Abstract
Solid-supported catalysts play efficient and crucial roles in organic synthesis. A solid-supported palladium(II) complex based on chitosan was synthesized and fully characterized using all possible tools (Fourier transform infrared spectroscopy, thermogravimetry analysis, differential scanning calorimetry, X-ray photoelectron spectroscopy, energy-dispersive X-ray spectroscopy, inductively coupled plasma atomic emission spectrometry, scanning electron microscopy, transmission electron microscopy, and Brunauer–Emmett–Teller analysis). The catalytic activity of the solid-phase catalyst in Suzuki cross-coupling reactions was evaluated in aqueous solvents under both conventional heating and microwave irradiation conditions. The recyclability and thermal stability of the prepared catalyst were also examined, and the catalyst was found to be active till five consecutive runs without a notable loss of activity under the microwave condition, with the turnover number and turnover frequency values reaching 19,019 and 114,114 h–1, respectively.
1. Introduction
Chitosan is a non-toxic, biodegradable, and eco-friendly biopolymer and is commonly obtained from the deacetylation reaction of chitin (a long-chain polymer of N-acetylglucosamine).1−4 Heterogeneous palladium catalysts have been characterized by their elevated catalytic sites, high selectivity, possibility to regulate the catalyst chemo-, regio-, and enantio-selectivities, ease of optimization of the catalytic systems, and better yields and are extensively used in Suzuki coupling reactions.5−8 In addition, heterogeneous catalysts are superior over homogeneous ones, which suffer from major problems such as difficult separation and recovery and sometimes result in non-acceptable contaminations in the reaction products.9−12 The expensive ligands employed in Suzuki coupling reactions were also problematic; therefore, numerous cheap materials, for example, silica, carbon, zeolite, cellulose, and chitosan had been involved as alternative support materials.13−15 Among these, chitosan was preferred due to its unique properties such as (1) low cost, (2) abundance, (3) environmental friendliness, (4) renewability, and (5) the presence of the reactive −NH2 and −OH functions capable of further modifications of chitosan.16,17 Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions were widely incorporated in organic chemistry.18−21 The use of a microwave (MW) irradiation platform had high impacts in the academia and industry.22−24 Furthermore, conducting organic reactions in aqueous solvents was advantageous compared to that in organic solvents.25,26 As part of our interest on the applications of various palladium(II) complexes in C–C cross-coupling reactions,27−38 the present work aimed at the facile synthesis of a chitosan-based palladium(II) biopolymer complex (denoted hereafter as ChsB-Pd 3) as a heterogeneous solid-nanosized catalyst and to study its catalytic activity in Suzuki cross-coupling reactions under the traditional heating mode as well as using the MW irradiation tool. In the presence of this heterogeneous catalyst, high conversion of different biaryls was achieved. The ChsB-Pd 3 solid-state catalyst is characterized by different techniques such as Fourier transform infrared (FTIR) spectroscopy, thermogravimetry analysis (TGA), energy-dispersive X-ray spectroscopy (EDX), X-ray photoelectron spectroscopy (XPS), and inductively coupled plasma atomic emission spectrometry (ICP-AES). In addition, scanning electron microscopy (SEM), the Brunauer–Emmett–Teller (BET) method, and transmission electron microscopy (TEM) imaging are also carried out to disclose the morphology, pores, and size of the prepared complex. The novelty of this work is the simple route to the complex and its robust catalytic activity, high efficiency, thermal stability, and recyclability under the MW irradiation condition in a water solvent.
2. Results
2.1. Catalyst Preparation
The chitosan-based Pd(II) complex (ChsB-Pd 3) was prepared as follows: chitosan (1.0 g) (Chs 1) was treated with 2-pyridinecarboxaldehyde (0.643 g) in methanol (10 mL) under the reflux condition in the presence of acetic acid (1%) to afford the chitosan–Schiff base (ChsB 2). Treatment of ChsB 2 with sodium tetrachloropalladate led to the formation of the chitosan–Pd(II) catalyst (ChsB–Pd 3), as shown in Scheme 1.
Scheme 1. Preparation of the Chitosan–Schiff Base ChsB 2 and Its Pd Complex ChsB–Pd 3.
2.2. Characterization of the Solid Catalyst (ChsB–Pd 3)
2.2.1. Vibrational Spectroscopy
The FTIR spectra in Figure 1 indicated the differences between chitosan (Chs 1), its Schiff base (ChsB 2), and its Pd(II) complex (ChsB–Pd 3). The FTIR spectrum of chitosan (Chs 1) disclosed the following bands: 3422 cm–1 (OH stretching vibration, which overlaps with NH2 stretching bands in the same region). A band representing C–H groups appeared at 2922 cm–1 besides the peaks representing C=O stretching and N–H bending vibrations of the NHCOCH3 group at 1657 and 1563 cm–1, respectively.39,40 Comparing the FTIR spectrum of chitosan (Chs 1) (Figure 1a) with that of the chitosan Schiff base (ChsB 2) (Figure 1b), ChsB 2 exhibited stretching vibrations of the C=N bonds at 1651 cm–1 and a new band at 777 cm–1, which is characteristic for the =CH band of pyridine. After complexation with Pd2+ ions, the stretching peak of the C=N group of ChsB–Pd 3 was shifted to a lower value than that of the Schiff base (ChsB 2) and appeared at 1644 cm–1, establishing the coordination of the imine-N to the Pd2+ ions.41,42
Figure 1.
FTIR spectra: (a) Chs, (b) ChsB, and (c) ChsB–Pd catalyst.
2.2.2. Thermal Analysis
TG–differential TG (DTG) diagrams of Chs 1, ChsB 2, and ChsB–Pd 3 are given in Figure 2. It was reported that chitosan (Chs 1) had better thermal stability than other natural biopolymers because of its high degree of crystallinity values.43 Inspection of Figure 2 revealed that the maximum thermal degradation points of Chs 1 and ChsB–Pd 3 were recorded at ca. 300 and 250 °C, respectively, leading to the ultimately loss of about 75 and 60% of their original masses (as recorded at 800 °C), respectively. The higher remaining residue of ChsB–Pd 3 was assigned to the metallic Pd contents, which was lacking in Chs 1.
Figure 2.
TGDTG analyses (a) Chs 1, (b) ChsB 2, and (c) ChsB–Pd 3 catalyst.
As shown in Table 1, the TG–DTG diagram of chitosan (Chs 1) disclosed three stages of mass loss. The first stage was attributed to the loss of water molecules physically adsorbed on the surface of chitosan; the second and third stages were mostly due to the chitosan degradation.44−46 The TG–DTG diagram of the Schiff base ligand (ChsB 2) exhibited three stages of decomposition. The first stage was caused via the loss of water molecules adsorbed on the surface; the second mass loss resulted from the depletion of free units of amino-chitosan; and the third one was assigned to the decomposition of the loaded chitosan unit to the pyridine motif. The deposit obtained at 650 °C was probably due to the carbon remains. In addition, the second considerable mass loss stage of the ligand (ChsB 2) occurred at a temperature of 210–375 °C, lower than that of chitosan (Chs 1) (245–440 °C), showing that the ligand (ChsB 2) was thermally unstable compared to chitosan (Chs 1). The observed result was attributed to the low number of primary amino functions in chitosan (Chs 1) after the modification to its Schiff base form (ChsB 2).
Table 1. Thermal Data from TG–DTG Analyses of Chs 1, ChsB 2, and ChsB–Pd 3.
| substrate | stage 1/°C (wt. loss %) | stage 2/°C (wt. loss %) | stage 3/°C (wt. loss %) | residue % (at 800 °C) |
|---|---|---|---|---|
| Chs 1 | 25–126 (∼12) | 220–338 (∼42) | 339–797 (∼24) | ∼22 |
| ChsB 2 | 25–160 (∼11) | 235–335 (∼36) | 336–791 (∼20) | ∼33 |
| ChsB–Pd 3 | 25–128 (∼12) | 180–276 (∼25) | 277–783 (∼29) | ∼34 |
Complex ChsB–Pd 3 also displayed three stages of mass loss. The first and second degradation steps originated from the evaporation of water molecules and loss of free amino-chitosan residues, respectively. The third stage of mass loss was mostly assigned to the distortion of the coordination junction between the imine-N and the metal ion.
2.2.3. Differential Scanning Calorimetry Analysis
The differential scanning calorimetry (DSC) thermograms of compounds Chs 1, ChsB 2, and ChsB–Pd 3 are shown in Figure 3. The endothermic signals at 145 °C for Chs 1, 122 °C for ChsB 2, and 153 °C for ChsB–Pd 3 are caused by the presence of water molecules in the samples, and the sharp and strong exothermic signals at 309.9 and 302.1 °C originate from the decomposition of the main chain of Chs 1 and ChsB 2, respectively. The DSC curves also showed that the evaporation temperatures of bounded water in Chs 1 were higher than that in ChsB 2, meaning that water molecules are more tightly connected to Chs 1 than to ChsB 2, where the insertion of an imine moiety into the polysaccharide structures disrupted the crystalline structure of Chs 1 via loss of hydrogen bonding.47 The endothermic peak temperature of ChsB–Pd 3 was sharp and greater than that of ChsB 2. As shown in Figure 3c, ChsB–Pd 3 exhibited higher water holding capacity than ChsB 2, which required high temperature and energy for complete evaporation of water.
Figure 3.
DSC thermograms of: (a) Chs 1, (b) ChsB 2, and (c) ChsB–Pd 3 catalyst.
