Abstract
An integrated approach to innovatively counter the transmission of various arthropod-borne diseases to humans would benefit from strategies that sustainably limit onward passage of infective life cycle stages of pathogens and parasites to the insect vectors and vice versa. Aiming to accelerate the impetus towards a disease-free world amid the challenges posed by climate change, discovery, mindful exploitation and integration of active natural products in design of pathogen transmission-blocking interventions is of high priority. Herein, we provide a review of natural compounds endowed with blockade potential against transmissible forms of human pathogens reported in the last 2 decades from 2000 to 2021. Finally, we propose various translational strategies that can exploit these pathogen transmission-blocking natural products into design of novel and sustainable disease control interventions. In summary, tapping these compounds will potentially aid in integrated combat mission to reduce disease transmission trends.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-022-05367-8.
Keywords: Human pathogen transmission-blocking, Natural products, Arthropod disease vectors, Disease control, Anti-infectives
Background
Haematophagous arthropod vectors—mainly mosquitoes, phlebotomine sand flies, triatomine bugs, simulid blackflies and tsetse flies—inadvertently transmit highly infectious pathogens to humans during blood meal acquisition. Arboviruses (chikungunya virus, CHIKV; Zika virus, ZIKV; dengue fever virus, DENV; West Nile virus, WNV; Rift Valley fever virus, RVFV; sand fly fevers, yellow fever virus, YFV, etc.), lymphatic filarial worms, Wuchereria bancrofti, Brugia spp., Plasmodium parasites, Onchocerca volvulus and kinetoplastids (leishmania and trypanosomes) that develop in these insects gravely afflict humans residing in tropical and subtropical regions. These vector-borne pathogens contribute to > 17% of all human infectious diseases, accounting for > 700,000 annual deaths estimated by the World Health Organization (WHO) (https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases). The successful transmission of these pathogens across vertebrate and insect hosts is facilitated by intricate multi-ecological factors, most of which remain poorly understood, yet intriguingly providing excellent opportunities for novel intervention designs. While the development of effective vaccines against these arthropod-borne diseases appears far beyond reach, translational aspects to counter the infectious bites especially through small molecule forms are warranted.
The attractiveness to infectious individuals in some insect vectors for pathogen uptake is the initial adaptive success towards sustained transmission. For instance, in malaria highly gametocytemic individuals have modulated host skin microbiota composition and chemistry that generates volatile organic compounds (VOCs), which attract more female Anopheles mosquitoes for blood feedings, and in the process acquire Plasmodium parasites [1–4]. A similar phenomenon has been reported for sand flies [5]. Such host-induced attractiveness is however lacking for many emerging and re-emerging arboviruses partly because these mosquito infections are incidental, but also other evolved viral mechanisms have been described to sustain their transmission cycle upon viral establishment [6]. These viral mechanisms include vertical (transovarial and trans-egg transmissions) and horizontal transmission, or both. However, other viruses find their way into mosquitoes through environmental acquisition; for instance, ZIKVs have been reported to potentially infect mosquito juveniles while breeding in contaminated sites [7]. Nevertheless, following the ingestion of infectious blood meals the developing pathogens must infect various tissues and evade physiological bottlenecks imposed by the vector immune defences to facilitate successful transformation into infective stages ready for transmission to the next human host. This infective passage from the vector to humans occurs during appetite-induced search for blood meal, but also as a result of enhanced manipulative feeding effects by the infective stages as in the case of malaria, leishmania and DENV [8]. A consequential worrying trend is the possible transmission of drug-resistant pathogens, exemplified by Plasmodium sporozoites, CHIKV variants and leishmania promastigotes that have been reported [9–12].
With current global elimination strategies for infectious diseases in focus, there is a dire need to find appropriate transmission-reducing interventions [13–16]. In fact, the UN Sustainable Development Goal 3 advocates for elimination of human infectious pathogens by 2030 anchored on target 3.3. Towards this goal, knowledge on transmission of insect-vectored human pathogens between host interfaces continues to grow rapidly in tandem with recent advancements of -omics technologies and potential drug targets discovered. Prioritizations have largely taken the form of disrupting either the development of transmissible stages in human and insect hosts or the activation factors of quiescent infectious forms, as well as targeting the host–pathogen interaction proteins that offer less drug pressure in the insect vector [17]. Opportunities, viz, (i) arbitrary screening for direct cidal activity against transmissible stages, (ii) targeting the host factors functionally identified to facilitate host cell invasion, replication and egression, (iii) exploitation of obligate endosymbionts, (iv) vector sugar feeding behaviour and endectocides, (v) enzyme inhibitions of essential pathogen proteins and (vii) Plasmodium liver stages following sporozoite inoculation have taken centre stage in the identification of transmission-blocking compounds [18–23].
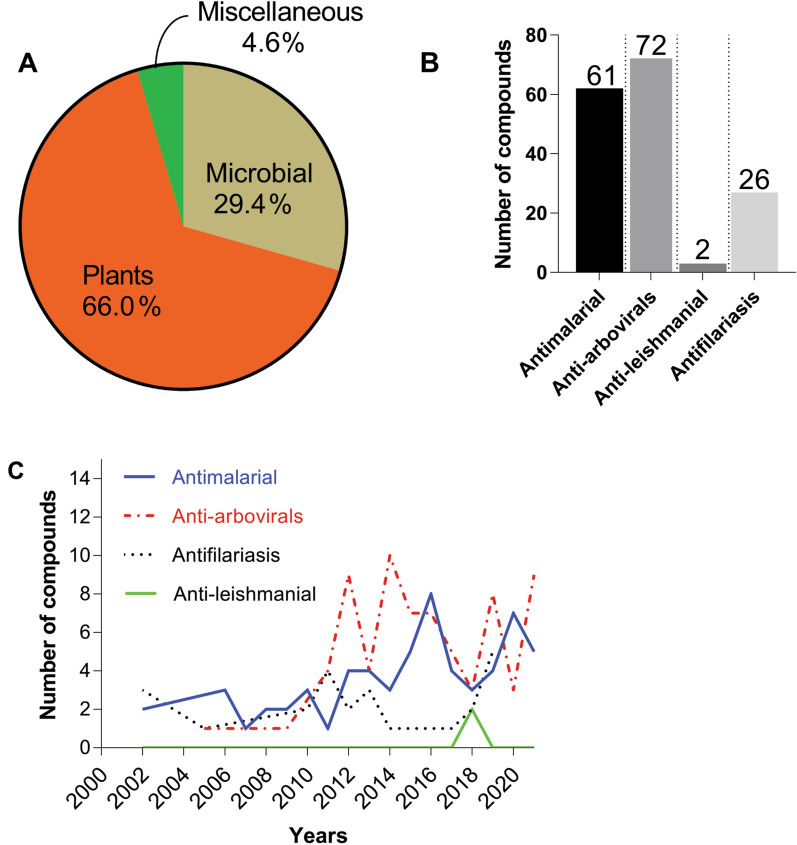
Despite a slow pace in their exploration, compared to chemotherapeutic counterparts, natural products (primarily from plants, microbial sources, marine organisms, insect microbiomes and higher invertebrates) and their synthetic derivatives as pathogen transmission-blocking agents could offer an integral pivot in disease control and prevention. Structural diversity and complexity provided by these compounds have been widely appreciated in drug discovery as novel lead scaffolds for various anti-infective drugs [24, 25]. Within this context, the identification and strategic use of natural products as new leads to combat further spread of vector-borne diseases to humans will complement the existing interventions. Our earlier studies have contributed to these efforts by documenting the vector control strategies utilizing natural compounds [26]. In this review, we provide a summary based on an extensive literature search on the promising anti-infective natural compounds (and their derivatives) discovered in the last 22 years (from 2000 to 2021). The scientific literature was searched from PubMed, Google Scholar, Wiley Online Library, ScienceDirect, ACS Publications, Royal Society of Chemistry (RSC), Web of Science and SpringerLink libraries. Relevant keywords, “transmission-blocking”, “natural products”, “human vector diseases”, “antivirals”, “procyclic and metacyclic trypomastigotes”, “arboviruses”, “antifilariasis”, “anti-wolbachial”, and “procyclic promastigotes” and appropriate combinations of the above terms, were used to retrieve the articles. Our analyses demonstrate a great emphasis on exploration of plant- (66.01%) and microbial-derived (29.41%) chemical space in pursuit of reducing the spread of malaria (61 compounds), arboviruses (72 compounds), lymphatic filariasis (26 compounds) and leishmania (2 compounds) transmissions over the 22-year period (Fig. 1A–C). Moreover, we present our perspectives on various prospective applicability strategies of these molecules towards impedance of transmission of infectious pathogens between humans and insect vectors.
Fig. 1.
Summarized analyses of the highlighted compounds retrieved from literature. A Overall distribution of sources of natural compounds highlighted in this review. B Disease target profile of the highlighted natural compounds. C Trends in exploration of natural products in pursuit of pathogen transmission-blocking between 2000 and 2021
Transmission-blocking in pursuit of human disease elimination and eradication agenda: the challenge meets nature?
Midgut microbiota and microbial explorations
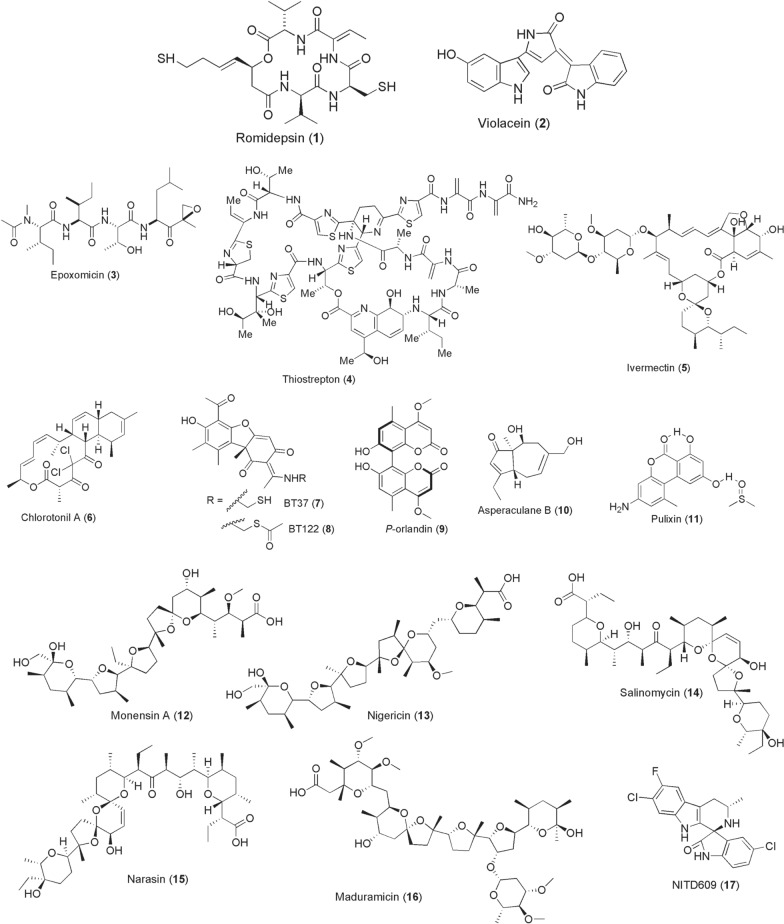
Naturally most if not all living organisms, including disease-transmitting insect vectors, are colonized by multigenera microbial communities existing in unresolved complex symbiotic interactions largely shaped by their proximate environment [27]. Wild-caught mosquitoes, for instance, are dominated by highly diverse and dynamic gram-negative bacteria, Pseudomonas, Aeromonas, Asaia, Comamonas, Elizabethkingia, Serratia, Acinetobacter, Enterobacter, Klebsiella and Pantoea, and symbiotic fungi; yeast, Candida, Penicillium, and Pichia (reviewed in [28]). Antiparasitic, antimicrobial and antiviral products secreted by such microbes (inclusive of insect-associated symbionts) are or have found interesting pharmaceutical applications [29–31]. Several studies published over the recent years have demonstrated the potential impact of gut bacterial products in impairing Plasmodium sporogonic development through direct killing, indirect immunomodulatory effects, or both. Chromobacterium spp. (Enterobacter bacterium) isolated from midguts of field-caught Zambian anophelines (Esp_Z) [32] and Panamanian Aedes aegypti mosquitoes (Csp_P) [33] inhibited Plasmodium ookinete development and also abrogated replication of dengue virus in mosquitoes. These inhibitory activities were later found to be mediated by a histone deacetylase inhibitor romidepsin (1; Fig. 2) and aminopeptidase secretion, respectively [34, 35]. The depsipeptide compound 1 has potent Plasmodium gametocytocidal activities. Besides, the bacterial isolate (Csp_P) kills mosquito larvae by its secreted hydrogen cyanide in addition to exerting adulticidal effects [36], underscoring its broad-spectrum activity window. A related Chromobacterium species C. violaceum has been isolated from soils [37]. These bacteria produce a broad-spectrum bisindole antimalarial agent, 3-[1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydeno]-1,3-dihydro-2H-indol-2-one (violacein (2)), reported to have gametocytocidal (EC50 1.25–2.5 µM) and Plasmodium transmission-blocking activity (43% oocysts reduction) [37]. In another more recent study, despite pending efforts to establish the identity of anti-Plasmodium mediating molecule(s), a novel sexually inherited microsporidian MB infecting Kenyan An. arabiensis population was discovered to block transmission of P. falciparum (undetectable levels of sporozoites) [38, 39]. Similar bacteria with P. vivax transmission-blocking activity are the Serratia spp. and Enterobacter spp. isolated from midguts of field-collected An. albimanus in southern Mexico, affording appreciable reductions in oocysts densities [40]. Further exploration of these bacterial species, and their bioactive compounds 1, 2 as potential leishmania, Trypanosoma, filarial and arboviral transmission-blocking interventions, is lacking in literature and presents an open path of scientific exploration. Transmission-blocking endosymbiotic “killer” yeast strains of Wickerhamomyces anomalus have been shown to undergo maternal inheritance in different insects, including Anopheles, Aedes, and Culex mosquitoes, and sand flies [41–43]. Secreted antimicrobial toxins such as WaF17.12 and WaATCC96603 induced in vitro killing of P. berghei ANKA sporogonic stages at LC50 64.6 µg/ml and 61.3 µg/ml, respectively, reducing 65.2% of early sporogonic parasites and oocysts in mosquitoes [44, 45]. Stable pathogen transmission-blocking compounds from symbionts in other disease vectors are yet to be identified.
Fig. 2.
Chemical structures of compounds 1–17. Romidepsin (1), Violacein (2), Epoxomicin (3), thiostrepton (4), ivermectin (5), chlorotonil a (6), bt37 (7), bt122 (8), p-orlandin (9), asperaculane b (10), pulixin (11), monensin a (12), nigericin (13), salinomycin (14), narasin (15), maduramicin (16), NITD609 (17)
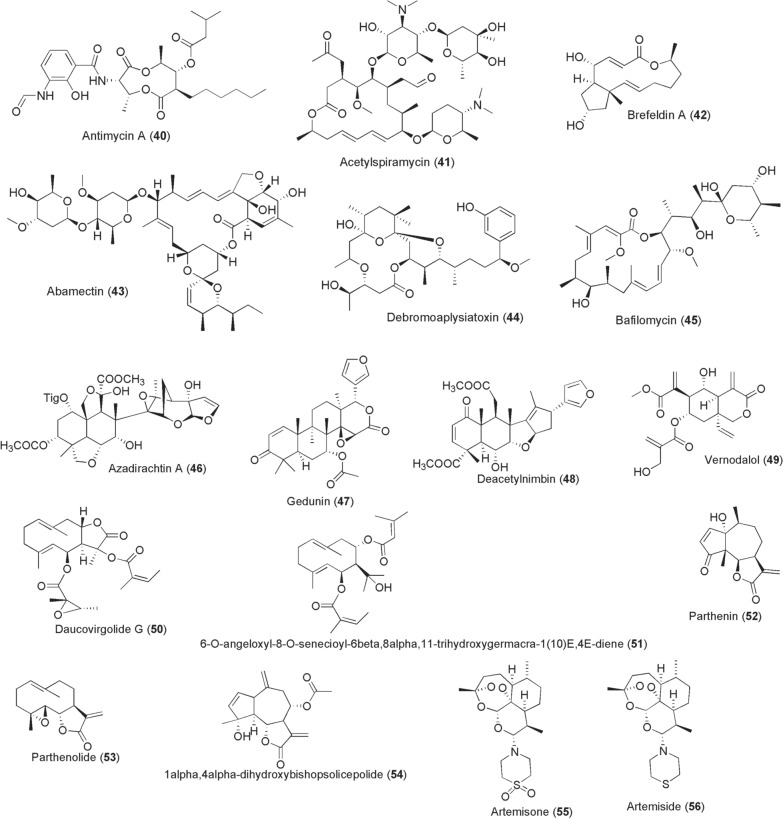
Equally important are the microbial products from ubiquitous bacteria and fungi. Exploitation of such microbial-derived compounds as human pathogen transmission-blockers is widely documented, but largely limited to malaria, filariasis, and arboviruses. Early in 2009, epoxomicin (3), a known proteosome degradation inhibitor discovered from an Actinomycetes strain Q996-17, emerged to potently kill mature Plasmodium stage V gametocytes at 100 or 10 nM 72-h post-treatment and blocked oocysts production in mosquitoes [46]. Subsequent work on plasmodial proteosome led to the identification of a Streptomycetes spp.-derived thiopeptide, thiostrepton (4), and its semisynthetic derivatives SS231/[14] and SS234/[05] with promising gametocytocidal activities in the IC50 range of 1.82–3.40 µM [47]. From Streptomyces avermectinius bacterium identified back in early 1970s by a Japanese microbiologist, Satoshi Omura, a macrocyclic lactone ivermectin (5) was pioneered and developed in 1983 by a Merck’s team led by William Campbell as an anthelmintic drug [48]. A veterinary drug trademarked as Mectizan® was later prioritized for mass drug administration (MDA) at an oral dose of 150–200 µg/kg to treat river blindness prevalent in West Africa, Yemen, and Latin America regions. Apart from this remarkable invention, compound 5 has been repurposed successfully in malaria exerting endectocidal, Plasmodium sporontocidal, and sporogonic inhibitions at both laboratory and field levels [49–52]. The systemic administration and/or topical application of compound 5 to cattle and humans reduces the survival, feeding frequencies, blood digestibility, locomotion, and fecundity of bloodsucking arthropod disease vectors including mosquitoes and tsetse flies [53–57]. As human malaria transmission greatly relies on the longevity of female mosquitoes, reduction of their survivorship rates from this intervention breaks parasite transmission especially by the outdoor feeding vectors.
