Abstract
Tree and shrub barks have been used as folk medicine by numerous cultures across the globe for millennia, for a variety of indications, including as vasorelaxants and antispasmodics. Here, using electrophysiology and myography we discovered that the KCNQ5 voltage-gated potassium channel mediates vascular smooth muscle relaxant effects of barks used in Native American folk medicine. Bark extracts (1%) from Birch, Cramp Bark, Slippery Elm, White Oak, Red Willow, White Willow and Wild Cherry each strongly activated KCNQ5 expressed in Xenopus oocytes. Testing of a subset including both the most and the least efficacious extracts revealed that Red Willow, White Willow and White Oak KCNQ-dependently relaxed rat mesenteric arteries; in contrast, Black Haw bark neither activated KCNQ5 nor induced vasorelaxation. Two compounds common to the active barks (gallic acid and tannic acid) had similarly potent and efficacious effects on both KCNQ5 activation and vascular relaxation, and this together with KCNQ5 modulation by other tannins provides a molecular basis for smooth muscle relaxation effects of Native American folk medicine bark extracts.
Keywords: bark, hypotensive, KCNQ, Kv7, tannin, vasorelaxant
Introduction
Plants have been used as medicines by humans for many thousands of years, probably extending to early hominids before Homo sapiens evolved (1–3). While the roots and aerial parts of true herbs form a substantial proportion of botanical folk medicines, many other parts and classes of plants play a significant role, including the barks of trees. Typical indications for tree barks include pain, cramps, fever, spasms, coughs, hypertension and congestive heart failure (4, 5). Preclinical and clinical studies support the therapeutic efficacy of many medicinal barks, but their mechanisms of action are in many cases incompletely understood (6). Barks have been used by indigenous peoples across the globe as an integral part of most folk medicine cultures, and they are a rich source of a plethora of medically important compounds. Perhaps the best-known is salicin, from willow bark, which led to the discovery in 1853 of the structurally related compound acetylsalicylic acid (aspirin), to this day one of the most important analgesics and antipyretics (7, 8).
We recently discovered that voltage-gated potassium (Kv) channels within the KCNQ (Kv7) subfamily (which in humans comprises 5 isoforms, KCNQ1-5) are common targets for secondary metabolites and other compounds found in medicinal plants utilized for a wide arrays of disorders ranging from seizures to hypertension to infectious diseases, by indigenous populations in Africa, Asia, Europe, the Americas and the Caribbean (9–12). KCNQ channels form as homomeric or heteromeric tetramers of pore-forming α subunits and are widely expressed and highly influential in tissues including the heart, vasculature, lungs, brain, gastrointestinal tract, and endocrine system (13, 14).
Relaxation of vascular smooth muscle via activation of KCNQ4 and KCNQ5 channels can result in a vasodilation. As such, KCNQ4 and KCNQ5 are considered potential targets for antihypertensive drugs (15–19). Previously, we found that KCNQ5 is an important target for antihypertensive botanical medicines, including chamomile, lavender, ginger, Ku Shen, fennel seed and green and black tea (11, 20). Barks from many different tree species are used in culturally and geographically distinct folk medicine systems across the globe to treat cardiac and vascular conditions, suggesting the possibility of common molecular mechanisms among different species (5, 21). Therefore, in this study, we tested the hypothesis that barks from a range of tree species used in Native American folk medicine practices and their constituents activate KCNQ channels and that this underlies specific therapeutic properties. Confirming this hypothesis, we identify KCNQ5-activating barks and their active components and demonstrate that bark extracts KCNQ-dependently relax arteries, providing a molecular rationale for their use in folk medicine as vasorelaxants and antispasmodics.
Materials and Methods
Preparation of bark extracts
Certified organic tree bark powders were purchased from Mountain Rose Herbs (Eugene, OR, USA). We resuspended the bark extracts in 80% methanol/20% water (100 ml per 5 g bark powder) and then incubated for 48 hours at room temperature, occasionally inverting the bottles to resuspend the extracts. We next filtered the bark extracts through Whatman filter paper #1 (Whatman, Maidstone, UK), and then removed the methanol using evaporation in a fume hood for 24–48 hours at room temperature. We then centrifuged the extracts for 10 minutes at 15 °C, 4000 RCF to remove the remaining particulate matter, followed by storage at −20 °C. On the day of electrophysiological recording, we thawed the extracts and diluted them 1:100 in bath solution (see below) immediately before use.
Chemical analysis of bark extracts
Chemicals:
(+)-Catechin hydrate, ellagic acid, gallic acid, propionyl chloride, tannic acid and vanillin were sourced from Sigma-Aldrich (St. Louis, MO, USA). Quercetin was obtained from Synaptent LLC (Chicago, IL, USA). Conc. hydrochloric acid (35–38% in water) was from Thermo Fisher Scientific (Waltham, MA, USA). Methanol (MeOH), ethyl acetate (EtOAc) and acetonitrile (ACN) were HPLC or LC/MS grade from Fisher or VWR International (Radnor, PA, USA). Water was 18.2 mΩ-cm from a Barnstead NANOpure Diamond™ system (Barnstead, NH, USA). Trifluoroacetic acid was obtained from MilliporeSigma (Burlington, MA, USA). Methyl gallate was synthesized from gallic acid as previously described (22). A solution of gallic acid in MeOH containing sulfuric acid was refluxed overnight. Once at room temperature, the reaction was added to ice-water and extracted with EtOAc (3 × 25 mL). The pooled organic layers were washed twice with water, once with brine and concentrated in vacuo affording the methyl ester as a light-yellow solid. MS/MS with negative ionization mode gave m/z 183 (M-H+ with daughter ions 168 and 124). Thin layer chromatography (TLC) employed Analtech GHLF UV254 Uniplate™ silica gel plates from Miles Scientific (Newark, DE, USA).
Chromatography:
Preparative HPLC separations were carried out using a Shimadzu (Kyoto, Japan) system consisting of two LC-8A pumps, a fraction collector (FRC-10A), a SIL-10AP auto sampler, a diode array detector (CPD-M20A) and a CBM-20A communication module. The separations employed a Waters (Milford, MA, USA) PREP Nova-Pak® HR C18 6 μM 60Å 40 × 100 mm reversed phase column with a 40 × 10 mm Guard-Pak insert and a Waters PrepLC Universal Base. The solvent systems employed were MeOH/water gradients both containing 0.1 % TFA or ACN/water gradients also with added 0.1 % TFA. Fractions were collected based on their response at 254 nm.
