Abstract
Activated carbon (AC) is a porous carbon-rich material that is widely used to remove pollutants, such as synthetic dyes, from contaminated water. Although quite efficient, the use of this technology is limited to the ability of the AC to be regenerated and/or reused. Conventional regeneration procedures are inefficient, requiring the development and/or implementation of new approaches. Advanced Oxidative Processes (AOP) have unique properties that result in high efficiency in wastewater treatment. The use of these technologies in the regeneration of AC has gained considerable prominence due to the ability to remove organic pollutants concentrated in the AC. During this process, the oxidizing species produced interact with the substrates adsorbed on the AC, in a non-selective way, mineralizing them and/or reducing their recalcitrance. Although widely used in wastewater treatment, few reviews focus on the use of AOP as AC regeneration technology, causing an insufficient exchange of information and ideas for strategic development in this area. Therefore, in this review, the authors present an overview of the use of some AOP (Photolysis, Peroxidation, Fenton reaction and Advanced electrochemical oxidative processes) when applied in regeneration of dye-saturated AC, including the mechanisms involved in the different processes, the general aspects that affect individual processes and the different methods established to quantify the effectiveness of regeneration.
Keywords: Adsorption, Dyes, Water treatment, Wastewater, Oxidation processes
Adsorption; Dyes; Water treatment; Wastewater; Oxidation processes.
1. Introduction
Contamination of natural waters has been one of the major problems of modern society and is an undesirable consequence of the rapid urbanization and industrialization. While industrialization playing an important role in the world economy, nevertheless contributes significantly to the production of contaminated wastewater, and threatens the environment [1, 2]. The textile industry, in particular, is considered one of the most polluting industries in the world, generating 637.3 MCM/year of contaminated water [3]. The main characteristic of the textile industrial effluents is color, from dyes used to produce the final products [4, 5, 6]. These colored compounds are detrimental to aquatic life, obstructing light penetration and preventing photosynthesis. In humans, synthetic dyes can cause health hazards including allergies, itching, skin sores, irritation of the mucous membrane and respiratory tract, and in extreme cases, cell mutation and cancer [7, 8, 9, 10, 11].
Textile dyes are organic molecules with high molecular weight and complex structures. These compounds are difficult to remove by conventional treatments, which includes chemical, physical, and biological methods. Because the new synthetic dyes developed are usually chemically and photolytically stable and therefore highly resistant to degradation [12, 13], and resulting in their accumulation in the environment [11, 14].
Numerous studies discuss alternative treatments for synthetic dyes removal including nanofiltration [5, 11, 12], biological approaches [15, 16], and systems based on electrochemical processes [17, 18]. Many of these techniques are limited because they are not economically viable, involve slow reactions, or do not achieve total removal of the pollutant. Adsorption processes are generally low cost, simple to operate, highly efficient (normally up to >90%) [19]. Compared to other techniques, adsorption provide an attractive alternative for the treatment of contaminated water, as it presents some important advantages such as: removal of a wide class of chemical contaminants, especially those that are little affected by conventional treatments, highly flexible, insensitivity to toxic pollutants, and do not produce toxic substances [20].
In an adsorption process, contaminants transfer from the aqueous phase to the surface of the adsorbents, where they preferentially accummulate, and then the treated effluent can be safely released or recycled [21]. A good adsorbent should generally possess a porous structure (resulting in high surface area of 500–2000 m2/g) to minimize the time to reach the equilibrium adsorption capacity. Many different adsorbent materials have been used but the most common material is activated carbon (AC) and, thus, this review focuses on the use of AC in water pollutant decontomination [22]. Over time, the adsorbent becomes saturated (i.e., the adsorption capacity of the is exhausted), and must be replaced [23, 24, 25]. Depending on the adsorbate, the spent adsorbent may become a potential pollutant and, thus, cannot be discarded in landfill [26]. The material may be combusted or alternatively regenerated to recover the adsorbate and the adsorption capacity of the adsorbent. The most commonly used methods for the regeneration of AC are biological treatments [27], solvent extraction [28] and heat treatment under oxidizing atmosphere [29]. Advanced oxidative processes (AOP), which include a combination of these technologies, have also been investigated. These processes are based predominantly (but not exclusively) on the in-situ generation of hydroxyl radicals (•OH), have proven efficiency (>90%) in the treatment of organic pollutants [30], are able to remove a wide range of organic pollutants [31], and convert the contaminants into biodegradable or inert substances, such as carbon dioxide, water and inorganic salts [32].
Although the use of AOPs in AC regeneration is reported in the literature [24, 33, 34, 35, 36], Therefore, a review of the current situation will be indispensable to direct the efforts of researchers. This review describes the dyes produced by the textile industry, their negative impacts and different treatment approaches. Further the potential use of the adsorption process, the importance of regeneration process and AC as an adsorbent, all as relate to using AOP for regenerating the material.
2. Adsorbent regeneration via AOPs
Although it is a viable alternative in wastewater treatment, adsorption presents in practice a concern problem related to the use of adsorbent agents since, after a specific period of time, the AC loses its adsorptive capacity, due to the high concentration of pollutants retained on its surface [24, 37, 38, 39, 40]. Saturated adsorbent materials are, in some cases, disposed in landfills without proper treatment, leading to the possibility of toxic contaminants infiltrating the environment, consequently causing secondary environmental problems. Saturated adsorbent can also be incinerated, but this process has some important disadvantages, such as operational cost, destruction in the structure of the adsorbent, as well as the emission of possibly toxic compounds and release of carbon dioxide [26, 41].
Thus, the economic and environmental capacity of the use of adsorbent for wastewater treatment is associated with its capacity of regeneration and/or reuse [42, 43]. The regeneration process seeks to return the original adsorptive capacity of adsorbent removing contaminants, damaging it as little as possible. Conventional techniques for regeneration are generally classified into three distinct classes that include physical, chemical and biological processes [43, 44].
There are several problems associated with the conventional treatments used in the regeneration of AC, such as, the non-economic viability, slow reactions, phase transfer of the pollutant, and the alterations of the properties of AC. These problems have caused, in recent years, numerous efforts to be dedicated to the proposition of treatments for the regeneration of activated carbon that allow not only the removal of adsorbed contaminants, but also decompose the desorbed pollutants. Among the processes currently studied, advanced oxidative processes (AOPs) have received considerable attention [22, 45, 46, 47]. AOPs are widely used in the treatment of wastewater containing a great diversity of contaminants due, mainly their efficiency in the removal of toxic and/or biorefractory pollutants and low cost [48]. The AOPs are based on the in-situ generation of the hydroxyl radical (•OH), as the main oxidizing species, a strong oxidizing agent, which has a reduction potential of 2.8 V vs. Standard Hydrogen Electrode (SHE), behind only fluorine (F2) (3.0 V vs. SHE, Table 1).
Table 1.
Standard reduction potential of different oxidants in acid solution [53].
| Oxidizing agent | Standard reduction potential (V vs SHE) |
|---|---|
| Fluorine (F2) | 3.03 |
| Radical Hydroxyl (•OH) | 2.80 |
| Atomic Oxygen | 2.42 |
| Ozone (O3) | 2.07 |
| Hydrogen Peroxide (H2O2) | 1.77 |
| Potassium Permanganate (KMnO4) | 1.67 |
| Chlorine Dioxide (ClO2) | 1.5 |
| Hypochlorosus Acid (HClO) | 1.49 |
| Chlorine (Cl2) | 1.36 |
| Oxygen (O2) | 1.23 |
| Bromine (Br2) | 1.09 |
These radicals react non-selectively with most organic contaminants, such as halogenated organic compounds, degrading them by electron transfer (redox reaction) (Eq. (1)), hydrogen removal (dehydrogenation) (Eq. (2)) or electron transfer (hydroxylation) (Eq. (3)) being able to mineralize them for CO2, water and inorganic ions [48, 49, 50].
| •OH + RX → RX+• + OH− | (1) |
| •OH + RH → R• + H2O | (2) |
| •OH + PhX →PhX(OH)• | (3) |
where RX and PhX represent aliphatic and aromatic halogens, respectively.