2.2.4. Powder X-ray Diffraction Spectroscopy
The X-ray diffraction (XRD) spectra of Chs 1, ChsB 2, and ChsB–Pd 3 are provided in Figure 4. Chitosan (Chs 1) had a characteristic peak at 2θ = 20° (Figure 4a) due to inter-molecular hydrogen bonding, leading to the high crystallinity character of Chs 1.48,49 The diffraction pattern of (ChsB 2)44 (Figure 4b) showed a characteristic peak at 2θ = 20.60°. The XRD pattern of (ChsB–Pd 3) showed new peaks at 2θ = 40.13, 46.24, and 68.27° corresponding to lattice plane (111), (200), and (220) reflections from fcc palladium (JCPD #00-005-0681), which are attributed to the Pd species (Figure 4c).50 These new peaks proved the coordination of the Pd atoms to the chitosan Schiff base (ChsB 2),51 and decreases in the intensity of the peak at 2θ = 20° were observed. The lower crystallinity indexes of chitosans were accompanied with the evolution of other Pd-based crystallites. In addition, this analysis established the formation of PdO as final decomposition product. The value (%) of the residue was 34.0% for the palladium compound Pd–Chs.52 The crystallinity index of chitosans was determined from the following equation53
where I110 refers to the maximum intensity at 2θ ∼20° and Iam is the intensity of the amorphous diffraction at 2θ ∼ 16°. The values of the crystallinity index were observed at 54.31, 62.18, and 30.51 for Chs 1, ChsB 2, and ChsB–Pd 3, respectively, and this lowering in the index of crystallinity disclosed the sensitivity of the Chs 1 crystal structure to modification.
Figure 4.
XRD spectra of (a) Chs 1 (b) ChsB 2, and (c) ChsB–Pd 3 catalyst.
2.2.5. Transmission Electron Microscopy
The morphologies of Chs 1, ChsB 2, and ChsB–Pd 3 were studied by TEM at two different magnifications. As shown in Figure 5, an obvious alteration in the morphology was noticed after loading palladium(II) on chitosan, as shown in Figure 5e, where aggregates in the nanoparticle scale were observable for the palladium(II) chitosan complex. The nanoparticle size was highly desirable from the catalysis point of view as it translated into a high surface-to-volume ratio and a greater number of accessible Pd sites, which was directly reflected on the catalyst utilization. Inspection of Figure 5f revealed that the average particle size of the ChsB–Pd 3 catalyst was about 36 nm, as estimated from the TEM images using ImageJ software.
Figure 5.
TEM pictures of (a,b) Chs 1 (c,d) ChsB 2, and (e,f) ChsB–Pd 3 at two different magnifications.
2.2.6. Scanning Electron Microscopy with Energy-Dispersive X-ray Analysis
The SEM imaging analyses of Chs 1, ChsB 2, and ChsB–Pd 3 were carried out at two different magnifications, as depicted in Figure 6. Chs 1 had a smooth surface morphology44 with no pores or semi-pores on the surface as it had a strong inter- and intra-hydrogen bonding types (Figure 6a,b), but ChsB 2 did not have a smooth surface and displayed a fibrous and a porous surface (Figure 6c,d), which confirmed that the desired modification was achieved.53
Figure 6.
SEM images: (a,b) Chs 1; (c,d) ChsB 2; and (e,f) ChsB–Pd 3 at two magnifications.
Following the metal ion coordination in ChsB–Pd 3, the catalyst surface (size and shape) (Figure 6e,f) did not exhibit the same structure as that of ChsB 2, and the fibrous structure disappeared. This difference might be assigned to the metal ions sites that coordinated to ChsB–Pd 3. Figure 7 displays the EDX spectrum of ChsB-Pd 3, indicating the presence of carbon, nitrogen, and traces of Pd, confirming the successful attachment of Pd to chitosan.
Figure 7.
EDX spectrum of ChsB–Pd 3.
2.2.7. UV–Vis Spectroscopy
In the UV–vis spectrum of ChsB 2, carried out in the chloroform solvent, the transitions below 300 nm were assigned to the pyridine ring (C=C) π–π* and imine (C=N) n−π* transitions. These transitions shifted to higher wavelength values for ChsB–Pd 3 (as shown in Figure 8), indicating the coordination of Pd(II) ions with nitrogen atoms of Chs in the complex ChsB–Pd 3.54
Figure 8.
UV–vis spectra of Chs 1, ChsB 2, and ChsB-Pd 3.
2.2.8. X-ray Photoelectron Spectroscopy Study
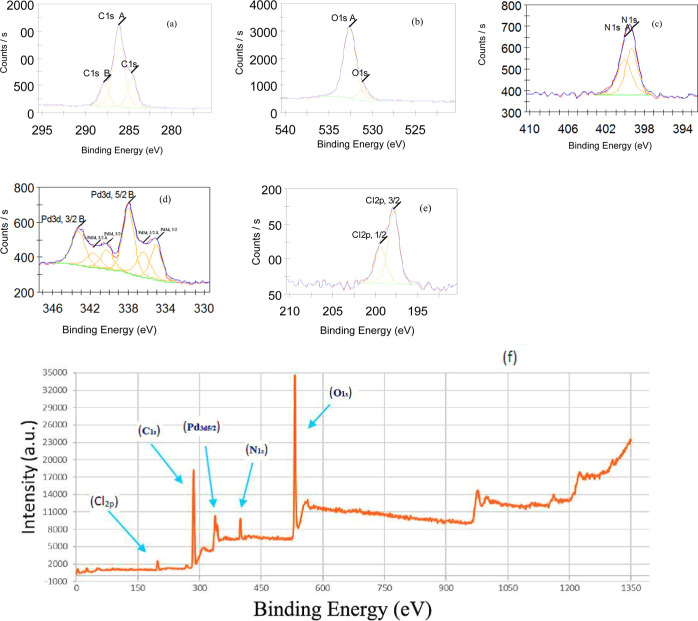
Chs 1, ChsB 2, and ChsB–Pd 3 were also characterized by XPS analysis. Figure 9 displays the XPS spectra of ChsB–Pd 3, where C 1s, O 1s, N 1s, Pd 3d, and Cl 2p spectra are all shown together with the wide scan spectrum. The obtained results for the three cases (i.e., Chs 1, ChsB 2, and ChsB–Pd 3) are listed in Table 2 for comparison. This table shows that the C 1s and N 1s binding energies (B.E.s) of ChsB 2 were lower by 0.04 and 0.11 eV compared to those of Chs 1, respectively, possibly due to the formation of C=N.55 Moreover, the B.E. of Pd 3d5/2 (in ChsB–Pd 3) was 337.99 eV, very close to the B.E. of Pd(II) in Na2PdCl4 (Pd3d5/2 = 338.07 eV) and higher than that of Pd0 (335.4 eV). This result confirmed that Pd species were present in the ionic form (Pd2+) rather than the elemental form (Pd0) in ChsB–Pd 3.
Figure 9.
XPS spectra of ChsB-Pd 3: (a) C 1s, (b) O 1s, (c) N 1s, (d) Pd 3d, (e) Cl 2p, and (f) wide scan.
Table 2. Data from XPS Studies of Chs 1, ChsB 2, and ChsB–Pd 3.
| binding
Energy (eV) |
||||
|---|---|---|---|---|
| sample | C 1s | O 1s | N 1s | Pd 3d5/2 |
| Chs 1 | 284.69 | 531.29 | 398.97 | |
| ChsB 2 | 284.65 | 531.35 | 398.86, 399.57 | |
| ChsB-Pd 3 | 284.62 | 531.09 | 399.34, 400.09 | 337.99 |
| Na2PdCl4 | 338.07 | |||
The slightly lower B.E. of Pd in the ChsB–Pd 3 complex (by 0.08 eV) compared to that of Pd(II) in Na2PdCl4 indicated the partial donation of electrons from N to Pd, consistent with the concurrent shifts of the B.E. of N1s from 398.86 to 399.34 eV and from 399.57 to 400.09 eV in ChsB 2 and ChsB–Pd 3, respectively (i.e., a positive shift of about 0.48 eV). These results concluded that coordination bonds were formed between two nitrogen atoms and one palladium ion in the ChsB–Pd 3 complex.
2.2.9. BET Analysis
The complex ChsB–Pd 3 catalyst was also characterized by BET and Barrett–Joyner–Halenda (BJH) analyses, as presented in Table 3. The pore diameter (DBJH), specific surface area (SBET), and total pore volume (Vtotal) were measured using the BET tool and BJH analysis employing the N2 adsorption/desorption. The surface area (m2 g–1), pore volume (cm3 g–1), and average pore diameter (nm) of the ChsB–Pd 3 catalyst were 0.2055, 0.00063,1 and 12.20439, respectively.
