Abstract
Uptake and release kinetics are investigated of a dilute aqueous polymeric-surfactant wetting agent, (ethylene oxide)45—(butylene oxide)10 copolymer, also referred to as poly(oxyethylene)-co-poly(oxybutylene), impregnated into a newly designed silicone-hydrogel lens material. Transient scanning concentration profiles of the fluorescently tagged polymeric surfactant follow Fick’s second law with a diffusion coefficient near 10–11 cm2/s, a value 3–4 orders smaller than that of the free surfactant in bulk water. The Nernst partition coefficient of the tagged polymeric wetting agent, determined by fluorescence microscopy and by methanol extraction, is near 350, a very large value. Back-extraction of the polymeric-surfactant wetting agent releases only ∼20% of the loaded amount after soaking the fully loaded lens for over 7 days. The remaining ∼80% is irreversibly bound in the lens matrix. Reverse-phase liquid chromatography of the lens-loaded and lens-extracted surfactant demonstrates that the released wetting agent is more hydrophilic with a higher polarity. Aqueous poly(oxyethylene)-co-poly(oxybutylene) is hypothesized to attach strongly to the lens matrix, most likely to the lens silicone domains. Strong binding leads to slow transient diffusion, to large uptake, and to significant irreversible retention. These characteristics indicate the suitability of using a poly(oxyethylene)-co-poly(oxybutylene) nonionic polymeric surfactant to maintain enhanced lens wettability over time. Methodology and findings from this study provide useful insights for designing sustained-release contact-lens wetting agents and materials.
Introduction
Silicone-hydrogel (SiHy) contact lenses are now the industry standard because of their high oxygen transmissibility and good mechanical properties compared to traditional hydrogel lens materials. During wear, soft contact lenses tend to contaminate and lose the desired surface wettability.1 Wetting agents are often relied upon to retain soft-contact-lens surface moisture, enhance surface-wetting characteristics, and improve wear comfort.1 Typical wetting agents include hydrophilic materials, such as PVA (poly(vinyl alcohol)),2−7 PEG (poly(ethylene glycol)),8,9 PVP (poly(vinylpyrrolidone)),10−14 PPO (poly(propylene oxide)),15 cellulose,16−18 and hyaluronic acid (HA).19−22 Various loading approaches have been tried, primarily polymer trapping during hydrogel lens fabrication,2,5,14 modification of lens-surface functional groups, such as hydroxyl reactive groups,2−4,13 and solution uptake.5,9−12,17,19,23 The simplest approach is solution loading, where the wetting agent diffuses from a supply solution into the contact-lens gel.5,9−12,17,19,23 However, during wear, wetting-agent release from the hydrogel matrix can be rapid, abrogating sustained effectiveness for many lower-molecular-weight hydrophilic wetting agents due to back-diffusion.24
To achieve slow release rates, larger-molecular-weight amphiphilic wetting agents, especially nonionic-block copolymers, have been introduced into the lens package saline or into the lens-care solutions. These include poloxamer (triblock copolymer, PEO-PPO-PEO), poloxamine (four-armed copolymer, PEO-PPO), and diblock PEO-PBO copolymer, among others.23,25 Release rates of wetting agents have been correlated against their molecular weight and/or against their hydrophilic/lipophilic balance (HLB).23,25
This work investigates quantitative uptake and release rates of the aqueous wetting agent (ethylene oxide)45–(butylene oxide)10 copolymer, also referred to as poly(oxyethylene)-co-poly(oxybutylene), preloaded into a newly designed surface-wettable SiHy contact-lens material. Poly(oxyethylene)-co-poly(oxybutylene) is an amphiphilic polymeric surfactant containing both ethylene glycol hydrophilic segments and butylene glycol lipophilic segments. Compared with other wetting agents, the butylene glycol segment is slightly more hydrophobic, leading to better wetting properties with silicone hydrogels.26,27 Uptake and release kinetics are experimentally measured and are interpreted theoretically to understand the underlying mechanisms.
Results and Discussion
Poly(oxyethylene)-co-poly(oxybutylene) Saturation Uptake
To gauge the final amount of poly(oxyethylene)-co-poly(oxybutylene) wetting agent absorbed by the SiHy material, a Nernst partition coefficient, k, was determined according to28−30
| 1 |
where Cg is the final concentration of the polymeric wetting agent in the SiHy lens per unit gel volume and Cb is the corresponding wetting-agent volume concentration in the surrounding aqueous solution. Strictly, eq 1 holds for reversible processes at equilibrium. The value reported here diagnoses poly(oxyethylene)-co-poly(oxybutylene) loading and is not restricted to an equilibrium process. Using the fluorescence-microscope method described in the Methods section, background fluorescence intensity was subtracted from both solution and lens signals. Because intensity is directly proportional to concentration, the ratio of the background-corrected lens intensity to the background-corrected solution intensity gave a partition coefficient of 505 ± 23. Using the extraction-plate-reader method, the concentration of poly(oxyethylene)-co-poly(oxybutylene) in the 4 μL lens was 39,659 ± 1782 ppm. Therefore, the partition coefficient from this method was k = 39,659 (±1782) ppm/150 ppm = 264 ± 12. The two measurement techniques differ by a factor of 2. Nevertheless, the magnitude of poly(oxyethylene)-co-poly(oxybutylene) partitioning into the SiHy material is large. Table 1 reports the average value of the partition coefficient (kavg) in the loading direction.