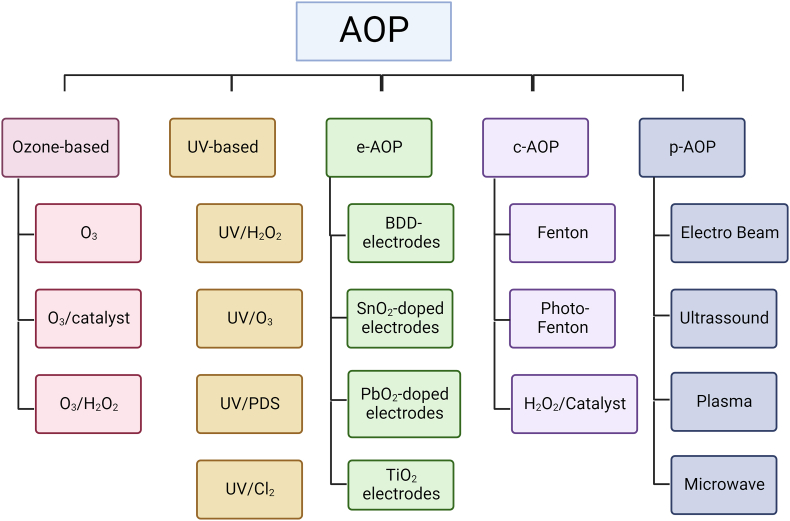
In recent decades, the emergence of several technologies classified as AOPs, which differ only in the mechanism used for the generation of oxidizing species, have been used in the degradation of organic contaminants in the literature. The main AOPs grouped based on the main source of hydroxyl radical generation: ozone-based, UV-based, electrochemical (eAOPs), catalytic (cAOPs) (such as Fenton reaction), and physical (pAOPs) (such as ultrasound and plasma) [49]. The different processes summarized in Figure 1.
Figure 1.
Broad overview and classification of different AOPs (based on Miklos et al. [49]).
Several AOPS are reported in the literature as regenerative techniques, this includes Microwave [51], plasma [52] and ultrasonic [35] methods. However, these methods are met with limitations such as high energy consumption and carbon attrition, need for secondary treatments and slow regeneration rates. In an attempt to overcome these restrictions, attention is being focused on relatively cheaper technologies that can be applied in situ and that present greater innovative potential, among which we can highlight the processes of Photolysis, Peroxidation, Fenton reaction and Advanced electrochemical oxidative processes.
2.1. Photolysis
Photolysis is widely used in potable water disinfection processes, due to its efficacy against a wide range of pathogens [54, 55]. This process can also degrade organic compounds when used in wastewater treatments [56]. Photolysis is based on the exposure of organic compounds of interest to light with different wavelength and can be classified into two distinct categories: direct photolysis and indirect photolysis. In the so-called direct photolysis, the contaminant itself absorbs the photons and is degraded, while in indirect photolysis the degradation occurs through the reaction of the compound with a reactive species generated by photosensitizers that can absorb radiation to reach an excited state [57].
In the case of direct photocatalysis, the process of photochemical degradation of the organic pollutant is initiated from an excited electronic state, achieved by the absorption of the radiation incident by the pollutant, as shown in the Eq. (4). Once excited, the molecule will undergo hemolytic bond scission, generating radicals that will react to the final products with the participation (or not) of molecular oxygen (Eq. (5)), or initiate a process of electron transfer to an oxygen molecule in its ground state generating radical cations and superoxide radical ions (O2•−) (Eq. (6)). The radical cation formed during these reactions may generate low molecular weight products through hydrolysis or mesolitic bond fission processes and superoxide radical ion (O2•−) will act on the degradation of aromatic molecules [58].
| R + hv → R∗ | (4) |
| R∗→ R1• + R2• →Products | (5) |
| R∗ + O2→ R•+ + O2•– | (6) |
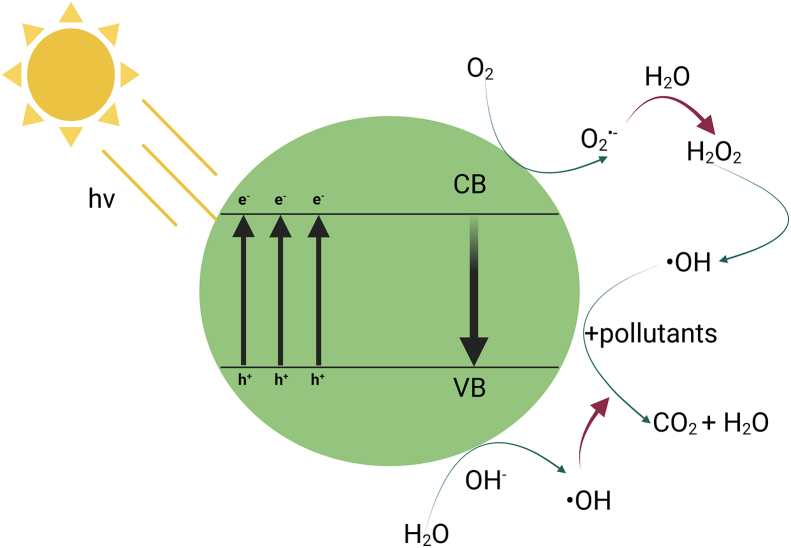
In the indirect photocatalysis, however, the irridiation of light transfers an electron from the valance band to the conduction band of a semiconductor such as TiO2 to generate an electron-hole pair (Eq. (7)). The electron reacts with the dissolved oxygen to generate superoxide radical ion, and the hole reacts with water molecules to produce hydroxyl radical for the oxidation of organic pollutants (Equations (8) and (9)). The organic pollutants may be also directly oxidized by the photogenerated hole (Eq. (10)) [59]. Figure 2 demonstrates the photocatalytic degradation mechanism using irradiation energy from a light source.
| TiO2 + hv → TiO2 (h+ + e–) | (7) |
| TiO2(e–) + O2 → TiO2 + O2•– | (8) |
| TiO2(h+) + H2O → TiO2 + •OH + H+ | (9) |
| TiO2(h+) + R → TiO2 + R+ | (10) |
Figure 2.
Scheme of photocatalytic degradation mechanism (based on [60, 61, 62]).
2.2. Peroxidation
Widely used in wastewater treatment, hydrogen peroxide is considered as a moderate oxidizing agent for most organic compounds. Moreover, with regard to the environmental issue, its use in water treatment processes is quite desirable, since the products of its decomposition are molecular oxygen (O2) and water (H2O) [63, 64].
The mechanism of degradation of organic pollutants through hydrogen peroxide (H2O2) involves its decomposition into •OH. Once generated, these species react with organic pollutants by oxidizing and/or mineralizing them to CO2 and H2O [65, 66]. Greater efficiency in the use of H2O2 in the treatment of contaminated waters can be obtained in the presence of a catalyst, for instance COD removal efficiency increased from 30% to ∼45% when a catalyst added during H2O2 oxidation of a dye intermediate [8]. Among the most commonly used catalysts we can mention Fe2+ in the so-called Fenton reaction. However, other materials such as transition metals and AC itself can be used as a catalyst in reactions called Fenton-like [67].
