Abstract
Group B Streptococcus (GBS) is the leading cause of bacterial sepsis and meningitis among neonates. While the capsular polysaccharide (CPS) is an important virulence factor of GBS, other cell surface components, such as C proteins, may also play a role in GBS disease. CPS production by GBS type III strain M781 was greater when cells were held at a fast (1.4-h mass-doubling time [td]) than at a slow (11-h td) rate of growth. To further investigate growth rate regulation of CPS production and to investigate production of other cell components, different serotypes and strains of GBS were grown in continuous culture in a semidefined and a complex medium. Samples were obtained after at least five generations at the selected growth rate. Cells and cell-free supernatants were processed immediately, and results from all assays were normalized for cell dry weight. All serotypes (Ia, Ib, and III) and strains (one or two strains per serotype) tested produced at least 3.6-fold more CPS at a td of 1.4 h than at a td of 11 h. Production of beta C protein by GBS type Ia strain A909 and type Ib strain H36B was also shown to increase at least 5.5-fold with increased growth rate (production at a td of 1.4 h versus 11 h). The production of alpha C protein by the same strains did not significantly change with increased growth rate. The effect of growth rate on other cell components was also investigated. Production of group B antigen did not change with growth rate, while alkaline phosphatase decreased with increased growth rate. Both CAMP factor and beta-hemolysin production increased fourfold with increased growth rate. Growth rate regulation is specific for select cell components in GBS, including beta C protein, alkaline phosphatase, beta-hemolysin, and CPS production.
Group B Streptococcus (GBS), also known as Streptococcus agalactiae, is part of the normal flora colonizing the respiratory, gastrointestinal, and urogenital tracts of humans. It is also the leading cause of bacterial sepsis and meningitis among neonates in the United States and is a major cause of endocarditis and fever in parturient women (6, 29). Most GBS strains that cause human infection in the United States are encapsulated by one of five antigenically distinct polysaccharides (serotype Ia, Ib, II, III, or V) (10, 11, 34, 37). The capsular polysaccharide (CPS) of GBS is an important virulence factor (1) and is the target of protective antibodies. The CPS has antiphagocytic properties (4, 5, 18), and the degree of encapsulation correlates directly with the virulence of the organism (28).
By definition, all strains of GBS produce Lancefield’s group B antigen, a cell wall-associated carbohydrate (23) that has been shown not to be a virulence factor of GBS (13, 19). Other surface antigens produced by human isolates of GBS include the C proteins alpha and beta and protein Rib (15, 22, 32, 38), which confer protective immunity and may have a role in GBS pathogenesis (3, 15–17, 21, 32). The relative production levels of CPS and surface proteins may affect their availability for antibody binding (8). GBS also produces a number of enzymes that may or may not be virulence factors, such as CAMP factor, a phospholipase secreted by GBS that causes complete lysis of sensitized sheep erythrocytes (SRBCs) (2). Partially purified CAMP factor was found to be lethal for rabbits (30), and purified CAMP factor, although not lethal in mice, did augment the virulence of various GBS isolates (7). Production of beta-hemolysin by GBS has been correlated with lung epithelial cell injury in vitro, suggesting a possible pathogenic role of this enzyme in the invasive step of early-onset GBS disease (24). The severe pneumonia associated with early-onset disease could be caused in part by damage of host cell membranes. In addition, increased hemolysin production was associated with a 50% decrease in the lethal dose and an earlier time of death in adult mice inoculated intranasally with GBS mutants producing a high level of beta-hemolysin compared with a nonhemolytic mutant (33).
Our laboratory has shown that production of CPS by GBS is regulated by growth rate (27). GBS serotype III strain M781 grown in continuous culture with modified chemically defined medium (MCDM) produced sixfold more cell-associated CPS at mass-doubling times (tds) of 0.8, 1.4, and 1.6 h than at tds of 2.3 and 11 h. Cell growth rate was determined to be the principal factor regulating CPS production, and growth rate-dependent production of type III CPS occurred independently of the growth-limiting nutrient. In this study we expand on these initial findings to determine the extent of growth rate regulation of (i) CPS of other GBS serotypes, (ii) different strains within selected serotypes, and (iii) potential virulence (alpha and beta C proteins, CAMP factor, and beta-hemolysin) and nonvirulence (group B antigen and alkaline phosphatase) factors.
MATERIALS AND METHODS
Bacterial strains, strain maintenance, and culture medium.
