Abstract
Women with breast cancer experience social disruption during and after treatment. Brief cognitive-behavioral (CBT) and relaxation (RT) interventions may improve social disruption by increasing positive affect. Using the Broaden-and-Build Theory as a framework, this study examined whether short-term CBT- and RT-related increases in positive affect mediate long-term reductions in social disruption in women with breast cancer undergoing treatment (N = 183). This secondary analysis used latent change score and growth models to test 6- and 12-month intervention effects on positive affect and social disruption, respectively; a parallel-process model assessed mediation. RT demonstrated larger reductions in social disruption across 12 months compared to CBT and a health education control. Six-month latent change in positive affect was significant but not driven by condition. There was a significant direct effect linking the latent slopes of positive affect and social disruption but meditation was not observed. These preliminary findings hint at the value of promoting positive affect and inform the development of brief behavioral interventions that aim to augment social functioning among women surviving breast cancer.
Keywords: breast cancer, Broaden-and-Build theory, social disruption, positive affect, relaxation training
There are nearly 4 million women in the United States with a history of breast cancer (DeSantis et al., 2019). This growing cohort of survivors is encouraging, yet women with breast cancer experience considerable psychosocial change during and after breast-conserving surgeries and adjuvant therapies (DeSantis et al., 2019; Runowicz et al., 2016). Commonly reported psychosocial challenges of breast cancer diagnosis and treatment include symptoms of depression and anxiety, fear of recurrence, disability, and reduced quality of life (Runowicz et al., 2016). Women with breast cancer also describe disruption to social functioning as an equally distressing aspect of their illness experience (Keesing et al., 2018; Trusson & Pilnick, 2017).
Illness-related social disruption (i.e., tendency to disengage from social activities and isolate from social networks due to a medical diagnosis; Carver et al., 2003) is prevalent among women with breast cancer (Choi et al., 2014; Leung et al., 2014; Runowicz et al., 2016). Fatigue and pain may render social outings more difficult in the months surrounding primary treatment; research shows that higher levels of fatigue and pain are associated with poorer social functioning throughout the breast cancer experience (Calderon et al., 2019; Leung et al., 2014; Thompson et al., 2013). Likewise, cosmetic changes postsurgery and chemotherapy may complicate efforts to participate in social interactions and activities (Runowicz et al., 2016). Choi and colleagues demonstrated that many survivors of breast cancer feel uncomfortable in public due to chemotherapy-induced alopecia, and thus avoid social outings (Choi et al., 2014). Finally, for patients with breast cancer experiencing symptoms of depression and anxiety, it is possible that social activities become unappealing during and after adjuvant treatment (Fong et al., 2017). A bidirectional relationship has been documented in the literature wherein symptoms of depression and anxiety relate to reduced social engagement with family members, friends, and work colleagues (Fong et al., 2017; Thompson et al., 2013). Given this, it is unsurprising that illness-related social disruption can undermine psychological adjustment after breast cancer diagnosis and has been linked to increased breast-cancer related mortality (Kroenke et al., 2017).
Conversely, enhanced social functioning is associated with improved physical and mental health outcomes among women with breast cancer (Calderon et al., 2019; Fong et al., 2017). As such, psychosocial interventions for women with breast cancer often incorporate content that targets illness-related social disruption. One such intervention is Cognitive Behavioral Stress Management (CBSM; Antoni, 2003), a 10-week, group-based protocol that combines specific strategies from cognitive-behavioral therapy (CBT; e.g., positive reappraisal, problem-focused coping, communication skills) and relaxation training (RT; e.g., diaphragmatic breathing, progressive muscle relaxation, imagery, mindfulness meditation). Participation in the 10-week CBSM protocol has been shown to reduce illness-related social disruption among women with breast cancer at posttreatment, 6-month, and 12-month follow-up time points (Antoni, Lechner, et al., 2006). Additionally, the 10-week CBSM protocol has been linked with attenuated symptoms of depression and anxiety, and increased positive affect during primary treatment for breast cancer and into survivorship (Antoni, Lechner, et al., 2006; Antoni et al., 2001; Antoni, Wimberly, et al., 2006; Stagl et al., 2015).
Yet, this 10-session, multicomponent intervention may be increasingly impractical in fast-paced oncology settings where time and resources are limited (Casellas-Grau et al., 2014). Psychosocial interventions comprised of only 4–6 sessions are potent and can significantly improve cancer symptom (i.e., distress, pain) management (Sheinfeld Gorin et al., 2012; Somers et al., 2016). For brief psychosocial interventions to be deployed effectively among women with breast cancer, it is necessary to understand the ingredients most critical to include in these protocols (Kelleher et al., 2017). This will allow researchers to develop concise protocols that are well-suited to specific patient concerns (Kelleher et al., 2017). Early research on the 10-session CBSM protocol was unable to elucidate which distinct cognitive-behavioral or relaxation strategies drove psychological outcomes in breast cancer samples (Antoni, Lechner, et al., 2006). When tested separately, brief cognitive-behavioral interventions, as well as relaxation interventions, have been shown to enhance social functioning among women with breast cancer (Carlson et al., 2013; Freeman et al., 2008; Henderson et al., 2013). Still, findings on the differential effects of cognitive-behavioral interventions versus relaxation interventions are lacking (Gudenkauf et al., 2015).
To this end, Gudenkauf et al. (2015) completed a dismantling trial of the traditional 10-week CBSM program wherein 5-week, group-based CBT (i.e., positive reappraisal, problem-focused coping, communication skills) and RT (i.e., diaphragmatic breathing, imagery, progressive muscle relaxation, mindfulness meditation) interventions were tested against each other and an attention-matched health education (HE) control group. Findings revealed that women who received RT reported significantly less illness-related social disruption at posttreatment compared to those randomized to the CBT or HE group conditions. These data have only been assessed immediately following the brief intervention period, warranting research on the longitudinal effects of RT (Gudenkauf et al., 2015). Furthermore, the underlying mechanism of this brief relaxation intervention remains unclear. Participation in a group intervention may, in itself, improve social connection via nonspecific processes such as group cohesion and collaboration (Schnur & Montgomery, 2010). Yet, such group processes existed across all RT, CBT, and HE interventions. Thus, it is unclear why only the 5-week RT protocol was linked to significant reductions in illness-related social disruption (Gudenkauf et al., 2015).
A potential mechanism that may explain the effect of relaxation interventions on illness-related social disruption is Fredrickson’s Broaden-and-Build theory of positive emotions. This theory posits that positive emotions broaden an individual’s perception of available behaviors and thoughts, and in turn build enduring social, emotional, and physical resources (Fredrickson, 2001, 2004). With respect to relaxation techniques, Fredrickson hypothesized that progressive muscle relaxation (PMR), imagery, and mindfulness meditation may cultivate positive emotions such as relief, calmness, and contentment. These positive emotions might then become self-reinforcing, encouraging one to savor the moment and relish in social connections, which can sustain the affective experience (Fredrickson et al., 2000). This aligns nicely with Carver’s (2003) account of affect and behavior; that is, within the behavioral avoidance system, PMR, imagery, and mindfulness meditation offer ways to avoid tension, and successful reduction of negative affect or subjective stress may simultaneously induce positive affect states (Carver, 2003). It is suggested that positive affective experiences of relief, calmness, and/or contentment may prompt a coasting response, wherein an individual slows down progress towards one focal goal to allow for attentional broadening and/or a reprioritization of alternative goals in line with current stress (e.g., breast cancer diagnosis and treatment; Carver, 2003). It is plausible that within the context of a focal goal to survive breast cancer, reprioritization of a broader array of goals could manifest as the creation of new, or resumption of old social roles, relationships, and activities.
