Abstract
Background
Hip fractures occurring in older patients often result in significant anemia, even hemodynamic disorders and hypoperfusion. The present study aims to investigate the general characteristics of hypotension following hip fracture surgery (HFHFS) and its effect on clinical outcomes.
Methods
A total of 168 patients aged ≥80 years who underwent hip fracture surgery at a tertiary orthopaedic hospital from January 1, 2020 to August 31, 2020 were enrolled and followed up for one year. Patients were divided into HFHFS and non-HFHFS cohorts according to blood pressure within 24 h after surgery. General difference comparison, univariate and multivariate regression, and survival analysis were applied to investigate the association between HSHSF and in-hospital and one-year clinical outcomes.
Results
The incidence of HFHFS was 23.8% (40/168), with a median time to onset of 8.0 (5.0–12.0) hours after surgery. The HFHFS group had more chronic heart disease before injury and experienced more positive fluid balance on the day of surgery (P values were 0.032 and 0.028, respectively). After adjustment for potential confounders, HFHFS was associated with prolonged length of hospital stay (B 2.66, 95% CI 0.22, 5.10; P = 0.033), postoperative cardiac dysfunction (OR 2.92, 95% CI 1.05, 8.11; P = 0.039), and postoperative brain dysfunction (OR 3.51, 95% CI 1.50, 8.23; P = 0.004). HFHFS had no effect on one-year modified Rankin Scale (mRS) (B 0.28, 95% CI -0.28, 0.84; P = 0.322) and one-year mortality (HR 1.07, 95% CI 0.29, 3.96; P = 0.917).
Conclusion
Many older patients develop hypotension several hours after hip fracture surgery, which may be related with preexisting decline in cardiac reserve in addition to postoperative hidden blood loss. Patients who experienced HFHFS were more likely to have postoperative cardiac and brain dysfunction and longer hospital stay. However, HFHFS had no significant effect on mRS and mortality at one year.
Keywords: Hypotension, Older patients, Hip fracture, Clinical outcomes
Hypotension, Older patients, Hip fracture, Clinical outcomes.
1. Introduction
Perioperative hypotension is a common clinical problem, which has been confirmed by a large number of studies to increase the risks of vital organ injury (heart, brain, kidneys, etc), and is associated with postoperative mortality [1, 2, 3]. Perioperative blood pressure (BP) regulation goals should be based on many considerations such as the type of surgery, patient's baseline BP, and risks of hypotension-related organ ischemia and hypertension related bleeding [4]. Especially in older patients, individualized perioperative BP management is more crucial because the reduced physiological reserve of older population leads to a more general propensity for hypotension and worse clinical outcomes [5].
Older patients with hip fracture, who typically experience both fracture and surgery, are clearly at high risk for hypotension and hypoperfusion. It has been shown that hip fracture in older patients caused a similar physiological insult as major trauma does in younger patients [6]. Meanwhile, hypotension during hip fracture surgery has also been shown to be associated with postoperative complications, even 5-day and 30-day mortality [7, 8, 9]. Hypoperfusion is bound to deteriorate the declining organ function in older population, so hypotension following hip fracture surgery (HFHFS) should also be recognized early and intervened appropriately. However, there is not enough evidence about HFHFS. Here we hypothesize that the occurrence of HFHFS is related to the preoperative basic condition of patients, which may increase in-hospital complications (postoperative acute cardiac, brain and renal dysfunction, prolonged hospital stay) and even cause adverse effects on long-term prognosis (living ability and mortality at one year). We intend to first explore the general clinical characteristics of HFHFS, and then separately investigate the association between HFHFS and different clinical outcomes.
2. Methods
2.1. Study design and population
This observational prospective cohort study included consecutive patients aged 80 years or older with hip fracture admitted to Sichuan Provincial Orthopedic Hospital (Chengdu, Sichuan) from January 1, 2020 to August 31, 2020. The inclusion and exclusion criteria, and the enrollment process of study participants have been described in previous study (https://pubmed.ncbi.nlm.nih.gov/33854308/). The total population was divided into HFHFS and non-HFHFS group according to the presence or absence of hypotension within 24 h after surgery (Figure 1). In this study, a systolic blood pressure (SBP) of 90 mmHg or 80% of the baseline value was set as the cut-off for defining hypotension. This study received approval from the Ethics Commission of Sichuan Provincial Orthopedic Hospital (KY2020-032-01). Because this was an observational study, the requirement for informed consent was waived. We conducted this research following the Declaration of Helsinki.
Figure 1.
Flow chart of study participants Abbreviations: HFHFS, hypotension following hip fracture surgery.