Table 1. Uptake and Release Model Parameters.
| kavg | 385 |
| Dload | 1.2 ×10–11 cm2/s |
| Dleach | 2.4 × 10–11 cm2/s |
Poly(oxyethylene)-co-poly(oxybutylene) Uptake Kinetics
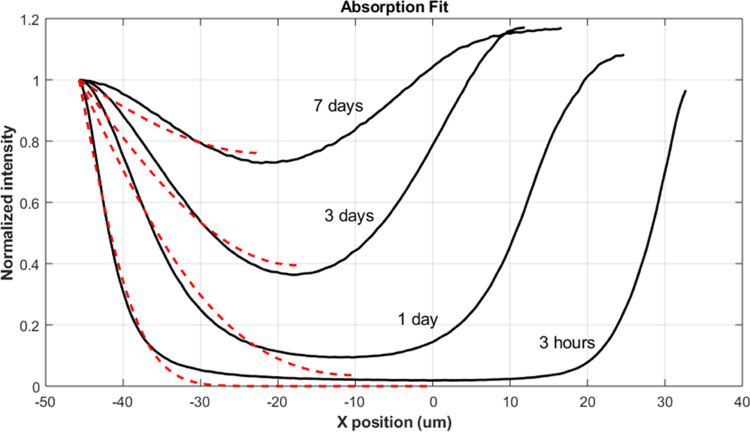
Typical fluorescence micrographs of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) cross-section transient-loading intensity profiles are illustrated in Figure 1. As time progresses, the fluorescently tagged polymeric surfactant clearly penetrates farther into the contact lens from each side. Figure 2 shows the tagged polymeric-surfactant loading cross-sectional intensity profiles (solid black lines) for the SiHy lens corresponding to the images in Figure 1. As noted, the normalized intensity profiles reflect the poly(oxyethylene)-co-poly(oxybutylene) concentration profiles in the lens. Figure 2 also reveals that the polymeric-surfactant concentration profiles are different between the right and left sides of the lens cross-section, suggesting that the cut lens material is asymmetric.
Figure 1.
Transient scanning-fluorescence micrographs of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) absorbed into a silicone-hydrogel contact lens. Yellow lines demark the cross scans shown in Figure 2.
Figure 2.
Transient-loading fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) fluorescence intensity profiles in the silicone hydrogel. Black solid lines are experimental. The red dashed lines are model fits to eqs 2 and 3. The external wetting-agent solution concentration was 150 ppm.
For the dilute concentrations studied, Fick’s second law was adopted to describe the poly(oxyethylene)-co-poly(oxybutylene) uptake dynamics into the lens material. Analytic solution appropriate to the rectangular geometry of the lenses is28,31
| 2 |
with
| 3 |
where t is time, x is the lens position relative to the cross-section centerline (0 ≤ x ≤ L), L is the half thickness of the lens, n is a summation index, λn ≡ (2n + 1)π/2, t* is the final initial loading time (14 days here), Cg∞ is the final loaded concentration in the gel, and D is the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) diffusivity in the SiHy lens matrix. To determine the wetting-agent diffusivity in the gel, eqs 2 and 3 were best fit to the intensity profiles of Figure 2 using the Levenberg–Marquardt algorithm in Matlab R2019b (Mathworks, Natick, MA) and n = 10,000 in the summations. Because the cut lens is not always symmetric and the thickness may vary, left and right halves of the lens cross-sections were fit separately, and the results were averaged. Maximum intensities at the lens edges were adjusted to be constant for the model fits. Red dashed lines in Figure 2 correspond to a best-fit diffusion coefficient of 7 × 10–12 cm2/s from the left-side fitting and 1.7 × 10–11 cm2/s from the right-side fitting (not shown). Table 1 lists the average value of 1.2 × 10–11 cm2/s for the diffusion coefficient (Dload) of the poly(oxyethylene)-co-poly(oxybutylene) wetting agent when loading into the gel.
Poly(oxyethylene)-co-poly(oxybutylene) Back-Extraction
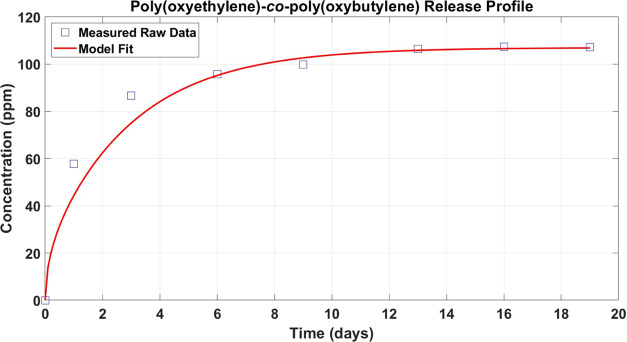
Figure 3 shows the measured polymeric-wetting-agent concentration in the saline bath at selected times (open squares) during back-extraction. Poly(oxyethylene)-co-poly(oxybutylene) continues to leach from the lenses over about 12 days. The final measured poly(oxyethylene)-co-poly(oxybutylene) concentration in the PBS bath was 107 ppm. This value assesses the amount of polymeric wetting agent in the lens that is finally released from the gel. Simple mass balance on the poly(oxyethylene)-co-poly(oxybutylene) in the lens during extraction gives the expression
| 4 |
Figure 3.
Back-extraction poly(oxyethylene)-co-poly(oxybutylene) release history after lens loading at 150 ppm. Open squares represent the measured poly(oxyethylene)-co-poly(oxybutylene) concentration in the PBS solution. The red line is theory fit to eq 6.
where Cgo is the concentration in the gel after initial saturation with the 150 ppm solution, Vg is the gel volume, and Vb is the extraction-bath volume. The initial polymeric wetting-agent concentration in the gel is Cg = kCbo, where Cb = 150 ppm is the bulk-solution initial-saturating concentration of the polymeric wetting agent. Fractional release of poly(oxyethylene)-co-poly(oxybutylene), ν, follows from eq 4 by definition
| 5 |
With a final bath concentration of 107 ppm and known values of the bath volume (4 L), lens volume (0.3 mL for 20 lenses), and k = 385, only about 10–20% of the initially absorbed poly(oxyethylene)-co-poly(oxybutylene) wetting agent eventually releases. Over 80% remains irreversibly trapped in the lens material.