S. agalactiae M781 (type III), A909 (type Ia), O90 (type Ia), and H36B (type Ib) were isolated from patients with GBS disease (14, 15). Strain D66-7 (type III) was recently isolated from the vagina of a healthy woman and identified by established criteria (25); it has been passed three to five times in vitro. All GBS strains were grown in MCDM under glucose limitation as described previously (27) except for the following changes: 5 g of Casitone (Difco Laboratories, Detroit, Mich.) per liter was substituted for vitamin assay Casamino Acids, tryptophan and glycine were omitted, and thiamine was used at 1 mg/liter. GBS strains M781 and H36B were also grown under unknown limitation in continuous culture with Columbia broth (Difco Laboratories) supplemented with 8% glucose.
Continuous culture.
All experiments were conducted as previously described, with a method of cultivating organisms in a stable growth environment that allows us to study the effect of a single environmental variable on the physiology and growth of the organism. A growth vessel volume of 500 ml was maintained in a 1-liter fermentor (Applikon, Foster City, Calif.). The sterile medium inflow rate was adjusted to obtain the desired dilution rate (D) and td. The tds used were 1.4 h (D = 0.49 h−1), 2.7 h (D = 0.26 h−1), 5.8 h (D = 0.12 h−1), and 11 h (D = 0.06 h−1). To ensure that a steady state had been reached, samples were taken after at least five generations had elapsed from initiation of fresh medium inflow.
Growth measurements.
Measurements were calculated as previously described (27), except for modifications to cell dry weight (CDW) determinations. After the final centrifugation, the supernatant was decanted and pelleted cells were resuspended in the residual liquid. The entire cell suspension was then transferred to a preweighed 0.22-μm-pore-size polycarbonate filter. The tube was rinsed four times (500 μl each) with water which was filtered through a 0.22-μm-pore-size membrane and added to the same 0.22-μm-pore-size polycarbonate filter.
Determination of GBS cell wall components.
Cells removed from the chemostat were washed, and cell walls were digested with mutanolysin as described previously (27). Inhibition enzyme-linked immunosorbent assay was used to quantify the amount of each cell component as previously described (27), with the following reagents: for cell-associated CPS types Ia, Ib, and III, homologous serotype-specific rabbit antiserum (diluted 1:100,000) (26, 35, 36), purified homologous CPS as the standard, and homologous CPS coupled to poly-l-lysine as the coating antigen; for group B antigen, rabbit serum (diluted 1:100,000) raised to group B antigen coupled to tetanus toxoid (19), purified group B antigen as the standard, and group B antigen coupled to human serum albumin as the coating antigen; for alpha and beta C proteins, mouse antiserum raised to alpha (diluted 1:1,000) and rabbit antiserum raised to beta (diluted 1:50,000), and purified antigens as the standards and coating antigens.
Determination of beta-hemolytic, alkaline phosphatase, and CAMP factor activity.
Enzyme activities in GBS strain M781 cells growing at tds of 1.4 and 11 h were compared. We used two chemostats to simultaneously measure enzyme activity at two different growth rates, one for each of the two tds. In all enzyme activity assays, samples from the two chemostats were taken simultaneously.