2.2. Medical procedures and data collection
Multi-disciplinary team and co-management mode are routine measures in our institution for senile hip fracture patients. Hip fracture surgery was performed under general anesthesia with laryngeal mask airway following fascia iliaca block. Intraoperative hypotension and vasopresors use were recorded by the anesthesiologist. After the surgery, the patient's vital signs were monitored continuously for at least 24 h. The same type of monitoring device was used for intraoperative and postoperative monitoring. The early rehabilitation was mainly carried out in bed. In addition to perioperative multidisciplinary assessment and optimization, the co-management mode was implemented by the medical team composed of orthopedics, critical care, geriatrics and rehabilitation medicine for all patients during hospitalization, and the main medical decisions were made after joint ward rounds. Regular follow-up after discharge was done in the joint outpatient department consisting of internal medicine, orthopedics and sports medicine, focusing on chronic disease management and exercise prescription formulation. In this study, the baseline characteristics at admission and information on the day of surgery for all patients were prospectively collected through an electronic medical record system.
2.3. Definition of HFHFS
We set the BP measured 5 min after fascia iliaca block as the basal value with reference to previous study [5]. HFHFS was defined as follows: SBP was ≤90 mmHg or 80% of the basal value within 24 h following hip fracture surgery [4,5], and lasting more than 10 min or vasopressors was administered. The BP in this study refers to non-invasive blood pressure obtained through the monitoring device.
2.4. Measurement of outcomes
Postoperative cardiac, brain and renal dysfunction and the length of hospital stay (LOS) were included and recorded as in-hospital clinical outcomes in this study. Postoperative organ dysfunction referred to the changes that occurred after fracture surgery or worsened on the basis of preoperative dysfunction. Diagnostic criteria for postoperative cardiac, brain and renal dysfunction were based on Diagnostic Criteria for Multiple Organ Dysfunction Syndrome in the Elderly (MODSE) (draft, 2003) [10] (Table 1). Meanwhile, the Confusion Assessment Method for Intensive Care Unit (CAM-ICU) was applied for the diagnosis of delirium during hospitalization [11]. We used modified Rankin Scale (mRS) and mortality at one year for long-term clinical outcomes. All study subjects were followed up through phone or outpatient visits until death or withdrawal from the program, and censored at one year if the patient was survival. The mRS from 0 to 6 represents no symptoms, no significant disability, slight disability, moderate disability, moderately severe disability, severe disability, and death in turn [12]. The one-year mortality was calculated from the date of hip fracture surgery.
Table 1.
Diagnostic criteria for MODSE.
| Pre-failure stage | Failure stage | |
|---|---|---|
| Heart† | i. Emerging arrhythmia, normal cardiac enzymes ii. Exertional dyspnea, no definite signs of heart failure iii. Increased PAWP (13–19 mmHg)‡ | i. Reduced stroke volume (EF ≤ 40%) ii. PAWP ≥20 mmHg§ iii. Definite signs and symptoms of heart failure |
| Lung | i. PaCO2 45–49 mmHg ii. SaO2 < 90% iii. pH 7.30–7.35 or 7.45–7.50 iv. 200 mmHg < PaO2/FiO2 ≤ 300 mmHg v. No MV requirement | i. PaCO2 > 50 mmHg ii. SaO2 < 80% iii. pH < 7.30 iv. PaO2/FiO2 ≤ 200 mmHg v. MV requirement |
| Kidney | i. Decreased UOP (20–40 mL/h), good response to diuretics ii. Scr 177.0–265.2 μmol/L (or >20% increase from baseline) iii. No dialysis requirement | i. Decreased UOP (<20 mL/h) and poor response to diuretics ii. Scr >265.2 μmol/L (or >20% increase from baseline) iii. Dialysis requirement |
| PC | i. Decreased UOP (20–40 mL/h) ii. MAP 50–60 mmHg or >20% decrease from baseline, good response to vasopressors iii. Exclude hypovolemia | i. Decreased UOP (<20 mL/h) complicated with cold limbs and cyanosis ii. MAP <50 mmHg, multiple vasopressors and inotropic agents dependence iii. Exclude hypovolemia |
| Liver | i. TBIL 35–102 μmol/L ii. ALT elevated <2 × normal value iii. Markedly increased bilirubin with normal or decreased transaminases | i. TBIL ≥102 μmol/L ii. ALT elevated >2 × normal value iii. Hepatic encephalopathy |
| GT | i. Abdominal distension ii. Hypoactive bowel sounds iii. Acalculous cholecystitis | i. Severe abdominal distension, disappeared bowel sounds ii. Stress ulceration complicated bleeding or perforation iii. Necrotizing enteritis v. Spontaneous gallbladder perforation |
| CNS | i. Obtundation ii. Disorientation iii. GCS 9–12 | i. Diffuse neurologic injury ii. No response to speech or voice iii. No response to pain v. GCS ≤8 |
| CS | i. PLT 51–99 × 109/L ii. FIB ≥2 ∼ 4 g/L iii. PT and TT prolonged < 3s iv. D-dimer increased <2 × normal value v. No obvious signs of bleeding | i. PLT ≤50 × 109/L with decreasing trend ii. FIB < 2 g/L iii. PT and TT prolonged > 3s iv. D-dimer increased >2 × normal value v. Obvious bleeding |
Note: † The criterion of PAWP is replaced by LUS findings, ‡ Replaced by ≤ 30 B-lines on 28 zone LUS, § Replaced by > 30 B-lines on 28 zone LUS.