Both Fredrickson and Carver note that a similar mechanism may also occur for cognitive-behavioral interventions, which cultivate positive emotions via approach-focused techniques such as behavioral activation that reinforce environmental contingencies by increasing engagement in value-driven behavior (Carver, 2003; Fredrickson, 2001, 2004). Positive affective states, such as joy, that are engendered by cognitive-behavioral strategies could in turn motivate social engagement. The dismantling trial of the 10-week CBSM protocol found that both RT and CBT groups exhibited significant reductions in depressed affect; however, changes to positive affect were not assessed (Gudenkauf et al., 2015). A review from Garland and colleagues (2010) highlights that mental imagery and mindfulness meditation are especially useful in generating positive emotions. Notably, in a randomized controlled trial of patients with comorbid depression and rheumatoid arthritis, mindfulness training increased positive affect significantly more than CBT and an education control (Zautra et al., 2008). Likewise, Fredrickson et al. (2008) observed increased positive affect in a sample of working adults after participation in a 7-week loving-kindness meditation workshop. More work is needed to clarify whether distinct RT and/or CBT interventions differentially enhance positive affect in cancer samples, and if this might drive reductions in illness-related social disruption.
To address these gaps in the literature, the present study utilized the sample of 183 women with breast cancer from Gudenkauf et al.’s dismantling trial (2015) to elucidate unique short- and long-term effects of a brief RT intervention in comparison to CBT and HE, and examine a potential mechanism through the framework of the Broaden-and-Build theory. First, we tested the effects of a 5-week, group-based RT intervention on illness-related social disruption over the initial 12 months of primary treatment for early-stage breast cancer. Based on prior work from Gudenkauf et al. (2015), we hypothesized that the RT intervention would decrease illness-related social disruption relative to CBT and HE group conditions. Second, we tested the effects of the RT intervention on change in positive affect over the initial 6 months of primary treatment for breast cancer. Given previous findings suggesting mental imagery and mindfulness meditation might increase positive effect (Fredrickson et al., 2008; Garland et al., 2010; Zautra et al., 2008), we hypothesized that the RT intervention would increase positive affect relative to CBT and HE group conditions. Finally, we tested whether the effect of the RT intervention on positive affect over 6 months mediated 12-month intervention effects on illness-related social disruption. We hypothesized that increase in positive affect over the initial 6-month period would mediate 12-month reduction to illness-related social disruption.
Methods
PARTICIPANTS
Women with nonmetastatic Stage 0–III breast cancer were recruited through physician referrals and advertising, and enrolled in a randomized controlled trial (RCT) between 2006–2013. Participants were required to have had surgery for primary breast cancer in the 2–10 weeks before enrollment. Exclusion criteria included: (1) diagnosis of Stage IV breast cancer or other cancer (except squamous or basal cell skin cancer); (2) ongoing neoadjuvant or postsurgical adjuvant treatment; (3) falling outside the ages of 21–75 years; (4) nonfluency in English; (5) past or current diagnosis of, or hospitalization for psychosis, suicidality, major depressive disorder, or panic disorder. The parent RCT (Gudenkauf et al., 2015) was approved by the appropriate Institutional Review Board and registered on ClinicalTrials.gov (NCT02103387).
PROCEDURES
Women with early-stage breast cancer were consented, enrolled, and completed a baseline assessment (T1) that occurred approximately 2–10 weeks postsurgery and prior to the initiation of adjuvant cancer treatment. Part of this initial assessment consisted of psychosocial questionnaires measuring demographic and medical variables, as well as illness-related social disruption and positive affect. After baseline, women were randomized into one of three group conditions: CBT, RT, or an HE time-matched control. Women were reassessed following the group interventions, approximately 2 months (T2), 6 months (T3), and 12 months (T4) postbaseline.
INTERVENTION CONDITIONS
Intervention groups were comprised of 3–7 women who met once weekly for 5 weeks. All sessions were approximately 1.5 hours and facilitated by master’s-level predoctoral psychology trainees. Interventionists were trained in all condition protocols and were available to lead any intervention, depending on group need and interventionist availability. Group sessions were video and audio recorded. All recordings were discussed during weekly, in-person supervision meetings with two licensed psychologists. Random segments of the recordings were reviewed; supervisors discussed notes regarding treatment fidelity with interventionists immediately after review of recordings. If cross-contamination was identified (e.g., delivery of CBT skill during RT protocol), supervisors provided additional training in CBT, RT, and HE content and reiterated instructions for distinguishing CBT, RT, and HE protocols. These procedures have been published elsewhere (Gudenkauf et al., 2015).
CBT
The CBT intervention condensed a 10-week group CBSM protocol (Antoni, 2003) into five sessions covering cognitive-behavioral strategies focused on stress awareness, positive reappraisal, problem-focused coping, assertiveness, and anger management. Women in the group had the opportunity to practice CBT skills with in-session demonstrations as well as at-home practice exercises. There was no content from the RT and/or HE interventions.
RT
The RT intervention was also adapted from Antoni’s (2003) 10-week CBSM protocol and taught techniques to reduce anxiety via diaphragmatic breathing, imagery, PMR, and mindfulness meditation over the course of five weekly sessions. Similar to the CBT intervention, women assigned to the RT group had the opportunity to practice RT skills in-session and at-home with audio recordings. There was no content from the CBT and/or HE interventions.
HE
The HE intervention provided a stringent group-based attention and time-matched control for CBT and RT. Over the course of five group sessions, women were provided with educational content related to breast cancer and its treatment, management of side effects, and healthy lifestyle behaviors. This educational content was obtained from the American Cancer Society, the National Cancer Institute, Susan G. Komen, Dr. Susan Love Research Foundation, and the Livestrong Foundation. Women randomized to this group were not exposed to CBT and/or RT techniques.
MEASURES
Demographic and Medical Characteristics
At the time of enrollment, self-reported information was collected regarding demographics (e.g., age, race/ethnicity, and partnered status) and socioeconomic status (e.g., income and education). Follow-up assessment time points similarly obtained self-reported medical and treatment-related information (e.g., stage of disease, hormonal status, number of positive nodes, surgical procedure, days since surgery, and type of adjuvant treatment received). Self-reported medical information was verified with chart review.
Social Disruption
The tendency to disengage from social activities and isolate from social networks was measured with the 11-item Social Disruption category of the Sickness Impact Profile (SIP-SD; Bergner et al., 1981). Participants were asked to determine how 11 statements applied to their activities over the “past few weeks,” and whether limitations are due to their current state of health (e.g., “I am going out less to visit people,” “I am avoiding social visits from others”). Two response options were provided: 1 = no or 2 = yes, this applies to me. Each item has a specific weight that reflects its relative severity of limitation; weighted scores were summed to obtain a total score. Per Pollard and Johnston (2001), the current study calculated a percentage limitation by dividing this total score by the maximum possible category score (1450) and multiplying by 100%. Higher percentage scores represented higher levels of social disruption (Pollard & Johnston, 2001). The full SIP has been validated on a variety of patient groups (Bergner et al., 1981) and the SIP-SD subscale has been used in breast cancer samples (Antoni, Wimberly, et al., 2006; Gudenkauf et al., 2015). Reliability for the current sample was adequate, ranging from alpha = .82–.89 across time points.
Positive Affect
Positive affect was measured using the 40-item Affect Balance Scale (ABS), which has been used in previous research on women with breast cancer (Derogatis, 1975). The ABS is a set of adjectives assessing aspects of positive (e.g., joy, contentment, affection, and vigor) and negative (e.g., depression, hostility, guilt, and anxiety) feelings separately. Women made ratings of the extent to which they had experienced the feeling “during the past week including today,” using a 5-point Likert-type scale ranging from 1 = never to 5 = always. The current study utilized the total positive affect score (ABS-POS), which is comprised of all four, 5-item positive affect dimensions. Items were averaged to obtain a total score, with higher scores indicating more positive affect. Alpha reliabilities of ABS-POS in the current sample were adequate, ranging from .78 to .86 across time points.
