface analysis. CrystEngComm. 2009;11:19–32. [Google Scholar]
- 16.Spackman M.A., McKinnon J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002;4:378–392. [Google Scholar]
- 17.Spackman M.A., McKinnon J.J., Jayatilaka D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm. 2008;10:377–388. [Google Scholar]
- 18.McKinnon J.J., Jayatilaka D., Spackman M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007:3814–3816. doi: 10.1039/b704980c. [DOI] [PubMed] [Google Scholar]
- 19.Gavezzotti A. Calculation of Intermolecular Interaction Energies by Direct Numerical Integration over Electron Densities. I. Electrostatic and Polarization Energies in Molecular Crystals. J. Phys. Chem. B. 2002;106:4145–4154. [Google Scholar]
- 20.McKinnon J.J., Spackman M.A., Mitchell A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. 2004;B60:627–668. doi: 10.1107/S0108768104020300. [DOI] [PubMed] [Google Scholar]
- 21.Cremer D. On the correct usage of the Cremer-Pople puckering parameters as quantitative descriptors of ring shapes - a reply to recent criticism by Petit, Dillen and Geise. Acta Crystallogr. 1984;B40:498–500. [Google Scholar]
- 22.Evans D.G., Boeyens J.C.A. Conformational analysis of ring pucker. Acta Crystallogr. 1989;B45:581–590. [Google Scholar]
- 23.Schiemenz G., Nāther C. peri-Interactions in Naphthalenes, 7 [1]. A Hetera-naphthalene as a Model Compound for 8-Dimethylaminonaphth- 1-yl Silicon and Phosphorus Compounds. Zeitschrift für Naturforschung B. 2002;57(3):309–318. [Google Scholar]
- 24.Sreenatha N.R., Lakshminarayana B.N., Ganesha D.P., Vijayshankar S., Nagaraju S. Crystal Structure and Hirshfeld Surfaces of (E)-1-(2-Hydroxyphenyl)-3-(5-methylthiophen-2-yl)prop-2-en-1-one. X-ray Struct. Anal. Online. 2018;34:23–24. [Google Scholar]
- 25.Nagaraju S., Sridhar M.A., Sreenatha N.R., Pradeepa Kumara C.S., Sadashiva M.P. Crystal Structure of (4-Fluorophenyl)(2-(methylthio)thiophen-3-yl)methanone. X-ray Struct. Anal. Online. 2018;34:13–14. [Google Scholar]
- 26.Braunstein P., Cura E., Herberich G.E. Heterometallic complexes and clusters with 2-boratanaphthalene ligands. J. Chem. Soc. Dalton Trans. 2001;(11):1754–1760. [Google Scholar]
- 27.Daoui Said, Berrin Cinar Emine, El Kalai Fouad, Saddik Rafik, Karrouchi Khalid, Benchat Noureddine, Baydereb Cemile, Dege Necmi. Crystal structure and Hirshfeld surface analysis of 4-(4-methylbenzyl)-6-phenylpyridazin-3(2H)-one. Acta Crystallogr. 2019;E75:1352–1356. doi: 10.1107/S2056989019011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serajul Haque Faizi Md., Berrin Cinar Emine, Erman Dogan Onur, Sema Aydin Alev, Agar Erbil, Dege Necmi, Mashrai Ashraf. Crystal structure, Hirshfeld surface analysis and DFT studies of 4-methyl-2-({[4-(trifluoromethyl)phenyl]imino}methyl)phenol. Acta Crystallogr. 2020;E76:1325–1330. doi: 10.1107/S2056989020009615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berrin Cinar Emine, Serajul Haque Faizi Md., Kahveci Yagci Nermin, Erman Dogan Onur, Sema Aydin Alev, Agar Erbil, Dege Necmi, Mashrai Ashraf. Crystal structure, Hirshfeld surface analysis and DFT studies of (E)-4-methyl-2-{[(2-methyl-3-nitrophenyl)imino]methyl}phenol. Acta Crystallogr. 2020;E76:1551–1556. doi: 10.1107/S2056989020011652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreenatha N.R., Lakshminarayana B.N., Ganesha D.P., Gnanendra C.R., Nagaraju S., Madan Kumar S. Structural and Hirshfeld surfaces of thiophene based isoxazole derivatives: 3-(3-Methylthiophen-2-yl)-5-(3,4,5-trimethoxyphenyl)isoxazole and 5-(3-Methylthiophen-2-yl)-3-(3,4,5-trimethoxyphenyl)isoxazole. Chem. Data Collect. 2018;17–18:394–403. [Google Scholar]
