Abstract
Background
Medulloblastoma is a malignant brain tumor that is common in children but very uncommon in adults, especially those older than 40 years, accounting for less than 1% of all primary brain tumors in adults. Although surgery and radiotherapy play an important role treatment of adult medulloblastoma, the use of chemotherapy is controversial. This is the first instance of adult medulloblastoma at the Ocean Road Cancer Institute in Tanzania.
Case description
We report the case of a 51-year-old female of African ethnicity who was diagnosed with high-risk hemispheric posterior cranial fossa medulloblastoma of classic type with World Health Organization central nervous system grade 4 and Chang stage M0. Immunohistochemistry, reticulin stain, and molecular subtyping could not be done because they were not available. She was treated by subtotal posterior cranial fossa tumor resection followed by adjuvant concurrent chemo-craniospinal radiation and adjuvant chemotherapy.
Conclusion
Even in adults over 50 years old, medulloblastoma should be included in the differential diagnosis of posterior fossa tumor. Adult medulloblastoma is a very rare and very heterogeneous tumor, but it has a good prognosis. Immunohistochemistry and molecular subclustering are difficult to implement in low-income countries such as Tanzania owing to cost. Treatment of adult medulloblastoma is highly heterogeneous among (and even within) facilities. There is no evidence that the extent of resection enhances survival. While craniospinal radiation therapy improves survival, there is controversy about the role of chemotherapy in managing adult MB.
Keywords: Case report, Chemotherapy, Craniospinal radiation, Medulloblastoma, Ocean Road Cancer Institute, Posterior cranial fossa
Introduction
Medulloblastoma (MB) is a highly heterogeneous, aggressive, and invasive malignant embryonic tumor that arises in the cerebellum [1]. It is the most common malignant brain tumor in children, accounting for nearly 20% of all pediatric brain tumors. Conversely, adult MB is extremely rare and accounts for less than 1% of intracranial tumors [2]. Smoll et al. showed that MB is 10 times more likely to be diagnosed in children than adults and that male gender is a risk factor for the development of MB in children only but not in adults [3].
The clinical symptoms and signs of MB in adults are associated with the location of the tumor in the posterior fossa, increased intracranial pressure, and/or obstruction of the cerebrospinal fluid pathway leading to headache, dizziness, nausea, ipsilateral cerebellar signs, and ataxia [4].
In adult MB, magnetic resonance imaging (MRI) of the brain shows hypointense T1 and hyper to hypointense T2 lesions. The intensity and extent of contrast improvement on T1 postcontrast imaging vary significantly, hence histopathological examination should be performed to confirm the diagnosis [5].
There are four major histological subtypes of MB with prognostic value: classical (CMB), desmoplastic/nodular (DNMB), large cell anaplasia (LCMB), and extensive nodularity/anaplastic (AMB). The most common histological variant is classic MB in children and adults (70–80%) [5]. Among adults, DNMB has the longest OS and PFS, followed by CMB and AMB with the worst treatment outcome [6].
There are four molecular subgroups of MB with prognostic value: wingless (WNT), sonic hedgehog (SHH), group 3, and group 4 [7]. In a study done by Zhao et al., it was shown that there is a preponderance of SHH-type tumors in adult MB (62%), followed by group 4 tumors (28%) and WNT-activated tumors (10%), with an absence of group 3 cases, suggesting that this subgroup may be restricted to pediatric MB [6]. Among adults, WNT has the longest OS and PFS followed by SHH, while group 4 has the worst prognosis. The age distribution of patients with SHH tumors is concentrated in the range of 20–40 years, whereas group 4 and WNT tumors have a wider distribution of age of onset (18–55 years). DNMBs are mainly found to be of SHH subtype, whereas LCMB/AMBs are only found in the SHH and group 4 subtypes. Classic histology is found in all molecular variants. Male patients predominantly have SHH subtype and group 4 tumors, whereas female patients predominantly have tumors of WNT subtype. SHH tumors tend to have a hemispheric location, and WNT tumors have a lower incidence of fourth ventricular floor involvement. Tumors with classic histology account for the largest proportion of all molecular variants, especially among those of WNT subtype, 95% of which tumors have classic histology. Most DNMB tumors belong to the SHH subtype. Anaplastic MBs and LCMBs are found to be either SHH or group 4 tumors [6]. Whether the histological categorization of MB retains its prognostic value once the molecular subgroup is widely available for all patients has yet to be determined [8].
