Abstract
Allergic airway disease models use laboratory mice housed in highly controlled and hygienic environments, which provide a barrier between the mice and a predetermined list of specific pathogens excluded from the facility. In this study, we hypothesized that differences in facility barrier level and, consequently, the hygienic quality of the environment that mice inhabit impact the severity of pulmonary inflammation and lung function. Allergen-naive animals housed in the cleaner, high barrier (HB) specific pathogen-free facility had increased levels of inflammatory cytokines and higher infiltration of immune cells in the lung tissue but not in the bronchoalveolar lavage compared with mice housed in the less hygienic, low barrier specific pathogen-free facility. In both genders, house dust mite–induced airway disease was more severe in the HB than the low barrier facility. Within each barrier facility, female mice developed the most severe inflammation. However, allergen-naive male mice had worse lung function, regardless of the housing environment, and in the HB, the lung function in female mice was higher in the house dust mite model. Severe disease in the HB was associated with reduced lung microbiome diversity. The lung microbiome was altered across housing barriers, gender, and allergen-exposed groups. Thus, the housing barrier level impacts microbial-driven disease and gender phenotypes in allergic asthma. The housing of laboratory mice in more clean HB facilities aggravates lung immunity and causes a more severe allergic lung disease. ImmunoHorizons, 2021, 5: 33–47.
INTRODUCTION
Asthma is a chronic inflammatory disease characterized by airway inflammation and bronchoconstriction (1, 2). Environmental factors and gender are known as determinants of asthma. Mouse models of allergic airway inflammation are fundamental to our understanding of this lung disease’s mechanisms, identification of new therapeutic targets, and preclinical testing. It is imperative that the research findings in mouse models are as close as possible to human asthma to translate bench discoveries into clinical therapies successfully.
Laboratory mice are frequently housed in hygienic facilities that are specific pathogen–free (SPF) and have immune systems that more closely resemble a newborn than an adult human (3). In the literature, 44% of publications mention housing of mice used in asthma research under SPF conditions, whereas in the remaining journals, the SPF status of housing facilities was not specified. Thus, it is unknown how SPF status and the various environmental and husbandry practices contributing to different hygienic conditions of individual housing facilities of laboratory mice affect allergic airway disease.
In addition to environmental factors, gender also plays an essential role in the pathogenesis of asthma. Before puberty, males are twice as likely as females to develop asthma (4–9). However, in adulthood, asthma is more prevalent and severe in women than in men (4–9). This is attributable to hormonal changes that occur during puberty. Indeed, several reports showed that ovarian hormones increase whereas testosterone decreases airway inflammation in asthma (4–10). Representation of the sexual dimorphism in mouse models of airway disease is thus essential. Several studies showed that female mice have higher OVA-induced allergic airway inflammation than male mice (11–15). However, gender effects in the commonly used house dust mite (HDM) extract (HDME) model of airway inflammation are unknown.
In this study, we hypothesized that isogenic and isobiotic mice shipped from the same commercial production facility and then housed at one institution but in separate facilities with different barrier levels, and hygiene practices would have different induced asthma phenotypic outcomes. Male and female mice were divided between two different facilities upon arrival. Then, we analyzed the effects of housing facility and gender on the development of allergic airway disease in a HDM model. We show that the housing facility affects basal lung cytokine and pulmonary inflammatory cell infiltration in both genders. Mice housed in a less clean low barrier (LB) facility had less activation of the basal lung immunity than mice housed in a more hygienic high barrier (HB) facility upon HDME exposure. Female mice in the HB facility developed the most severe allergic airway disease.
MATERIALS AND METHODS
Animals and barrier housing
Male and female C57BL/6J mice (aged 12 wk) were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in a maximum barrier production facility under highly hygienic SPF conditions while at The Jackson Laboratory during the critical period early in life in which exposure to a wide range of microbes promotes the development of immunological maturity. Upon arrival, both male and female mice were housed in either a more hygienic HB SPF facility or a less clean LB SPF facility for 1 wk before the onset of experiments.
In both facilities, mice were housed in polycarbonate microisolator cages with sterile corncob bedding (Andersons) and sterile cotton nestlets (Ancare) or paper towels on racks that provide high-efficiency particulate air–filtered air ventilation to individual cages with ad libitum access to irradiated mouse chow (Harlan Teklad) and acidified, filtered water under a 14:10 light/dark cycle. There were only minor variations in ambient light levels, high/low average daily temperatures, and humidity. In both facilities, cages were changed every 14 d inside of a Class IIA biological safety cabinet.
Sterilized individually ventilated cages were used in both facilities. Health monitoring for the absence of specific pathogens involves the complete or partial exclusion from mouse facilities of infectious agents according to a predetermined list. Current health monitoring of mouse facilities is limited to particular agents only. Mice from different hygienic barriers may vary considerably in their responses to experimental treatments, making the phenotypic outcome of established asthma models unpredictable.
In the less clean LB facility, access was less restricted, and the presence of more infectious agents (e.g., murine norovirus, murine species of Helicobacter, Pasteurella pneumotropica) were tolerated than in the HB facility. Only gloves and a clean laboratory coat needed to be put on to handle mice in the LB, whereas clean scrubs, entry through an air shower, a clean laboratory coat, hair bonnet, surgical mask, shoe covers, gloves, and Tyvek sleeves were required for handling mice in the HB facility. In the LB, cages were washed and autoclaved between uses, whereas the HB cages were irradiated and single use. Mice housed in the facility were either directly imported from a commercial vendor or research facilities at academic research institutions. Mice in the HB were imported directly from a commercial vendor or rederived through Caesarean section and cross-fostering if coming from an academic institution.
Literature screening for housing conditions of mouse asthma models
PubMed was searched using the keywords HDM and mouse and asthma for publications in 2018 and 2019. In total, 45 original research papers were screened for housing conditions.
Mouse HDM model of allergic inflammation
On day 8, animals were anesthetized by isoflurane inhalation and sensitized intranasally with 100 μg HDME (Dermatophagoides pteronyssinus, XPB82D3A2.5; Greer Laboratories) resuspended in 50 μl saline. The control group was sensitized with 50 μl of saline only. On day 13, animals were anesthetized by isoflurane inhalation and intranasally challenged daily with 10 μg HDME in 50 μl saline for five consecutive days. The control group was challenged with 50 μl of sterile saline only. The mice were sensitized and challenged within their assigned facility. The same batch of HDME was used for all studies. On day 20, the animals were processed for airway hyperresponsiveness (AHR) and negative pressure–driven forced expiration (NPFE) measurements in each facility. A schematic representation of HMDE sensitization and challenges is provided in Supplemental Fig. 1.
AHR and NPFE
Mice were kept in their specific facilities for all measurements on live animals. Mice were anesthetized by i.p. injection with 40–50 mg/kg of pentobarbital. A tracheotomy was performed, followed by tracheal intubation with an 18-g cannula. The mouse was placed on the flexiVent FX2 system (SCIREQ, Montreal, QC, Canada), and pancuronium bromide (P1918; Sigma) 0.8 mg/kg was administered i.p. as a paralytic. Baseline (no saline) and control (saline only) airway mechanics were taken before increasing concentrations of nebulized methacholine doses. The NPFE were taken at cycle 9 of 16 for baseline and control to mimic the peaks in the methacholine challenges. Three concentrations, 25, 50, and 100 mg/ml of methacholine, were nebulized, and measurements were performed after each dose. NPFE were taken after the peak (highest total respiratory resistance) of each concentration. Following NPFE, two deep inflations were administered to reinflate the lungs before the next methacholine dose.
Plasma, bronchoalveolar lavage, and tissue collection
After the flexiVent measurements, blood was collectedin K2EDTA tubes via cardiac puncture for isolation of plasma. The tubes were centrifuged at 500 × g for 10 min, and the plasma was collected. The airways were lavaged with 0.7 ml of 2% FBS in saline to collect bronchoalveolar lavage (BAL) fluid. The BAL was centrifuged (300 × g, 10 min) to collect the supernatant. The cell pellets were resuspended in 0.5 ml of 2% FBS, and the total cell count was determined using a hemocytometer. Ten microliters of cell suspension was combined with 10 μl of 2× LIVE/DEAD dye. The 2× dilution was made of 20 μl of 100× LIVE/DEAD dye plus 980 μl of 1× PBS. The 100× stock solution contains 100 μl of 10 mg/ml ethidium bromide (E1510; Sigma), 100 μl of 10 mg/ml Acridine orange (A3568; Thermo Fisher Scientific), and 800 μl of 1×PBS. A fluorescence microscope was used to count the live cells. Epithelial cells, cells that were clumped together, were excluded from the count. After the total cell count was determined, cells were resuspended at a concentration of 200 × 103 cells/ml in 2% FBS in saline. The 200 μl of cell suspension was pipetted into a Cytofunnel (A78710020; Thermo Fisher Scientific). Samples were spun down at 162.58 × g (1200 Rpm) for 4 min in the Cytospin. The slides were stained with Kwik-Diff Kit (9990700; Thermo Fisher Scientific) reagent, following the enclosed instructions. Two hundred cells were scored using a light microscope to determine the inflammatory cell types using standard cytology criteria. The left lung lobes were harvested, fixed in 10% formalin, and processed for histology. The right lungs were harvested and divided for single cell digestion for flow cytometry analysis and protein extraction for ELISAs.
Single cell suspension
Preparation of a single cell suspension from the lung tissue was performed as described (16). Briefly, the inferior and intermediate lobes of the right lungs were dissected and minced with scissors. Lung pieces were incubated with a mixture of digestive enzymes containing 10 mg/ml dispase II, 100 mg/ml collagenase A, and 150,000 KU/ml DNase I at 37°C for 30 min. The tissue was pipetted and placed at 37°C for an additional 20 min. The cell suspension was filtered through a 40-μm cell strainer and stained with an amine-reactive viability dye and fixed with 4% paraformaldehyde. Aliquots were frozen at −80°C in FBS with 10% DMSO for batch flow cytometry.
