Abstract

Excitonic coupling of bacteriochlorophyll (BChl) a in light-harvesting (LH) proteins of purple photosynthetic bacteria is key for efficient photon capture and energy transfer. Environmental factors can affect the spectral features of these BChl a pigments and investigating these effects can provide insight into the molecular mechanisms underlying the photosynthetic spectral tuning. The present study analyzes the spectral alterations of the Qy band of B820 BChl a within the LH3 protein in relation to the type and concentration of detergents in the buffer. Changing the detergent from lauryl dimethylamine N-oxide (LDAO) to n-dodecyl-β-d-maltoside (DDM) caused a red shift in the B820 Qy band accompanied by hyperchromism; these spectral alterations were completely reversed by exchanging back from DDM to LDAO. These results reflect the different effects of harsh vs mild detergents on the perturbation of LH3. The B820 Qy band did not change when LDAO or NaCl concentration was altered, suggesting that electrostatic effects by external components have little influence on the spectral features of B820 BChl a in LH3.
Introduction
Naturally occurring cyclic tetrapyrroles such as bacteriochlorophyll (BChl) and chlorophyll are crucial for collecting photon energy in the initial stage of photosynthesis. These compounds are arranged within light-harvesting (LH) proteins to enable efficient photon capture and excitation energy transfer to reaction center proteins. The electronic states of cyclic tetrapyrroles in LH proteins are optimized by their excitonic coupling and interactions with polypeptides.1,2
Purple photosynthetic bacteria generally express two LH proteins, namely, core LH1 and peripheral LH proteins.3,4 While LH2 is a major LH protein in purple photosynthetic bacteria, some species produce an alternative peripheral LH antenna termed LH3 under low-light conditions.5−8 Both LH2 and LH3 are formed via symmetric circular oligomerization of pigment–protein subunits (Figure 1), each containing three BChl a pigments and one or two carotenoids within a pair of transmembrane α- and β-polypeptides.9−14 The BChl a pigments are found in two states in these proteins. One is a peripherally located monomer within the subunit, termed B800 BChl a because of the red-most absorption band (Qy band) at ca. 800 nm.15,16 The other is an excitonically coupled dimer found in the hydrophobic region of the subunit. The Qy band of the dimeric BChl a (B850) in LH2 absorbs at ca. 850 nm.15,16 In contrast, the Qy band of the dimeric BChl a in LH3 is positioned between 820 and 830 nm and thus termed B820. The blue shift of the B820 Qy band in LH3 compared with the B850 Qy band in LH2 is thought to be due to differences in the hydrogen-bonding patterns of the 3-acetyl group of BChl a with amino acid residues, as well as in the deformations of the bacteriochlorin ring in the protein matrix between B820 and B850.16−23
Figure 1.
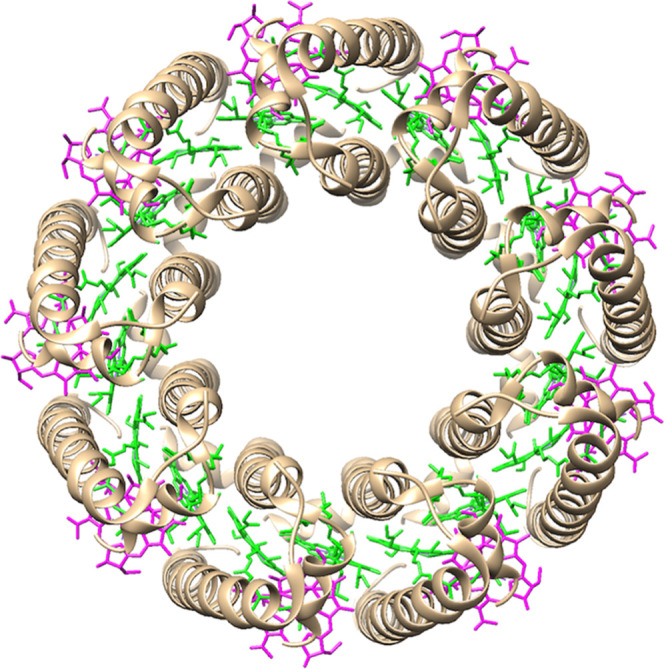
Top view structures of LH3 from Rhodoblastus acidophilus (Protein Data Bank entry 1IJD). B800 and B820 BChl a are colored magenta and green, respectively, and the carotenoids are omitted.