Abbreviations: MODSE, multiple organ dysfunction syndrome in the elderly; PAWP, pulmonary artery wedge pressure; EF, ejection fraction; PaCO2, partial pressure of carbon dioxide; SaO2, arterial oxygen saturation; PaO2, arterial partial pressure of oxygen; FiO2, inspired oxygen concentration; MV, mechanical ventilation; UOP, urine output; Scr, serum creatinine; PC, Peripheral circulation; MAP, mean arterial pressure; TBIL, total bilirubin; ALT, alanine aminotransferase; GT, gastrointestinal tract; CNS, central nervous system; GCS, Glasgow score; CS, coagulation system; PLT, platelet; FIB fibrinogen; PT, prothrombin time; TT, thrombin time; LUS, lung ultrasound.
2.5. Statistical analysis
All numerical variables were presented as mean and standard deviation (SD) or median and interquartile range (IQR), and the categorical variables were presented as frequency and percentages. Patients were divided into HFHFS and non-HFHFS groups according to whether BP fluctuations reached a predetermined criterion within 24 h after surgery. We used the Student's t test or Mann-Whitney U test to compare differences between HFHFS and non-HFHFS group where appropriate; The chi-square test or Fisher's exact test was utilised to compare categorical variables. 0.05 was regarded as the significance level.
Univariate and multivariate regression models were used to investigate the associations between HFHFS and clinical outcomes. Firstly, we examined the associations between HFHFS and clinical outcomes without adjustment for any covariates. In multivariate regression analysis, age, gender, number of comorbidities, time-to-surgery, Stage of Heart Failure, and systolic blood pressure and hemoglobin at admission were included in the models as potential confounders. The screening of covariates was according to the results of univariate analysis (P < 0.05), and the parameters that were confirmed to be associated with prognosis by previous studies [13, 14, 15]. To avoid overfitting in the regression models, we excluded the collinear indicators based on statistical and clinical relevance. A backward stepwise regression was used to sequentially eliminate factors that did not contribute significantly to the risks of clinical outcomes from the multivariate models [16]. The results are expressed as partial regression coefficient (linear regression), odds ratio (logistic regression), and hazard ratio (COX regression) with 95% confidence interval (CI). The one-year survival curves were calculated using the Kaplan-Meier method. All data were analyzed by SPSS 22.0.
3. Results
3.1. Characteristics of HFHFS
Of the 208 patients hospitalized during this study period, 168 were finally included. 40 patients were excluded as follows: nonoperative treatment (n = 6), multiple fractures (n = 9), periprosthetic fracture (n = 8), excessively prolonged time-to-surgery (n = 13), and discharge against medical advice (n = 4) (Figure 1). The incidence of HFHFS was 23.8% (40/168), with a median time to onset of 8.0 (5.0–12.0) hours after ICU admission. Emerging arrhythmia occurred in 7 of 40 patients with HFHFS, including four cases of rapid atrial fibrillation, one case of left bundle branch block and two cases of bradycardia. After assessment by bedside cardiopulmonary ultrasound, eight cases received vasoactive drugs (dopamine in five cases and dobutamine in three cases) for 2–96 h. On admission, SBP was lower in the HFHFS group compared to the non-HFHFS group, more chronic heart diseases, worse Stage of Heart Failure, and higher pro-brain natriuretic peptide (pro-BNP) appeared in the HFHFS group. On the day of surgery, the HFHFS group had a higher acute physiology and chronic health evaluation II score and received more intravenous fluid volumes, blood transfusions, and human albumin administrations, with a corresponding more significant positive fluid balance. In addition, a higher proportion of patients in the HFHFS group received continuous sedation with dexmedetomidine due to postoperative agitation (Table 2).
Table 2.