The full transient back-extraction data in Figure 3 permit the quantitative assessment of the polymeric-wetting-agent release kinetics. For diffusion-based release, the bath concentration increases according to the expression32,33
| 6 |
where Cb(t = ∞) = 107 ppm is the final back-extracted polymeric wetting-agent solution concentration. As above, a Levenberg–Marquardt least-squares error minimization of eq 6 is applied to the data in Figure 3. The red solid line in Figure 3 corresponds to the best-fitting procedure and gives a desorption diffusion coefficient of 2.4 × 10–11 cm2/s (reported as Dleach in Table 1), surprisingly a value not very different from the loading diffusivity.
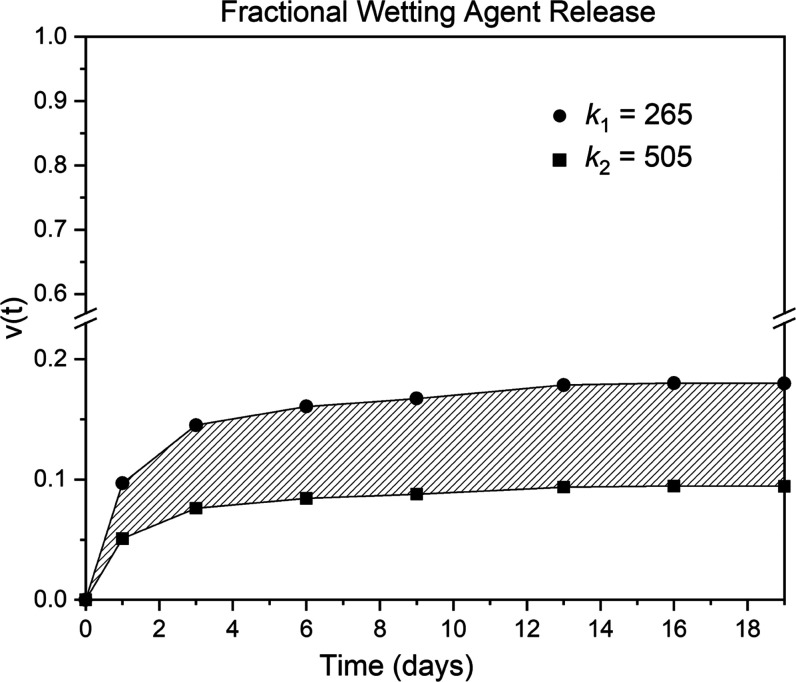
Equation 5 applies also to the transient data in Figure 3 with the measured poly(oxyethylene)-co-poly(oxybutylene) extracting bath concentration, Cb, and hence the release fraction, varying in time. Figure 4 graphs the result of this calculation using the two measured values of the loading partition coefficient. Hatched lines drawn between the calculated points guide the eye; the shaded region demarks the uncertainty in the partition coefficient. Release of the 10% reversibly absorbed and mobile poly(oxyethylene)-co-poly(oxybutylene) takes over 10 days to migrate out of the lenses into the well-stirred bath. In one day of soaking, less than 10% of the polymeric wetting agent in the lens leaches. Equivalently, 90% remains in the lens.
Figure 4.
Fractional release of poly(oxyethylene)-co-poly(oxybutylene) from the poly(oxyethylene)-co-poly(oxybutylene)-saturated lens from Figure 3.
Effective diffusion coefficients of poly(oxyethylene)-co-poly(oxybutylene) in the studied SiHy material listed in Table 1 are dramatically smaller than the free diffusion coefficient of a 3 kDa aqueous polymeric molecule, which typically is in the range of 10–7 cm2/s.32,33 Diffusivities of noninteracting solutes in gels are the product of a hydrodynamic resistance factor and a steric or obstruction factor.31,32 Typical reductions in the gel diffusion coefficient are somewhat larger than a factor of 10.28,31 The diffusivities reported in Table 1, however, are 5 orders of magnitude smaller than that for unrestricted aqueous polymer diffusion. The hypothesis is that poly(oxyethylene)-co-poly(oxybutylene) molecules specifically interact with the SiHy lens matrix. That is, the polymeric surfactant adsorbs specifically onto the polymeric domains of the cross-linked hydrogel.31 During loading, poly(oxyethylene)-co-poly(oxybutylene) molecules partition to the polymer strands, slowing diffusion depending on the amount adsorbed.33,34
Examination of the loading partition coefficient in Table 1 reveals a similar picture. Ideal solute uptake into a gel gives a partition coefficient equal to the gel porosity, here approximately 0.5. If present, size exclusion and electrostatic repulsion reduce this value further.29 Hence, the very large partition coefficient reported in Table 1 is explained by strong adsorption of the nonionic polymeric surfactant to the gel matrix. Similarly, the observation in Figures 3 and 4 of significant irreversible polymeric-wetting-agent attachment to the silicone-hydrogel matrix gives further evidence for strong polymeric-surfactant uptake. Less than 20% of the impregnated poly(oxyethylene)-co-poly(oxybutylene) wetting agent leaches from the gel even after two weeks of extraction.