Mass Spectrometry:
Mass spectrometry employed a Thermo Fisher Scientific TSQ Quantum Ultra triple stage quadrupole mass spectrometer. Heated-electrospray ionization (H-ESI) was used in negative or positive ionization mode depending on the structure of the analyte. Automatic methods for the optimization of instrument parameters were used to maximize sensitivity except for capillary temperature which was kept at 275 °C. Samples were analyzed by direct injection in MeOH or MeOH/water (TFA conc kept at 0.01% or less) using a syringe pump. Aqueous bark extracts were diluted 100-fold with MeOH before analysis. Gallic acid was identified from m/z 169 (M-H+) in negative ionization mode. Ellagic acid showed m/z 301 (M-H+) in negative ionization mode and its presence was confirmed by daughter ion analysis (m/z 284, 245, 229, 201 and 173) to distinguish it from quercetin, also m/z 301 (M-H+), daughter ions 179 and 151.
Transesterification Analysis for Hydrolysable Tannins:
Methanolysis was initially accomplished by treating samples with a 10% v/v sulfuric acid solution in MeOH as described by Harztfeld et al. (23) Aqueous bark extracts (1 mL) were concentrated in vacuo with a Büchi (Flawil, Switzerland) Rotavapor R-205 (water bath temp 40°C) connected to a DryFast Ultra® pump, model 2031B-01, from Welch Rietschle Thomas (Fürstenfeldbruck Germany). The residues obtained were dissolved in the H2SO4/MeOH solution (1–2 mL) and heated at 85°C in a sealed tube under N2 overnight. Once at room temperature, the reactions were added to ice-water/EtOAc. The organic layer was separated and washed twice with water and concentrated to dryness. This method was replaced with the operationally simpler method of Newsome et al. (24) except that acetyl chloride was replaced with an equivalent molar amount of propionyl chloride. Bark extracts were concentrated to dryness as above and the residues were treated with 1–2 mL of the 2.75 M methanol-HCl solution under N2. The resulting solutions were heated at 85°C for 6 h. Once at room temperature, the solvent was removed in vacuo and the residues were reconstituted in MeOH and injected onto the preparative HPLC system. The methanolysis method was tested with tannic acid. Heating as above gave methyl gallate that was identified by HPLC retention time (RT), TLC Rf and mass spectrum (Supplementary Figure 1).
Vanillin Assay for Condensed Tannins.
The concentration of condensed tannins in the bark samples was determined using the vanillin hydrochloric acid assay as described by Makkar et al. (25). Aqueous bark extracts were diluted with an equal volume of MeOH. In the case of the aqueous slippery elm bark extract, addition of an equal volume of MeOH gave a precipitate and 1:1 MeOH/water was added to give a clear solution. 100 μL of the resulting solutions (in triplicate) were diluted with 0.6 mL of a 4% w/v solution of vanillin in MeOH and 0.3 mL of conc hydrochloric acid. The samples were vortexed briefly and then allowed to stand in the dark for 20 min. The absorbance at 490 nm was measured in a 96-well plate using a KC Junior plate reader (Bio-Tek Instruments, Vermont, USA). (+)-Catechin hydrate was used to generate the standard curve (114 to 1153 μg/mL, n = 5) (Figure 1). The standard curve was fit to a linear regression using GraphPad Prizm: Absorbance at 490nm = 0.001713[conc. catechin] + 0.1405, r2 = 0.9778. The condensed tannin content of the barks is expressed as (+)-catechin equivalents (μg/mL and μg/mg). The absorbance of a blank with no (+)-catechin added was subtracted from the response of the bark samples and the standard curve. The cramp bark and the white willow bark extracts in 1:1 MeOH/water were diluted 10-fold and 5-fold with 1:1 MeOH/water, respectively, to put their absorbances on the standard curve.
Figure 1. Standard Curve for (+)-Catechin in the Vanillin Assay.
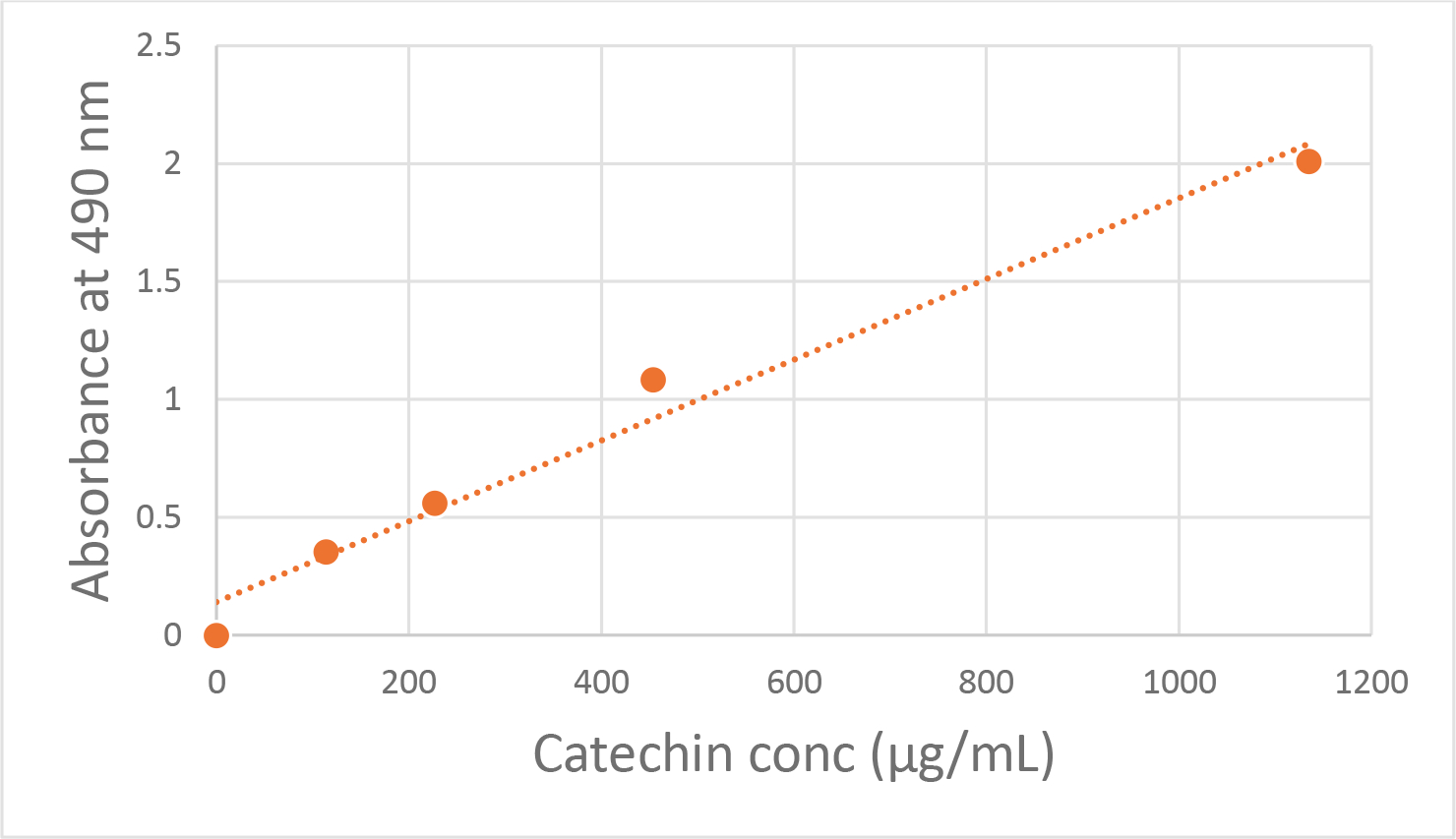
Standard curve generated from (+)-Catechin hydrate (114 to 1153 μg/mL, n = 5) for the purpose of quantifying condensed tannins in the bark samples using the vanillin hydrochloric acid assay (see Methods). The absorbance at 490 nm was measured in a 96-well plate using a KC Junior plate reader (Bio-Tek Instruments, Vermont, USA). The standard curve was fit to a linear regression using GraphPad Prizm: Absorbance at 490nm = 0.001713[conc. catechin] + 0.1405, r2 = 0.9778.