Environmental factors can affect the spectral features of LH proteins.24−33 Therefore, understanding their precise effects could enable us to elucidate the underlying molecular mechanism of photosynthetic spectral tuning. The present paper focuses on the effects of buffer conditions on the spectral properties of peripheral LH proteins from purple photosynthetic bacteria. In the case of LH2 proteins, spectral changes of B850 BChl a were evaluated using LH2 from a purple nonsulfur bacterium (Rhodobacter sulfidophilus) and purple sulfur bacteria (Ectothiorhodospira sp. and Thermochromatium tepidum); their B850 Qy bands were blue-shifted with hypochromism at high concentrations of lauryl dimethylamine N-oxide (LDAO, a detergent) and in low cationic concentrations.31−33 There is currently little information available in the literature relating to the spectral changes of LH3 in response to buffer conditions.
This study investigates the effects of detergent types and concentrations, as well as NaCl concentration, on the spectral features of B820 BChl a in LH3 from a purple bacterium Rhodoblastus acidophilus DSM137 (formerly known as Rhodopseudomonas acidophila 7050). The Qy band of B820 BChl a in LH3 was red-shifted with hyperchromism by exchanging LDAO for n-dodecyl-β-d-maltoside (DDM), and the absorption spectrum was completely recovered by reverse exchange from DDM to LDAO. In contrast to these reversible spectral changes, the B820 Qy band was insensitive to detergents or NaCl concentration. The tolerance of the B820 Qy band of LH3 to detergent and NaCl concentration is in contrast to the previously reported B850 Qy shifts of LH2.31−33
Materials and Methods
Materials
Rhodoblastus acidophilus DSM137 was cultivated at 28 °C under low-light (5 μmol s–1 m–2) and high-light conditions (c.a. 100 μmol s–1 m–2) to obtain LH3 and LH2, respectively. Grown cells (Figure S1) were disrupted using a Stansted pressure cell homogenizer FPG12800 operated at ca. 125 MPa, and LH proteins were solubilized from the collected photosynthetic membranes (Figure S2) using 0.35% LDAO. Proteins were purified by sucrose gradient ultracentrifugation and anion-exchange column chromatography using a DEAE Sephacel resin (GE Healthcare, Little Chalfont, U.K.) according to previous reports.34,35 We used LDAO in the LH purification process because LDAO has been generally used for purification of LH proteins.5,6,8,29,31,33 LDAO and DDM were purchased from FUJIFILM Wako Chemical Industries (Osaka, Japan) and Dojindo Laboratories (Kumamoto, Japan), respectively, and used without further purification.
Detergent Exchange
Purified LH proteins in 20 mM Tris buffer containing 0.1% LDAO (pH 8.0) were adsorbed onto a DEAE Sephacel resin, and more than six column volumes of 20 mM Tris buffer containing 0.02% DDM (pH 8.0) were eluted. Then, LH proteins were eluted using a 20 mM Tris buffer containing 0.02% DDM and 150 mM NaCl (pH 8.0). Detergent exchange from DDM to LDAO was carried out by adsorbing the LH proteins onto a DEAE Sephacel resin, eluting more than six column volumes of 20 mM Tris buffer containing 0.1% LDAO (pH 8.0), and collecting LH proteins with 20 mM Tris buffer containing 0.1% LDAO and 100 mM NaCl (pH 8.0).
Evaluation of the Effect of Detergent Concentration
To adjust the LDAO concentration, a solution of LH3 in 20 mM Tris buffer containing LDAO (pH 8.0) was diluted with 20 mM Tris buffer (pH 8.0). To adjust the DDM concentration, a solution of LH3 protein in 20 mM Tris buffer containing DDM and 150 mM NaCl (pH 8.0) was diluted with 20 mM Tris buffer containing 150 mM NaCl (pH 8.0). The final solutions were incubated for 30 min at 25 °C in the dark prior to spectral measurements.
Evaluation of the Effect of NaCl Concentration
A 3 mL solution of LH3 in 20 mM Tris buffer containing either 0.1% LDAO or 0.02% DDM (pH 8.0) was mixed with a 1 mL solution of 20 mM Tris buffer containing the same detergent and NaCl (pH 8.0) to adjust the NaCl concentration. The solutions were incubated for 30 min at room temperature in the dark prior to spectral measurements.