Activated carbon (AC) has been widely used in catalytic reactions, both as support for the active species, as well as a catalyst itself due to its physical properties and functional surface groups containing mainly oxygen. The texture and chemical composition of the surface determine the performance and final application of the carbon material. Hydroxyl radicals produced during the activation of hydrogen peroxide by AC provide an oxidizing agent so strong that it can oxidize organic compounds from aqueous matrices [68].
The mechanism of the AC-H2O2 reaction involves the reduced (AC) and oxidized (AC+) catalyst states (Eq. (11) and (12)) and occurs, according to the Haber-Weiss mechanism, by electron transfer. Parallel reactions of free radicals generated at the carbon surface (Eq. (13),(14)) or scavenging reactions (Eq. (15)) inefficient for process efficiency are also reported. The decomposition of hydrogen peroxide produces oxygen and water by exchanging the hydroxyl groups generated on the AC surface with hydrogen peroxide anions and subsequent reactivity of the peroxide groups, thus regenerating the carbon surface (Eq. (16) and (17)).
| AC + H2O2→ AC+ + HO• +−OH | (11) |
| AC+ + H2O2→ AC + HO2• + H+ | (12) |
| AC + HO• → AC+ + −OH | (13) |
| AC+ + HO2• → AC + O2 + H+ | (14) |
| HO• + HO2• → H2O + O2 | (15) |
| AC–OH + H+–OOH-→ AC–OOH + H2O | (16) |
| AC–OOH + H2O2 → AC–OH + H2O + O2 | (17) |
2.3. Fenton reaction
The Fenton reaction was discovered by Fenton in 1894 in the study that investigated the catalytic oxidation of tartaric acid in the presence of H2O2and ferrous salt. This reaction is still a widely used in the treatment of organic pollutants, mainly due to its high efficiency in the degradation/mineralization of organic pollutants, operational simplicity (occurs at atmospheric pressure and room temperature), and low cost, because cheap, moderately reactive and relatively easy to handle reagents are applied in the reaction [53, 65, 72].
These reactions are based on the activation of H2O2 by ferrous ions (Fe2+) for the generation of •OH radicals (Eq. (18)). However, it is traditionally accepted that other processes, such as those indicated in Eqs. (19), (20), (21), (22), (23), (24), (25), and (26), occur simultaneously catalyzing or inhibiting and competing with the process [53, 73, 74, 75]. The mechanisms involved in Fenton reaction shows that this is a very complex process. The reaction begins with the generation of •OH radicals by activating H2O2 using ferrous ions (Eq. (18)). The ferric ions generated at this stage react with H2O2 regenerating ferrous ions and hydroperoxyl radicals (HO2•) (Eq. (12)) to complete the catalytic cycle. From there, the formed radicals can be consumed by iron species (equations (20), (21) and (22)), H2O2 (Eq. (24)), HO2• radicals (equations (25) and (26)) present in solution, and even self-eliminated (Eq. (23)) [53]. The Fenton reaction occurs under necessarily acidic conditions, because in these circumstances the species Fe2+ and Fe3+ will be present in the ionic form and in the appropriate proportions in order to obtain a maximum efficiency of the process. At neutral or basic pH, Fe2+ and Fe3+ precipitate, decreasing process efficiency [65].
| Fe2+ + H2O2 → Fe3+ +•OH + OH− | (18) |
| Fe3+ + H2O2 → Fe2+ + HO2• + H+ | (19) |
| Fe2+ + •OH→ Fe3+ + OH− | (20) |
| Fe2+ + HO2•→ Fe3+ + HO2− | (21) |
| Fe3+ + HO2• → Fe2+ + O2 + H+ | (22) |
| •OH + •OH → H2O2 | (23) |
| •OH + H2O2 → HO2• + H2O | (24) |
| HO2• + HO2• → H2O2 + O2 | (25) |
| •OH + HO2• → H2O + O2 | (26) |
Santos and collaborators [70] studied the regeneration of AC used in methylene blue adsorption was evaluated through the processes of photolysis, oxidation with H2O2 and Fenton reaction. The efficiency of these processes was evaluated by the recovery of the adsorption capacity of AC, measured by the amount of MB adsorbed in the successive adsorption/oxidation cycles and by the physical properties of the material obtained through SEM analysis, X-Ray Diffraction (XRD), Energy Scattering X-Ray Spectroscopy (EDS) and Fourier Transform Infrared Spectroscopy (FTIR) before and after the regeneration process. During the photolysis process, the AC saturated with MB was irradiated with a UV light source for different time intervals, the results showed that a maximum of 30% of the adsorption capacity of the AC was recovered after 2 h of reaction. For the oxidation tests performed with H2O2, a maximum of 40% of the adsorption capacity of the AC was recovered when the concentration of the oxidizing agent was 50 mmol/L. As for the tests in which the Fenton reaction was used, a maximum of 71% of regeneration was observed, the efficiency of this process was attributed to a synergistic AC-Fe effect. The stability of the material through adsorption-regeneration cycles and the characterization results of the AC showed that, in general, the Fenton reaction provided greater stability to the material, causing the adsorption capacity to be recovered for 7 consecutive cycles [70].
3. Advanced electrochemical oxidative processes
Developed during the last decade, advanced electrochemical oxidative processes (EAOPs) generated great interest because they have high efficiency in the oxidation of several persistent organic pollutants. In these processes, •OH radicals are produced electrolytically or through electrochemically generated reagents [76]. Oxidation of organic pollutants through EAOPs can occur directly or indirectly. In direct oxidation the •OH radicals are produced on the electrode surface by water oxidation. The production rate and extent depend on the nature (catalytic activity) of the anodic material. Boron-Doped Diamond (DDB), Platinum (Pt) and Dimensionally Stable Anodes (ADE) are reported as effective electrode materials for water oxidation and subsequent •OH radical production in aqueous medium [50].
The mechanism of degradation (Figure 3) of organic pollutant in metal oxide anodes (MOx) described by Comninellis and De Battisti (1996) classify electrodes as "active" (RuO2, IrO2 and PtOx) and "non-active" (e.g., SnO2 and PbO2). For both categories of electrodes, the first step is the formation of the hydroxyl radical MOx(HO•) by the water oxidation (Eq. (27)). These adsorbed radicals can react with oxygen resulting in the production of higher oxides (Eq. (28). Thus, we can consider that on the surface of the anode are present two states of " activated oxygen": chemisorbeds (in the form of oxide) and physisorbeds (in the form of • OH). In the absence of organic compounds that can be oxidized, the two types of "active oxygen" present on the surface of the anodes can produce gaseous oxygen (equations (29) and (30)) [77]. When there are oxidisable organic compounds, both MOX(HO•) and MOx +1 oxidize the organic pollutant as shown in Eqs. (31) and (32). In indirect oxidation, the production of hydroxyl radicals is performed in situ through the electrogeneration of reagents that generate the oxidizing agents (•OH or active chlorine). Within the most common indirect oxidation methods we can mention the processes of electro-Fenton, sonoeletro-Fenton, among others [50].
| MOx + H2O → MOx (HO•) + H+ + e− | (27) |
| MOx (HO•) → MOx + 1 + e− | (28) |
| MOx (HO•) → MOx + H+ + e− + ½ O2 | (29) |
| MOx + 1 → MOx + ½ O2 | (30) |
| MOx (HO•) + RH → MOx + H2O + R• | (31) |
| MOx + 1 + RH → MOx + RHO | (32) |
Figure 3.