Channel subunit cRNA preparation and Xenopus laevis oocyte injection
As previously described (9), we generated cRNA transcripts encoding human KCNQ4 and KCNQ5 by in vitro transcription using the mMessage mMachine kit (Thermo Fisher Scientific), after vector linearization, from cDNA sub-cloned into plasmids incorporating Xenopus laevis β-globin 5’ and 3’ UTRs flanking the coding region to enhance translation and cRNA stability. We injected defolliculated stage V and VI Xenopus laevis oocytes (Xenoocyte, Dexter, MI, US) with KCNQ cRNAs (5–10 ng). We incubated the oocytes at 16 °C in ND96 oocyte storage solution containing penicillin and streptomycin, with daily washing, for 3–4 days prior to two-electrode voltage-clamp (TEVC) recording.
Two-electrode voltage clamp (TEVC)
We performed TEVC at room temperature using an OC-725C amplifier (Warner Instruments, Hamden, CT) and pClamp10 software (Molecular Devices, Sunnyvale, CA) 2–4 days after cRNA injection as described in the section above. For recording, oocytes we placed in a small-volume oocyte bath (Warner) and viewed with a dissection microscope. We sourced chemicals from Sigma. We studied effects of bark extracts and of compounds previously identified in barks, solubilized directly in bath solution (in mM): 96 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES (pH 7.6). We introduced extracts or compounds into the oocyte recording bath by gravity perfusion at a constant flow of 1 ml per minute for 3 minutes prior to recording. Pipettes were of 1–2 MΩ resistance when filled with 3 M KCl. We recorded currents in response to voltage pulses between −80 mV and +40 mV at 20 mV intervals from a holding potential of −80 mV, to yield current-voltage relationships and examine activation kinetics. We analyzed data using Clampfit (Molecular Devices) and Graphpad Prism software (GraphPad, San Diego, CA, USA), stating values as mean ± SEM. We plotted raw or normalized tail currents versus prepulse voltage and fitted with a single Boltzmann function:
| Eq. 1 |
where g is the normalized tail conductance, A1 is the initial value at −∞, A2 is the final value at +∞, V1/2 is the half-maximal voltage of activation and Vs the slope factor.
Mesenteric artery myography
In accordance with the methods of killing animals described in annex IV of the EU Directive 2010/63EU on the protection of animals used for scientific purposes, male Wistar rats, 12 weeks old (Janvier Labs, Le Genest-Saint-Isle, France), were made unconscious by a single, percussive blow to the head. Immediately after the onset of unconsciousness, cervical dislocation was performed. Rats were group-housed with regular 12-hour light/dark cycles, in clear plastic containers with ad libitum access to food and water and underwent at least one week of habituation. After euthanasia, the intestines were removed, and third-order mesenteric arteries were dissected in ice-cold physiological saline solution containing (in mM): 121 NaCl, 2.8 KCl, 1.6 CaCl2, 25 NaHCO3, 1.2 KH2HPO4, 1.2 MgSO4, 0.03 EDTA, and 5.5 glucose. Segments, 2 mm in length, of mesenteric artery were mounted on 40 μm stainless steel wires in a myograph (Danish Myo Technology, Aarhus, Denmark) for isometric tension recordings. The chambers of the myograph contained PSS maintained at 37°C and aerated with 95% O2/5% CO2. Changes in tension were recorded by PowerLab and Chart software (ADInstruments, Oxford, United Kingdom). The arteries were equilibrated for 30 minutes and normalized to passive force. Artery segments were precontracted with 10 μM methoxamine (Sigma; Copenhagen, Denmark) in the absence or presence of linopirdine (10 μM) (Sigma; Copenhagen, Denmark), before application of bark extracts at various dilutions as described in the text (Mountain Rose Herbs).
Statistics and Reproducibility
All values are expressed as mean ± SEM. One-way ANOVA was applied for all tests; all p values were two-sided.