Flow cytometry
Lung single cells were thawed and washed in PBS to remove DMSO. Samples from each mouse were stained with varying concentrations of Pacific Blue succinimidyl ester (0, 20, 80, 320, and 1280 ng/ml) to barcode the cells so that five samples could be combined in a single tube (17). After barcoding and subsequent washes, cells were incubated in50 μl of Fc block for 15 min to block nonspecific binding of Abs to Fc receptors. In a volume of 50 μl, diluted cell surface Abs were added to the cells in 50 μl of Fc block and incubated for 30 min on ice. All Abs were titrated to determine optimal staining concentrations (Supplemental Table I). Because multiple BD Horizon Brilliant Violet dyes were used, both the Fc block and Ab dilutions were prepared in BD Horizon Brilliant Stain Buffer. After the incubation, samples were washed with 1% BSA in PBS. Data were acquired using the LSRFortessa (BD Biosciences) equipped with five lasers (355, 407, 488, 561, and 641 nm). SPHERO Ultra Rainbow Calibration Kit (Spherotech, Lake Forest, IL) was used to standardize the instrument’s fluorescence channels for day-to-day variation. AbC Total Ab Compensation Bead Kit (Life Technologies) was used to prepare single-color compensation controls. Cells were used for single-color controls for the LIVE/DEAD dye and Pacific Blue succinimidyl ester. Data analysis, including compensation, was performed postacquisition using FlowJo software V.10.6 (Tree Star, Ashland, OR). Subsets were identified as described (18). The gating strategy is shown in Supplemental Fig. 2.
Protein extraction
Protein was extracted by placing a small piece of the lung (5 mm × 1 mm) in 200 μl cold PBS + 1:100 protease and phosphatase inhibitor (catalog no. 1861281; Thermo Fisher Scientific). The tissue was homogenized by mincing with scissors first and then using a pellet pestle. The tissue was frozen on dry ice and then thawed at room temperature twice. The tissue was centrifuged (18,000 × g for 30 min) at 4°C. The supernatant was collected, and the volume was raised to 200 μl with PBS.
MSD U-PLEX assay and ELISA
We used the Th1/Th2 U-PLEX (K15069L-2; Meso Scale Discovery [MSD]) platform to analyze cytokines in protein extract and BAL supernatant. This platform uses a 10-spot U-PLEX plate and unique linkers to analyze samples. Biotinylated capture Abs are assigned to linkers, which correspond to unique spots in the U-PLEX plate. The analytes tested were IFN-γ, IL-1β, IL-2, IL-4, IL-5, KC, IL-10, IL-12p70, TNF-α, and IL-13. The MSD instrument applies a voltage to the plate electrodes, causing the capture labels to emit light. The intensity of the emitted light is measured. The BAL supernatant was at 1:1 dilution, and the protein extraction was at 1:2 dilution. Dilutions were used in the assay and then corrected for in the analysis. Blood was collected via cardiac puncture and stored in K2EDTA tubes. The tubeswere centrifuged at 500 × g for 10 min, and the plasma was collected. We used the Mouse IgE ELISA Kit (EMIGHE; Thermo Fisher Scientific) to analyze for IgE. The plasma was diluted 10,000-fold as suggested by the vendor and corrected for in the analysis. The lung protein was extracted from homogenized lung tissue. We used the IL-17 ELISA Kit (GR3332177–1; Abcam) to analyze for IL-17 cytokine. The lung protein extract was diluted 1:1. The dilution was corrected for in the analysis.
Lung microbiome
DNA extraction and 16S rRNA amplicon sequencing was performed on lung tissue samples. First, DNA isolation was performed using QIAGEN’s MagAttract Kit. Next, the isolated microbial guide DNA was checked for signs of degradation and quantified to ensure accurate sample input for the initial PCR step. A nested PCR method was used to amplify the V4 region of the 16S rRNA gene and the addition of Illumina Nextera Unique Dual Indexes. Afterward, each library underwent standard quality control procedures checking for sample concentration and sample quality. Each library was pooled together, ensuring equal sample distribution among sequencing reads. Amplicon sequencing was then performed on Illumina’s iSeq 100 with a 2 × 150 read length.
Lung microbiome bioinformatics and statistical analysis
Demultiplexed fastq files without nonbiological nucleotides were processed using the Divisive Amplicon Denoising Algorithm pipeline (19). The output of the dada2 pipeline (feature table of amplicon sequence variants [ASV] [an ASV table]) was processed for an α- and β-diversity analysis using phyloseq (20) and micro-biomeSeq(http://www.github.com/umerijaz/microbiomeSeq)packages in R. α-Diversity estimates were measured within group categories using estimate_richness function of the phyloseq package (20). Multidimensional scaling (also known as principal coordinate analysis) was performed using the Bray–Curtis dissimilarity matrix (21) between groups and visualized by using the ggplot2 package (22). We assessed the statistical significance (P < 0.05) throughout, and whenever necessary, we adjusted p values for multiple comparisons according to the Benjamini and Hochberg method to control false discovery rate (23) while performing multiple testing on ASV abundance according to sample categories. We performed an ANOVA among sample categories while measuring α-diversity measures using plot_ anova_diversity function in the microbiomeSeq package (http://www.github.com/umerijaz/microbiomeSeq). Permutational multivariate ANOVA with 999 permutations was performed on all principal coordinates obtained during principal coordinate analysis with the ordination function of the microbiomeSeq package. Pairwise two-group analysis was performed using White’s nonparametric t test (24).
Statistical analysis
JMP 13.1.0 software program (SAS Institute) was used for comparisons between two groups. Student t test for parametric and a Wilcoxon test for nonparametric data were used as appropriate. Airway hyperreactivity data were analyzed using a mixed-effects regression model, as described (25).
RESULTS
Gender and housing effects on BAL inflammation in allergen-naive and HDME-exposed mice
We investigated whether differences in environmental and husbandry practices intended to prevent exposure of mice to specific pathogenic and opportunistic infectious organisms in an LB or HB SPF mouse housing facility impact the gender effects and development of asthma in an established mouse model. Histological imaging of airways showed no apparent housing or gender effects inallergen-naive mice (Fig. 1A–D). Absolute cell numbers in BAL and differential cell counts for neutrophils, eosinophils, macrophages, and lymphocytes were similar across groups (Fig. 1E–I).
FIGURE 1. Airway histology and BAL cell counts.
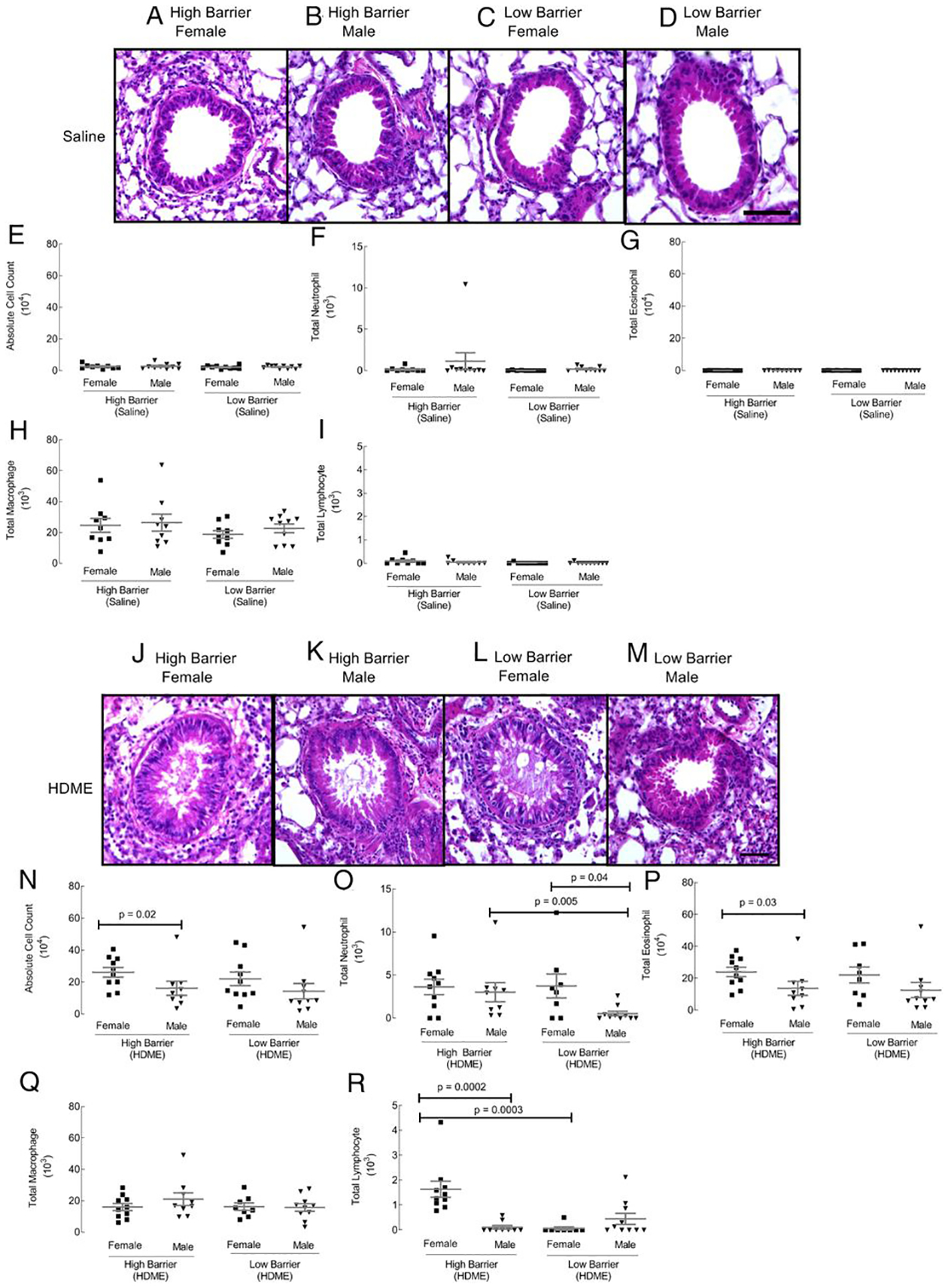
Paraffin-embedded lung tissue sections were stained with H&E (A–D control groups; J–M HDME groups). Scale bar, 50 μm. Airways were lavaged with 0.7 ml of 2% FBS in saline. BAL cytospins were quantified for absolute cell numbers (E and N), neutrophils (F and O), eosinophils (G and P), macrophages (H and Q), and lymphocytes (I and R). Each dot represents data from one mouse. Ten mice in each group were used.