Another tricyclic macrolide, chlorotonil A (6), that structurally resembles the antibiotic anthracimycin was discovered from a soil-dwelling myxobacterium Sorangium cellulosum ce1525 in Germany. Chlorotonil A exerts nanomolar potency against late-stage IV/V gametocytes (IC50 29.6 nM; IC90 123.2 nM), besides its antimalarial activity against all intraerythrocytic stages [58]. This notable bioactivity has however not been extended for investigations against either the Plasmodium sporogonic stages or repurposed to target other arthropod transmissible human pathogens. Furthermore, lichen-derived (+)-usnic acid derivatives of dibenzofurandione class, BT37 (7) and BT122 (8), prevent Plasmodium zygote-to-ookinete maturation achieving 100% inhibition of oocyst production at 250 µg/ml [59]. (+)-Usnic acid also inhibits P. berghei liver stages at IC50 value of 2.3 µM, but is less active against P. falciparum blood stages [60]. It has also been demonstrated that fibrinogen-related proteins (FREPs) from An. gambiae midgut epithelium, and specifically FREP1, facilitate Plasmodium ookinete invasion through surface anchorage [61]. They observed that silencing of FREP1 gene expression reduces P. falciparum infection in mosquitoes. Inspired by these findings, through a systematic screening of the fungal library by ELISA-based method, a bicoumarin p-orlandin (9) from Aspergillus niger emerged as a potential candidate that prevents either Plasmodium gametocytes or ookinetes from interacting with FREP1 at 92% inhibition [62]. Consequently, such disruption, as at low dose of 3 µg/ml, effectively reduced P. falciparum infection load in mosquitoes by 56.7% oocyst numbers and 35% infection prevalence. In continuation with this work by Jun Li’s group, other fungal compounds, Asperaculane B (10) and [3-amino-7,9-dihydroxy-1-methyl-6H-benzo[c]chromen-6-one (Pulixin, (11)], with Plasmodium transmission-blocking activities have been identified [63, 64]. Compound 10 derived from Aspergillus aculeatus inhibits Plasmodium transmission at IC50 7.89 µM, while 11 isolated from Purpureocillium lilacinum exerts its activity in mosquitoes at EC50 11 µM, without notable host cytotoxicity.
A chemical class of polyether ionophores comprising monensin A (12), nigericin (13), salinomycin (14), narasin (15), and maduramicin (16), to mention a few, is reportedly derived from Actinobacteria of Streptomyces spp. These specific lipid-soluble monovalent compounds tend to bind metal cations reversibly at high affinities, transporting them across the cell membrane and disrupting parasite intracellular ionic homeostasis. Apart from exhibiting nanomolar activity against asexual stages of Plasmodium, the repurposed ionophores 12–16 preferentially kill transmissible stage IV/V gametocytes and liver stages more rapidly and impair sporogonic development in mosquitoes at nanomolar doses [65–69]. Such potent susceptibility of gametocytes, in addition to parasite transmission-blocking activity, to ionic balance perturbation is akin to PfATP4 targeting by spiroindolone NITD609 (17) [70, 71]. NITD609 (also referred to as cipargamin or KAE609) is a clinical trial phase 2 candidate discovered and developed from a screen of ~ 12,000 Novartis microbial product library.
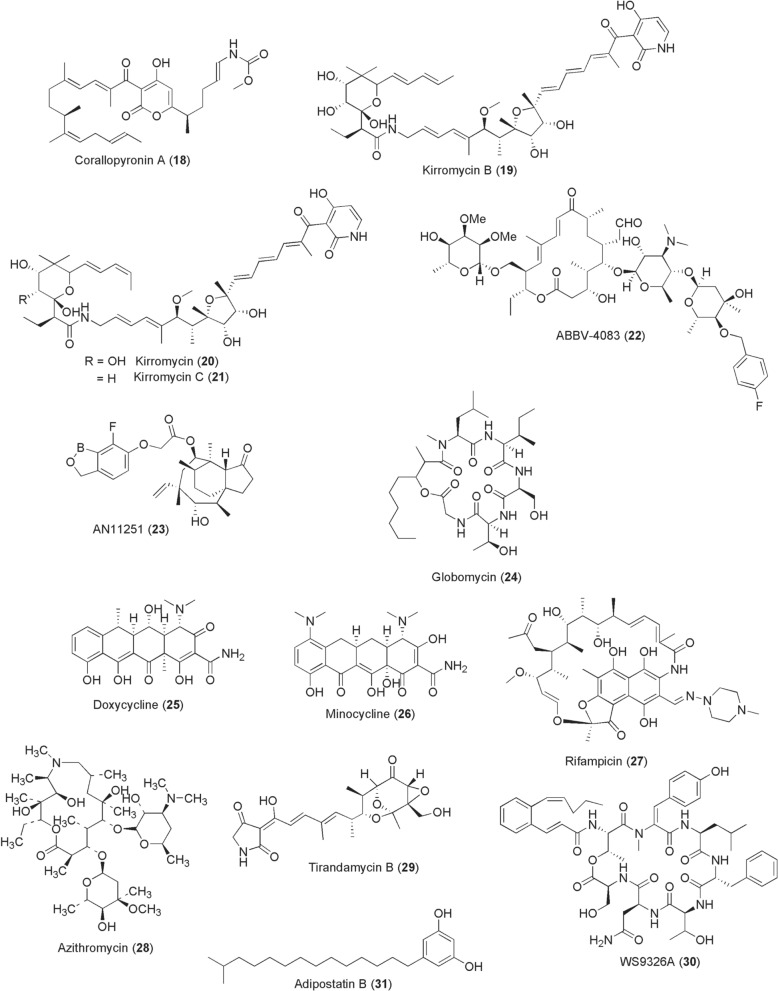
For lymphatic filariasis, efforts to discover new treatment options with transmission-blocking capability to complement ivermectin (5) are to date targeting the obligate filarial endosymbiotic bacterium Wolbachia by utilizing suitable platforms of insect cell lines. Chemical depletion of Wolbachia from filarial worms renders them infertile and nonviable, blocks embryogenesis, and inhibits their development, potentially blocking parasite transmission. In this regard, adult worm-sterilizing compounds such as corallopyronin A (CorA) (18; Fig. 3) from Corallococcus coralloides B035 that specifically depletes Wolbachia from filarial nematodes are showing promising preclinical candidature [72, 73]. CorA effectively targets bacterial RNA polymerase. When offered to infected Litomosoides sigmodontis rodent model for 14 days, > 90% of Wolbachia from filarial worms were cleared and development of adult worms abrogated, exerting a short-course efficacy in combination with albendazole for only 7 days at 10 mg/kg CorA [72]. Elsewhere, Xu et al. [74] discovered kirromycins–kirromycin B (19) and congeners kirromycin (20) and kirromycin C (21) from Streptomyces sp. CB00686 through a high-throughput screening of a natural product library consisting of 348 compounds isolated from 65 bacterial strains at The Scripps Research Institute. The three kirromycins 19–21 potently depleted Wolbachia in LDW1 Drosophila cells (IC50 0.58, 0.25, 1.08 nM, respectively) and Brugia pahangi ovaries ex vivo (65–95% efficiency at 1 µM). Such anti-Wolbachia activity of 19–21 is believed to originate from inhibition of protein synthesis through interaction with prokaryotic elongation factor Tu (EF-Tu). From a discovered macrolide tylosin A (TylA) of Streptomyces fradiae, von Geldern et al. [75] esterified the 4″-OH on mycarose sugar to develop ABBV-4083 (22) with potent anti-Wolbachia activity (EC50 0.019 nM), in vivo efficacy of > 99.9% at 150 mg/kg for 14 days, and superior pharmacokinetic profile. Another modified antibiotic boron-pleuromutilin, AN11251 (23), exerts enhanced in vitro anti-Wolbachia activity of EC50 15 nM compared to less active pleuromutilin itself (EC50 > 1 µM) [76]. Oral administration of compound 23 at 50 mg/kg to L. sigmodontis mouse infection model for 14 days effectively clears > 99% of Wolbachia from adult female worms. Globomycin (24) is yet another filaricidal agent, first isolated in 1978 from fermentation of Streptomyces spp. [77]. Globomycin is known for its lipoprotein signal peptidase II (LspA) inhibitory activity. By inhibiting lipoprotein biosynthesis, globomycin depletes Wolbachia from B. malayi and kills the adult worms at 100 µg/ml [78].
Fig. 3.
Chemical structures of compounds 18–31. Corallopyronin A (CorA) (18), kirromycin b (19), kirromycin (20), kirromycin c (21), abbv-4083 (22), an11251 (23), globomycin (24), doxycycline (25), minocycline (26), rifampicin (27), azithromycin (28), tirandamycin b (29), WS9326a (30), adipostatin compound (31)
Other compounds with anti-Wolbachia and antifilarial activity are the antibiotics doxycycline (25; Fig. 3), minocycline (26): two synthetic derivatives of Streptomyces-derived tetracycline, rifampicin (27): a product of a soil bacterium Amycolatopsis rifamycinica, and azithromycin (28): a semisynthetic macrolide derivative of Streptomyces derived erythromycin [79]. In a recent study report by Bullman et al. [80], however, the antibiotics 25–27 displayed inverse treatment effects on Wolbachia, increasing titres with dose increment, despite rendering Brugia worms moribund. Besides these advances, microbial compounds of Streptomyces spp. origin targeting asparaginyl-tRNA synthetase activity are able to kill adult Brugia worms. These include tirandamycin B (29), WS9326A (30), and adipostatins A-D [structurally represented by (31)] identified via high throughput screening [81–83]. Notably, the aforementioned compounds 18–31 have only been tested in vitro and using mouse infection models, raising a concern as to whether the observed activity is also extrapolatable to insect vector level from an infectious human blood meal. This arises owing to a compendium of interactive factors influencing transmissibility of human pathogens that are potentially missed by in vitro assay conditions.
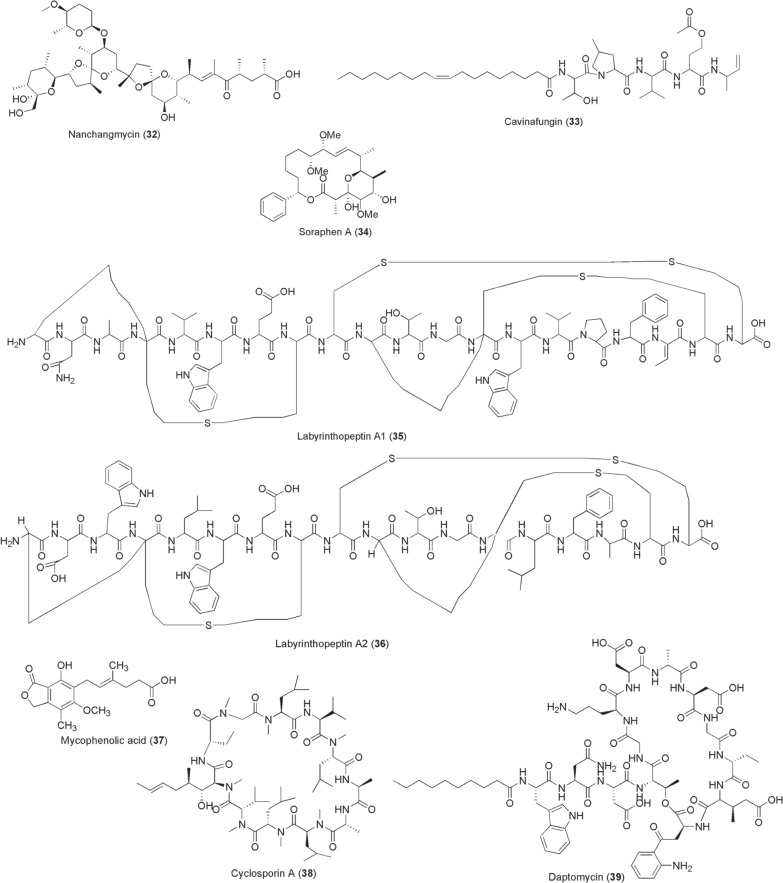
Reduction of arboviral titres at the points of acquisition and inoculation through chemical inhibition of host factors required for viral replication is a promising approach for blocking transmissions of arboviruses. However, only a few examples of comparable inhibitors of microbial origin are known at present and they are highlighted below. Nanchangmycin [32; isolated from Streptomyces nanchangensis (Fig. 4)] was identified through screening a 2000 compound library and established to block ZIKV infection at EC50 0.97 µM by targeting the human viral attachment factor AXL [84]. In addition, this ionophore nanchangmycin was shown to actively inhibit entry of CHIKV, DENV and WNV into human cells. During the same year of its discovery in 2017, Estoppey et al. identified a fungal lipopeptide, cavinafungin (33), from Colispora cavincola with potent activity of nanomolar range against ZIKV and DENV 1–4 serotypes, albeit with less inhibitory efficacy against CHIKV [85]. The selective antiviral activity of compound 33 was demonstrated to largely stem from its inhibition of endoplasmic reticulum host signal peptidase (ER-SPase) activity that consequently impairs viral polyprotein processing. Another screen of myxobacteria extracts, which was conducted in Germany, led to isolation of a polyketide soraphen A (SorA) (34) that targeted host acetyl-CoA carboxylase, a key lipid biosynthetic enzyme. SorA inhibits DENV in vitro at EC50 of 4.7 nM and reduces the viral load in vivo with promising pharmacological profile [86]. Prochnow et al., from the same research group, recently isolated labyrinthopeptins A1 (35) and A2 (36) from actinomycete Actinomadura namibiensis DCM 6313 [87]. These compounds 35 and 36, which bind to lipid phosphatidylethanolamine on the viral membranes, exert broad-spectrum antiviral activities against diverse human viruses including DENV, CHIKV, ZIKV and WNV at low micromolar to nanomolar ranges. A high-throughput screening of 774 FDA-approved drugs for anti-ZIKV chemotherapy by repurposing resulted in identification of; the antiparasitic ivermectin (5), an inosine-5′-monophosphate (IMPDH) inhibitor mycophenolic acid (MPA, 37), the cyclophilin inhibitor cyclosporine A (38), and a lipopeptide daptomycin (39) [88]. Besides the aforementioned activities as anthelmintic, antimalarial, antifilarial and mosquitocidal, it is not surprising for the wonder drug ivermectin (5) to inhibit arboviruses, including not only ZIKV (EC50 1–10 µM) but also DENV, WNV and CHIKV (EC50 0.6 µM) by interacting with non-structural (ns) helicase protein 3 [89, 90]. The IMPDH inhibitor, MPA (37), was first discovered from Penicillium stoloniferum during 1893–1896 and approved for reducing transplantation rejection. In 2002, Diamond and colleagues reported the anti-DENV activity of MPA whose mechanism of action is through impairment of viral genome replication; in addition, a similar antiviral activity was later reported for CHIKV and ZIKV (EC50 0.1–1 µM) [88, 91]. Likely blockage to directly access purines from the host cells through IMPDH inhibition slowing viral replication is perhaps the possible mechanism of action of MPA. Like MPA, cyclosporine A (38; a fermentation product of Trichoderma polysporum) inhibits viral RNA synthesis, but through interference of viral NS5 protein interaction with human cyclophilins. Such protein–protein interaction interference perturbs viral replication affording cyclosporine A antiviral activity against DENV and ZIKV [92]. In the case of daptomycin (39) from Streptomyces roseosporus, its antiviral mechanisms against ZIKV are yet unknown but postulated to interfere with viral cell entry. Whilst Barrows et al. in vitro screen for daptomycin’s anti-ZIKV activity was exciting at EC50 0.1–1 µM, this efficacy was unfortunately lost and invalidated in infected Aedes mosquitoes [93].
Fig. 4.
Chemical structures of compounds 32–39. Nanchangmycin (32), cavinafungin (33), soraphen a (34), labyrinthopeptins a1 (35), labyrinthopeptins a2 (36), mycophenolic acid (mpa) (37), cyclosporine a (38), daptomycin (39)
Additional compounds with anti-arboviral activity from microbes are acknowledged. Antimycin A1a [40; produced by Streptomyces kaviengensis (Fig. 5)] is a potential anti-DENV agent identified by high-throughput screening of microbial compounds. Antimycin A exerts antiviral activity at IC50 80 nM [94]. Whilst its known cellular mechanism is through binding the Qi site of cytochrome c reductase and inhibition of oxidative phosphorylation, the precise antiviral mechanism is to date unclear. Further interrogation of the same screen yielded acetylspiramycin (41; from Streptomyces ambofaciens) with anti-DENV activity at IC50 0.91 µM. A polyketone from Penicillium sp., brefeldin A (42), inhibits DENV at IC50 54.6 nM [95]. Apart from ivermectin, a related macrolide abamectin (43) from fermentation of Streptomyces avermitilis identified by a high-throughput screen performed by Varghese et al. was shown to inhibit CHIKV replication, besides other flaviviruses, at EC50 1.5 µM [90]. Antibiotics, doxycycline (25) and azithromycin (28), are also potential anti-arbovirals targeting CHIKV and ZIKV infections, respectively [96, 97]. Debromoaplysiatoxin (44), and its 3-methoxy derivative have been isolated from Singaporean Trichodesmium erythraeum (TLTY/PSK/001). These compounds inhibit CHIKV replications in vitro at IC50 1.3 and 2.7 µM [98].
Fig. 5.
Chemical structures of compounds 40–56. Antimycin A1a (40), acetylspiramycin (41), brefeldin a (42), abamectin (43), debromoaplysiatoxin (44), bafilomycin (45), azadirachtin a (46), gedunin (47), deacetylnimbin (48), vernodalol (49), daucovirgolide g (50), 6-o-angeloxyl-8-o-senecioyl-6β,8α,11-trihydroxygermacra-1(10)e,4e-diene (51), parthenin (52), parthenolide (53), 1α,4α -dihydroxybishopsolicepolide (54), artemisone (55), artemiside (56)
Perhaps the more exciting bioactivity exhibited by these compounds is the blockade of viral passage through insect vectors after ingestion of viraemic blood meals, in addition to their in vitro antiviral activity in mammalian cells. Here we should highlight the works by Dimopoulos’ group. For instance, Kang et al., demonstrated that thoracic microinjection of a macrolide bafilomycin [45; an inhibitor of vacuolar H + -ATPase (vATPase) from fermentation products of Streptomyces spp.] and mycophenolic acid (37; MPA) block DENV-2 infection in Ae. aegypti [99]. The authors demonstrated that microinjection of either 45 (5 µM) or 37 (250 µM) a day prior to ingestion of DENV-2 viraemic blood meal led to inhibition of viral titres in the salivary glands by 90% and 83%, respectively, at day 14 post-infection. These findings are not different from the recently reported inhibitory efficacy of these compounds against ZIKV in C6/36 cells and mosquito midguts [93].
Plant-derived compounds
Over the years plants have been indispensable sources of drug-like molecules that are sought as curatives and/or novel scaffolds for drug lead development against various human disease pathogens. Apart from their use in clinical treatment, innovative exploration of these bioactives as promising pathogen transmission blockers has lately gained remarkable traction. Moreover, an emerging translational approach to control disease transmission motivated by exploitation of female insect vectors’ sugar foraging behaviour from randomly selected host plants is explorable for novel interventions [20]. From this ecological perspective, the female disease vectors are evidently reported to feed on particular plant families, harbour host plant tissue DNA as foraging evidence or be manipulated by developing pathogens for increased plant sugar uptake [100, 101]. Laboratory and field studies [102–109] show both native and invasive alien host plant tissues or secretions being ingested by phlebotomine sand flies, arboviral Aedes mosquitoes, Anopheles gambiae and triatomine bug Rhodnius prolixus. Table 1 summarizes these identified host plants foraged by various insect vectors.