Results
Medicinal barks are highly effective KCNQ5 openers
We first screened 1:100 dilutions of bark extracts from 8 species (Birch, White Willow, Red Willow, White Oak, Wild Cherry, Cramp Bark, Black Haw, and Slippery Elm) by applying them to Xenopus laevis oocytes expressing KCNQ5 and recording the effects using two-electrode voltage-clamp (TEVC) electrophysiology. We compared the bark extract effects to those of 10 μM retigabine, an anticonvulsant that activates KCNQ2-5 (Figure 2). Retigabine increased current magnitude relatively voltage-independently, without altering the voltage dependence of activation (Figure 2A–C). Two of the barks – Birch (Betula lenta) and Cramp Bark (Viburnum opulus) negative-shifted the voltage dependence of activation, leading to constitutive activation at −120 mV, without altering maximal current magnitude at depolarized potentials. One bark (Wild Cherry; Prunus serotina) negative-shifted the voltage dependence of activation, leading to constitutive activation at −120 mV, but reduced maximal current at membrane potentials positive to −60 mV (by up to 50%). Only one bark, (Black Haw; Viburnum prunifolium) failed to activate KCNQ5 (Figure 2A–C). Maximal increases in current for this group of barks reached tenfold, for Cramp Bark and Wild Cherry Bark at hyperpolarized potentials (Figure 2C). Retigabine, Birch Bark and Cramp Bark hyperpolarized the resting membrane potential (EM) of oocytes expressing KCNQ5, while Wild Cherry and Black Haw Bark had no effect on EM (Figure 2D).
Figure 2. A subset of 1% medicinal bark extracts each negative-shift KCNQ5 voltage-dependence of activation.

Error bars indicate SEM. n indicates number of oocytes. Statistical comparisons by one-way ANOVA. BHB, Black Haw Bark; BB, Birch Bark; CB, cramp bark; WCB, Wild Cherry Bark.
A. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence of retigabine (10 μM) or the bark extracts indicated. Dashed lines indicate zero current line here and throughout. Scale bars lower left for each trace; voltage protocol upper inset used here and throughout; n = 5 per group.
B. Mean tail current (left) and normalized tail current (G/Gmax) (right) for traces as in A; n = 5 per group.
C. Mean current fold increase versus membrane potential for traces as in A; n = 5 per group.
D. Mean unclamped oocyte membrane potential for KCNQ5-expressing oocytes as in A; n = 5 per group.
Strikingly, the remaining four medicinal barks tested - White Willow (Salix alba), Red Willow (Salix laevigata), White Oak (Quercus alba) and Slippery Elm (Ulmus rubra) conferred constitutive activation at −120 mV and increased current magnitude across the voltage range tested (Figure 3A, B). Fold-increases in current were greatest at hyperpolarized potentials (Figure 3C). Red Willow bark, which induced 50% of KCNQ5 maximal activity at −120 mV, was the most efficacious with respect to augmenting KCNQ5 across the voltage range and especially at the most negative membrane potentials (Figure 3C). Maximal current fold-increases were 50–75-fold compared to baseline at select membrane potentials for Red Willow Bark and White Willow Bark (Figure 3C). Accordingly, all four barks in this group hyperpolarized the resting membrane potential of oocytes expressing KCNQ5 (Figure 3D). Results for the bark effects are summarized in Supplementary Data.
Figure 3. A subset of 1% medicinal bark extracts each lock open KCNQ5 at −120 mV and augment current across the voltage range.

Error bars indicate SEM. n indicates number of oocytes. Statistical comparisons by one-way ANOVA. RWB, Red Willow Bark; SEB, Slippery Elm Bark; WHB, White Willow Bark; WOB, White Oak Bark.
A. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence the bark extracts indicated; n = 5–6 per group.
B. Mean tail current (left) and normalized tail current (G/Gmax) (right) for traces as in A; n = 5–6 per group.
C. Mean current fold increase versus membrane potential for traces as in A; n = 5–6 per group.
D. Mean unclamped oocyte membrane potential for KCNQ5-expressing oocytes as in A; n = 5–6 per group.
Medicinal bark extracts are highly effective vasorelaxants
We next studied the effects of three of the most KCNQ5-active bark extracts (White Oak, Red Willow, White Willow) and the KCNQ5-inactive Black Haw bark (0.1–2.5% solutions) on ex vivo segments of rat mesenteric arteries preconstricted with methoxamine. All bark extracts tested, except Black Haw, caused relaxations of the arterial segments (Figure 4A, B) which were attenuated by the relatively specific KCNQ channel inhibitor, linopirdine (Figure 4B, C). The EC50 for the active bark extracts (i.e., all those except Black Haw) in the absence of linopirdine was <0.5% (Figure 4D), while both the efficacy and the potency of the active bark extracts were reduced by linopirdine (Figure 4C, E). Thus, similar to the KCNQ5 effects observed in oocytes, the myography data demonstrated that the barks tested, with the exception of Black Haw, are KCNQ-dependent vasodilators.
Figure 4. Vasorelaxant effects of different bark extracts and their dependence on KCNQ channel activation.

A. Representative traces of the concentration-dependent vasorelaxations produced in segments of rat mesenteric artery by bark extracts from red willow, white willow, white oak and black haw.
B. Mean concentration-effect curves for the different bark extracts in the absence (black) and presence (red) of the KCNQ channel inhibitor, linopirdine (n = 5).
C. Mean EC50 values for the different bark extracts in the absence (black) and presence (red) of the KCNQ channel inhibitor, linopirdine (n = 5). P values, from an unpaired Students t test, are denoted.
D and E. Comparison of the EC50 values for each bark extract under control conditions or in the presence of linopirdine.
Detection of hydrolysable tannins in medicinal barks
Mass spectrometry analysis of the bark extracts after dilution 100-fold with methanol was used to initially detect hydrolysable tannins, based on the appearance of gallic acid (m/z 169 M-H+) and ellagic acid (m/z 301 M-H+). Ellagic acid was distinguished from isomeric quercetin by daughter ion analysis. Red willow and white oak bark extracts showed both gallic acid and ellagic acids, suggesting that the samples contain ellagitannins (Table 1). None of the other extracts were found to contain gallic acid at baseline. It was generally difficult to identify single components in the extracts. In the case of black haw extract the most intense ion observed was m/z 353 with daughter ion 191. Loss of 160 indicated that black haw bark extract likely contains a hexose of scopoletin or quinic acid (MW 192). Daughter ion analysis indicated that the ion with m/z 191 is scopoletin. This would be consistent with presence of the glucoside scopolin (MW 354) and is supported by prior identification of the coumarin scopoletin (26) in black haw extract. We also detected scopoletin in wild cherry bark extract.
Table 1.