For assessment of beta-hemolysin production, a 1-ml culture sample was serially diluted in phosphate-buffered saline (PBS; 40 mM phosphate [pH 7.0], 0.15 M NaCl) and plated on 5% sheep blood agar plates to determine initial bacterial concentrations. In addition, a 120-ml culture sample was taken, and the cells were sedimented by centrifugation (20 min at 13,689 × g and 6°C). The pellet was resuspended in residual liquid and transferred to a microcentrifuge tube. The centrifuge bottle was washed with PBS, and the wash volume was added to the microcentrifuge tube (approximately 1.5 ml). Cells were sedimented in a microcentrifuge (4 min at maximum speed), washed once in 1.0 ml of PBS, sedimented, and then resuspended in filter-sterilized lysis buffer (60 mM phosphate [pH 7.0], 0.15 M NaCl, 146 mg of MgCl2 [20 mM Mg2+], 1% soluble starch [Difco Laboratories], 1% T-500 dextran [Sigma Company, St. Louis, Mo.] 3% Tween 20, 1% glucose). Acid-washed glass beads (0.5 ml; Sigma) were added to microcentrifuge tubes, which were then shaken with a dental amalgamator (Foremost Dental Manufacturing, Englewood, N.J.) (3 min at high speed) (39). Samples were incubated for 30 min at 37°C to achieve maximum bacterial lysis. Cell debris was sedimented in a microcentrifuge (4 min at maximum speed). The pellet was resuspended in PBS to a final volume of 1.0 ml and processed as described above to determine bacterial concentration. The lysate was used to prepare two sets of diluted supernatants (serial twofold dilutions in PBS). Defibrinated SRBCs were prepared in a 96-well microtiter plate. Two hundred microliters of 1% SRBC was sedimented by centrifugation (10 min at 2,800 rpm [model TJ-6 centrifuge; Beckman Instruments, Inc., Palo Alto, Calif.]), and the supernatant was decanted. One set of serially diluted bacterial extracts (150 μl/dilution) was mixed with SRBCs until the pellets were resuspended. The mixture was transferred to another 96-well microtiter plate (incubation plate). The other set of serially diluted bacterial extracts was transferred (150 μl/well) to the incubation plate. Controls, both positive (0.1% sodium dodecyl sulfate in PBS) and negative (PBS alone), were prepared in duplicate with and without blood, transferred to the incubation plate (150 μl/well), and incubated at 37°C for 1 h before debris was sedimented (10 min at 2,800 rpm [TJ-6 centrifuge]) and 100 μl of the supernatant was transferred to a fresh microtiter plate. The absorbance of each sample was determined at 540 nm. A hemolytic titer was defined as the reciprocal of the dilution of sample producing 50% hemoglobin release. The absorbance value for 50% hemoglobin release was calculated for each assay as the average A540 of the positive control (100% hemoglobin release) minus the average A540 of the negative control (background absorbance) divided by 2. Sample absorbance readings were adjusted for background with the absorbances from the matched serially diluted samples without SRBCs. Efficiency of bacterial lysis was measured by comparison of the bacterial concentration before and after lysis.
For alkaline phosphatase and CAMP factor activity, culture samples were normalized for CDW by dilution with Tris buffer (0.01 M Tris HCl, 0.01 M MgCl2, 0.15 M NaCl [pH 7.4]). One milliliter of each normalized sample was centrifuged (10 min at 2,800 rpm [TJ-6 centrifuge]). Pelleted cells were analyzed for alkaline phosphatase activity, and the supernatant was used in CAMP factor analysis. Enzyme assays were performed as described below.
Alkaline phosphatase activity was measured with a modification of a previously described method (31). Cells were resuspended in 1 ml of 0.85% NaCl, and 0.5 ml of this suspension was added to 0.5 ml of a fresh substrate solution and mixed. A negative control was prepared by the addition of 0.5 ml of 0.85% NaCl to 0.5 ml of a fresh substrate solution that consisted of p-nitrophenyl phosphate (1 mg/ml) in 0.04 M glycine (pH 10.5). A standard curve was generated with commercial alkaline phosphatase solution (50 U/ml; Sigma) in 0.04 M glycine (pH 10.5). All samples, including the standard, were incubated at 37°C for 15 min without shaking. Cells were removed by centrifugation, and the supernatants were filtered through a 0.22-μm-pore-size membrane. Test samples and the negative control were transferred to the microtiter plate (200 μl/well), and the absorbance at 405 nm was measured. Alkaline phosphatase activity is reported as units of enzyme activity per milligram of CDW and is the mean of four samples.
The amount of CAMP factor produced by GBS grown in continuous culture at different growth rates was determined by a modification of a published method (9). SRBCs (2 × 104) were washed three times and resuspended in 100 μl of PBS. A sphingomyelinase solution was prepared by soaking one Beta Lysin disk (Remel, Lenexa, Kans.) in 0.5 ml of Tris buffer. The SRBC suspension (20 μl) was added to 4 ml of Tris buffer and 40 μl of the sphingomyelinase solution, mixed, and incubated 5 min at 30°C. Supernates from CDW-normalized samples were filtered through a 0.22-μm-pore-size membrane, and 50 μl was transferred to microtiter plate wells containing 50 μl of sensitized SRBCs, mixed, and incubated 30 min at 30°C. For the negative control, 50 μl of Tris buffer was used. Absorbance at 490 nm was measured immediately after 3 s of high-speed shaking. The absorbance values reported are the mean of 12 replicates. The titer was defined as the reciprocal of the dilution resulting in the A490 value at the midpoint of the exponential portion of the A490 curve (0.25 for both curves).
Statistics.
Instat version 2.0 software (Graphpad Software, Inc., San Diego, Calif.) was used to analyze data to determine significance (P < 0.05).