Table 3. BET and BJH Analytical Data of the Chitosan-Based Pd(II) Complex (ChsB–Pd 3).
| surface area (m2/g) | single-point surface area | 0.2055 |
| BET surface area | 0.2067 | |
| BJH adsorption cumulative surface area of pores with a 17.000–3000.000 Å width | 0.243 | |
| Langmuir surface area | 0.7270 | |
| pore volume (cm3/g) | single-point adsorption total pore volume | 0.000631 |
| BJH adsorption cumulative volumes of pores with a 17.000–3000.000 Å width | 0.0000637 | |
| pore size (nm) | adsorption average pore size (4 V/A according to the BET method) | 12.20439 |
| BJH adsorption average pore width (4 V/A) | 10.5040 |
2.3. Optimization of Catalytic Conditions of ChsB–Pd 3 for Suzuki Coupling
The catalytic efficiency of the Pd(II) complex (ChsB-Pd 3) in the coupling reaction of 4-bromoanisole (4) with 4-hydroxyphenylboronic acid (5b) in varied solvents using various bases was examined under two heating modes; MW irradiation conditions at 80 °C (for 10 min) and traditional heating (for 2 h), as illustrated in Table 4. Thus, when 5.1 × 10–3 mol % of the solid-phase Pd(II) complex (ChsB–Pd 3) was employed using sodium bicarbonate as the base and different solvents (DMF, toluene, ethanol, or water), it resulted in moderate conversion into 4′-methoxy-1,1′-biphenyl-4-ol (6b) in 55–75% yields (under thermal heating) and 60–85% yields (under MW heating), respectively, where the higher yield was obtained in ethanol, while in toluene, the product yield was lower (Table 4, entries 1–4). The cross-coupling reaction was repeated in an ethanol/water mixed solvent (1:1) using different inorganic and organic bases (e.g., KOH, K2CO3, Cs2CO3, Na2CO3, NaHCO3, Et3N, DBU, and DABCO) (Table 4, entries 5–19). Using the EtOH/water solvent and 5.1 × 10–3 mol % of the Pd(II) complex (ChsB–Pd 3), the bicarbonate base was the most effective among the inorganic bases utilized under both thermal heating (90% yield) and MW irradiation (97% yield) (Table 4, entry 6), with turnover frequency (TOF) values of 8823 and 114,114 h–1, respectively, (measured as moles of the product per mole of the catalyst), much better than that of all the organic bases used (Et3N, DBU, and DABC) (Table 4, entries 11–13). Moreover, temperature played a vital role in the reaction productivities (Table 4, entries 6–8), where conducting the cross-coupling reaction at ambient temperature (25–30 °C) in an EtOH–H2O mixed solvent (1:1) employing the ChsB–Pd 3 catalyst and NaHCO3 led to the formation of the cross-coupled product 6b in 48% yield (thermal heating) [turnover number (TON) = 9411 and TOF = 4705 h–1] and 60% yield (MW condition) (TON = 11,764 and TOF = 70,584 h–1) (Table 4, entry 7). However, performing the reaction at 120 °C furnished product 6b in 84% (TOF = 8240 h–1) and 90% yields (TOF = 105,882 h–1) under thermal and MW conditions, respectively (Table 4, entry 8). The effect of concentration of the Pd complex ChsB-Pd 3 for coupling of 4 with 5b was also examined, where catalyst loading experiments were carried out in the range of 2.55 × 10–3 to 1.02 × 10–2 mol % (Table 4, entries 5, 6, 9, and 10). From this, 5.1 × 10–3 mol % of the catalyst (Table 4, entry 6), produced the maximum yield of the cross-coupled product 6b in 90% yield after 2 h with TOF = 8823 h–1 (thermal heating) and in 97% yield after 10 min with TOF = 114,114 h–1 (MW condition). Therefore, it was decided to use EtOH/H2O (v/v = 1:1) as the solvent, NaHCO3 as the base, and 5.1 × 10–3 mol % catalyst at 80 °C as the optimum condition in further studies.
Table 4. Optimization of Catalytic Activity of ChsB–Pd 3 for Suzuki Couplinga.
| thermal (2 h) | MW (10 min) | |||||
|---|---|---|---|---|---|---|
| entry | mol % Pd catalyst | base | solvent | temp. °C | yieldb (%)/TON/TOF (h–1) | yieldb (%)/TON/TOF (h–1) |
| 1 | 5.1 × 10–3 | NaHCO3 | DMF | 80 | 55/10784/5392 | 60/11764/70584 |
| 2 | 5.1 × 10–3 | NaHCO3 | toluene | 80 | 60/11764/5882 | 68/13333/79998 |
| 3 | 5.1 × 10–3 | NaHCO3 | ethanol | 80 | 75/14705/7352 | 85/16666/99996 |
| 4 | 5.1 × 10–3 | NaHCO3 | H2O | 80 | 68/13333/6666 | 76/14901/89406 |
| 5 | 2.55 × 10–3 | NaHCO3 | H2O + EtOH | 80 | 80/31372/15686 | 86/33725/202350 |
| 6 | 5.1 × 10–3 | NaHCO3 | H2O + EtOH | 80 | 90/17647/8823 | 97/19019/114114 |
| 7 | 5.1 × 10–3 | NaHCO3 | H2O + EtOH | rt | 48/9411/4705 | 60/11764/70584 |
| 8 | 5.1 × 10–3 | NaHCO3 | H2O + EtOH | 120 | 84/16470/8240 | 90/17647/105882 |
| 9 | 7.65 × 10–3 | NaHCO3 | H2O + EtOH | 80 | 83/10849/5424 | 93/12156/72936 |
| 10 | 1.02 × 10–2 | NaHCO3 | H2O + EtOH | 80 | 81/7941/3971 | 90/8823/52938 |
| 11 | 5.1 × 10–3 | Et3N | H2O + EtOH | 80 | 62/12156/12156 | 69/13529/81174 |
| 12 | 5.1 × 10–3 | DBU | H2O + EtOH | 80 | 65/12745/6372 | 75/14705/88230 |
| 13 | 5.1 × 10–3 | DABCO | H2O + EtOH | 80 | 64/12549/6274 | 72/14117/84702 |
| 14 | 5.1 × 10–3 | KOH | H2O + EtOH | 80 | 67/13137/6568 | 76/14901/89406 |
| 15 | 5.1 × 10–3 | Cs2CO3 | H2O + EtOH | 80 | 71/13921/6960 | 80/15686/94116 |
| 16 | 5.1 × 10–3 | K2CO3 | H2O + EtOH | 80 | 74/14509/7254 | 82/16078/96468 |
| 17 | 5.1 × 10–3 | Na2CO3 | H2O + EtOH | 80 | 75/14705/7352 | 84/16470/98820 |
Reaction conditions: 4-bromoanisole (4) (1 mmol), 4-hydroxylphenylboronic acid 5b (1.2 mmol), Pd catalyst (2.55 × 10–3 to 1.02 × 10–2 mol %), and base (2 mmol) in a solvent [3 mL, and in the case of EtOH/water (3 mL, 1:1 v/v)], with MW (10 min, Pmax = 200 W) and conventional heating (2 h).
Isolated yield. TON = turnover number: yield of the product/mol of Pd. TOF = turn over frequency: TON/time of the reaction (hour).
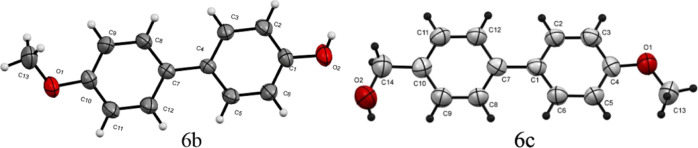
Under the optimum conditions described above, the efficiency of the ChsB–Pd 3 catalyst was also evaluated in the Suzuki reaction of 4-bromoanisole 4 with various arylboronic acids 5a–e, (more details are presented in Table 5). In all cases, high yields were obtained under both thermal heating (79–90%) and MW irradiation (89–97%) conditions, respectively. The reaction proceeded more efficiently with the coupling of 4-hydroxyphenylboronic acid 5b resulting in 97% yield (TOF = 114,114 h–1 and TON = 19,019) after 12 min of MW irradiation and in 90% yield (TOF = 8823 h–1 and TON = 17,647) after 2 h of thermal heating, respectively (Table 5, entry 5). However, the low efficacy of ChsB–Pd 3 was noticed in the coupling of 4-hydroxymethylphenylboronic acid 5c under both MW irradiation (TOF = 52,350 h–1 and TON = 17,058) and thermal heating (TOF = 3872 h–1 and TON = 15,490), respectively (Table 5, entry 3). Thus, electron-rich groups accelerated the coupling of arylboronic acids with 4-bromoanisole 4. The structural formulae of the obtained products were confirmed by the spectroscopic data and single-crystal X-ray analysis of the two products 6b (CCDC 2035844) and 6c (CCDC 2035841) (Figure 10).56
Table 5. Suzuki Coupling of Arylboronic Acids 5a–e with 4-Bromoanisole 4 Using ChsB–Pd 3.
Reaction conditions: 4-bromoanisole 4 (1 mmol), arylboronic acids 5a–e (1.2 mmol), Pd catalyst (5.1 × 10–3 mol %), NaHCO3 (2 mmol) in EtOH/H2O (1:1, 3 mL), MW setting: Pmax = 200 W.
Isolated yield.
Figure 10.
ORTEP plot of the X-ray crystallographic data determined for 6b and 6c.
In addition, the efficiency of the Pd catalyst (ChsB–Pd 3) was examined for the Suzuki reaction of a number of arylboronic acids bearing electron-donating or electron-withdrawing groups with 5-iodovanillin 7 for the synthesis of the corresponding coupled products 8a–e. As summarized in Table 6, the products yields ranged between 90 and 97% under MW conditions, compared to 87–95% yields under conventional thermal heating conditions (Table 6, entries 1–5). Variation of the reaction time for the cross-coupling reaction of phenylboronic acid with 5-iodovanillin 7 was also examined, where high yields of the biaryl derivative 8a (Table 6, entry 1) was obtained after 12 min of MW irradiation (97% yield) and after 52 h of traditional heating (95% yield) (Table 6, entry 1), with TOF values of 95,095 and 3725 h–1, respectively, and total TON values of 19019 and 18627, respectively. In addition, coupling of p-tolylboronic acid 6e with 5-iodovanillin 7 gave the corresponding product (8b) in 93% yield after 18 min of MW irradiation and in 89% yield after 6 h of thermal heating (Table 6, entry 2), which corresponds to TOF values of 60,783 and 2908 h–1, respectively, and total TON values of 18,235 and 17,450, respectively. A suggested mechanism for the cross-coupling reaction of arylboronic acids with aryl halides is presented in Scheme 2. The structural formulae of the obtained products were substantiated from all possible spectroscopic data and single-crystal X-ray analysis of two products; 8a (CCDC 2153120) and 8c (CCDC 2153130) (Figure 11).56
Table 6. Suzuki Coupling of 5-Iodovanillin 7 with Arylboronic Acids 5a–e Using ChsB–Pd 3a.