Chang staging for MB considers tumor size and disease spread to determine low- and high-risk groups. Patients with tumors over 3 cm with unequivocal propagation in the brainstem or beyond the aqueduct of Sylvius and/or foramen magnum noted during surgery or on imaging, or any metastasis outside of the brain parenchyma, are high risk [9]. Adult patients with high-risk MB have lower survival compared with standard-risk patients. However, unlike children, for adults, the prognostic value of the extent of resection is controversial [2]. Metastatic disease at onset is far less common in adults than in children (7% versus 30%) [10]. These differences show that clinical staging of MB may not be as relevant in adults as in children, and the prognosis is likely driven mostly by molecular subgroups and not the extent of the disease [8].
Current management of adult MB is often extrapolated from pediatric protocols and includes maximum safe resections [11], followed by craniospinal radiation therapy (CSRT), with or without concurrent and/or adjuvant chemotherapy depending on clinical risk stratification. Even though the treatment of adult MB is very heterogeneous across (and even within) institutions, a common practice for the last several decades has been to treat patients with complete resection and nonmetastatic dissemination with CSRT alone, whereas patients with incomplete resections and/or metastatic dissemination are treated also with upfront chemotherapy [2]. Over the past two decades, MB survival has been improved by considerable advancements in therapeutic strategies based on clinical risk stratification. However, MB is still associated with a poor outcome overall, and most survivors are left with long-term disabilities secondary to treatment [6].
We report herein the case of a 51-year-old woman with a cerebellum mass who was diagnosed with adult MB. This is the first case to be reported in Tanzania.
Case presentation
A 51-year-old female of African ethnicity was referred to our oncology center for adjuvant therapy. She had a chief complaint of occipital headache which was associated with dizziness and projectile vomiting for over a year. She had no history of chronic illness. She had three children, and all childbirths were done by cesarean section. She had no previous history of blood transfusion. She had no history of malignancy or cancer within her family. She worked as a nurse. She was married. She denied a history of use of tobacco, alcohol, or other drugs. All vitals were within normal limits. Physical examination was essentially normal. Neurological findings showed that all cranial nerves were within normal limits, and there was no motor or sensory deficit, but she had poor coordination of movement. Basic laboratory investigations were done and included: complete blood count (WBC-4.92 × 103/uL, Hb-12.3 g/dl and platelet-165.0 × 103/uL), urea (5.5 mmol/L), serum creatinine (85.19 micromol/L), alanine aminotransferase (23.1 IU/L), Aspartate aminotransferase (30.1 IU/L), total bilirubin (0.19 mg/dl), and albumin (38.45 g/L) essentially normal with negative human immunodeficiency virus (HIV) test. Brain MRI was conducted (Fig. 1) and showed a left posterior fossa mass, which had an iso-hypointense appearance on T1-weighted images, an inhomogeneously enhanced appearance on T1-weighted contrast images and iso-hyperintensity on T2-weighted images (Fig. 2).
Fig. 1.
A timeline of important events
Fig. 2.