Comparison of characteristics of patients with and without HFHFS.
| Characteristics | Non-HFHFS (n = 128) | HFHFS (n = 40) | P |
|---|---|---|---|
| Baseline characteristics at admission | |||
| Age (years) | 85.0 (82.0, 89.0) | 86.0 (84.0, 90.0) | 0.251 |
| Male gender | 39 (30.5) | 7 (17.5) | 0.154 |
| Type of fracture (femoral neck) | 53 (41.4) | 17 (42.5) | 1.000 |
| Comorbidities | 105 (82.0) | 31 (77.5) | 0.645 |
| Hypertension | 59 (46.1) | 19 (47.5) | 1.000 |
| Diabetes | 30 (23.4) | 8 (20.0) | 0.675 |
| Chronic heart disease | 18 (14.1) | 12 (30.0)※ | 0.032 |
| Chronic lung disease | 15 (11.7) | 15 (12.5) | 1.000 |
| Chronic kidney disease | 6 (4.7) | 1 (2.6) | 0.687 |
| Chronic CNS disease | 28 (21.9) | 7 (17.5) | 0.659 |
| Stage of Heart Failure (≥ grade B) | 76 (59.4) | 32 (80.0)※ | 0.023 |
| Left ventricular ejection fraction (%) | 67 (63, 70) | 65 (60, 70) | 0.233 |
| Systolic blood pressure (mmHg) | 136.6 ± 19.3 | 127.3 ± 20.6※ | 0.009 |
| Diastolic blood pressure (mmHg) | 74.1 ± 10.9 | 70.5 ± 10.9 | 0.109 |
| Heart rate (beats/min) | 84.5 (77.1, 93.0) | 82.0 (68.3, 98.5) | 0.437 |
| Red blood cell count (×109 per L) | 3.7 ± 0.7 | 3.7 ± 0.6 | 0.878 |
| Haemoglobin (g/L) | 110.0 ± 21.1 | 111.0 ± 19.6 | 0.810 |
| High-sensitivity cardiac troponin T (pg/mL) | 16.0 (10.3, 22.0) | 16.0 (11.0, 33.0) | 0.283 |
| Pro-brain natriuretic peptide (pg/mL) | 362.3 (215.1, 700.0) | 484.4 (287.7, 1419.0)※ | 0.040 |
| Albumin (g/L) | 36.0 (32.9, 38.5) | 36.1 (32.5, 38.9) | 0.885 |
| Serum creatinine (μmol/L) | 67.4 (54.0, 87.0) | 63.5 (52.3, 82.8) | 0.668 |
| Glucose (mmol/L) | 6.6 (5.8, 8.0) | 6.7 (5.6, 8.2) | 0.829 |
| Potassium (mmol/L) | 4.0 (3.7, 4.3) | 4.0 (3.7, 4.3) | 0.597 |
| Sodium (mmol/L) | 140.0 (138.0, 142.0) | 139.5 (137.0, 141.0) | 0.198 |
| Calcium (mmol/L) |
2.2 ± 0.1 |
2.2 ± 0.1 |
0.546 |
| Clinical characteristics on the day of surgery | |||
| Time-to-surgery (days) | 6.0 (4.0, 9.0) | 6.0 (4.0, 12.0) | 0.576 |
| Operation time (min) | 49.0 (40.0, 60.0) | 50.0 (40.0, 68.8) | 0.865 |
| Estimated intraoperative blood loss (mL) | 100.0 (80.0, 117.5) | 100.0 (80.0, 100.0) | 0.470 |
| Postoperative hemoglobin (g/L)† | 94.8 ± 15.7 | 94.2 ± 18.8 | 0.836 |
| Total venous input (mL) | 3068.6 ± 580.1 | 3309.5 ± 496.4※ | 0.019 |
| Positive fluid balance (mL) | 1971.5 ± 824.7 | 2289.2 ± 668.5※ | 0.028 |
| Average hourly urine output (mL) | 58.9 (42.3–82.9) | 56.7 (39.1–67.2) | 0.192 |
| Acute physiology and chronic health evaluation II score | 9.0 (8.0, 11.0) | 11.5 (8.0, 14.0)※ | 0.002 |
| Blood transfusion | 50 (39.1) | 25 (62.5)※ | 0.011 |
| Human albumins infusion | 43 (33.6) | 22 (55.0)※ | 0.017 |
| Diuretic injection | 20 (15.6) | 11 (27.5) | 0.105 |
| Sedatives injection | 15 (11.7) | 13 (32.5)※ | 0.004 |
Abbreviations: HFHFS, hypotension following hip fracture surgery.
Note: ※Compared with non-HFHFS group, P < 0.05; † Based on the results of arterial blood gas analysis performed immediately after surgery.
3.2. HFHFS on in-hospital clinical outcomes
The LOS for all patients was 15.0 (11.0, 19.0) days, and the incidence of postoperative cardiac, brain and renal dysfunction was 13.7%, 20.2% and 11.3%, respectively. Of the 34 patients who developed postoperative brain dysfunction, three were identified as acute stroke, and the remaining 31 were diagnosed as postoperative delirium after CAM-ICU assessment. Compared with the non-HFHFS group, the HFHFS group had prolonged LOS and a significantly higher incidence of postoperative cardiac and brain dysfunction. There was no difference in the incidence of postoperative renal dysfunction between the two groups. After adjustment for potential confounders, HFHFS was associated with prolonged LOS (B 2.66, 95% CI 0.22, 5.10; P = 0.033), postoperative cardiac dysfunction (OR 2.92, 95% CI 1.05, 8.11; P = 0.039), and postoperative brain dysfunction (OR 3.51, 95% CI 1.50, 8.23; P = 0.004) (Table 3).