- 31.Sreenatha N.R., Lakshminarayana B.N., Ganesha D.P., Gnanendra C.R. Crystal structure and Hirshfeld surface analysis of (E)-1-(3,5-dichloro-2-hydroxyphenyl)-3-(5-methylfuran-2-yl)prop-2-en-1-one. Acta Crystallogr. 2018;E74:1451–1454. doi: 10.1107/S2056989018012173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daoui Said, Berrin Cinar Emine, Dege Necmi, Chelfi Tarik, El Kalai Fouad, Abudunia Abdulmalik, Karrouchi Khalid, Benchat Noureddine. Crystal structure and Hirshfeld surface analysis of 4-(2,6-dichlorobenzyl)-6-[(E)-2-phenylethenyl]pyridazin-3(2H)-one. Acta Crystallogr. 2021;E77:23–27. doi: 10.1107/S205698902001573X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeşilbağ Semanur, Berrin Çınar Emine, Dege Necmi, Ağar Erbil, Saif Eiad. Crystal structure and Hirshfeld surface analysis of dimethyl 3,3′-{[(1E,2E)-ethane-1,2-diylidene]bis(azanylylidene)}bis(4-methylbenzoate) Acta Crystallogr. 2022;E78:1–6. doi: 10.1107/S2056989022002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain R., Ernst J.T., Kutzki O., Park H.S., Hamilton A.D. Protein recognition using synthetic surface-targeted agents. Mol. Divers. 2014;8:89–100. doi: 10.1023/b:modi.0000025652.55320.16. [DOI] [PubMed] [Google Scholar]
- 35.Turner M.J., Thomas S.P., Shi M.W., Jayatilaka D., Spackman M.A. Energy frameworks: insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015;51:3735–3738. doi: 10.1039/c4cc09074h. [DOI] [PubMed] [Google Scholar]
- 36.Elejalde N-R., Butassi E., Zacchino S., Macías M.A., Portilla J. Intermolecular interaction energies and molecular conformations in N-substituted 4-aryl-2-methylimidazoles with promising in vitro antifungal activity. Acta Crystallogr. 2019;B75:1197–1207. doi: 10.1107/S2052520619013271. [DOI] [PubMed] [Google Scholar]
- 37.Sreenatha N.R., Harisha A.S., Ganesha D.P., Mahadeva Prasad T.N., Thippeswamy G.B., Lakshminarayana B.N. Structural Investigation, Hirshfeld Surfaces and 3D Interaction Energy Analysis of the Compound 3-aryl-2-cyanoprop-2-enoic Acid. Eur. J. Appl. Phys. 2022;4:12–23. [Google Scholar]
- 38.Lakshminarayana B.N., Sreenatha N.R., Jeevan Chakravarthy A.S., Suchithra B., Hariprasad S. Structural, Computational and 3D Interaction Energy Calculations of the Compound 2-chloro-3-(1-napthyl)-5,5-dimethyl-2-cyclohexenone. Crystallogr. Rep. 2022;67(2):201–208. [Google Scholar]
- 39.Dilshad Sumra, Berrin Çınar Emine, Ali Arif, Ahmed Adeeba, Alam Mohd Jane, Ahmad Musheer, Ahmad Aiman, Dege Necmi, Saif Eiad. Crystal structure and Hirshfeld surface analysis of 2,4,6-triaminopyrimidine-1,3-diium dinitrate. Acta Crystallogr. 2022;E78:669–674. doi: 10.1107/S2056989022005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreenatha N.R., Jeevan Chakravarthy A.S., Lakshminarayana B.N., Hariprasad S. Structural characterization, computational, charge density studies of 2-chloro-3-(2’-methoxy)-5,5-dimethyl-2-cyclohexenone. J. Mol. Struct. 2021;1225:1–8. [Google Scholar]
- 41.Sreenatha N.R., Jeevan Chakravarthy A.S., Suchithra B., Lakshminarayana B.N., Hariprasad S., Ganesha D.P. Crystal, spectral characterization, molecular docking, Hirshfeld computational studies and 3D-energy framework analysis of a novel puckered compound (C14H15Cl O): 2-chloro-3-phenyl-5,5-dimethylcyclohex-2-en-1-one. J. Mol. Struct. 2020;1210:1–14. [Google Scholar]
- 42.Ganesha D.P., Sreenatha N.R., Gnanendra C.R., Lakshminarayana B.N. Structural, Hirshfeld surface studies and computation of interaction energies of 4-Amino-N-(3-chloropyrazin-2-yl)benzene-1-Sulfonamide organic compound. Mater. Today Proc. 2022;49:817–823. [Google Scholar]
- 43.Mackenzie C.F., Spackman P.R., Jayatilaka D., Spackman M.A. CrystalExplorer model energies and energy frameworks: extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ. 2017;4(5):575–587. doi: 10.1107/S205225251700848X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at CCDC under the accession number 2064704.