Table 1.
Examples of the host plants ingested by various disease vectors
| Serial number | Host plantsa | Disease vector | References |
|---|---|---|---|
| 1 | Prosopis juliflora (Fabaceae), Vachellia tortilis (Fabacae), V. nilotica (Fabacae), Senegalia laeta (Fabacae), Cannabis sativa (Cannabaceae) | Phlebotomine sand flies | [104, 105] |
| 2 | Pithecellobium dulce (Fabaceae), Senna uniflora (Fabaceae), Hibiscus heterophyllus (Malvaceae), Opuntia ficus-indica (Cactaceae), Prosopis juliflora (Fabaceae), Hibiscus rosasinensis (Malvaceae), Azadirachta indica (Meliacae), Zea mays (Poaceae), Vigna unguiculata (Fabaceae), Malva parviflora (Malvaceae), Acacia spp. (Fabaceae) | Aedes spp. | [100, 103, 110] |
| 3 | Solanum lycopersicum (Solanaceae) | Triatomine Rhodnius prolixus | [108] |
| 4 |
Prosopis juliflora (Fabaceae), Parthenium hysterophorus (Asteraceae), Senna occidentalis (Fabaceae), Senna alata (Fabaceae), Senna tora, (Fabaceae), Ricinus communis (Euphorbiaceae), Leonotis nepetifolia (Lamiaceae), Bidens pilosa (Asteraceae), Senna didymobotrya (Fabacae), Tecoma stans (Bignoniaceae), Acacia macrostachya (Fabaceae), Faidherbia albida (Fabaceae), Boscia angustifolia (Capparaceae), Ziziphus jujuba (Rhamnaceae), Mangifera indica (Anacardiaceae), Delonix regia (Fabaceae), Thevetia neriifolia, Senna siamea (Fabaceae), Cassia sieberiana (Fabaceae) |
An. gambiae | [100, 109, 111, 112] |
aHost plants were validated by PCR targeting chloroplast DNA using gene-specific primers: matK, rbcL and trnH-psbA, and also through chemical olfactory attractiveness
Besides the pervasive quest for plant sugars, ingestion of other bioactive secondary metabolites is likely to occur and consequently have variable detrimental effects on the development of infectious pathogens harboured in vector’s midguts and salivary glands [105, 113–115]. Although the above list in Table 1 is not exhaustive, because geographical sites and seasons of insect vector collection could influence plant foraging diversity, these studies inform a feasible starting point in the search for chemoprotective compounds. Exemplar agents and others are described below.
Terpene derivatives
Natively, neem trees (Azadirachta indica; Meliaceae) are among the most sought sources of plant-derived remedies at primary care level. Neem has been widely characterized and known for its bioactive terpene derivatives, azadirachtin A (46), gedunin (47), nimbolide, nimbin, salannin, azadirone, azadiradione, deacetylnimbin (48), etc., as well as its standardized alcoholic formulation, NeemAzal® [116, 117]. In 2002, Billker et al. reported remarkable distortions of mitotic microtubule arrays and axonemes in activated male gametes of P. berghei by compound 46 [118]. This cytoskeleton assembly disruption impaired exflagellations, subsequent fertilization and ookinete development. Subsequent studies by Annete Habluetzel’s team have demonstrated in vitro and in vivo Plasmodium transmission-blocking activities by neem terpene compounds [119–121]. In this regard, the standardized NeemAzal® (34% azadirachtin A, 4% salannin, 2% nimbins) reduced the number of zygotes developing into mature ookinetes and exerted a 100% blockade of oocysts in An. stephensi at 50 mg/kg in vivo dose [121]. Synergistic action of NeemAzal® constituents afforded a stronger activity against early sporogonic stages of Plasmodium compared to azadirachtin A alone [120]. From the seed kernels, Tapanelli et al. isolated various limonoids. A thermally and chemically stable deacetylnimbin (48, an analogue of nimbin) was highlighted as a potential inhibitor of early sporogonic stages achieving a 100% parasite clearance at 100 µM [119]. Gedunin (47) is a potent plasmodial Hsp90 inhibitor and has been identified among the promising inhibitors of Plasmodium liver stages with prospective prophylactic efficacy [69]. Other compounds with potential malaria transmission-blocking activity have also emerged from Habluetzel’s research team. Abay et al. [122] identified a sesquiterpene lactone, vernodalol (49), from Vernonia amygdalina (Asteraceae) leaves modestly acting against P. berghei zygotes and ookinetes at IC50 18.7 µM, but did not impair microgamete formation even when tested at high concentration of 50 µM. Germacranolide sesquiterpenoids, daucovirgolides A–L and polyoxygenated germacranes have been isolated from Tunisian plants of Daucus genera (Apiaceae), D. virgatus and D. carota [123–125]. Remarkable P. berghei ookinete formation inhibitory activities were noted for daucovirgolide G (50) (92% at 50 µg/ml; IC50 17.5 µM) and 6-O-angeloxyl-8-O-senecioyl-6β,8α,11-trihydroxygermacra-1(10)E,4E-diene (51) (86.4% at 50 mM; IC50 96.4 µM), without a general cytotoxicity. Whilst no apparent defined mechanism or biological target has been identified yet, the observed activity is hypothesized to result from the intact endocyclic double-bond system of these compounds [124].
Parthenin (52), a major sesquiterpene lactone from a mosquito preferred host plant, the invasive Parthenium hysterophorus (Asteraceae), is well tolerated by female mosquito vectors without any apparent tissue toxicity [109]. Motivated in part by this initial finding, Balaich and colleagues examined parthenin’s inhibitory effects against transmissible sporogony stages of P. falciparum alongside a structurally related parthenolide (53) from Tanacetum parthenium (Asteraceae). The authors noted decreased Plasmodium oocyst densities of 40–80% on offering mosquitoes 6.25 µg/ml parthenin in gametocytemic blood meal and a complete clearance at doses between 50–100 µg/ml. Similar activity was exerted by parthenolide at 40 nM to 4 µM and poised to occur through inactivation of stage V gametocytes, inhibition of microgamete exflagellation and impaired ookinete maturation [126]. Another gametocytocidal guaianolide sesquiterpenoid, 1α,4α -dihydroxybishopsolicepolide (54), was recently isolated from a South African plant of Asteraceae family Artemisia afra (Asteraceae). Compared to its activity against early gametocytes, compound 54 was demonstrated to exert better cidal activity against the late-stage IV/V gametocytes (IC50 6.3 µM) [127]. This is however in contrast to derivatives of artemisinin from Artemisia annua (Asteraceae): dihydroartemisinin (DHA), artemether and artesunate with relatively poor activity against late-stage IV/V gametocytes [128]. Besides their rapid clearance of asexual stages, they also potently kill early stage I-III gametocytes reducing gametocyte carriage. But, failure to clinically clear stage IV/V gametocytes by these artemisinin derivatives promotes the transmission of Plasmodium to mosquitoes, including parasites from artemisinin-based combination therapy (ACT) resistance backgrounds [129, 130]. Inspired to reverse this challenge into better antimalarials, Coertzen et al. [131] and Wong et al. [132] have developed artemisone (55), artemiside (56) and 10-aminoartemisinins 57–60 designed from artemisinin skeleton (Fig. 6). These compounds 55–60 exhibit preferential nanomolar activity against late-stage IV/V gametocytes (IC50 0.04–42.4 nM), without being overshadowed by artemisinin’s PfKelch-13 C580Y mutation genotypes.
Fig. 6.
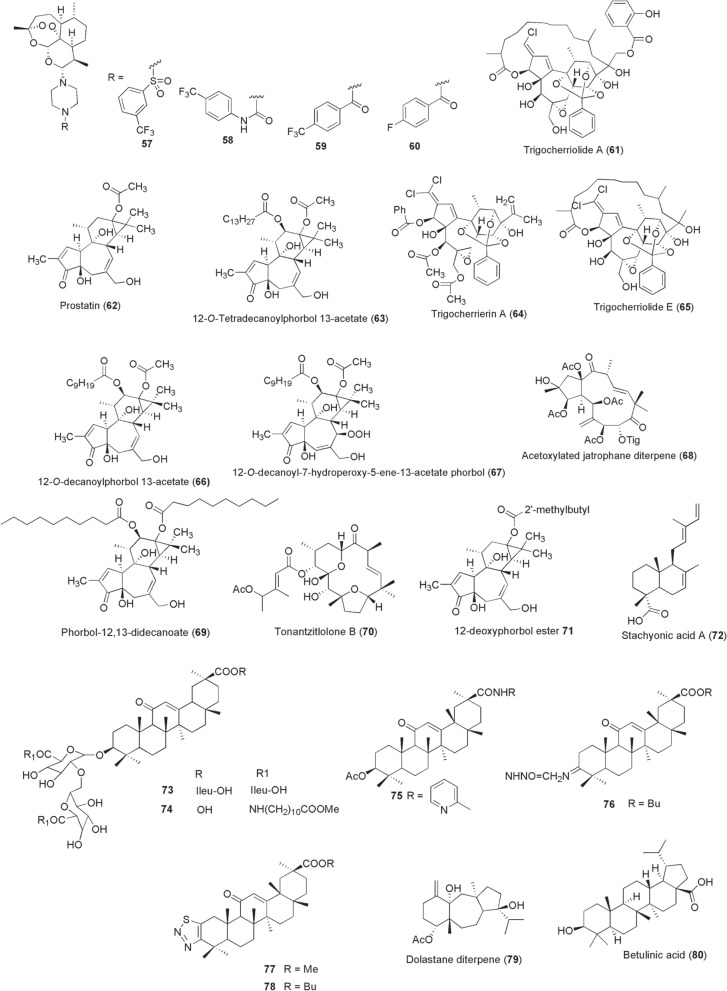
Chemical structures of compounds 57–80. 10-Aminoartemisinin compound (57), 10-aminoartemisinin compound (58), 10-aminoartemisinin compound (59), 10-aminoartemisinin compound (60), trigocherriolide A (61), prostratin (62), 12-O-tetradecanoylphorbol 13-acetate (63), trigocherrierin A (64), trigocherriolide E (65), 12-O-decanoylphorbol 13-acetate (66), 12-O-decanoyl-7-hydroperoxy-phorbol-5-ene-13-acetate (67), (2R,3R,4S,5R,7S,8R,13R,15R)-3,5,7,15-tetraacetoxy-2-hydroxy-8-tigloyloxy-9,14-dioxojatropha-6(17),11E-diene (68), phorbol-12,13-didecanoate (69), tonantzitlolone B (70), 12-deoxyphorbol-13(2"-methyl)butyrate (71), stachyonic acid a (72), compound 73, compound 74, compound 75, compound 76, compound 77, compound 78, ((4r,9s,14s)-4α-acetoxy-9β,14α-dihidroxydolast-1(15),7-diene (79), betulinic acid (80)
Antiviral activities of terpene derivatives against various arboviruses are reported, particularly in experiments utilizing in vitro conditions. An oxygenated diterpenoid, trigocherriolide A (61), was isolated alongside other compounds from the bark of a New Caledonian plant Trigonostemon cherrieri (Euphorbiaceae) in 2012 [133]. A relatively strong inhibitory activity of compound 61 (IC50 3.1 µM) for DENV NS5 RdRp was reported. In the same year, two plant-derived phorbol esters, prostratin [62; from Homalanthus nutans (Euphorbiaceae)] and 12-O-tetradecanoylphorbol 13-acetate (63), were reported to selectively inhibit CHIKV replication at EC50 values of 2.6 µM and 2.9 nM, respectively [134]. Bourjot et al. later isolated unusually chlorinated daphane diterpenoid orthoesters (DDO) from the leaves of Trigonostemon cherrieri, among them trigocherrierin A (64) and trigocherriolide E (65). Using a viral cell-based assay, the authors reported potent inhibition of CHIKV by compound 64 (EC50 0.6 µM), with similar bioactivity exhibited by 65 (EC50 0.7 µM) [135]. From the leaves of another Euphorbiaceae plant, Croton mauritianus, Corlay et al. isolated alongside other compounds two promising anti-CHIKV tigliane diterpenes, 12-O-decanoylphorbol 13-acetate (66) and 12-O-decanoyl-7-hydroperoxy-phorbol-5-ene-13-acetate (67). Compounds 66 and 67 inhibited CHIKV-induced cell death at EC50s of 2.4 µM and 4 µM, respectively [136]. In the same spirit of finding anti-CHIKV inhibitors, Nothias-Scaglia et al. identified a potent acetoxylated jatrophane diterpene (2R,3R,4S,5R,7S,8R,13R,15R)-3,5,7,15-tetraacetoxy-2-hydroxy-8-tigloyloxy-9,14-dioxojatropha-6(17),11E-diene (68) from a Mediterranean Euphorbia amygdaloides (EC50 0.76 µM) [137]. In a follow-up study from the same group in 2015, 29 commercially available natural diterpenoids were screened against CHIKV and HIV replications. This effort led to identification of a potent inhibitor agent phorbol-12,13-didecanoate (69) with anti-CHIKV activity (EC50 6 nM) [138].
In addition, tonantzitlolone-type diterpenes were isolated from stem barks of Euphorbiaceae plant Stillingia lineata collected in Reunion Island and screened against CHIKV. Among the compounds, Techer et al. [139] reported 4′-acetoxytonantzitlolone (70; tonantzitlolone B) endowed with a promising anti-CHIKV activity (EC50 7 µM). From the leaves of the same plant, a more potent tigliane diterpenoid 12-deoxyphorbol-13(2"-methyl)butyrate (71) was isolated (anti-CHIKV, EC50 1.2 µM) [140]. A labdane diterpene stachyonic acid A (72) was isolated in 2019 from Basilicum polystachyon (Lamiaceae) [141]. By using a DENV plaque-reduction neutralization (PRNT) assay, compound 72 exerted an antiviral activity of IC50 1.4 µM relative to less potent andrographolide (IC50 51 µM). Elsewhere, antiviral triterpenoid compounds have been reported from the roots of licorice herb Glycyrrhiza glabra (Fabaceae). Unlike the parent compound glycyrrhizic acid that exerts anti-DENV-2 activity (IC50 8.1 µM), its derivatization through chemical conjugation with isoleucine and 11-aminoundecanoic acid methyl ester resulted in potent compounds 73 (IC50 1.3 µM) and 74 (IC50 1.2 µM) [142]. Through a similar strategy, a more recent study reported derivatives of a pentacyclic triterpenoid, glycyrrhetinic acid from G. gabra, with potent anti-ZIKV replication activity [143]. The resultant compounds 75–78 had IC50 values of 0.13 µM, 0.55 µM, 0.29 µM and 0.56 µM, respectively. From a marine brown seaweed (Canistrocarpus cervicornis) collected from Praia do Velho in Angra dos Reis (Brazil), a dolastane diterpene ((4R,9S,14S)-4α-acetoxy-9β,14α-dihidroxydolast-1(15),7-diene; 79) was isolated and reported to inhibit ZIKV (EC50 0.95 µM) and CHIKV (EC50 1.3 µM) [144]. Elsewhere the triterpenoid betulinic acid (80) displayed an anti-DENV-2 activity at IC50 0.95 µM, with a specific inhibition exerted at viral RNA replication step of other DENV serotypes (DENV-1,3,4; IC50 0.9–1.84 µM) and ZIKV (IC50 2.45 µM) [145].
The compounds gedunin (47) and photogedunin (81; Fig. 7) were isolated from ethyl acetate fractions derived from the fruits of naturally growing Xylocarpus granatum (Meliaceae). Evaluation of these compounds against filarial worms, B. malayi, resulted in complete immobilization and macrofilaricidal activity at IC50 values of 0.239 µg/ml and 0.213 µg/ml, respectively [146]. Kalani et al. isolated and derivatized glycyrrhetinic acid that exhibited potential antifilarial activity against the microfilariae (IC50 1.2 µM) but was inactive against the adult worms of B. malayi. The authors reported an improved amide analogue 82 with lesser potency against microfilariae (IC50 2.2 µM) but active against adult worms at IC50 of 8.8 µM [147]. At a concentration of 10 µg/ml ursolic acid [83; isolated from ethyl acetate fraction of Nyctanthes arbortristis (Oleaceae)], about 84.15% reduction of W. bancrofti microfilariae viability was achieved through redox imbalance [148]. In 2016, antifilarial activity of compounds isolated from Taxodium distichum (Cupressaceae) collected from Palampur, India, was investigated [149]. Among the compounds identified, the diterpenoid labda-8(20),13-diene-15-oic acid (84) exerted a 100% reduction in motility of B. malayi microfilariae and adult worms, killed > 80% adult worms in an infected mouse model (dose: 100 mg/kg for 5 days) and sterilized > 36% female worms.
Fig. 7.
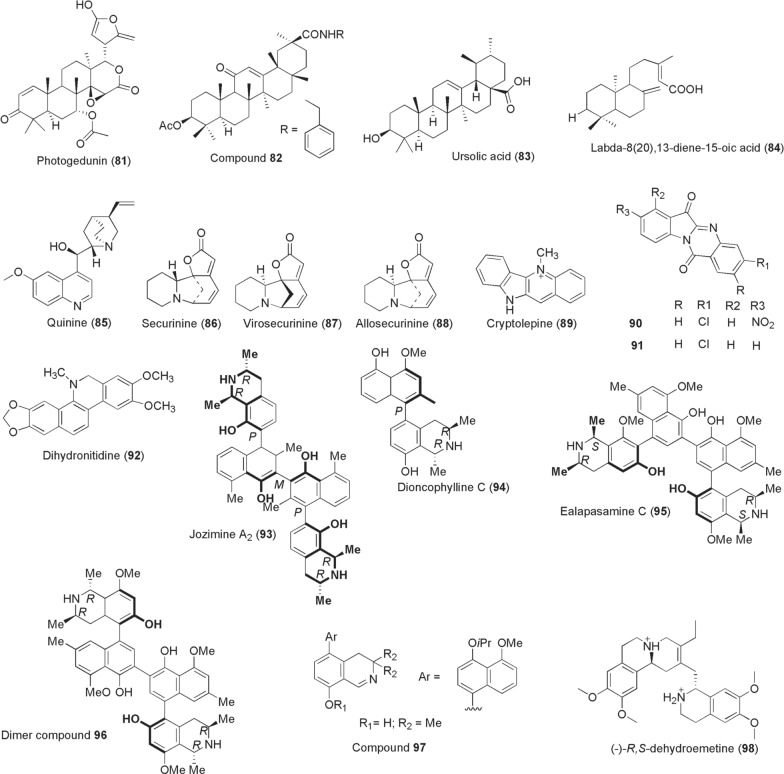
Chemical structures of compounds 81–98. Photogedunin (81), analog compound 82, ursolic acid (83), labda-8(20),13-diene-15-oic acid (84), quinine (85), securinine (86), virosecurinine (87), allosecurinine (88), cryptolepine (89), 3-chloro-8-nitro-tryptanthrin, 3-chloro-8-nitro-indolo [2,1-b] quinazoline-6,12-dione (nt1) (90), 3-chloro-indolo [2,1-b] quinazoline-6,12-dione (t8) (91), dihydronitidine (92), jozimine a2 (93), dioncophylline c (94), ealapasamine c (95), dimer compound 96, compound 97, (−)-R,S-dehydroemetine (98)
Alkaloids
Various plant-derived compounds of the alkaloid class show profound activities against infectious pathogens in the context of blocking disease transmissions between hosts. In view of this, the first ever discovered antimalarial compound quinine (85) has shown cidal effects on the early gametocytes, but weak activity against mature gametocytes of Plasmodium falciparum from various screening platforms [128, 150]. Yet, quinine could effectively kill mature gametocytes of P. vivax and P. malariae, as well as reduce P. falciparum oocysts numbers when provided at higher concentrations of EC50 642 ng/ml [151, 152]. Attempts to find other potential Plasmodium transmission-blocking agents utilizing a fragment-based screening approach of natural products afforded the identification of three compounds based on securinine [153]. (−)-Securinine is an alkaloid sourced from two Phyllanthaceae plants Securinega suffruticosa and Phyllanthus niruri. Securinine-related compounds 86–88 from the fragment screen inhibited > 80% Plasmodium gametocyte viability at 100 µM through an allosteric binding of 2′-deoxyuridine 5′-triphosphate nucleotidohydrolase (PfdUTPase). From the West African antimalarial herbal plant Cryptolepis sanguinolenta (Periplocaceae), its main alkaloid constituent cryptolepine (89) was demonstrated to exert late-stage NF54 gametocytocidal activity at IC50 1.97 µM [154].