HDME exposure induced airway inflammation in all groups, as expected (Fig. 1J–M). Inflammatory cells around airways and mucus in the airway lumen were present in all groups. No apparent differences were observed with light microscopy in the HDME-exposed mice. In the LB facility, absolute cell counts were similar between males and females (Fig. 1N). There was an increase in neutrophils in female mice compared with male mice in the LB facility (Fig. 1O). However, in the HB facility, females had a higher absolute cell count than males (Fig. 1N). This was due to increased infiltration of eosinophils and lymphocytes (Fig. 1P, 1R). When facilities were compared, HB males showed an increased neutrophilic, and HB females had higher lymphocytes in the lungs (Fig. 1N–R). Macrophages in the BAL were similar across groups (Fig. 1Q). Overall, the data show gender and housing facility–specific differences in airway inflammation in HDME-exposed mice but not in allergen-naive animals.
Gender and housing effects on inflammation in the lung tissue
Lungs from all groups were harvested, enzymatically digested into single cell suspension, and analyzed for inflammatory cells using flow cytometry. In contrast to the BAL examination, saline-exposed mice showed marked differences in lung tissue inflammation. Males housed in the HB facility had increased infiltration of hematopoietic cells in lung tissue than males in the LB facility (Fig. 2A). Both genders in the LB facility had higher alveolar macrophages than the HB facility (Fig. 2B). Eosinophils and interstitial macrophages were similar across all saline groups (Fig. 2C, 2D). Females in both facilities recruited more lymphocytes compared with males (Fig. 2E). Females housed in the HB facility showed an increase in lymphocytes and neutrophils than females in the LB facility (Fig. 2E, 2F). In the HDME groups, all quantified immune cells in lung tissue were affected. Males housed in the HB facility continued to show an increase in inflammation in the lung tissue compared with males in the standard facility (Fig. 2G). However, males housed in the LB facility show a significant increase in alveolar and interstitial macrophages compared with males in the HB (Fig. 2H, 2J). Females in the LB facility showed an increase in eosinophils compared with females in the HB facility (Fig. 2I). Within the LB facility, males had higher alveolar macrophages compared with females (Fig. 2H). However, females within the LB facility had more elevated amounts of eosinophils than males (Fig. 2I). Lymphocytes and neutrophils were increased in HB mice (Fig. 2K, 2L). Female mice had significantly higher neutrophil infiltration within the HB facility than males (Fig. 2L). In general, gender and housing conditions affected immune cell infiltration in the lung tissue in allergen-naive and HDME-exposed groups.
FIGURE 2. Inflammation in lung tissue.
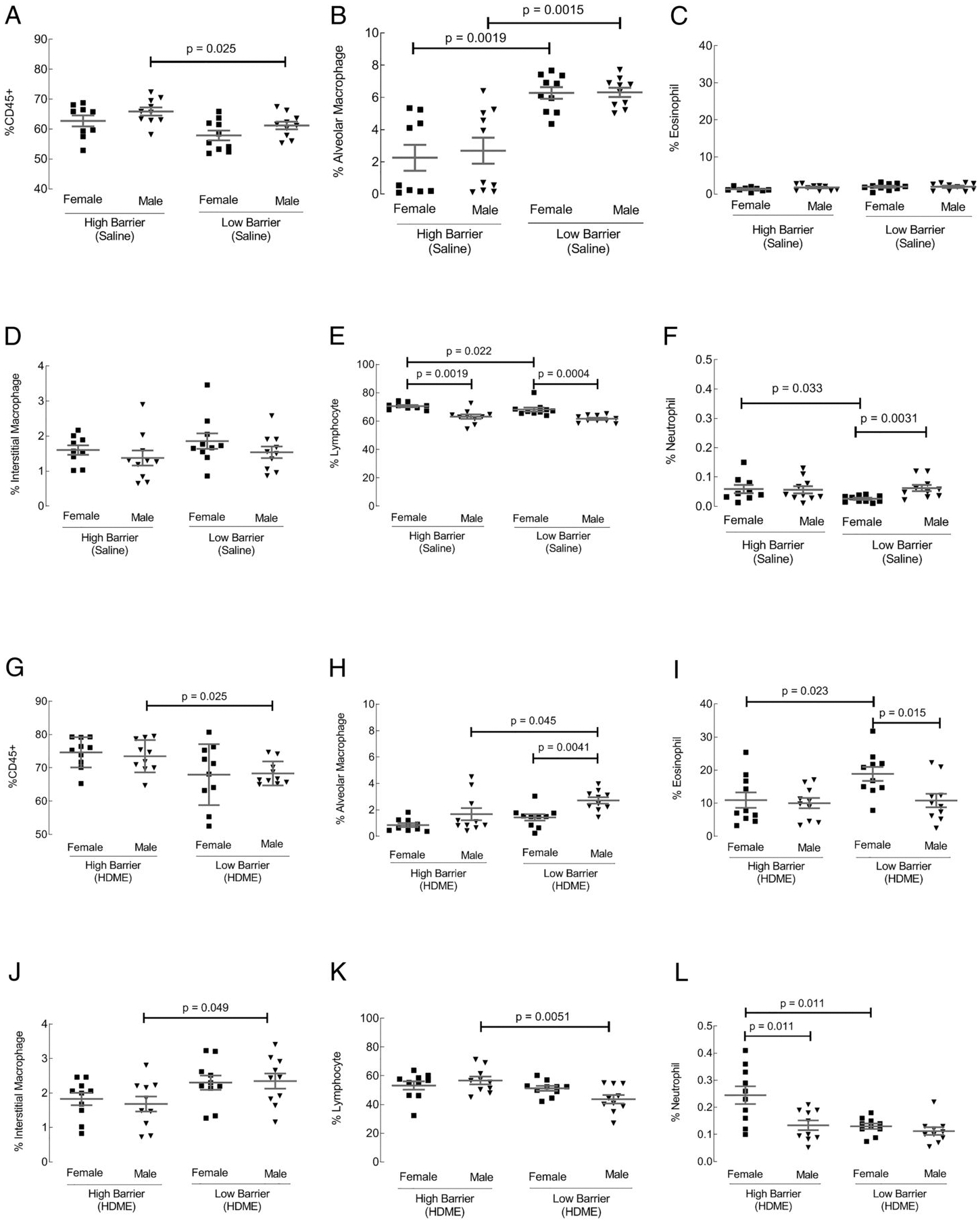
Lungs from control (A–F) and HDME-exposed (G–L) groups were harvested from each mouse and digested into a single cell suspension for flow cytometric analysis. Percentages of immune cells (CD45), alveolar macrophages, eosinophils, interstitial macrophages, lymphocytes, and neutrophils were determined. Each dot represents data from one mouse. Ten mice in each group were used.
AHR and NPFE
Lung functions determined by flexiVent experiment showed no significance in AHR (total respiratory resistance) between genders or facilities among saline- or allergen-exposed groups (Supplemental Fig. 3A–D). Male mice in both facilities had worse lung function, as indicated by a lower forced expiratory volume at 0.1 s (FEV0.1) than females at baseline or after allergen exposure (Fig. 3A–C). However, HB females had lower FEV0.1 compared with LB females after allergen exposure (Fig. 3D). The data showed that allergen-naive male mice and allergen-exposed female mice in the HB facility have a worse lung function.
FIGURE 3. NPFE lung function measurements.