Mass spectral analysis of bark samples for the presence of hydrolysable tannins.
| Bark Extract | Gallic Acid (m/z 169) | Methyl gallate (m/z 183) | Ellagic Acid (m/z 301) |
|---|---|---|---|
| Birch | No | Yes | No |
| Black Haw | No | No | No |
| Cramp | No | No | No |
| Red Willow | Yes | NT | Yes |
| Slippery Elm | No | Yes | No |
| White Oak | Yes | NT | Yes |
| White Willow | No | Yes | No |
| Wild Cherry | No | No | No |
NT = Not tested. Methyl gallate formation after samples treated with methanol/sulfuric acid or methanol/propionyl chloride. Compounds were analyzed in negative ionization mode and all show m/z MW – H+
The bark extracts that did not contain detectable, monomeric gallic acid at baseline were subjected to transesterification with acidic methanol (methanol/propionyl chloride or 10% sulfuric acid/methanol), a process in which the formation of methyl gallate (m/z 183 M-H+) can be used as evidence for the presence of larger, gallic acid-containing hydrolysable tannins, as previously described (23, 24). For reference, commercially available tannic acid (Sigma) was heated with acidic methanol (methanol/propionyl chloride) and concentrated in vacuo. The residue was subjected to reversed phase HPLC (ACN/water containing 0.1% TFA) and showed one major peak (retention time 2.3 min) that co-eluted with commercially sourced methyl gallate (Sigma). Thin-layer chromatography (100% EtOAc) gave one major UV-active spot with an Rf = 0.7 identical to methyl gallate. We identified methyl gallate by mass spectrometry in birch bark, slippery elm and white willow extracts after transesterification, indicating that these barks contain hydrolysable tannins (likely gallotannins). We did not detect methyl gallate in black haw bark, cramp bark and wild cherry bark extracts (Table 1).
Detection of condensed tannins in medicinal barks
We next used the vanillin assay (see Methods section and Figure 1) to determine condensed tannin content of the bark extracts, expressed as (+)-catechin equivalents. The results are provided as both μg/mL and μg/mg (Table 2). All the barks contained condensed tannins except for the very low concentration found in black haw bark (<1 μg/mg). The highest levels were found in white willow bark (126 μg/mg) followed by slippery elm bark (65 μg/mg). The other extracts showed condensed tannins between 30–42 μg/mg (cramp bark, wild cherry, birch bark and white oak), while red willow bark showed 15 μg/mg.
Table 2.
(+)-Catechin Equivalents in Bark Extracts.
| Bark Extract | Catechin equivalents (μg/mL) | Catechin equivalents (μg/mg) |
|---|---|---|
| Birch | 514±31 | 40±2 |
| Black Haw | 20±2 | 0.8±0.07 |
| Cramp | 3850±660 | 30±5 |
| Red Willow | 535±66 | 15±2 |
| Slippery Elm | 846±17 | 65±1 |
| White Oak | 500±46 | 42±4 |
| White Willow | 3950±130 | 126±4 |
| Wild Cherry | 1100±175 | 35±6 |
The concentrations of condensed tannins were determined using the vanillin assay. Values are given with 95% confidence intervals (n = 3).
Bark phenolic acids constitutively activate KCNQ5
Our data show that the KCNQ5-activating bark extracts we tested contain 15–126 μg/mg condensed tannins, while the KCNQ5-inactive bark extract (black haw) contained only 0.8 μg/mg condensed tannins (Table 2). These data are consistent with our previous finding that condensed tannins contribute to the KCNQ5-activating effects of green and black tea (20).
Our chemical analyses herein (Table 1) also indicated the presence in the medicinal barks tested of hydrolysable gallotannins such as tannic acid, and related compounds gallic acid and ellagic acid. We therefore tested the ability of the above to modulate KCNQ5 activity, and also tested gentisic acid and salicyclic acid (both phenolic acids also found in tree bark), the alcoholic β-glucoside salicin (present in willow bark (7)) and the structurally related synthetic drug acetylsalicyclic acid (aspirin) (Figure 5). At 100 μM (10 μM for ellagic acid due to poor aqueous solubility), neither acetylsalicyclic acid, salicyclic acid, ellagic acid, nor gentisic acid altered KCNQ5 maximal current, voltage dependence of activation, nor the effect of KCNQ5 on resting membrane potential when heterologously expressed in Xenopus oocytes (Figure 5A–D). In contrast, salicin negative-shifted KCNQ5 voltage dependence of activation moderately (by 7.2 ± 0.7 mV), while gallic acid and especially tannic acid each negative-shifted KCNQ5 voltage dependence of activation (by 7.7 ± 0.9 and −12.4 ± 2.5 mV, respectively) and induced constitutive activation at −80 mV (Figure 5A, B). Gallic acid and tannic acid each increased KCNQ5 current >5-fold at −80 mV (Figure 5C). Accordingly, only salicin, gallic acid and tannic acid hyperpolarized the resting membrane potential of KCNQ5-expressing oocytes (Figure 5D). In contrast to its augmenting effect at hyperpolarized potentials, tannic acid (100 μM) inhibited the tail current following prepulses at potentials positive to −50 mV (Figure 5A, B). Results are summarized in Supplementary Data.
Figure 5. Tannic acid, gallic acid and salicin each increase KCNQ5 activity at hyperpolarized potentials.

Error bars indicate SEM. n indicates number of oocytes. Statistical comparisons by one-way ANOVA.
A. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence of the compounds indicated (100 μM); n = 4–5 per group.
B. Mean tail current (left) and normalized tail current (G/Gmax) (right) for traces as in A; n = 4–5 per group.
C. Mean current fold increase versus membrane potential for traces as in A; n = 4–5 per group.
D. Mean unclamped oocyte membrane potential for KCNQ5-expressing oocytes as in A; n = 4–5 per group.
Tannic acid exerts dual effects on KCNQ5
Interestingly, while the negative shift in voltage dependence of KCNQ5 activation was less substantial at 10 μM than at 100 μM tannic acid, the opposite applied for inhibition of KCNQ5 at membrane potentials positive to −50 mV (Figure 6A, B; Figure 5A, B). Dose response studies revealed a sigmoidal relationship for the tannic acid dose-dependent shift in the half-maximal voltage dependence of KCNQ5 activation (EC50 = 6.3 ± 1.4 μM) (Figure 6C). There was also a “locking open” of KCNQ5 channels at the most hyperpolarized potentials such that the current did not return to baseline even at the most hyperpolarized potentials studied (Figure 5B, 6B). This new pedestal current is reflected as a large fold-increase especially at −80 mV and becomes prominent with 100 μM tannic acid (Figure 6D). Tannic acid also dose-dependently increased KCNQ5 activation rate (Figure 6E).