Onambele et al. [155] designed and synthesized various derivatives of a (indolo-2,1-b)-quinazoline-6,12-dione [tryptanthrin; derived from Isatis tinctoria (Brassicaceae)]. Among the synthesized compounds, two derivatives designated as NT1 (90; 3-chloro-8-nitro-tryptanthrin, 3-chloro-8-nitro-indolo [2,1-b] quinazoline-6,12-dione) and T8 (91; 3-chloro-indolo [2,1-b] quinazoline-6,12-dione) emerged to confer 100% inhibition of gametocyte maturation when tested at their IC90 concentrations. Despite this promising gametocytocidal activity, the compounds unfortunately had weak inhibition of microgamete exflagellations with only 20% for NT1 [155]. Goodman et al. isolated dihydronitidine (92) alongside other compounds from the stem bark of Zanthoxylum heitzii (Rutaceae). When tested for in vitro P. berghei ANKA ookinete conversion inhibitions, dihydronitidine exerted a more potent activity at IC50 0.59 µg/ml compared to heitziquinone at IC50 6.2 µg/ml [156]. Moreover, following the successful isolation of various potent anti-infective naphthylisoquinoline alkaloids from rare lianas of Ancistrocladaceae and Dioncophyllaceae by a team led by Gerhard Bringmann, Moyo et al. subsequently tested for their gametocytocidal activity profiles. As a result, Jozimine A2 (93), dioncophylline C (94), ealapasamine C (95), dimer compound (96) and compound 97 tested at 2 µM inhibited male gamete exflagellations between 73 and 100% [157]. With exception of compounds 94 and 97 (not tested), potent gametocytocidal activity was reported against early and late gametocytes: jozimine A2: early gametocytes IC50 0.375, late gametocytes IC50 0.511 µM; ealapasamine C: early gametocytes IC50 0.545, late gametocytes IC50 0.889 µM; dimer compound 96: early gametocytes IC50 0.542, late gametocytes IC50 0.623 µM [157]. In the same period in 2020, Panwar et al. reported a synthetic analogue of emetine dihydrochloride, (-)-R,S-dehydroemetine (98), identified through a drug repositioning strategy and lead optimization. Emetine dihydrochloride hydrate is derived from Psychotria ipecacuanha (Rubiaceae). The emetine derivative compound 98 was demonstrated to possess potent inhibition against asexual parasite stages and prevented activated P. falciparum NF54 gametocytes from progressing into gametes in a dual gamete formation assay at IC50 0.43 µM (male gametocytes) and 1.04 µM (female gametocytes) [158].
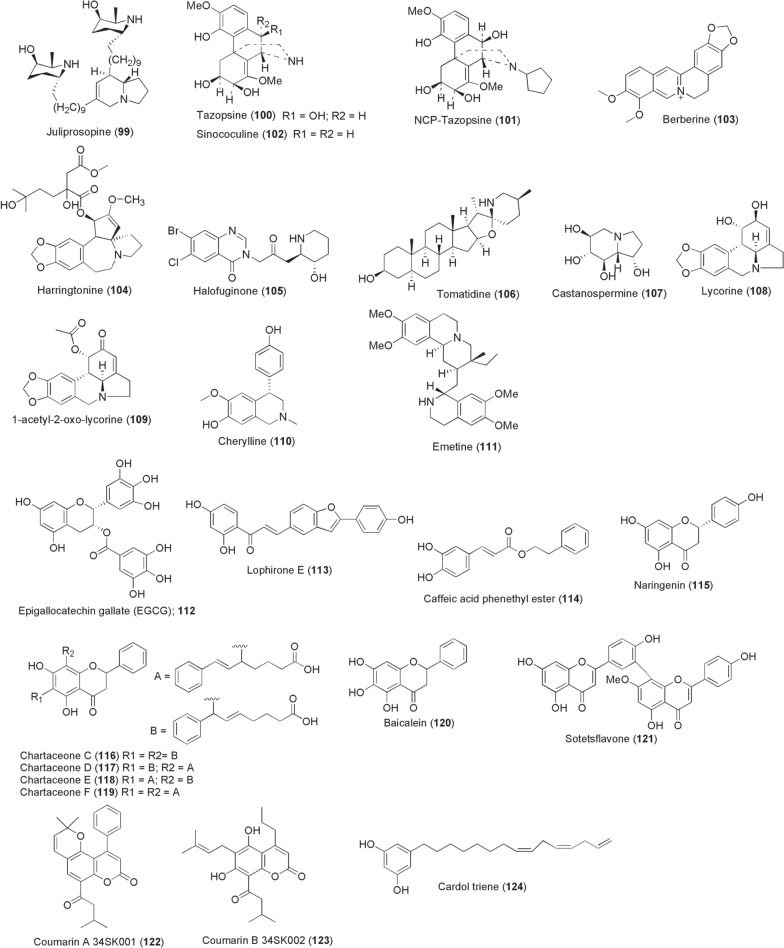
Mosquitoes have been reported to commonly feed on the invasive plant Prosopis juliflora (Fabaceae) (Table 1) for sugar acquisition. The Prosopis plant is a reliable source of indolizidine alkaloids, majorly the juliprosopine (99; Fig. 8). We recently reported findings from our study, which demonstrated potent gametocytocidal activity of juliprosopine against late-stage IV/V gametocytes of Plasmodium clinical isolates (IC50 < 1 µM) (patent no. KE/P/2020/3643) [159]. Compound 99 further strongly impaired sexual conversions, with no observable young NF54 gametocytes on day 5–7 post-induction, and killed developing ookinetes in vitro without lethal effects on survival of female mosquitoes (patent no. KE/P/2020/3643). Elsewhere, Carraz et al. reported a morphinan alkaloid from stem bark of a Madagascan Menispermaceae plant Strychnopsis thouarsii. Evaluations performed against Plasmodium yoelii and P. falciparum liver stages led to the identification of tazopsine (100), with a promising cidal activity (P. yoelii; IC50 3.1 µM, IC90 6.3 µM; P. falciparum IC50 4.2 µM, IC90 18.3 µM). Following an establishment of its toxicity in mice and cultured cells, modification through N-alkylation of tazopsine resulted in NCP-tazopsine (101) with improved therapeutic index, low cellular toxicity and IC50 values, P. yoelii (3.3 µM) and P. falciparum (42.4 µM) [160]. Follow-up study on S. thouarsii yielded, among other morphinan compounds, sinococuline (102), displaying slightly less but comparable activity against P. yoelii liver stages to tazopsine (IC50 4.5 µM) [161].
Fig. 8.
Chemical structures of compounds 99–124. Juliprosopine (99), tazopsine (100), NCP-tazopsine (101), sinococuline (102), berberine (103), harringtonine (104), halofuginone (105), tomatidine (106), castanospermine (107), lycorine (108), 1-acetyllycorine analogue (109), cherylline (110), emetine (111), epigallocatechin gallate (112), lophirone e (113), caffeic acid phenethyl ester (114), naringenin (115), chartaceone c (116), chartaceone d (117), chartaceone e (118), chartaceone f (119), baicalein (120), sotetsflavone (121), coumarin a 34sk001 (122), coumarin b 34sk002 (123), cardol triene (124)
In vitro studies highlight plant-derived alkaloids as excellent antiviral scaffolds despite not being tested in arboviral mosquito vectors. The antimalarial quinine (85) is a ten-fold more potent inhibitor of CHIKV replication (IC50 0.1 µg/ml) compared to its derivative chloroquine (CQ) (IC50 1.1 µg/ml) [162]. A relatively nontoxic isoquinoline berberine (103) identified through a high-throughput screen targets virus-induced mitogen-activated protein kinase (MAPK) signalling to inhibit CHIKV replication (EC50 1.8 µM) [90, 163]. Furthermore, a recent study [164] reported the ability of berberine to impair CHIKV nucleocapsid assembly at later stages of the viral life cycle, with decreased infectivity of viral particles produced from the treated cells suggesting dual mechanisms of its antiviral activity. Kaur et al. screened a 502 natural product compound library and identified the highly potent anti-CHIKV agent harringtonine [104; derived from Cephalotaxus harringtonia (Taxaceae)] (EC50 0.24 µM) [165]. Harringtonine inhibited CHIKV after cell entry 6 h post-infection by targeting viral protein synthesis. Through targeting host translational machinery hijacked by invading viruses, Hwang et al. demonstrated potent inhibition of DENV and CHIKV by a synthetic derivative of plant-derived febrifugine (halofuginone; 105) at 100 nM [166]. A recently identified steroidal alkaloid tomatidine (106; isolated from leaves and stem of unripe tomatoes) reduced CHIKV particle production (93.7%) in various mammalian cells at additional 2 h post-infection [167]. Tomatidine achieved its anti-CHIKV activity at EC50s range 1.3–3.9 µM. Also tomatidine inhibits DENV 1–4 in vitro at micromolar EC50 range of between 0.82 and 4.87 µM, but is more active against DENV-2 independent of ATF4 transcription factor activation [168].
Among the earliest inhibitors of DENV, the indolizidine alkaloid castanospermine (107) isolated from seeds of Castanospermum australe (Fabaceae) is known to be a potent inhibitor of all DENV 1–4 serotypes [169]. Its antiviral activity stems from inhibiting host cell α-glucosidase activity reducing secretion and infectivity of viral particles. However, when later administered into female Aedes mosquitoes via microinjection, castanospermine failed to suppress DENV-2 infectivity [99]. Another potent inhibitor of DENV, as well as YFV, ZIKV, RVFV and WNV, has been derived from the Amaryllidaceae family, lycorine (108) and its 1-acetyllycorine analog (109). Compound 108 reduces flaviviral titres by up to 104-fold at 1.2 µM and IC50 0.24 µM, while its derivative 109 exerts EC50 0.4 µM [170]. In a recent study by Chen et al. [171], compound 108 compromised ZIKV replication in vitro and in vivo by inhibiting viral RNA replication and protein synthesis at EC50 0.22–0.39 µM. Whilst mechanistic antiviral actions of these compounds 108 and 109 are still unclear, targeted NS4B is possibly the direct interaction protein [172] but also binding of ZIKV RdRp has been postulated [171]. From another Amaryllidaceae plant, namely Crinum jagus collected from Senegal, antiviral alkaloid cherylline (110) was isolated. Cherylline efficiently inhibited DENV and ZIKV at EC50 values of 8.8 µM and 20.3 µM by interfering with viral RNA synthesis post-entry step [173]. Elsewhere in a drug repurposing study, the antiprotozoal emetine (111) emerged to potently inhibit ZIKV African prototype (ZIKV MR766) infection with an IC50 52.9 nM and completely suppressed ZIKV replication at IC50 8.74 nM. Emetine was identified to exert its antiviral activity by inhibiting ZIKV NS5 polymerase activity and disrupting lysosomal function [174].
Only berberine (103) has been reported to be active against lymphatic filarial worms. Li et al. [175] demonstrated that berberine targets Wolbachial FtsZ, a cell division protein, inhibiting its GTPase activity. When treated with 10 – 40 µM berberine for 2 days, adult female B. malayi worms were immobilized and subsequently the microfilariae production was completely stopped.
Flavonoids and phenolic derivatives
Flavonoids and phenolics from various plants have been exploited as potential pathogen-blocking agents, with most interrupting arboviral replication cycles. However, very few molecules of this chemical class are presently known to inhibit transmissible stages of Plasmodium and lymphatic filarial worms. With reference to malaria, the abundant green tea polyphenol EGCG (epigallocatechin gallate; 112) was demonstrated in 2010 to kill infective Plasmodium sporozoites achieving IC50 values of 1.1 µM (6 h) and 0.12 µM (12 h). Mechanistically, EGCG impaired sporozoite gliding motility (IC50 0.14 µM), affecting their infectivity to liver cells through unknown intracellular targets. The sporozoite kill effect of EGCG was reported to be more pronounced through a synergistic addition of membrane permeant digitoxin that decreased overall IC50 values to 0.044 µM (6 h) and 0.035 µM (12 h) [176]. From the stem bark of Lophira lanceolata (Ochnaceae) collected from Burkina Faso, the bioflavonoid lophirone E (113) was isolated alongside other compounds from the ethyl acetate fraction phase. Whilst the compound 113 exerted moderate activity against asexual stages of Plasmodium, a selectively potent activity against 3D7elo1CBG99 stage V gametocytes at IC50 0.14 µM was reported [177].
Regarding anti-lymphatic filariasis, flavonoids have been investigated for their capacity to abrogate macrofilarial viability and microfilarial productions. Al-Abd et al. reported antifilarial activity of caffeic acid phenethyl ester (114) isolated from Melaleuca cajuputi (Myrtaceae) flowers against B. pahangi adult worms. In their evaluations, these authors indicated that compound 114 kills adult worms and microfilariae at IC50 values of 3.9 µg/ml and 7.5 µg/ml, respectively, while administration of 50 mg/kg compound 114 for 14 days to an infected mouse model reduced circulating microfilariae by 60% and 58% for adult worms. Depletion of Wolbachia, demonstrated by reduced WolbachiaftsZ gene copy number on treatment, could underlie the observed antifilarial activity [178]. From a screen of six flavonoids against B. malayi naringenin (115) appeared as the most potent filaricidal, immobilizing female adult worms at IC50 2.5 µg/ml and killing 73% of transplanted worms in vivo at 50 mg/kg dose. The molecule was however less effective against microfilariae (IC50 297.3 µg/ml) [179].
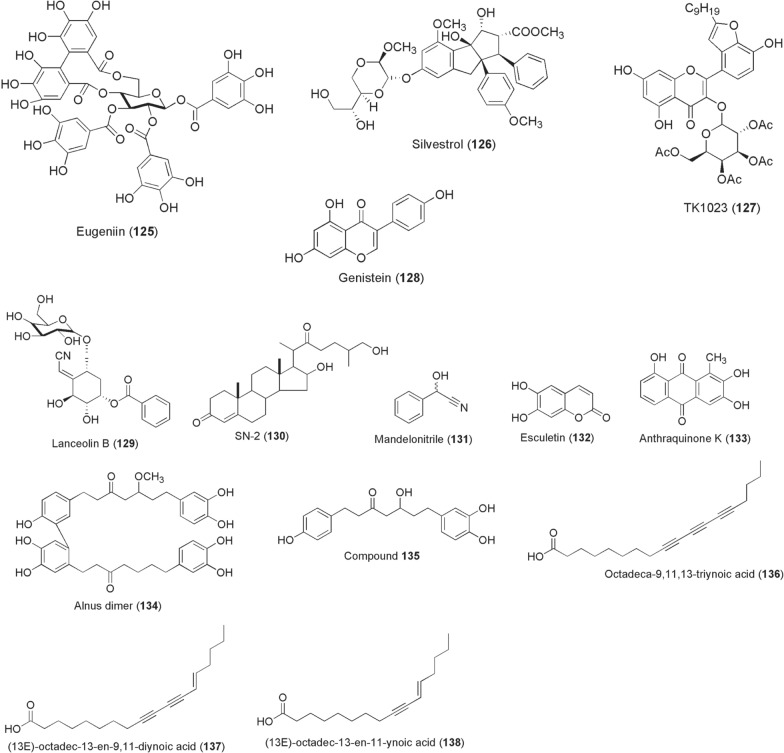
One of the most widely investigated bioactivites of flavonoids and phenolics in this context is that of anti-arbovirals. However, for the purpose of this review, we focussed on the most potent reported compounds with IC50/EC50 of < 10 µg/ml and 10 µM. In 2011, Allard et al. isolated various dialkylated flavanones (chartaceones A–F) from the stem bark of Cryptocarya chartacea (Lauraceae) collected from New Caledonia. Screening these compounds against DENV-2 NS5 RNA-dependent RNA polymerase (RdRp) activity identified chartaceones C–F (116–119) as the most potent inhibitors with IC50 1.8–4.2 µM [180]. The bioflavonoid baicalein [120; derived from roots of Scutellaria baicalensis (Labiatae)] exerts potent anti-DENV-2 activity at IC50 1.55 µg/ml [181]. Besides, compound 120 inhibited DENV-3 replication in a virus foci reduction assay at 100 µg/ml (99.78%) and IC50 12.7 µg/ml. The study demonstrated that compound 120 required a short time of contact (0 min) to exert its virucidal activity (62.45%) by blocking viral attachment and cell entry, interfering with infectivity of all DENV 1–4 serotypes [182]. Elsewhere, compounds inhibiting DENV NS5 RdRp activity were isolated from leaves of another New Caledonian plant, Dacrydium araucarioides (Podocarpaceae), and structure–activity relationships analyzed alongside other bioflavonoids previously obtained from a related plant D. balansae (Podocarpaceae). From this analysis, the authors pointed out that the number and position of methyl groups on the bioflavonoid moiety as well as the degree of oxygenation of flavonoid monomers influence the anti-DENV bioactivity. The 7"-O-methylamentoflavone, sotetsflavone (121), from D. araucarioides with an IC50 of 0.16 µM emerged as the strongest DENV-NS5 RdRp inhibitor [183]. Coumarins A 34SK001 (122) and B 34SK002 (123) isolated from seeds of Mammea americana (Clusiaceae) collected in the Colombian Caribbean Region (Colombia) were reported to potently inhibit both DENV-2/NG and CHKV-ACol at EC50 values: 9.6 and 10.7, 2.6 and 0.5 µg/ml, respectively [184]. Compounds 122 and 123 acted strongly by inhibiting replication of viral genome. Recent studies have further highlighted other potential DENV inhibitors. In 2018, a phenolic lipid cardol triene (124) was identified from a structure–activity relationship study of cashew nutshell phenolics as a potential anti-DENV inhibitor. Compound 124 inhibited DENV-2 (EC50 7.13 µM), but also displayed pan-dengue inhibition at EC50 values of 5.35–8.89 µM by targeting envelope protein kl loops preventing fusion and infectivity [185]. The compounds 5,7-dihydroxy-2-methylchromone-8C-β-d-glucopyranoside (isobiflorin), 5,7-dihydroxy-2-methylchromone-6C-β-d-glucopyranoside (biflorin) and eugeniin (125; Fig. 9) have been isolated from flower buds of cloves [Syzygium aromaticum (Myrtaceae)]. Only the ellagitannin compound 125 potently inhibited recombinant DENV-2 and -3 NS2BNS3pro complex (IC50 94.7 nM and 7.43 µM, respectively) through a competitive inhibitory mechanism [186].