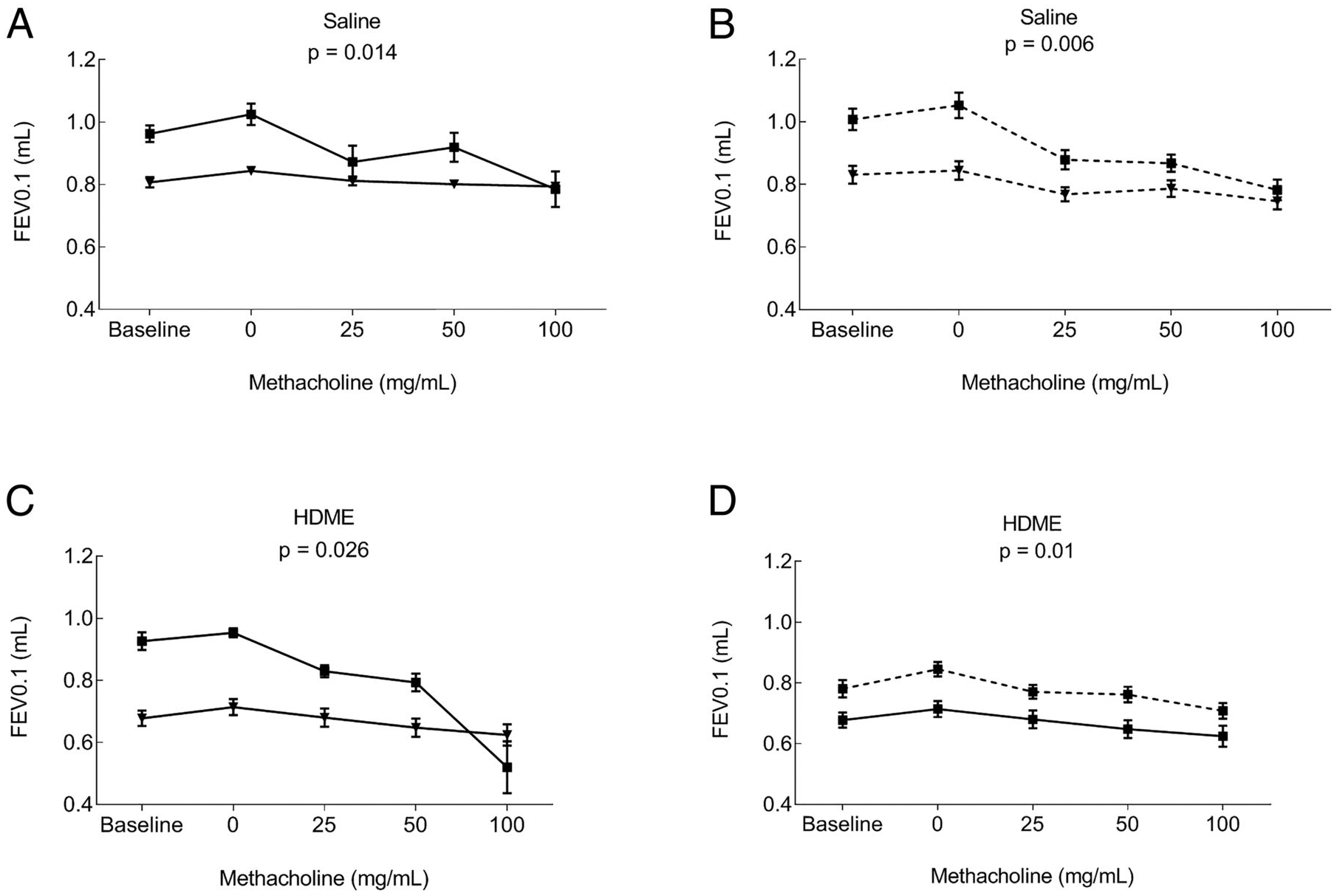
Forced expiration volumes were determined in control and HDME groups. FEV0.1 graphs that were significantly different are shown. (A) Comparison of female and male mice in the HB in saline group. (B) Comparison of female and male mice in the LB saline group. (C) Comparison of female and male mice in the HB after allergen exposure. (D) Comparison of female mice between the HB and LB after allergen exposure. Mean ± SE of 10 mice/group is shown.
Th1/Th2/Th17 cytokines in lung tissue protein extract
Th1 and Th2 cytokines were measured using the MSD U-PLEX assay in lung tissue protein extract. The lung tissue protein extract was analyzedto detect the following Th1 cytokines: TNF-α, INF-γ, IL-1β, KC, Il-12p70, and IL-2 (26). Only TNF-α, INF-γ, IL-1β, and KC showed differences in either housing conditions or gender (Fig. 4). In the control group, males housed in the HB facility had higher levels of TNF-α, INF-γ, and IL-1β compared with males housed in the LB facility (Fig. 4A–C). In both facilities, males overall have increased TNF-α, IL-1β, and KC (Fig. 4A, 4C, 4D). However, after exposure to HDME, Th1 cytokines were comparable across groups (Fig. 4E–H). The lung tissue protein extract was analyzed to detect the following Th2 cytokines IL-4, IL-10, IL-13, and IL-5 (26). Only IL-4, IL-10, and IL-13 cytokines showed an effect on housing conditions or gender. In the control groups, IL-4 and IL-13 showed no differences between housing conditions and gender (Fig. 4I, 4K). However, IL-10 was increased in females housed in the HB compared with females in the standard facility (Fig. 4J). HDME-exposed female mice, in both facilities, increased in IL-4 (Fig. 4L). Although IL-10 was not significantly different (Fig. 4M) across groups, LB facility males showed an increase in IL-13 (Fig. 4N). These data indicated that housing and gender affect Th1 and Th2 cytokine profiles in lung tissue for allergen-naive and HDME-exposed mice. Levels of IL-17 were detected in lung tissue protein extracts in both genders and housing. Saline-exposed males housed in the HB displayed higher levels of IL-17 compared with males in the LB facility (Fig. 4O), but there were no differences in HDME-exposed mice (Fig. 4P).
FIGURE 4. Th1, Th2, and IL-17 levels in lung tissue.
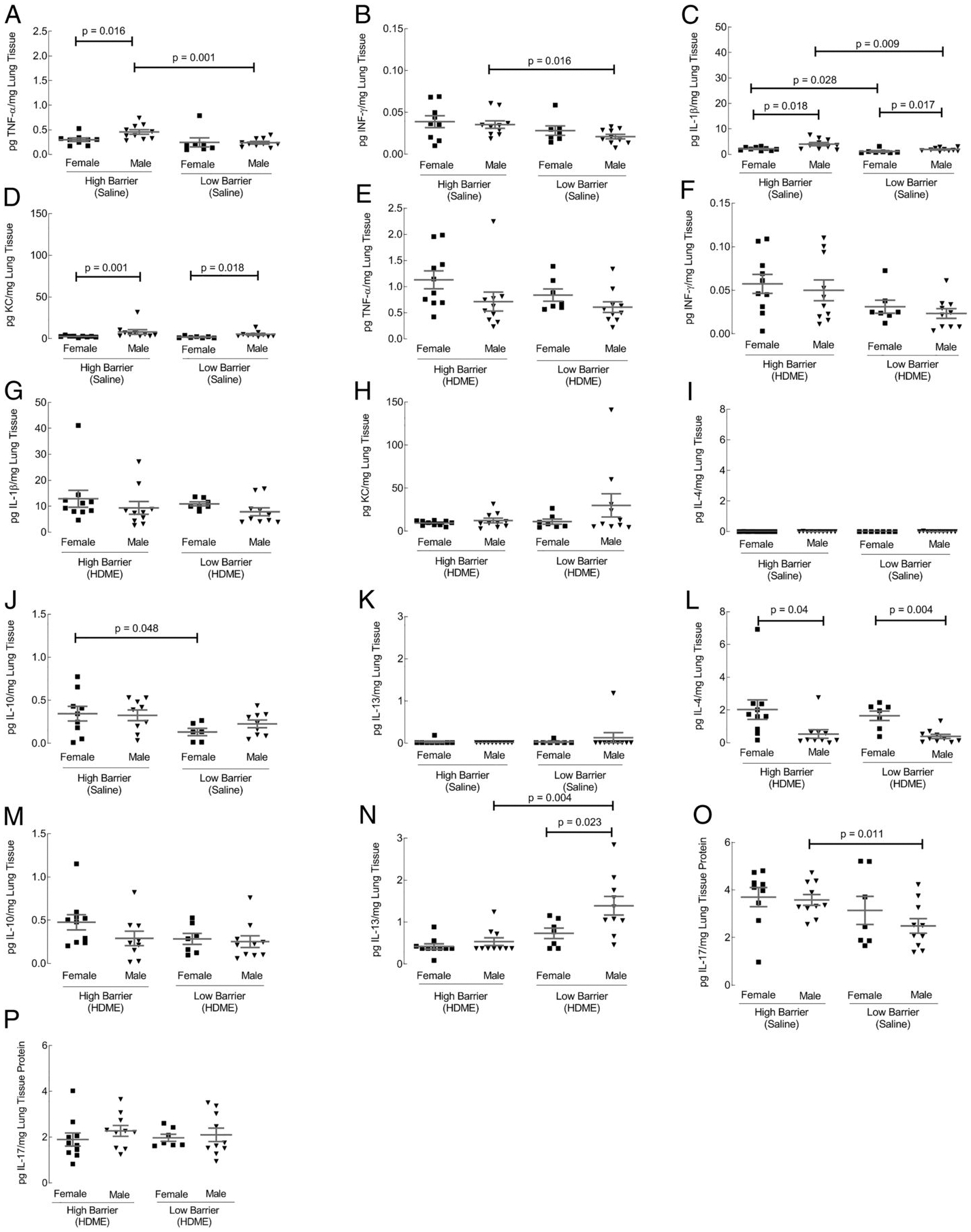
Protein was extracted from lung tissue, and cytokines were quantified using an MSD U-PLEX Platform. Using the 10-spot MSD U-PLEX Platform, the protein was analyzed for Th1 cytokines (IFN-γ, IL-1β, IL-2, KC, IL-12p70, and TNF-α), Th2 cytokines (IL-4, IL-5, IL-10, and IL-13), and IL-17. From the Th1 cytokines, only IFN-γ, IL-1β, KC, and TNF-α were significantly different in control groups (A–D) but similar after HDME exposure (E–H). Among Th2 cytokines, IL-4, IL-10, and IL-13 cytokines were affected by housing or gender (I–N). IL-17 levels are shown in (O and P). Each dot represents data from one mouse. Ten mice in each group were used.