RESULTS
Biomass production.
The mean CDW values (± standard errors of the means [SEM]) determined for each serotype and strain grown in MCDM with glucose limitation are presented in Table 1. The mean CDW values for GBS grown in Columbia broth supplemented with 8% glucose at tds of 11 h and 1.4 h were 1.69 ± 0.17 and 2.34 ± 0.11 mg/ml, respectively, for strain M781 and 2.16 ± 0.01 and 2.37 ± 0.01 mg/ml, respectively, for strain H36B.
TABLE 1.
Biomass produced by different GBS serotypes and strains during continuous culture growth in MCDM and glucose limitation
| Serotype | Strain | td (h) | Mean CDW ± SEMa (mg/ml) |
|---|---|---|---|
| III | M781 | 11.0 | 0.31 ± 0.01 |
| 1.4 | 0.32 ± 0.03 | ||
| D66-7 | 11.0 | 0.43 ± 0.08 | |
| 1.4 | 0.46 ± 0.01 | ||
| Ia | A909 | 11.0 | 0.68 ± 0.17 |
| 5.8 | 0.39 ± 0.02 | ||
| 2.7 | 0.34 ± 0.01 | ||
| 1.4 | 0.31 ± 0.01 | ||
| O90 | 11.0 | 0.19 ± 0.00 | |
| 1.4 | 0.35 ± 0.01 | ||
| Ib | H36B | 11.0 | 0.29 ± 0.00 |
| 5.8 | 0.39 ± 0.00 | ||
| 2.7 | 0.41 ± 0.02 | ||
| 1.4 | 0.29 ± 0.01 |
Calculated with three or more samples.
Production of CPS.
All five GBS strains grown in MCDM with glucose limitation produced significantly more CPS (P < 0.01, unpaired t test) at the fast td of 1.4 h than at the slower td of 11 h (Table 2). The increase in net CPS production ranged from 3.6-fold (strain D66-7) to 14.9-fold (strain A909). Columbia broth supplemented with 8% glucose (growth limitation unknown) produced a similar pattern of increase in specific CPS production with a td of 1.4 h: strain M781 (serotype III) produced 7.5-fold more CPS and strain H36B (serotype Ib) 4.4-fold more than at the slower growth rate (data not shown).
TABLE 2.
Production of specific CPS by different GBS serotypes and strains grown in continuous culture using MCDM and glucose limitation
| Serotype | Strain | Mean specific CPS ± SEMa (μg/mg of CDW) at td of:
|
Fold increaseb | |
|---|---|---|---|---|
| 11 h | 1.4 h | |||
| III | M781 | 18.5 ± 5.5 | 88.3 ± 19.4 | 4.8 |
| D66-7 | 10.7 ± 1.7 | 38.0 ± 6.5 | 3.6 | |
| Ia | A909 | 2.0 ± 1.2 | 29.7 ± 7.7 | 14.9 |
| O90 | 11.0 ± 1.1 | 90.7 ± 4.3 | 8.2 | |
| Ib | H36B | 7.0 ± 6.2 | 80.8 ± 10.7 | 11.5 |
Calculated with three or more samples.
All values are considered significant (two-tailed P value is < 0.01).
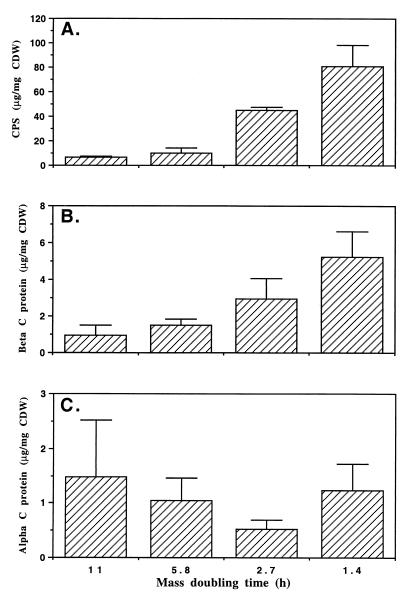
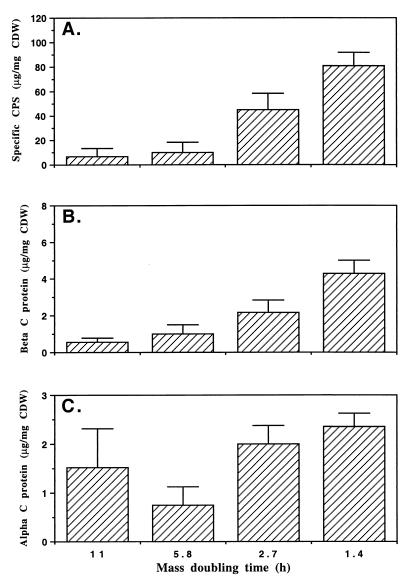
For GBS type Ia strain A909 (Fig. 1A) and type Ib strain H36B (Fig. 2A) examined over a range of increasing growth rates, CPS production increased with increasing growth rate. Both strains exhibited a significant linear trend in the change in the amount of CPS produced with increasing td (P < 0.01). In addition to both strains producing significantly more CPS at a td of 1.4 h than 11 h, strain H36B produced significantly more CPS at a td of 1.4 h than 5.8 h (P < 0.01, Tukey-Kramer multiple comparison test) that was not exhibited by strain A909.