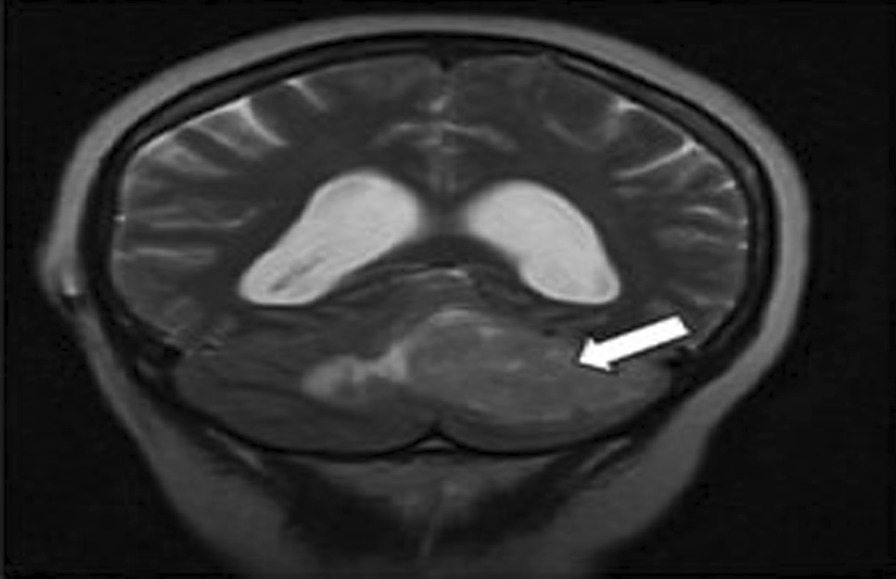
T1W image showing hypointense left lateral posterior cranial fossa lesion as indicated by the arrow
A ventriculoperitoneal shunt procedure was conducted before surgery to overcome hydrocephalus. Craniotomy for the posterior fossa tumor was done (Fig. 1). The patient was placed in prone position with her head supported on the Mayfield head holder. A posterior midline suboccipital approach was used. Through the displaced left cerebellar, a grayish solid mass, with an undefined border, was identified and dissected carefully with cauterization of the feeder vessels. Subtotal tumor resection (STR) was done, and hemostasis was achieved. Dura closure was achieved with a dura patch, and the bone flap was returned. Wound closure was done in layers. A histopathology sample from the mass was obtained. The postsurgery phase was uneventful.
Histopathology revealed cells with minimal cytoplasm and dense basophilic nuclei present in diffuse sheets in keeping with MB of classic type (CMB) with WHO CNS grade 4 (Figs. 3 and 4). Reticulin staining was not done because it was not available. Immunohistochemical analysis and molecular subgroup were also not performed because they were not available.
Fig. 3.
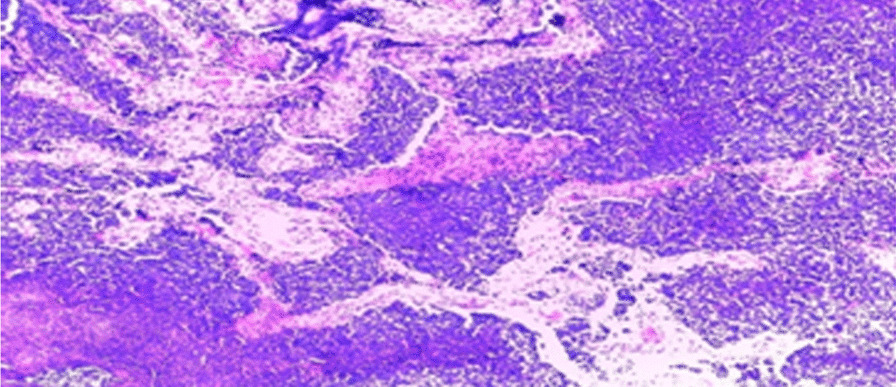
Diffuse and solid sheets with focal areas of nodularity, but desmoplasia was not evident
Fig. 4.
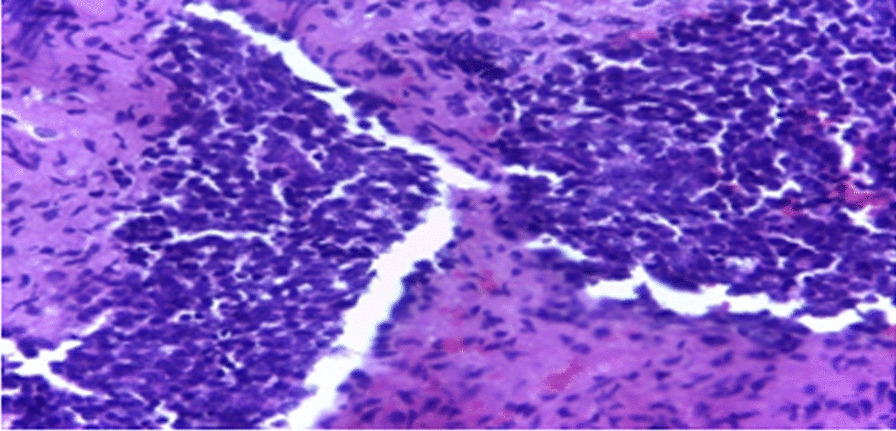
Small round cells with dense nuclear chromatin, scant and indistinct cytoplasm
We did not carry out CSF analysis to investigate the leptomeningeal spread of the tumor due to concerns about transtentorial herniation. We conducted MRI of the brain and spine at 1 month after surgery (Fig. 1), which revealed a residual posterior fossa tumor without leptomeningeal spread. We stratified the tumor as high risk, and the staging was modified to Chang stage M0. We treated the patient with adjuvant concurrent chemoradiotherapy and adjuvant chemotherapy.