ANALYTICAL STRATEGY
Preliminary descriptive analyses were conducted using Statistical Package for the Social Sciences Version 24 (SPSS 24). Outcome variables (SIP-SD and ABS-POS) were inspected for skewness and kurtosis, and violations to assumptions of normality and homoscedasticity (Kline, 2005). Subsequent analyses were performed using Mplus Version 7 (Muthén & Muthén, 2012).
Model Specification
The RT intervention effect on SIP-SD from baseline to 12-month follow-up was specified using a latent-growth model with treatment included as a predictor. Group condition was dummy coded to allow for comparisons of all intervention conditions. In Model A, the HE intervention was coded as the referent group (i.e., 0), while the CBT and RT interventions were coded as 1 to form two separate dummy vectors (i.e., CBT vs. HE and RT vs. HE). In Model B, the CBT intervention was coded as the referent group (i.e., 0), while the RT and HE were coded as 1 to form two separate dummy vectors (i.e., RT vs. CBT and HE vs. CBT). The intercept (i.e., starting value of the trajectory) and slope (i.e., rate of change over repeated measures) were represented as latent variables capturing data from time points of interest. Loadings for the intercept latent variable were constrained to one and loadings for the slope latent variable were set equal to the number of months after baseline at which measurements were obtained (i.e., 0, 2, 6, 12 months; Muthén & Muthén, 2012). The path from the dummy vector to the latent slope variable reflected the change over time in SIP-SD that was attributable to group condition.
The trajectory of ABS-POS from baseline to 6-month follow-up was modeled using a latent-change score model (McArdle, 2009). To fit the model, a latent variable representing change in ABS-POS was specified by the single indicator of T3 ABS-POS, and then regressed on T1 ABS-POS with indicator loadings constrained to one. The estimate of the latent variable regressed on the dummy vectors (CBT vs. HE and RT vs. HE) provided a measure of error-free, treatment-related residualized change in ABS-POS from T1 to T3.
To evaluate for mediation, the latent-change score model for the proposed mediator, positive affect (ABS-POS), and the latent growth model for the outcome variable (SIP-SD) were entered into parallel-process latent growth/change models with two dummy vectors to compare group conditions (CBT vs. HE and RT vs. HE). Advantages of parallel-process growth modeling include the ability to model simultaneous change processes over time and to estimate residual variance components that are crucial to understanding change (Curran et al., 2014). Growth modeling also offers flexibility with missing data by using full information maximum likelihood (FIML; Kline, 2005). FIML uses all available data for each person, estimating missing information from relations among variables in the full sample (Kline, 2005). Four indices were estimated and interpreted for model fit: chi-square test (χ2) p > .05, confirmatory fit index (CFI) > .95, root-mean-squared-error of approximation (RMSEA) < .06, and standardized-root-mean-square residual (SRMR) < .08 (Kline, 2005). Significant standardized coefficients were interpreted as measures of effect sizes in accordance with the following levels: .1 = small; .3 = medium; .5 = large (Cohen, 1988).
Covariates
Based on use in prior literature, demographic (age) and cancer-related (stage of disease and type of adjuvant therapy received) factors served as covariates (Stagl et al., 2015). Stage of disease was categorized as noninvasive (Stage 0 = 0) or invasive (Stage I, II, or III = 1). Adjuvant (radiation, and/or chemotherapy) and anti-hormonal therapy was categorized dichotomously (No = 0 vs. Yes = 1) to capture whether women received radiation, chemotherapy and/or hormone therapy throughout their treatment course. Models were estimated both with and without covariates.
Results
PARTICIPANT CHARACTERISTICS
Between 2006 and 2013, 739 women with newly diagnosed early-stage breast cancer were screened for participation. From this initial screening sample, 556 women were excluded or withdrew before randomization. Informed consent was obtained from 183 women who then completed their T1 assessment and were randomized to CBT (n = 55), RT (n = 70), or HE (n = 58). Of these, 138 women (75.4%) completed the T2 post-intervention assessment, 130 (71.0%) completed the T3 assessment, and 136 (74.3%) completed the T4 assessment. Data was analyzed on an intent-to-treat basis, thus all 183 enrolled participants were included in longitudinal analyses. Number of sessions attended did not differ based on group assignment (CBT M = 3.98, SD = 1.47; RT M = 3.61, SD = 1.58; HE M = 4.29, SD = 1.08; p > .05).
Within the entire sample women were an average of 54.28 (SD = 10.06) years old and the majority were partnered at the time of enrolment (63.9%). There was a similar representation of women who self-identified as Hispanic (44.8%) and non-Hispanic White (41.5%), while the remainder of the sample identified as Black/African-American (8.7%) or other racial/ethnic categories (4.4%). Women completed 15.70 years (SD = 2.55) of education on average, and reported an average household income of $101, 236 per year (SD = 67.03).
Most women were diagnosed with Stage I breast cancer (51.4%). The remainder were diagnosed with ductal carcinoma in-situ (DCIS; 19.1%), Stage II (24.0%), or Stage III (4.9%) disease. Type of surgical procedure was distributed evenly, with 48.6% of women undergoing a lumpectomy and 51.4% undergoing a mastectomy. Per medical records, 77.0% of the sample had estrogen receptor-positive breast cancer, 66.7% had progesterone receptor-positive disease, and a minority of women (20.2%) had positive lymph nodes. On average, 37.42 days (SD = 22.30) elapsed between surgery and study enrollment. A large portion of women (80.9%) received some form of adjuvant treatment after surgical intervention. Medical and demographic characteristics compared by group condition are reported in Table 1.
Table 1.
Means, Standard Deviations, and Frequencies for Baseline Demographic and Medical Variables by Group Condition
| Variable | CBT (n = 55) | RT (n = 70) | HE (n = 58) | Statistic | p |
|---|---|---|---|---|---|
| Age (yrs) | 54.62 (9.15) | 53.69 (11.51) | 54.67 (9.09) | F(2,180) = .20 | .82 |
| Race/Ethnicity | χ2(6) = 4.69 | .59 | |||
| NHW | 25 (45.5%) | 24 (34.3%) | 27 (46.6%) | ||
| Hispanic | 20 (36.4%) | 35 (50.0%) | 23 (39.7%) | ||
| African-American | 4 (7.3%) | 6 (8.6%) | 6 (10.3%) | ||
| Other | 5 (9.1%) | 5 (7.1%) | 2 (3.4%) | ||
| Income (thousands) | 99.76 (67.48) | 83.40 (63.78) | 120.29 (83.49) | F(2,155) = 3.58 | .03* |
| Education (yrs) | 16.11 (2.56) | 15.25 (2.34) | 15.82 (2.72) | F(2,173) = 1.82 | .17 |
| Married/partnered | 39 (70.9%) | 40 (57.1%) | 38 (65.5%) | χ2(2) = 2.39 | .30 |
| Stage | χ2(6) = 2.36 | .88 | |||
| 0 | 11 (20.0%) | 12 (17.1%) | 12 (20.7%) | ||
| I | 31 (56.4%) | 34 (48.6%) | 29 (50.0%) | ||
| II | 11 (20.0%) | 20 (28.6%) | 13 (22.4%) | ||
| III | 2 (3.6%) | 3 (4.3%) | 4 (6.9%) | ||
| Positive Nodes (yes) | 6 (10.9%) | 16 (22.9%) | 15 (25.9%) | χ2(2) = 4.23 | .12 |
| Hormonal Status | |||||
| ER positive | 44 (80.0%) | 56 (80.0%) | 41 (70.7%) | χ2(2) = 3.33 | .19 |
| PR positive | 36 (65.5%) | 49 (70.0%) | 37 (63.8%) | χ2(2) = .31 | .86 |
| Surgical Procedure | χ2(2) = .82 | .66 | |||
| Lumpectomy | 24 (43.6%) | 35 (50.0%) | 30 (51.7%) | ||
| Mastectomy | 31 (56.4%) | 35 (50.0%) | 28 (48.3%) | ||
| Days Since Surgery | 35.80 (22.04) | 38.40 (18.47) | 35.48 (17.95) | F(2,180) = .44 | .65 |
| Adjuvant Treatment | |||||
| Chemotherapy | 17 (30.9%) | 26 (37.1%) | 20 (34.5%) | χ2(2) = .83 | .66 |
| Radiation | 25 (45.5%) | 28 (40.0%) | 34 (58.6%) | χ2(2) = 2.96 | .23 |
| Anti-hormonal | 43 (78.2%) | 47 (67.1%) | 42 (72.4%) | χ2(2) = 1.65 | .44 |
Note. CBT = Cognitive Behavioral Training; RT = Relaxation Training; HE = Health Education; NHW = Non-Hispanic White; ER = Estrogen Receptor; PR = Progesterone Receptor;
p < .05.