Table 3.
Association between HFHFS and clinical outcomes in older patients with hip fracture.
| Outcomes | Non-HFHFS (n = 128) | HFHFS (n = 40) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| B, OR or HR (95% CI) | P | B, OR or HR (95% CI) | P | |||
| In-hospital clinical outcomes | ||||||
| Length of hospital stay | 14.5 (11.0, 19.0) | 17.0 (14.0, 21.0)※ | 2.76 (0.51, 5.00)† | 0.016 | 2.66 (0.22, 5.10)† | 0.033 |
| Postoperative cardiac dysfunction | 13 (10.2) | 10 (25.0)※ | 2.95 (1.18, 7.38)‡ | 0.021 | 2.92 (1.05, 8.11)‡ | 0.039 |
| Postoperative brain dysfunction | 19 (14.8) | 15 (37.5)※ | 3.44 (1.54, 7.70)‡ | 0.003 | 3.51 (1.50, 8.23)‡ | 0.004 |
| Postoperative renal dysfunction |
15 (11.7) |
4 (10.0) |
0.84 (0.26, 2.68)‡ |
0.765 |
- |
- |
| Modified Rankin Scale at one year§ | ||||||
| No symptoms | 10 (8.1) | 1 (2.6) | 0.28 (−0.28, 0.84)† |
0.322 |
- |
- |
| No significant disability | 23 (18.7) | 8 (20.5) | ||||
| Slight disability | 31 (25.2) | 8 (20.5) | ||||
| Moderate disability | 35 (28.5) | 11 (28.2) | ||||
| Moderately severe disability | 10 (8.1) | 6 (15.4) | ||||
| Severe disability | 5 (4.1) | 2 (5.1) | ||||
| Death |
9 (7.3) |
3 (7.7) |
||||
| Death within one year | 9 (7.0) | 3 (7.5) | 1.07 (0.29, 3.96)¶ | 0.917 | - | - |
Abbreviations: HFHFS, hypotension following hip fracture surgery; B, partial regression coefficient; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
Note: ※Compared with non-HFHFS group, P < 0.05; † Linear regression analysis, the effect measure was expressed as a partial regression coefficient; ‡ Logistic regression analysis, the effect measure was expressed as a odds ratio; § One-year modified Rankin Scale was obtained in 162 cases due to 6 cases lost to follow-up, 123 in non-HFHFS group and 39 in HFHFS group; ¶ COX regression analysis, the effect measure was expressed as a hazard ratio.
4. HFHFS on long-term clinical outcomes
Among the 168 patients included, six cases were lost to follow-up within one year. Only 25.9% (42/162) regained pre-injury function (mRS ≤1), and 44.4% (72/162) developed moderate or greater disability (mRS ≥3). There was no difference in mRS at one year between the two groups (B 0.28, 95% CI -0.28, 0.84; P = 0.322) (Table 3, Figure 2). Three patients died within 30 days after surgery, and finally a total of 12 patients died within one year, with a one-year mortality of 7.1% (12/168). One-year mortality did not differ between the two groups (HR 1.07, 95% CI 0.29, 3.96; P = 0.917) (Table 3, Figure 3).
Figure 2.
Distribution of Modified Rankin Scale at one year in the two groups. Six patients were lost to follow-up, and a total of 162 patients received a final score. The scores from 0 to 6 represents no symptoms, no significant disability, slight disability, moderate disability, moderately severe disability, severe disability, and death in turn. Abbreviations: HFHFS, hypotension following hip fracture surgery.
Figure 3.
Survival curve of patients in the two groups. Within one year, six patients were lost to follow-up and 12 died. Abbreviations: HFHFS, hypotension following hip fracture surgery.
5. Discussion
In the context of global aging, the incidence of osteoporosis-related hip fracture is increasing year by year. Hip fracture, as a mediator of low-energy injury, causes a high level of stress in the older population, and most of whom will suffer a second stress response from surgery and anesthesia in the following hours or days. It is clear that anemia, hypotension, and hypoperfusion due to fracture, anesthesia, and surgery are prominent pathophysiological features in the early stage of hip fracture. The presence of hypotension and hypoperfusion before and during hip fracture surgery has been widely concerned. Kumar et al showed that the hemoglobin concentration of patients with subtrochanteric and intertrochanteric fracture decreased by 2.23 and 1.1 g per deciliter, respectively, from admission to pre-surgery [17]. Hypoperfusion on admission was found in 19% of senile hip fracture patients and was associated with a higher 30-day mortality [6,18]. Even though the reported incidence of hypotension during hip fracture surgery varies widely (18.1–81.6%), the occurrence and cumulative time of intraoperative hypotension was definitely demonstrated to be associated with postoperative complications [9,19,20]. However, little attention has been paid to HFHFS, and its pathogenesis, clinical features and prognosis have rarely been reported.
