Abstract
Xanthoxyline (1), a small natural methyl ketone, was previously reported as a plant growth inhibitor. In this research, related methyl ketones bearing electron-donating and electron-withdrawing groups, together with heteroaromatics, were investigated against seed germination and seedling growth of Chinese amaranth (Amaranthus tricolor L.) and barnyard grass [Echinochloa crus-galli (L.) Beauv]. The structure–activity relationships (SARs) of methyl ketone herbicides were clarified, and which types and positions of substituents were crucially important for activity were also clarified. Indole derivatives, namely, 3-acetylindole (43) and 3-acetyl-7-azaindole (44) were found to be the most active methyl ketones that highly suppressed plant growth at low concentrations. The molecular docking on the 4-hydroxyphenylpyruvate dioxygenase (HPPD) enzyme indicated that carbonyl, aromatic, and azaindole were key interactions of HPPD inhibitors. This finding would be useful for the development of small ketone herbicides.
1. Introduction
Small molecules, or low-molecular-weight organic compounds, have been widely used in various fields and industries.1 Most patented drugs and pesticides are small molecules, and sometimes these compounds exhibit a wide range of biological functions.2 Generally, small-molecule bioactivities depend largely on their chemical structures and functional groups presented.3 Because of their small size, these compounds are designed to engage biological targets through various mechanisms of action.4
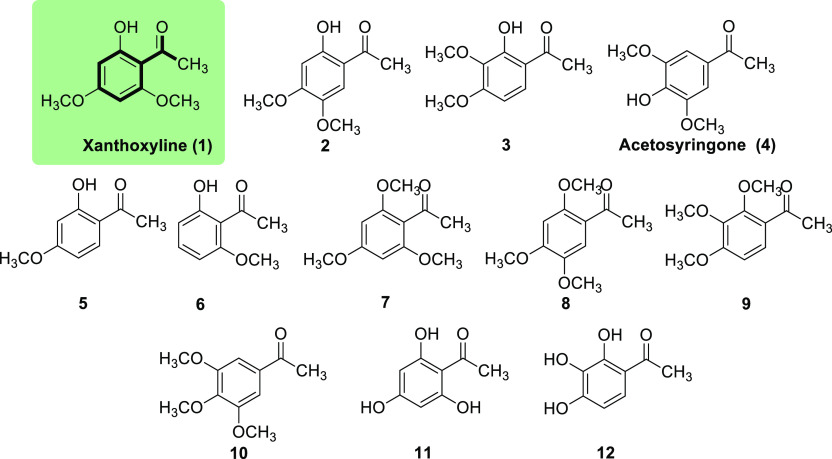
In agriculture, small molecules have been applied to several pesticide products, for example, fungicides, herbicides, insecticides, and so forth. Regarding herbicides, our research group is also interested in utilizing small molecules as plant growth inhibitors. We successfully isolated a methyl ketone herbicide, xanthoxyline (1) (Figure 1), from the dried fruits of a traditional herb, Zanthoxylum limonella Alston.5 Furthermore, we converted xanthoxyline (1) (and other related methyl ketones) to chalcones (an α,β-unsaturated ketone) and tested their herbicidal activity.6 We found that the structures or substituents of the chalcones had a crucial role in controlling plant growth. Apart from these ketones, we have also investigated the inhibitory effects of numerous aldehydes and α-amino acids on plant growth.7,8 Clearly, many tested compounds exhibited great weed control properties, and the herbicidal activity relied very much on the chemical structures, absolute configurations, and applied concentrations of the molecules.
Figure 1.
Chemical structures of xanthoxyline (1) and its derivatives.
In fact, several studies have found that ketones are a good herbicide.9−12 Triketones,13−17 in particular, have been extensively investigated and strongly inhibit the germination and growth of weeds. Many of these compounds are commercially produced for use as herbicides, and their well-known mechanism of action is to inhibit 4-hydroxyphenylpyruvate dioxygenase (HPPD). Diketones were also reported as plant growth inhibitors.18−21 Even though their modes of action are likely to be complex, some diketones, such as benzobicyclon, are also HPPD inhibitors.22 Monoketones, or compounds containing one ketonic carbonyl, are reported to exhibit weed-killing properties.23−33 In addition, the study of their mechanisms of action varies according to their main structure, for example, ACCase inhibitor (cyclohexenones),33 Hill reaction inhibitor (flavones),30 HPPD inhibitor (pyrazoles and isoxaflutoles),23,24,29,31,32 mitotic disrupter (sindone B),25 PEPCase inhibitor (chalcones),26,28 and PSII electron transport inhibitor (2,4-quinolinediones).27 Although their herbicidal activities have been widely investigated, the comprehensive study of some types of monoketones remains limited. Methyl ketones, such as xanthoxyline (1), are a good example. As mentioned earlier, we have found that xanthoxyline (1) inhibits tested plants.5,34 However, the weed control ability of other related methyl ketones has been barely investigated.19 To the best of our knowledge, so far, only a few methyl ketones, such as usnic acid,35 have a reported mode of mechanism, which is also an HPPD inhibitor. We hereby assume that understanding the growth-regulating properties and the mode of action of methyl ketones would be necessary for the development of small-molecule herbicides.
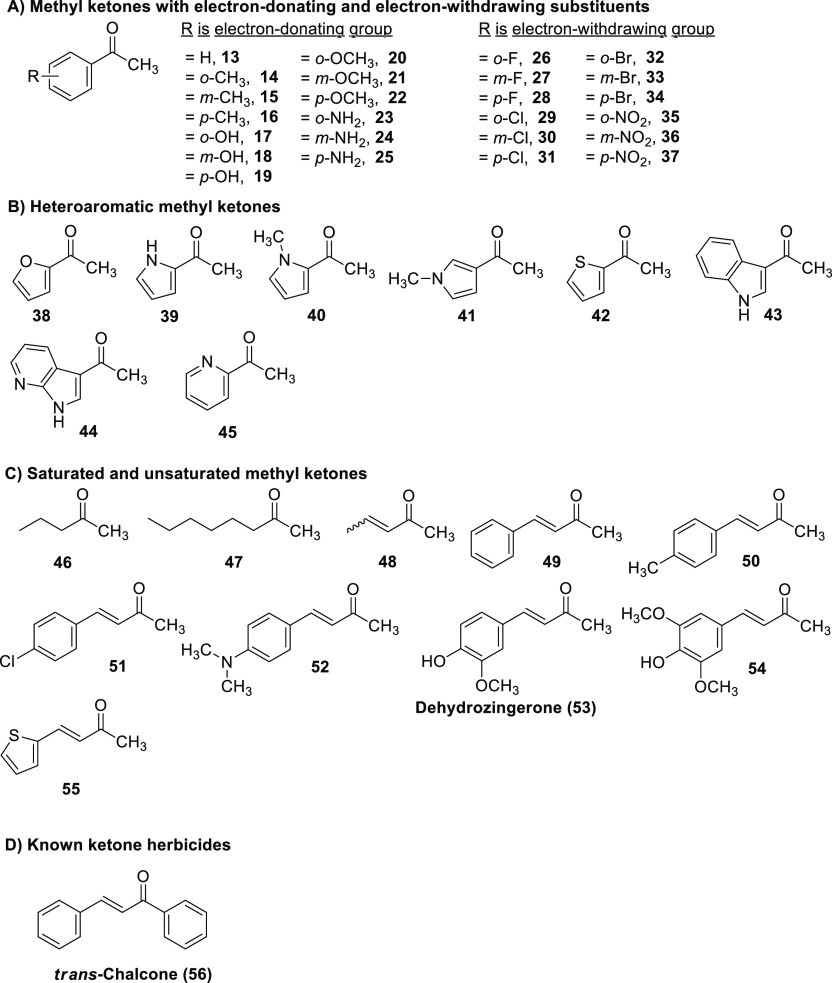
In this research, we investigated the structure–activity relationship (SAR) of xanthoxyline (1) and small methyl ketone herbicides (Figures 1 and 2) using Chinese amaranth (Amaranthus tricolor L.) and barnyard grass (Echinochloa crus-galli (L.) Beauv.) as representative dicot and monocot species. Some active compounds were also subjected to a molecular docking simulation to determine their enzymatic targets in plants. The enzyme receptor, HPPD, was used as it is crucially important for normal growth of plants.36 The effects of substituents on activity were also discussed.