Means and standard deviations for main study variables across all women are shown in Table 2. These statistics are in line with previous reports on this sample (Gudenkauf et al., 2015), as well as prior work by this group and others on survivors of early-stage breast cancer (Antoni, Lechner, et al., 2006; Antoni et al., 2001). Zero-order correlations suggest that all study outcomes are significantly correlated across study time points (ps < .01).
Table 2.
Means, Standard Deviations, and Correlation Matrix for Main Study Variables
| Variable | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|---|---|
| ABS-POS | ABS-POS | ABS-POS | ABS-POS | SIP-SD | SIP-SD | SIP-SD | SIP-SD | |
| Mean | 3.41 | 3.56 | 3.58 | 3.64 | 60.60 | 58.96 | 56.53 | 55.33 |
| (SD) | (.69) | (.61) | (.60) | (.64) | (11.72) | (10.89) | (9.65) | (6.95) |
| T1 ABS-POS | 1 | – | – | – | – | – | – | – |
| T2 ABS-POS | .72** | 1 | – | – | – | – | – | – |
| T3 ABS-POS | .59** | .67** | 1 | – | – | – | – | – |
| T4 ABS-POS | .66** | .64** | .79** | 1 | – | – | – | – |
| T1 SIP-SD | −.54** | −.41** | −.36** | −.38** | 1 | – | – | – |
| T2 SIP-SD | −.55** | −.59** | −.46** | −.50** | .63** | 1 | – | – |
| T3 SIP-SD | .29** | −.33** | −.55** | −.40** | .49** | .54** | 1 | – |
| T4 SIP-SD | −.35** | −.33** | −.37** | −.40** | .36** | .59** | .63** | 1 |
Note. ABS-POS = total positive affect subscale of Affect Balance Scale; SIP-SD = social disruption category of Sickness Impact Profile;
p < .01.
INTERVENTION EFFECTS ON ILLNESS-RELATED SOCIAL DISRUPTION (SIP-SD)
Group trajectories of mean values for SIP-SD suggested a linear decline in percent disruption for all conditions. At baseline, those in the HE group had significantly lower SIP-SD scores compared to those in the RT group (HE: M = 57.56; RT: M = 62.94, p < .05). An unconditional linear latent growth model had good model fit, χ2(5) = 5.20, p = .39; CFI = 1.00 (RMSEA = 0.02; SRMR = 0.07) and showed a significant time effect across the entire sample, wherein women demonstrated an average decrease in SIP-SD of .37 from time point to time point (β = −.54, SE = .11, z = −5.67, 95% CI [−.75, −.34], p < .001; Figure 1). Intercept and slope factors were observed to be significantly associated, with higher initial values of SIP-SD being correlated with smaller rates of linear decrease in SIP-SD.
FIGURE 1.
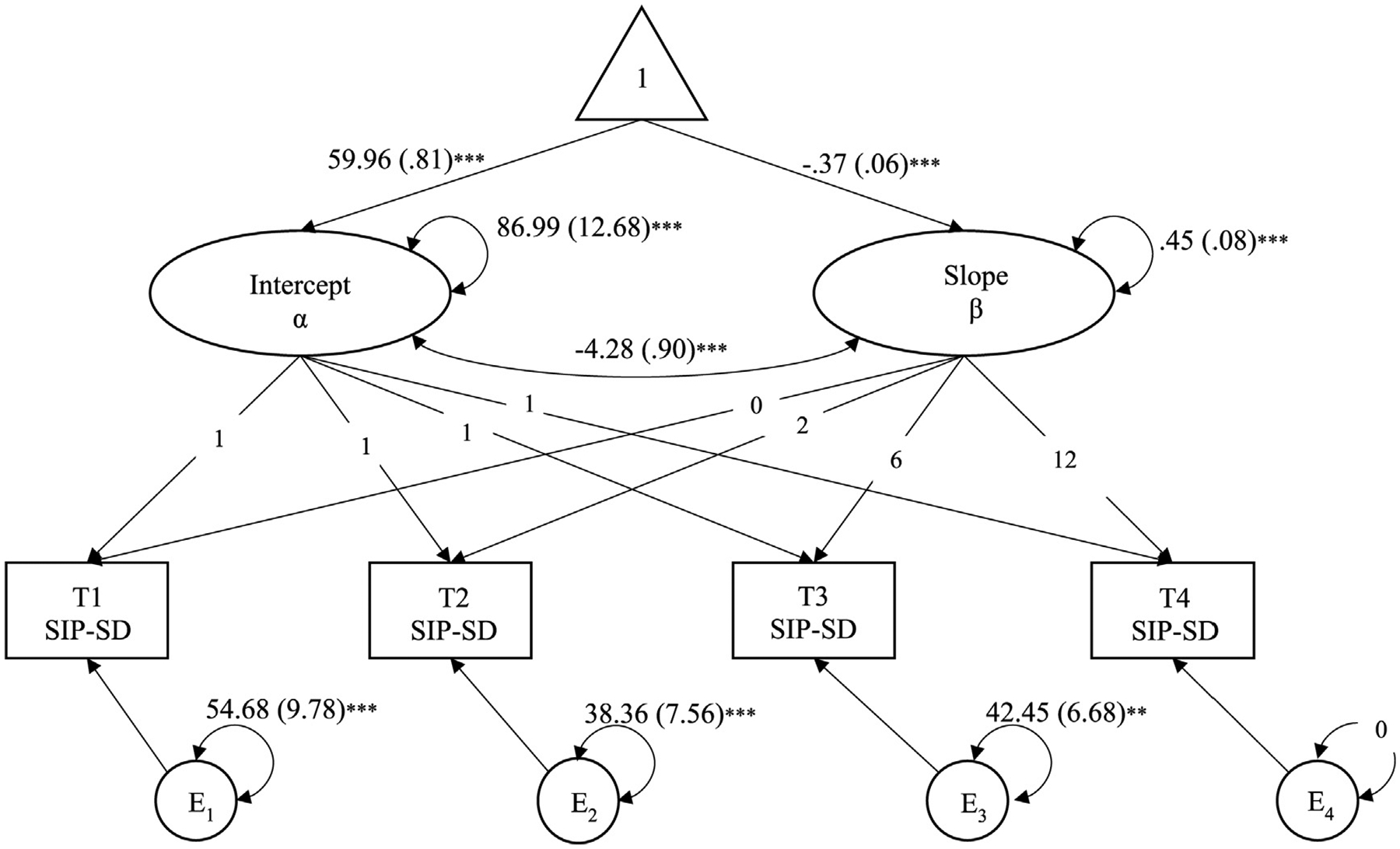
Unconditional Latent Growth Model of SIP-SD. Note. SIP-SD = social disruption category of Sickness Impact Profile; Correlations not shown for simplicity; Unstandardized estimates (standard error) shown; **p < .01; ***p < .001
When dummy vectors and covariates were added to the model a small-to-medium intervention effect was observed, such that those in the RT group experienced .32 (B = −.32, β = −.23, SE = .11, z = −2.10, 95% CI [−.43, −.02], p < .05) and .30 (B = −.30, β = −.21, SE = .11, z = −1.93, 95% CI [−.42, .002], p < .05) more decrease in SIP-SD across T1-T4 time points compared to women in HE (Model A; shown in Table 3) and CBT (Model B; shown in Table 3), respectively. Treatment effects on SIP-SD did not differ between the CBT and HE conditions (B = −.02, β = −.01, SE = .10, z = −.14, 95% CI [−.21, .19], p = .89). Covariances between the intercept and slope remained significant in fully adjusted models. With the addition of covariates to the model, baseline differences in SIP-SD were no longer significant; thus, a direct path adjustment from the intercept to the slope factor was not warranted. There were no significant relationships between control variables and intercept and/or slope factors. Fit statistics for fully controlled models were good and residuals for means and covariances were small (Table 3).