This prospective cohort study revealed a 23.1% incidence of HFHFS occurring 8.0 (5.0–12.0) hours after surgery. The effect of postoperative hidden blood loss should be taken into account, as demonstrated by previous study [21]. However, we found in clinical practice that although adequate volume therapy was given after surgery, a proportion of patients still inevitably suffered from hypotension. In present study, the comparison of preoperative baseline status showed significant differences in cardiac-related parameters (chronic heart diseases, Stage of Heart Failure, and pro-BNP) between the two groups. These differences suggest a possible effect of declining cardiac reserve on HFHFS. Hemodynamic alterations during acute stress in the elderly differ markedly from those in younger patients as a result of physiological degeneration or pathological abnormality of the heart [22]. Older patients with acute blood loss have weaker self-regulation, poorer response to fluid treatment and a more pronounced tendency to hypotension, because both restricted ventricular filling and diminished myocardial contraction inevitably lead to reduced cardiac output. In addition, sedative therapy based on postoperative agitation was significantly increased in the HFHFS group, cardiovascular inhibition of dexmedetomidine also played a role in the development of HFHFS in our research.
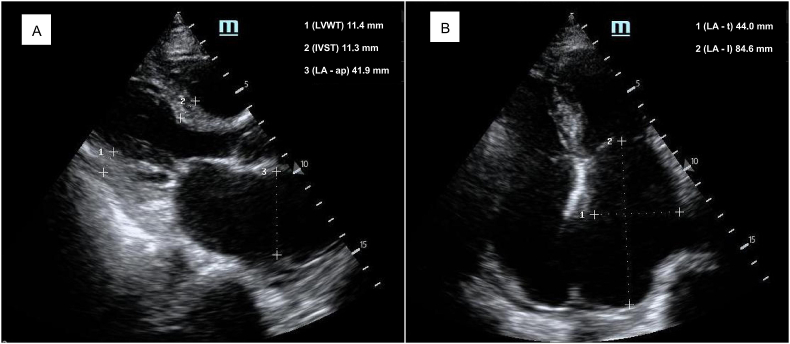
Acute organ dysfunction in older patients is often a continuation and exacerbation on the basis of chronic degeneration or injury [10]. In this study, the HFHFS group had more chronic heart disease preoperatively, while the significant association between HFHFS and postoperative cardiac dysfunction was also confirmed by subsequent multivariate regression analysis. Changes in the structure and function of the heart complicate fluid treatment after bleeding, making it no longer a simple matter of “loss and supplement”. Figure 4 shows images of bedside cardiac ultrasound of a 85-year-old woman with hypertension who developed HFHFS 20 h after surgery, where significantly enlarged left atrium indicates increased left ventricular filling pressure. Intravenously infused fluid may become congested in the pulmonary or even systemic circulation, while the left ventricle with diastolic filling restrictions has difficulty obtaining and accommodating effective blood volume. This patient developed acute heart failure characterized by the coexistence of congestion and hypoperfusion after surgery. Similar findings are not uncommon in senile hip fracture patients (especially those with hypertension and atrial fibrillation), but the clinical presentation of cardiac dysfunction may vary by reason of differences in the severity of congestion and hypoperfusion [22]. Delirium, as a sign of acute brain dysfunction, has been extensively studied, but its mechanism remains difficult to fully clarify. Perioperative BP is closely related to postoperative delirium, in which intraoperative and postoperative hypotension and increased BP fluctuations all contribute to delirium development [20,23,24]. Our observations showed the same results. Furthermore, heart failure itself plays an important role in the development of delirium in patients with cardiac dysfunction [25]. Finally, although the findings of the study showed no association between HFHFS and postoperative renal dysfunction, we presume that this may be an uncertain conclusion. Because of the reduction in muscle mass and the prevalence of chronic kidney disease, the rate and magnitude of increase in serum creatinine levels may be attenuated in older patients who develop acute kidney injury [26]. We may therefore have underestimated the incidence of postoperative renal dysfunction, and the relationship between HFHFS and postoperative renal dysfunction needs further investigation. The presence of hypotension as well as vital organ dysfunction complicated postoperative management, which inevitably led to a significantly prolonged length of hospital stay.
Figure 4.
Bedside cardiac ultrasound images of a patient with HFHFS. A 85-year-old woman with hypertension developed HFHFS 20 h after surgery. The enlarged atria suggest preoperative chronic changes of the heart, which may contribute to HFHFS and subsequent acute cardiac dysfunction. A, Parasternal long-axis view; B, Apical four-chamber view. Abbreviations: LVWT, left ventricular wall thickness; IVST, interventricular septal thickness; LA-ap, left atrial anteroposterior diameter; LA-t, left atrial transverse diameter; LA-l, left atrial length.