Fig. 9.
Chemical structures of compounds 125–138. Eugeniin (125), silvestrol (126), houttuynoid B derivative (tk1023) (127), genistein (128), lanceolin b (129), sn-2 (130), mandelonitrile (131), esculetin (132), anthraquinone k (133), alnus dimer (134), (5 s)-5- hydroxy-1-(4-hydroxyphenyl)-7-(3,4-dihydroxyphenyl)-3-heptanone (135), octadeca-9,11,13-triynoic acid (136), (13e)-octadec-13-en-9,11-diynoic acid (137), (13E)-octadec-13-en-11-ynoic acid (138)
The polyphenol EGCG (112) is a potent viral cell entry inhibitor of CHIKV (IC50 6.54 µg/ml) [187] and inhibits viral infection of CHIKV S27 in U2OS cells at IC50 1.99 µg/ml [188]. Synergistic anti-CHIKV action with suramin against various strains was demonstrated. However, EGCG exerts relatively weak anti-DENV and anti-ZIKV activities. By inhibiting a host cell DEAD-box helicase eIF4A, silvestrol (126; derived from Aglaia foveolata) abrogates CHIKV replication through delayed synthesis of nonstructural and structural proteins in 293 T and NIH3T3 cells at IC50 1.89 nM and 5.06 nM, respectively [189]. Another potent ZIKV cell entry inhibitor was derived from a flavonoid glycoside isolated from Houttuynia cordata (Sauraceae). A tetra-O-acetylated houttuynoid B derivative (TK1023; 127) strongly reduced ZIKV intracellular and extracellular viral genomes within 48 h post-treatment, achieving EC50 values of 1.68 and 1.55 µM against Polynesia and Ugandan strains, respectively [190].
Pre-treatment, and not post-entry treatment, of Aedes C6/36 cells with genistein (128) at 60 µM impaired WNV replication. Only ~ 25% cells were detected positive for the viral antigen. The anti-WNV activity by compound 128 was through disruption of focal adhesion kinase (FAK) functionality [191].
Quinones, steroids, cardiac glycosides and other chemical classes from plants
Motivated by the selective gametocytocidal potency of compound 117 from Lophira lanceolata, Sore et al. further isolated alongside other compounds two lanceolins of cyanoglucosides class, lanceolin A and B (129), containing a cyanomethylene group. Lanceolin B exerted considerable inhibitory activity against P. berghei early sporogonic stages and ookinete development at IC50 values of 12.75 and 10.95 µM, respectively [192]. Besides, steroidal compounds isolated from Solanum nudum (Solanaceae), SN-1, SN-2 and SN-4, were evaluated for their sporontocidal effects on P. vivax isolates in An. albimanus. Administered at doses between 50 and 200 µg/ml in infectious blood meals, SN-2 (130) reduced Plasmodium infection in mosquitoes by 90% and mean oocyst numbers by 60% [193].
Ferreira et al. [194] investigated the physiological effects of various plant-derived beta-glycosides and their aglycones on Leishmania spp. viability in female sand fly Lutzomyia longipalpis and in vitro culture. Oral administration of the toxic aglycone mandelonitrile (131) in sugar diets reduced infection prevalence and L. mexicana parasite numbers in sand fly guts, and both mandelonitrile (131) and esculetin (132) had strong anti-Leishmania activities in in vitro cell cultures [194].
Substituted anthraquinones inspired by potently active antischistosomial compounds isolated from Hemerocallis fulva (Asphodelaceae) roots were synthesized into anthraquinones A-S and evaluated for filaricidal activity against microfilariae and adult worms of B. malayi. From these derivatizations, anthraquinone K (133) exerted 100% worm mortality at 5 ppm in 3 days and caused marked distortions in intrauterine embryos [195]. In 2013, Yadav et al. isolated five diarylheptanoid compounds from the leaves of Alnus nepalensis (Betulaceae) and tested for their anti-filariasis activity. Their analyses led to the identification of two potentially active agents, alnus dimer (134) and (5S)-5-hydroxy-1-(4-hydroxyphenyl)-7-(3,4-dihydroxyphenyl)-3-heptanone (135), exhibiting both macrofilaricidal (IC50 6.57–10.31 µg/ml) and microfilaricidal (IC50 11.05–22.10 µg/ml) effects [196].
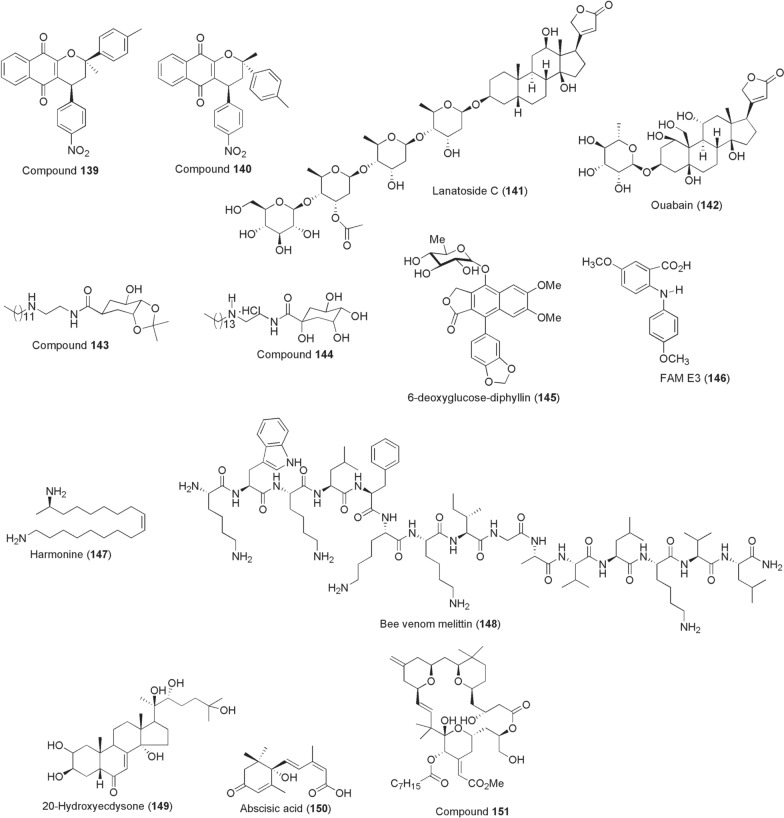
Three acetylenic acids, octadeca-9,11,13-triynoic acid (136), (13E)-octadec-13-en-9,11-diynoic acid (137) and (13E)-octadec-13-en-11-ynoic acid (138), were isolated alongside other compounds from the leaves of a Madagascan plant, Anacolosa pervilleana (Olacaceae). The compounds 136–138 selectively inhibited the DENV RdRp (IC50s ~ 3 µM) at lower micromolar concentrations, were moderately potent against WNV RdRp and inactive against CHIKV [197]. Inspired by the structural framework of lapachol, a plant-derived naphthoquinone, several 1,4-pyran naphthoquinone derivatives were synthesized in the search for potential DENV inhibitors by da Costa et al. [198]. As a result, two diastereoisomers 139 and 140 (Fig. 10) potently inhibited DENV-2 replications in Vero cells achieving 99.0 and 99.6% blockade at IC50 values of 1.64 and 0.31 µM, respectively. Elsewhere, two independent drug repurposing studies have reported the antiviral activities of a number of FDA-approved plant-derived cardiac glycosides functionally acting through blockade of Na+/K+ ATPase pump channel. An earlier study screening a US drug collection library showed lanatoside C (141; derived from Digitalis lanata) exerted pan-DENV antiviral activity at an IC50 0.19 µM in Huh-7 cells through inhibition of viral RNA and protein synthesis. However, the compound 141 weakly inhibits CHIKV by 38.66% at 1 µM [199]. More recently, Guo et al. screened two FDA-approved Na+/K+-ATPase inhibitors, digoxin and ouabain (142), against ZIKV under in vitro and in vivo conditions. Whilst both compounds displayed nanomolar IC50 anti-ZIKV inhibitory values, twice-potent ouabain (IC50 48.39 nM) was found to block viral RNA synthesis by targeting Na+/K+-ATPase and reduced viral loads in mouse tissues [200]. In 2015, Zanello et al. synthesized a number of derivatives based on quinic acid backbone usually found in tomatoes, potatoes, coffee, carrots, etc. Quinic acid has previously been modified into potential antivirals and inspired synthesis of amide derivatives 143 and 144, which inhibited replication of all DENV 1–4 serotypes at IC50 ≤ 10 µM [201].
Fig. 10.
Chemical structures of compounds 139–151. Diastereoisomers compound 139, diastereoisomers compound 140, lanatoside c (141), ouabain (142), quinic acid amide derivative 143, quinic acid amide derivative 144, diphyllin (145), anthranilic acid (fam e3) (146), (17r,9z)-1,17-diaminooctadec-9-ene (harmonine) (147), melittin (148), ecdysteroid (20e) (149), abscisic acid (150), compound 151
Other reported anti-arboviral compounds include glycosylated diphyllin (145) and a diarylamine synthetic derivative of anthranilic acid (FAM E3; 146). The former antiviral agent 145, also known as patentiflorin A, is a naphthalene-derived compound isolated from Justicia gendarussa (Acanthaceae). Martinez-Lopez et al. reported its antiviral activity, demonstrating that the diphyllin component was the active principle against ZIKV and other flaviviruses, DENV, WNV, etc. Compound 145 potently blocked ZIKV infection (100% inhibition) at concentrations ~ 0.25–0.5 µM achieving an IC50 between 0.01 and 0.03 µM. The antiviral activity was mediated through prevention of endosomal acidification [202]. FAM E3 reduced up to 86% ZIKV replications and 96% infectivity at 3 µM during post-entry phase. The compound achieved an anti-ZIKV EC50 of 2.59 µM by binding to and stabilizing NS3 helicase [203].
Miscellaneous sources
Apart from plants, insect gut microbiota and terrestrial microbes, exploration of other natural sources for bioactives has been reported especially from invertebrates. For instance, in 2012 Röhrich et al. identified (17R,9Z)-1,17-diaminooctadec-9-ene (harmonine; 147) from the haemolymph of harlequin ladybird betele Harmonia axyridis. In addition to its activity against Mycobacterium tuberculosis, compound 147 reduced P. falciparum NF54 gametocytes at 4.8 µM to 18%, completely killing all gametocytes at 50 µM. Microgametogenesis was inhibited at IC50 value of 5.8 µM, reducing zygote formation to 17% and 1.2% at 4.8 and 50 µM, respectively. Offering harmonine at 10 µM in gametocytaemic blood meals to Anopheles stephensi mosquitoes resulted in 45/91 infected females with mean oocyst numbers of 1 [204].
A screen of 33 antimicrobial peptides from various sources, including toxins from bacteria, invertebrate stings and venoms, amphibian skin secretions, fish mucus and vertebrate antimicrobial peptides against Plasmodium sporogony in 2013 led to the identification of a bee killer effector peptide (melittin; 148). At 50 µM, melittin consistently reduced P. berghei oocyst parasite prevalence by a mean 10% and intensity by 68% in mosquito midguts. Also the peptide reduced P. falciparum by an average of 57% [205]. Additional bee venom constituent, phospholipase A2, transgenically expressed in An. stephensi midguts effectively impaired P. berghei oocyst formation by 87% and blocked parasite transmissions to naïve mice [206]. Another potential source of bioactive peptides with Plasmodium transmission-blocking activity is from spiders. In this regard, an antimicrobial peptide gomesin isolated from haemocytes of spider Acanthoscurria gomesiana was reported. In addition to its antimalarial activity against asexual stages, the peptide at 50 µM inhibited P. berghei exflagellation of male gametes by 58% and formation of ookinetes by 100%. In mosquitoes the peptide reduced Plasmodium oocyst numbers without inducing noticeable toxicity effects [207].
Studies have implicated host cell lipid hijacking by vector-borne pathogens as a unifying strategy to complete their transmission life cycle and development (reviewed in [208]). But as a feasible intervention target to combat infectious transmissions, its translational applicability is still at infancy stages. In mosquitoes, for instance, lipid metabolism is controlled by pathways heavily dependent upon by developing sporogonic Plasmodium parasites, including the ecdysteroid (20E; 149) hormone signalling [209, 210]. Topical application of steroid/non-steroid agonists, halofenozide, dibenzylhydrazines (e.g. methoxyfenozide) or microinjection of 20E itself into female Anopheles mosquitoes prior to infection manipulates steroid hormone titres, reducing susceptibility to Plasmodium [211–213]. In part, 20E manipulations boost basal innate immune responses that render mosquito midgut unfavourable for sporogonic parasite development.
The human-derived stress signalling isoprenoid molecule and phytohormone, abscisic acid (150), have a calcium signalling function essential in Apicomplexans [214, 215]. While compound 150 itself is inactive against gametocytes and asexual stages in vitro, Glennon et al. have demonstrated that either oral supplementation or pre-treatment of mature Plasmodium gametocytes reduces transmission to mosquitoes [216, 217]. In the mechanistic principle of its transmission-blocking, abscisic acid primes innate immune activation of infected hosts and appears to increase expression of mosquito nitric oxide synthase levels, in consequence mediating reduced infection prevalence in a nitric oxide- and NF-kB-dependent manner [216, 218].
Bryostatin 1 is a macrolide isolated from marine algae in 1982 and largely associated with pan-protein kinase C (PKC) modulation. While the compound itself was inactive against CHIKV (EC50 > 96 µM), structural simplification of the scaffold at A- and B-ring fragments gave 151 that outperformed other anti-CHIKV inhibitors mediating activity through PKC such as prostatin and reported jatrophanes (EC50 0.8 µM). Compound 151 possessing C-8 gem-dimethyl + C-13-methylenyl substitutions exerted anti-CHIKV with an EC50 of 0.35 µM [219].
A summary of the discussed compounds has been provided in “Additional file 1”.
Filling the gap in applicability
Leveraging of the existing and innovative approaches in delivery of the highlighted natural compounds is poised to expand their use beyond human treatments through disruption of pathogen transmission by insect vectors. Notably, the deployability of the suggested approaches could be crosscutting for control of various vector-borne diseases. Among these delivery mechanisms of transmission-blocking technologies would be by treatment of surfaces as reported by Paton et al. [220], where efficacious doses of atovaquone were delivered directly to the mosquito through contact exposure. Modelling studies also suggested that atovaquone would be effective when applied to long-lasting insecticide-treated nets, providing a potential route of administration for similar transmission-blocking chemistries.
Molecules can also be administered to animals as endectocides especially where less anthropogenic vectors such as Anopheles arabiensis mosquitoes are targeted. However, this approach will require drug properties that confer high residual activity, systemic activity and safety to both animals and humans such as topical and feed-through mosquito control systems using fipronil and ivermectin [221, 222]. Attractive sugar baits or other baited surfaces treated with transmission-blocking compounds may also provide a feasible route of administration as demonstrated in laboratory testing, semi-field and field application of disease vectors and harboured pathogens over the last decade [223–226]. For instance, the development of ivermectin-based attractive toxic sugar baits (ATSBs) in 10% sucrose solutions (0.01% ivermectin) against An. arabiensis resulted in > 95% mosquitoe knock-down 48 h post-feeding, suggesting its potential use for outdoor implementation [227].
Another route of administration of mosquito-stage drugs would be through nanoparticle formulations as sprays or biolarvicides, with engineered technology to ensure bioavailability of the drug in the adult mosquito. To our knowledge such robust technologies have not been developed yet and form a subject of further research. The use of adult vector artificial diets treated with the formulated natural compounds can also be explored to be deployed as auto-dissemination stations of the mosquito-stage transmission-blocking chemistries. Based on studies with electrostatic nettings for application of mosquito adulticides [228], another approach that we suggest is utilizing electrostatic dust treated with the drug and applied as wall lining, bed nets, electrostatic netting fitted eave-tubes or indoor wall sprays, which would pile on the adult mosquito body upon wing vibrations during flight.
Agonistic proteins aiding pathogen invasions and vector tissue colonization are potential candidates for drug targeting and transmission-blocking intervention designs. In view of this, small molecule mimetics were designed to target glycosaminoglycan anchorage of Plasmodium-impaired mosquito midgut invasions, reducing oocyst development by 99% [229, 230]. Other screening attempts have been subsequently directed against the Anopheles carboxypeptidase B [231]. Whether through enzymatic inhibition screens, polymeric conjugation with such exemplar proteins or nanoformulated as drug delivery carriers [232], the potentially active natural products could be applied as anchorage inhibitors and/or immune boosters of basal antipathogen responses. Also, advancements in synthetic biology and bioengineering [233] could be adopted for sustainable release and delivery of effector antipathogen products on trigger by pathogen-induced mechanisms during vector infection. This approach is best suited when the biosynthetic gene cluster(s) of a given bioactive natural compound are known and utilized to bioengineer the stable obligate microbiomes and viromes in a paratransgenic manner. This novelty in effector release could offer unprecedented routes of transmission-blocking interventions.
Besides that, natural products could be chemically modified by molecular hybridization with clinically approved chemistries for improved in vivo potencies and pharmacokinetics profiles.
Conclusions
In this review, we have provided a list of natural compounds reported to have potential arthropod-borne pathogen transmission-blocking activities and a contemporary perspective on how such molecules could be integrated into design of control interventions. These molecules provide a blueprint towards (i) scaffold advancement to better drug-like pathogen transmission-blocking leads with improved therapeutic indices, (ii) spurring the continuous search for other potent compounds from various natural sources and (iii) providing a roadmap for translating the laboratory findings and innovatively lead to designing novel community-viable interventions that aid in reducing disease endemicity. For chemical groups that share similar activity, we propose that further chemical modelling is needed to establish structural scaffolds that can be used to inspire synthetic analogues of multiple modes of action to help combat one or more diseases. The provided strategies, in addition to other upcoming next-generation approaches, could be followed in focused design of sustainable delivery systems of these molecules towards acceleration for reduced disease spread amongst vulnerable human hosts. We however noted with mindful concern the lack of compounds investigated against the vector infective forms of Leishmania metacyclic promastigotes and trypanosome metacyclic trypomastigotes. Like other diseases of public health concern, there is need to address the existing gap through revitalized discovery efforts. We believe this review will inspire more discovery efforts within the field of natural products for development of vector-based approaches from the potent molecules endowed with pathogen transmission incapacitations.