FIG. 1.
Effect of growth rate on production of specific CPS (A) and beta (B) and alpha (C) C proteins by GBS type Ia strain A909. Cells were grown in continuous culture in MCDM with glucose limitation. Shown in the bar graph are means of three samples, with error bars representing SEM. CPS and beta C protein produced at a td of 1.4 h compared with 11 h, P < 0.05; all other combinations were not significant at P > 0.05.
FIG. 2.
Effect of growth rate on production of specific CPS (A) and beta (B) and alpha (C) C proteins by GBS type Ib strain H36B. Cells were grown in continuous culture in MCDM with glucose limitation. Shown in the bar graph are means of three samples, with error bars representing SEM. CPS produced at a td of 1.4 h compared with production at 5.8 or 11 h, P < 0.01; beta C protein produced at a td of 1.4 h compared with production at 5.8 or 11 h, P < 0.05; all other combinations were not significant at P > 0.05.
Production of alpha and beta C proteins.
The production of alpha and beta C proteins with changing growth rate was investigated in strains A909 and H36B. We found that 5.5-fold more beta C protein was produced by strain A909 cells (Fig. 1B) at the fast td of 1.4 h than at the slower td of 11 h (P < 0.05, Tukey-Kramer multiple comparison test). Similarly, strain H36B (Fig. 2B) produced 7.9-fold more beta C protein at the fast than at the slower growth rate (P < 0.05, Tukey-Kramer multiple comparison test). Both strains exhibited a significant linear trend in the change in the amount of CPS produced with increasing td (P < 0.01).
In contrast to the production of beta C protein, production of alpha C protein by strains A909 and H36B did not change significantly (P > 0.05, Tukey-Kramer multiple comparison test) between any of the growth rates tested (tds of 1.4, 2.7, 5.8, and 11 h) (Fig. 1C and 2C). A Western blot of these samples confirmed the relative levels of alpha C protein detected by enzyme-linked immunosorbent assay (data not shown).
Group B antigen production.
Growth rate did not alter the production of group B antigen in type III strain M781 (Table 3). The mean ± SEM values of specific group B antigen produced at tds of 11 and 1.4 h were 26.7 ± 3.5 and 30.9 ± 4.9 μg of antigen/mg of CDW, respectively (P = 0.52, unpaired t test, n = 10 and 12, respectively).
TABLE 3.
Production of cell components by GBS grown in continuous culture using MCDM and glucose limitation
| td (h) | Strain M781
|
Mean specific value (μg/mg of CDW) ± SEM
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean group B antigen (μg/mg of CDW) ± SEM | Mean alkaline phosphatase (U/mg of CDW) ± SEM | Titer
|
Alpha C protein
|
Beta C protein
|
||||
| Beta-hemolysin (mean ± SEM) | CAMP factor | Strain H36B | Strain A909 | Strain H36B | Strain A909 | |||
| 11 | 26.7 ± 3.5 | 1.48 ± 0.03 | 12 ± 6 | 12 | 1.53 ± 0.79 | 1.46 ± 1.02 | 0.54 ± 0.21 | 0.95 ± 0.54 |
| 1.4 | 30.9 ± 4.9 | 1.34 ± 0.03 | 50 ± 7 | 48 | 2.35 ± 0.28 | 1.22 ± 0.47 | 4.28 ± 0.74 | 5.23 ± 1.37 |
| P | NSa | 0.01 | <0.05 | NDb | NS | NS | <0.05 | <0.05 |
NS, not significant.