Reaction conditions: 5-iodovanillin 7 (1 mmol), arylboronic acids 5a–e (1.2 mmol), 5.1 × 10–3 mol % Pd catalyst (5.1 × 10–3 mol %) and NaHCO3 (2 mmol) in EtOH/H2O (1:1, 3 mL), 10 min under MW setting: Pmax = 200 W and (2 h) under the conventional heating method.
Isolated yield. TON: turnover number. TOF: turnover frequency.
Scheme 2. Plausible Mechanism for the Cross-Coupling Reactions.
Figure 11.
ORTEP plot of the X-ray crystallographic data determined for 8a and 8c.
2.4. Recyclability and Heterogeneity Studies of the Pd Catalyst
The lifetime of heterogeneous catalysts was an essential factor to be considered while using them, particularly for pharmaceutical and industrial applications of the Suzuki reaction. Thus, reusability test of the solid catalyst ChsB–Pd 3 in the cross-coupling of 4-bromoanisole 4 (1 equiv) with phenylboronic acid 5a (1.2 equiv) in the presence of ChsB-Pd 3 (5.1 × 10–3 mol %) in EtOH/water (1:1, 3 mL) was performed under the MW condition (10 min at 80 °C and 200 W) using NaHCO3 (2 equiv) to give 4-methoxybiphenyl 6a in 94% isolated yield. After the reaction was complete, the catalyst was filtered off, then cleaned by stirring for 10 min in ethyl acetate (15 mL), filtered again, washed with water followed by ethanol, and dried. The used catalyst ChsB–Pd 3 was then involved in the next run for the same coupling partners as those above for the same time and was found to be highly active for five consecutive runs with minor depletion of its activity, providing excellent yields of the product even at the fifth run (90%) with a TOF of 105,882 h–1 and a TON of 17,647. The reaction yields and TOF and TON values for each run are listed in Table 7 and Figure 12. The obtained results proved the high thermal stability and reusability of the solid-supported ChsB–Pd 3 catalyst under MW irradiation conditions.
Table 7. Recyclability of the ChsB–Pd 3 Catalysta.
| number of cycle | yield (%) | TON | TOF (h–1) |
|---|---|---|---|
| 1st | 94 | 18431 | 110,586 |
| 2nd | 94 | 18431 | 110,586 |
| 3rd | 92 | 18039 | 108,234 |
| 4th | 92 | 18039 | 108,234 |
| 5th | 90 | 17647 | 105,882 |
Reaction conditions: 4-bromoanisole 4 (1 mmol), phenylboronic acid 5a (1.2 mmol), Pd catalyst (5.1 × 10–3 mol %), and NaHCO3 (2 mmol) in EtOH/H2O (1:1, 3 ml), 80 °C for 10 min under the MW condition with Pmax = 200 W. Isolated yield.
Figure 12.
Recyclability of the catalyst ChsB–Pd 3 in Suzuki coupling.
The XRD analysis of the recycled catalyst ChsB–Pd 3 was performed to establish its structural stability, as shown in Figure 13. The XRD results confirmed that the catalyst ChsB–Pd 3 maintained its structure to be similar to that of the freshly unused catalyst, confirming its high stability. Additionally, the heterogeneity of the catalyst ChsB–Pd 3 was also confirmed via filtration of the solid catalyst, while the reaction mixture was hot under the optimum condition for the Suzuki model reaction after 1 h, followed by the reaction being run for an additional 1 h without the solid catalyst. It was noticed that the yield of the product remained fixed with no further increase.
Figure 13.

XRD patterns of the reused catalyst (red) and fresh catalyst (blue).
3.5. Comparative Study Related to the ChsB–Pd 3 Catalyst
The TON and TOF values of a number of previously reported solid-phase supported Pd catalysts are shown in Table 8. As demonstrated in the table, the TOF number in the presence of the Pd(II) complex (ChsB–Pd 3) was higher than that of the other reported catalysts. In this study, we used a small amount of catalyst loading (5.1 × 10–3 mol %), and the reactions were accomplished within 10 min under the MW irradiation, leading to highly efficient TOFs and TONs. Thus, the chitosan-based palladium catalyst ChsB–Pd 3 was the superior one.
Table 8. Comparison of the Activity of the ChsB–Pd 3 Catalyst with That of Other Previously Related Catalysts for Suzuki Cross-Coupling.
| catalyst (mol %) | reaction conditions | time (h) | TON | TOF(h–1) | yield (%)Ref |
|---|---|---|---|---|---|
| nano Pd(0) supported on cellulose (0.3 mol %) | H2O, K2CO3, TBAB, 100 °C | 5.5 | 320 | 58 | 9657 |
| PdCl2 (complex with chitosan-supported diimine) 0.015% mol | K2CO3, 50 °C, MW, 400 W, solvent free | 4 min | 6467 | 107,783 | 9758 |
| Pd NPs@microcapsules (CAP) (0.1 mol %), | K2CO3, MW, (400 W) | 6 min | 920 | 9200 | 9259 |
| Pd NPs@Fe3O4/lignin/chitosan nanocatalyst (0.08 mol %) | K2CO3, MW, 400 W | 5 min | 1113 | 13,356 | 8960 |
| Schiff base-functionalized pectin-supported Pd(II) catalyst (6 × 10–2 mol %) | K2CO3, MW, 400 W | 8 min | 1583 | 12,664 | 9561 |
| Chitosan–Ulva-supported Pd(II) catalyst (0.02 mol %) | K2CO3, 50 °C, MW, 400 W | 4 min | 4950 | 75,000 | 9962 |
| GG-Pd (2 × 10–2 mol %) | K2CO3, MW, solvent-free | 5 min | 4400 | 52,800 | 8863 |
| Ca, Pd/alginate (0.4 mol %) | DMF, K2CO3, 70 °C | 5 | 243 | 49 | 9764 |
| Cellulose-supported Pd(0) nanoparticles (0.5 mol %) | H2O, K2CO3, MW: 400 W, 80 °C | 5 min | 188 | 2256 | 9465 |
| biopolymer–metal complex wool–Pd (0.45 mol %) | H2O, K2CO3, 75 °C | 5 | 188 | 38 | 8566 |
| chitosan-supported Pd nanoparticles (0.1 mol %) | H2O, K2CO3, TBAB, 70 °C | 5 | 980 | 196 | 9843 |
| chitosan-supported heterogeneous Pd catalysts (0.25 mol %) | H2O, K2CO3, MW: 400 W, 150 °C | 10 min | 220 | 1330 | 5567 |
| palladium-supported sepiolite (0.25 μmol%) | DMF, K2CO3, 130 °C | 24 | 65,000 | 39,000 | 6568 |
| palladacycle (10–3 mol %) | H2O, K2CO3, TBAB, reflux | 100 min | 92,000 | 52,600 | 9269 |
| dendrimer-stabilized Pd nanoparticles (0.001 mol %) | CHCl3/MeOH (2:1), NaOAc, 25 °C | 48 | 30,000 | 6000 | 3070 |
| chitosan–Schiff base-supported heterogeneous Pd (5.1 × 10–3 mol %) | EtOH/H2O (1:1), NaHCO3, MW, 200 W, 80 °C | 10 min | 19,019 | 114,114 | 97this work |
3. Experimental Section
3.1. General Part
All starting materials were highly pure and purchased from Sigma-Aldrich. Chitosan has a high molecular weight (mol. wt. 310,000–375,000) with a degree of deacetylation of >75% and viscosities of 800–2000 cPa, 1 wt % in 1% acetic acid (419419 Aldrich). Melting points were measured using a Griffin apparatus for the melting point and were uncorrected. IR spectra were carried out using KBr disks in the spectrophotometer; PerkinElmer System 2000 FTIR. 1H- and 13C NMR spectra were measured at 600 and 150 MHz, respectively, at 25 °C using the deuterated solvent DMSO-d6 and tetramethylsilane as the internal standard (with chemical shifts given in parts per million) on a Bruker DPX 400 or 600 super-conducting NMR spectrometer. Low-resolution electron impact mass spectroscopy [MS (EI)] and high-resolution electron impact mass spectroscopy [HRMS (EI)] were carried out using a high resolution thermo-spectrometer [GC–MS (DFS)] using a magnetic sector mass analyzer at 70.1 eV. MW experiments were conducted using a Discover LabMate CEM microwave instrument (300 W with CHEMDRIVER software; Matthews, NC). Reactions were conducted under MW irradiation in closed pressured Pyrex tubes fitted with PCHS caps. The BET method was used to perform the surface area analysis based on adsorption data obtained by measuring the isotherms of nitrogen sorption of the samples at −195 °C using a model Gemini VII ASAP 2020 Automatic Micromeritics sorptometer (Micromeritics, USA). The samples were degassed for 12 h at 110 °C prior to the analysis. The X-ray single crystal data were measured employing a Bruker X8 Prospector and a Rigaku R-AXISRAPID diffractometer, and the single-crystal data collection was performed using Cu Kα radiation at room temperature. The Bruker SHELXTL software package (structure solution program-SHELXS-97 and refinement program-SHELXL97) was used for solving and refining the structures.