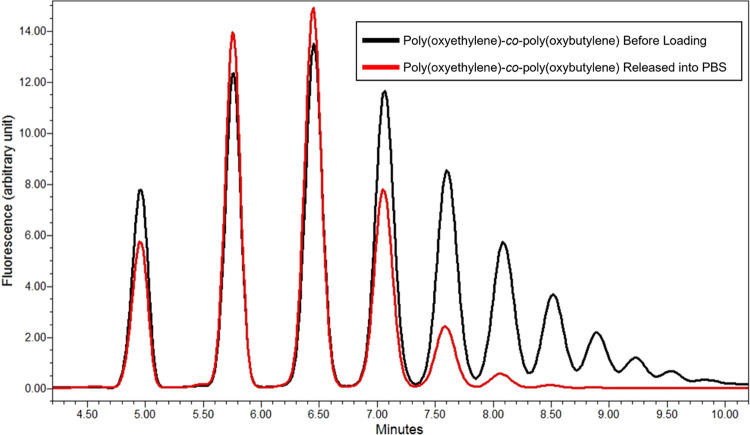
The black-line ultra-performance liquid chromatography (UPLC) chromatogramin Figure 5 displays a measured chromatogram of the purified fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) before loading into the silicone hydrogel. Conversely, the red-line UPLC chromatogram in Figure 5 indicates the chromatogram of the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) released from the lens into PBS after 7 days of soaking using the same process as in Figure 3. Reversed-phase liquid chromatography of poly(oxyethylene)-co-poly(oxybutylene) elutes polar analytes first (shorter retention time), ahead of nonpolar analytes.35 Thus, as shown by the red curve in Figure 5, only the more hydrophilic (more polar) poly(oxyethylene)-co-poly(oxybutylene) molecules escape from the gel during the 7 day extraction. The less hydrophilic (more nonpolar) copolymer surfactant conveys through the gel at a slow pace, as reflected by the small unloading diffusion coefficient and by the large partition coefficient in Table 1, and accordingly provides further support for the strong irreversible binding of poly(oxyethylene)-co-poly(oxybutylene) to the SiHy lens matrix.
Figure 5.
UPLC chromatograms of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene). The black trace is the poly(oxyethylene)-co-poly(oxybutylene) before loading to the silicone hydrogel, and the red trace is poly(oxyethylene)-co-poly(oxybutylene) released during back-extraction.
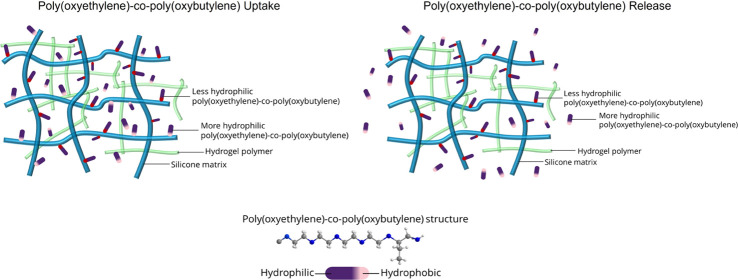
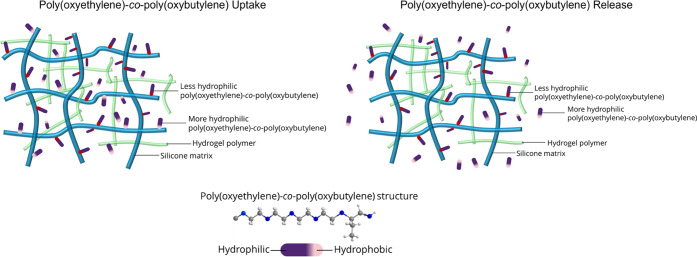
Apparently, the more hydrophobic segments of poly(oxyethylene)-co-poly(oxybutylene) bind strongly with the silicone-rich domains of the contact-lens matrix. The larger are the butylene oxide segments of the polymeric wetting agent, the stronger is the binding and the larger is the fraction of irreversible attachment. Poly(oxyethylene)-co-poly(oxybutylene) binding to the hydrogel matrix gives rise to the very large loading partition coefficients, to the very low transient loading and unloading diffusion coefficients, and to irreversible attachment. The schematic in Figure 6 demonstrates the preferential binding of the more hydrophobic segments poly(oxyethylene)-co-poly(oxybutylene) wetting agent to silicone domains of the hydrogel. Interaction of the wetting agent with the more hydrophilic domains of the gel matrix is apparently weaker.
Figure 6.
Schematic of poly(oxyethylene)-co-poly(oxybutylene) uptake and release from the contact lens gel. During loading, the partition coefficient is high and the diffusion rate is low. During the desorption experiment, the diffusion rate slightly increases since the more hydrophilic poly(oxyethylene)-co-poly(oxybutylene) molecules preferentially release.
Conclusions
Wetting agents are important toward establishing enhanced surface-wetting characteristics of soft contact lenses during wear. To achieve this goal, the presence of the wetting agent remaining at the anterior lens surface is requisite. However, many wetting surfactants release quickly from the lens, which diminishes their effectiveness. This study establishes that the dilute aqueous poly(oxyethylene)-co-poly(oxybutylene) block-copolymer surfactant is ∼80% irreversibly bound in a SiHy soft contact lens (serafilcon A). The remaining polymeric surfactant is reversibly attached. The reversible portion of the wetting agent releases slowly over 7 days. Over 90% of the poly(oxyethylene)-co-poly(oxybutylene) surfactant remains in the lens after 1 day of leaching.
Theoretical analysis of the uptake and release kinetics of aqueous poly(oxyethylene)-co-poly(oxybutylene) in the lens material reveals a very high partition coefficient (i.e., near 350) and very low diffusion coefficients (i.e., about 10–11 cm2/s) for both loading and leaching. Determined diffusion coefficients are many orders of magnitude smaller than that of the bulk-solution diffusion coefficient. These two results suggest strong specific binding of poly(oxyethylene)-co-poly(oxybutylene) to the gel matrix. Most likely, the more hydrophobic butylene oxide segments bind to the silicone domains. Prolonged back-extraction recovers only about 20% of the initially loaded polymeric surfactant, confirming strong irreversible binding to the lens matrix. Reverse-phase liquid chromatography of the back-extracted surfactant reveals that the more hydrophilic poly(oxyethylene)-co-poly(oxybutylene) molecules are mobile and leach, albeit slowly.