ND, not determined.
Beta-hemolysin.
A faster growth rate had a positive effect on beta-hemolysin production by GBS strain M781. Cells grown at a td of 1.4 h produced a significant 4.2-fold greater amount of beta hemolysin activity (titer of 50 ± 7 [mean ± SEM]) than cells grown at a td of 11 h (12 ± 6) (P < 0.05, Mann-Whitney test) (Table 3).
CAMP factor.
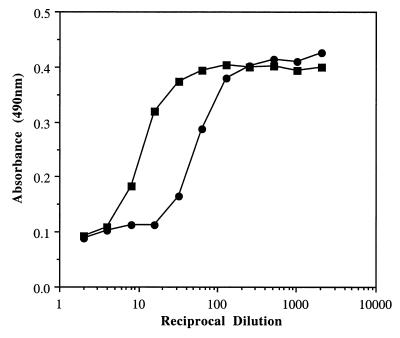
Strain M781 produced 4.1-fold more CAMP factor when grown at a td of 1.4 h than when grown at a td of 11 h (Fig. 3 and Table 3), a result confirmed by a cohemolysis assay in which the zones of cohemolysis on a blood agar plate for the samples from cells grown at tds of 1.4 h and 11 h were 3 to 3.5 mm and 1 to 1.5 mm, respectively. However, there was no difference in CAMP factor activity at different growth rates as determined by the slope of the linear portion of their titration curves (0.31 absorbance units/reciprocal dilution of sample) (Fig. 3).
FIG. 3.
Lysis of SRBCs by CAMP factor from GBS type III strain M781 grown in continuous culture. Amounts and activities of CAMP factor produced at growth rates of 1.4 h (open squares) and 11 h (closed diamonds) were compared.
Alkaline phosphatase.
The amount of alkaline phosphatase produced by strain M781 increased with decreasing growth rate (Table 3). There was an approximately 1.1-fold increase in the amount of enzyme produced by cells grown at a td of 11 h compared with those grown at a td of 1.4 h. The mean ± SEM values of alkaline phosphatase at tds of 1.4 and 11 h were 1.34 ± 0.03 and 1.48 ± 0.03 U/mg of CDW (P = 0.01, unpaired t test), respectively. This change, though statistically significant, is unlikely to be biologically significant.
DISCUSSION
In our initial investigation of the regulation of CPS production, we concluded that growth rate regulates CPS production in GBS type III strain M781 (27). We have continued these studies to determine the scope and specificity of growth rate regulation of CPS production and the production of other GBS cellular factors, both virulent and nonvirulent. By using continuous culture methods to study the physiology of GBS, we have been able to separate and define parameters that are interdependent during batch culture growth, such as nutrient concentration and growth rate. Continuous culture permits cells to grow indefinitely in an unchanging environment, resulting in a metabolically homogeneous population of cells. The range of growth rates studied (td from 1.4 to 11 h) cannot be directly correlated with in vivo growth rates for GBS because these values are not known, but they can be indirectly correlated with the range of growth rates for bacteria growing in the oral cavity (20).
Regulation of CPS production by growth rate was common to all serotypes and strains of GBS tested and was not dependent on the growth medium used. Various serotypes and strains of GBS, in addition to type III strain M781, were tested with a chemostat. For all serotypes and strains tested (type III strains M781 and D66-7, type Ia strains A909 and O90, and type Ib strain H36B), there was a significant increase in CPS production when cells were grown at a td of 1.4 rather than 11 h (Table 2). Even a recent vaginal isolate (strain D66-7) produced more specific CPS at a td of 1.4 h, an indication that an increase in CPS production is not restricted to laboratory strains that have been passed a number of times on laboratory medium. It is interesting that a GBS strain believed to be a constitutive high producer of CPS, strain O90, produced significantly more CPS at a td of 1.4 h than at a td of 11 h. The results from continuous culture studies using supplemented Columbia broth indicated that increased production of specific CPS with increased growth rate is not dependent on a component of MCDM.