She was simulated by using CT–Simulator in supine position. The headrest and a thermoplastic head and neck mask were used to restrain the head. Contouring was done for the target and the organs at risk. Laterally parallel opposed fields with photon energy of 6 MV were used to treat the brain (Fig. 5), and two direct posterior adjacent fields were used to treat the spinal cord (Fig. 6). Collimator rotation was used to match the cranial and spinal fields, and a feathering maneuver was used in the two adjusted spine fields to avoid hotspots. The radiotherapy technique was 3D conformal radiotherapy (3DCRT) using a linear accelerator (LINAC). The patient underwent two phases of treatment. The first phase was craniospinal radiation therapy (CSRT) whereby 36 Gy in 20 fractions was delivered, and the second phase was the posterior cranial fossa boost, whereby a boost dose of 18 Gy in 10 fractions was delivered. A weekly dose of 2 mg vincristine was administered intravenously with radiation therapy. She tolerated the treatment very well apart from fatigue, nausea, and dizziness.
Fig. 5.
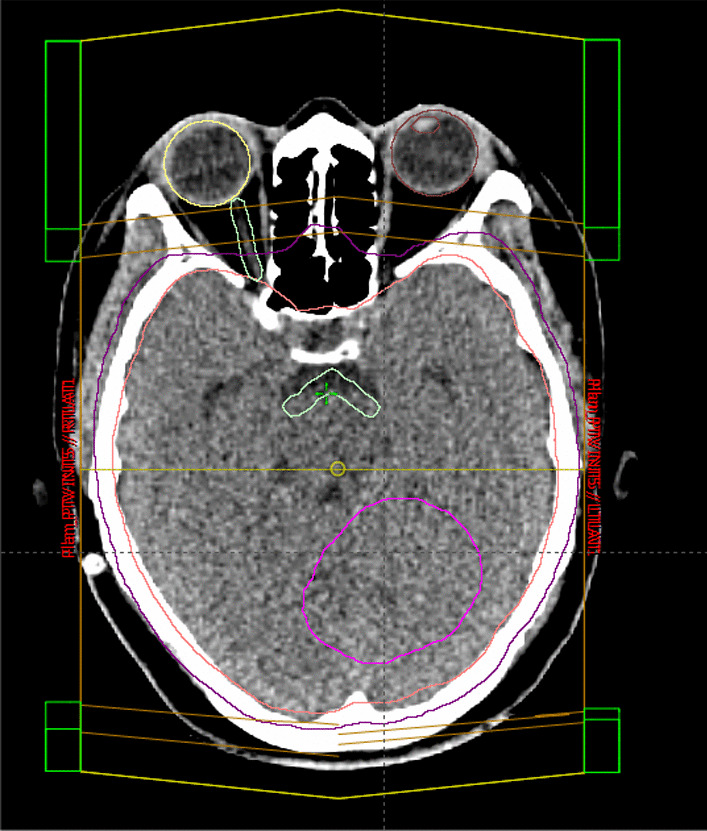
Whole-brain lateral fields
Fig. 6.