Results
Solubilized LH3 protein in a buffer containing 0.1% LDAO showed two Qy absorption bands at 805 and 823 nm (black curve in Figure 2A), which are assigned to B800 BChl a and B820 BChl a, respectively. The relative ratio of the Qy absorbance of B820 to that of B800 (B820/B800 ratio) was 0.93. This spectral feature was analogous to that of LH3 reported previously.5−8 When the detergent was exchanged with 0.02% DDM, the Qy band of B820 BChl a was red-shifted to 826 nm, and the B820/B800 ratio increased to 1.01 (red curve in Figure 2A). The peak positions and relative absorbance of the B800 Qy band, Qx and Soret bands of BChl a, and carotenoid absorption were unaffected by detergent exchange (Figure S3A). The Qy band of B820 BChl a was completely recovered by reverse exchange from 0.02% DDM to 0.1% LDAO, namely, the B820 Qy peak position was blue-shifted to 823 nm and the B820/B800 ratio decreased to 0.92 (blue curve in Figures 2B and S3B). These results indicate that the spectral features of the Qy band of B820 BChl a in LH3 depend on detergent types and that spectral changes induced by the detergent exchange are reversible.
Figure 2.
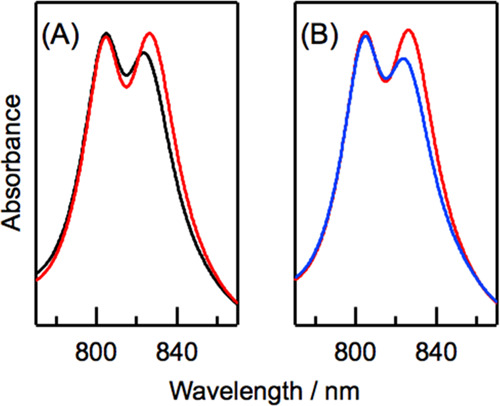
(A) Electronic absorption spectra of B800 and B820 BChl a in LH3 that was solubilized with 0.1% LDAO (black curve) and after exchange from 0.1% LDAO to 0.02% DDM (red curve) in 20 mM Tris buffer (pH = 8.0). (B) Electronic absorption spectra of LH3 that was solubilized with 0.02% DDM (red curve) and after exchange from 0.02% DDM to 0.1% LDAO (blue curve) in 20 mM Tris buffer (pH = 8.0). Spectra are normalized by absorbance at 528 nm (see Figure S3).
The effects of detergent exchanges on LH2 from the same species were also investigated. LH2 solubilized with 0.1% LDAO showed the Qy bands of B800 and B850 BChl a at 803 and 855 nm, respectively (black curve in Figure 3A). Exchanging the detergent from 0.1% LDAO to 0.02% DDM did not affect the peak positions of both the B800 and B850 Qy bands as well as the B850/B800 absorbance ratio (red curve in Figure 3A). The other absorption bands of BChl a and carotenoid in LH2 were also unaffected by the detergent exchange (Figure S4A). The spectral features of LH2 were unaffected by reverse exchange of the detergent from 0.02% DDM to 0.1% LDAO (Figures 3B and S4B).
Figure 3.

(A) Electronic absorption spectra of B800 and B850 BChl a in LH2 that was solubilized with 0.1% LDAO (black curve) and after exchange from 0.1% LDAO to 0.02% DDM (red curve) in 20 mM Tris buffer (pH = 8.0). (B) Electronic absorption spectra of LH2 that was solubilized with 0.02% DDM (red curve) and after exchange from 0.02% DDM to 0.1% LDAO (blue curve) in 20 mM Tris buffer (pH = 8.0). Spectra are normalized by absorbance at 524 nm (see Figure S4).
The Qy bands of B800 and B820 BChl a in LH3 did not change when LDAO concentration was changed from 0.025 to 0.1% (Figure 4A). The insensitivity of LH3 toward LDAO concentration is in sharp contrast to LH2 from three purple bacteria Rhodobacter sulfidophilus, Ectothiorhodospira sp., and Thermochromatium tepidum.31−33 The spectral features of the two Qy bands in LH3 were not also changed when DDM concentration was changed (Figure 4B). The lack of change in the LH3 B820 Qy band in response to changing DDM concentration is in line with previous results for LH2 from Thermochromatium tepidum.33
Figure 4.