This work confirms that aqueous poly(oxyethylene)-co-poly(oxybutylene) is an effective sustained-release wetting agent for SiHy soft contact lenses. The combination of very large and strongly irreversible uptake of aqueous poly(oxyethylene)-co-poly(oxybutylene) with extremely small effective diffusion coefficients of the leached mobile portion of the surfactant retains the wetting agent in the lens for many days. This unique combination of behaviors provides guidance for future development of improved sustained-release wetting agents in soft contact lenses.
Methods
Materials
(Ethylene oxide)45–(butylene oxide)10 was synthesized with an average molecular weight of Mn ∼ 3000 g/mol by procedures published previously.25,36 NBD-COCl (4-(N-Chloroformylmethyl-N-methylamino)-7-nitro-2,1,3-benzoxadiazole, reagent grade, >92%) was purchased from TCI (Japan) and used as received. N-hexane (≥97%, HPLC grade), methylene chloride (≥98.5% purity grade), and potassium carbonate (K2CO3, reagent grade) were purchased from VWR and used as received. Phosphate-buffered saline (PBS) (composition: 0.044 wt % NaH2PO4·H2O, 0.388 wt % Na2HPO4·2H2O, 0.79 wt % NaCl, pH = 7.4) was prepared in-house using VWR-supplied chemicals.
Synthesis and Characterization of Fluorescently Tagged Poly(oxyethylene)-co-poly(oxybutylene)
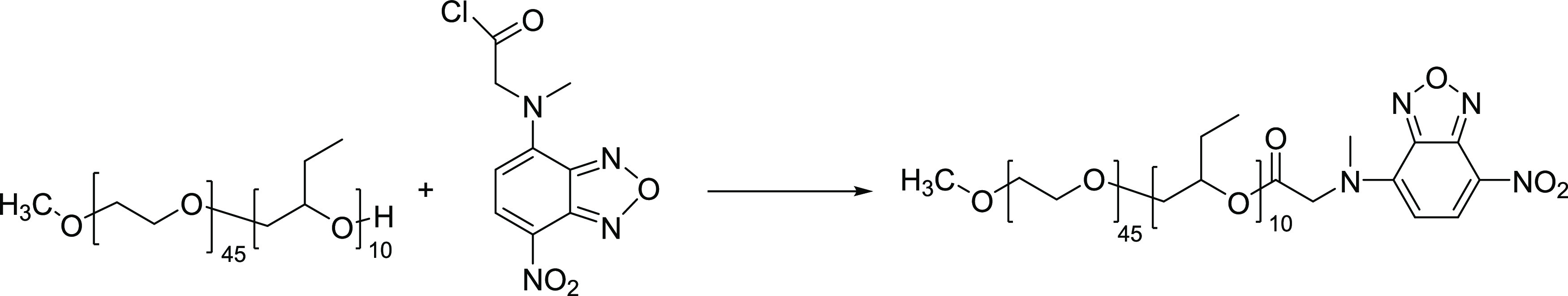
As illustrated in Scheme 1, the synthetic procedures for fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) were as follows:37 100 mg of NBD-COCl was dissolved in 0.5 mL of hexane and 2.5 mL of methylene chloride. Then, ∼1 g of (ethylene oxide)45–(butylene oxide)10 and 0.7 g of dry potassium carbonate were added. The reaction was performed under N2 for more than 48 h at room temperature. K2CO3 was centrifuged, and the solvent was evaporated. Purification of the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) was achieved by dialysis with a membrane molecular weight cutoff of 1 kDa. Further purification by UPLC from Waters (Acquity H-Class, Milford, MA) with column-fraction collection removed the remaining free dye. Purified fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) polymer was characterized by reverse-phase UPLC with a fluorescence detector from Waters (Acquity H-Class, Milford, MA). Polymer separation was achieved based on molecular polarity.35 Fluorescence emission was measured at 510 nm with excitation at 460 nm. A step gradient separated the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) peak envelopes from that of the saline solution. Mobile phases were as follows: A—50:30:19.8:0.2 (v/v) methanol/acetonitrile/water/formic acid; B—59.8:40:0.2 (v/v) methanol/acetone/formic acid. The stationary phase (column) used was a reverse-phase C4 column (i.e., nonpolar four carbon chains on the silica column packing). Because the mobile phases are more polar than the stationary phase, the less hydrophilic (less polar) species of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) exhibit longer retention times. Method precision (repeatability) for six replicate preparations at a target concentration was less than ≤10% RSD (relative standard deviation).
Scheme 1. Synthetic Scheme of Fluorescently Tagged Poly(oxyethylene)-co-poly(oxybutylene).
Silicone-Hydrogel Contact Lens Material (Serafilcon A)
The SiHy contact-lens material (serafilcon A) was prepared by free-radical polymerization of a monomer mixture containing polydimethylsiloxane mono-methacrylate, glycerol-functionalized polydimethylsiloxane dimethacrylate, N-vinylpyrrolidone, tri(ethylene glycol) dimethacrylate, methyl methacrylate, and 2,2-azobisisobutyronitrile. After polymerization, the lens material was extracted in the alcohol, packaged in a blister shell with phosphate-buffered saline (PBS) containing 150 ppm poly(oxyethylene)-co-poly(oxybutylene), and autoclaved. The finished lens had ∼55 wt % water content and an oxygen permeability of 119 Barrer.