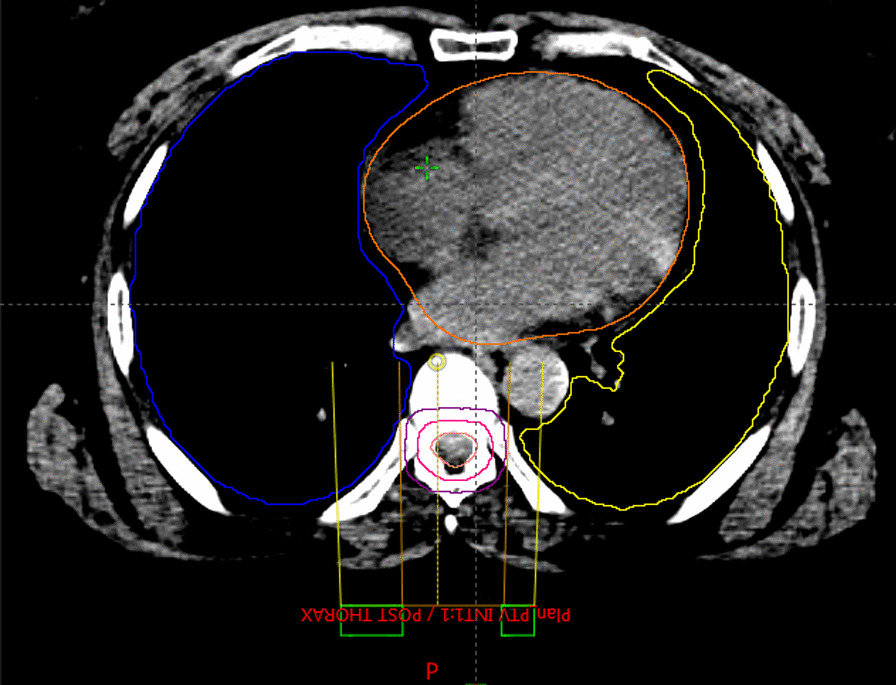
Posterior radiotherapy field to the spinal cord
Upon completion of radiotherapy, she was put on a monthly course of adjuvant intravenous chemotherapy (etoposide 50 mg/m2 D1–D4 and cisplatin 20 mg/m2 D1–D4) for a total of six cycles from June to September 2021.
Post-treatment brain MRI showed a significant decrease in the posterior fossa tumor (Fig. 7).
Fig. 7.
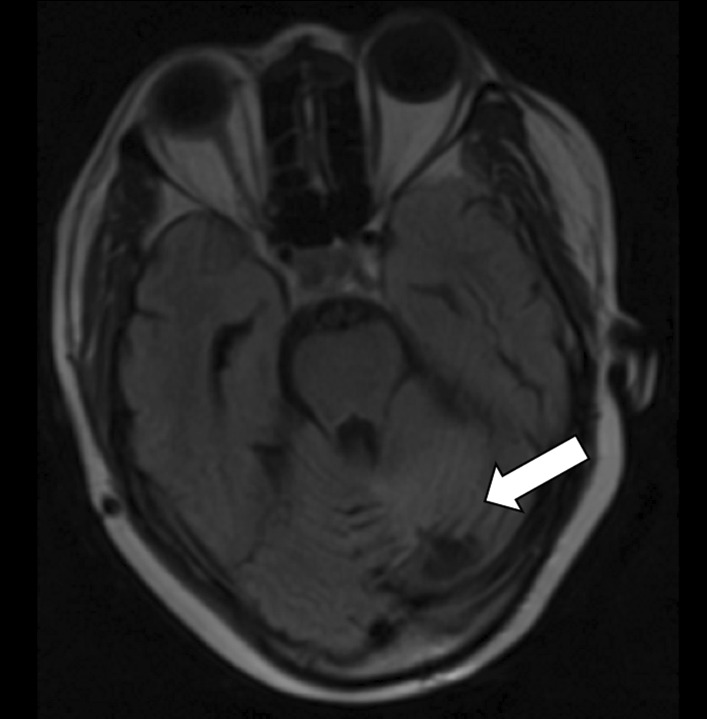
T1W brain MRI 9 months after finishing treatment showing a significant reduction in the posterior cranial fossa lesion as indicated by the arrow
She is currently doing well, apart from mild dizziness.
Discussion
We report the case of a 51-year-old woman with a high-risk hemispheric posterior cranial fossa MB of classic type (CMB) with WHO CNS grade 4 and Chang stage M0. MB is extremely rare in adults, accounting for less than 1% of intracranial tumors [2]. This is the first documented case of adult MB to be reported in Sub-Saharan Africa.
The tumor in this case report was hemispheric and located in the left side posterior cranial fossa. In adults, MB most frequently involves the cerebellar hemisphere; thus, it is usually more lateral than the midline vermis, which commonly occurs in the pediatric population [12]. Poorer PFS and OS have been observed among adults with tumors located in the midline than in those with tumors located in the hemispheres [6].