Figure 6. Tannic acid exerts dual effects on KCNQ5 activity but does not summate or synergize with gallic acid.

Error bars indicate SEM. n indicates number of oocytes. Statistical comparisons by one-way ANOVA. GA, gallic acid; TA, tannic acid.
A. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence of the compounds indicated (10 μM tannic acid, middle; 10 μM tannic acid and 10 μM gallic acid, right); n = 4–5 per group.
B. Mean tail current (left) and normalized tail current (G/Gmax) (right) for TA traces as in A; n = 5 per group.
C. Dose response showing activation V50 versus TA concentration, calculated using recordings generated as in A, fitted with a sigmoidal relationship; n = 4–5 per group.
D. Mean current fold increase versus membrane potential for traces as in A; n = 4–5 per group.
E. Mean KCNQ5 activation rate (tau, calculated from a single exponential fit) versus tannic acid concentration (n = 4–5 per group).
F. Mean tail current (left) and normalized tail current (G/Gmax) (right) for KCNQ5 in the absence (Ctrl) or presence of 10 μM TA + 10 μM GA, traces as in A; n = 5 per group.
G. Mean current fold change versus membrane potential for traces as in A; n = 5 per group.
H. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence of the compounds indicated; n = 5 per group.
I. Mean unclamped oocyte membrane potential for KCNQ5-expressing oocytes as in G; n = 5 per group.
J. Mean tail current (left) and normalized tail current (G/Gmax) (right) for traces as in G; n = 5 per group.
K. Mean current fold change versus membrane potential for traces as in G; n = 5 per group.
L. Mean traces for KCNQ5 expressed in oocytes in the absence (Control) or presence of the compounds indicated; n = 5 per group.
M. Mean unclamped oocyte membrane potential for KCNQ5-expressing oocytes as in K; n = 5 per group.
N. Mean tail current (left) and normalized tail current (G/Gmax) (right) for traces as in K; n = 5 per group.
O. Mean current fold change versus membrane potential for traces as in K; n = 5 per group.
We previously found that in some cases, multiple compounds in a given plant extract can summate or synergize to produce greater effects than each individual compound in terms of KCNQ channel activation (9, 11, 27). Here, the situation is complicated by the fact that tannic acid effects vary both qualitatively and dose-dependently in their nature, in addition to their efficacy, i.e., tannic acid inhibits at 10 μM, but not 100 μM, as well as its activating effects at hyperpolarized potentials. Addition of 10 μM gallic acid did not rescue KCNQ5 from the inhibitory effects of 10 μM tannic acid (Figure 6A, F, G), neither did addition of 100 μM gallic acid (Figure 6H–K). Further, addition of 100 μM gallic acid did not augment the effects of 100 μM tannic acid (Figure 6L–O). The data indicate that effects of tannic acid and gallic acid do not summate or synergize with respect to KCNQ5 activation. Results are summarized in Supplementary Data.
As KCNQ4 is also expressed in vascular smooth muscle, where it can form heteromers with KCNQ5 (28–31), we tested whether KCNQ4 or KCNQ4/5 channels showed sensitivity to tannic and gallic acids, and whether the two compounds synergized with respect to modulation of either channel. However, both these channel types showed lower sensitivity than homomeric KCNQ5 and also failed to show synergy for activation by tannic acid and gallic acid in combination (Supplementary Figure 2).
Bark phenolic acids are KCNQ-dependent vasorelaxants
We next studied the effects of tannic acid, gallic acid and salicin on ex vivo segments of rat mesenteric arteries, alone or together with linopirdine. Both gallic and tannic acid induced vasorelaxation, while salicin, the less efficacious KCNQ5 activator, did not (Figure 7A). The relative efficacy for vasorelaxation of the three compounds mirrored that of the KCNQ5 activation effects (Figure 5). The relaxations to tannic and gallic acid were linopirdine-sensitive, indicating the underlying mechanism was KCNQ-dependent (Figure 7A, B). Interestingly, when added in combination at a concentration that had no effect for each acid alone (60 μM), gallic and tannic acid induced almost complete vasorelaxation, regardless of the order in which they were applied, while salicin (350 μM) had minimal effect in this combination (Figure 7C, D). The vasorelaxation induced by the low-concentration tannic and gallic acid combination was linopirdine-sensitive, indicating KCNQ-dependence of the effect, which appears to be synergistic (Figure 7E), unlike the lack of synergy we observed in the cellular electrophysiology studies (Figure 6L–O; Supplementary Figure 2).
Figure 7. Tannic acid and gallic acid relax rat mesenteric artery segments in a KCNQ-dependent manner.

(A) Mean concentration-effect curves for relaxations produced in segments of preconstricted rat mesenteric artery by tannic acid, gallic acid and salicin in the absence (black) and presence (grey) of the KCNQ inhibitor, linopirdine (n=5–7).
(B) Mean EC50 values for tannic acid, gallic acid and salicin in the absence (circles) and presence (squares) of the KCNQ channel inhibitor, linopirdine (n=5–7). P values, from an unpaired Students t test, are denoted.
(C) (D) and (E) Combinations of tannic acid (60 μM), gallic acid (60 μM) and salicin (350 μM) were applied to segments of mesenteric artery in different orders (n=6). (E) shows that only tannic acid and gallic acid are required to produce a full relaxation (black), which is attenuated by linopirdine (grey) (n=5). Significance is shown according to a two-way ANOVA, followed by a Bonferroni multiple comparison posttest.
Discussion
Barks have a long history in folk medicine traditions. Evidence exists, for example, that Homo neanderthalensis medicated with willow bark (Salix sp.) to relieve the pain from tooth abscesses (2, 3). Willow bark contains salicin, which is metabolized into a range of salicylate derivatives including salicyclic acid, the principal metabolite of aspirin (acetylsalicyclic acid). A standard dose of willow bark extract containing, e.g., 240 mg of salicin, provides neither the quantity of salicyclic acid nor the COX inhibition equivalent to even a single aspirin dose (32, 33). However, the combination of salicin, polyphenols and flavonoids in willow bark produces broad-spectrum anti-inflammatory and analgesic effects. Furthermore, willow bark has the additional advantage that, unlike aspirin, it does not inhibit blood clotting and can therefore be used by those for whom aspirin is contraindicated (33). The barks that we tested, including both red and white willow, contain an overlapping spectrum of compounds and are represented in Native American folk medicine traditions (5, 6).