Figure 2.
Chemical structures of other small ketones used in this study.
2. Experimental Section
2.1. Chemicals and Instrument
Sinon Corporation (Bangkok, Thailand) supplied commercial butachlor (60% w/v). Xanthoxyline (1) was isolated from dried fruits of Z. limonella, as previously described.5 Tween 80, glyphosate, mesotrione, and ketones 2–3, 5–10, 12, 17, 22, 24, 26–27, 30, 37–38, 40–41, 44–41, and 55–56 were purchased from Sigma-Aldrich (Singapore). Ketones 4, 11, 13–16, 18–21, 25, 28–29, 31–36, 39, and 43 were purchased from Across (Belgium). However, ketones 23 and 42 were available from Tokyo Chemical Industry (Japan). Infra-red (IR) spectra were recorded on a Perkin Elmer 8900 at the Department of Chemistry, School of Science, KMITL. 1H and 13C NMR spectra were recorded on a JEOL JNM-ECZ-500R/S1 (500 MHz) at the Scientific Instruments Center, School of Science, KMITL, using residual protonated chloroform (CDCl3, 7.26 ppm for 1H NMR and 77.00 ppm for 13C NMR) as an internal standard. Melting points were recorded on a Gallenkamp melting point apparatus. High-resolution mass spectra (HRMS) were recorded on a Bruker Daltonics (micrOTOF) at the Faculty of Science, Mahidol University.
2.2. Synthesis of Unsaturated Methyl Ketones
Unsaturated methyl ketones (52–54) were synthesized by the aldol condensation reaction between acetone and commercial aldehydes, according to the general procedure shown below.
2.2.1. General Procedure
To a solution of aromatic aldehydes (1.0 mmol) in acetone (4 mL), potassium hydroxide (168 mg, 3.0 mmol) was slowly added. The mixture was stirred at room temperature until the aldehyde was consumed (monitored by TLC). The mixture was then acidified with 1 N HCl (aq.) and left to precipitate. The precipitated compound was filtrated and recrystallized from methanol to afford the unsaturated methyl ketone product.
2.3. Preparation of an Aqueous Solution of Butachlor at 400 μM
Similar to the previous work,6 40 μmol of butachlor was thoroughly mixed with distilled water in a 100 mL volumetric flask to produce a 400 μM solution of butachlor. This solution was used as a positive control experiment.
2.4. 0.10% (v/v) Aqueous Solution of Tween 80
Following our previous procedure,6 1.0 mL of Tween 80 was dissolved in distilled water in a 1000 mL volumetric flask to produce a stock solution of 0.10% (v/v) Tween 80. This solution was used as a negative control and also for the dilution step.
2.5. Aqueous Solutions of Methyl Ketones, Glyphosate, and Mesotrione at 400 μM
Following our previous work,6 40 μmol of pure methyl ketone and 0.1 mL of Tween 80 were homogeneously mixed in a 50 mL beaker, followed by adding 40 mL of distilled water. This solution was then transferred to a 100 mL volumetric flask. The volume of the solution was adjusted by adding distilled water to produce a 400 μM solution of methyl ketone containing 0.10% (v/v) Tween 80. A similar procedure was used for the 400 μM solution of glyphosate and mesotrione. Both solutions were used as positive control experiments.
2.6. Aqueous Solutions of Methyl Ketones at 13, 25, 50, 100, 200, 400, 800, and 1600 μM
Stock solutions of active ketones at 1600 μM were prepared using the same procedure as the 400 μM solution. The stock solution was then diluted to 800, 400, 200, 100, 50, 25, and 13 μM by mixing with the 0.10% (v/v) solution of Tween 80.
2.7. Tested Plants
Thai Seed & Agriculture Co. Ltd. (Bangkok, Thailand) sold seeds of Chinese amaranth (A. tricolor L), and seeds of barnyard grass [E. crus-galli (L.) Beauv.] were collected annually from paddy fields in Phitsanulok Province (16°48′ 57″N 100°15′49″E, Thailand). Both plants showed >80% germination.
2.8. Seed Germination and Seedling Growth Bioassay6
Similar to our previous report6 for the vial test, a 0.5 mL of aqueous solution of small methyl ketones was added to a small vial (45 mm × 20 mm) lined with the germination paper. Each treatment was replicated four times. 10 seeds of the tested plant were placed on the paper. The vials were sealed with Parafilm and kept in a growth chamber (cool white 840 Climacell 707, Munich, Germany) for 7 days. The growth conditions were at 28–30 °C with a photoperiod of 12 h (light intensity of 100 μmol m–2·s–1), and 80% relative humidity. After the specified time, germinated seeds were counted, and the lengths of shoot and root were measured. Percent inhibition of seed germination and seedling growth were calculated using the following equation
2.9. Molecular Docking
The molecular docking approach is known as one of the powerful tools used to predict the orientation of ligands in the binding pocket of a protein. Herein, the suitable conformation of herbicidal inhibitors was investigated at the binding site of the HPPD enzyme from the plant Arabidopsis thaliana (plant HPPD). For molecular docking studies, the 3D crystal structures of the HPPD enzyme with co-crystallized inhibitors were taken from the protein data bank [PDB: 1TFZ(https://www.rcsb.org/structure/1TFZ)] and hydrogen atoms were added to the protein and all water molecules were removed using the Discovery Studio Visualizer 2017 program.37 All the 3D structures of herbicidal inhibitors were created and optimized in the gas phase using DFT calculations at the M062X/6-31G(d) level of theory in the Gaussian09 D.01 program.38 The binding mode of the herbicidal inhibitors in the binding pocket of plant HPPD was investigated using the GOLD molecular docking program,39 which works based on the empirical free-energy function and applies the standard protocol of the Genetic Algorithm (GA). All default parameters were computed for docking processes with a scoring function of 100 GA run.