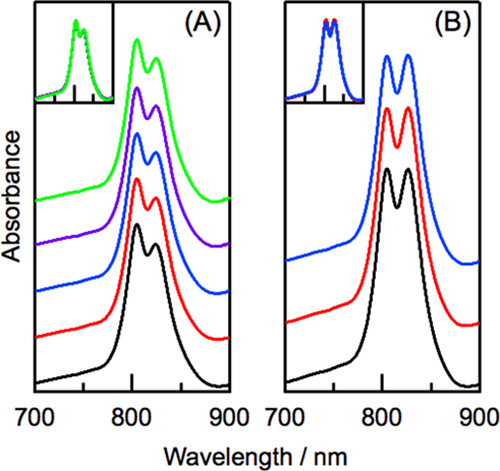
Effects of detergent concentration on electronic absorption spectra of LH3 in 20 mM Tris buffer (pH = 8.0) containing LDAO (A) and DDM (B). In panel (A), the LDAO concentrations were 0.1% (black), 0.075% (red), 0.05% (blue), 0.033% (purple), and 0.025% (green). In panel (B), the DDM concentrations were 0.05% (black), 0.038% (red), and 0.025% (blue). Spectra are normalized by absorbance at 524 nm. Insets show overlapped spectra.
There were no changes in the peak positions and relative absorbance of LH3 B800 and B820 BChl a in a buffer containing LDAO or DDM when NaCl concentration was changed from 0 to 500 mM (Figure 5). These results conflict with previous studies on LH2,31−33 which reported that increased NaCl concentration induced a red shift in the Qy position for B850 BChl a.
Figure 5.

Effects of NaCl concentration on electronic absorption spectra of LH3 in 20 mM Tris buffer (pH = 8.0) containing 0.1% LDAO (A) and 0.02% DDM (B). The NaCl concentrations were 0 mM (black), 10 mM (red), 20 mM (blue), 50 mM (sky blue), 100 mM (purple), 250 mM (green), and 500 mM (yellow). Insets show overlapped spectra.
Discussion
The spectral features of excitonically coupled BChl a in circularly arranged LH proteins from purple photosynthetic bacteria are majorly tuned by interactions among BChl a pigments and between BChl a and polypeptides. Furthermore, external components such as detergents and salts affect the spectral features of the BChl a pigments. For example, the peak positions and relative absorbance of the B850 Qy band of LH2 isolated from some species of purple bacteria have been reported to vary in response to the concentration of the detergent and salts in the buffer.31−33
The present study demonstrates that the Qy band of B820 BChl a in LH3 changes reversibly in response to detergent type, while spectral features of the B800 Qy band are unchanged. The inclusion of LDAO in the buffer induced a blue shift with hypochromism of the B820 Qy band, whereas DDM had an opposite effect. These responses cannot be explained by electrostatic effects alone since the B820 Qy band was insensitive to LDAO, a cationic detergent, and sodium ion concentration. Because LDAO has been reported to be a harsher detergent for membrane proteins compared with DDM,36 it could be expected that LDAO destabilizes the structure of LH3, including the microenvironment around B820 BChl a in the hydrophobic domain. In addition to this nonspecific perturbation, specific interactions of detergents with the vicinity of B820 BChl a in LH3 could affect spectral features. Indeed, the adsorption of n-octyl-β-d-glucoside (OG), which is used in crystallization, onto the outside hydrophobic surface close to B820 BChl a of LH3 has been confirmed from the crystal structure (Figure S5).14 It is possible that DDM and LDAO interact specifically in this position and perturb the local structure of B820 BChl a in LH3.
In contrast to the response of B820 in LH3 to detergent types, the spectral features of B850 BChl a in LH2 from the same species were unaffected by detergent types. These results suggest that the effect of detergent types on the B820 Qy band is not characteristic of both the peripheral LH proteins from this bacterium but originates from an inherent property of LH3. It would be also possible that the difference in the adsorption position of detergents to the LH proteins induces different perturbations to excitonically coupled BChl a (B820 or B850), since OG used in crystallization can be found on the inner surface of LH2, which differs from the adsorption position of OG in LH3, in the crystal structure of LH2 from Rhodoblastus acidophilus DSM145 (formerly known as Rhodopseudomonas acidophila 10050).11
The difference in response of the LH3 B820 Qy band from the LH2 B850 Qy band31−33 in relation to LDAO/NaCl concentration is ascribed to the structural robustness of the peripheral LH proteins from Rhodoblastus acidophilus compared with those from Rhodobacter sulfidophilus, Ectothiorhodospira sp., and Thermochromatium tepidum, although the molecular origin of this robustness is unclear.