The patient had a classical variant of MB, but immunohistochemical analysis and molecular subgroup were not performed because they were not available. Immunohistochemistry and molecular subgroups are costly and difficult to perform in low-income countries such as Tanzania [13]. Moreover, reticulin staining could also not be carried out because it was not available. Reticulin staining could help to rule out the DNMB subtype, which is the most common histologic associated with hemispheric adult MB. The age, hemispheric location, and good prognosis in this case suggest a SHH subtype.
The patient was treated by subtotal resection (STR), and a ventriculoperitoneal (VP) shunt was inserted. According to Majd et al., about 20% of adult MB patients required VP shunts to relieve hydrocephalus [8]. Maximum safe resection has also been the goal of surgical resection in adults [11]. Nayak et al. evaluated the survival benefit of extent of resection (gross tumor resection or GTR versus STR) in pediatric MB. Interestingly, no significant survival benefit was found with greater extent of resection for patients with WNT, SHH, or group 3 tumors, questioning the benefit of “second-look” surgeries to remove small residual portions of tumor given the potential for neurological deficits associated with posterior fossa tumor resection [13].
The patient was treated with CSRT concurrently with chemotherapy and adjuvant chemotherapy, as reported previously [14]. CSRT is a favorable prognostic factor, according to a large meta-analysis of literature in adult MB patients [15]. MB is a chemotherapy-sensitive tumor, and chemotherapy plays an established role in treating pediatric MB patients, especially those younger than 3 years of age, to delay radiotherapy [16]. Exact cytotoxic agents and timing of upfront chemotherapy with CSRT (before and after RT) have been controversial in both the pediatric and adult populations, although at present, adjuvant chemotherapy is favored in standard practice [17].
It remains unknown whether chemotherapy plays a role in adult MB. In 2012, Frederick et al. published a prospective study in which they followed 70 adult patients with MB treated with postoperative CSRT that was followed (in 49 of these patients) by chemotherapy according to a pediatric protocol. They did not find any prognostic difference between the groups treated with or without chemotherapy [18]. In another retrospective analysis of the national database, it was shown that concurrent postoperative chemotherapy and CSRT are associated with superior survival for patients with adult MB compared with radiotherapy alone [19].
This patient has a good prognosis despite STR. The Surveillance, Epidemiology and End Results (SEER) database with 454 patients with adult MB showed relative survival rates at 2, 5, and 10 years of 79.9%, 64.9%, and 52.1%, respectively [20].
Conclusion
Even in adults over 50 years old, MB should be included in the differential diagnosis of posterior fossa tumor. Adult MB is a very rare tumor, but with a relatively good prognosis. Immunohistochemistry and molecular subgroup analyses are challenging to implement in low-income countries such as Tanzania because of the cost. Treatment for adult MB is highly heterogeneous between institutions. There is no evidence that the extent of resection enhances survival. While CSRT improves survival, there is controversy about the role of chemotherapy in the management of MB.
Acknowledgements
We would like to acknowledge the support from Ocean Road Cancer Institute and members of the faculty department of clinical oncology of Muhimbili University of Health and Allied Sciences, Prof. James Kitinya and Dr. Daniel Tarimo.
Abbreviations
- ECOG
Eastern Cooperative Oncology Group
- CNS
Central nervous system
- CSRT
Craniospinal radiation therapy
- GCS
Glasgow Coma Scale
- LINAC
Linear accelerator
- MB
Medulloblastoma
- ORCI
Ocean Road Cancer Institute
- OS
Overall survival
- RT
Radiotherapy
- VP
Ventriculoperitoneal
- PFS
Progression-free survival
- CSF
Cerebrospinal fluid
- GTR
Gross tumor resection
- STR
Subtotal tumor resection
- 3DCT
3D conformal radiotherapy
Author contributions
Concept and design: EM, ELL, AA. Collection and assembly of data: EM, ELL, AA. Data analysis and interpretation: EM, ELL, AA. Financial support: EM, ELL, AA. Manuscript writing: ELL, EM. Final approval of manuscript: EM, ELL, AA. Agree to be accountable for all aspects of work: EM, ELL, AA. All authors read and approved the final manuscript.