3. Results and Discussion
3.1. Herbicidal Activity of Xanthoxyline (1) and Related Small Methyl Ketones on Chinese Amaranth
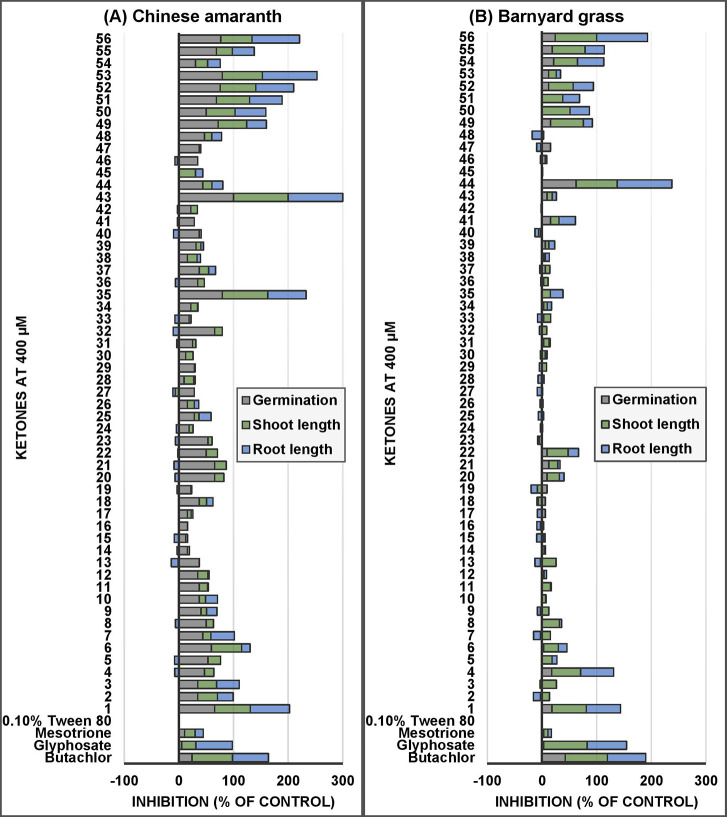
Initially, we compared the effects of xanthoxyline (1) and its derivatives (2–12), at a concentration of 400 μM, on Chinese amaranth (Figure 3A) to see whether altering the type and position of substituents improved the inhibitory activity. In order to know the level of herbicidal activity of these small ketones, a commercial pre-emergence herbicide (butachlor),6,40 a commercial post-emergence herbicide (glyphosate),41,42 a commercial HPPD inhibitor (mesotrione),15,16 and a common unsaturated ketone herbicide [trans-chalcone (56)]28 were used as positive control experiments. Among this group, xanthoxyline (1) was retained the most active compound, and a similar effect was observed with compound 6. The derivatives containing both hydroxyl and methoxy groups (2–5), and with only methoxy group (7–10), showed comparable activity. However, derivatives containing only hydroxyl group (11 and 12) had little effect on plant growth. This result indicated that both hydroxyl–methoxy and methoxy alone were crucial for activity but not hydroxyl alone.
Figure 3.
Inhibitory effects of xanthoxyline (1) and related small methyl ketones on seed germination, shoot and root growth of Chinese amaranth (A), and barnyard grass (B). An aqueous solution of Tween 80 was used as a negative reference, and aqueous solutions of butachlor, glyphosate, mesotrione, and trans-chalcone (56) were used as positive references.
For acetophenone (13) and its monosubstituted derivatives (26–37), most compounds showed weak activity. Among compounds with those electron-donating groups, compounds with methoxy (20–22) were apparently more active than those with methyl (13–15) or hydroxyl (17–19). Herbicidal properties were also affected by the substituent position, with a compound containing hydroxyl at meta position (18) having a greater effect than compounds containing hydroxyl at ortho (17) and para (19). However, for the amino group (23–25), the opposite effect was observed. In terms of ketones bearing electron-withdrawing groups (26–37), o-nitroacetophenone (35) was the most active substance. For most derivatives, the substituents in the ortho position showed stronger activity than those in the meta and para positions.
Heteroaryl methyl ketones (38–45) were also studied, and almost all compounds had low herbicidal activity against Chinese amaranth. Fortunately, however, 3-acetylindole (43) completely inhibited seed germination of the plant. For saturated and unsaturated methyl ketones (46–55), it was revealed that α,β-unsaturated compounds (48–55) were slightly more potent than saturated ketones (46–47), and their herbicidal effects were similar to those of monoketone herbicide (56).6,43,44 Moreover, among unsaturated derivatives, compounds with aromatics (49–53 and 55) were more damaging than those with aliphatic (48), and overall, for this group, dehydrozingerone (53) had the best activity. Overall, some active ketones (1, 35, 52, and 53) were more active than the reference herbicides [butachlor, glyphosate, mesotrione, and trans-chalcone (56)]. The effects of concentrations and molecular docking studies of the active ketones were discussed further in the next section.
3.2. Herbicidal Activity of Xanthoxyline (1) and Related Methyl Ketones on Barnyard Grass
The herbicidal effects of methyl ketones on barnyard grass, a representative monocot plant, were studied at a concentration of 400 μM (Figure 3B). Among xanthoxyline (1) and its derivatives (2–12), xanthoxyline (1) remained the most potent, followed by acetosyringone (4). For acetophenone (13) and its derivatives (14–37), the compounds with methoxy (20–22) slightly suppressed shoot and root elongation of the plant, while other ketones had no effects.
In terms of heteroaromatic ketones (38–45), almost all compounds showed no activity. However, 3-acetyl-7-azaindole (44) highly inhibited the plant. Although its level of weed suppression was quite similar to that of butachlor, it was more potent than glyphosate, mesotrione, and trans-chalcone (56). Furthermore, saturated (46–47) and unsaturated methyl ketones (48–55) were compared and found that most unsaturated compounds were active, while the saturated ketones were unreactive, and the effects of these ketones were lower than those of the unsaturated ketone herbicide (56). The effects of the concentration and the molecular docking study of some active compounds were further investigated in the following sections.
3.3. Herbicidal Activity of the Most Active Methyl Ketones on Chinese Amaranth and Barnyard Grass
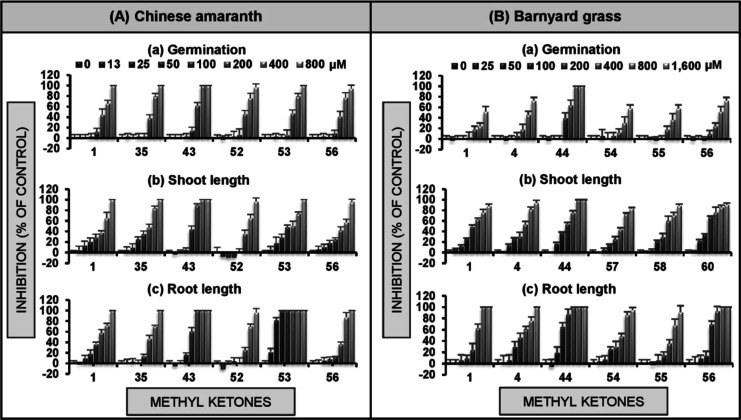
To understand the effect of the applied concentration, the five most active ketones (1, 35, 43, and 52–53) were further investigated at concentrations of 13–800 μM (Figure 4A). A known monoketone herbicide (56) was used to compare the levels of activity, and the solution of Tween 80 was again used as a negative control (0 μM). Obviously, compound 43 affected seed germination and shoot growth of Chinese amaranth the most. At the concentration range of 400–800 μM, the germination of the plant was completely inhibited by ketone 43. The effects on the root revealed that dehydrozingerone 53 highly suppressed root elongation. At a concentration of 50 μM, this ketone completely inhibited the root growth of barnyard grass. Overall, most of the active ketones (Figure 4A) were more potent than the monoketone herbicide (56) and their herbicidal properties relied on the applied concentrations.
Figure 4.
Inhibitory effects of the most effective methyl ketones on seed germination (a), shoot (b), and root (c) growth of Chinese amaranth (A) and barnyard grass (B). Aqueous solutions of Tween 80 were used as a negative reference (0 μM).
The dose–response of six small ketones (1, 4, 44, and 54–56) on barnyard grass was also investigated at concentrations of 25–1600 μM (Figure 4B). Apparently, compound 44 strongly inhibited seed germination and seedling growth of the plant. At a concentration range of 800–1600 μM, ketone 44 completely inhibited grass germination. For shoot growth, ketone 44 had similar activity to known ketone 56. However, in terms of root growth, this ketone showed greater herbicidal property. At a concentration of 400 μM, ketone 44 completely inhibited the root growth of barnyard grass. Again, the inhibitory effect of all active ketones relies very much on the applied concentrations.