Production of other cell surface antigens varied, and no pattern related to their properties as virulence factors was noted (Table 3). Production of group B antigen, not considered a virulence factor (13, 19), did not significantly change with growth rate as was the case with alkaline phosphatase. Although the change in production was statistically significant, a 1.1-fold change for this enzyme most likely is not biologically significant. For both strains H36B and A909, beta C protein production followed a pattern similar to that for CPS production in the same samples (Fig. 1A, 1B, 2A, and 2B). However, this was not the case for production of alpha C protein by the same strains (Fig. 1C and 2C). These results indicate that the mechanisms of regulation of alpha and beta C protein production are different. Alpha and beta C proteins have been shown by others to have unrelated amino acid sequences and to be produced independent of each other (12). From these results, we conclude that beta C protein production is regulated by growth rate, while production of alpha C protein, alkaline phosphatase, and group B antigen is not. Furthermore, growth rate regulation of production of GBS cell surface antigens is specific, since not all surface antigens were produced optimally at a fast td nor were they all virulence factors.
Another interesting finding was the growth rate regulation of CAMP factor. Cells grown at a td of 1.4 h produced more CAMP factor than those grown at a td of 11 h (Fig. 3), but the activity of the enzyme produced at each td did not vary. Beta-hemolysin activity also increased significantly with increased growth rate. These results suggest a different type of growth rate regulation for the production of these components than for the production of CPS, CAMP factor, beta-hemolysin, and beta C protein.
In summary, growth rate regulation of CPS by GBS is not unique to strain M781 serotype III but is exhibited by other clinically important serotypes as well. This type of regulation affects production of some (beta C protein, CAMP factor, and beta hemolysin) but not all (alkaline phosphatase, group B antigen, and alpha C protein) GBS components, demonstrating that GBS can specifically modulate cellular components in response to growth rate. Future studies will determine the molecular mechanisms by which growth rate regulates GBS antigen production.
ACKNOWLEDGMENTS
We thank Claudia Gravekamp for her contributions to the work involving alpha and beta C proteins and Ken Johnson for his technological expertise.
This work was supported by NIH-NIAID grant AI-25152.
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1990. pp. 742–811. [Google Scholar]
- 2.Bernheimer A W, Linder R, Avigad L S. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect Immun. 1979;23:838–844. doi: 10.1128/iai.23.3.838-844.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevanger L, Naess A I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand. 1985;93:121–124. doi: 10.1111/j.1699-0463.1985.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 4.Edwards M S, Kasper D L, Jennings H J, Baker C J, Nicholson W A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 5.Edwards M S, Nicholson W A, Baker C J, Kasper D L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980;151:1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faro S. Group B beta-hemolytic streptococci and puerperal infections. Am J Obstet Gynecol. 1981;139:686–689. doi: 10.1016/0002-9378(81)90486-5. [DOI] [PubMed] [Google Scholar]
- 7.Fehrenbach F J, Jurgens D, Ruhlmann J, Sterzik B, Ozel M. Role of CAMP-factor (factor B) for virulence. Zentbl Bakteriol Hyg Suppl. 1988;17:351–357. [Google Scholar]
- 8.Gravekamp C, Rosner B, Madoff L C. Deletion of repeats in the alpha C protein enhances the pathogenicity of group B streptococci in immune mice. Infect Immun. 1998;66:4347–4354. doi: 10.1128/iai.66.9.4347-4354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huser, H., L. Goeke, G. Karst, and F. J. Fehrenbach. 1983. Fermenter growth of Streptococcus agalactiae and large-scale production of CAMP factor. J. Gen. Microbiol. 1295–1300. [DOI] [PubMed]
- 10.Jennings H J, Katzenellenbogen E, Lugowski C, Kasper D L. Structure of the native polysaccharide antigens of type Ia and type Ib group B Streptococcus. Biochemistry. 1983;22:1258–1263. doi: 10.1021/bi00274a042. [DOI] [PubMed] [Google Scholar]
- 11.Jennings H J, Rosell K G, Katzenellenbogen E, Kasper D L. Structural determination of the capsular polysaccharide antigen of type II group B Streptococcus. J Biol Chem. 1983;258:1793–1798. [PubMed] [Google Scholar]
- 12.Johnson D R, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984;19:506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancefield R C. Antigens of group B streptococci relation to mouse-protective antibodies and immunity. In: Robbins J B, et al., editors. New approaches for inducing natural immunity to pyogenic organisms. Bethesda, Md: National Institutes of Health; 1975. pp. 145–179. [Google Scholar]