Supplementary Information
Additional file 1. Summarized details of the highlighted compounds 1 - 151: their chemical names, class, pathogens tested, and described mode of action.
Acknowledgements
We extend our sincere gratitude to Dr. Godfrey Nattoh for his important review suggestions on this write up.
Author contributions
JMM reviewed the literature and wrote the manuscript with the assistance of the co-authors. All the authors edited the manuscript and approved the final version. All authors read and approved the final manuscript.
Funding
We appreciate the support of Higher Education Loans Board (HELB) Postgraduate Scholarship Award to J.M. Muema*. The study was partly funded by the International Foundation for Science (IFS) under Individual Research Grant number I-1-F-6349-1 awarded to J.M. Muema*.
Availability of data and materials
All datasets generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jackson M. Muema, Email: Jackson_mbithi@yahoo.com
Joel L. Bargul, Email: jbargul@jkuat.ac.ke
Meshack A. Obonyo, Email: obonyom@gmail.com, Email: meshack.obonyo@egerton.ac.ke
Sospeter N. Njeru, Email: snjeru@kemri.go.ke
Damaris Matoke-Muhia, Email: dmatoke@kemri.go.ke.
James M. Mutunga, Email: mutungajames@gmail.com
References
- 1.Debebe Y, Hill SR, Birgersson G, Tekie H, Ignell R. Plasmodium falciparum gametocyte-induced volatiles enhance attraction of Anopheles mosquitoes in the field. Malar J. 2020;19:327. doi: 10.1186/s12936-020-03378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Moraes CM, Wanjiku C, Stanczyk NM, Pulido H, Sims JW, Betz HS, et al. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc Natl Acad Sci. 2018;115:5780–5785. doi: 10.1073/pnas.1801512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busula AO, Bousema T, Mweresa CK, Masiga D, Logan JG, Sauerwein RW, et al. Gametocytemia and attractiveness of Plasmodium falciparum–infected Kenyan children to Anopheles gambiae mosquitoes. J Infect Dis. 2017;216:291–295. doi: 10.1093/infdis/jix214. [DOI] [PubMed] [Google Scholar]
- 4.Batista EPA, Costa EFM, Silva AA. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasit Vectors. 2014;7:251. doi: 10.1186/1756-3305-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Tavares DS, Salgado VR, Miranda JC, Mesquita PRR, de Rodrigues FM, Barral-Netto M, et al. Attraction of phlebotomine sandflies to volatiles from skin odors of individuals residing in an endemic area of tegumentary leishmaniasis. PLoS ONE. 2018;13:e0203989. doi: 10.1371/journal.pone.0203989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lequime S, Paul RE, Lambrechts L. Determinants of arbovirus vertical transmission in mosquitoes. PLOS Pathog. 2016;12:e1005548. doi: 10.1371/journal.ppat.1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du S, Liu Y, Liu J, Zhao J, Champagne C, Tong L, et al. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat Commun. 2019;10:1324. doi: 10.1038/s41467-019-09256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Xiang BW, Saron WAA, Stewart JC, Hain A, Walvekar V, Missé D, et al. Dengue virus infection modifies mosquito blood-feeding behavior to increase transmission to the host. Proc Natl Acad Sci. 2022;119:e2117589119. doi: 10.1073/pnas.2117589119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeno Y, Quang NT, Culleton R, Kawai S, Masuda G, Hori K, et al. Detection of the Plasmodium falciparum Kelch-13 gene P553L mutation in sporozoites isolated from mosquito salivary glands in South-Central Vietnam. Parasit Vectors. 2017;10:308. doi: 10.1186/s13071-017-2247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell AS, Huijben S, Paaijmans KP, Sim DG, Chan BHK, Nelson WA, et al. Enhanced transmission of drug-resistant parasites to mosquitoes following drug treatment in rodent malaria. PLoS ONE. 2012;7:e37172. doi: 10.1371/journal.pone.0037172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Bockstal L, Hendrickx S, Maes L, Caljon G. Sand fly studies predict transmission potential of drug-resistant Leishmania. Trends Parasitol. 2020;36:785–795. doi: 10.1016/j.pt.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Delang L, Yen P-S, Vallet T, Vazeille M, Vignuzzi M, Failloux A-B. Differential transmission of antiviral drug-resistant chikungunya viruses by Aedes mosquitoes. MSphere Am Soc Microbiol. 2018;3:e00230–e318. doi: 10.1128/mSphere.00230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schorderet-Weber S, Noack S, Selzer PM, Kaminsky R. Blocking transmission of vector-borne diseases. Int J Parasitol Drugs Drug Resist. 2017;7:90–109. doi: 10.1016/j.ijpddr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonçalves D, Hunziker P. Transmission-blocking strategies: the roadmap from laboratory bench to the community. Malar J. 2016;15:95. doi: 10.1186/s12936-016-1163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinden RE. Developing transmission-blocking strategies for malaria control. PLoS Pathog. 2017;13:e1006336. doi: 10.1371/journal.ppat.1006336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway MJ, Colpitts TM, Fikrig E. Role of the vector in arbovirus transmission. Annu Rev Virol Annu Rev. 2014;1:71–88. doi: 10.1146/annurev-virology-031413-085513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitner WW, Wali T, Kincaid R, Costero-Saint DA. Arthropod vectors and disease transmission: translational aspects. PLoS Negl Trop Dis. 2015;9:e0004107. doi: 10.1371/journal.pntd.0004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston KL, Hong WD, Turner JD, O’Neill PM, Ward SA, Taylor MJ. Anti-Wolbachia drugs for filariasis. Trends Parasitol. 2021;37:1068–1081. doi: 10.1016/j.pt.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Dong S, Dimopoulos G. Antiviral compounds for blocking arboviral transmission in mosquitoes. Viruses. 2021;13:108. doi: 10.3390/v13010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torto B. Innovative approaches to exploit host plant metabolites in malaria control. Pest Manag Sci. 2019;75:2341–2345. doi: 10.1002/ps.5460. [DOI] [PubMed] [Google Scholar]
- 21.Delves MJ, Angrisano F, Blagborough AM. Antimalarial transmission-blocking interventions: past, present, and future. Trends Parasitol. 2018;34:735–746. doi: 10.1016/j.pt.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Birkholtz L-M, Coetzer TL, Mancama D, Leroy D, Alano P. Discovering new transmission-blocking antimalarial compounds: challenges and opportunities. Trends Parasitol. 2016;32:669–681. doi: 10.1016/j.pt.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues T, Prudêncio M, Moreira R, Mota MM, Lopes F. Targeting the liver stage of malaria parasites: a yet unmet goal. J Med Chem. 2012;55:995–1012. doi: 10.1021/jm201095h. [DOI] [PubMed] [Google Scholar]
- 24.Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 26.Muema JM, Bargul JL, Njeru SN, Onyango JO, Imbahale SS. Prospects for malaria control through manipulation of mosquito larval habitats and olfactory-mediated behavioural responses using plant-derived compounds. Parasit Vectors. 2017;10:184. doi: 10.1186/s13071-017-2122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muturi EJ, Lagos-Kutz D, Dunlap C, Ramirez JL, Rooney AP, Hartman GL, et al. Mosquito microbiota cluster by host sampling location. Parasit Vectors. 2018;11:468. doi: 10.1186/s13071-018-3036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao H, Cui C, Wang L, Jacobs-Lorena M, Wang S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020;36:98–111. doi: 10.1016/j.pt.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraiva RG, Dimopoulos G. Bacterial natural products in the fight against mosquito-transmitted tropical diseases. Nat Prod Rep. 2020;37:338–354. doi: 10.1039/C9NP00042A. [DOI] [PubMed] [Google Scholar]
- 30.Lacerda AF, Pelegrini PB, de Oliveira DM, Vasconcelos ÉAR, Grossi-de-Sá MF. Anti-parasitic peptides from arthropods and their application in drug therapy. Front Microbiol. 2016;7:91. doi: 10.3389/fmicb.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, et al. Antiviral and antitumor peptides from insects. Proc Natl Acad Sci. 2002;99:12628–12632. doi: 10.1073/pnas.192301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014;10:e1004398. doi: 10.1371/journal.ppat.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saraiva RG, Huitt-Roehl CR, Tripathi A, Cheng Y-Q, Bosch J, Townsend CA, et al. Chromobacterium spp. mediate their anti-Plasmodium activity through secretion of the histone deacetylase inhibitor romidepsin. Sci Rep. 2018;8:6176. doi: 10.1038/s41598-018-24296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva RG, Fang J, Kang S, Angleró-Rodríguez YI, Dong Y, Dimopoulos G. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl Trop Dis. 2018;12:e0006443–e0006443. doi: 10.1371/journal.pntd.0006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short SM, van Tol S, MacLeod HJ, Dimopoulos G. Hydrogen cyanide produced by the soil bacterium Chromobacterium sp. Panama contributes to mortality in Anopheles gambiae mosquito larvae. Sci Rep. 2018;8:8358. doi: 10.1038/s41598-018-26680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavella TA, da Silva NSM, Spillman N, Kayano ACAV, Cassiano GC, Vasconcelos AA, et al. Violacein-induced chaperone system collapse underlies multistage antiplasmodial activity. ACS Infect Dis. 2021;7:759–776. doi: 10.1021/acsinfecdis.0c00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattoh G, Maina T, Makhulu EE, Mbaisi L, Mararo E, Otieno FG, et al. Horizontal transmission of the symbiont Microsporidia MB in Anophelesarabiensis. Front Microbiol. 2021;12. [DOI] [PMC free article] [PubMed]
- 39.Herren JK, Mbaisi L, Mararo E, Makhulu EE, Mobegi VA, Butungi H, et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat Commun. 2020;11:2187. doi: 10.1038/s41467-020-16121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol. 2003;40:371–374. doi: 10.1603/0022-2585-40.3.371. [DOI] [PubMed] [Google Scholar]
- 41.Steyn A, Roets F, Botha A. Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb Ecol. 2016;71:747–760. doi: 10.1007/s00248-015-0709-1. [DOI] [PubMed] [Google Scholar]
- 42.Cappelli A, Ulissi U, Valzano M, Damiani C, Epis S, Gabrielli MG, et al. A Wickerhamomyces anomalus killer strain in the malaria vector Anopheles stephensi. PLoS ONE. 2014;9:e95988. doi: 10.1371/journal.pone.0095988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci I, Damiani C, Scuppa P, Mosca M, Crotti E, Rossi P. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Env Microbiol. 2011;13:911–921. doi: 10.1111/j.1462-2920.2010.02395.x. [DOI] [PubMed] [Google Scholar]
- 44.Cappelli A, Valzano M, Cecarini V, Bozic J, Rossi P, Mensah P, et al. Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit Vectors. 2019;12:329. doi: 10.1186/s13071-019-3587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valzano M, Cecarini V, Cappelli A, Capone A, Bozic J, Cuccioloni M. A yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malar J. 2016;15. [DOI] [PMC free article] [PubMed]
- 46.Czesny B, Goshu S, Cook JL, Williamson KC. The proteasome inhibitor Epoxomicin has potent Plasmodium falciparum gametocytocidal activity. Antimicrob Agents Chemother. 2009;53:4080–4085. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aminake MN, Schoof S, Sologub L, Leubner M, Kirschner M, Arndt H, et al. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite. Antimicrob Agents Chemother. 2011;55:1338–1348. doi: 10.1128/AAC.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campell CW, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983;221:823–828. doi: 10.1126/science.6308762. [DOI] [PubMed] [Google Scholar]
- 49.Pinilla YT, Lopes CP, Sampaio VS, Andrade FS, Melo GC, Orfanó AS, et al. Promising approach to reducing Malaria transmission by ivermectin: Sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Negl Trop Dis. 2018;12:e0006221–e0006221. doi: 10.1371/journal.pntd.0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobylinski KC, Escobedo-Vargas KS, López-Sifuentes VM, Durand S, Smith ES, Baldeviano GC, et al. Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi. Malar J. 2017;16:474. doi: 10.1186/s12936-017-2125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendes AM, Albuquerque IS, Machado M, Pissarra J, Meireles P, Prudêncio M. Inhibition of Plasmodium liver infection by ivermectin. Antimicrob Agents Chemother. 2017;61:e02005–e2016. doi: 10.1128/AAC.02005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobylinski KC, Foy BD, Richardson JH. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malar J. 2012;11:381. doi: 10.1186/1475-2875-11-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Sampaio VS, da Rivas GBS, Kobylinski K, Pinilla YT, Pimenta PFP, Lima JBP, et al. What does not kill it makes it weaker: effects of sub-lethal concentrations of ivermectin on the locomotor activity of Anopheles aquasalis. Parasit Vectors. 2017;10:623. doi: 10.1186/s13071-017-2563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyimo IN, Kessy ST, Mbina KF, Daraja AA, Mnyone LL. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar J. 2017;16:239. doi: 10.1186/s12936-017-1885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pooda HS, Rayaisse J-B, de Hien DFS, Lefèvre T, Yerbanga SR, Bengaly Z, et al. Administration of ivermectin to peridomestic cattle: a promising approach to target the residual transmission of human malaria. Malar J. 2015;13:496. doi: 10.1186/s12936-015-1001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pooda SH, Mouline K, De Meeûs T, Bengaly Z, Solano P. Decrease in survival and fecundity of Glossina palpalis gambiensis vanderplank 1949 (Diptera: Glossinidae) fed on cattle treated with single doses of ivermectin. Parasit Vectors. 2013;6:165. doi: 10.1186/1756-3305-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Held J, Gebru T, Kalesse M, Jansen R, Gerth K, Müller R, et al. Antimalarial activity of the myxobacterial macrolide chlorotonil A. Antimicrob Agents Chemother. 2014;58:6378–6384. doi: 10.1128/AAC.03326-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastrana-mena R, Mathias DK, Delves M, Rajaram K, King JG, Yee R, et al. A malaria transmission-blocking (+)-Usnic acid derivative prevents Plasmodium zygote-to-ookinete maturation in the mosquito midgut. ACS Chem Biol. 2016;11:3461–3472. doi: 10.1021/acschembio.6b00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauinger IL, Vivas L, Perozzo R, Stairiker C, Tarun A, Zloh M, et al. Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target. J Nat Prod. 2013;76:1064–1070. doi: 10.1021/np400083k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang G, Niu G, Franca CM, Dong Y, Wang X, Butler NS, et al. Anopheles midgut FREP1 mediates Plasmodium invasion. J Biol Chem. 2015;290:16490–16501. doi: 10.1074/jbc.M114.623165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niu G, Wang B, Zhang G, King JB, Cichewicz RH, Li J. Targeting mosquito FREP1 with a fungal metabolite blocks malaria transmission. Sci Rep. 2015;5:14694. doi: 10.1038/srep14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niu G, Wang X, Hao Y, Kandel S, Niu G, Raptis RG, et al. A novel fungal metabolite inhibits Plasmodium falciparum transmission and infection. Parasit Vectors. 2021;14:177. doi: 10.1186/s13071-021-04677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niu G, Hao Y, Wang X, Gao J-M, Li J. Fungal metabolite Asperaculane B inhibits malaria infection and transmission. Molecules. 2020;25:3018. doi: 10.3390/molecules25133018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Douglas RG, Reinig M, Neale M, Frischknecht F. Screening for potential prophylactics targeting sporozoite motility through the skin. Malar J. 2018;17:319. doi: 10.1186/s12936-018-2469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, et al. Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep. 2014;4:3743. doi: 10.1038/srep03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maron MI, Magle CT, Czesny B, Turturice BA, Huang R, Zheng W, et al. Maduramicin rapidly eliminates malaria parasites and potentiates the gametocytocidal activity of the pyrazoleamide PA21A050. Antimicrob Agents Chemother. 2015;60:1492–1499. doi: 10.1128/AAC.01928-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Alessandro S, Corbett Y, Ilboudo DP, Misiano P, Dahiya N, Abay SM, et al. Salinomycin and other ionophores as a new class of antimalarial drugs with transmission-blocking activity. Antimicrob Agents Chemother. 2015;59:5135–5144. doi: 10.1128/AAC.04332-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Derbyshire ER, Prudêncio M, Mota MM, Clardy J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc Natl Acad Sci. 2012;109:8511–8516. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, et al. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother. 2012;56:3544–3548. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, et al. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schiefer A, Hübner MP, Krome A, Lämmer C, Ehrens A, Aden T, et al. Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections. PLoS Negl Trop Dis. 2020;14:e0008930. doi: 10.1371/journal.pntd.0008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiefer A, Schmitz A, Schäberle TF, Specht S, Lämmer C, Johnston KL, et al. Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J Infect Dis. 2012;206:249–257. doi: 10.1093/infdis/jis341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z, Fang S-M, Bakowski MA, Rateb ME, Yang D, Zhu X, et al. Discovery of kirromycins with anti-Wolbachia activity from Streptomyces sp. CB00686. ACS Chem Biol. 2019;14:1174–1182. doi: 10.1021/acschembio.9b00086. [DOI] [PubMed] [Google Scholar]
- 75.von Geldern TW, Morton HE, Clark RF, Brown BS, Johnston KL, Ford L, et al. Discovery of ABBV-4083, a novel analog of Tylosin A that has potent anti-Wolbachia and anti-filarial activity. PLoS Negl Trop Dis. 2019;13:e0007159. doi: 10.1371/journal.pntd.0007159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacobs RT, Lunde CS, Freund YR, Hernandez V, Li X, Xia Y, et al. Boron-pleuromutilins as anti-Wolbachia agents with potential for treatment of onchocerciasis and lymphatic filariasis. J Med Chem. 2019;62:2521–2540. doi: 10.1021/acs.jmedchem.8b01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inukai M, Nakajima M, Osawa M, Haneishi T, Arai M. Globomycin, a new peptide antibiotic with spheroplast-forming activity. II. Isolation and physico-chemical and biological characterization. J Antibiot (Tokyo). 1978;31:421–425. doi: 10.7164/antibiotics.31.421. [DOI] [PubMed] [Google Scholar]
- 78.Johnston KL, Wu B, Guimarães A, Ford L, Slatko BE, Taylor MJ. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasit Vectors. 2010;3:99. doi: 10.1186/1756-3305-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao R, Weil GJ. In vitro effects of antibiotics on Brugia malayi worm survival and reproduction. J Parasitol. 2002;88:605–611. doi: 10.1645/0022-3395(2002)088[0605:IVEOAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 80.Bulman CA, Chappell L, Gunderson E, Vogel I, Beerntsen B, Slatko BE, et al. The Eagle effect in the Wolbachia-worm symbiosis. Parasit Vectors. 2021;14:118. doi: 10.1186/s13071-020-04545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rateb ME, Yang D, Vodanovic-Jankovic S, Yu Z, Kron MA, Shen B. Adipostatins A-D from Streptomyces sp. 4875 inhibiting Brugia malayi asparaginyl-tRNA synthetase and killing adult Brugia malayi parasites. J Antibiot (Tokyo). 2015;68:540–542. doi: 10.1038/ja.2015.22. [DOI] [PubMed] [Google Scholar]