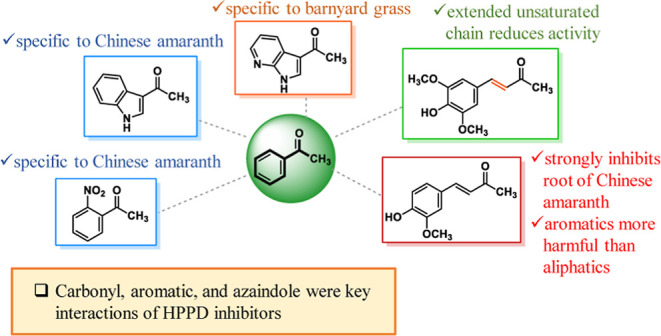
Results from vial assay indicated that the type and position of the substituents affected the herbicidal properties of methyl ketones. This is consistent with various studies showing the effects of different substituents45−47 and positions15,16,48 on activity. Among xantholyline (1) and its polysubstituted derivatives (2–12), xanthoxyline (1) and acetosyringone (4) were the most potent. Xantholyline (1) was an effective natural herbicide isolated by our research group,5,34 while acetosyringone (4) was reported previously as an active allelochemical in some plants.49−51 Even though we found that xantholyline (1) caused genetic changes in root meristematic cells of Allium cepa L. (onion), other modes of action of both compounds are still worth investigating.
In terms of acetophenone (13) and its derivatives (14–37), obviously, o-nitroacetophenone (35) was the only compound that exhibited remarkable activity against seed germination of Chinese amaranth. The mechanism of action of ketone 35 against tested plants is still unknown, but structurally similar compounds, such as mesotrione and analogues, have been widely investigated. The known mode of action of mesotrione and its derivatives is to inhibit HPPD.13,14,29
For heterocyclic ketones, we found that 3-acetylindole (43) strongly inhibited Chinese amaranth, but its closely related derivative, 3-acetyl-7-azaindole (44), was the most effective inhibitor against barnyard grass. The reason behind the specificity of both compounds is still unknown and requires further investigation. Generally, indole derivatives have a crucial role in plants. Indole-3-acetic acid (IAA), in particular, is the main auxin regulating growth and developmental processes in plants.52 However, for other roles, IAA and other indole derivatives, produced by some plant endophytes, have been reported to inhibit plant growth and development.53,54 Besides, our current finding suggests that tryptophan,8 an indole containing α-amino acid, could suppress plant growth, and its mode of action would be as a pigment synthesis inhibitor. Not only natural indoles but synthetic indoles are also effective against weeds. Several reports revealed that synthetic indoles were promising plant growth suppressors.55−57 The known mechanisms of action of these compounds include photosystem II (PSII) inhibitors and protoporphyrinogen oxidase (PPO) inhibitors.
Our findings here indicate that α,β-unsaturated ketones (48–55) were slightly more active than saturated ketones (46–47). Among these, dehydrozingerone (53) was the most potent substance. Although dehydrozingerone (53) has rarely been reported as a herbicide, other α,β unsaturated carbonyls, for example, cinnamic acid and derivatives,58,59 ferulic acid and derivatives,60−62 other unsaturated carboxylic acids,63,64 lactones,65−67 chalcones,6,26,28 and other unsaturated ketones,68 have been widely investigated as herbicides or algicides. Moreover, a similar trend was found in our previous study that unsaturated aldehydes, such as cinnamaldehyde and crotonaldehyde, were much more active to plants than saturated aldehydes.7 The known modes of action of chalcones and ferulic acids were phosphoenolpyruvate carboxylase (PEPCase) inhibitors and acetolactate synthase (ALS) inhibitors, respectively, while the mechanism of other ketones was barely studied.
Other factors affecting the herbicidal activity of ketones were applied concentrations and tested species. Methyl ketones were more effective at suppressing plants when used in higher concentrations, and Chinese amaranth was more affected by ketones than barnyard grass. This finding is similar to those reported that the herbicide is effective at high concentrations, but at low concentrations, it may promote plant growth.69
3.4. Case Study
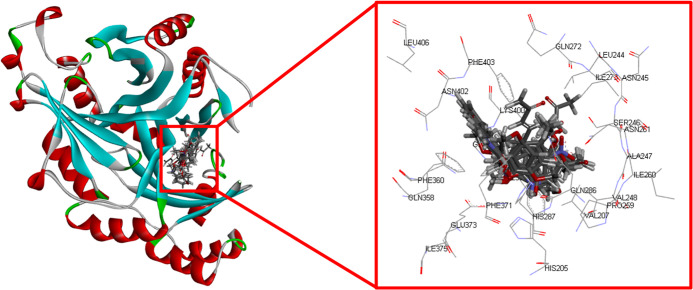
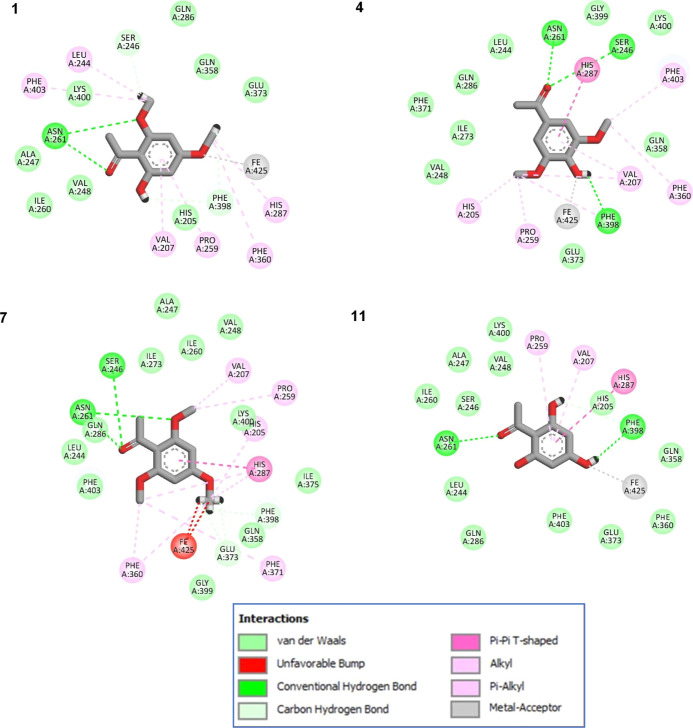
In this section, molecular docking was performed using GOLD molecular docking software39 in order to investigate how candidate inhibitors are bound to plant HPPD. Even though there are various possible mechanisms of action of herbicides, for example, ALS inhibitor, PEPCase inhibitor, PSII inhibitor, PPO inhibitor, and so forth, we are still interested in the HPPD enzyme receptor because it is crucially important for normal growth of plants.36 As can be seen in Table 1, the GOLD fitness scores (GoldScore) for selected inhibitors (compounds 1, 4, 7, 11, 43, 44, and 54) as bound to the binding pocket of plant HPPD were reported. All selected compounds exhibited a GoldScore in the range of 42.39–51.55 when bound to the pocket of the HPPD enzyme. As shown in Figure 5, the pose between selected ligands and plant HPPD revealed the interaction sites within the binding pocket, which comprised His205, Val207, Leu244, Asn245, Ser246, Ala247, Val248, Pro259, Ile260, Asn261, Gln272, Ile273, Gln286, His287, Gln358, Phe360, Phe371, Glu373, Ile375, Phe398, Gly399, Lys400, Asn402, Phe403, Leu406, and Fe425. From molecular docking analysis, it was noticed that the carbonyl moiety was a key molecular substructure of active compounds (Figure 6). By comparing xanthoxyline (1) and its derivatives (4, 7, and 11), it was clear that xanthoxyline (1) and acetosyringone (4) exhibited stronger herbicidal activity than the others. Both xanthoxyline (1) and acetosyringone (4) formed H-bonds with Asn261 and the metal acceptor with Fe425. Moreover, acetosyringone (4) also formed H-bonds with Phe398 and Ser246 as well as formed π–π interaction with His287, while the inactive ketone 7 showed unfavorable bump interactions with Fe425. For ketone 11, although it could form the same kinds of interactions as acetosyringone (4), its herbicidal properties were much weaker than that of acetosyringone (4). These results suggest that to increase the HPPD activity, the substituents should consist of both the hydroxyl group and the methoxy group but not hydroxyl or methoxy alone.