Th1/Th2 cytokines in BAL
Th1 and Th2 cytokines were also quantified in the BAL. Only TNF-α and KC cytokines samples showed differences in housing or gender (Fig. 5). Saline-exposed mice showed higher TNF-α and KC in males housed in the LB facility (Fig. 5A, 5B). HDME-exposed females in both facilities showed an increase in TNF-α compared with males (Fig. 5C). However, females housed in the HB exhibited the highest TNF-α concentration (Fig. 5C). KC was not significantly different after HDME exposure (Fig. 5D). Among the Th2 cytokines, housing or gender effects were only observed for IL-4, IL-13, and IL-5 cytokines. In the control groups there were no differences in the Th2 cytokines, expect for a higher IL-5 among females in the LB facility compared with males (Fig. 5E–G). After HDME exposure, females showed an increase in IL-4 compared with males in both facilities (Fig. 5H). Males housed in the LB facility had significantly higher IL-13 compared with males in the HB (Fig. 5I). Within the HB facility, female mice showed elevated levels of IL-5 compared with males (Fig. 5J). IL-17 in the BAL was not analyzed because previous work showed no significant levels of IL-17 in BAL (27). Overall, the data showed both Th1 and Th2 cytokine profiles in the BAL were affected by both gender and housing.
FIGURE 5. Th1 and Th2 cytokines in BAL and MSD U-PLEX Platform BAL Th1.
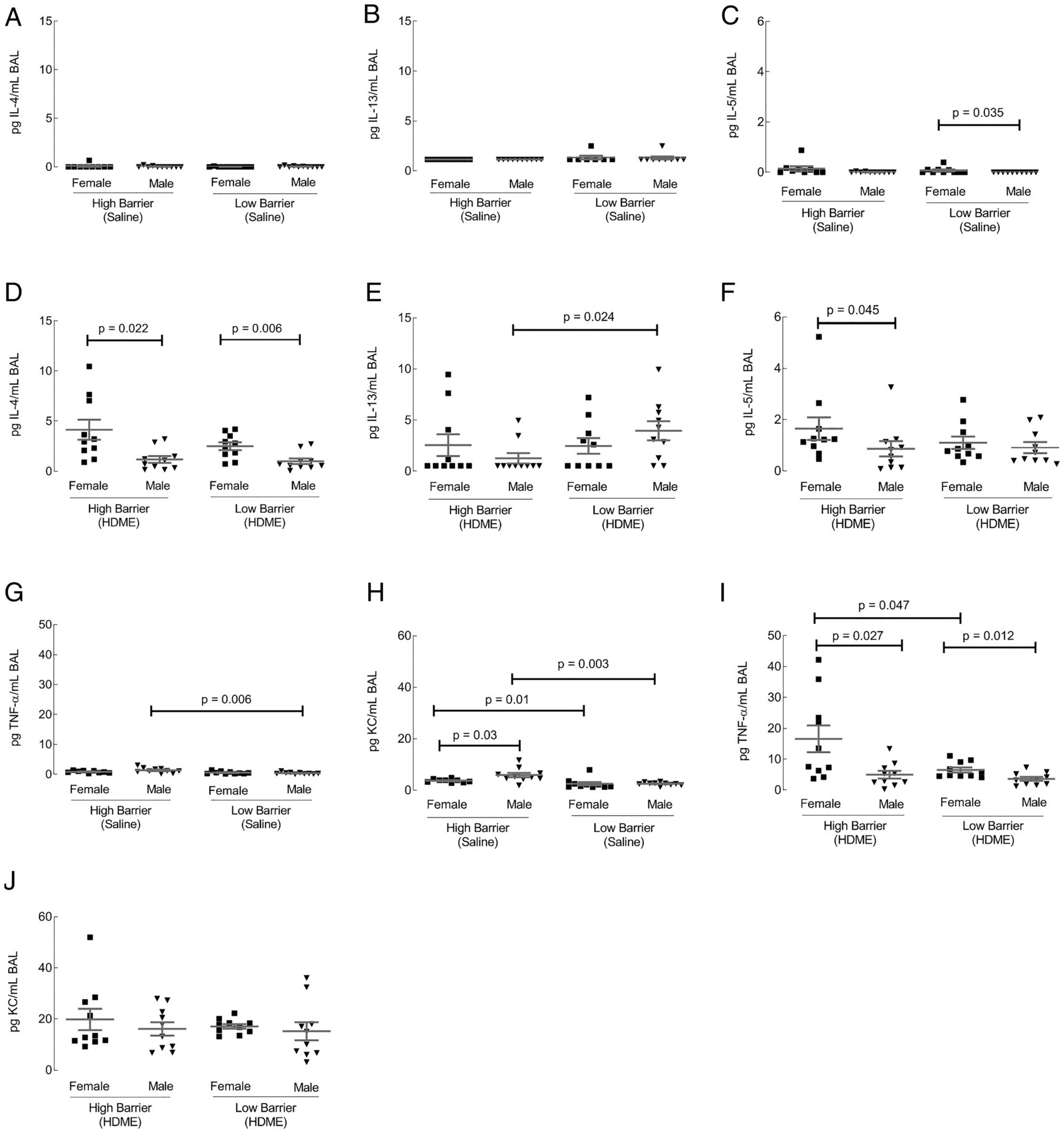
Using the 10-spot MSD U-PLEX Platform, the BAL was analyzed for Th1 and Th2 as described for the lung tissue. Th1 cytokines TNF-α and KC were significantly altered by housing or gender. (A–D) In the Th2 chemokine group, IL-4, IL-13, and IL-5 were affected (E–J). Each dot represents data from one mouse. Ten mice in each group were used.
IgE levels in plasma
At baseline, total IgE levels within each facility were higher in female mice compared with male mice (Fig. 6A). HB facility females had more elevated IgE compared with LB facility females (Fig. 6A). After allergen exposure, IgE levels increased and remained higher in female mice than male mice in both facilities (Fig. 6B). The findings showed that females have higher IgE levels than males at baseline and after HDME exposure.
FIGURE 6. Effect of housing and gender on plasma IgE levels.
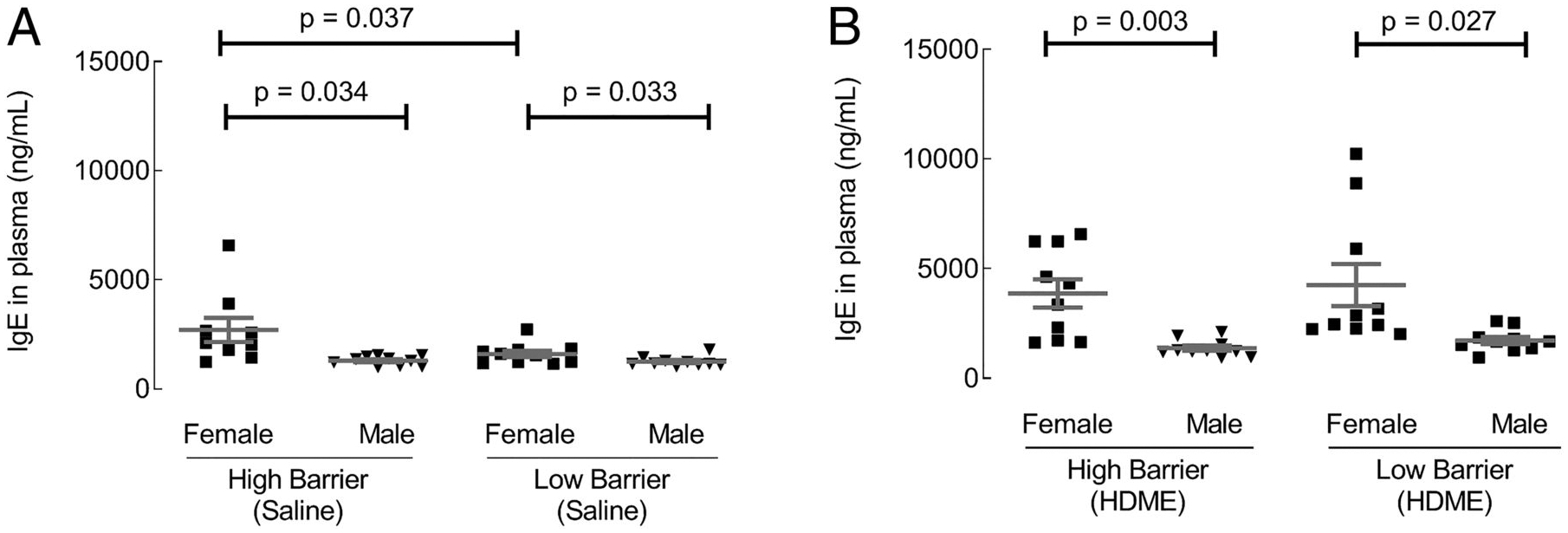
Total IgE levels in plasma were quantified by ELISA. (A and B) IgE levels in saline and HDM exposed groups, respectively. Each dot represents data from one mouse. Ten mice in each group were used.
Effect of barrier housing on lung microbiome
Because environmental microbiome is associated with asthma development (28–30), we analyzed the barrier, gender, and allergen exposure effects on the microbiome in the lung tissue. Overall, the lung microbiome was altered across gender, housing barrier, and allergen-exposed groups (Fig. 7A). Compared with LB housing, both male and female mice in the HB facility had reduced amounts of specific bacteria, as shown in Fig. 7B, 7C. However, after allergen exposure, the housing barrier effects were dramatically reduced (Fig. 7D). Gender differences were observed in allergen-naive mice in both barrier facilities (Fig. 8A, 8B) but were reduced in the HB housing. In the latter facility, allergen-exposed female mice had higher proportions of lung germs than males (Fig. 8C, 8D). Thus, HB housing was associated with reducing lung microbiome diversity (Fig. 9).
FIGURE 7. Microbial abundance and diversity patterns across LB and HB facilities.