Conclusions
This study indicates that the exchange between LDAO and DDM reversibly alters the Qy absorption band of B820 BChl a in LH3. In contrast, the spectral features of B820 BChl a are not influenced by changing detergent concentration. In addition, NaCl concentration did not influence the spectral features of B820 BChl a. The lack of response to changing detergent and sodium ion concentration sharply contrasts with the spectral changes of the Qy band of B850 BChl a observed for LH2 from some purple photosynthetic bacteria. Our results suggest that harsh detergents such as LDAO can cause destabilization of the LH3 structure, including the microenvironment of B820 BChl a, and/or perturbations due to specific interactions of detergent molecules with the hydrophobic surface of the cylindrical structure of LH3. Our results will deepen the current understanding of the spectral tuning mechanisms of excitonically coupled BChl a in circular arrangements of LH proteins from purple photosynthetic bacteria.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03323.
Supplementary data on electronic absorption spectra of grown cells (Figure S1), photosynthetic membranes (Figure S2), and LH3 (Figures S3 and S4) and the structure of LH3 with n-octyl-β-d-glucoside (Figure S5) (PDF)
This work was partially supported by a Grant-in-Aid for Scientific Research (C) (JP21K06104) from the Japan Society for the Promotion of Science.
The authors declare no competing financial interest.
Supplementary Material
References
- Croce R.; van Amerongen H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. 10.1038/nchembio.1555. [DOI] [PubMed] [Google Scholar]
- Saer R. G.; Blankenship R. E. Light-harvesting in phototrophic bacteria: structure and function. Biochem. J. 2017, 474, 2107–2131. 10.1042/BCJ20160753. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J.; Gall A.; Köhler J. The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes. Q. Rev. Biophys. 2006, 39, 227–324. 10.1017/S0033583506004434. [DOI] [PubMed] [Google Scholar]
- Niederman R. A. Development and dynamics of the photosynthetic apparatus in purple photosynthetic bacteria. Biochim. Biophys. Acta 2016, 1857, 232–246. 10.1016/j.bbabio.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J.; Durant I.; Valentine J.; Lindsay J. G.; Schmidt K. The isolation and partial characterization of the light-harvesting pigment-protein complement Rhodopseudomonas acidophila. Biochim. Biophys. Acta 1983, 722, 427–435. 10.1016/0005-2728(83)90058-0. [DOI] [Google Scholar]
- Angerhofer A.; Cogdell R. J.; Hipkins M. F. A spectral characterization of the light-harvesting pigment-protein complexes from Rhodopseudomonas acidophila. Biochim. Biophys. Acta 1986, 848, 333–341. 10.1016/0005-2728(86)90208-2. [DOI] [Google Scholar]
- Gardiner A. T.; Cogdell R. J.; Takaichi S. The effect of growth conditions on the light-harvesting apparatus in Rhodopseudomonas acidophila. Photosynth. Res. 1993, 38, 159–167. 10.1007/BF00146415. [DOI] [PubMed] [Google Scholar]
- Gardiner A. T.; Niedzwiedzki D. M.; Cogdell R. J. Adaptation of Rhodopseudomonas acidophila strain 7050 to growth at different light intensities: what are the benefits to changing the type of LH2?. Faraday Discuss. 2018, 207, 471–489. 10.1039/C7FD00191F. [DOI] [PubMed] [Google Scholar]
- McDermott G.; Prince S. M.; Freer A. A.; Hawthornthwaite-Lawless A. M.; Papiz M. Z.; Cogdell R. J.; Isaacs N. W. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 1995, 374, 517–521. 10.1038/374517a0. [DOI] [Google Scholar]