Table 1. GoldScore of the Candidate Ligand in Active Site of 4-Hydroxyphenylpyruvate Dioxygenase (HPPD) Enzyme vs Percent Inhibition of Barnyard Grass Seed Germination, Shoot Lengths, and Root Lengths.
| % inhibitiona |
||||
|---|---|---|---|---|
| compounds | GoldScore | germination | shoot lengths | root lengths |
| 1 | 48.70 | 18.18 ± 6.06 | 63.02 ± 1.46 | 62.68 ± 7.27 |
| 4 | 51.55 | 15.63 ± 6.25 | 42.77 ± 1.17 | 60.29 ± 5.63 |
| 7 | 49.04 | –3.13 ± 6.25 | 15.37 ± 3.27 | –12.26 ± 9.43 |
| 11 | 42.39 | 0 ± 10.21 | 15.41 ± 3.27 | 1.37 ± 6.12 |
| 43 | 42.48 | 9.38 ± 6.25 | 9.38 ± 7.25 | 7.62 ± 8.34 |
| 44 | 43.04 | 62.50 ± 10.21 | 75.10 ± 3.25 | 100 |
| 54 | 46.17 | 21.21 ± 6.25 | 53.75 ± 3.28 | 48.01 ± 5.31 |
Methyl ketones were tested at concentration of 400 μM.
Figure 5.
Docking poses analysis in the binding site between the candidate ligand and the HPPD enzyme (PDB ID: 1TFZ).
Figure 6.
Representative ligand–protein interactions for active (ketones 1,4) and inactive compounds (7,11) in the active site of the HPPD enzyme.
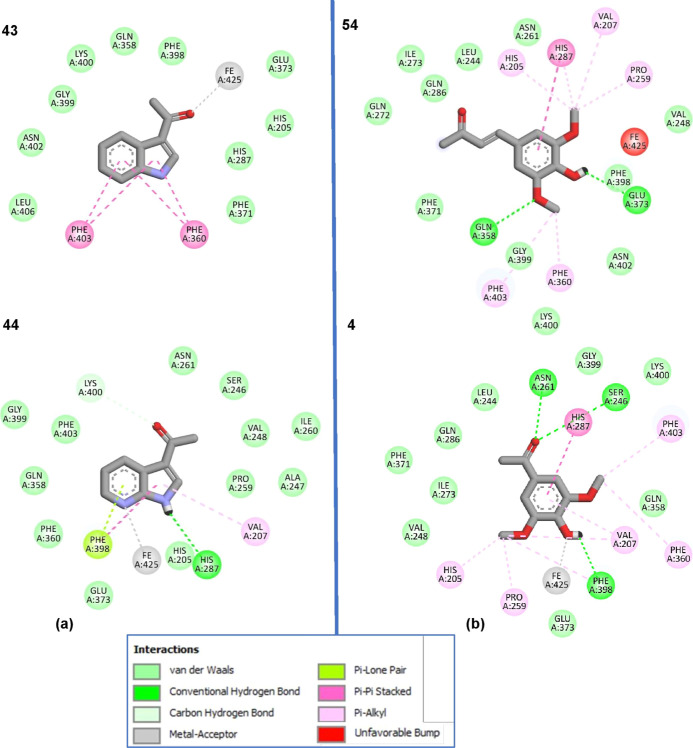
For 3-acetylindole (43) and 3-acetyl-7-azaindole (44), clearly, the pyridine moiety of ketone 44 formed the π–lone pair interaction with Phe398 and the metal acceptor interaction with Fe425. Furthermore, its pyrrole ring could interact with His287 via H-bonding as well as π–π interaction via Phe398, whereas compound 43 only exhibited π–π interactions (Figure 7). This result confirmed that the pyridine ring helped increase HPPD activity greater than benzene.
Figure 7.
Representative ligand–protein interactions for active (ketones 4 and 44) and inactive compounds (43 and 54) in the active site of the HPPD enzyme.
To explain why α,β-unsaturated ketone (54) showed weaker herbicidal activity than acetosyringone (4), molecular docking simulations of the two compounds were investigated (Figure 7). The result found that acetosyringone (4) had H-bond interactions with key amino acids (Asn261 and Ser264) and the metal acceptor (Fe), while ketone 54 poorly contacted Fe.
Altogether, the molecular docking results for plant HPPD indicated that carbonyl, aromatic, and azaindole groups were key interactions of HPPD inhibitors. Despite the fact that our methyl ketone derivatives’ binding mode differs from that of nitisinone, sulcotione, mesotrinoe,70 4-hydroxyphenylpyruvate acid (HPPA), quinotrione (Y13161),71 and hydroxyphenylacetate (HPA),72 which demonstrate metal atom interactions with the diketone group, whereas our methyl ketone derivatives demonstrate metal atom interactions with other functional groups. However, the mode of action of methyl ketone derivatives derived from molecular docking may provide guidance for designing more potent HPPD inhibitors prior to synthesis.
4. Conclusions
In summary, the SAR study of xanthoxyline (1) and related methyl ketone herbicides has been reported against Chinese amaranth and barnyard grass. The herbicidal properties of this chemical class depend largely on the type and position of substituents as well as their applied concentrations. Both tested species respond differently to the herbicides, and Chinese amaranth is relatively more susceptible to the herbicides than the grass. Xanthoxyline (1) and acetosyringone (4) are more active than other polysubstituted ketones. Among monosubstituted ketones, 2-nitroacetophenone (35) is the most potent compound, specifically, with Chinese amaranth. Overall, indole derivatives, namely, 3-acetylindole (43) and 3-acetyl-7-azaindole (44), show greater activity than other tested ketones, and their effects are species-dependent. Results from the molecular docking study indicated that carbonyl, aromatic, and azaindole were key interactions of HPPD inhibitors. This novel finding would be beneficial in the development of small-molecule pesticides.
Acknowledgments
This study was funded by the King Mongkut’s Institute of Technology Ladkrabang (grant number 2563-02-05–40). The GOLD license was supported by the Kasetsart University Research and Development Institute, KURDI (grant no. FF (KU) 11.64). Also, we would like to thank the Department of Chemistry, School of Science, KMITL for laboratory facilities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02704.