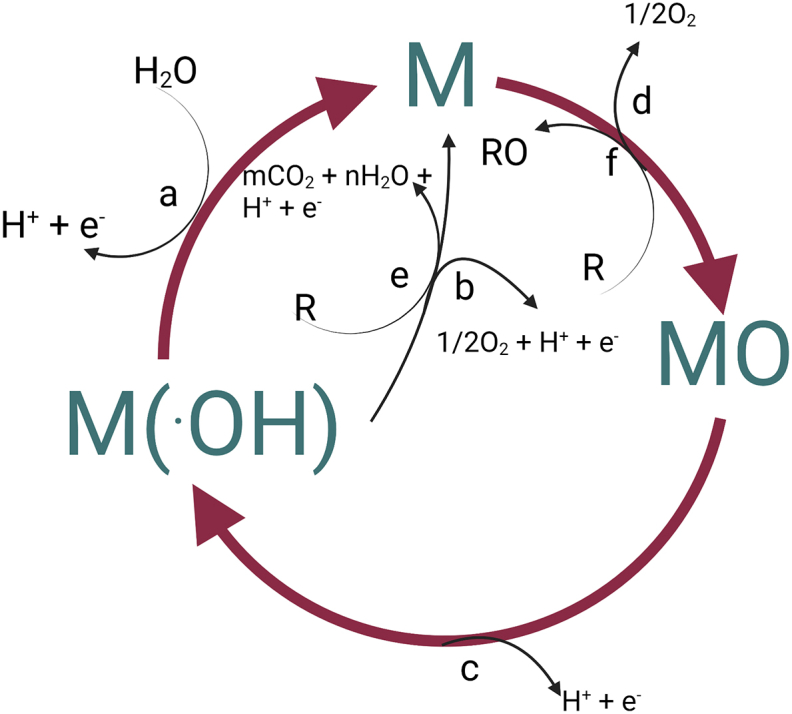
Electrochemical degradation mechanism (based on [78]).
4. Application of AOPs for dye-activated saturated carbon regeneration
In the course of the adsorption process, the performance efficiency of AC progressively diminishes, and requires replenishment or treatment after exhaustion [70, 79, 80]. Replenishment is expensive, and the economic viability of the adsorption process is determined by the reusability of spent AC [51, 81, 82]. Commonly, the spent AC is landfilled or incinerated to minimize environmental safety risks caused by secondary pollution [83, 84]. Landfilling requires large expanses of land, and pollutant-laden AC could contaminate groundwater through leaching. Thus, regeneration for reuse is a viable option, and this has been traditionally achieved through thermal methods. Despite having a high regeneration efficiency, thermal regeneration could cause 5–15% attrition due to friction, carbon burn-off, and washing, and this challenge could be more severe after several regeneration cycles [51, 83, 84, 85, 86].
When organic pollutants are subjected to heat, there is a possibility of forming toxic by-products that can be emitted into the environment [70, 80]. Besides, thermal regeneration cycles are long, and causes a deterioration in the microporous structure of AC, consequently reducing the adsorption efficiency in subsequent adsorption runs [23, 79, 87, 88]. The high energy demand discourages the uptake of AC adsorption technology in large scale water treatment [85, 86]. Moreover, some countries have laws that prohibit the disposal of toxic sludge into the environment [89]. There is thus need to develop environmentally sustainable regeneration methods to allow reuse of the AC. In this respect, a number of alternative regeneration methods including microbiological, microwave, electrochemical, and temperature swing (using inert gases, hot air, and steam), and chemical methods (acid-base pH swing, solvent leaching, Fenton oxidation, wet oxidation) have been explored [45, 81, 82, 84, 85].
Advanced oxidation processes (AOPs), including photocatalysis, Fenton processes, and wet oxidation, have been used to degrade various refractory organic pollutants in aqueous systems [79]. Their major advantage lies in their capacity to mineralize pollutants to less harmful compounds, usually carbon dioxide, water and mineral acids. Because of this, AOPs have recently been investigated for the regeneration of spent AC.
Basically, AOPs generate reactive oxygen species (ROS) in situ, e.g., the superoxide (O2•) and the hydroxyl (•OH) radicals, which are nonselective and highly oxidizing [70, 79, 90, 91]. Techniques for generating the ROS include ultrasound cavitation, ozone, the Fenton, Fenton-like, UV/Fenton processes and various combinations of these [90, 92]. The catalytic generation of ROS can be accomplished by the AC surface on its own, or by homogeneous or heterogeneous catalysis using Fenton reagents, which significantly enhance the production efficiency [91]. These radicals convert organic pollutants into water, carbon dioxide, and inorganic ions which are environmentally benign [70, 85]. The overall mechanism of Fenton oxidation follows the typical Fenton reaction (Equation 18) and by side reactions where the reduction of Fe3+ regenerates Fe2+ (Eqs. (19) and (22)) [93]. The reaction between •OH radicals and organic pollutants is rapid, and requires H2O2, Fe, and the pollutant to coexist within the same site on the AC surface for effective oxidation [94].
While AOPs add oxidants to the aqueous system to degrade pollutants, a variation of this method, electrochemical AOPs (EAOPs) depends on the in-situ generation of oxidants [88]. EAOPs generally comprise electro-Fenton oxidation and its variations. The EAOPs exploit electro-desorption and electro-oxidation processes to eliminate organic pollutants adsorbed on the AC surface [87, 88]. Through in-situ production of •OH radicals, an oxidizing environment is created and the organic pollutants on AC surface and in solution are transformed, regenerating the AC surface [88]. A typical EAOP example is the electro-Fenton oxidation process, which was designed to circumvent the requirement of H2O2 in the aqueous system and instead generate it in-situ via a green reagent [87, 88]. The reaction proceeds via the following steps (Eqs. 18, 36, and 37): (1) the electro-generation of H2O2 from H2O, (2) the conversion of H2O2 into •OH radicals using Fe2+, and (3) the reduction of Fe3+ to regenerate the catalytic Fe2+ [87, 88].
| O2 + 2H+ + e− → H2O2 | (36) |
| Fe3+ + e− → Fe2+ | (37) |
To maintain Fe2+ and Fe3+ in solution, low pH (∼3) is necessary. Above pH 4, Fe(OH)3 precipitates out. Normally, air is bubbled through the solution to supply the O2, but recently in-situ O2 generation has been achieved using specialized electrodes [88]. Since electrons are also reactants in the process, the AC acts as a cathode.
Fenton processes depend on the use of iron salts to generate ROS. However, in cases where the use of iron is not possible (e.g.), treatment of iron-complexing compounds), other metal salts can be used via processes such as the electro-Fenton-like oxidation process. In such cases, other transition metals can be used to catalyse the generation of •OH radicals from H2O2. The generation of H2O2 still follows Equation (4), and a transition metal with an Mn/Mn+1 redox pair catalyses the generation of •OH radicals (Equation 38). Reduction of the transition metal ion back to Mn is achieved by electrons supplied from the electric current (Equation 39) [88].
| Mn + H2O2 → Mn+1 + •OH + OH− | (38) |
| Mn+1 + e− → Mn | (39) |
In electro-Fenton oxidation, the catalytic metal can either be homogenous in the aqueous phase or heterogeneous as a solid phase catalyst. The latter is called heterogeneous electro-Fenton-like oxidation. In this case a number of configurations can be used: the cathode can be made of iron only, a composite cathode made of a combination of iron with other materials can be used, or the cathode can be made of iron on a suitable support [88]. Keeping the catalyst in the solid phase the requirement to dissolve the iron, and this is beneficial in that: there is no precipitation of Fe(OH)3 sludge, avoiding the extra step of separating the Fe(OH)3 before discharging into the environment, regeneration of the catalyst is easier since it remains in the system, and there is no requirement for an acid environment to maintain the iron in solution, minimizing the use of toxic chemicals [88].