- Koepke J.; Hu X.; Muenke C.; Schulten K.; Michel H. The crystal structure of the light-harvesting complex II (B800–850) from Rhodospirillum molischianum. Structure 1996, 4, 581–597. 10.1016/S0969-2126(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Papiz M. Z.; Prince S. M.; Howard T.; Cogdell R. J.; Issacs N. W. The structure and thermal motion of the B800–B850 LH2 complex from Rps. acidophila at 2.0 Å resolution and 100 K: new structural features and functionally relevant motions. J. Mol. Biol. 2003, 326, 1523–1538. 10.1016/S0022-2836(03)00024-X. [DOI] [PubMed] [Google Scholar]
- Gardiner A. T.; Naydenova K.; Castro-Hartmann P.; Nguyen-Phan T. C.; Russo C. J.; Sader K.; Hunter C. N.; Cogdell R. J.; Qian P. The 2.4 Å cryo-EM structure of a heptameric light-harvesting 2 complex reveals two carotenoid energy transfer pathways. Sci. Adv. 2021, 7, eabe4650 10.1126/sciadv.abe4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P.; Swainsbury D. J. K.; Croll T. I.; Castro-Hartmann P.; Divitini G.; Sader K.; Hunter C. N. Cryo-EM structure of the Rhodobacter sphaeroides light-harvesting 2 complex at 2.1 Å. Biochemistry 2021, 60, 3302–3314. 10.1021/acs.biochem.1c00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLuskey K.; Prince S. M.; Cogdell R. J.; Isaacs N. W. The crystallographic structure of the B800-820 LH3 light-harvesting complex from the purple bacteria Rhodopseudomonas acidophila strain 7050. Biochemistry 2001, 40, 8783–8789. 10.1021/bi010309a. [DOI] [PubMed] [Google Scholar]
- Sundström V.; Pullerits T.; van Grondelle R. Photosynthetic light-harvesting: reconciling dynamics and structure of purple bacterial LH2 reveals function of photosynthetic unit. J. Phys. Chem. B 1999, 103, 2327–2346. 10.1021/jp983722+. [DOI] [Google Scholar]
- Cogdell R. J.; Howard T. D.; Isaacs N. W.; McLuskey K.; Gardiner A. T. Structural factors which control the position of the Qy absorption band of bacteriochlorophyll a in purple bacterial antenna complexes. Photosynth. Res. 2002, 74, 135–141. 10.1023/A:1020995224156. [DOI] [PubMed] [Google Scholar]
- Fowler G. J. S.; Visschers R. W.; Grief G. G.; van Grondelle R.; Hunter C. N. Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature 1992, 355, 848–850. 10.1038/355848a0. [DOI] [PubMed] [Google Scholar]
- Fowler G. J. S.; Sockalingum G.; Robert B.; Hunter C. N. Blue shifts in bacteriochlorophyll absorbance correlate with changed hydrogen bonding patterns in light-harvesting 2 mutants of Rhodobacter sphaeroides with alterations at α-Tyr-44 and α-Tyr-45. Biochem. J. 1994, 299, 695–700. 10.1042/bj2990695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgis J. N.; Jirsakova V.; Reiss-Husson F.; Cogdell R. J.; Robert B. Structure and properties of the bacteriochlorophyll binding site in peripheral light-harvesting complexes of purple bacteria. Biochemistry 1995, 34, 517–523. 10.1021/bi00002a016. [DOI] [PubMed] [Google Scholar]
- Sturgis J. N.; Robert B. Pigment-binding site and electronic properties in light-harvesting proteins of purple bacteria. J. Phys. Chem. B 1997, 101, 7227–7231. 10.1021/jp963363n. [DOI] [Google Scholar]
- De Vico L.; Anda A.; Osipov V. A. I.; Madsen A. Ø.; Hansen T. Macrocycle ring deformation as the secondary design principle for light-harvesting complexes. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, E9051–E9057. 10.1073/pnas.1719355115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. C.; Nottoli M.; Cupellini L.; Mennucci B. The molecular mechanisms of light adaption in light-harvesting complexes of purple bacteria revealed by a multiscale modeling. Chem. Sci. 2019, 10, 9650–9662. 10.1039/C9SC02886B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottoli M.; Jurinovich S.; Cupellinil L.; Gardiner A. T.; Cogdell R. J.; Mennucci B. The role of charge-transfer states in the spectral tuning of antenna complexes of purple bacteria. Photosynth. Res. 2018, 137, 215–226. 10.1007/s11120-018-0492-1. [DOI] [PubMed] [Google Scholar]