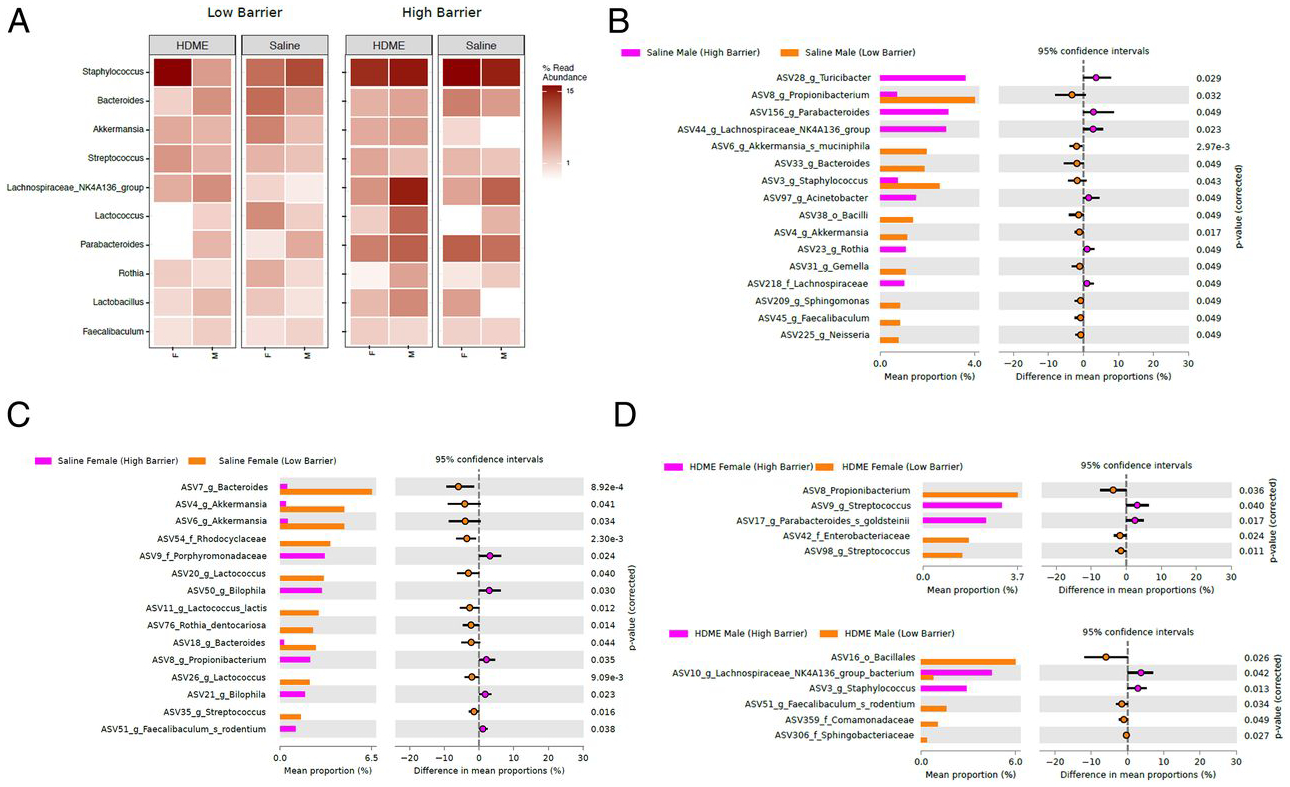
(A) Total microbial diversity patterns in saline and HDME groups sampled across LB and HB facilities. White’s nonparametric t test analysis (p value correction for false discovery rate using Benjamini/Hochberg) identified differentially abundant taxa (ASVs) between (B) males from saline group samples from HB facility versus males from saline group samples from LB facility, (C) females from saline group samples from HB facility versus females from saline group samples from LB facility, (D) females from HDME group samples from HB facility versus females from HDME group samples from LB facility, and males from HDME group samples from HB facility versus males from HDME group samples from LB facility.
FIGURE 8. Differential microbial abundance across LB and HB facility.
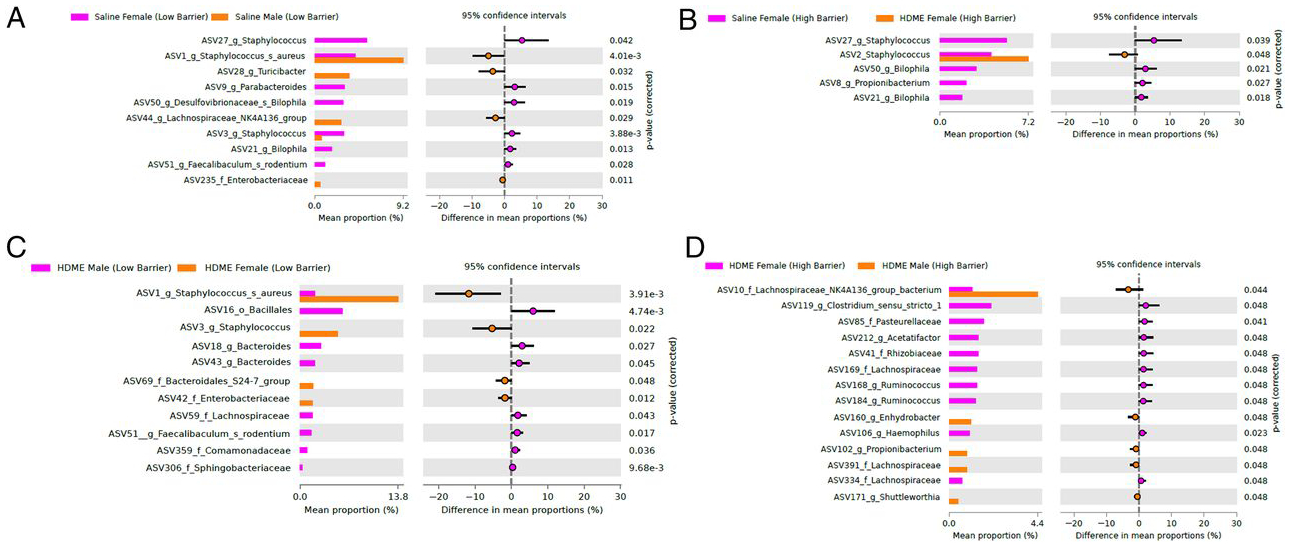
White’s nonparametric t test analysis (p value correction for false discovery rate using Benjamini/Hochberg) identified differentially abundant taxa (ASVs) between (A) females from saline group samples from LB facility versus males from saline group samples from LB facility, (B) females from saline group samples from HB facility versus females from HDME group samples from HB facility, (C) males from HDME group samples from LB facility versus females from HDME group samples from LB facility, and (D) females from HDME group samples from HB facility versus males from HDME group samples from HB facility.
FIGURE 9. Summary of housing barrier and gender effects.
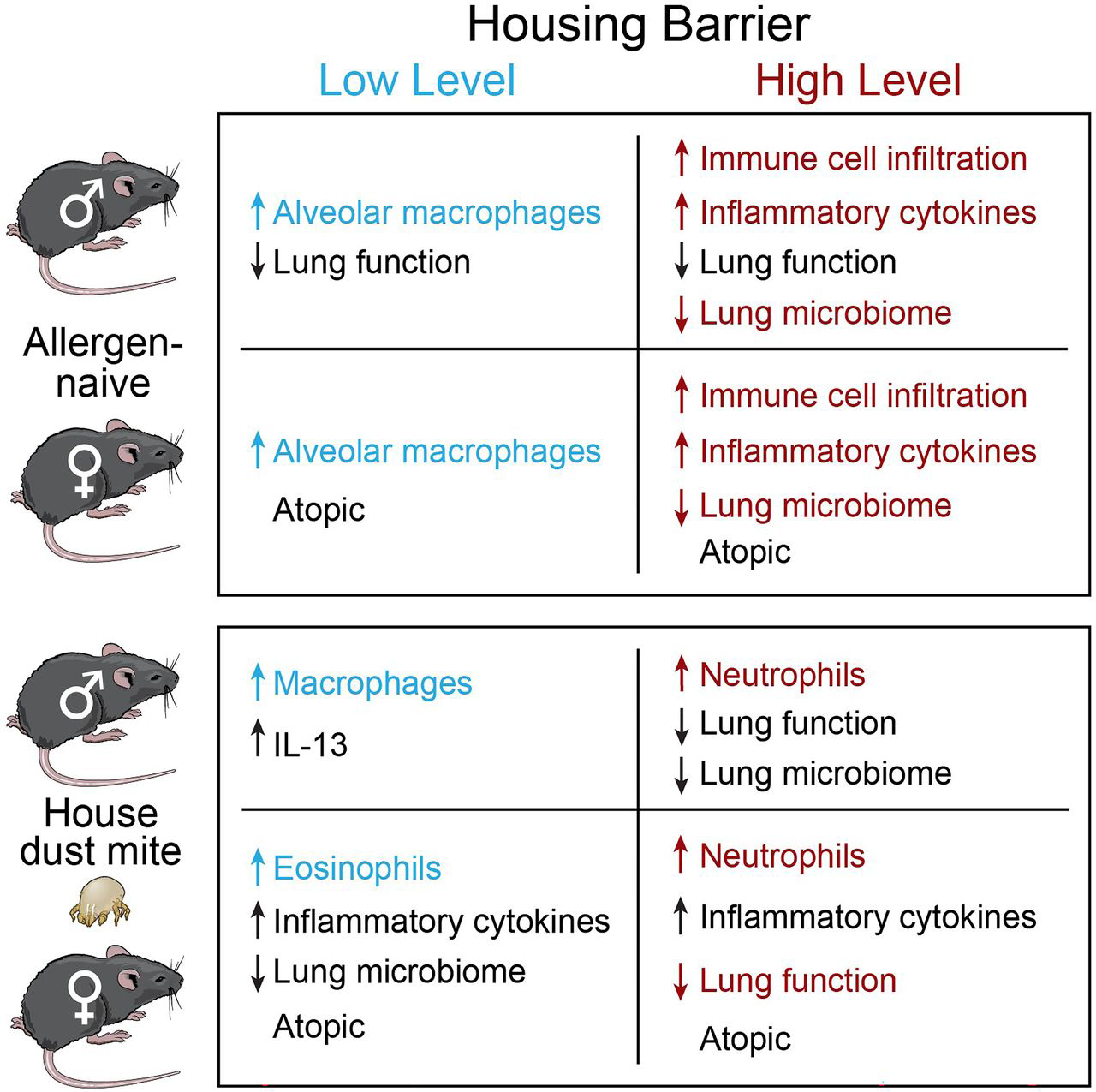
Gender effects within each barrier are in black font color, and barrier effects between each gender are in color.