Research on the degradation of organic compounds using electro-Fenton and electro-Fenton-like oxidation processes is not well established. The few compounds investigated are generally pharmaceuticals, aromatics, and pesticides/herbicides [88]. The reusability of the regenerated AC depends on its retention of physicochemical properties. In this regard, Fenton-based regeneration does not cause a significant a reduction in the pore volume, surface area, and adsorption capacity of the AC [92]. Since these AOPs generate •OH radicals, it has potential for use in regenerating spent AC.
Photocatalytic regeneration is a heterogeneous catalysis technique that uses photoactive nanoparticles loaded onto carbon matrices are used to transform light energy to chemical energy and degrade organic pollutants on the AC surface [79]. The organic pollutants desorb from the inner adsorption sites of AC and are transported to the outer surface via diffusion. Regeneration is then achieved through the degradation of pollutants by the photocatalyst on the exterior surface of AC after excitation with radiation. Throughout this process, the AC desorption sites are pivotal in attaining effective desorption and pollutant degradation. The efficiency of photocatalytic regeneration is determined by the nature of the pollutant, and experimental conditions such as intensity of radiation source, concentration of the spent adsorbent, and solution temperature. Overall, photocatalytic regeneration has high efficiency, is ecofriendly, and is economically efficient, accomplishing both adsorption and in-situ regeneration [79].
Apart from •OH radicals, other ROS such as the sulphate free radical (•SO4-) have been explored in the degradation of various organic pollutants. The benefits of using the •SO4- radical include a strong oxidation ability arising from a high redox potential, excellent selectivity, and a long half-life [86, 95]. Generally, •SO4- can be generated via the activation of peroxymonosulphate, which is a relatively stable and ecofriendly oxidant [96]. Nonetheless, •SO4--based AOPs have limited efficiency in the regeneration of spent AC [160]. Other AOPs that can potentially be used to regenerate spent AC include catalytic ozonation, which can use AC as a catalyst to generate •OH radicals [97], and the electro-peroxone (Eperoxone) process, which can degrade ozone-recalcitrant organic pollutants in aquatic systems [98]. The rapid conversion of selective O3 into non-selective •OH radicals via in situ electrochemical generation of H2O2 accounts for the high degradation efficiency of the Eperoxone process [98]. Besides these AOPs, ionizing radiation such as gamma radiation, UV radiation, or solar radiation holds great promise in degrading organic pollutants in aquatic environments [85, 88] However, extremely high radiation doses are required for complete mineralization. Nevertheless, AC regeneration using gamma irradiation can be performed at ambient-temperature, there is no need for chemical reagents, and the adsorbed organic pollutants are decomposed by ROS generated in situ during radiolysis [85]. Generally, AOPs have high degradation efficiency (>90%) for eliminating recalcitrant organic pollutants, are simple to design and operate, and have low operation cost; consequently, they have great potential for the regeneration of exhausted AC [83, 85, 92].
5. Main challenges
The contamination of aquatic systems by dyes poses an environmental and human health risk. Of the many methods that have been used to remove dyes from the aquatic environment, the use of AC represents an efficient approach. However, spend AC can potentially cause secondary environmental pollution through release of adsorbed dye compounds. Besides, the replenishment of spent AC has cost implications. Thus, pollutant-laden AC requires regeneration for multiple reuses. Among the methods used for regeneration, AOPs constitute an effective regeneration technique. This review has discussed the various benefits of regenerating exhausted AC using AOPs.
Although the mechanisms involved in the degradation of dyes using AOPs have been widely studied, mechanisms for the degradation of specific dyes require further study. This is especially due to the rapid development of dyes with novel characteristics. Cost is a major driver in the large-scale adoption of water treatment technologies. For a better understanding of the cost-effectiveness of this approach, economic feasibility studies deserve further investigation. This will generate data that will inform the large-scale up-take of the technology for industrial application. Moreover, the recovery of catalysts in the case of Fenton and Fenton-like AOPs should be investigated. The comparison of these processes with others AOPS used in the regeneration of the ACare summarized in Table 2.
Table 2.
Comparison of different AOPs applied in the regeneration of dye-saturated AC.
| Types of AC | Adsorbed dyes | AOP used | Analyze used for Efficiency | limitation | reference |
|---|---|---|---|---|---|
| commercial | Reactive blue 19 | Electrochemical |
|
- | [45] |
| agricultural-waste | methylene blue | microwave-irradiation |
|
- | [99] |
| commercial | acid orange dye 7 | Pulsed Discharge Plasma (PDP) |
|
|
[52] |
| commercial | methylene blue | -photolysis; -oxidation with hydrogen peroxide - Fenton reaction |
|
|
[70] |
| Commercial | methylene blue | Electrochemical |
|
- | [100] |
| Commercial | Rhodamine B | Ultrasonic (Sono-Fenton) |
|
association of two techniques | [35] |
| commercial | Rhodamine B | electro-peroxone |
|
ozonation regeneration could not effectively mineralize the desorbed pollutants due to the selective oxidation characteristics of ozone (O3) | [101] |
| Commercial | Basic Blue 9 and Acid Blue 93 | microwave |
|
- | [84] |
| Commercial | Acid orange 7 | Microwave |
|
changes on the structural properties, surface chemistry and the AO7 adsorption capacities of AC | [102] |
6. Conclusion
Despite being a viable technology for the treatment of textile effluents, adsorption processes that use AC as an adsorbent, are generally expensive, and their commercial use depends on a regeneration process. Conventional regeneration processes have low efficiency, requiring the development and/or application of alternative methods for this purpose. This article presented a comprehensive review of the use of different technologies associated with AOPs with special emphasis on the mechanisms and factors that affect regeneration processes.