Table 3.
Model Fit and Parameter Estimates for Latent Growth Models of SIP-SD
| Model A: CBT vs. HE and RT vs. HE | ||
|---|---|---|
| Model Fit | ||
| Chi-square | χ2(21) = 17.32, p = .69 | |
| RMSEA | 0.00 | |
| CFI | 1.00 | |
| SRMR | .04 | |
| Statistic | Estimate (SE) | 95% CI |
| SIP-SD α | 63.35 (10.74)*** | [42.30, 84.39] |
| SIP-SD β | −.77 (.90) | [−2.53, .99] |
| CBT vs. HE → α | 3.60 (1.96) | [−.24, 7.44] |
| CBT vs. HE → β | −.02 (.16) | [−.33, .28] |
| RT vs. HE → α | 3.52 (1.87) | [−.15, 7.18] |
| RT vs. HE → β | −.32 (.15)* | [−.62, −.02] |
| Model B: RT vs. CBT and HE vs. CBT | ||
| Model Fit | ||
| Chi-square | χ2(21) = 17.32, p = .69 | |
| RMSEA | 0.00 | |
| CFI | 1.00 | |
| SRMR | .04 | |
| Statistic | Estimate (SE) | 95% CI |
| SIP-SD α | 66.95 (10.72)*** | [45.93, 87.97] |
| SIP-SD β | −.79 (.89) | [−2.54, .96] |
| RT vs. CBT → α | −.09 (1.91) | [−3.83, 3.66] |
| RT vs. CBT → β | −.30 (.16)* | [−.61, .01] |
| HE vs. CBT → α | −3.60 (1.96) | [−7.44, .24] |
| HE vs. CBT → β | .02 (.16) | [−.28, .33] |
Note. SIP-SD = social disruption category of Sickness Impact Profile; CI = confidence interval; RMSEA = root-mean-square error of approximation; CFI = comparative fit index; SRMR = standardized root-mean-square residual; CBT = cognitive behavioral therapy; RT = relaxation therapy; HE = health education; α = intercept factor; β = slope factor; Unstandardized estimates (standard error) shown;
p < .05;
p < .01;
p < .001.
INTERVENTION EFFECTS ON POSITIVE AFFECT (ABS-POS)
Fit statistics for the latent change score model were not computed since the model was just-identified (i.e., number of free parameters exactly equals the number of known values; Kline, 2005). The unstandardized parameter estimate for the unconditional model revealed a pattern wherein higher initial values of ABS-POS were associated with smaller latent change scores from T1 to T3 (B = −.50, SE = .06, z = −8.36, 95% CI [−.70, −.48], p < .001). The mean latent change score for ABS-POS suggested statistically significant residual change in ABS-POS from T1- to T3 (B = .16, SE = .05, z = 3.24, 95% CI [.06, .26], p < .001). When dummy vectors comparing CBT vs. HE (B = −.08, SE = .11, z = −.80, 95% CI [−.29, 12], p = .43) and RT vs. HE (B = −.14, SE = .10, z = −1.32, 95% CI [−.34, .07], p = .19) were added to the unconditional models, there were no treatment effects on the slope of ABS-POS. The path relating initial value of ABS-POS to its respective change score remained significant in the conditional models (B = −.52, SE = .06, z = −8.50, 95% CI [−.64, −.40], p < .001), but with the addition of predictors the mean latent change score for ABS-POS became non-significant (B = .12, SE = .08, z = 1.46, 95% CI [−.04, .29], p = .14).
MEDIATION ANALYSES
Once the latent growth and latent change models were tested for direct treatment effects and to ensure adequate model fit, they were combined into a parallel-process latent growth/latent change score model (Figure 2). Significant paths were observed relating group condition to initial values of ABS-POS, such that those in the CBT and RT groups had significantly lower baseline values of ABS-POS (ps < .05) compared to those in the HE group. The CBT vs. HE and RT vs. HE dummy vectors were not significantly related to the latent change of ABS-POS. A small-to-medium treatment effect was observed for SIP-SD: those in the RT group demonstrated .32 more decrease in SIP-SD across time, compared to those in the HE group (β = −.23, SE = .12, z = −2.04, 95% CI [−.47, −.001], p < .05). A significant direct path emerged relating the latent change in ABS-POS to the rate of linear decrease in SIP-SD, suggesting that larger increases in ABS-POS predicted larger decreases in SIP-SD (B = −.65, β = −.58, SE = .22, z = −2.00, 95% CI [−1.00, .15], p < .05). Yet, the indirect mediation path between treatment-related change in SIP-SD through change in ABS-POS was not significant. Fit indices and unstandardized estimates for all specified paths are reported in Table 4.
FIGURE 2.