Poly(oxyethylene)-co-poly(oxybutylene) Uptake Kinetics
To determine the rate of poly(oxyethylene)-co-poly(oxybutylene) uptake into the SiHy material, six contact-lens pieces previously swollen in PBS, each with 8 mm diameter and 80–100 μm thickness, were placed in a 600-mL aqueous-PBS bath containing 150 ppm of the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) wetting agent. Solution volume was chosen so that the polymeric-surfactant concentration remained unchanged or only slightly decreased (<3%) during the uptake process. The bath was kept at 35 °C under gentle magnetic stirring at 100 rpm. At selected times (i.e., 3 h, 1 day, 3 days, 7 days, and 14 days), a single gel piece was removed from the bath, lightly blotted on both surfaces to remove surface moisture, and manually cross-sectioned into three strips for fluorescence-microscope imaging. Each strip was placed on a microscope slide and covered with a coverslip, and intensity profiles were recorded in cross section on a Nikon Eclipse Ti2-E inverted fluorescence microscope (Tokyo, Japan) equipped with a Semrock 32 mm GFP filter (excitation wavelength of 466 nm, emission wavelength of 525 nm, and dichroic beam splitter of 495 nm). Background fluorescence intensity was subtracted from the solution and the lens, and ∼five representative line scan images of each sample were taken. Fluorescence intensities were then averaged into a single dynamic intensity curve with a precision of about ±15%. Because intensity is proportional to concentration, the resulting concentration profiles were used to quantify polymeric-surfactant uptake dynamics, as discussed above.
Poly(oxyethylene)-co-poly(oxybutylene) Saturation Uptake
To measure k, the same procedure as for the uptake dynamics was followed, except that the gel-soaking time was 14 days (see Figure 1) or longer to allow complete polymeric-wetting-agent saturation. Gel pieces were then removed from the solution, lightly blotted, and analyzed to determine Cg by two independent measurements: fluorescence microscopy and fluorescence plate reading.29,30
For fluorescence microscopy, pre-equilibrated gel pieces were cut into strips, individually placed on a microscope slide, and covered with a coverslip, and intensity was recorded on a Nikon Eclipse Ti2-E inverted fluorescence microscope (Tokyo, Japan) equipped with a Semrock 32 mm GFP filter (excitation wavelength of 466 nm, emission wavelength of 525 nm, and dichroic beam splitter of 495 nm). Bulk solution [150 ppm fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) in PBS] intensity was determined by capillary imbibition into the gap between two parallel microscope slides of the same gap thickness as the gel samples. Fluorescence intensity was assessed at the same microscope exposure settings as those for the corresponding gel pieces. For each lens, the scanning area was ∼400 μm2 with over 1500 intensity readings averaged to a single number for each lens. After correction for background intensities, the partition coefficient followed from the ratio of the two corrected intensities, as outlined in the Supporting Information.
For the fluorescence plate-reading method, the fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) surfactant in the pre-equilibrated lens pieces was first extracted by methanol with a lens-to-solvent ratio of one lens piece (4 μL) to 4 mL of methanol. After agitation for 3 days at room temperature, the lens piece was removed, the methanol was evaporated, and 3 mL of PBS was added back to the methanol residue reconstituting the extracted surfactant in aqueous PBS. An EnVision plate reader (Model 2105, PerkinElmer, Waltham, MA) with FITC 485 as the excitation filter and FITC 535 as the emission filter then measured the fluorescence intensity. The fluorescence plate reader was precalibrated against 200 μL of standard solutions (37.5, 75, 150, and 300 ppm) of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) surfactant in PBS. Once the extracted mass of surfactant was known from the calibrated plate-reader fluorescence, the polymeric-wetting-agent partition coefficient was then ascertained after dividing by the plate-reader intensity corresponding to 200 μL of 150 ppm of fluorescently tagged poly(oxyethylene)-co-poly(oxybutylene) in PBS. More detail is available in the Supporting Information.
Poly(oxyethylene)-co-poly(oxybutylene) Back-Extraction
Back-extraction experiments were performed to determine both the rate and final amount of poly(oxyethylene)-co-poly(oxybutylene) that was extracted from the lens material by PBS exposure. Wetting-agent release conditions were representative of the ocular environment: 35 °C and physiological pH of 7.4. Twenty pieces of whole contact lenses (∼14 mm in diameter) were first soaked in 4 L of PBS of 150 ppm poly(oxyethylene)-co-poly(oxybutylene) under magnetic stirring at 35 °C for 14 days to guarantee complete saturation. After saturation, the twenty lenses saturated with poly(oxyethylene)-co-poly(oxybutylene) were placed in 20 mL of PBS at 35 °C with agitation. At selected release times, 250 μL of the solution was sampled and subjected to analysis. Static sessile-drop contact-angle measurements on fully hydrated and immediately blot-dried contact lenses confirmed that the silicone-hydrogel surface wettability is preserved during sustained release of the wetting agent. A Waters Acquity UPLC H-Class system equipped with ELSD (evaporative light-scattering detector) measured the transient back-extracted poly(oxyethylene)-co-poly(oxybutylene) solution concentrations. The column used was a 5 μm C4 300Å; 50 mm × 4.6 mm (e.g., Phenomenex Jupiter or equivalent), and the ELSD detector settings were nitrogen gas (50 psi), nebulizer (mode–heating), and sample-compartment temperature at 25°C. Column conditions were as follows: 60 °C; ∼2000 PSI; mobile phase A: 0.5 M ammonium formate; and mobile phase B: 0.04% trifluoric acid /methanol. The total run time was about 30 min. Five replicate preparations at the target concentration exhibited less than 10.0% RSD. No analyte was detected in the blank solvent.
Acknowledgments
The authors would like to thank Drs. Charlie Shi, Ye Hong, and John Pruitt for their insightful comments and scientific discussions and Dr. Steve Zhang for providing the testing materials. This work was funded by Alcon Research, LLC.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03310.