Funding
There was no external source of funding for the study.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The case presentation was reviewed and approved by the ORCI Institutional Review Committee.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors do not disclose any conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuzan-Fischer CM, Juraschka K, Taylor MD. Medulloblastoma in the molecular era. J Korean Neurosurg Soc. 2018;61(3):292–301. doi: 10.3340/jkns.2018.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majd NK, Mastall M, Lin H. Characterization of adult medulloblastoma patients at recurrence: retrospective review of the MD Anderson Cancer Center experience. Neuro-Oncology Adv. 2021;3(1):1–12. doi: 10.1093/noajnl/vdab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumors in adults and children. J Clin Neurosci. 2012;19:1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Mahapatra S, Amsbaugh MJ. Medulloblastoma. 2021. p. 1–9. https://www.ncbi.nlm.nih.gov/books/NBK431069/. Accessed 19 Feb 2022.
- 5.Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M. Long-term Results of a Prospective Study on the Treatment of Medulloblastoma in Adults. www.interscience.wiley.com. [DOI] [PubMed]
- 6.Zhao F, Ohgaki H, Xu L, Giangaspero F, Li C, Li P, et al. Molecular subgroups of adult medulloblastoma: a long-term single-institution study. Nuero-Oncology. 2016;18(February):982–990. doi: 10.1093/neuonc/now050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho Y-J, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majd N, Penas-Prado M. Updates on management of adult medulloblastoma. Curr Treat Options Oncol. 2019;20(8):1–24. doi: 10.1007/s11864-019-0663-0. [DOI] [PubMed] [Google Scholar]
- 9.Chang CH, Housepian EM, Herbert C. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 10.Carrie C, Lasset C, Alapetite C, Maire J-P, Haie-Meder C, Hoffstetter S, et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74(8):2352–2360. doi: 10.1002/1097-0142(19941015)74:8<2352::AID-CNCR2820740821>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.DelCharco JO, Bolek TW, McCollough WM, Maria BL, Kedar A, Braylan RC, et al. Medulloblastoma: time–dose relationship based on a 30-year review. Int J Radiat Oncol Biol Phys. 1998;42(1):147–154. doi: 10.1016/S0360-3016(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 12.Brandes AA, Franceschi E, Tosoni A, Reni M, Gatta G, Vecht C, et al. Adult neuroectodermal tumors of posterior fossa (medulloblastoma) and of supratentorial sites (stPNET) Crit Rev Oncol Hematol. 2009;71(2):165–179. doi: 10.1016/j.critrevonc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Thompson et al. Prognostic Value of Medulloblastoma Extent of Resection After Accounting for Molecular Subgroup: An Integrated Clinical and Molecular Analysis. Lancet Oncol. 2016;17(4):484–95. [DOI] [PMC free article] [PubMed]
- 14.Franceschi E, Minichillo S, Mura A, Tosoni A, Mascarin M, Tomasello C, et al. Adjuvant chemotherapy in average-risk adult medulloblastoma patients improves survival: a long term study. BMC Cancer. 2020;20(1):1–6. doi: 10.1186/s12885-020-07237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocakaya S, Beier CP, Beier D. Chemotherapy increases long-term survival in patients with adult medulloblastoma—a literature-based meta-analysis. Neuro Oncol. 2016;18(3):408–416. doi: 10.1093/neuonc/nov185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21(8):1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 17.Kortmann RD, Kühl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ’91. Int J Radiat Oncol Biol Phys. 2000;46(2):269–279. doi: 10.1016/S0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich C, Von Bueren AO, Von Hoff K, Kwiecien R, Pietsch T, Warmuth-Metz M, et al. Treatment of adult nonmetastatic medulloblastoma patients according to the pediatric HIT 2000 protocol: a prospective observational multicentre study. Eur J Cancer. 2013;49(4):893–903. doi: 10.1016/j.ejca.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Kann BH, Lester-Coll NH, Park HS, Yeboa DN, Kelly JR, Baehring JM, et al. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017;19(2):259–269. doi: 10.1093/neuonc/now150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai R. Survival of patients with adult medulloblastoma. Cancer. 2008;112(7):1568–1574. doi: 10.1002/cncr.23329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.