DISCUSSION
In this study, we show that 1) allergen-naive animals housed in an HB facility have increased levels of inflammatory cytokines and higher infiltration of immune cells in the lung tissue compared with mice housed in an LB facility; 2) in both genders, HDME exposure in HB facility caused a more severe airway inflammation than mice housed in the LB facility, and within each housing facility, female mice developed the most severe inflammation; 3) HDME allergen exposure in the HB facility induced a predominantly neutrophilic inflammation and, in the LB facility, an eosinophilic inflammation; 4) naive male mice were predisposed to worse lung function, regardless of housing environment, and in the HB facility, lung function in male mice was the lowest in the HDM model; 5) there were gender, barrier, and HMDE allergen exposure–dependent differences in lung microbiota; and 6) the quantification of immune cells and inflammatory cytokines in BAL and pulmonary tissue, two different compartments of the lungs, provides complementary insight, and perhaps both should be performed to obtain a comprehensive overview of lung inflammation in mouse models of asthma. Thus, housing mice in more clean HB facilities aggravates basal lung immunity and causes a more severe allergic lung disease. Protective effects of the male gender on lung function are abolished in a more hygienic environment. The findings suggest that a microbial-diverse environment mitigates basal lung immunity, and male sex hormones adversely affect lung function in allergen-naive animals. Mice exposed to HDME allergen and bystander pathogens developed less severe inflammation and had better lung function. Similarly, male sex hormones attenuated the allergic airway inflammation in both housing facilities but were unable to improve lung function in the HB SPF facility (Fig. 9). This observational study contributes to the growing literature demonstrating facility-dependent impacts of environmental and husbandry factors on mouse models’ microbial-driven disease phenotypes.
Traditionally, lung function in preclinical models was measured using the forced oscillation technique (FOT), based on changes in pressure, volume, and airflow in response to oscillatory airflow waves (31, 32). However, in humans, forced expiration lung volume quantification is the standard for assessing lung function. The newer flexiVent system, flexiVent FX, which was used in our study, allows simultaneous FOT and forced expiration parameters in murine models (31, 32). This new application uses NPFE to provide spirometric-like measures in rodents. FEV0.1 is one such measurement characterizing functional obstruction. Our findings show that gender and barrier housing affects FEV0.1, without apparent changes to traditional FOT-based measures.
Environmental effects and gender disparity in allergic asthma is well established. Female patients have a more severe phenotype, whereas exposure to a farm environment has a protective impact on the development of asthma in both genders. Murine models of asthma are essential tools in the investigation of mechanisms and preclinical studies. However, most allergic airway disease models use laboratory mice housed in highly controlled and hygienic environments, which structurally and functionally provide a barrier between the mice and a predetermined list of specific pathogens and infectious agents that are excluded from the facility. Structural features of barrier facilities often include filtered ambient air, filtered air workstations, and autoclaved caging. Husbandry practices include the provision of sterilized food and water and the use of clean gloves when handling the mice. The combination of individual mouse facilities’ structural and functional features varies widely from low- to high-level barriers, depending on the desired SPF status. Mice housed in HB facilities are exposed to less pathogenic and opportunistic organisms and typically have less complex microbiota than mice housed in LB facilities.
Standard contemporary practices in mouse housing facilities that standardize light intensity and duration cycle, water and food treatment, cage sanitation methods, and ambient temperature and humidity minimize variability in rodent research outcomes. Similarly, environmental factors and husbandry practices in many mouse housing facilities are designed to limit mice exposure to certain specified pathogenic and opportunistic infectious organisms to safeguard the health and avoid phenotypic deviation due to differences in microbial exposure. However, this raises the question of whether laboratory mice housed in very hygienic facilities are relevant models for asthma patients who inhabit pathogen-rich environments. The effect of facility barrier level on the genesis of allergic airway disease in murine models was not studied before this work. One general study showed that the mouse immune system changes early with exposure to different pathogens (3). Cohousing wild pet store mice with laboratory mice strengthened the laboratory mice’s immune system, bringing it closer to more human-like (3). Our findings showed that mice housed in a very clean facility were predisposed to neutrophilic asthma associated with an increase in TNF-α, INF-γ, IL-1β, and KC cytokines in the lungs. HB SPF-housed mice likely developed this more severe asthma because of an overactive immune system in the absence of opportunistic pathogens. Animals housed in the LB facility were predisposed to eosinophilic asthma associated with an increase in alveolar macrophages and IL-4, IL-10, IL-13, and IL-5 cytokines. Several reports have shown that highly exposed individuals to environmental farm microbial conditions are 25% less likely to develop asthma (28, 33, 34). The lack of bystander microbial/pathogen exposure is possibly why mice housed in the SPF facility developed more severe inflammation.
Gender disparity in asthma patients has been well documented. Throughout childhood, males tend to be more susceptible to wheezing, AHR, and increased eosinophils (4–9). However, after puberty, females are more likely to develop asthma and more severe symptoms (4–9). Many studies have shown a link between increases in estrogen levels and the development of a more severe phenotype. Estrogen enhances IL-4–induced M2 macrophage gene expression and caused an increase in airway inflammation (35–40), whereas testosterone downregulated allergic airway inflammation (11–13, 41, 42). Other studies showed that gonadectomized male mice, which lack testosterone, had increased eosinophils and lymphocytes (42–44).
In the OVA model, female mice had a higher risk of allergic airway inflammation than male mice. This was driven by a predominately Th2 cytokine, IL-4, and IL-5, response in females (12, 35). Female mice also had higher infiltration of inflammatory cells and higher IgE levels (12, 13, 41). Similarly, our study showed that HDM-induced allergic airway inflammation increased the secretion of IL-4 and IL-5 cytokines, infiltration of eosinophils and lymphocytes, and elevated IgE levels in female compared with male mice. These parameters lead to female mice developing worse airway inflammation compared with male mice.
Several studies have shown that IL-17 contributes to the development of airway inflammation and AHR in wild-type mice (45–48). Reported IL-17 levels were elevated in homogenized lung tissue; however, the values were not corrected for total protein in these studies (45–48). We reported previously that IL-17 was present in saline and HDME wild-type groups, and the levels were elevated in Arg2-deficient mice (27). In the current study with wild-type mice, IL-17 was detected in lung tissue homogenates and not affected by housing or gender.
Housing barrier–related lung microbiome diversity in our study confirms findings in several reports documenting an association between microbial exposure and asthma severity (28–30). Patients with less diverse bacteria have neutrophilic asthma, whereas a rich microbial diversity was associated with eosinophilic airway inflammation (49–51). Our observation of predominantly neutrophilic asthma in the HB versus eosinophilic inflammation in the LB is also in line with these reports. Allergen exposure increased gender lung microbiome differences in HB housing, suggesting that female sex hormones may promote specific, potentially pathogenic bacteria under hygienic conditions. These findings add to the growing body of evidence that lung microbiota may play a critical role in the education, regulation, and activation of lung immune cells, thus predisposing disease severity (52, 53).
Together, these data underscore the impact of a housing facility and gender effects in allergic airway disease models. Future studies in asthma mouse models should consider the impact of barrier housing environments on severity and gender bias in airway disease. Every mouse housing facility has its unique combination of factors that account for a high degree of interfacility variability in phenotypes. Much of mouse models’ phenotypic variability may be driven by differences in microbes that arise when environmental conditions vary. Careful documentation of variability between facilities is essential. More study is needed to determine how variability between facilities lead to phenotypic differences and how these differences can be minimized through standardization of barrier facility conditions. The diversity of phenotypes may also provide a valuable opportunity for looking into the specific microbial exposures responsible for different phenotypes. Consideration must be given to the questionable value of ultraclean mice housed in very hygienic HB facilities as models of human disease. Maintaining diversity and complexity of the microbial exposures of mouse asthma models and using both genders in studies will likely increase mouse models’ relevance, as suggested previously (54). Also, further studies on sex hormone–microbial interactions in asthma would be advantageous. By understanding the housing variables affecting outcomes in mouse asthma models, we will improve reproducibility and promote the translation of research findings into clinical applications.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Lerner Research Institute Imaging Core for assistance with histology and immunohistochemistry and the Flow Cytometry Core for assistance with immunophenotyping of lung samples. We also thank Jeff Hammel for service with statistical analyses.
This work was supported by National Institutes of Health Grants HL103453, HL081064, HL60917, and HL109250 and the Alfred Lerner Memorial Chair in Innovative Biomedical Research at the Cleveland Clinic.