Percent yields, melting points, retardation factor (Rf), IR spectra, 1H NMR and 13C NMR spectra, and HRMS results of synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Robert A.; Benoit-Vical F.; Liu Y.; Meunier B. Small molecules: the past or the future in drug innovation?. Met Ions Life Sci 2019, 19, 17–48. 10.1515/9783110527872-002. [DOI] [PubMed] [Google Scholar]
- Makurvet F. D. Biologics vs. small molecules: drug costs and patient access. Med. Drug Discov. 2021, 9, 100075. 10.1016/j.medidd.2020.100075. [DOI] [Google Scholar]
- Harrold M.; Zavod R.. Chapter 2 Functional group characteristics and roles. In Basic Concepts in Medicinal Chemistry; American Society of Health-System Pharmacists: Maryland, United States, 2013, pp 15–50. [Google Scholar]
- A big future for small molecules: targeting the undruggable. 2022, (accessed 15 June 2022).https://www.astrazeneca.com/r-d/next-generation-therapeutics/small-molecule.html
- Charoenying P.; Teerarak M.; Laosinwattana C. An allelopathic substance isolated from Zanthoxylum limonella Alston fruit. Sci. Hortic. (Amsterdam, Neth.) 2010, 125, 411–416. 10.1016/j.scienta.2010.04.045. [DOI] [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Herbicidal activity of flavokawains and related trans-chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. 10.1021/acsomega.9b03144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Inhibitory effects of a variety of aldehydes on Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. Molecules 2018, 23, 471. 10.3390/molecules23020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Enantioselective and synergistic herbicidal activities of common amino acids against Amaranthus tricolor and Echinochloa crus-galli. Molecules 2021, 26, 2071. 10.3390/molecules26072071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens H.; Lange G.; Müller T.; Rosinger C.; Willms L.; van Almsick A. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: solutions for modern and sustainable agriculture. Angew. Chem., Int. Ed. 2013, 52, 9388–9398. 10.1002/anie.201302365. [DOI] [PubMed] [Google Scholar]
- Beaudegnies R.; Edmunds A. J. F.; Fraser T. E. M.; Hall R. G.; Hawkes T. R.; Mitchell G.; Schaetzer J.; Wendeborn S.; Wibley J. Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors–a review of the triketone chemistry story from a Syngenta perspective. Bioorg. Med. Chem. 2009, 17, 4134–4152. 10.1016/j.bmc.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Santucci A.; Bernardini G.; Braconi D.; Petricci E.; Manetti F. 4-Hydroxyphenylpyruvate dioxygenase and its inhibition in plants and animals: small molecules as herbicides and agents for the treatment of human inherited diseases. J. Med. Chem. 2017, 60, 4101–4125. 10.1021/acs.jmedchem.6b01395. [DOI] [PubMed] [Google Scholar]
- Ndikuryayo F.; Moosavi B.; Yang W.-C.; Yang G.-F. 4-Hydroxyphenylpyruvate dioxygenase inhibitors: from chemical biology to agrochemicals. J. Agric. Food Chem. 2017, 65, 8523–8537. 10.1021/acs.jafc.7b03851. [DOI] [PubMed] [Google Scholar]
- Dayan F. E.; Duke S. O.; Sauldubois A.; Singh N.; McCurdy C.; Cantrell C. p-Hydroxyphenylpyruvate dioxygenase is a herbicidal target site for β-triketones from. Leptospermum scopariumPhytochemistry 2007, 68, 2004–2014. 10.1016/j.phytochem.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Zhang S.-Q.; Liu Y.-X.; Wang J.-Y.; Gao S.; Zhao L.-X.; Ye F. Design, synthesis, SAR and molecular docking of novel green niacin-triketone HPPD inhibitor. Ind. Crops Prod. 2019, 137, 566–575. 10.1016/j.indcrop.2019.05.070. [DOI] [Google Scholar]
- Nan J.-X.; Yang J.-F.; Lin H.-Y.; Yan Y.-C.; Zhou S.-M.; Wei X.-F.; Chen Q.; Yang W.-C.; Qu R.-Y.; Yang G.-F. Synthesis and herbicidal activity of triketone-aminopyridines as potent p-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem. 2021, 69, 5734–5745. 10.1021/acs.jafc.0c07782. [DOI] [PubMed] [Google Scholar]
- Wang D. W.; Lin H. Y.; Cao R. J.; Ming Z. Z.; Chen T.; Hao G. F.; Yang W. C.; Yang G. F. Design, synthesis and herbicidal activity of novel quinazoline-2, 4-diones as 4-hydroxyphenylpyruvate dioxygenase inhibitors. Pest Manage. Sci. 2015, 71, 1122–1132. 10.1002/ps.3894. [DOI] [PubMed] [Google Scholar]
- Yamamoto S.; Tanetani Y.; Uchiyama C.; Nagamatsu A.; Kobayashi M.; Ikeda M.; Kawai K. Mechanism of action and selectivity of a novel herbicide, fenquinotrione. J. Pestic. Sci. 2021, 46, 249–257. 10.1584/jpestics.d21-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalinova A.; Dubovik V.; Chisty L.; Kochura D.; Ivanov A.; Smirnov S.; Petrova M.; Zolotarev A.; Evidente A.; Berestetskiy A. Stagonolides J and K and stagochromene A, two new natural substituted nonenolides and a new disubstituted chromene-4,5-dione Isolated from Stagonospora cirsii S-47 proposed for the biocontrol of Sonchus arvensis. J. Agric. Food Chem. 2019, 67, 13040–13050. 10.1021/acs.jafc.9b04573. [DOI] [PubMed] [Google Scholar]
- Macías F. A.; Varela R. M.; Torres A.; Oliva R. M.; Molinillo J. G. Bioactive norsesquiterpenes from Helianthus annuus with potential allelopathic activity. Phytochemistry 1998, 48, 631–636. 10.1016/s0031-9422(97)00995-3. [DOI] [Google Scholar]
- Potapovich M.; Eremin A.; Rubinov D.; Metelitza D. Effect of herbicide tralkoxydim and 2-acylcyclohexane-1, 3-diones on peroxidase activity. Appl. Biochem. Microbiol. 2008, 44, 19–27. 10.1134/s0003683808010031. [DOI] [PubMed] [Google Scholar]
- Yilimulati M.; Jin J.; Wang X.; Wang X.; Shevela D.; Wu B.; Wang K.; Zhou L.; Jia Y.; Pan B.; Govindjee G.; Zhang S. Regulation of photosynthesis in bloom-forming cyanobacteria with the simplest β-diketone. Environ. Sci. Technol. 2021, 55, 14173–14184. 10.1021/acs.est.1c04683. [DOI] [PubMed] [Google Scholar]
- Komatsubara K.-i.; Sekino K.; Yamada Y.; Koyanagi H.; Nakahara S. Discovery and development of a new herbicide, benzobicyclon. J. Pestic. Sci. 2009, 34, 113–114. 10.1584/jpestics.j09-01. [DOI] [Google Scholar]
- Aubert S.; Pallett K. E. Combined use of 13C-and 19F-NMR to analyse the mode of action and the metabolism of the herbicide isoxaflutole. Plant Physiol. Biochem. 2000, 38, 517–523. 10.1016/s0981-9428(00)00771-3. [DOI] [Google Scholar]
- Fu Q.; Cai P. P.; Cheng L.; Zhong L. K.; Tan C. X.; Shen Z. H.; Han L.; Xu T. M.; Liu X. H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manage. Sci. 2020, 76, 868–879. 10.1002/ps.5591. [DOI] [PubMed] [Google Scholar]