3.2. Synthesis of the Chitosan-Based Palladium(II) Complex (ChsB–Pd 3)
The chitosan-based Schiff base (ChsB 2) was prepared by dropwise addition of a solution of 2-pyridinecarbaldehyde (0.643 g, 6 mmol) dissolved in methanol (10 mL) to a solution of chitosan (Chs 1) (1.0 g, equivalent to 6 mmol NH2) dissolved in 1% acetic acid (300 mL) and refluxed with stirring for 30 min to obtain a gel solution, and the reaction process was controlled via color change from colorless to yellow. The reaction mixture was then stirred for 1.5 h, and drops of NaOH solution were added till the reaction solution turned into a basic medium by pH. Thereafter, the obtained Chitosan schiff base (ChsB 2) was dissolved in dioxane/methanol (1:1, 10 mL) and then treated with a solution of sodium tetrachloropalladate (Na2PdCl4) dissolved in methanol (5 mL) with stirring and heating under the reflux condition in a water bath. The crude complex (ChsB–Pd 3) was isolated by filtration, washed with water and then ethanol several times, and finally dried at 80 °C under vacuum. The structure of the ChsB–Pd 3 catalyst was established using the facilities at the analytical center of Kuwait University such as FTIR spectroscopy, UV spectroscopy, XRD, XPS, ICP-AES, TGA, DTG, TEM, BET, and EDX analyses. The concentration of palladium in ChsB–Pd 3 was found to be 0.541 wt % (5.1 × 10–2 mmol/g), as determined by ICP-AES.
3.3. Suzuki–Miyaura Cross-Coupling Reaction of 4-Bromoanisole or 5-Iodovanillin with Arylboronic Acids
A mixture of 4-bromoanisole or 5-iodovanillin (1.0 mmol) and the appropriate arylboronic acid (1.2 mmol) in a 1:1 ratio of water and ethanol mixture (3 mL), NaHCO3 (0.17 g, 2 mmol), and Pd complex ChsB–Pd 3 (1 mg, 5.1 × 10–3 mol % Pd) were thermally heated at 80 °C till the substrates were completely consumed, as examined by thin-layer chromatography (TLC). Extraction of the reaction mixture with ethyl acetate (3 × 15 mL) and drying over MgSO4 were performed. Removal of the solvent under reduced pressure afforded the desired products 6a–e and 8a–e, respectively.
3.3.2. General Method B
A mixture of 4-bromoanisole or 5-iodovanillin (0.125 mL, 1.0 mmol) and arylboronic acid (1.2 mmol) in a 1:1 ratio of water/ethanol mixture (3 mL), NaHCO3 (0.17 g, 2 mmol), and Pd complex ChsB–Pd 3 (1 mg, 5.1 × 10–3 mol % Pd) were mixed in a process Pyrex glass tube. The tube was capped properly, and the mixture was then heated under MW conditions at 80 °C and 200 W for the required reaction time, as shown in Tables 5 and 6. After the confirmation of the complete consumption of the starting compounds into products (TLC-monitored), extraction of the reaction mixture with ethyl acetate (3 × 15 mL), followed by drying over MgSO4, was performed, and the solvent was then removed under reduced pressure to afford the desired products 6a–e and 8a–e, respectively.
3.3.2.1. 4-Methoxy-1,1′-biphenyl (6a)
Pale yellow crystals, mp 88–90 °C;71 IR (KBr) ν/cm–1: 3033, 1606, 1581; 1H NMR (DMSO-d6): δ 7.59–7.61 (m, 4H, Ar-H); 7.41–7.43 (m, 2H, Ar-H), 7.29–7.31 (m, 1H, Ar-H), 7.02 (dd, J = 1.8, 1.8 Hz, 2H, Ar-H), 3.79 (s, 1H, OCH3), 13C NMR (DMSO-d6): δ 158.86, 139.81, 134.04, 132.50, 128.82, 127.72, 126.66, 116.14, 114.33 (Ar-C), 55.13 (OCH3). MS (EI): m/z 184.09 [M]+. HRMS: calcd for C13H12O, 184.0888; found, 184.0883.
3.3.2.2. 4′-Methoxy-1,1′-biphenyl-4-ol (6b)
White crystals, mp 183–184 °C (Lit.72 mp 183.5 °C); IR (KBr) ν/cm–1: 3391, 3014, 1609, 1597. 1H NMR (DMSO-d6): δ 9.42 (s, 1H, OH), 7.49 (d, J = 9 Hz, 2H, Ar-H), 7.40 (d, J = 8.4 Hz, 2H, Ar-H), 6.97 (d, J = 9 Hz, 2H, Ar-H), 6.81 (d, J = 8.4 Hz, 2H, Ar-H), 3.76 (s, 1H, CH3); 13C NMR (DMSO-d6): δ 158.07, 156.48, 132.07, 130.70, 129.33, 127.19, 126.97, 116.20, 115.60, 114.19 (Ar-C), 55.07 (OCH3). MS (EI): m/z 200.13 [M]+; HRMS: calcd for C13H12O2, 200.0837; found, 200.0832. Crystal Data, C13H12O2, M = 200.23, monoclinic, a = 23.1408(12) Å, b = 5.4177(3) Å, c = 8.0330(5) Å, α = 90°, β = 91.016(4)°, γ = 90°, T = 296(2) K, V = 1006.94(10) Å3, calculated density = 1.321 g/cm3, space group: P121/c 1, Z = 4, number of reflections measured 7951, R1 = 0.0952, θmax = 66.56° (CCDC 2035844) [54].
3.3.2.3. (4′-Methoxy-1,1′-biphenyl-4-yl)methanol (6c)
White crystals, mp 159–160 °C (Lit.73 mp 158–159 °C); IR (KBr) ν/cm–1: 3325, 1607, 1531, 1499; 1H NMR (DMSO-d6): δ 7.55–7.59 (m, 4H, Ar-H), 7.36 (d, J = 8.4 Hz, 2H, Ar-H), 7.00 (dd, J = 2.40 Hz, J = 2.40 Hz, 2H, Ar-H), 5.19 (t, J = 5.70 Hz, 1H, OH), 4.52 (d, J = 5.40 Hz, 2H, CH2), 3.78 (s, 1H, CH3); 13C NMR (DMSO-d6): δ 158.74, 140.98, 138.21, 132.45, 127.58, 126.99, 125.83, 114.31, (Ar-C), 62.63 (CH2) 55.13 (OCH3). MS (EI): m/z 214.2 [M]+; HRMS: calcd for C14H14O2, 214.0993; found, 214.0989. Crystal Data: C14H14O2, monoclinic, a = 6.1263(6) Å, b = 48.858(5) Å, c = 7.4272(8) Å, α = 90°, β = 90.130(6)°, γ = 90°, T = 293 K, V = 2223.1(4) Å3, space group: P21/c, Z = 4, calcd density = 1.280 g/cm3, reflections measured 3920, R1 = 0.0824, θmax = 25.021° (CCDC 2035841) [54].
3.3.2.4. 4-Chloro-4′-methoxy-1,1′-biphenyl (6d)
Pale yellow crystals, mp 109–110 °C (Lit.74 mp 106–108 °C); IR (KBr) ν/cm–1: 3009, 1605, 1524; 1H NMR (DMSO-d6): δ 3.80 (s, 3H, OCH3), 7.03 (d, J = 9 Hz, 2H, Ar-H), 7.47–7.48 (m, 2H, Ar-H), 7.61–7.65 (m, 4H, Ar-H); 13C NMR (DMSO-d6): δ 159.13, 138.62, 132.07, 131.47, 131.11, 128.75, 128.42, 127.86, 127.74, 116.20, 114.42 (Ar-C), 55.18 (OCH3), 20.59 (CH3). MS (EI): m/z 218.10 [M]+; HRMS: calcd for C13H11ClO, 218.04984; found, 218.0493.
3.3.2.5. 4-Methoxy-4′-methyl-1,1′-biphenyl (6e)
White crystals, mp 107–108 °C (Lit.75 mp 107–108 °C); IR (KBr) ν/cm–1: 3020, 1608, 1582,1499, 1440,1288, 807; 1H NMR (DMSO-d6): δ 7.57 (dd, J = 2.4 Hz, J = 1.8 Hz, 2H, Ar-H), 7.50 (d, J = 7.8 Hz, 2H, Ar-H), 7.24 (d, J = 6.6 Hz, 2H, Ar-H), 7.01 (dd, J = 1.8 Hz, J = 1.8 Hz, 2H, Ar-H), 3.79 (s, 3H, OCH3), 2.33 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 158.64, 136.95, 135.84, 134.17, 132.47, 129.42, 128.01, 127.44, 125.97, 114.28 (Ar-C), 55.12 (OCH3). MS (EI): 198.10 [M]+. HRMS: calcd for C14H14O, 198.1044; found, 198.1038.
3.3.2.6. 6-Hydroxy-5-methoxy-[1,1′-biphenyl]-3-carbaldehyde (8a)
White solid, mp 112–113 °C;76 IR (KBr) ν/cm–1: 3413, 3232, 1670, 1590; 1H NMR (DMSO-d6): δ 9.85 (s, 1H, CHO), 7.59–7.54 (m, 3H, Ar-H), 7.46–7.33 (m, 4H, Ar-H), 3.95 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 56.09 (OCH3), 108.70, 127.18, 127.32, 127.40, 127.87, 128.08, 128.13, 129.07, 134.04, 137.14, 148.40, 149.86 (Ar-C), 191.26 (CHO). MS (EI): for C14H12O3m/z, 228.24; found, 228.1 [M]+; HRMS: calcd for C14H12O3m/z, 228.0786; found, 228.0782. Crystal Data, C14H12O3, M = 585.04, orthorhombic, a = 7.6875(6) Å, b = 22.0075(13) Å, c = 7.0719(6) Å, α = 90°, β = 90°, γ = 90°, V = 1196.44(15) Å3, T = 296(2) K, space group: Pna21, Z = 4, calculated density = 1.267 g/cm3, no. of reflection measured 5000, θmax = 66.06°, R1 = 0.0740.