Of the barks that we found to hyperpolarize the voltage dependence of KCNQ5 activation without increasing maximal conductance at depolarized potentials (Figure 2), Wild Cherry (Prunus serotina) bark was used by the Cherokee (34) and Delaware (35) Native American tribes, sometimes in combination with other barks and other plant parts, to treat fever, rheumatism, dysentery, and coughs. The Iroquois used Cramp Bark (Viburnum opulus) for emesis to treat a fever and as a gastrointestinal and pulmonary aid; a ‘decoction of the branches’ was taken as a gynecological aid, and they used a decoction of the roots as ‘heart medicine’ (36). The Micmac treated swollen glands and mumps with Cramp bark (37), while the Ojibwa used it to relieve stomach cramps (38). Cramp bark is also known to produce hypotension and bradycardia, with one active component being the sesquiterpene viopudial, which also contributes to its smooth muscle antispasmodic qualities (39). The Cherokee (34) and Chippewa (40) used Birch (Betula lenta) bark as a gastrointestinal and pulmonary aid, and to treat cloudy urine. Black Haw (Viburnum prunifolium) bark, which we found to not activate KCNQ5, had a different therapeutic profile, being used by the Cherokee as a tonic and as a wash for sore tongues (34), and by the Delaware as a reproductive aid (35). There is no recorded history of significant Native American use of the above barks specifically for cardiovascular medicine, although many are listed as “tonics”, a generic term that may encompass cardiovascular ailments. It remains possible, however, that the action of these barks on other systems is mediated by KCNQ5 and/or other KCNQ isoforms. KCNQ5 is, for example, also expressed in the brain, gastrointestinal tract, peripheral nociceptive neurons, skeletal muscle, placenta and uterine smooth muscle (17, 19, 41–45), potentially rationalizing some of the uses described above. Other KCNQ isoforms are expressed in the GI tract, heart, thyroid, respiratory tract and kidneys (13, 14, 17, 18, 46), and the action of barks and their components on KCNQ1-4 merits further study for this reason.
The final category includes the most potent and/or efficacious barks, which we found to both increase maximal KCNQ5 conductance at depolarized voltages and lock open KCNQ5 at −120 mV (Figure 3). Of these, willow (Salix sp.) bark was used by the Cherokee as a tonic, a poultice, for hoarseness, as a gastrointestinal aid and to encourage hair growth (34, 47). The Chippewa and Ojibwa used bark from Quercus species for cardiac and bronchial medicine, among many other indications (38, 48). Slippery Elm (Ulmus rubra) was used as a gastrointestinal, pulmonary and/or gynecological aid by the Alabama, Cherokee, Chippewa, and Dakota peoples (34, 40, 48). Unlike the less KCNQ5-active species described in the preceding paragraph, preclinical evidence, albeit limited, suggests the ability of Ulmus (49, 50) and Quercus (51) species in ameliorating hypertension.
KCNQ4 and KCNQ5 channels are expressed in the smooth muscle of various arteries from rodents, swine and humans. In vascular smooth muscle cells, activation of KCNQ channels leads to a vasorelaxation or prevents vasoconstriction, whereas inhibition of these channels can attenuate relaxations produced by endogenous signaling pathways, such as cAMP, cGMP and adipose-derived relaxing factor (16, 17, 30, 52–54). Additionally, these channels were described recently in endothelial cells of rat mesenteric arteries where they contribute to nitric oxide release, which leads to vasorelaxations (55). Thus, KCNQ4 and KCNQ5 channels have a crucial physiological role in determining vessel tone in both a voltage- and ligand-dependent manner.
In a prior study, we showed that certain plant extracts previously found to have antihypertensive effects, including fennel seed, chamomile and lavender, activate KCNQ5 channels, and for one, Sophora flavescens root (Ku Shen) extract, we demonstrated both KCNQ5 activation and relaxation of arterial segments in a KCNQ-sensitive manner (11, 20). The current study further highlights the important functional role of KCNQ channels in vascular smooth muscle and identifies these channels as functional targets for active compounds in specific bark extracts from species used in Native American folk medicine. When comparing the KCNQ5-activating barks with the black haw bark extract, which was unable to activate KCNQ5, hydrolysable and condensed tannins were noticeably absent or in much lower concentrations from black haw compared to the KCNQ5-activating barks (Table 1; Table 2), supporting the premise that one or more tannins provide the molecular basis for KCNQ5-activating properties of the bark extracts.
Tannic acid has been previously described to relax rat mesenteric arteries in a KCNQ-sensitive manner, but gallic acid was not tested in that study, neither were effects tested of tannic acid on homomeric KCNQ5 or heteromeric KCNQ4/5 (instead, homomeric KCNQ4 and heteromeric KCNQ3/5 were studied (56). In addition, it was previously reported that tannic acid activates KCNQ2/3 channels and that this contributes to the analgesic properties of tannic acid when topically applied (57). Furthermore, we previously found that both tannic and gallic acids can activate KCNQ2/3 channels, while tannic acid also augments KCNQ1 and KCNQ1/KCNE1 currents at hyperpolarized potentials yet inhibits KCNQ1/KCNE3 – properties that together underlie the dual use of some Californian native plants by Native Americans as both analgesics and gastrointestinal aids (58). Here, we found that both tannic and gallic acid were able to activate KCNQ5 and relax rat mesenteric artery segments through activation of KCNQ channels. Interestingly, when tannic acid and gallic acid were applied to mesenteric arteries together at concentrations that had no effect by themselves, a complete relaxation was observed, which was attenuated by the KCNQ channel inhibitor, linopirdine. In contrast, we did not detect synergy between these two compounds in activation of KCNQ5 in oocytes, suggesting there may be additional targets in mesenteric arteries that contribute to the synergy between tannic and gallic acids – possibly channels formed by other KCNQ isoforms, including KCNQ1. However, KCNQ5 is more sensitive to tannic acid (EC50 = 6.3 μM; Figure 6C) than either KCNQ2/3 (EC50 = 132 μM in oocytes) or KCNQ1 (potentiating effect did not saturate at 250 μM in oocytes) (58); therefore, one would expect KCNQ5 to be the primary target for tannic acid among the KCNQ subfamily in the vasculature.