Calibration curve for the fluorescence plate-reader method and the loading partition coefficient obtained from fluorescence imaging (PDF)
This work was funded by Alcon Research, LLC.
The authors declare no competing financial interest.
Notes
Y.Z., J.D., Y.W., L.Z., G.Y., Y.H.K., and J.Y.W. are employees of Alcon Research LLC. The authors declare no competing financial interest.
Supplementary Material
References
- Willcox M.; Keir N.; Maseedupally V.; Masoudi S.; McDermott A.; Mobeen R.; Purslow C.; Santodomingo-Rubido J.; Tavazzi S.; Zeri F.; Jones L. BCLA CLEAR - Contact lens wettability, cleaning, disinfection and interactions with tears. Contact Lens Anterior Eye 2021, 44, 157–191. 10.1016/j.clae.2021.02.004. [DOI] [PubMed] [Google Scholar]
- Fonn D. Targeting contact lens induced dryness and discomfort: What properties will make lenses more comfortable. Optom. Vision Sci. 2007, 84, 279–285. 10.1097/OPX.0b013e31804636af. [DOI] [PubMed] [Google Scholar]
- Hyon S.-H.; Cha W.-I.; Ikada Y.; Kita M.; Ogura Y.; Honda Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci., Polym. Ed. 1994, 5, 397–406. 10.1163/156856294X00103. [DOI] [PubMed] [Google Scholar]
- Kita M.; Ogura Y.; Honda Y.; Hyon S.-H.; Cha W. II; Ikada Y. Evaluation of polyvinyl alcohol hydrogel as a soft contact lens material. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 533–537. 10.1007/BF00918486. [DOI] [PubMed] [Google Scholar]
- Phan C. M.; Walther H.; Riederer D.; Lau C.; Lorenz K. O.; Subbaraman L. N.; Jones L. Analysis of polyvinyl alcohol release from commercially available daily disposable contact lenses using an in vitro eye model. J. Biomed. Mater. Res., Part B 2019, 107, 1662–1668. 10.1002/jbm.b.34259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterton L. C.; Lally J. M.; Sentell K. B.; Chapoy L. L. The elution of poly (vinyl alcohol) from a contact lens: The realization of a time release moisturizing agent/artificial tear. J. Biomed. Mater. Res., Part B 2007, 80B, 424–432. 10.1002/jbm.b.30613. [DOI] [PubMed] [Google Scholar]
- Wolffsohn J. S.; Hunt O. A.; Chowdhury A. Objective clinical performance of ’comfort-enhanced’ daily disposable soft contact lenses. Contact Lens Anterior Eye 2010, 33, 88–92. 10.1016/j.clae.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Jee J.-P.; Kim H.-J. Development of hydrogel lenses with surface-immobilized PEG layers to reduce protein adsorption. Bull. Korean Chem. Soc. 2015, 36, 2682–2687. 10.1002/bkcs.10545. [DOI] [Google Scholar]
- Phan C.-M.; Walther H.; Smith R. W.; Riederer D.; Lau C.; Osborn Lorenz K.; Subbaraman L. N.; Jones L. Determination of the release of PEG and HPMC from nelfilcon A daily disposable contact lenses using a novel in vitro eye model. J. Biomater. Sci., Polym. Ed. 2018, 29, 2124–2136. 10.1080/09205063.2018.1514192. [DOI] [PubMed] [Google Scholar]
- Maulvi F. A.; Patel P. J.; Soni P. D.; Desai A. R.; Desai D. T.; Shukla M. R.; Ranch K. M.; Shah S. A.; Shah D. O. Novel poly(vinylpyrrolidone)-coated silicone contact lenses to improve tear volume during lens wear: In vitro and in vivo studies. ACS Omega 2020, 5, 18148–18154. 10.1021/acsomega.0c01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J.; Reindel W.; Steffen R.; Mosehauer G.; Chinn J. Use of a novel extended blink test to evaluate the performance of two polyvinylpyrrolidone-containing, silicone hydrogel contact lenses. Clin. Ophthalmol. 2018, 12, 819–825. 10.2147/OPTH.S162233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yañez F.; Concheiro A.; Alvarez-Lorenzo C. Macromolecule release and smoothness of semi-interpenetrating PVP–pHEMA networks for comfortable soft contact lenses. Eur. J. Pharm. Biopharm. 2008, 69, 1094–1103. 10.1016/j.ejpb.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Hoteling A. J.; Nichols W. F.; Harmon P. S.; Conlon S. M.; Nuñez I. M.; Hoff J. W.; Cabarcos O. M.; Steffen R. B.; Hook D. J. Characterization and quantitation of PVP content in a silicone hydrogel contact lens produced by dual-phase polymerization processing. J. Biomed. Mater. Res., Part B 2018, 106, 1064–1072. 10.1002/jbm.b.33904. [DOI] [PubMed] [Google Scholar]
- Menzies K. L.; Jones L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vision Sci. 2010, 87, 387–399. 10.1097/OPX.0b013e3181da863e. [DOI] [PubMed] [Google Scholar]
- Kading D. A two-week clinical evaluation of the safety of Systane Ultra in contact lens-wearing patients. Clin. Ophthalmol. 2010, 4, 27–32. 10.2147/OPTH.S8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho F.; do Vale Braido G. V.; Cavicchioli M.; Mendes L. S.; Specian S. S.; Franchi L. P.; Lima Ribeiro S. J.; Messaddeq Y.; Scarel-Caminaga R. M.; O Capote T. S. Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency. Contact Lens Anterior Eye 2019, 42, 512–519. 10.1016/j.clae.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Vehige J. G.; Simmons P. A.; Anger C.; Graham R.; Tran L.; Brady N. Cytoprotective properties of carboxymethyl cellulose (CMC) when used prior to wearing contact lenses treated with cationic disinfecting agents. Eye Contact Lens 2003, 29, 177–180. 10.1097/01.ICL.0000074106.82322.17. [DOI] [PubMed] [Google Scholar]