- 82.Yu Z, Vodanovic-Jankovic S, Kron M, Shen B. New WS9326A congeners from Streptomyces sp. 9078 inhibiting Brugia malayi asparaginyl-tRNA synthetase. Org Lett. 2012;14:4946–4949. doi: 10.1021/ol302298k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu Z, Vodanovic-Jankovic S, Ledeboer N, Huang S-X, Rajski SR, Kron M, et al. Tirandamycins from Streptomyces sp. 17944 inhibiting the parasite Brugia malayi asparagine tRNA synthetase. Org Lett. 2011;13:2034–2037. doi: 10.1021/ol200420u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, et al. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against zika virus. Cell Rep. 2017;18:804–815. doi: 10.1016/j.celrep.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Estoppey D, Lee CM, Janoschke M, Lee BH, Wan KF, Dong H, et al. The natural product cavinafungin selectively interferes with zika and dengue virus replication by inhibition of the host signal peptidase. Cell Rep. 2017;19:451–460. doi: 10.1016/j.celrep.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 86.Rox K, Heyner M, Krull J, Harmrolfs K, Rinne V, Hokkanen J, et al. Physiologically based pharmacokinetic/pharmacodynamic model for the treatment of dengue infections applied to the broad spectrum antiviral Soraphen A. ACS Pharmacol Transl Sci. 2021;4:1499–1513. doi: 10.1021/acsptsci.1c00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hans P, Katharina R, Suryanarayana BNV, Loreen W, Sven-Kevin H, Philipp K, et al. Labyrinthopeptins exert broad-spectrum antiviral activity through lipid-binding-mediated virolysis. J Virol. 2021;94:e01471–e1519. doi: 10.1128/JVI.01471-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, et al. A screen of FDA-approved drugs for inhibitors of zika virus infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012;67:1884–1894. doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varghese FS, Kaukinen P, Gläsker S, Bespalov M, Hanski L, Wennerberg K, et al. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res. 2016;126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Diamond MS, Zachariah M, Harris E. Mycophenolic Acid inhibits dengue virus infection by preventing replication of viral RNA. Virology. 2002;304:211–221. doi: 10.1006/viro.2002.1685. [DOI] [PubMed] [Google Scholar]
- 92.Min Q, Feng Y, Bo Z, Gang Z, Robida JM, Zhiming Y, et al. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother. 2009;53:3226–3235. doi: 10.1128/AAC.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong S, Kang S, Dimopoulos G. Identification of anti-flaviviral drugs with mosquitocidal and anti-Zika virus activity in Aedes aegypti. PLoS Negl Trop Dis. 2019;13:e0007681. doi: 10.1371/journal.pntd.0007681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raveh A, Delekta PC, Dobry CJ, Peng W, Schultz PJ, Blakely PK, et al. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS ONE. 2013;8:e82318. doi: 10.1371/journal.pone.0082318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raekiansyah M, Mori M, Nonaka K, Agoh M, Shiomi K, Matsumoto A, et al. Identification of novel antiviral of fungus-derived brefeldin A against dengue viruses. Trop Med Health. 2017;45:32. doi: 10.1186/s41182-017-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chunfeng L, Shulong Z, Yong-Qiang D, Dapei L, Kislay P, Natalie Q, et al. Azithromycin protects against zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob Agents Chemother. 2021;63:e00394–e419. doi: 10.1128/AAC.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rothan HA, Bahrani H, Mohamed Z, Teoh TC, Shankar EM, Rahman NA, et al. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS ONE. 2015;10:e0126360. doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta DK, Kaur P, Leong ST, Tan LT, Prinsep MR, Chu JJ. Anti-chikungunya viral activities of Aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar Drugs. 2014;12:115–127. doi: 10.3390/md12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang S, Shields AR, Jupatanakul N, Dimopoulos G. Suppressing dengue-2 infection by chemical inhibition of Aedes aegypti host factors. PLoS Negl Trop Dis. 2014;8:e3084. doi: 10.1371/journal.pntd.0003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nyasembe VO, Tchouassi DP, Pirk CWW, Sole CL, Torto B. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl Trop Dis. 2018;12:e0006185. doi: 10.1371/journal.pntd.0006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nyasembe VO, Peter EA, Sawa P, Tumlinson JH, Borgemeister C, Torto B. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr Biol. 2014;24:217–221. doi: 10.1016/j.cub.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agha SB, Alvarez M, Becker M, Fèvre EM, Junglen S, Borgemeister C. Invasive alien plants in Africa and the potential emergence of mosquito-borne arboviral diseases—a review and research outlook. Viruses. 2020;13:32. doi: 10.3390/v13010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wanjiku C, Tchouassi DP, Sole CL, Pirk C, Torto B. Plant sugar feeding patterns of wild-caught Aedes aegypti from dengue endemic and non-endemic areas of Kenya. Med Vet Entomol. 2021;35:417–425. doi: 10.1111/mve.12514. [DOI] [PubMed] [Google Scholar]
- 104.Hassaballa IB, Sole CL, Cheseto X, Torto B, Tchouassi DP. Afrotropical sand fly-host plant relationships in a leishmaniasis endemic area. Kenya PLoS Negl Trop Dis. 2021;15:e0009041. doi: 10.1371/journal.pntd.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abbasi I, TrancosoLopo de Queiroz A, Kirstein OD, Nasereddin A, Horwitz BZ, Hailu A, et al. Plant-feeding phlebotomine sand flies, vectors of leishmaniasis, prefer Cannabis sativa. Proc Natl Acad Sci. 2018;115:11790–11795. doi: 10.1073/pnas.1810435115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stone CM, Witt ABR, Walsh GC, Foster WA, Murphy ST. Would the control of invasive alien plants reduce malaria transmission? A review. Parasit Vectors. 2018;11:76. doi: 10.1186/s13071-018-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muller GC, Junnila A, Traore MM, Traore SF, Doumbia S, Sissoko F, et al. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: a habitat manipulation experiment. Malar J. 2017;16:237. doi: 10.1186/s12936-017-1878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Díaz-Albiter HM, Ferreira TN, Costa SG, Rivas GB, Gumiel M, Cavalcante DR, et al. Everybody loves sugar: first report of plant feeding in triatomines. Parasit Vectors. 2016;9:114. doi: 10.1186/s13071-016-1401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nyasembe VO, Cheseto X, Kaplan F, Foster WA, Teal EA, Tumlinson JH, et al. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS ONE. 2015;10:e0137836. doi: 10.1371/journal.pone.0137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sissoko F, Junnila A, Traore MM, Traore SF, Doumbia S, Dembele SM, et al. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB) PLoS ONE. 2019;14:e0214170. doi: 10.1371/journal.pone.0214170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 2010;9:262. doi: 10.1186/1475-2875-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 2007;6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hien DFDS, Paré PSL, Cooper A, Koama BK, Guissou E, Yaméogo KB, et al. Contrasting effects of the alkaloid ricinine on the capacity of Anopheles gambiae and Anopheles coluzzii to transmit Plasmodium falciparum. Parasit Vectors. 2021;14:479. doi: 10.1186/s13071-021-04992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hien DFS, Dabiré KR, Roche B, Diabaté A, Yerbanga RS, Cohuet A, et al. Plant-mediated effects on mosquito capacity to transmit human malaria. PLOS Pathog. 2016;12:e1005773. doi: 10.1371/journal.ppat.1005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Almire F, Terhzaz S, Terry S, McFarlane M, Gestuveo RJ, Szemiel AM, et al. Sugar feeding protects against arboviral infection by enhancing gut immunity in the mosquito vector Aedes aegypti. PLOS Pathog. 2021;17:e1009870. doi: 10.1371/journal.ppat.1009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gualtieri MJ, Malafronte N, Vassallo A, Braca A, Cotugno R, Vasaturo M, et al. Bioactive limonoids from the leaves of Azaridachta indica (Neem) J Nat Prod. 2014;77:596–602. doi: 10.1021/np400863d. [DOI] [PubMed] [Google Scholar]
- 117.Chianese G, Yerbanga SR, Lucantoni L, Habluetzel A, Basilico N, Taramelli D, et al. Antiplasmodial triterpenoids from the fruits of Neem, Azadirachta indica. J Nat Prod. 2010;73:1448–1452. doi: 10.1021/np100325q. [DOI] [PubMed] [Google Scholar]
- 118.Billker O, Shaw MK, Jones IANW, Ley SV, Luntz AJM, Sinden RE. Azadirachtin disrupts formation of organised microtubule arrays during microgametogenesis of Plasmodium berghei. J Eukaryot Microbiol. 2002;49:489–497. doi: 10.1111/j.1550-7408.2002.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 119.Tapanelli S, Chianese G, Lucantoni L, Yerbanga RS, Habluetzel A, Taglialatela-Scafati O. Transmission blocking effects of neem (Azadirachta indica) seed kernel limonoids on Plasmodium berghei early sporogonic development. Fitoterapia. 2016;114:122–126. doi: 10.1016/j.fitote.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Dahiya N, Chianese G, Abay SM, Taglialatela-Scafati O, Esposito F, Lupidi G, et al. In vitro and ex vivo activity of an Azadirachta indica A.Juss. seed kernel extract on early sporogonic development of Plasmodium in comparison with azadirachtin A, its most abundant constituent. Phytomedicine. 2016;23:1743–1752. doi: 10.1016/j.phymed.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 121.Lucantoni L, Yerbanga RS, Lupidi G, Pasqualini L, Esposito F, Habluetzel A. Transmission blocking activity of a standardized neem (Azadirachta indica) seed extract on the rodent malaria parasite Plasmodium berghei in its vector Anopheles stephensi. Malar J. 2010;9:66. doi: 10.1186/1475-2875-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abay SM, Lucantoni L, Dahiya N, Dori G, Dembo EG, Esposito F, et al. Plasmodium transmission blocking activities of Vernonia amygdalina extracts and isolated compounds. Malar J. 2015;14:288. doi: 10.1186/s12936-015-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sirignano C, Hammami S, El Mokni R, Blagborough AM, Luciano P, Rigano D, et al. Polyoxygenated germacranes from Daucus carota and their antimalarial transmission blocking activity. Phytochemistry. 2021;183:112632. doi: 10.1016/j.phytochem.2020.112632. [DOI] [PubMed] [Google Scholar]
- 124.Sirignano C, Snene A, Tenoh AR, El Mokni R, Rigano D, Habluetzel A, et al. Daucovirgolides I-L, four congeners of the antimalarial daucovirgolide G from Daucus virgatus. Fitoterapia. 2019;137:104188. doi: 10.1016/j.fitote.2019.104188. [DOI] [PubMed] [Google Scholar]
- 125.Sirignano C, Snene A, Rigano D, Tapanelli S, Formisano C, Luciano P, et al. Angeloylated germacranolides from Daucus virgatus and their Plasmodium transmission blocking activity. J Nat Prod. 2017;80:2787–2794. doi: 10.1021/acs.jnatprod.7b00603. [DOI] [PubMed] [Google Scholar]
- 126.Balaich JN, Mathias DK, Torto B, Jackson BT, Tao D, Ebrahimi B, et al. The nonartemisinin sesquiterpene lactones parthenin and parthenolide block Plasmodium falciparum sexual stage transmission. Antimicrob Agents Chemother. 2016;60:2108–2117. doi: 10.1128/AAC.02002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moyo P, Kunyane P, Selepe MA, Eloff JN, Niemand J, Louw AI, et al. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae) Malar J. 2019;18:65. doi: 10.1186/s12936-019-2694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lelièvre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “Transmission Blocking” assay. PLoS ONE. 2012;7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Witmer K, Dahalan FA, Delves MJ, Yahiya S, Watson OJ, Straschil U, et al. Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob Agents Chemother. 2020;19:952. doi: 10.1128/AAC.00898-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–2024. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coertzen D, Reader J, Van Der Watt M, Nondaba SH, Gibhard L, Wiesner L, et al. Artemisone and artemiside are potent panreactive antimalarial agents that also synergize redox imbalance in Plasmodium falciparum transmissible gametocyte stages. Antimicrob Agents Chemother. 2018;62:e02214–e2217. doi: 10.1128/AAC.02214-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wong HN, Padín-Irizarry V, Van der Watt ME, Reader J, Liebenberg W, Wiesner L, et al. Optimal 10-Aminoartemisinins with potent transmission-blocking capabilities for new artemisinin combination therapies activities against blood stage P.falciparum including PfKI3 C580Y mutants and liver stage P.berghei parasites. Front Chem. 2020; 901. [DOI] [PMC free article] [PubMed]
- 133.Allard P-M, Leyssen P, Martin M-T, Bourjot M, Dumontet V, Eydoux C, et al. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry. 2012;84:160–168. doi: 10.1016/j.phytochem.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 134.Bourjot M, Delang L, Nguyen VH, Neyts J, Guéritte F, Leyssen P, et al. Prostratin and 12-O-tetradecanoylphorbol 13-acetate are potent and selective inhibitors of chikungunya virus replication. J Nat Prod. 2012;75:2183–2187. doi: 10.1021/np300637t. [DOI] [PubMed] [Google Scholar]
- 135.Bourjot M, Leyssen P, Neyts J, Dumontet V, Litaudon M, Trigocherrierin A. a potent inhibitor of chikungunya virus replication. Molecules. 2014;19:3617–3627. doi: 10.3390/molecules19033617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corlay N, Delang L, Girard-Valenciennes E, Neyts J, Clerc P, Smadja J, et al. Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. Fitoterapia. 2014;97:87–91. doi: 10.1016/j.fitote.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 137.Nothias-Scaglia L-F, Retailleau P, Paolini J, Pannecouque C, Neyts J, Dumontet V, et al. Jatrophane diterpenes as inhibitors of chikungunya virus replication: Structure–activity relationship and discovery of a potent lead. J Nat Prod. 2014;77:1505–1512. doi: 10.1021/np500271u. [DOI] [PubMed] [Google Scholar]
- 138.Nothias-Scaglia L-F, Pannecouque C, Renucci F, Delang L, Neyts J, Roussi F, et al. Antiviral activity of diterpene esters on chikungunya virus and HIV replication. J Nat Prod. 2015;78:1277–1283. doi: 10.1021/acs.jnatprod.5b00073. [DOI] [PubMed] [Google Scholar]
- 139.Techer S, Girard-Valenciennes E, Retailleau P, Neyts J, Guéritte F, Leyssen P, et al. Tonantzitlolones from Stillingia lineata ssp. lineata as potential inhibitors of chikungunya virus. Phytochem Lett. 2015;12:313–319. doi: 10.1016/j.phytol.2015.04.023. [DOI] [Google Scholar]
- 140.Olivon F, Palenzuela H, Girard-Valenciennes E, Neyts J, Pannecouque C, Roussi F, et al. Antiviral activity of flexibilane and tigliane diterpenoids from Stillingia lineata. J Nat Prod. 2015;78:1119–1128. doi: 10.1021/acs.jnatprod.5b00116. [DOI] [PubMed] [Google Scholar]
- 141.Tan YP, Houston SD, Modhiran N, Savchenko AI, Boyle GM, Young PR, et al. Stachyonic acid: a dengue virus inhibitor from Basilicum polystachyon. Chem A Eur J. 2019;25:5664–5667. doi: 10.1002/chem.201900591. [DOI] [PubMed] [Google Scholar]
- 142.Baltina LA, Tasi Y-T, Huang S-H, Lai H-C, Baltina LA, Petrova SF, et al. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg Med Chem Lett. 2019;29:126645. doi: 10.1016/j.bmcl.2019.126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baltina LA, Lai H-C, Liu Y-C, Huang S-H, Hour M-J, Baltina LA, et al. Glycyrrhetinic acid derivatives as Zika virus inhibitors: synthesis and antiviral activity in vitro. Bioorg Med Chem. 2021;41:116204. doi: 10.1016/j.bmc.2021.116204. [DOI] [PubMed] [Google Scholar]
- 144.Cirne-Santos CC, Souza barros de C, Oliveira de MC, Rabelo VW-H, Azevedo RC, Teixeira VL, et al. In vitro studies on the inhibition of replication of Zika and Chikungunya viruses by dolastane isolated from seaweed Canistrocarpus cervicornis. Sci Rep. 2020;10:8263. doi: 10.1038/s41598-020-65357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loe MWC, Hao E, Chen M, Li C, Lee RCH, Zhu IXY, et al. Betulinic acid exhibits antiviral effects against dengue virus infection. Antiviral Res. 2020;184:104954. doi: 10.1016/j.antiviral.2020.104954. [DOI] [PubMed] [Google Scholar]
- 146.Misra S, Verma M, Mishra SK, Srivastava S, Lakshmi V, Misra-Bhattacharya S. Gedunin and photogedunin of Xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite Brugia malayi in experimental rodent host. Parasitol Res. 2011;109:1351. doi: 10.1007/s00436-011-2380-x. [DOI] [PubMed] [Google Scholar]
- 147.Kalani K, Kushwaha V, Verma R, Murthy PK, Srivastava SK. Glycyrrhetinic acid and its analogs: a new class of antifilarial agents. Bioorg Med Chem Lett. 2013;23:2566–2570. doi: 10.1016/j.bmcl.2013.02.115. [DOI] [PubMed] [Google Scholar]
- 148.Saini P, Gayen P, Kumar D, Nayak A, Mukherjee N, Mukherjee S, et al. Antifilarial effect of ursolic acid from Nyctanthes arbortristis: molecular and biochemical evidences. Parasitol Int. 2014;63:717–728. doi: 10.1016/j.parint.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 149.Kushwaha V, Saxena K, Verma R, Verma SK, Katoch D, Kumar N, et al. Antifilarial activity of diterpenoids from Taxodium distichum. Parasit Vectors. 2016;9:312. doi: 10.1186/s13071-016-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar J. 2012;11:34. doi: 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NPJ, Coleman RE, et al. Transmission-blocking activities of Quinine, Primaquine, and Artesunate. Antimicrob Agents Chemother. 2006;50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vu H, Roullier C, Campitelli M, Trenholme KR, Gardiner DL, Andrews KT, et al. Plasmodium gametocyte inhibition identified from a natural-product-based fragment library. ACS Chem Biol. 2013;8:2654–2659. doi: 10.1021/cb400582b. [DOI] [PubMed] [Google Scholar]
- 154.Forkuo AD, Ansah C, Mensah KB, Annan K, Gyan B, Theron A, et al. In vitro anti-malarial interaction and gametocytocidal activity of cryptolepine. Malar J. 2017;16:496. doi: 10.1186/s12936-017-2142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Onambele LA, Riepl H, Fischer R, Pradel G, Prokop A, Aminake MN. Synthesis and evaluation of the antiplasmodial activity of tryptanthrin derivatives. Int J Parasitol Drugs drug Resist. 2015;5:48–57. doi: 10.1016/j.ijpddr.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goodman CD, Austarheim I, Mollard V, Mikolo B, Malterud KE, Mcfadden GI, et al. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malar J. 2016;15:481. doi: 10.1186/s12936-016-1533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moyo P, Shamburger W, van der Watt ME, Reader J, de Sousa ACC, Egan TJ, et al. Naphthylisoquinoline alkaloids, validated as hit multistage antiplasmodial natural products. Int J Parasitol Drugs Drug Resist. 2020;13:51–58. doi: 10.1016/j.ijpddr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Priyanka P, Burusco KK, Muna A, Holly M, Andrey G, Elena F-Á, et al. Lead optimization of dehydroemetine for repositioned use in malaria. Antimicrob Agents Chemother. 2021;64:e01444–e1519. doi: 10.1128/AAC.01444-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muema JM, Bargul JL, Mutunga JM, Obonyo MA, Mwakubambanya RS. Process of blocking Plasmodium gametocytogenesis and transmission using juliprosopine from Prosopisjuliflora. Kenya Intellectual Property Institute; 2020; KE/P/2020/3643.