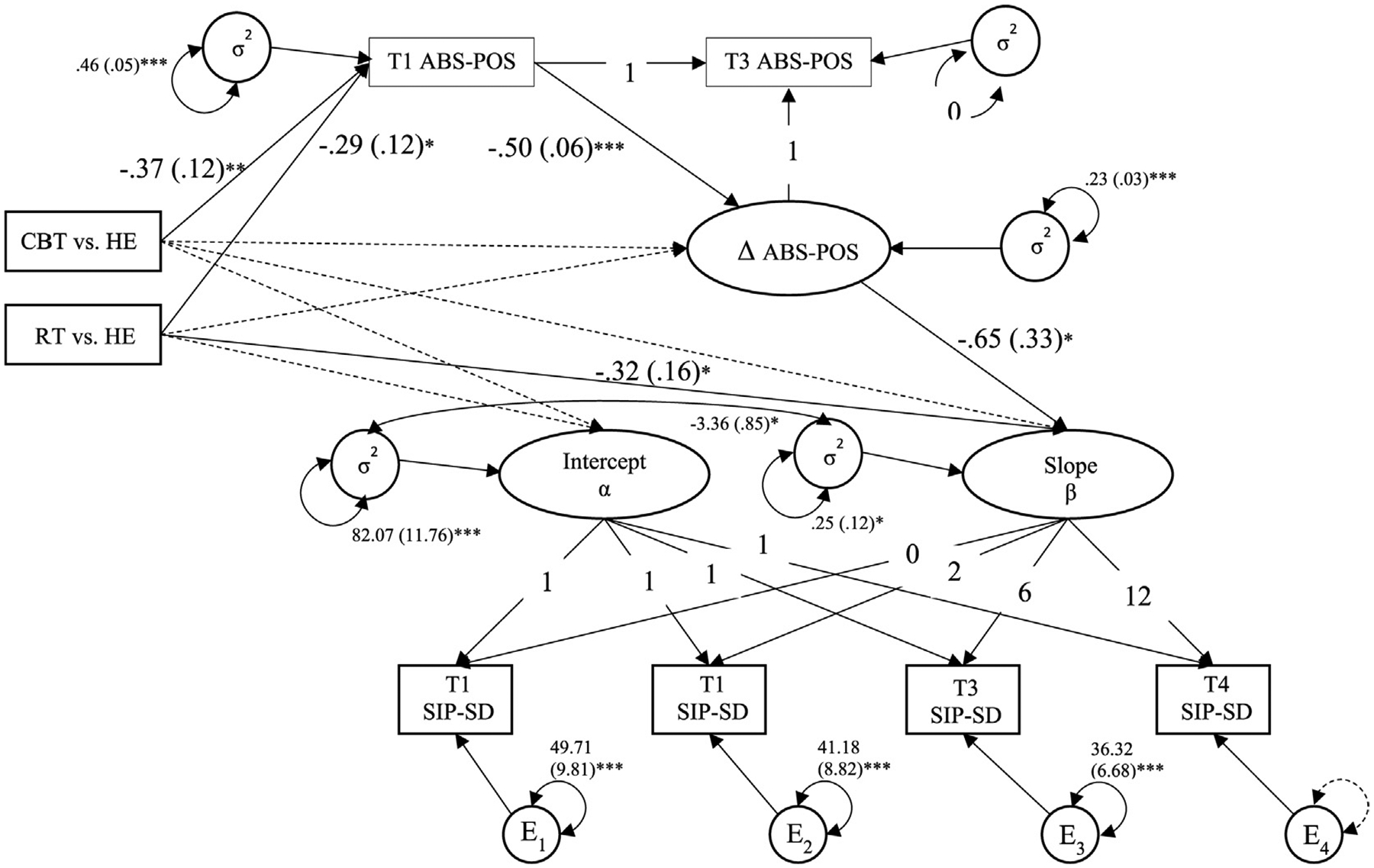
Model of Latent Growth of SIP-SD and Indirect Effect from Group Condition to Outcome Variables via ABS-POS. Note. SIP-SD = social disruption category of Sickness Impact Profile; ABS-POS = total positive affect subscale of Affect Balance Scale; CBT = cognitive behavioral therapy; RT = relaxation therapy; HE = health education; Correlations and paths from covariates age, stage, receipt of chemotherapy, radiation, and hormonal treatment not shown for simplicity; Unstandardized parameter estimates (standard error) shown; Dotted line = non-significant path; *p < .05; **p < .01; ***p < .001
Table 4.
Model Fit and Parameter Estimates for Latent Growth of SIP-SD and Indirect Effect from Group Condition to Outcome Variables via ABS-POS
| Model Fit | ||
|---|---|---|
| Chi-square | χ2(30) = 26.56, p = .65 | |
| RMSEA | .00 | |
| CFI | 1.00 | |
| SRMR | .04 | |
| Statistic | Estimate (SE) | 95% CI |
| Intercept α | 64.18 (5.05)*** | [54.29, 74.07] |
| Slope β | −.71 (.42) | [−1.53, .11] |
| ABS-POS T1 | 3.63 (.09)*** | [3.46, 3.80] |
| Δ ABS-POS | 1.90 (.23)*** | [1.45, 2.36] |
| CBT vs. HE → α | 3.76 (1.97) | [−.10, 7.63] |
| CBT vs. HE → β | .06 (.16) | [−.26, .38] |
| RT vs. HE → α | 3.75 (1.88) | [.06, 7.44] |
| RT vs. HE → β | −.32 (.16)* | [−.63, −.01] |
| CBT vs. HE → ABS-POS T1 | −.37 (.12)** | [−.61, −.13] |
| RT vs. HE → ABS-POS T1 | −.29 (.12)* | [−.52, −.06] |
| CBT vs. HE → Δ ABS-POS | −.06 (.09) | [−.25, .12] |
| RT vs. HE → Δ ABS-POS | −.15 (.09) | [−.33, .03] |
| ABS-POS T1 → Δ ABS-POS | −.50 (.06)*** | [−.62, −.38] |
| Δ ABS-POS → β | −.65 (.33)* | [−1.29, −.01] |
| CBT vs. HE Indirect Effect | .04 (.06) | [−.08, .16] |
| RT vs. HE Indirect Effect | .10 (.09) | [−.05, .25] |
Note. SIP-SD = social disruption subscale of Sickness Impact Profile; ABS-POS = total positive affect subscale of Affect Balance Scale; CI = confidence interval; RMSEA = root-mean-square error of approximation; CFI = comparative fit index; SRMR = standardized root-mean-square residual; CBT = cognitive behavioral therapy; RT = relaxation therapy; HE = health education; α = intercept factor; β = slope factor; Unstandardized estimates (standard error) shown;
p < .05;
p < .01;
p < .001.
Discussion
This secondary analysis is the first from our research team exploring the longitudinal effect of brief, group-based behavioral stress management interventions on illness-related social disruption, and the role of short-term change in positive affect in driving this effect among women with breast cancer undergoing treatment. We observed several noteworthy findings. First, a main time effect across the entire sample emerged for illness-related social disruption. Second, those assigned to the relaxation intervention (RT) demonstrated larger reductions in illness-related social disruption compared to those in both cognitive-behavioral therapy (CBT) and health education (HE) groups. Third, despite the absence of mediation, an increase in positive affect from baseline to 6-month follow-up did occur, and this latent change factor was related to the slope of illness-related social disruption across the full study timeframe.
12-MONTH INTERVENTION EFFECTS ON ILLNESS-RELATED SOCIAL DISRUPTION
The first aim of this study was to test the effect of 5-week, group-based CBT and RT interventions on illness-related social disruption across the initial year postdiagnosis. Women in the RT group demonstrated a small-to-medium treatment effect on illness-related social disruption compared to those in both the CBT and HE group conditions, lending support for Hypothesis 1. This finding is consistent with Antoni, Lechner, et al.’s (2006) work showing that receipt of the 10-week CBSM protocol is associated with reduced illness-related social disruption among women with nonmetastatic breast cancer; however, the effect size here was less than that observed for the full, 10-week protocol (d = .21 vs d = .47). The smaller effect size seen here may be explained by dosing (i.e., 5- vs. 10-sessions) and/or a fading of the intervention effect over time. If the latter, this might indicate the need for booster sessions deployed after the completion of the intervention. Sequential multiple assignment randomized trials offer a novel way to explore optimal dose and sequence of psychosocial interventions and are becoming increasingly prevalent in psycho-oncology research (Kelleher et al., 2017).
This finding also mirrors Gudenkauf and colleagues’ (2015) observation of significant pre- to post-intervention decreases in illness-related social disruption for women randomized to the 5-week RT group, compared to those in CBT and HE group conditions. Although the effect size for the present study is modest, our preliminary results extend the literature by suggesting that the RT treatment effect for illness-related social disruption persists out to 12-months post-enrollment. It is noteworthy that a brief, group-based RT intervention can result in long-term change in illness-related social disruption, above and beyond the effect of CBT and HE group interventions, as well as relevant sociodemographic and medical covariates. Other research teams have shown that components included in our RT intervention may improve social functioning (e.g., imagery, mindfulness meditations; Carlson et al., 2013; Henderson et al., 2013; Zautra et al., 2008), yet explanations for this observation are underdeveloped. It appears that some aspect of relaxation training helps motivate women to resume social activities.