Abbreviations used in this article:
- AHR
airway hyperresponsiveness
- ASV
amplicon sequence variant
- BAL
bronchoalveolar lavage
- FEV0.1
forced expiratory volume at 0.1 s
- FOT
forced oscillation technique
- HB
high barrier
- HDM
house dust mite
- HDME
HDM extract
- LB
low barrier
- MSD
Meso Scale Discovery
- NPFE
negative pressure–driven forced expiration
- SPF
specific pathogen–free
Footnotes
The online version of this article contains supplemental material.
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, and Vignola AM. 2000. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med 161: 1720–1745. [DOI] [PubMed] [Google Scholar]
- 2.Davies DE, Wicks J, Powell RM, Puddicombe SM, and Holgate ST. 2003. Airway remodeling in asthma: new insights. J. Allergy Clin. Immunol 111: 215–225, quiz 226. [DOI] [PubMed] [Google Scholar]
- 3.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma DS 2007. Gender differences in asthma development and progression. Gend. Med 4 Suppl. B: S133–S146. [DOI] [PubMed] [Google Scholar]
- 5.Schaubel D, Johansen H, Dutta M, Desmeules M, Becker A, and Mao Y. 1996. Neonatal characteristics as risk factors for preschool asthma. J. Asthma 33: 255–264. [DOI] [PubMed] [Google Scholar]
- 6.Larsson L 1995. Incidence of asthma in Swedish teenagers: relation to sex and smoking habits. Thorax 50: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunyer J, Antó JM, Kogevinas M, Barceló MA, Soriano JB, Tobías A, Muniozguren N, Martínez-Moratalla J, Payo F, and Maldonado JA. 1997. Risk factors for asthma in young adults. Spanish Group of the European Community Respiratory Health Survey. Eur. Respir. J 10: 2490–2494. [DOI] [PubMed] [Google Scholar]
- 8.1997. Asthma and respiratory symptoms in 6–7 yr old Italian children: gender, latitude, urbanization and socioeconomic factors. SIDRIA (Italian Studies on Respiratory Disorders in Childhood and the Environment). Eur. Respir. J 10: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 9.Venn A, Lewis S, Cooper M, Hill J, and Britton J. 1998. Questionnaire study of effect of sex and age on the prevalence of wheeze and asthma in adolescence. BMJ 316: 1945–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuseini H, and Newcomb DC. 2017. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep 17: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T, Adachi Y, Hasegawa K, and Morimoto M. 2003. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand. J. Immunol 57: 562–567. [DOI] [PubMed] [Google Scholar]
- 12.Takeda M, Tanabe M, Ito W, Ueki S, Konnno Y, Chihara M, Itoga M, Kobayashi Y, Moritoki Y, Kayaba H, and Chihara J. 2013. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology 18: 797–806. [DOI] [PubMed] [Google Scholar]
- 13.Blacquière MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, and Melgert BN. 2010. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int. Arch. Allergy Immunol 153: 173–181. [DOI] [PubMed] [Google Scholar]
- 14.Seymour BW, Friebertshauser KE, Peake JL, Pinkerton KE, Coffman RL, and Gershwin LJ. 2002. Gender differences in the allergic response of mice neonatally exposed to environmental tobacco smoke. Dev. Immunol 9: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corteling R, and Trifilieff A. 2004. Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC Pharmacol 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichard A, Wanner N, Stuehr E, Alemagno M, Weiss K, Queisser K, Erzurum S, and Asosingh K. 2018. Quantification of airway fibrosis in asthma by flow cytometry. Cytometry A 93: 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutzik PO, Clutter MR, Trejo A, and Nolan GP. 2011. Fluorescent cell barcoding for multiplex flow cytometry. Curr. Protoc. Cytom Chapter 6: Unit 6.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, and Perlman H. 2013. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol 49: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, and Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurdie PJ, and Holmes S. 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8: e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurdie PJ, and Holmes S. 2014. Waste not, want not: why rarefying microbiome data is inadmissible. PLOS Comput. Biol 10: e1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickham H 2009. ggplot2: Elegant Graphics for Data Analysis Springer Publishing Company, Incorporated, New York. [Google Scholar]
- 23.Benjamini Y 2010. Discovering the false discovery rate. J. R. Stat. Soc. Series B Stat. Methodol 72: 405–416. [Google Scholar]
- 24.White JR, Nagarajan N, and Pop M. 2009. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLOS Comput. Biol 5: e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asosingh K, Weiss K, Queisser K, Wanner N, Yin M, Aronica M, and Erzurum S. 2018. Endothelial cells in the innate response to allergens and initiation of atopic asthma. J. Clin. Invest 128: 3116–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raphael I, Nalawade S, Eagar TN, and Forsthuber TG. 2015. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asosingh K, Lauruschkat CD, Alemagno M, Frimel M, Wanner N, Weiss K, Kessler S, Meyers DA, Bennett C, Xu W, and Erzurum S. 2020. Arginine metabolic control of airway inflammation. JCI Insight 5: e127801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Mutius E 2016. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol 137: 680–689. [DOI] [PubMed] [Google Scholar]
- 29.Hufnagl K, Pali-Schöll I, Roth-Walter F, and Jensen-Jarolim E. 2020. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol 42: 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barcik W, Boutin RCT, Sokolowska M, and Finlay BB. 2020. The role of lung and gut microbiota in the pathology of asthma. Immunity 52: 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devos FC, Maaske A, Robichaud A, Pollaris L, Seys S, Lopez CA, Verbeken E, Tenbusch M, Lories R, Nemery B, et al. 2017. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir. Res 18: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalaby KH, Gold LG, Schuessler TF, Martin JG, and Robichaud A. 2010. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir. Res 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genuneit J, Strachan DP, Büchele G, Weber J, Loss G, Sozanska B, Boznanski A, Horak E, Heederik D, BraunFahrländer C, and von, Mutius E; GABRIELA study group. 2013. The combined effects of family size and farm exposure on childhood hay fever and atopy. Pediatr. Allergy Immunol 24: 293–298. [DOI] [PubMed] [Google Scholar]
- 34.Radon K, Windstetter D, Eckart J, Dressel H, Leitritz L, Reichert J, Schmid M, Praml G, Schosser M, von Mutius E, and Nowak D. 2004. Farming exposure in childhood, exposure to markers of infections and the development of atopy in rural subjects. Clin. Exp. Allergy 34: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 35.Keselman A, Fang X, White PB, and Heller NM. 2017. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J. Immunol 199: 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteis M, Polverino F, Spaziano G, Roviezzo F, Santoriello C, Sullo N, Bucci MR, Rossi F, Polverino M, Owen CA, and D’Agostino B. 2014. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm. Med 14: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macsali F, Svanes C, Sothern RB, Benediktsdottir B, Bjørge L, Dratva J, Franklin KA, Holm M, Janson C, Johannessen A, et al. 2013. Menstrual cycle and respiratory symptoms in a general Nordic-Baltic population. Am. J. Respir. Crit. Care Med 187: 366–373. [DOI] [PubMed] [Google Scholar]
- 38.Tam A, Wadsworth S, Dorscheid D, Man SF, and Sin DD. 2014. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One 9: e100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly EA, et al. 2015. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol 52: 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vink NM, Postma DS, Schouten JP, Rosmalen JGM, and Boezen HM. 2010. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J. Allergy Clin. Immunol 126: 498–504.e1–e6. [DOI] [PubMed] [Google Scholar]
- 41.Yu CK, Liu YH, and Chen CL. 2002. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinaesensitized mice. J. Microbiol. Immunol. Infect 35: 199–202. [PubMed] [Google Scholar]
- 42.Warren KJ, Sweeter JM, Pavlik JA, Nelson AJ, Devasure JM, Dickinson JD, Sisson JH, Wyatt TA, and Poole JA. 2017. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann. Allergy Asthma Immunol 118: 233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laffont S, Blanquart E, Savignac M, Cénac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, and Guéry JC. 2017. Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med 214: 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, et al. 2017. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep 21: 2487–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vroman H, Das T, Bergen IM, van Hulst JAC, Ahmadi F, van Loo G, Lubberts E, Hendriks RW, and Kool M. 2018. House dust mite-driven neutrophilic airway inflammation in mice with TNFAIP3-deficient myeloid cells is IL-17-independent. Clin. Exp. Allergy 48: 1705–1714. [DOI] [PubMed] [Google Scholar]
- 46.Wonnenberg B, Jungnickel C, Honecker A, Wolf L, Voss M, Bischoff M, Tschernig T, Herr C, Bals R, and Beisswenger C. 2016. IL-17A attracts inflammatory cells in murine lung infection with P. aeruginosa. Innate Immun 22: 620–625. [DOI] [PubMed] [Google Scholar]
- 47.Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, Peebles RS Jr., and Newcomb DC. 2018. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J. Immunol 201: 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chenuet P, Fauconnier L, Madouri F, Marchiol T, Rouxel N, Ledru A, Mauny P, Lory R, Uttenhove C, van Snick J, et al. 2017. Neutralization of either IL-17A or IL-17F is sufficient to inhibit house dust mite induced allergic asthma in mice. Clin. Sci. (Lond.) 131: 2533–2548. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SL, Leong LEX, Mobegi FM, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. 2019. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am. J. Respir. Crit. Care Med 200: 309–317. [DOI] [PubMed] [Google Scholar]
- 50.Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Hugenholtz P, Willner D, and Gibson PG. 2016. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur. Respir. J 47: 792–800. [DOI] [PubMed] [Google Scholar]
- 51.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, and Howarth PH. 2014. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 9: e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickson RP 2018. The lung microbiome and ARDS. It is time to broaden the model. Am. J. Respir. Crit. Care Med 197: 549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, and Marsland BJ. 2014. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med 20: 642–647. [DOI] [PubMed] [Google Scholar]
- 54.Tao L, and Reese TA. 2017. Making mouse models that reflect human immune responses. Trends Immunol 38: 181–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