- Lehnen L. P. Jr; Vaughn K. C. The herbicide sindone B disrupts spindle microtubule organizing centers. Pestic. Biochem. Physiol. 1992, 44, 50–59. 10.1016/0048-3575(92)90008-n. [DOI] [Google Scholar]
- Liu X.; Chen Y.; Deng Y.; Xiao C.; Luan S.; Huang Q. Novel galactosyl moiety-conjugated furylchalcones synthesized facilely display significant regulatory effect on plant growth. J. Agric. Food Chem. 2022, 70, 1766–1775. 10.1021/acs.jafc.1c05240. [DOI] [PubMed] [Google Scholar]
- Liu Y.-X.; Zhao H.-P.; Wang Z.-W.; Li Y.-H.; Song H.-B.; Riches H.; Beattie D.; Gu Y.-C.; Wang Q.-M. The discovery of 3-(1-aminoethylidene) quinoline-2, 4 (1H, 3H)-dione derivatives as novel PSII electron transport inhibitors. Mol. Diversity 2013, 17, 701–710. 10.1007/s11030-013-9466-6. [DOI] [PubMed] [Google Scholar]
- Nguyen G.; Erlenkamp G.; Jäck O.; Küberl A.; Bott M.; Fiorani F.; Gohlke H.; Groth G. Chalcone-based selective inhibitors of a C4 plant key enzyme as novel potential herbicides. Sci. Rep. 2016, 6, 27333. 10.1038/srep27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallett K. E.; Cramp S. M.; Little J. P.; Veerasekaran P.; Crudace A. J.; Slater A. E. I. the background to its discovery and the basis of its herbicidal properties. Pest Manage. Sci. 2001, 57, 133–142. . [DOI] [PubMed] [Google Scholar]
- Rios M. Y.; Córdova-Albores L. C.; Ramírez-Cisneros M. Á.; King-Díaz B.; Lotina-Hennsen B.; León Rivera I.; Miranda-Sánchez D. Phytotoxic potential of Zanthoxylum affine and its major compound linarin as a possible natural herbicide. ACS Omega 2018, 3, 14779–14787. 10.1021/acsomega.8b02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.-M.; Huang H.; Shu L.; Liu J.-M.; Zhang J.-Q.; Yan Y.-L.; Zhang D.-Y. Synthesis and herbicidal activities of aryloxyacetic acid derivatives as HPPD inhibitors. Beilstein J. Org. Chem. 2020, 16, 233–247. 10.3762/bjoc.16.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.-L.; Lin H.-Y.; Ruan X.; Yang S.-G.; Hao G.-F.; Yang W.-C.; Yang G.-F. Synthesis and bioevaluation of pyrazole-benzimidazolone hybrids as novel human 4-hydroxyphenylpyruvate dioxygenase inhibitors. Eur. J. Med. Chem. 2015, 92, 427–438. 10.1016/j.ejmech.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Ye F.; Ma P.; Zhang Y.-Y.; Li P.; Yang F.; Fu Y. Herbicidal activity and molecular docking study of novel ACCase inhibitors. Front. Plant Sci. 2018, 9, 1850. 10.3389/fpls.2018.01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotsaeng N.; Laosinwattana C.; Charoenying P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. 10.3390/molecules22111841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagni J. G.; Meazza G.; Nanayakkara N. D.; Dayan F. E. The phytotoxic lichen metabolite, usnic acid, is a potent inhibitor of plant p-hydroxyphenylpyruvate dioxygenase. FEBS lett 2000, 480, 301–305. 10.1016/s0014-5793(00)01907-4. [DOI] [PubMed] [Google Scholar]
- Alam M.; Kim Y.; Park S. Synthesis, characterization (IR, 1H, 13C & 31P NMR), fungicidal, herbicidal and molecular docking evaluation of steroid phosphorus compounds. Open Chem. 2019, 17, 621–628. 10.1515/chem-2019-0069. [DOI] [Google Scholar]
- BIOVIA . Dassault Systèmes, Discovery Studio Visualizer 2017; Dassault Systèmes:FEBS Lett. San Diego, 2017. [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams L.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16; Rev. C.01: Wallingford, CT, 2016. [Google Scholar]
- Verdonk M. L.; Chessari G.; Cole J. C.; Hartshorn M. J.; Murray C. W.; Nissink J. W. M.; Taylor R. D.; Taylor R. Modeling water molecules in protein–ligand docking using GOLD. J. Med. Chem. 2005, 48, 6504–6515. 10.1021/jm050543p. [DOI] [PubMed] [Google Scholar]
- Ji Z.; Zhou F.; Wei S. Synthesis and herbicidal activities of benzothiazole N,O-acetals. Bioorg. Med. Chem. Lett. 2015, 25, 4065–4068. 10.1016/j.bmcl.2015.08.051. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen Y.; Xu S.; Wang J.; Dong H.; Zhao Z.; Jiang J. Design, synthesis, and herbicidal activity of sec-p-menthane-7-amine derivatives as botanical herbicides. RSC Adv. 2021, 11, 27207–27214. 10.1039/d1ra04910k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Xu S.; Jing W.; Zhao Z.; Jiang J. Synthesis and herbicidal activities of p-menth-3-en-1-amine and its schiff base derivatives. J. Agric. Food Chem. 2016, 64, 9702–9707. 10.1021/acs.jafc.6b03977. [DOI] [PubMed] [Google Scholar]
- Chen W. J.; Yun M. S.; Deng F.; Yogo Y. Effects of root-applied naringenin and chalcone on the growth of annual plants. Weed Biol. Manage. 2004, 4, 235–238. 10.1111/j.1445-6664.2004.00143.x. [DOI] [Google Scholar]
- Díaz-tielas C.; Graña E.; Sotelo T.; Reigosa M. J.; Sánchez-moreiras A. M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant, Cell Environ. 2012, 35, 1500–1517. 10.1111/j.1365-3040.2012.02506.x. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Wang K.; Wang P.; Kang J.-X.; Gao S.; Zhao L.-X.; Ye F. Design, synthesis, and herbicidal activity evaluation of novel aryl-naphthyl methanone derivatives. Front. Chem. 2019, 7, 2. 10.3389/fchem.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.; Wang M.; Zhao L.-X.; Zhang S.-Q.; Liu Y.-X.; Guo Y.-Y.; Zhang D.; Gao S.; Ye F. Design, synthesis, herbicidal activity and CoMFA of aryl-formyl piperidinone HPPD inhibitors. Pestic. Biochem. Physiol. 2021, 174, 104811. 10.1016/j.pestbp.2021.104811. [DOI] [PubMed] [Google Scholar]
- Li K.-J.; Qu R.-Y.; Liu Y.-C.; Yang J.-F.; Devendar P.; Chen Q.; Niu C.-W.; Xi Z.; Yang G.-F. Design, synthesis, and herbicidal activity of pyrimidine–biphenyl hybrids as novel acetohydroxyacid synthase inhibitors. J. Agric. Food Chem. 2018, 66, 3773–3782. 10.1021/acs.jafc.8b00665. [DOI] [PubMed] [Google Scholar]
- Jampilek J.; Kralova K.; Pesko M.; Kos J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. 10.1016/j.bmcl.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Fernandez C.; Lelong B.; Vila B.; Mévy J.-P.; Robles C.; Greff S.; Dupouyet S.; Bousquet-Mélou A. Potential allelopathic effect of Pinus halepensis in the secondary succession: an experimental approach. Chemoecology 2006, 16, 97–105. 10.1007/s00049-006-0334-z. [DOI] [Google Scholar]