6-MONTH INTERVENTION EFFECTS ON POSITIVE AFFECT
We leveraged a latent change score model to explore 6-month intervention effects on positive affect. The mean latent change value for positive affect was significant in the unconditional model, such that within the entire sample there was residualized change in positive affect from baseline to 6-month follow-up. When dummy vectors comparing RT vs. HE and RT vs. CBT were added, the latent change score for positive affect became nonsignificant, suggested that no intervention (RT, CBT, or HE) was linked with a significant increase to positive affect. This finding is in contrast to our second hypothesis. It is conceivable that positive affect did not exhibit a robust latent change in the current study because it subsumes four different affective subscales (i.e., joy, contentment, vigor, and affection), which may have experienced differential change from T1 to T3.1 Though not observed here, it is possible that specific types of positive emotion (e.g., relief, calmness, contentment) might be better cultivated by RT versus CBT interventions.
These data contrast with previous findings observed by Antoni and colleagues (2006) wherein women who received a 10-week course in CBSM exhibited significant increases to positive affect at 6-month follow-up compared to those randomized to attend a 1-day psycho-education control seminar. It is possible that the 5-week, group-based interventions in the present study may have been too short to exert an enduring influence on positive affect. Likewise, the comparison condition in the current study was stronger than the 1-day psychoeducation control seminar used by Antoni, Lechner, et al. (2006) and, as such, might have concealed significant intervention effects. It is possible that all women across the RT, CBT, and HE interventions benefited from nonspecific (common) group factors (i.e., group cohesion and collaboration); as such, positive affect increased consistently across all intervention protocols. Finally, improvements in positive affect might be contingent on receipt of both cognitive-behavioral and relaxation training together, rather than just one set of skills.
MEDIATION VIA THE BROADEN-AND-BUILD THEORY
Parallel-process latent growth/latent change models were employed to test the Broaden-and-Build theory as a change mechanism underpinning 5-week RT and CBT group interventions. The fully adjusted model assessing latent change in positive affect as a mediator linking treatment to the slope factor of illness-related social disruption exhibited good fit. This suggested that on a broad level, our theoretical model was consistent with the data. Although the 12-month RT effects for illness-related social disruption were retained, latent change in positive affect was not driven by group assignment. Thus, complete mediation was not viable and our third hypothesis regarding the Broaden-and-Build theory as an underlying mechanism for the 5-week RT intervention was not upheld.
There was a significant direct effect relating the slopes of positive affect across 6 months and illness-related social disruption across 12 months. Thus, it appears that as women feel more positive affect they might become more motivated and better able to resume social roles and activities after breast cancer diagnosis and treatment. This is the first study to reveal such a relationship using parallel-process latent growth/latent change models within the context of randomized controlled trial of stress management for women with early-stage breast cancer. This observation is especially novel in that it highlights how increases in positive affect trend with decreases in illness-related social disruption, lending tentative support for Fredrickson’s “undoing hypothesis” (p. 3, Fredrickson et al., 2000). Specifically, these data suggest that increases in positive affect may trend with the undoing of social disruption caused by breast cancer, rather than simply increase social behavior from a set baseline. This is relevant for how we understand the role of positive affect and how we can leverage positive affect in psychosocial interventions.
Still, our findings do not clearly support our hypothesis that the Broaden-and-Build theory might underpin intervention effects on positive affect and illness-related social disruption. It remains unclear why women in the RT group exhibited larger reductions in illness-related social disruption compared to those in the CBT and HE group conditions. Positive affect did not emerge as a mechanistic variable, thus alternative explanations must be considered. It may be that women in the RT group felt more confident in their ability to use PMR, imagery, and mindfulness, and this increased self-efficacy in relaxation skills yielded a more robust reduction in long-term outcomes (i.e., illness-related social disruption). Indeed, previous work has shown that the 10-week CBSM program increased confidence in being able to relax at will, and this effect mediated improvements to well-being (Antoni et al., 2006).
STRENGTHS AND LIMITATIONS
These findings should be considered in light of several limitations. First, this was a secondary analysis of a dataset from a trial conducted several years ago, and thus may not generalize to contemporary samples of women receiving newer treatments for nonmetastatic breast cancer. Second, only English-speaking women with nonmetastatic disease were enrolled, limiting generalizability to beyond this specific subset of cancer survivors. Finally, given the complexity of the proposed models, it is likely that this observational study was underpowered to adequately test for intervention effects. Guidelines for determining sample size for structural models suggest that for every free parameter there should be 5 participants (Kline, 2005). The latent growth model used here had 5 parameters, necessitating a sample size of 275 women. The larger parallel-process latent growth/latent change model included 69 parameters, calling for a sample size of 350 participants. The current sample size was below these requirements, limiting confidence in parameter estimates. As such, results presented here should be considered preliminary. Future work on intervention mechanisms using latent growth modeling should recruit larger and more diverse samples to ensure adequate power.
There are many strengths of this work. Our study leveraged a latent growth/latent change model framework to establish temporal precedence of a mechanistic variable and better reflect the dynamic nature of the Broaden-and-Build theory. This study is the first to examine whether such a change theory is occurring within the context of a brief psychosocial intervention for women with breast cancer. Retention rates in the current study were mostly in line with those observed in prior trials of breast cancer survivors (Antoni, Lechner, et al., 2006); yet, the use of a robust statistical method within a structural modeling framework provided the flexibility to account for missing data at follow-up assessments. Another strength of this study was its use of the ABS to index positive affect. Alternative measures of positive affect (e.g., positive affect subscale of the CES-D) are often contaminated by items that reflect social and cognitive constructs (e.g., self-esteem, life satisfaction etc.).
CONCLUSION
The preliminary results presented here hint at the relevance of relaxation skills for women with breast cancer who are struggling to resume social roles and activities. Increases to positive affect appear correlated with decreases to illness-related social disruption, but more research is warranted to uncover a clear mechanism driving the effect of RT interventions on illness-related social disruption. Some aspect of relaxation training appears to motivate women to resume social activities, and this finding may be useful in the development of brief psychosocial interventions that aim to enhance social functioning among women surviving breast cancer.
Acknowledgments
This work was supported by National Institute of Health (2R01CA064710).
Footnotes
Conflict of Interest Statement
Michael Antoni, Ph.D. reports receiving royalties from a book on stress management and breast cancer. The other authors have no relevant financial or non-financial interests to disclose.
Exploratory analyses showed that while joy, t(126) = −3.06, p < .01), contentment, t(126) = −2.52, p < .05, and vigor, t(126) = −3.93, p < .001, increased significantly from T1 to T3, affection did not, t(126) = −.49, p = .62.
Contributor Information
Hannah M. Fisher, Duke University Medical Center
Chloe J. Taub, Northwestern University Feinberg School of Medicine
Suzanne C. Lechner, Research Advisor LLC, Hallandale Beach, FL
Aaron S. Heller, University of Miami Sylvester Cancer Center, University of Miami Miller School of Medicine.
David J. Lee, University of Miami Miller School of Medicine
Michael H. Antoni, University of Miami Sylvester Cancer Center, University of Miami Miller School of Medicine.