The widespread use of AOPs for this purpose is related to the unique characteristics of these technologies, which result in a high regeneration efficiency, making the use of these processes more both economically and environmentally viable than other common regeneration processes such as steam and chemical regeneration, which are energy intensive and often lead to deterioration of the AC structure. Although studies on the use of different AOPs in AC regeneration are reported in the literature, further research needs to be carried out to better understand the factors that affect the regeneration process, the mechanisms involved in different technologies and the quantification of regeneration, especially the reversibility of adsorption, validity and efficiency of the different processes. There is room for innovation and improvement of these technologies, such as the development and improvement of reactor designs, since most of the works reported in literature were carried out on a laboratory scale.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kumar Verma A., Dash R., Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2011 doi: 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 2.He J., Sun H., Indrawirawan S., Duan X., Tade M.O., Wang S. Novel polyoxometalate@g-C 3 N 4 hybrid photocatalysts for degradation of dyes and phenolics. J. Colloid Interface Sci. 2015 doi: 10.1016/j.jcis.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Mungray A., Agarwal S., Ali J., Chandra Garg M. Performance optimisation of forward-osmosis membrane system using machine learning for the treatment of textile industry wastewater. J. Clean. Prod. 2020 [Google Scholar]
- 4.Ene Manai I., Miladi B., Mselmi A.E., Smaali I., Hassen A.B., Hamdi M., et al. Industrial textile effluent decolourization in stirred and static batch cultures of a new fungal strain Chaetomium globosum IMA1 KJ472923. J. Environ. Manag. 2016 doi: 10.1016/j.jenvman.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Zhijiang C., Cong Z., Ping X., Jie G., Kongyin Z. Calcium alginate-coated electrospun polyhydroxybutyrate/carbon nanotubes composite nanofibers as nanofiltration membrane for dye removal. J. Mater. Sci. 2018;53:14801–14820. [Google Scholar]
- 6.Asses N., Ayed L., Hkiri N., Hamdi M. Congo red decolorization and detoxification by Aspergillus Niger: removal mechanisms and dye degradation pathway. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/3049686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma S., Meng J., Li J., Zhang Y., Ni L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J. Membr. Sci. 2013 [Google Scholar]
- 8.Zhu R., Xu Y., Bai Q., Wang Z., Guo X., Kimura H. Direct degradation of dyes by piezoelectric fibers through scavenging low frequency vibration. Chem. Phys. Lett. 2018 [Google Scholar]
- 9.Mahmoudi K., Hosni K., Hamdi N., Srasra E. Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits-A comparative study. Environ. Eng. 2015;32:274–283. [Google Scholar]
- 10.Tang A.Y.L., Lo K.Y., Kan C. Textile dyes and human health : a systematic and citation network analysis review. Color. Technol. 2018:245–257. [Google Scholar]
- 11.Cardoso N.F., Lima E.C., Pinto I.S., Amavisca C.V., Royer B., Pinto R.B., et al. Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J. Environ. Manag. 2011;92:1237–1247. doi: 10.1016/j.jenvman.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Meghlaoui F.Z., Merouani S., Hamdaoui O., Bouhelassa M., Ashokkumar M. Rapid catalytic degradation of refractory textile dyes in Fe(II)/chlorine system at near neutral pH: radical mechanism involving chlorine radical anion (Cl 2 %−)-mediated transformation pathways and impact of environmental matrices. Sepration and Purification Technol. 2019 [Google Scholar]
- 13.Mohammed Al-Sakkaf B., Nasreen S., Ejaz N., Editor G., Feng F. Degradation pattern of textile effluent by using bio and sono chemical reactor. J. Chem. 2020 [Google Scholar]
- 14.Peláez-cid A.A., Velázquez-ugalde I., Herrera-gonzález A.M., García-serrano J. Textile dyes removal from aqueous solution using Opuntia fi cus-indica fruit waste as adsorbent and its characterization. J. Environ. Manag. 2013;130:90–97. doi: 10.1016/j.jenvman.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 15.Paz A., Carballo J., Jos erez M.P., Manuel Domínguez J. Biological treatment of model dyes and textile wastewaters. Chemosphere. 2017 doi: 10.1016/j.chemosphere.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Shabbir S., Faheem M., Ali N., Kerr P.G., Wu Y. Periphyton biofilms: a novel and natural biological system for the effective removal of sulphonated azo dye methyl orange by synergistic mechanism. Chemosphere. 2016 doi: 10.1016/j.chemosphere.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Migliorini F.L., Steter J.R., Rocha R.S., Lanza M.R.V., Baldan M.R., Ferreira N.G. Efficiency study and mechanistic aspects in the Brilliant Green dye degradation using BDD/Ti electrodes. Diam. Relat. Mater. 2015 [Google Scholar]
- 18.Salazar R., Soledad Ureta-Za M., Gonz Alez-Vargas C., Do C., Brito N., Martinez-Huitle C.A. Electrochemical degradation of industrial textile dye disperse yellow 3: role of electrocatalytic material and experimental conditions on the catalytic production of oxidants and oxidation pathway. Chemosphere. 2017 doi: 10.1016/j.chemosphere.2017.12.092. [DOI] [PubMed] [Google Scholar]
- 19.Liang X., Zang Y., Xu Y., Tan X., Hou W., Wang L., et al. Sorption of metal cations on layered double hydroxides. Physicochem. Eng. Asp. 2013;433:122–131. [Google Scholar]
- 20.Yagub M.T., Kanti Sen T., Afroze S., Ang H. Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 2014 doi: 10.1016/j.cis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Kausar A., Javed A., Aftab K., Nazli Z.-I.-H., Bhatti N., Nouren S. Dyes adsorption using clay and modified clay: a review. J. Mol. Liq. 2018 [Google Scholar]
- 22.Trellu C.C., Oturan N., Kaba Keita F., Chloéfourdrin C., Pechaud Y., Oturan M.A. Regeneration of activated carbon fiber by the electro-fenton process. ACS Publ. 2018 doi: 10.1021/acs.est.8b01554. [DOI] [PubMed] [Google Scholar]
- 23.Foo K.Y., Hameed B.H. Bioresource Technology Microwave-assisted regeneration of activated carbon. Bioresour. Technol. 2012;119:234–240. doi: 10.1016/j.biortech.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Bañuelos J.A., Rodríguez F.J., Rocha J.M., Bustos E., Rodríguez A., Cruz J.C., et al. Novel electro-fenton approach for regeneration of activated carbon. Environ. Sci. Technol. 2013;47:7927–7933. doi: 10.1021/es401320e. [DOI] [PubMed] [Google Scholar]
- 25.Sarah S., Frank-Dieter K., Weiner B. Hydrothermal treatment for regeneration of activated carbon loaded with organic micropollutants. Sci. Total Environ. 2018:854–861. doi: 10.1016/j.scitotenv.2018.06.395. [DOI] [PubMed] [Google Scholar]
- 26.Salvador F., Martin-Sanchez N., Sanchez-Hernandez R., Sanchez-Montero M.J., Izquierdo C. Regeneration of carbonaceous adsorbents. Part II: chemical, microbiological and vacuum regeneration. Microporous Mesoporous Mater. 2015;202:277–296. [Google Scholar]
- 27.El Gamal M., Mousa H.A., El-Naas M.H., Zacharia R., Judd S. Bio-regeneration of activated carbon: a comprehensive review. Separ. Purif. Technol. 2018;197:345–359. [Google Scholar]
- 28.Li Q., Qi Y., Gao C. Chemical regeneration of spent powdered activated carbon used in decolorization of sodium salicylate for the pharmaceutical industry. J. Clean. Prod. 2015;86:424–431. [Google Scholar]
- 29.Ledesma B., Román S., Álvarez-Murillo A., Sabio E., González J.F. Cyclic adsorption/thermal regeneration of activated carbons. J. Anal. Appl. Pyrolysis. 2014;106:112–117. [Google Scholar]