In terms of long-time prognosis, HFHFS had no effect on one-year mRS and mortality. The mRS, introduced by Dr John Rankin in 1957, is currently used as primary outcome scale for many acute stroke trials [27]. This scale covers the entire range of functional outcomes from no symptoms to death [12], and also reflects both the limb function and living ability. We used mRS to evaluate the one-year prognosis and found that recovery of the patient's self-care ability was not optimistic. However, the lower 30-day and one-year mortality (1.8% and 7.1%, respectively) were found in this study. These lower mortality rates may be largely attributable to the selection of participants. Because patients treated conservatively had to be excluded for study purposes, and they were more likely to die within one year. On the other hand, the multidisciplinary and co-management model were concretized, standardized, and individualized by joint ward rounds and outpatient follow-up. Comprehensive, rational and continuous medical intervention may improve the clinical outcomes of senile hip fracture patients.
We acknowledge some limitations of the present study. First, because of the low number of 30-day deaths in this study, the association between HFHFS and 30-day mortality was not explored. Second, since left ventricular diastolic dysfunction was not routinely classified at admission, we used Stage of Heart Failure to assess basic cardiac function, while the former may be more accurate and objective for patients with cardiac diastolic dysfunction. Third, we included the mRS and mortality at one year as the long-term clinical outcomes, but HFHFS had no effect on them. Whether the positive results will occur with the extension of follow-up time? Does HFHFS impact long-term cardiovascular events, cognitive ability, and vital organ function? These issues need to be explored in future studies. Finally, despite the use of multivariate regression to adjust for confounders, bias in observational studies is difficult to avoid.
6. Conclusions
Many older patients develop hypotension several hours after hip fracture surgery, which may be related with preexisting decline in cardiac reserve in addition to postoperative hidden blood loss. Patients who experienced HFHFS were more likely to have postoperative cardiac and brain dysfunction and longer hospital stay. However, HFHFS had no significant effect on mRS and mortality at one year.
Declarations
Author contribution statement
Xi Yang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zhijun Qin: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yi Li: Contributed reagents, materials, analysis tools or data.
Yang Deng: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Man Li: Performed the experiments; Analyzed and interpreted the data.
Funding statement
Dr. Zhijun Qin was supported by Scientific Research Project of Sichuan Medical Association [S20034].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the patients who participated in this study.
References
- 1.Ahuja S., Mascha E.J., Yang D., Maheshwari K., Cohen B., Khanna A.K., et al. Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2020;132(2):291–306. doi: 10.1097/ALN.0000000000003048. [DOI] [PubMed] [Google Scholar]
- 2.Gu W.J., Hou B.L., Kwong J.S.W., Tian X., Qian Y., Cui Y., et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int. J. Cardiol. 2018;258:68–73. doi: 10.1016/j.ijcard.2018.01.137. [DOI] [PubMed] [Google Scholar]
- 3.Salmasi V., Maheshwari K., Yang D., Mascha E.J., Singh A., Sessler D.I., et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 4.Meng L., Yu W., Wang T., Zhang L., Heerdt P.M., Gelb A.W. Blood pressure targets in perioperative care. Hypertension. 2018;72(4):806–817. doi: 10.1161/HYPERTENSIONAHA.118.11688. [DOI] [PubMed] [Google Scholar]
- 5.White S.M., Griffiths R. Problems defining 'hypotension' in hip fracture anaesthesia. Br. J. Anaesth. 2019;123(6):e528–e529. doi: 10.1016/j.bja.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Rocos B., Whitehouse M.R., Kelly M.B. Resuscitation in hip fractures: a systematic review. BMJ Open. 2017;7(4) doi: 10.1136/bmjopen-2017-015906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alghanem S.M., Massad I.M., Almustafa M.M., Al-Shwiat L.H., El-Masri M.K., Samarah O.Q., et al. Relationship between intra-operative hypotension and post-operative complications in traumatic hip surgery. Indian J. Anaesth. 2020;64(1):18–23. doi: 10.4103/ija.IJA_397_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beecham G., Cusack R., Vencken S., Crilly G., Buggy D.J. Hypotension during hip fracture surgery and postoperative morbidity. Ir. J. Med. Sci. 2020;189(3):1087–1096. doi: 10.1007/s11845-020-02175-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White S.M., Moppett I.K., Griffiths R., Johansen A., Wakeman R., Boulton C., et al. Secondary analysis of outcomes after 11,085 hip fracture operations from the prospective UK Anaesthesia Sprint Audit of Practice (ASAP-2) Anaesthesia. 2016;71(5):506–514. doi: 10.1111/anae.13415. [DOI] [PubMed] [Google Scholar]