3.3.2.7. 6-Hydroxy-5-methoxy-4′-methyl-[1,1′-biphenyl]-3-carbaldehyde (8b)
White solid, mp 137–138 °C; IR (KBr) ν/cm–1: 3332, 3023, 2919, 1679, 1514, 1463; 1H NMR (DMSO-d6): δ 9.83 (s, 1H, CHO), 7.52 (d, 1H, J = 2.40 Hz, Ar-H), 7.47 (d, 2H, J = 7.80 Hz, Ar-H), 7.40 (d, 1H, J = 1.80 Hz, Ar-H), 7.24 (d, 2H, J = 7.80 Hz, Ar-H), 3.93 (s, 3H, OCH3), 2.34 (s, 3H, CH3); 13C NMR (DMSO-d6): δ 20.76 (CH3), 56.09 (OCH3), 108.55, 127.20, 127.81, 128.19, 128.67, 128.91, 134.15, 136.42, 148.35, 149.70, (Ar-C), 191.31 (C=O); MS (EI): for C15H14O3m/z, 242.27; found, 242.10 [M]+. HRMS: calcd for C15H14O3m/z, 242.0942; found, 242.0937.
3.3.2.8. 6-Hydroxy-4′-(hydroxymethyl)-5-methoxy-[1,1′-biphenyl]-3-carbaldehyde (8c)
White solid, mp 132–135 °C; IR (KBr) ν/cm–1: 3340, 1679, 1589, 1153; 1H NMR (DMSO-d6): δ 9.85 (s, 1H, CHO), 7.55–7.54 (m, 3H, Ar-H), 7.42 (d, 1H, J = 1.80 Hz, Ar-H), 7.38 (d, 2H, J = 8.40 Hz, Ar-H), 5.22 (br, 2H, OH), 4.55 (s, 2H, CH2), 3.94 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 56.10 (OCH3), 62.68 (CH2), 149.70, 148.34, 141.53, 135.40, 128.77, 128.21, 127.82, 127.22, 126.16 (Ar-C), 191.31 (C=O). MS (EI): for C15H14O4m/z, 258.27; found m/z, 258.1 [M]+. HRMS: calcd for C15H14O4m/z, 258.0892; found, 258.0886.
3.3.2.9. 4′-Chloro-6-hydroxy-5-methoxy-[1,1′-biphenyl]-3-carbaldehyde (8d)
White solid, mp 137–139 °C; IR (KBr) ν/cm–1: 3310, 1675, 1590, 1152; 1H NMR (DMSO-d6): δ 10.06 (br, 1H, OH), 9.84 (s, 1H, CHO), 7.61–7.59 (m, 2H, Ar-H), 7.55 (d, 1H, J = 1.80 Hz, Ar-H), 7.50–7.49 (m, 2H, Ar-H), 7.43 (d, 1H, J = 1.80 Hz, Ar-H), 3.93 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 56.13 (OCH3), 109.02, 126.51, 127.11, 128.10, 128.28, 130.86, 131.95, 135.88, 148.41, 149.69 (Ar-C), 191.23 (C=O). MS (EI): for C14H11ClO3m/z, 262.69; found m/z, 262.10 [M]+. HRMS: calcd for C14H11ClO3m/z, 262.0396; found, 262.0391.
3.3.2.10. 4′,6-Dihydroxy-5-methoxy-[1,1′-biphenyl]-3-carbaldehyde (8e)
White solid, mp 140–142 °C; IR (KBr) ν/cm–1: 3420, 3256, 1667, 1589, 1150; 1H NMR (DMSO-d6): δ 9.83 (s, 1H, CHO), 9.75 (s, 1H, OH), 9.51 (s, 1H, OH), 7.49 (d, 1H, J = 1.80 Hz, Ar-H), 7.46–7.33 (m, 3H, Ar-H), 6.83 (dd, 2H, J = 1.80 Hz, Ar-H), 3.93 (s, 3H, OCH3); 13C NMR (DMSO-d6): δ 56.06 (OCH3), 108.07, 114.89, 126.61, 127.07, 127.95, 128.15, 130.17, 130.36, 148.28, 149.45, 156.67, (Ar-C), 191.36 (C=O). MS (EI): for C14H12O4m/z, 244.24 [M]+; found m/z, 244.10. HRMS: calcd for C14H12O4m/z, 244.0735; found, 244.0730.
3.4. Recyclability Test of ChsB–Pd 3
A mixture of 4-bromoanisole 4 (0.125 mL, 1.0 mmol) with phenylboronic acid 5a (1.2 mmol) in a water/ethanol mixture (3 mL, 1:1 v/v), NaHCO3 (0.17 g, 2 mmol), and Pd complex ChsB-Pd 3 (1 mg, 5.1 × 10–3 mol %) were mixed in a Pyrex glass tube. The glass tube was capped properly, then the mixture was heated for 10 min at 80 °C and 200 W under MW accelerating conditions. After the Suzuki reaction was completed, the ChsB–Pd 3 catalyst was filtered off, then cleaned well by stirring in ethyl acetate (15 mL) for 10 min, refiltered again, washed with water followed by ethanol, and then dried at 100 °C before its reuse in the next run. This regeneration process for the ChsB–Pd 3 catalyst was repeated after every run.
4. Conclusions
A solid-phase supported chitosan-based palladium(II) complex was reported and thoroughly characterized using all possible elucidation tools (FTIR spectroscopy, TGA, DSC, XPS, EDX, and ICP-AES, SEM, BET analysis, and TEM). The solid-phase catalyst was found to have an excellent catalytic activity for Suzuki–Miyaura cross-coupling reactions. Three green tools were employed in the current study, namely, aqueous solvent, MW irradiation technology, and solid-phase palladium catalysis. The catalyst proved itself as robust and recyclable under MW conditions. The biaryl cross-coupled products were obtained in high isolated yields and fully characterized by all spectroscopic data.
Acknowledgments
The RSP unit general facilities of the Faculty of Science GFS supported by research grants GS01/05, GS01/03, GS03/01, GS02/01, and GS03/08 were greatly appreciated. The Nanoscopy Science Centre is also gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01809.
1H- and 13C NMR, MS, and HRMS spectra (PDF)
Author Contributions
A.M.A., K.M.D., H.M.A.-M., and W.M.T. proposed the research work and prepared, wrote, and edited the manuscript for publication. A.M.A and K.M.D. were in charge of any correspondence of the manuscript. W.M.T. carried out the experimental work. All authors approved the final revised form.
The present research work was funded by the University of Kuwait; grant number (SC01/15).
The authors declare no competing financial interest.
Supplementary Material
References
- Baskar D.; Sampath Kumar T. S. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohydr. Polym. 2009, 78, 767–772. 10.1016/j.carbpol.2009.06.013. [DOI] [Google Scholar]
- Jin X.; Wang J.; Bai J. Synthesis and antimicrobial activity of the Schiff base from chitosan and citral. Carbohydr. Res. 2009, 344, 825–829. 10.1016/j.carres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R. A.; Ilari P. Chitosans carrying the methoxyphenyl functions typical of lignin. Carbohydr. Polym. 1994, 23, 155–160. 10.1016/0144-8617(94)90097-3. [DOI] [Google Scholar]
- Kumar M. R.; Muzzarelli R. A.; Muzzarelli C.; Sashiwa H.; Domb A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- Çalışkan M.; Baran T.; Nasrollahzadeh M. Facile preparation of nanostructured Pd-Sch-δ-FeOOH particles: A highly effective and easily retrievable catalyst for aryl halide cyanation and p-nitrophenol reduction. J. Phys. Chem. Solid. 2021, 152, 109968. 10.1016/j.jpcs.2021.109968. [DOI] [Google Scholar]
- Çalışkan M.; Baran T. Immobilized palladium nanoparticles on Schiff base functionalized ZnAl layered double hydroxide: A highly stable and retrievable heterogeneous nanocatalyst towards aryl halide cyanations. Appl. Clay Sci. 2022, 219, 106433. 10.1016/j.clay.2022.106433. [DOI] [Google Scholar]
- Uozumi Y.Recent progress in polymeric palladium catalysts for organic synthesis. Immob. Catal. 2004, 77–112. 10.1007/b96874. [DOI] [PubMed] [Google Scholar]
- Miyaura N.; Yanagi T.; Suzuki A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synth. Commun. 1981, 11, 513–519. 10.1080/00397918108063618. [DOI] [Google Scholar]
- Kantam M. L.; Srinivas P.; Yadav J.; Likhar P. R.; Bhargava S. Trifunctional N, N, O-terdentate amido/pyridyl carboxylate ligated Pd (II) complexes for Heck and Suzuki reactions. J. Org. Chem. 2009, 74, 4882–4885. 10.1021/jo900361c. [DOI] [PubMed] [Google Scholar]
- Huang Y. T.; Tang X.; Yang Y.; Shen D. S.; Tan C.; Liu F. S. Efficient pyridylbenzamidine ligands for palladium-catalyzed Suzuki–Miyaura reaction. Appl. Organomet. Chem. 2012, 26, 701–706. 10.1002/aoc.2913. [DOI] [Google Scholar]
- Hajipour A. R.; Rahimi H.; Rafiee F. Dimeric ortho-palladated homoveratrylamine as an efficient homogeneous catalyst for copper-free Sonogashira cross-coupling reaction. Appl. Organomet. Chem. 2012, 26, 727–730. 10.1002/aoc.2920. [DOI] [Google Scholar]
- Dawood K. M. Microwave-assisted Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions of aryl chlorides and bromides in water using stable benzothiazole-based palladium (II) precatalysts. Tetrahedron 2007, 63, 9642–9651. 10.1016/j.tet.2007.07.029. [DOI] [Google Scholar]
- Das P.; Bora U.; Tairai A.; Sharma C. Triphenylphosphine chalcogenides as efficient ligands for room temperature palladium (II)-catalyzed Suzuki–Miyaura reaction. Tetrahedron Lett. 2010, 51, 1479–1482. 10.1016/j.tetlet.2010.01.032. [DOI] [Google Scholar]
- Linninger C. S.; Herdtweck E.; Hoffmann S. D.; Herrmann W. A.; Kühn F. E. A new palladium (II) complex of a functionalized N-heterocyclic carbene: Synthesis, characterization and application in Suzuki–Miyaura cross-coupling reactions. J. Mol. Struct. 2008, 890, 192–197. 10.1016/j.molstruc.2008.05.037. [DOI] [Google Scholar]