- White C. J.; McBride M. K.; Pate K. M.; Tieppo A.; Byrne M. E. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 2011, 32, 5698–5705. 10.1016/j.biomaterials.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Fagnola M.; Pagani M. P.; Maffioletti S.; Tavazzi S.; Papagni A. Hyaluronic acid in hydrophilic contact lenses: Spectroscopic investigation of the content and release in solution. Contact Lens Anterior Eye 2009, 32, 108–112. 10.1016/j.clae.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Singh A.; Li P.; Beachley V.; McDonnell P.; Elisseeff J. H. A hyaluronic acid-binding contact lens with enhanced water retention. Contact Lens Anterior Eye 2015, 38, 79–84. 10.1016/j.clae.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks A.; Boone A.; Luensmann D.; Jones L.; Sheardown H. The effects of hyaluronic acid incorporated as a wetting agent on lysozyme denaturation in model contact lens materials. J. Biomater. Appl. 2013, 28, 323–333. 10.1177/0885328212446936. [DOI] [PubMed] [Google Scholar]
- Weeks A.; Subbaraman L. N.; Jones L.; Sheardown H. Physical entrapment of hyaluronic acid during synthesis results in extended release from model hydrogel and silicone hydrogel contact lens materials. Eye Contact Lens 2013, 39, 179–185. 10.1097/ICL.0b013e318281ae06. [DOI] [PubMed] [Google Scholar]
- Ketelson H. A.; Meadows D. L.; Stone R. P. Dynamic wettability properties of a soft contact lens hydrogel. Colloids Surf., B 2005, 40, 1–9. 10.1016/j.colsurfb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Peng C.-C.; Kim J.; Chauhan A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. 10.1016/j.biomaterials.2010.01.113. [DOI] [PubMed] [Google Scholar]
- Huo Y.; Rudy A.; Wang A.; Ketelson H.; Perry S. Impact of ethylene oxide butylene oxide copolymers on the composition and friction of silicone hydrogel surfaces. Tribol. Lett. 2012, 45, 505–513. 10.1007/s11249-011-9902-7. [DOI] [Google Scholar]
- Nace V. M. Contrasts in the surface activity of polyoxypropylene and polyoxybutylene-based block copolymer surfactants. J. Am. Oil Chem. Soc. 1996, 73, 1–8. 10.1007/BF02523440. [DOI] [Google Scholar]
- Huo Y.; Ketelson H.; Perry S. Ethylene oxide-block-butylene oxide copolymer uptake by silicone hydrogel contact lens materials. Appl. Surf. Sci. 2013, 273, 472–477. 10.1016/j.apsusc.2013.02.064. [DOI] [Google Scholar]
- Kotsmar C.; Sells T.; Taylor N.; Liu D. E.; Prausnitz J. M.; Radke C. J. Aqueous solute partitioning and mesh size in HEMA/MAA hydrogels. Macromolecules 2012, 45, 9177–9187. 10.1021/ma3018487. [DOI] [Google Scholar]
- Dursch T. J.; Taylor N. O.; Liu D. E.; Wu R. Y.; Prausnitz J. M.; Radke C. J. Water-soluble drug partitioning and adsorption in HEMA/MAA hydrogels. Biomaterials 2014, 35, 620–629. 10.1016/j.biomaterials.2013.09.109. [DOI] [PubMed] [Google Scholar]
- Guan L.; Jiménez M. G.; Walowski C.; Boushehri A.; Prausnitz J. M.; Radke C. J. Permeability and partition coefficient of aqueous sodium chloride in soft contact lenses. J. Appl. Polym. Sci. 2011, 122, 1457–1471. 10.1002/app.33336. [DOI] [Google Scholar]
- Liu D. E.; Kotsmar C.; Nguyen F.; Sells T.; Taylor N. O.; Prausnitz J. M.; Radke C. J. Macromolecule sorption and diffusion in HEMA/MAA hydrogels. Ind. Eng. Chem. Res. 2013, 52, 18109–18120. 10.1021/ie402148u. [DOI] [Google Scholar]
- Brady J. F.Hindered Diffusion. In Extended Abstracts; AIChE Annual Meeting: San Francisco, CA, 1994; p 320. [Google Scholar]
- Liu D. E.; Dursch T. J.; Taylor N. O.; Chan S. Y.; Bregante D. T.; Radke C. J. Corrigendum to “Diffusion of water-soluble sorptive drugs in HEMA/MAA hydrogels [J. Control. Release, 239,(October 10, 2016), 242-248,]”. J. Controlled Release 2017, 249, 197 10.1016/j.jconrel.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Liu D. E.; Dursch T. J.; Taylor N. O.; Chan S. Y.; Bregante D. T.; Radke C. J. Diffusion of water-soluble sorptive drugs in HEMA/MAA hydrogels. J. Controlled Release 2016, 239, 242–248. 10.1016/j.jconrel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Dong M. W.HPLC and UHPLC for Practicing Scientists, 2nd ed.; Wiley: Hoboken, 2019; pp 45–78. [Google Scholar]
- Davis J. W.; Ketelson H. A.; Meadows D. L.. Ethyleneoxide butyleneoxide block copolymer compositions, Alcon Research, Ltd., US9,175,249, 2015.
- Dou J.Modulation of Cell Behaviors on Photo-Crosslinked Polymer Networks and Polymer Spherulites, 2017, University of Tennessee: Knoxville, https://trace.tennessee.edu/utk_graddiss/4457 (accessed January 02, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.