- 14.Lancefield R C. Two serological types of group B hemolytic streptococci with related, but not identical, type-specific substances. J Exp Med. 1938;67:25–40. doi: 10.1084/jem.67.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancefield R C, McCarty M, Everly W N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975;142:165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Kasper D L, Ausubel F M, Rosner B, Michel J L. Inactivation of the alpha C protein antigen, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B Streptococcus. Proc Natl Acad Sci USA. 1997;94:13251–13256. doi: 10.1073/pnas.94.24.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madoff L C, Michel J L, Gong E W, Kling D E, Kasper D L. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc Natl Acad Sci USA. 1996;93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques M B, Kasper D L, Pangburn M K, Wessels M R. Prevention of C3 deposition is a virulence mechanism of type III group B Streptococcus capsular polysaccharide. Infect Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marques M B, Kasper D L, Shroff A, Michon F, Jennings H J, Wessels M R. Functional activity of antibodies to the group B streptococci elicited by a polysaccharide-protein conjugate vaccine. Infect Immun. 1994;62:1593–1599. doi: 10.1128/iai.62.5.1593-1599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh P, Martin M. Oral microbiology. 3rd ed. London, United Kingdom: Chapman & Hall; 1992. [Google Scholar]
- 21.Michel J L, Madoff L C, Kling D E, Kasper D L, Ausubel F M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991;59:2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel J L, Madoff L C, Kling D K, Kasper D L, Ausubel F M. The C proteins of group B Streptococcus. In: Dunny G M, Cleary P C, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 214–218. [Google Scholar]
- 23.Michon F, Katzenellenbogen E, Kasper D L, Jennings H J. Structure of the complex group-specific polysaccharide of group B Streptococcus. Biochemistry. 1987;26:476–486. doi: 10.1021/bi00376a020. [DOI] [PubMed] [Google Scholar]
- 24.Nizet V, Gibson R L, Chi E Y, Framson P E, Hulse M, Rubens C E. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3926. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onderdonk A B, Zamarchi G R, Walsh J A, Mellor R D, Munoz A, Kass E H. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl Environ Microbiol. 1986;51:333–339. doi: 10.1128/aem.51.2.333-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paoletti L, Wessels M, Rodewald A, Shroff A, Jennings H, Kasper D. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by maternal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1994;62:3236–3243. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoletti L C, Ross R A, Johnson K D. Cell growth rate regulates expression of group B Streptococcus type III capsular polysaccharide. Infect Immun. 1996;64:1220–1226. doi: 10.1128/iai.64.4.1220-1226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubens C E, Wessels M R, Heggen L M, Kasper D L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci USA. 1987;84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuchat A, Wenger J D. Epidemiology of group B streptococcal disease. Risk factors, prevention strategy, and vaccine development. Epidemiol Rev. 1994;16:374–402. doi: 10.1093/oxfordjournals.epirev.a036159. [DOI] [PubMed] [Google Scholar]
- 30.Skalka B, Smola J. Lethal effect of CAMP-factor and UBERIS-factor: a new finding about diffusible exosubstances of Streptococcus agalactiae and Streptococcus uberis. Zentbl Bakteriol. 1981;249:190–194. [PubMed] [Google Scholar]
- 31.Smibert R M, Krieg N R. General characterization. In: Gerhardt P, editor. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 409–443. [Google Scholar]
- 32.Stalhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wennerstrom D E, Tsaihong C J, Crawford J T. Evaluation of the role of hemolysin and pigment in the pathogenesis of early onset group B streptococcal infection. In: Kimura Y, Kotami S, Shiokawa Y, editors. Recent advances in streptococcal diseases. Bracknell, United Kingdom: Reedbooks; 1985. pp. 155–156. [Google Scholar]
- 34.Wessels M R, DiFabio J L, Benedi V-J, Kasper D L, Michon F, Brisson J-R, Jelinkova J, Jennings H J. Structural determination and immunochemical characterization of the type V group B Streptococcus capsular polysaccharide. J Biol Chem. 1991;266:6714–6719. [PubMed] [Google Scholar]
- 35.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wessels M R, Paoletti L C, Rodewald A K, Michon F, DiFabio J, Jennings H J, Kasper D L. Stimulation of protective antibodies against types Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–4766. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessels M R, Pozsgay V, Kasper D L, Jennings H J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]
- 38.Wilkinson H W, Eagon R G. Type-specific antigens of group B Streptococcus. Infect Immun. 1971;4:596–604. doi: 10.1128/iai.4.5.596-604.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yim H, Rubens C. Use of a dental amalgamator to extract RNA from the Gram-positive bacterium Streptococcus agalactiae. BioTechniques. 1997;23:229–231. doi: 10.2144/97232bm11. [DOI] [PubMed] [Google Scholar]