- 30.Dewil R., Mantzavinos D., Poulios I., Rodrigo M.A. New perspectives for advanced oxidation processes. J. Environ. Manag. 2017;195:93–99. doi: 10.1016/j.jenvman.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Patel S., Mondal S., Majumder S.K., Das P., Ghosh P. Treatment of a pharmaceutical industrial effluent by a hybrid process of advanced oxidation and adsorption. ACS Omega. 2020;5:32305–32317. doi: 10.1021/acsomega.0c04139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santana R.M.D.R., Charamba L.C.V., do Nascimento G.E., de Oliveira J.G.C., Sales D.C.S., Duarte M.M.M.B., et al. Degradation of textile dyes employing advanced oxidative processes: kinetic, equilibrium modeling, and toxicity study of seeds and bacteria. Water Air Soil Pollut. 2019;230 [Google Scholar]
- 33.Cheng S., Wu J., Xia H., Peng J., Wang S., Zhang L. Microwave-assisted regeneration of spent activated carbon from paracetamol wastewater plant using response surface methodology. Desalination Water Treat. 2016;57:18981–18991. [Google Scholar]
- 34.Tang S., Li N., Qi J., Yuan D., Li J. Degradation of phenol using a combination of granular activated carbon adsorption and bipolar pulse dielectric barrier discharge plasma regeneration. Plasma Sci. Technol. 2018;20 [Google Scholar]
- 35.Parsa J.B., Jafari F. Sono-Fenton regeneration of granular activated carbon saturated with Rhodamine B: optimization using response surface methodology. Chem. Eng. Commun. 2017;204:1070–1081. [Google Scholar]
- 36.Alvarez-Pugliese C.E., Acuña-Bedoya J., Vivas-Galarza S., Prado-Arce L.A., Marriaga-Cabrales N. Electrolytic regeneration of granular activated carbon saturated with diclofenac using BDD anodes. Diam. Relat. Mater. 2019;93:193–199. [Google Scholar]
- 37.Leong K.Y., Loo S.L., Bashir M.J.K., Oh W.D., Rao P.V., Lim J.W. Bioregeneration of spent activated carbon: review of key factors and recent mathematical models of kinetics. Chin. J. Chem. Eng. 2018;26:893–902. [Google Scholar]
- 38.Baccar R., Bouzid J., Feki M., Montiel A. Preparation of activated carbon from Tunisian olive-waste cakes and its application for adsorption of heavy metal ions. J. Hazard Mater. 2009;162:1522–1529. doi: 10.1016/j.jhazmat.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 39.Duan X., Kannan S. Thermal regeneration of spent coal-based activated carbon using carbon dioxide : process optimisation , Methylene Blue decolorisation isotherms and kinetics. Coloration Technology. 2012:464–472. [Google Scholar]
- 40.Montilla F., Lillo-ro M.A., A-oto M.G. Electrochemical regeneration of activated carbon saturated with toluene. J. Appl. Electrochem. 2005:319–325. [Google Scholar]
- 41.Muranaka N.T., Polit E. Combinação de adsorção por carvão ativado com Processo Oxidativo Avançado (POA) para tratamento de efluentes contendo. Fenol. 2010:165. [Google Scholar]
- 42.Román S., Ledesma B., González J.F., Al-Kassir A., Engo G., Álvarez-Murillo A. Two stage thermal regeneration of exhausted activated carbons. Steam gasification of effluents. J. Anal. Appl. Pyrolysis. 2013;103:201–206. [Google Scholar]
- 43.Mcquillan R.V., Stevens W., Mumford K.A. The electrochemical regeneration of granular activated carbons : A review. J. Hazardous Mater. 2018;355:34–49. doi: 10.1016/j.jhazmat.2018.04.079. [DOI] [PubMed] [Google Scholar]
- 44.Álvarez P.M., Beltrán F.J., Gómez-Serrano V., Jaramillo J., Rodríguez E.M. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol. Water Res. 2004;38:2155–2165. doi: 10.1016/j.watres.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W., Meng X., Ding Y., Rajic L., Gao J., Qin Y., et al. Self-cleaning” electrochemical regeneration of dye-loaded activated carbon. Electrochem. Commun. 2019;100:85–89. doi: 10.1016/j.elecom.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanella O., Bilibio D., Priamo W.L., Tessaro I.C. Electrochemical regeneration of phenol-saturated activated carbon – proposal of a reactor. Environ. Technol. 2016:3330. doi: 10.1080/09593330.2016.1202327. [DOI] [PubMed] [Google Scholar]
- 47.Bouaziz I., Hamza M., Abdelhedi R., Serrano K.G. A comparative study for the electrochemical regeneration of adsorbents loaded with methylene blue. J. Water Environ. Nanotechnol. 2017;2:17–25. [Google Scholar]
- 48.Brillas E. A review on the degradation of organic pollutants in waters by UV photoelectro-Fenton and solar photoelectro-Fenton. J. Braz. Chem. Soc. 2014;25:393–417. [Google Scholar]
- 49.Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment – a critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 50.N P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: a review. Environ. Sci. Pollut. Res. Int. 2017;24:27047–27069. doi: 10.1007/s11356-017-0481-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q., Cheng S., Xia H., Zhang L., Zhou J., Jiang X., et al. Removal of Congo red and methylene blue using H2O2 modified activated carbon by microwave regeneration: isotherm and kinetic studies. Mater. Res. Express. 2019;6 [Google Scholar]
- 52.Wang H., Guo H., Liu Y., Yi C. Regeneration of acid orange 7 exhausted granular activated carbon using pulsed discharge plasmas. Plasma Sci. Technol. 2015;17:881–886. [Google Scholar]
- 53.Babuponnusami A., Muthukumar K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014;2:557–572. [Google Scholar]
- 54.Abusallout I., Hua G. Natural solar photolysis of total organic chlorine, bromine and iodine in water. Water Res. 2016;92:69–77. doi: 10.1016/j.watres.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 55.W J., Y J., P H., W M., S W., L Y., et al. Solar photolysis of soluble microbial products as precursors of disinfection by-products in surface water. Chemosphere. 2018;201:66–76. doi: 10.1016/j.chemosphere.2018.02.185. [DOI] [PubMed] [Google Scholar]
- 56.Pereira Vanessa J., Weinberg Howard S., Linden Karl G., Singer P.C. UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Environ. Sci. Technol. 2007;41:1682–1688. doi: 10.1021/es061491b. [DOI] [PubMed] [Google Scholar]
- 57.Andreozzi R., Marotta R., Paxéus N. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere. 2003;50:1319–1330. doi: 10.1016/s0045-6535(02)00769-5. [DOI] [PubMed] [Google Scholar]
- 58.Gomes da Silva C., Faria J.L. Photochemical and photocatalytic degradation of an azo dye in aqueous solution by UV irradiation. J. Photochem. Photobiol. Chem. 2003;155:133–143. [Google Scholar]
- 59.Ameta S.C., Ameta R. Advanced oxidation processes for wastewater treatment: emerging green chemical technology. Adv. Oxid Process Wastewater Treat Emerg. Green Chem. Technol. 2018:1–412. [Google Scholar]
- 60.Nasrollahi N., Ghalamchi L., Vatanpour V., Khataee A. Photocatalytic-membrane technology: a critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021;93:101–116. [Google Scholar]
- 61.Majumdar A., Pal A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy. 2020;22 Springer Berlin Heidelberg. [Google Scholar]
- 62.Lv T., Li D., Hong Y., Luo B., Xu D., Chen M., et al. Facile synthesis of CdS/Bi4V2O11 photocatalysts with enhanced visible-light photocatalytic activity for degradation of organic pollutants in water. Dalton Trans. 2017;46:12675–12682. doi: 10.1039/c7dt02151h. [DOI] [PubMed] [Google Scholar]
- 63.Zuo G., Li B., Guo Z., Wang L., Yang F., Hou W., et al. Efficient photocatalytic hydrogen peroxide production over TiO2 passivated by SnO2. Catal. 2019;9:623. [Google Scholar]
- 64.Rebelo S.L.H., Pereira M.M., Simões M.M.Q., Neves M.G.P.M.S., Cavaleiro J.A.S. Mechanistic studies on metalloporphyrin epoxidation reactions with hydrogen peroxide: evidence for two active oxidative species. J. Catal. 2005;234:76–87. [Google Scholar]
- 65.Bokare A.D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard Mater. 2014;275:121–135. doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 66.Toijer E., Jonsson M. H2O2 and γ-radiation induced corrosion of 304L stainless steel in aqueous systems. Radiat. Phys. Chem. 2019;159:159–165. [Google Scholar]