References
- Antoni MH (2003). Stress management intervention for women with breast cancer. American Psychological Association. [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, Phillips K, Gluck S, & Carver CS (2006). How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology, 74(6), 1143–1152. 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, Yount SE, McGregor BA, Arena PL, Harris SD, Price AA, & Carver CS (2001). Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology, 20(1), 20–32. 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S, Wells KA, Blomberg B, & Carver CS (2006). Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry, 163(10), 1791–1797. 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner M, Bobbitt RA, Carter WB, & Gilson BS (1981). The Sickness Impact Profile: Development and final revision of a health status measure. Medical Care, 19(8), 787–805 https://www.ncbi.nlm.nih.gov/pubmed/7278416. [DOI] [PubMed] [Google Scholar]
- Calderon C, Carmona-Bayonas A, Hernandez R, Ghanem I, Castelo B, Martinez de Castro E, Ferreira E, Ciria L, Muniz M, & Jimenez-Fonseca P (2019). Effects of pessimism, depression, fatigue, and pain on functional health-related quality of life in patients with resected non-advanced breast cancer. Breast, 44, 108–112. 10.1016/j.breast.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Doll R, Stephen J, Faris P, Tamagawa R, Drysdale E, & Speca M (2013). Randomized controlled trial of Mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. Journal of Clinical Oncology, 31(25), 3119–3126. 10.1200/JCO.2012.47.5210. [DOI] [PubMed] [Google Scholar]
- Carver C (2003). Pleasure as a sign you can attend to something else: Placing positive feelings within a general model of affect. Cognition & Emotion, 17(2), 241–261. 10.1080/02699930302294. [DOI] [PubMed] [Google Scholar]
- Carver CS, Lehman JM, & Antoni MH (2003). Dispositional pessimism predicts illness-related disruption of social and recreational activities among breast cancer patients. Journal of Personality and Social Psychology, 84 (4), 813–821. 10.1037/0022-3514.84.4.813. [DOI] [PubMed] [Google Scholar]
- Casellas-Grau A, Font A, & Vives J (2014). Positive psychology interventions in breast cancer. A systematic review. Psycho-Oncology, 23(1), 9–19. 10.1002/pon.3353. [DOI] [PubMed] [Google Scholar]
- Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, Lee SK, Im YH, Park YH, Yang JH, & Cho J (2014). Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-Oncology, 23(10), 1103–1110. 10.1002/pon.3531. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences ((2nd ed.).). Academic Press. [Google Scholar]
- Curran PJ, Howard AL, Bainter SA, Lane ST, & McGinley JS (2014). The separation of between-person and within-person components of individual change over time: A latent curve model with structured residuals. Journal of Consulting and Clinical Psychology, 82(5), 879–894. 10.1037/a0035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR (1975). The Affects Balance Scale. Clinical Psychometric Research. [Google Scholar]
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, & Siegel RL (2019). Breast cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(6), 438–451. 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Scarapicchia TMF, McDonough MH, Wrosch C, & Sabiston CM (2017). Changes in social support predict emotional well-being in breast cancer survivors. Psycho-Oncology, 26(5), 664–671. 10.1002/pon.4064. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. American Psychologist Journal, 56(3), 218–226 https://www.ncbi.nlm.nih.gov/pubmed/11315248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2004). The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society B: Biological Sciences, 359(1449), 1367–1378. 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, & Tugade MM (2000). The Undoing Effect of Positive Emotions. Motivation and Emotion, 24(4), 237–258. 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, & Finkel SM (2008). Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology, 95(5), 1045–1062. 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E, Barker C, & Pistrang N (2008). Outcome of an online mutual support group for college students with psychological problems. CyberPsychology & Behavior, 11 (5), 591–593. 10.1089/cpb.2007.0133. [DOI] [PubMed] [Google Scholar]
- Garland EL, Fredrickson B, Kring AM, Johnson DP, Meyer PS, & Penn DL (2010). Upward spirals of positive emotions counter downward spirals of negativity: insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review, 30(7), 849–864. 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, Blomberg BB, Gluck S, Derhagopian RP, Giron GL, Avisar E, Torres-Salichs MA, & Carver CS (2015). Brief cognitive-behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(4), 677–688. 10.1037/ccp0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VP, Massion AO, Clemow L, Hurley TG, Druker S, & Hebert JR (2013). A randomized controlled trial of mindfulness-based stress reduction for women with early-stage breast cancer receiving radiotherapy. Integrative Cancer Therapies, 12(5), 404–413. 10.1177/1534735412473640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing S, Rosenwax L, & McNamara B (2018). The implications of women’s activity limitations and role disruptions during breast cancer survivorship 1745505718756381. Women’s Health, 14. 10.1177/1745505718756381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher SA, Dorfman CS, Plumb Vilardaga JC, Majestic C, Winger J, Gandhi V, Nunez C, Van Denburg A, Shelby RA, Reed SD, Murphy S, Davidian M, Laber EB, Kimmick GG, Westbrook KW, Abernethy AP, & Somers TJ (2017). Optimizing delivery of a behavioral pain intervention in cancer patients using a sequential multiple assignment randomized trial SMART. Contemporary Clinical Trials, 57, 51–57. 10.1016/j.cct.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2005). Principles and practice of structural equation modeling ((2nd ed.).). Guildford Press. [Google Scholar]
- Kroenke CH, Michael YL, Poole EM, Kwan ML, Nechuta S, Leas E, Caan BJ, Pierce J, Shu XO, Zheng Y, & Chen WY (2017). Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer, 123(7), 1228–1237. 10.1002/cncr.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Pachana NA, & McLaughlin D (2014). Social support and health-related quality of life in women with breast cancer: A longitudinal study. Psycho-Oncology, 23 (9), 1014–1020. 10.1002/pon.3523. [DOI] [PubMed] [Google Scholar]
- McArdle JJ (2009). Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology, 60, 577–605. 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Muthén L, & Muthén B (2012). MPlus user’s guide ((6th ed.).). Muthén & Muthén. [Google Scholar]
- Pollard B, & Johnston M (2001). Problems with the sickness impact profile: A theoretically based analysis and a proposal for a new method of implementation and scoring. Social Science & Medicine, 52(6), 921–934. 10.1016/s0277-9536(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, & Ganz PA (2016). American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. Journal of Clinical Oncology, 34(6), 611–635. 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- Schnur JB, & Montgomery GH (2010). A systematic review of therapeutic alliance, group cohesion, empathy, and goal consensus/collaboration in psychotherapeutic interventions in cancer: Uncommon factors? Clinical Psychology Review, 30(2), 238–247. 10.1016/j.cpr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinfeld Gorin S, Krebs P, Badr H, Janke EA, Jim HS, Spring B, Mohr DC, Berendsen MA, & Jacobsen PB (2012). Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. Journal of Clinical Oncology, 30(5), 539–547. 10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers TJ, Kelleher SA, Westbrook KW, Kimmick GG, Shelby RA, Abernethy AP, & Keefe FJ (2016). A small randomized controlled pilot trial comparing mobile and traditional pain coping skills training protocols for cancer patients with pain. Pain Research and Treatment, 2016, 2473629. 10.1155/2016/2473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagl JM, Antoni MH, Lechner SC, Bouchard LC, Blomberg BB, Gluck S, Derhagopian RP, & Carver CS (2015). Randomized controlled trial of cognitive behavioral stress management in breast cancer: A brief report of effects on 5-year depressive symptoms. Health Psychology, 34(2), 176–180. 10.1037/hea0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Rodebaugh TL, Perez M, Schootman M, & Jeffe DB (2013). Perceived social support change in patients with early stage breast cancer and controls. Health Psychology, 32(8), 886–895. 10.1037/a0031894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusson D, & Pilnick A (2017). Between stigma and pink positivity: Women’s perceptions of social interactions during and after breast cancer treatment. Sociology of Health & Illness, 39(3), 458–473. 10.1111/1467-9566.12486. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, Kratz A, Parrish B, & Irwin MR (2008). Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. Journal of Consulting and Clinical Psychology, 76(3), 408–421. 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]