- Xu Q.; Hao W.; Cai M. Mercapto-functionalized MCM-41 anchored palladium (0) complex as an efficient catalyst for the heterogeneous Suzuki reaction. Catal. Lett. 2007, 118, 98–102. 10.1007/s10562-007-9157-y. [DOI] [Google Scholar]
- Tong J.; Li Z.; Xia C. Highly efficient catalysts of chitosan-Schiff base Co (II) and Pd (II) complexes for aerobic oxidation of cyclohexane in the absence of reductants and solvents. J. Mol. Catal. A: Chem. 2005, 231, 197–203. 10.1016/j.molcata.2005.01.011. [DOI] [Google Scholar]
- Hardy J. J.; Hubert S.; Macquarrie D. J.; Wilson A. J. Chitosan-based heterogeneous catalysts for Suzuki and Heck reactions. Green Chem. 2004, 6, 53–56. 10.1039/B312145N. [DOI] [Google Scholar]
- Han F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. 10.1039/C3CS35521G. [DOI] [PubMed] [Google Scholar]
- Bonin H.; Fouquet E.; Felpin F. X. Aryl Diazonium versus Iodonium Salts: Preparation, Applications and Mechanisms for the Suzuki–Miyaura Cross-Coupling Reaction. Adv. Synth. Catal. 2011, 353, 3063–3084. 10.1002/adsc.201100531. [DOI] [Google Scholar]
- Knappke C. E.; Jacobi von Wangelin A. A Synthetic Double Punch: Suzuki–Miyaura Cross-Coupling Mates with C-H Functionalization. Angew. Chem., Int. Ed. 2010, 49, 3568–3570. 10.1002/anie.201001028. [DOI] [PubMed] [Google Scholar]
- Alonso F.; Beletskaya M.; Yus I. Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling: A critical overview. Part 2: The Suzuki reaction. Tetrahedron 2008, 64, 3047–3101. 10.1016/j.tet.2007.12.036. [DOI] [Google Scholar]
- Prieto P.; de la Hoz A.; Díaz-Ortiz A.; Rodríguez A. M. Understanding MAOS through computational chemistry. Chem. Soc. Rev. 2017, 46, 431–451. 10.1039/c6cs00393a. [DOI] [PubMed] [Google Scholar]
- Gawande M. B.; Shelke S. N.; Zboril R.; Varma R. S. Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. 10.1021/ar400309b. [DOI] [PubMed] [Google Scholar]
- de la Hoz A.; Díaz-Ortiz A.; Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. 10.1039/B411438H. [DOI] [PubMed] [Google Scholar]
- Kitanosono T.; Masuda K.; Xu P.; Kobayashi S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2018, 118, 679–746. 10.1021/acs.chemrev.7b00417. [DOI] [PubMed] [Google Scholar]
- Chanda A.; Fokin V. V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748. 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood A. F.; Darweesh M. R.; Shaaban A. M.; Farag P.; Metz K. M. Facile access to biaryls and 2-acetyl-5-arylbenzofurans via Suzuki coupling in water under thermal and microwave conditions. Synthesis 2010, 3163–3173. 10.1055/s-0030-1258181. [DOI] [Google Scholar]
- Dawood K. M.; Darweesh A. F.; Shaaban M. R.; Farag A. M. Microwave-promoted Heck and Suzuki coupling reactions of new 3-(5-bromobenzofuranyl)pyrazole in aqueous media. Arkivoc 2018, 2018, 348–358. 10.24820/ark.5550190.p010.609. [DOI] [Google Scholar]
- Dawood K. M.; Elamin M. B.; Farag A. M. Microwave-Assisted Synthesis of Arylated Pyrrolo [2,1-a] Isoquinoline Derivatives via Sequential [3+2] Cycloadditions and Suzuki-Miyaura Cross-Couplings in Aqueous Medium. J. Heterocycl. Chem. 2016, 53, 1928–1934. 10.1002/jhet.2508. [DOI] [Google Scholar]
- Dawood K. M.; Shaaban M. R.; Elamin M. B.; Farag A. M. Catalytic activity of some oxime-based Pd (II)-complexes in Suzuki coupling of aryl and heteroaryl bromides in water. Arab. J. Chem. 2017, 10, 473–479. 10.1016/j.arabjc.2013.06.004. [DOI] [Google Scholar]
- Dawood K. M.; El-Deftar M. M. Microwave-assisted CC cross-coupling reactions of aryl and heteroaryl halides in water. Arkivoc 2010a, 2010, 319–330. 10.3998/ark.5550190.0011.930. [DOI] [Google Scholar]
- Dawood K. M.; El-Deftar M. M. Microwave-assisted synthesis of 2-substituted 4-biarylyl-1, 3-thiazoles by carbon-carbon cross-coupling in water. Synthesis 2010, 2010, 1030–1038. 10.1055/s-0029-1218662. [DOI] [Google Scholar]
- Dawood K. M.; Kirschning A. Combining enabling techniques in organic synthesis: solid-phase-assisted catalysis under microwave conditions using a stable Pd (II)-precatalyst. Tetrahedron 2005, 61, 12121–12130. 10.1016/j.tet.2005.07.113. [DOI] [Google Scholar]
- Dawood K. M.; Solodenko W.; Kirschning A. Microwave-accelerated Mizoroki-Heck and Sonogashira cross-coupling reactions in water using a heterogeneous palladium (II)-precatalyst. Arkivoc 2007, 2007, 104–124. [Google Scholar]
- Dawood K. M.; Elamin M. B.; Faraga A. M. Microwave-assisted synthesis of 2-acetyl-5-arylthiophenes and 4-(5-arylthiophen-2-yl) thiazoles via Suzuki coupling in water. Arkivoc 2015, 2015, 50–62. 10.3998/ark.5550190.p009.018. [DOI] [Google Scholar]
- Dawood K. M.; Farag A. M.; El-Deftar M. M.; Gardiner M.; Abdelaziz H. A. Microwave-assisted synthesis of 5-arylbenzofuran-2-carboxylates via Suzuki coupling using a 2-quinolinealdoxime-Pd (II)-complex. Arkivoc 2013, 2013, 210–226. 10.3998/ark.5550190.0014.317. [DOI] [Google Scholar]
- Dawood K. M.; Fayed M. S.; Elkhalea M. M. Heck and Suzuki cross-couplings of aryl and heteroaryl bromides in water using a new palladium (II)-complex. Arkivoc 2009, 2009, 324–341. 10.3998/ark.5550190.0010.d28. [DOI] [Google Scholar]
- Shaaban M. R.; Darweesh A. F.; Dawood K. M.; Farag A. Mizoroki–Heck cross-couplings of 2-acetyl-5-bromobenzofuran and aryl halides under microwave irradiation. Arkivoc 2010, 2010, 208–225. 10.3998/ark.5550190.0011.a18. [DOI] [Google Scholar]
- Cárdenas G.; Díaz J.; Meléndrez M.; Cruzat C. Physicochemical properties of edible films from chitosan composites obtained by microwave heating. Polym. Bull. 2008, 61, 737–748. 10.1007/s00289-008-0994-7. [DOI] [Google Scholar]
- Sutirman Z. A.; Sanagi M. M.; Abu Naim A.; Abd Karim K. J.; Wan Ibrahim W. A. Ammonium persulfate-initiated graft copolymerization of methacrylamide onto chitosan: synthesis, characterization and optimization. Sains Malays. 2017, 46, 2433–2440. 10.17576/jsm-2017-4612-19. [DOI] [Google Scholar]
- Demetgül C.; Karakaplan M.; Serin S. Synthesis, characterization and thermal properties of oligo-N, N′-bis (2, 4-dihydroxybenzylidene) ethylenediamine and its cobalt (II) and manganese (II) complexes. Des. Monomers Polym. 2008, 11, 565–579. 10.1163/156855508X363852. [DOI] [Google Scholar]
- Kwaskowska-Chęć E.; Kubiak M.; Głowiak T.; Ziółkowski J. J. Synthesis, crystal and molecular structure of the novel complex [dinitrato {N, N-bis (1–methylbenzimidazol-2–ylmethyl) methylamine}] cobalt (II). Transition Met. Chem. 1998, 23, 641–644. 10.1023/A:1006909528805. [DOI] [Google Scholar]
- Cotugno P.; Casiello M.; Nacci A.; Mastrorilli P.; Dell’Anna M. M.; Monopoli A. Suzuki coupling of iodo and bromoarenes catalyzed by chitosan-supported Pd-nanoparticles in ionic liquids. J. Organomet. Chem. 2014, 752, 1–5. 10.1016/j.jorganchem.2013.11.033. [DOI] [Google Scholar]
- Baran T.; Menteş A. Cu (II) and Pd (II) complexes of water soluble O-carboxymethyl chitosan Schiff bases: Synthesis, characterization. Int. J. Biol. Macromol. 2015, 79, 542–554. 10.1016/j.ijbiomac.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Baran T.; Menteş A.; Arslan H. Synthesis and characterization of water soluble O-carboxymethyl chitosan Schiff bases and Cu (II) complexes. Int. J. Biol. Macromol. 2015, 72, 94–103. 10.1016/j.ijbiomac.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Tirkistani F. A. Thermal analysis of some chitosan Schiff bases. Polym. Degrad. Stab. 1998, 60, 67–70. 10.1016/s0141-3910(97)00020-7. [DOI] [Google Scholar]
- Zong Z.; Kimura Y.; Takahashi M.; Yamane H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. 10.1016/s0032-3861(99)00270-0. [DOI] [Google Scholar]