- 67.Z Y., C J.W., L S., K J.A., C C.W., Y Y.S. Experimental and QSAR studies on adsorptive interaction of anionic nonsteroidal anti-inflammatory drugs with activated charcoal. Chemosphere. 2018;212:620–628. doi: 10.1016/j.chemosphere.2018.08.115. [DOI] [PubMed] [Google Scholar]
- 68.Yu G., Lu S., Chen H., Zhu Z. Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon. Carbon N Y. 2005;43:2285–2294. [Google Scholar]
- 70.Santos D.H.S., Duarte J.L.S., Tonholo J., Meili L., Zanta C.L.P.S. Separation and Puri fi cation Technology Saturated activated carbon regeneration by UV-light , H 2 O 2 and Fenton reaction. Separ. Purif. Technol. 2020;250 [Google Scholar]
- 72.Pignatello J.J., Oliveros E., MacKay A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006;36:1–84. [Google Scholar]
- 73.Navalon S., Alvaro M., Garcia H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010;99:1–26. [Google Scholar]
- 74.Pupo Nogueira R.F., Trovó A.G., Da Silva M.R.A., Villa R.D., De Oliveira M.C. Fundaments and environmental applications of Fenton and photo-Fenton processes. Quim. Nova. 2007;30:400–408. [Google Scholar]
- 75.Youssef N.A., Shaban S.A., Ibrahim F.A., Mahmoud A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt J Pet. 2016;25:317–321. [Google Scholar]
- 76.Dong H., Su H., Chen Z., Yu H., Yu H. Fabrication of electrochemically reduced graphene oxide modified gas diffusion electrode for in-situ electrochemical advanced oxidation process under mild conditions. Electrochim. Acta. 2016;222:1501–1509. [Google Scholar]
- 77.Manuel J., Ramos P., Pereira-queiroz N.M., Santos D.H.S., Nascimento J.R., Monteiro C., et al. Printing ink e ffl uent remediation : a comparison between electrochemical and Fenton treatments. J. Water Proc. Eng. 2019;31 [Google Scholar]
- 78.Simond O., Schaller V., Comninellis C. Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochim. Acta. 1997;42:2009–2012. [Google Scholar]
- 79.Shang Y., Li X., Yang Y., Wang N., Zhuang X., Zhou Z. Optimized photocatalytic regeneration of adsorption-photocatalysis bifunctional composite saturated with Methyl Orange. J. Environ. Sci. 2020;94:40–51. doi: 10.1016/j.jes.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 80.Larasati A., Fowler G.D., Graham N.J.D. Insights into chemical regeneration of activated carbon for water treatment. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.chemosphere.2021.131888. [DOI] [PubMed] [Google Scholar]
- 81.Shende R.V., Mahajani V.V. Wet oxidative regeneration of activated carbon loaded with reactive dye. Waste Manag. 2002;22:73–83. doi: 10.1016/s0956-053x(01)00022-8. [DOI] [PubMed] [Google Scholar]
- 82.Xin-hui D., Srinivasakannan C., Jin-sheng L. Process optimization of thermal regeneration of spent coal based activated carbon using steam and application to methylene blue dye adsorption. J. Taiwan Inst. Chem. Eng. 2014;45:1618–1627. [Google Scholar]
- 83.Wang Y., Lin C., Liu X., Ren W., Huang X., He M., et al. Efficient removal of acetochlor pesticide from water using magnetic activated carbon: adsorption performance, mechanism, and regeneration exploration. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146353. [DOI] [PubMed] [Google Scholar]
- 84.Durán-jiménez G., Stevens L.A., Hodgins G.R., Uguna J., Ryan J., Binner E.R., et al. Fast regeneration of activated carbons saturated with textile dyes : textural , thermal and dielectric characterization. Chem. Eng. J. 2019;378 [Google Scholar]
- 85.Chu L., Wang J. Regeneration of sulfamethoxazole-saturated activated carbon using gamma irradiation. Radiat. Phys. Chem. 2017;130:391–396. [Google Scholar]
- 86.Liu Z., Ren B., Ding H., He H., Deng H., Zhao C., et al. Simultaneous regeneration of cathodic activated carbon fiber and mineralization of desorbed contaminations by electro-peroxydisulfate process: advantages and limitations. Water Res. 2020;171 doi: 10.1016/j.watres.2019.115456. [DOI] [PubMed] [Google Scholar]
- 87.Trellu C., Gibert-Vilas M., Pechaud Y., Oturan N., Oturan M.A. Clofibric acid removal at activated carbon fibers by adsorption and electro-Fenton regeneration – modeling and limiting phenomena. Electrochim. Acta. 2021;382 [Google Scholar]
- 88.Bury N.A., Mumford K.A., Stevens G.W. The electro-Fenton regeneration of Granular Activated Carbons: degradation of organic contaminants and the relationship to the carbon surface. J. Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125792. [DOI] [PubMed] [Google Scholar]
- 89.Lu P.J., Lin H.C., Yu W.T., Chern J.M. Chemical regeneration of activated carbon used for dye adsorption. J. Taiwan Inst. Chem. Eng. 2011;42:305–311. [Google Scholar]
- 90.Krishnamoorthy S., Ajala F., Mohammed S.M., Asok A., Shukla S. High adsorption and high catalyst regeneration kinetics observed for Flyash-Fe3O4-Ag magnetic composite for efficient removal of industrial azo reactive dyes from aqueous solution via persulfate activation. Appl. Surf. Sci. 2021;548 [Google Scholar]
- 91.Cabrera-Codony A., Gonzalez-Olmos R., Martín M.J. Regeneration of siloxane-exhausted activated carbon by advanced oxidation processes. J. Hazard Mater. 2015;285:501–508. doi: 10.1016/j.jhazmat.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 92.Cai Q.Q., Wu M.Y., Hu L.M., Lee B.C.Y., Ong S.L., Wang P., et al. Organics removal and in-situ granule activated carbon regeneration in FBR-Fenton/GAC process for reverse osmosis concentrate treatment. Water Res. 2020;183 doi: 10.1016/j.watres.2020.116119. [DOI] [PubMed] [Google Scholar]
- 93.Huling S.G., Jones P.K., Ela W.P., Arnold R.G. Fenton-driven chemical regeneration of MTBE-spent GAC. Water Res. 2005;39:2145–2153. doi: 10.1016/j.watres.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 94.Huling S.G., Hwang S. Iron amendment and Fenton oxidation of MTBE-spent granular activated carbon. Water Res. 2010;44:2663–2671. doi: 10.1016/j.watres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 95.Jatta S., Huang S., Liang C. A column study of persulfate chemical oxidative regeneration of toluene gas saturated activated carbon. Chem. Eng. J. 2019;375 [Google Scholar]
- 96.Su L., Chen M., Zhuo G., Ji R., Wang S., Zhang L., et al. Comparison of biochar materials derived from coconut husks and various types of livestock manure, and their potential for use in removal of h2s from biogas. Sustain. Times. 2021;13:6262. [Google Scholar]
- 97.Bakht Shokouhi S., Dehghanzadeh R., Aslani H., Shahmahdi N. Activated carbon catalyzed ozonation (ACCO) of Reactive Blue 194 azo dye in aqueous saline solution: experimental parameters, kinetic and analysis of activated carbon properties. J. Water Proc. Eng. 2020;35 [Google Scholar]
- 98.Mustafa M., Kozyatnyk I., Gallampois C., Oesterle P., Östman M., Tysklind M. Regeneration of saturated activated carbon by electro-peroxone and ozonation: fate of micropollutants and their transformation products. Sci. Total Environ. 2021;776 [Google Scholar]
- 99.Foo K.Y. Effect of microwave regeneration on the textural network, surface chemistry and adsorptive property of the agricultural waste based activated carbons. Process Saf. Environ. Protect. 2018;116:461–467. [Google Scholar]
- 100.Santos D.H.S., Santos J.P.T.S., Duarte J.L.S., Oliveira L.M.T.M., Tonholo J., Meili L., et al. Regeneration of activated carbon adsorbent by anodic and cathodic electrochemical process. Process Saf. Environ. Protect. 2022;159:1150–1163. [Google Scholar]
- 101.Liu S., Wang Y., Wang B., Huang J., Deng S., Yu G. Regeneration of Rhodamine B saturated activated carbon by an electro-peroxone process. J. Clean. Prod. 2017;168:584–594. [Google Scholar]
- 102.Q X., L X., B L., C S., Z Y., C X. Regeneration of acid orange 7-exhausted granular activated carbons with microwave irradiation. Water Res. 2004;38:4484–4490. doi: 10.1016/j.watres.2004.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.