- 160.Carraz M, Jossang A, Franetich J-F, Siau A, Ciceron L, Hannoun L, et al. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLOS Med. 2006;3:e513. doi: 10.1371/journal.pmed.0030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carraz M, Jossang A, Rasoanaivo P, Mazier D, Frappier F. Isolation and antimalarial activity of new morphinan alkaloids on Plasmodium yoelii liver stage. Bioorg Med Chem. 2008;16:6186–6192. doi: 10.1016/j.bmc.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 162.de Lamballerie X, Ninove L, Charrel RN. Antiviral treatment of chikungunya virus infection. Infect Disord Targets. 2009;9:101–104. doi: 10.2174/187152609787847712. [DOI] [PubMed] [Google Scholar]
- 163.Varghese SF, Bastian T, Naqiah AS, Diane S, Kai R, Nyman TA, et al. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J Virol. 2021;90:9743–9757. doi: 10.1128/JVI.01382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wan JJ, Brown RS, Margaret K. Berberine chloride is an alphavirus inhibitor that targets nucleocapsid assembly. MBio. 2021;11:e01382–e1420. doi: 10.1128/mBio.01382-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parveen K, Meerra T, Hua LRC, Huixin C, Caiyun CK, Lee NM, et al. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57:155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hwang J, Jiang A, Fikrig E. A potent prolyl tRNA synthetase inhibitor antagonizes Chikungunya and Dengue viruses. Antiviral Res. 2019;161:163–168. doi: 10.1016/j.antiviral.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Troost B, Mulder LM, Diosa-Toro M, van de Pol D, Rodenhuis-Zybert IA, Smit JM. Tomatidine, a natural steroidal alkaloid shows antiviral activity towards chikungunya virus in vitro. Sci Rep. 2020;10:6364. doi: 10.1038/s41598-020-63397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diosa-Toro M, Troost B, van de Pol D, Heberle AM, Urcuqui-Inchima S, Thedieck K, et al. Tomatidine, a novel antiviral compound towards dengue virus. Antiviral Res. 2019;161:90–99. doi: 10.1016/j.antiviral.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 169.Kevin W, Pierson CT, Brian G, Kelly L, Michael E, Yi Z, et al. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 2005;79:8698–8706. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang P, Li L-F, Wang Q-Y, Shang L-Q, Shi P-Y, Yin Z. Anti-dengue-virus activity and structure–activity relationship studies of lycorine derivatives. ChemMedChem. 2014;9:1522–1533. doi: 10.1002/cmdc.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen H, Lao Z, Xu J, Li Z, Long H, Li D, et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology. 2020;546:88–97. doi: 10.1016/j.virol.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zou G, Puig-Basagoiti F, Zhang B, Qing M, Chen L, Pankiewicz KW, et al. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology. 2009;384:242–252. doi: 10.1016/j.virol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ka S, Merindol N, Sow AA, Singh A, Landelouci K, Plourde MB, et al. Amaryllidaceae alkaloid cherylline inhibits the replication of dengue and zika viruses. Antimicrob Agents Chemother. 2021;65:e0039821–e0039821. doi: 10.1128/AAC.00398-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:31. doi: 10.1038/s41421-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li Z, Garner AL, Gloeckner C, Janda KD, Carlow CK. Targeting the Wolbachia cell division protein FtsZ as a new approach for antifilarial therapy. PLoS Negl Trop Dis. 2011;5:e1411–e1411. doi: 10.1371/journal.pntd.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hellmann JK, Munter S, Wink M, Frischknecht F. Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility. PLoS ONE. 2010;5:e8682. doi: 10.1371/journal.pone.0008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lopatriello A, Soré H, Habluetzel A, Parapini S, D’Alessandro S, Taramelli D, et al. Identification of a potent and selective gametocytocidal antimalarial agent from the stem barks of Lophira lanceolata. Bioorg Chem. 2019;93:103321. doi: 10.1016/j.bioorg.2019.103321. [DOI] [PubMed] [Google Scholar]
- 178.Al-Abd NM, Nor ZM, Junaid QO, Mansor M, Hasan MS, Kassim M. Antifilarial activity of caffeic acid phenethyl ester on Brugia pahangi in vitro and in vivo. Pathog Glob Health. 2017;111:388–394. doi: 10.1080/20477724.2017.1380946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lakshmi V, Joseph SK, Srivastava S, Verma SK, Sahoo MK, Dube V, et al. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop. 2010;116:127–133. doi: 10.1016/j.actatropica.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 180.Allard P-M, Dau ETH, Eydoux C, Guillemot J-C, Dumontet V, Poullain C, et al. Alkylated flavanones from the bark of Cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J Nat Prod. 2011;74:2446–2453. doi: 10.1021/np200715v. [DOI] [PubMed] [Google Scholar]
- 181.Zandi K, Teoh B-T, Sam S-S, Wong P-F, Mustafa MR, AbuBakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Low ZX, OuYong BM, Hassandarvish P, Poh CL, Ramanathan B. Antiviral activity of silymarin and baicalein against dengue virus. Sci Rep. 2021;11:21221. doi: 10.1038/s41598-021-98949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Coulerie P, Nour M, Maciuk A, Eydoux C, Guillemot J-C, Lebouvier N, et al. Structure-activity relationship study of biflavonoids on the Dengue virus polymerase DENV-NS5 RdRp. Planta Med. 2013;79:1313–1318. doi: 10.1055/s-0033-1350672. [DOI] [PubMed] [Google Scholar]
- 184.Gómez-Calderón C, Mesa-Castro C, Robledo S, Gómez S, Bolivar-Avila S, Diaz-Castillo F, et al. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on Dengue and Chikungunya virus infections. BMC Complement Altern Med. 2017;17:57. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kanyaboon P, Saelee T, Suroengrit A, Hengphasatporn K, Rungrotmongkol T, Chavasiri W, et al. Cardol triene inhibits dengue infectivity by targeting kl loops and preventing envelope fusion. Sci Rep. 2018;8:16643. doi: 10.1038/s41598-018-35035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saleem HN, Batool F, Mansoor HJ, Shahzad-ul-Hussan S, Saeed M. Inhibition of dengue virus protease by eugeniin, isobiflorin, and biflorin isolated from the flower buds of Syzygium aromaticum (Cloves) ACS Omega. 2019;4:1525–1533. doi: 10.1021/acsomega.8b02861. [DOI] [Google Scholar]
- 187.Weber C, Sliva K, von Rhein C, Kümmerer BM, Schnierle BS. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res. 2015;113:1–3. doi: 10.1016/j.antiviral.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 188.Lu J-W, Hsieh P-S, Lin C-C, Hu M-K, Huang S-M, Wang Y-M, et al. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochem Biophys Res Commun. 2017;491:595–602. doi: 10.1016/j.bbrc.2017.07.157. [DOI] [PubMed] [Google Scholar]
- 189.Henss L, Scholz T, Grünweller A, Schnierle BS. Silvestrol inhibits chikungunya virus replication. Viruses. 2018;10:592. doi: 10.3390/v10110592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Basic M, Elgner F, Bender D, Sabino C, Herrlein M-L, Roth H, et al. A synthetic derivative of houttuynoid B prevents cell entry of Zika virus. Antiviral Res. 2019;172:104644. doi: 10.1016/j.antiviral.2019.104644. [DOI] [PubMed] [Google Scholar]
- 191.Chu JJH, Leong PWH, Ng ML. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology. 2006;349:463–475. doi: 10.1016/j.virol.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 192.Soré H, Lopatriello A, Ebstie YA, TenohGuedoung AR, Hilou A, Pereira JA, et al. Plasmodium stage-selective antimalarials from Lophira lanceolata stem bark. Phytochemistry. 2020;174:112336. doi: 10.1016/j.phytochem.2020.112336. [DOI] [PubMed] [Google Scholar]
- 193.Arango E, Londoño B, Segura C, Solarte Y, Herrera S, Saez J, et al. Prevention of sporogony of Plasmodium vivax in Anopheles albimanus by steroids of Solanum nudum Dunal (Solanaceae) Phyther Res. 2006;20:444–447. doi: 10.1002/ptr.1874. [DOI] [PubMed] [Google Scholar]
- 194.Ferreira TN, Pita-Pereira D, Costa SG, Brazil RP, Moraes CS, Díaz-Albiter HM, et al. Transmission blocking sugar baits for the control of Leishmania development inside sand flies using environmentally friendly beta-glycosides and their aglycones. Parasit Vectors. 2018;11:614. doi: 10.1186/s13071-018-3122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dhananjeyan MR, Milev YP, Kron MA, Nair MG. Synthesis and activity of substituted anthraquinones against a human filarial parasite, Brugia malayi. J Med Chem. 2005;48:2822–2830. doi: 10.1021/jm0492655. [DOI] [PubMed] [Google Scholar]
- 196.Yadav D, Singh SC, Verma RK, Saxena K, Verma R, Murthy PK, et al. Antifilarial diarylheptanoids from Alnus nepalensis leaves growing in high altitude areas of Uttarakhand. India Phytomedicine. 2013;20:124–132. doi: 10.1016/j.phymed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 197.Bourjot M, Leyssen P, Eydoux C, Guillemot J-C, Canard B, Rasoanaivo P, et al. Chemical constituents of Anacolosa pervilleana and their antiviral activities. Fitoterapia. 2012;83:1076–1080. doi: 10.1016/j.fitote.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 198.da Costa ECB, Amorim R, da Silva FC, Rocha DR, Papa MP, de Arruda LB, et al. Synthetic 1,4-Pyran naphthoquinones are potent inhibitors of dengue virus replication. PLoS ONE. 2013;8:e82504. doi: 10.1371/journal.pone.0082504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cheung YY, Chen KC, Chen H, Seng EK, Chu JJH. Antiviral activity of lanatoside C against dengue virus infection. Antiviral Res. 2014;111:93–99. doi: 10.1016/j.antiviral.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 200.Guo J, Jia X, Liu Y, Wang S, Cao J, Zhang B, et al. Inhibition of Na+/K+ ATPase blocks Zika virus infection in mice. Commun Biol. 2020;3:380. doi: 10.1038/s42003-020-1109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zanello PR, Koishi AC, de Rezende Júnior CO, Oliveira LA, Pereira AA, de Almeida MV, et al. Quinic acid derivatives inhibit dengue virus replication in vitro. Virol J. 2015;12:223. doi: 10.1186/s12985-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Martinez-Lopez A, Persaud M, Chavez MP, Zhang H, Rong L, Liu S, et al. Glycosylated diphyllin as a broad-spectrum antiviral agent against Zika virus. EBioMedicine. 2019;47:269–283. doi: 10.1016/j.ebiom.2019.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Silva S, Shimizu JF, de Oliveira DM, de Assis LR, Bittar C, Mottin M, et al. A diarylamine derived from anthranilic acid inhibits ZIKV replication. Sci Rep. 2019;9:17703. doi: 10.1038/s41598-019-54169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rohrich CR, Ngwa CJ, Wiesner J, Schmidtberg H, Degenkolb T, Kollewe C, et al. Harmonine, a defence compound from the harlequin ladybird, inhibits mycobacterial growth and demonstrates multi-stage antimalarial activity. Biol Lett. 2012;8:308–311. doi: 10.1098/rsbl.2011.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carter V, Underhill A, Baber I, Sylla L, Baby M, Larget- I, et al. Killer bee molecules: antimicrobial peptides as effector molecules to target sporogonic stages of Plasmodium. PLoS Pathog. 2013;9:e1003790. doi: 10.1371/journal.ppat.1003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 207.Moreira CK, Rodrigues FG, Ghosh A, de Varotti FP, Miranda A, Daffre S, et al. Effect of the antimicrobial peptide gomesin against different life stages of Plasmodium spp. Exp Parasitol. 2007;116:346–353. doi: 10.1016/j.exppara.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.O’Neal AJ, Butler LR, Rolandelli A, Gilk SD, Pedra JHF. Lipid hijacking: a unifying theme in vector-borne diseases. Elife. 2020;9:e61675. doi: 10.7554/eLife.61675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Werling K, Shaw WR, Itoe MA, Westervelt KA, Marcenac P, Paton DG, et al. Steroid hormone function controls non-competitive Plasmodium development in Anopheles. Cell. 2019;177:315–325.e14. doi: 10.1016/j.cell.2019.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.van Schaijk BC, Santha KTR, Vos WVM, Adam R, van Geert-Jan G, Tao L, et al. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell. 2014;13:550–559. doi: 10.1128/EC.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reynolds RA, Kwon H, Alves E Silva TL, Olivas J, Vega-Rodriguez J, Smith RC. The 20-hydroxyecdysone agonist, halofenozide, promotes anti-Plasmodium immunity in Anopheles gambiae via the ecdysone receptor. Sci Rep. 2020;10:21084. doi: 10.1038/s41598-020-78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reynolds RA, Hyeogsun K, Smith CR, Photini S. 20-Hydroxyecdysone primes innate immune responses that limit bacterial and malarial parasite survival in Anopheles gambiae. mSphere. 2021;5:e00983–e1019. doi: 10.1128/mSphere.00983-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Childs LM, Cai FY, Kakani EG, Mitchell SN, Paton D, Gabrieli P, et al. Disrupting mosquito reproduction and parasite development for malaria Control. PLoS Pathog. 2016;12:e1006060. doi: 10.1371/journal.ppat.1006060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Olds CL, Glennon EKK, Luckhart S. Abscisic acid: new perspectives on an ancient universal stress signaling molecule. Microbes Infect. 2018;20:484–492. doi: 10.1016/j.micinf.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 215.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Glennon EKK, Torrevillas BK, Morrissey SF, Ejercito JM, Luckhart S. Abscisic acid induces a transient shift in signaling that enhances NF-κB-mediated parasite killing in the midgut of Anopheles stephensi without reducing lifespan or fecundity. Parasit Vectors. 2017;10:333. doi: 10.1186/s13071-017-2276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Glennon EKK, Adams LG, Hicks DR, Dehesh K, Luckhart S. Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. Am J Trop Med Hyg. 2016;94:1266. doi: 10.4269/ajtmh.15-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Glennon EKK, Megawati D, Torrevillas BK, Ssewanyana I, Huang L, Aweeka F, et al. Elevated plasma abscisic acid is associated with asymptomatic falciparum malaria and with IgG-/caspase-1-dependent immunity in Plasmodium yoelii-infected mice. Sci Rep. 2018;8:8896. doi: 10.1038/s41598-018-27073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Staveness D, Abdelnabi R, Near KE, Nakagawa Y, Neyts J, Delang L, et al. Inhibition of Chikungunya Virus-induced cell death by salicylate-derived bryostatin analogues provides additional evidence for a PKC-independent pathway. J Nat Prod. 2016;79:680–684. doi: 10.1021/acs.jnatprod.5b01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Paton DG, Childs LM, Itoe MA, Holmdahl IE, Buckee CO, Catteruccia F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature. 2019;567:239–243. doi: 10.1038/s41586-019-0973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dreyer SM, Leiva D, Magaña M, Pott M, Kay J, Cruz A, et al. Fipronil and ivermectin treatment of cattle reduced the survival and ovarian development of field-collected Anopheles albimanus in a pilot trial conducted in northern Belize. Malar J. 2019;18:296. doi: 10.1186/s12936-019-2932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Makhanthisa TI, Braack L, Lutermann H. The effect of cattle-administered ivermectin and fipronil on the mortality and fecundity of Anopheles arabiensis Patton. Parasit Vectors. 2021;14:349. doi: 10.1186/s13071-021-04846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Müller G, Junnila A, Qualls W, Revay EE, Kline DL, Allan S, et al. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med Vet Entomol. 2010;24:346–351. doi: 10.1111/j.1365-2915.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- 224.Junnila A, Revay EE, Müller GC, Kravchenko V, Qualls WA, Xue R, et al. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015;152:195–200. doi: 10.1016/j.actatropica.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Revay EE, Schlein Y, Tsabari O, Kravchenko V, Qualls W, De-Xue R, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015;150:29–34. doi: 10.1016/j.actatropica.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali. West Africa Malar J. 2020;19:72. doi: 10.1186/s12936-020-3132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tenywa FC, Kambagha A, Saddler A, Maia MF. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar J. 2017;16:338. doi: 10.1186/s12936-017-1994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Andriessen R, Snetselaar J, Suer RA, Osinga AJ, Deschietere J, Lyimo IN. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc Natl Acad Sci USA. 2015;112:12081. doi: 10.1073/pnas.1510801112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mathias DK, Pastrana-mena R, Ranucci E, Tao D, Ferruti P, Ortega C, et al. A small molecule glycosaminoglycan mimetic blocks Plasmodium invasion of the mosquito midgut. PLoS Pathog. 2013;9:e1003757. doi: 10.1371/journal.ppat.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lantero E, Fernandes J, Aláez-Versón CR, Gomes J, Silveira H, Nogueira F, et al. Heparin administered to Anopheles in membrane feeding assays blocks Plasmodium development in the mosquito. Biomolecules. 2020;10:1136. doi: 10.3390/biom10081136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mongkol W, Arunyawat U, Surat W, Kubera A. Active compounds against Anopheles minimus carboxypeptidase B for malaria transmission-blocking strategy. J Med Entomol. 2015;52:1322–1332. doi: 10.1093/jme/tjv133. [DOI] [PubMed] [Google Scholar]
- 232.Urbán P, Ranucci E, Fernàndez-busquets X. Polyamidoamine nanoparticles as nanocarriers for the drug delivery to malaria parasite stages in the mosquito vector. Nanomedicine. 2015;10:3401–3414. doi: 10.2217/nnm.15.174. [DOI] [PubMed] [Google Scholar]
- 233.Cubillos-Ruiz A, Guo T, Sokolovska A, Miller PF, Collins JJ, Lu TK, et al. Engineering living therapeutics with synthetic biology. Nat Rev Drug Discov. 2021;20:941–960. doi: 10.1038/s41573-021-00285-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Summarized details of the highlighted compounds 1 - 151: their chemical names, class, pathogens tested, and described mode of action.
Data Availability Statement
All datasets generated or analysed during this study are included in this published article.