- Anan N. A.; Hassan S. M.; Saad E. M.; Butler I. S.; Mostafa S. I. Preparation, characterization and pH-metric measurements of 4-hydroxysalicylidenechitosan schiff-base complexes of Fe (III), Co (II), Ni (II), Cu (II), Zn (II), Ru (III), Rh (III), Pd (II) and Au (III). Carbohydr. Res. 2011, 346, 775–793. 10.1016/j.carres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Li-xia W.; Zi-wei W.; Guo-song W.; Xiao-dong L.; Jian-guo R. Catalytic performance of chitosan-Schiff base supported Pd/Co bimetallic catalyst for acrylamide with phenyl halide. Polym. Adv. Tech. 2010, 21, 244–249. 10.1002/pat.1420. [DOI] [Google Scholar]
- Makhubela B. C.; Jardine A.; Smith G. S. Pd nanosized particles supported on chitosan and 6-deoxy-6-amino chitosan as recyclable catalysts for Suzuki–Miyaura and Heck cross-coupling reactions. Appl. Catal., A 2011, 393, 231–241. 10.1016/j.apcata.2010.12.002. [DOI] [Google Scholar]
- Zhang Y.; Xue C.; Xue Y.; Gao R.; Zhang X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. 10.1016/j.carres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Barbosa H. F. G.; Attjioui M.; Ferreira A. P. G.; Dockal E. R.; El Gueddari N. E.; Moerschbacher B. M.; Cavalheiro É.T. G. Synthesis, characterization and biological activities of biopolymeric schiff bases prepared with chitosan and salicylaldehydes and their Pd (II) and Pt (II) complexes. Molecules 2017, 22, 1987. 10.3390/molecules22111987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony R.; Theodore David Manickam S. T. D.; Saravanan K.; Karuppasamy K.; Balakumar S. Synthesis, spectroscopic and catalytic studies of Cu (II), Co (II) and Ni (II) complexes immobilized on Schiff base modified chitosan. J. Mol. Struct. 2013, 1050, 53–60. 10.1016/j.molstruc.2013.07.006. [DOI] [Google Scholar]
- Huang G.; Cai C.-C.; Luo J.; Zhou H.; Guo Y.-A.; Liu S.-Y. Highly selective oxidation of toluene using air over [Fe (III) TPP] Cl supported on chitosan. Can. J. Chem. 2008, 86, 199–204. 10.1139/v08-002. [DOI] [Google Scholar]
- Xu X.; Liu P.; Li S.-h.; Zhang P.; Wang X.-y. Chitosan-supported imine palladacycle complex and its catalytic performance for Heck reaction. React. Kinet. Catal. Lett. 2006, 88, 217–223. 10.1007/s11144-006-0055-x. [DOI] [Google Scholar]
- The Crystallographic Data for compounds 6b (ref. CCDC 2035844), 6c (ref. CCDC 2035841), 8a (ref. CCDC 2153120), and 8c (ref. CCDC 2153130) can be obtained on request from the Director; Cambridge Crystallographic Data Center: 12 Union Road, Cambridge CB2 1EW, UK.
- Jamwal N.; Sodhi R. K.; Gupta P.; Paul S. Nano Pd(0) supported on cellulose: A highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int. J. Biol. Macromol. 2011, 49, 930–935. 10.1016/j.ijbiomac.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Baran T.; Menteş A. Microwave assisted synthesis of biarlys by CC coupling reactions with a new chitosan supported Pd (II) catalyst. J. Mol. Struct. 2016, 1122, 111–116. 10.1016/j.molstruc.2016.05.091. [DOI] [Google Scholar]
- Baran T.; Nasrollahzadeh M. Cyanation of aryl halides and Suzuki- Miyaura coupling reaction using palladium nanoparticles anchored on developed biodegradable microbeads. Int. J. Biol. Macromol. 2020, 148, 565–573. 10.1016/j.ijbiomac.2020.01.157. [DOI] [PubMed] [Google Scholar]
- Baran T.; Sargin I. Green synthesis of a palladium nanocatalyst anchored on magnetic lignin-chitosan beads for synthesis of biaryls and aryl halide cyanation. Int. J. Biol. Macromol. 2020, 155, 814–822. 10.1016/j.ijbiomac.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Baran T. Highly recoverable, reusable, cost-effective, and Schiff base functionalized pectin supported Pd (II) catalyst for microwave-accelerated Suzuki cross-coupling reactions. Int. J. Biol. Macromol. 2019, 127, 232–239. 10.1016/j.ijbiomac.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Baran T.; Sargin I.; Kaya M.; Menteş A. An environmental catalyst derived from biological waste materials for green synthesis of biaryls via Suzuki coupling reactions. J. Mol. Catal. A: Chem. 2016, 420, 216–221. 10.1016/j.molcata.2016.04.025. [DOI] [Google Scholar]
- Baran T.; Yılmaz Baran N. Y.; Menteş A. Highly active and recyclable heterogeneous palladium catalyst derived from guar gum for fabrication of biaryl compounds. Int. J. Biol. Macromol. 2019, 132, 1147–1154. 10.1016/j.ijbiomac.2019.04.042. [DOI] [PubMed] [Google Scholar]
- Primo A.; Liebel M.; Quignard F. Palladium coordination biopolymer: A versatile access to highly porous dispersed catalyst for suzuki reaction. Chem. Mater. 2009, 21, 621–627. 10.1021/cm8020337. [DOI] [Google Scholar]
- Baruah D.; Das R. N.; Hazarika S.; Konwar D. Biogenic synthesis of cellulose supported Pd (0) nanoparticles using hearth wood extract of Artocarpus lakoocha Roxb—A green, efficient and versatile catalyst for Suzuki and Heck coupling in water under microwave heating. Catal. Commun. 2015, 72, 73–80. 10.1016/j.catcom.2015.09.011. [DOI] [Google Scholar]
- Ma H.-c.; Cao W.; Bao Z.-k.; Lei Z.-Q. Biopolymer–metal complex wool–Pd as a highly active catalyst for Suzuki reaction in water. Catal. Sci. Tech. 2012, 2, 2291–2296. 10.1039/c2cy20126g. [DOI] [Google Scholar]
- Leonhardt S. E.; Stolle A.; Ondruschka B.; Cravotto G.; Leo C.; Jandt K. D.; Keller T. F. Chitosan as a support for heterogeneous Pd catalysts in liquid phase catalysis. Appl. Catal. A 2010, 379, 30–37. 10.1016/j.apcata.2010.02.029. [DOI] [Google Scholar]
- Shimizu K.-i.; Kan-no T.; Kodama T.; Hagiwara H.; Kitayama Y. Suzuki cross-coupling reaction catalyzed by palladium-supported sepiolite. Tetrahedron Lett. 2002, 43, 5653–5655. 10.1016/s0040-4039(02)01132-2. [DOI] [Google Scholar]
- Botella L.; Nájera C. Cross-coupling reactions with boronic acids in water catalysed by oxime-derived palladacycles. J. Organomet. Chem. 2002, 663, 46–57. 10.1016/s0022-328x(02)01727-8. [DOI] [Google Scholar]
- Diallo A. K.; Ornelas C.; Salmon L.; Ruiz Aranzaes J.; Astruc D. Homeopathic” Catalytic Activity and Atom-Leaching Mechanism in Miyaura–Suzuki Reactions under Ambient Conditions with Precise Dendrimer-Stabilized Pd Nanoparticles. Angew. Chem., Int. Ed. 2007, 46, 8644–8648. 10.1002/anie.200703067. [DOI] [PubMed] [Google Scholar]
- Luo S.; Zhang X.; Zeng Z.; Wei D.; Zhao T.; Kang W.; Zhang M.; Yan M. Desulfitative Suzuki cross-couplin gs of arylsulfonyl chlorides and boronic acids catalyzed by a recyclable polymer-supported N-heterocyclic carbene-palladium complex catalyst. Synlett 2006, 1891–1894. 10.1055/s-2006-948163. [DOI] [Google Scholar]
- Razler T. M.; Hsiao Y.; Qian F.; Fu R.; Khan R. K.; Doubleday W. A Preparatively Convenient Ligand-Free Catalytic PEG 2000 Suzuki–Miyaura Coupling. J. Org. Chem. 2009, 74, 1381–1384. 10.1021/jo802277z. [DOI] [PubMed] [Google Scholar]
- Sato T. Estrogenic Biphenyls. VIII. Preparation and Estrogenic Activity of 4′-(1-Hydroxyalkyl)-4-methoxybiphenyls. Bull. Chem. Soc. Jpn. 1960, 33, 5–7. 10.1246/bcsj.33.5. [DOI] [Google Scholar]
- Rao M. L.; Banerjee D.; Dhanorkar R. J. Pd-catalyzed coupling of aryl iodides with triarylbismuths as atom-economic multi-coupling organometallic nucleophiles under mild conditions. Tetrahedron Lett. 2010, 51, 6101–6104. 10.1016/j.tetlet.2010.09.053. [DOI] [Google Scholar]
- Molander G. A.; Petrillo D. E.; Landzberg N. R.; Rohanna J. C.; Biolatto B. Palladium-catalyzed Suzuki-Miyaura reactions of potassium aryl-and heteroaryltrifluoroborates with aryl-and heteroaryl triflates. Synlett 2005, 1763–1766. 10.1055/s-2005-871544. [DOI] [Google Scholar]
- Silva T.; Bravo J.; Summavielle T.; Remião F.; Pérez C.; Gil C.; Martínez A.; Borges F. Biology-oriented development of novel lipophilic antioxidants with neuroprotective activity. RSC Adv. 2015, 5, 15800–15811. 10.1039/c4ra15164j. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.