- 10.Qin Z.J., Wu Q.Y., Deng Y., Li X., Wei X.D., Tang C.J., et al. Association between high-sensitivity troponin T on admission and organ dysfunction during hospitalization in patients aged 80 Years and older with hip fracture: a single-centered prospective cohort study. Clin. Interv. Aging. 2021;16:583–591. doi: 10.2147/CIA.S303246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koskderelioglu A., Onder O., Gucuyener M., Altay T., Kayali C., Gedizlioglu M. Screening for postoperative delirium in patients with acute hip fracture: assessment of predictive factors. Geriatr. Gerontol. Int. 2017;17(6):919–924. doi: 10.1111/ggi.12806. [DOI] [PubMed] [Google Scholar]
- 12.Broderick J.P., Adeoye O., Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke. 2017;48(7):2007–2012. doi: 10.1161/STROKEAHA.117.017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang W., Lv H., Feng C., Yuwen P., Wei N., Chen W., et al. Preventable risk factors of mortality after hip fracture surgery: systematic review and meta-analysis. Int. J. Surg. 2018;52:320–328. doi: 10.1016/j.ijsu.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 14.Marco-Martínez J., Bernal-Sobrino J.L., Fernández-Pérez C., Elola-Somoza F.J., Azaña-Gómez J., García-Klepizg J.L., et al. Impact of heart failure on in-hospital outcomes after surgical femoral neck fracture treatment. J. Clin. Med. 2021;10(5):969. doi: 10.3390/jcm10050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan K.J., Guerrero E.M., Tainter D., Dial B., Milton-Cole R., Blair J.A., et al. Prognostic factors of in-hospital complications after hip fracture surgery: a scoping review. Osteoporos. Int. 2019;30(7):1339–1351. doi: 10.1007/s00198-019-04976-x. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell R., Harvey L., Brodaty H., Draper B., Close J. One-year mortality after hip fracture in older individuals: the effects of delirium and dementia. Arch. Gerontol. Geriatr. 2017;72:135–141. doi: 10.1016/j.archger.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D., Mbako A.N., Riddick A., Patil S., Williams P. On admission haemoglobin in patients with hip fracture. Injury. 2011;42(2):167–170. doi: 10.1016/j.injury.2010.07.239. [DOI] [PubMed] [Google Scholar]
- 18.Venkatesan M., Smith R.P., Balasubramanian S., Khan A., Uzoigwe C.E., Coats T.J., et al. Serum lactate as a marker of mortality in patients with hip fracture: a prospective study. Injury. 2015;46(11):2201–2205. doi: 10.1016/j.injury.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Küpeli İ., Subaşı F., Eren N., Arslan Y.K. Evaluating the relationship between the pleth variability index and hypotension and assessing the fluid response in geriatric hip fracture under spinal anaesthesia: an observational study. Turk J Anaesthesiol Reanim. 2020;48(3):208–214. doi: 10.5152/TJAR.2019.59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tantardini V., Roca F., Bahri O., Compere V., Dujardin F., Chassagne P. Hypotension per-opératoire et syndrome confusionnel chez les patients présentant une fracture de l’extrémité supérieure du fémur [Intraoperative hypotension and delirium in patients with hip fracture] Geriatr Psychol Neuropsychiatr Vieil. 2020;18(1):25–33. doi: 10.1684/pnv.2019.0824. [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Wu Q., Wang X. Investigation of perioperative hidden blood loss of unstable intertrochanteric fracture in the elderly treated with different intramedullary fixations. Injury. 2017;48(8):1848–1852. doi: 10.1016/j.injury.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J., et al. Wytyczne ESC dotyczące diagnostyki i leczenia ostrej i przewlekłej niewydolności serca w 2016 roku [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure] Kardiol. Pol. 2016;74(10):1037–1147. doi: 10.5603/KP.2016.0141. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari K., Ahuja S., Khanna A.K., Mao G., Perez-Protto S., Farag E., et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth. Analg. 2020;130(3):636–643. doi: 10.1213/ANE.0000000000004517. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J., DePalma G., Tsai T.T., Sands L.P., Leung J.M. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br. J. Anaesth. 2015;115(3):418–426. doi: 10.1093/bja/aeu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vellone E., Chialà O., Boyne J., Klompstra L., Evangelista L.S., Back M., et al. Cognitive impairment in patients with heart failure: an international study. ESC Heart Fail. 2020;7(1):46–53. doi: 10.1002/ehf2.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coca S.G. Acute kidney injury in elderly persons. Am. J. Kidney Dis. 2010;56(1):122–131. doi: 10.1053/j.ajkd.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn T.J., Dawson J., Walters M. Dr John Rankin; his life, legacy and the 50th anniversary of the Rankin stroke scale. Scot. Med. J. 2008;53(1):44–47. doi: 10.1258/RSMSMJ.53.1.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.