- Yoneyama K.; Natsume M.. 4.13-Allelochemicals for Plant–Plant and Plant–Microbe Interactions. In Comprehensive Natural Products II Chemistry and Biology; Liu H.-W., Mander L., Eds.; Elsevier: Oxford, United Kingdom, 2010, pp 539–561. 10.1016/b978-008045382-8.00105-2 [DOI] [Google Scholar]
- Zaman F.; Iwasaki A.; Suenaga K.; Kato-Noguchi H. Allelopathic potential and identification of two allelopathic substances in Eleocharis atropurpurea. Plant Biosyst. 2021, 155, 510–516. 10.1080/11263504.2020.1762779. [DOI] [Google Scholar]
- Fu S.-F.; Wei J.-Y.; Chen H.-W.; Liu Y.-Y.; Lu H.-Y.; Chou J.-Y. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signaling Behav. 2015, 10, e1048052 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.-M.; Bilal S.; Shahzad R.; Kim Y.-N.; Park C.-W.; Lee K.-E.; Lee J.-R.; Lee I.-J. Effect of ammonia and indole-3-acetic acid producing endophytic Klebsiella pneumoniae YNA12 as a bio-herbicide for weed inhibition: special reference with evening primroses. Plants 2020, 9, 761. 10.3390/plants9060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Wang S.-Q.; Tang H.-Y.; Li X.-J.; Zhang L.; Xiao J.; Gao Y.-Q.; Zhang A.-L.; Gao J.-M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain–many compounds method. J. Agric. Food Chem. 2013, 61, 11447–11452. 10.1021/jf403200g. [DOI] [PubMed] [Google Scholar]
- Mendes M. C.; Fazolo B. R.; de Souza J. M.; de Vasconcelos L. G.; Dall’Oglio P. T.; Soares E. L.; Sampaio M. A.; Vieira O. M.; Vieira L. C. C. Synthesis and evaluation of indole derivatives as photosynthesis and plant growth inhibitors. Photochem. Photobiol. Sci. 2019, 18, 1350–1358. 10.1039/c8pp00506k. [DOI] [PubMed] [Google Scholar]
- Souza J. M.; Fazolo B. R.; Lacerda J. W. F.; Moura M. d. S.; Santos A. C. R.; Vasconcelos L. G.; Dall’Oglio P. T.; Ali E. L.; Sampaio A.; Vieira O. M. Rational design, synthesis and evaluation of indole nitrogen hybrids as photosystem II inhibitors. Photochem. Photobiol. 2020, 96, 1233–1242. 10.1111/php.13295. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang Q.; Ning X.; Hang H.; Ma J.; Yang X.; Lu X.; Zhang J.; Li Y.; Niu C.; Song H.; Wang X.; Wang P. G. Synthesis and biological evaluations of a series of thaxtomin analogues. J. Agric. Food Chem. 2015, 63, 3734–3741. 10.1021/jf506153t. [DOI] [PubMed] [Google Scholar]
- Abe M.; Nishikawa K.; Fukuda H.; Nakanishi K.; Tazawa Y.; Taniguchi T.; Park S.-y.; Hiradate S.; Fujii Y.; Okuda K.; Shindo M. Key structural features of cis-cinnamic acid as an allelochemical. Phytochemistry 2012, 84, 56–67. 10.1016/j.phytochem.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Vishnoi S.; Agrawal V.; Kasana V. K. Synthesis and structure–activity relationships of substituted cinnamic acids and amide analogues: a new class of herbicides. J. Agric. Food Chem. 2009, 57, 3261–3265. 10.1021/jf8034385. [DOI] [PubMed] [Google Scholar]
- Orcaray L.; Igal M.; Zabalza A.; Royuela M. Role of exogenously supplied ferulic and p-coumaric acids in mimicking the mode of action of acetolactate synthase inhibiting herbicides. J. Agric. Food Chem. 2011, 59, 10162–10168. 10.1021/jf2025538. [DOI] [PubMed] [Google Scholar]
- Wang R.; Hua M.; Yu Y.; Zhang M.; Xian Q.-M.; Yin D.-Q. Evaluating the effects of allelochemical ferulic acid on Microcystis aeruginosa by pulse-amplitude-modulated (PAM) fluorometry and flow cytometry. Chemosphere 2016, 147, 264–271. 10.1016/j.chemosphere.2015.12.109. [DOI] [PubMed] [Google Scholar]
- Zhou Z.-Y.; Liu W.-X.; Pei G.; Ren H.; Wang J.; Xu Q.-L.; Xie H.-H.; Wan F.-H.; Tan J.-W. Phenolics from Ageratina adenophora roots and their phytotoxic effects on Arabidopsis thaliana seed germination and seedling growth. J. Agric. Food Chem. 2013, 61, 11792–11799. 10.1021/jf400876j. [DOI] [PubMed] [Google Scholar]
- Grisi P. U.; Forim M. R.; Costa E. S.; Anese S.; Franco M. F.; Eberlin M. N.; Gualtieri S. C. J. Phytotoxicity and identification of secondary metabolites of Sapindus saponaria L. leaf extract. J. Plant Growth Regul. 2015, 34, 339–349. 10.1007/s00344-014-9469-2. [DOI] [Google Scholar]
- Isaac B. G.; Ayer S. W.; Stonard R. J. Arabenoic acid, a natural product herbicide of fungal origin. J. Antibiot. 1991, 44, 793–794. 10.7164/antibiotics.44.793. [DOI] [PubMed] [Google Scholar]
- Macías F. A.; Simonet A. M.; Pacheco P. C.; Barrero A. F.; Cabrera E.; Jiménez-González D. Natural and synthetic podolactones with potential use as natural herbicide models. J. Agric. Food Chem. 2000, 48, 3003–3007. 10.1021/jf990321y. [DOI] [PubMed] [Google Scholar]
- Macías F. A.; Velasco R. F.; Álvarez J. A.; Castellano D.; Galindo J. C. Synthesis of melampolides and cis,cis-germacranolides as natural herbicide models. Tetrahedron 2004, 60, 8477–8488. 10.1016/j.tet.2004.06.128. [DOI] [Google Scholar]
- Xuan T.; Elzaawely A.; Fukuta M.; Tawata S. Herbicidal and fungicidal activities of lactones in Kava (Piper methysticum). J. Agric. Food Chem. 2006, 54, 720–725. 10.1021/jf0519461. [DOI] [PubMed] [Google Scholar]
- Bagchi S. Structure and site of action of an algicide from a cyanobacterium, Oscillatoria late-virens. J. Plant Physiol. 1995, 146, 372–374. 10.1016/s0176-1617(11)82072-9. [DOI] [Google Scholar]
- Velini E. D.; Alves E.; Godoy M. C.; Meschede D. K.; Souza R. T.; Duke S. O. Glyphosate applied at low doses can stimulate plant growth. Plant Signaling Behav. 2008, 64, 489–496. 10.1002/ps.1562. [DOI] [PubMed] [Google Scholar]
- Lin H.-Y.; Yang J.-F.; Wang D.-W.; Hao G.-F.; Dong J.-Q.; Wang Y.-X.; Yang W.-C.; Wu J.-W.; Zhan C.-G.; Yang G.-F. Molecular insights into the mechanism of 4-hydroxyphenylpyruvate dioxygenase inhibition: enzyme kinetics, X-ray crystallography and computational simulations. The FEBS Journal 2019, 286, 975–990. 10.1111/febs.14747. [DOI] [PubMed] [Google Scholar]
- Lin H.-Y.; Chen X.; Chen J.-N.; Wang D.-W.; Wu F.-X.; Lin S.-Y.; Zhan C.-G.; Wu J.-W.; Yang W.-C.; Yang G.-F. Crystal Structure of 4-hydroxyphenylpyruvate dioxygenase in complex with substrate reveals a new starting point for herbicide discovery. Research 2019, 2019, 2602414. 10.34133/2019/2602414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-Y.; Chen X.; Dong J.; Yang J.-F.; Xiao H.; Ye Y.; Li L.-H.; Zhan C.-G.; Yang W.-C.; Yang G.-F. Rational redesign of enzyme via the combination of quantum mechanics/molecular mechanics, molecular dynamics, and structural biology study. J. Am. Chem. Soc. 2021, 143, 15674–15687. 10.1021/jacs.1c06227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.