- Wu H.-M.; Ratsep M.; Jankowiak R.; Cogdell R. J.; Small G. J. Comparison of the LH2 antenna complexes of Rhodopseudomonas acidophila (strain 10050) and Rhodobacter sphaeroides by high-pressure absorption, high-pressure hole burning, and temperature-dependent absorption spectroscopies. J. Phys. Chem. B 1997, 101, 7641–7653. 10.1021/jp9715134. [DOI] [Google Scholar]
- Timpmann K.; Ellervee A.; Pullerits T.; Ruus R.; Sundström V.; Freiberg A. Short-range exciton couplings in LH2 photosynthetic antenna proteins studied by high hydrostatic pressure absorption spectroscopy. J. Phys. Chem. B 2001, 105, 8436–8444. 10.1021/jp003496f. [DOI] [Google Scholar]
- Gall A.; Ellervee A.; Sturgis J. N.; Fraser N. J.; Cogdell R. J.; Freiberg A.; Robert B. Membrane protein stability: high pressure effects on the structure and chromophore-binding properties of the light-harvesting complex LH2. Biochemistry 2003, 42, 13019–13026. 10.1021/bi0350351. [DOI] [PubMed] [Google Scholar]
- Kangur L.; Timpmann K.; Freiberg A. Stability of integral membrane proteins under high hydrostatic pressure: the LH2 and LH3 antenna pigment-protein complexes from photosynthetic bacteria. J. Phys. Chem. B 2008, 112, 7948–7955. 10.1021/jp801943w. [DOI] [PubMed] [Google Scholar]
- Zerlauskiene O.; Trinkunas G.; Gall A.; Robert B.; Urboniene V.; Valkunas L. Static and dynamic protein impact on electronic properties of light-harvesting complex LH2. J. Phys. Chem. B 2008, 112, 15883–15892. 10.1021/jp803439w. [DOI] [PubMed] [Google Scholar]
- Shibuya Y.; Itoh T.; Matsuura S.; Yamaguchi A. Structural stability of light-harvesting protein LH2 adsorbed on mesoporous silica supports. Anal. Sci. 2015, 31, 1069–1074. 10.2116/analsci.31.1069. [DOI] [PubMed] [Google Scholar]
- Rätsep M.; Muru R.; Freiberg A. High temperature limit of photosynthetic excitons. Nat. Commun. 2018, 9, 99 10.1038/s41467-017-02544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zarate I. O.; Picorel R. Spectral changes of the B800-B850 antenna complex from Ectothiorhodospira sp. induced by detergent and salt treatment. Photosynth. Res. 1994, 41, 339–347. 10.1007/BF00019411. [DOI] [PubMed] [Google Scholar]
- Tadros M. H.; Hagemann G. E.; Katsiou E.; Dierstein R.; Schiltz E. Isolation and complete amino acid sequence of the β- and α-polypeptides from the peripheral light-harvesting pigment-protein complex II of Rhodobacter sulfidophilus. FEBS Lett. 1995, 368, 243–247. 10.1016/0014-5793(95)00645-P. [DOI] [PubMed] [Google Scholar]
- Sekine F.; Horiguchi K.; Kashino Y.; Shimizu Y.; Yu L.-J.; Kobayashi M.; Wang Z.-Y. Gene sequencing and characterization of the light-harvesting complex 2 from thermophilic purple sulfur bacterium Thermochromatium tepidum. Photosynth. Res. 2012, 111, 9–18. 10.1007/s11120-011-9658-9. [DOI] [PubMed] [Google Scholar]
- Saga Y.; Hirota K. Determination of the molar extinction coefficients of the B800 and B850 absorption bands in light-harvesting complexes 2 derived from three purple photosynthetic bacteria Rhodoblastus acidophilus, Rhodobacter sphaeroides, and Phaeospirillum molischianum by extraction of bacteriochlorophyll a. Anal. Sci. 2016, 32, 801–804. 10.2116/analsci.32.801. [DOI] [PubMed] [Google Scholar]
- Saga Y.; Hirota K.; Asakawa H.; Takao K.; Fukuma T. Reversible changes in the structural features of photosynthetic light-harvesting complex 2 by removal and reconstitution of B800 bacteriochlorophyll a pigments. Biochemistry 2017, 56, 3484–3491. 10.1021/acs.biochem.7b00267. [DOI] [PubMed] [Google Scholar]
- Odahara T. Stability and solubility of integral membrane proteins from photosynthetic bacteria solubilized in different detergents. Biochim. Biophys. Acta 2004, 1660, 80–92. 10.1016/j.bbamem.2003.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