Finally, regarding the dual action of tannic acid on KCNQ5, which involves both augmentation of current at hyperpolarized potentials (with locking open at higher concentrations) and inhibition at depolarized potentials, we previously observed similar effects for tannic acid on KCNQ2/3, KCNQ1 and KCNQ1-KCNE1 (58). We suggest that in addition to hyperpolarizing the voltage dependence of KCNQ channel activation, tannic acid also inhibits (perhaps by direct channel block). Indeed, we previously found that tannic acid inhibits KCNQ1/KCNE3, which is a largely voltage-independent channel (58). This suggested that the inhibition is independent of effects on voltage dependence of gating, although caution should be observed when extrapolating effects between different channel types, even when in the same subfamily.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health, National Institute of General Medical Sciences (GM130377) and a Samueli Scholarship from the University of California, Irvine, Susan Samueli Integrative Health Institute to GWA, the National Institute of Neurological Disorders and Stroke (T32NS045540) to KR, the Lundbeck Foundation (R323-2018-3674) to TAJ, and JH received funding from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement No. 801199.
Footnotes
Competing Interests: The authors declare no competing interests.
Data and Materials Availability:
All datasets are available upon reasonable request.
References
- 1.Inskeep RR (1969) Health Hazards and Healing in Antiquity. South African Archaeological Bulletin 24, 21–29 [Google Scholar]
- 2.Hardy K, Buckley S, Collins MJ, Estalrrich A, Brothwell D, Copeland L, Garcia-Tabernero A, Garcia-Vargas S, de la Rasilla M, Lalueza-Fox C, Huguet R, Bastir M, Santamaria D, Madella M, Wilson J, Cortes AF, and Rosas A (2012) Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften 99, 617–626 [DOI] [PubMed] [Google Scholar]
- 3.Weyrich LS, Duchene S, Soubrier J, Arriola L, Llamas B, Breen J, Morris AG, Alt KW, Caramelli D, Dresely V, Farrell M, Farrer AG, Francken M, Gully N, Haak W, Hardy K, Harvati K, Held P, Holmes EC, Kaidonis J, Lalueza-Fox C, de la Rasilla M, Rosas A, Semal P, Soltysiak A, Townsend G, Usai D, Wahl J, Huson DH, Dobney K, and Cooper A (2017) Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 544, 357–361 [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi S (2007) Terminalia arjuna Wight & Arn.--a useful drug for cardiovascular disorders. J Ethnopharmacol 114, 114–129 [DOI] [PubMed] [Google Scholar]
- 5.Moerman DE (2009) Native American Medicinal Plants - an Ethnobotanical Dictionary. Timber Press, Portland, Oregon, USA [Google Scholar]
- 6.Miller AL (1998) Botanical influences on cardiovascular disease. Altern Med Rev 3, 422–431 [PubMed] [Google Scholar]
- 7.Desborough MJR, and Keeling DM (2017) The aspirin story - from willow to wonder drug. Br J Haematol 177, 674–683 [DOI] [PubMed] [Google Scholar]
- 8.Levesque H, and Lafont O (2000) [Aspirin throughout the ages: a historical review]. Rev Med Interne 21 Suppl 1, 8s–17s [DOI] [PubMed] [Google Scholar]
- 9.Manville RW, and Abbott GW (2018) Ancient and modern anticonvulsants act synergistically in a KCNQ potassium channel binding pocket. Nat Commun 9, 3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manville RW, and Abbott GW (2019) Cilantro leaf harbors a potent potassium channel-activating anticonvulsant. FASEB J 33, 11349–11363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manville RW, van der Horst J, Redford KE, Katz BB, Jepps TA, and Abbott GW (2019) KCNQ5 activation is a unifying molecular mechanism shared by genetically and culturally diverse botanical hypotensive folk medicines. Proc Natl Acad Sci U S A 116, 21236–21245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matschke V, Piccini I, Schubert J, Wrobel E, Lang F, Matschke J, Amedonu E, Meuth SG, Strunker T, Strutz-Seebohm N, Greber B, Scherkenbeck J, and Seebohm G (2016) The Natural Plant Product Rottlerin Activates Kv7.1/KCNE1 Channels. Cell Physiol Biochem 40, 1549–1558 [DOI] [PubMed] [Google Scholar]
- 13.Abbott GW (2014) Biology of the KCNQ1 potassium channel. New Journal of Science 2014, 26 [Google Scholar]
- 14.Abbott GW (2020) KCNQs: Ligand- and Voltage-Gated Potassium Channels. Front Physiol 11, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, and Byron KL (2007) Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 292, H1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, and Greenwood IA (2007) Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol 151, 758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, and Byron KL (2008) Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325, 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung S, Schwake M, Pucovsky V, and Greenwood I (2008) Bimodal effects of the Kv7 channel activator retigabine on vascular K+ currents. Br J Pharmacol 155, 62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung SY, Lange W, Schwake M, and Greenwood IA (2008) Expression profile and characterisation of a truncated KCNQ5 splice variant. Biochem Biophys Res Commun 371, 741–746 [DOI] [PubMed] [Google Scholar]
- 20.Redford KE, Rognant S, Jepps TA, and Abbott GW (2021) KCNQ5 Potassium Channel Activation Underlies Vasodilation by Tea. Cell Physiol Biochem 55, 46–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grieve M (1971) A Modern Herbal: The Medicinal, Culinary, Cosmetic and Economic Properties, Cultivation and Floklore of Herbs, Grasses, Fungi, Shrubs, & Trees with All Their Modern Scientific Uses, Dover Publications, New York [Google Scholar]
- 22.Zhao C, Chen J, Cao D, Wang J, and Ma W (2019) Novel coumarin-based containing dendrons selective fluorescent chemosensor for sequential recognition of Cu2+ and PPi. Tetrahedron 75, 1997–2003 [Google Scholar]
- 23.Hartzfield PW, Forkner R, Hunter MD, Hagermann AE (2002) Determination of Hydrolyzable Tannins (Gallotannins and Elagitannins) after Reaction with Potassium Iodate. J. Agric. Food Chem. 50, 1785–1790 [DOI] [PubMed] [Google Scholar]
- 24.Newsome AG, Li Y, and van Breemen RB (2016) Improved Quantification of Free and Ester-Bound Gallic Acid in Foods and Beverages by UHPLC-MS/MS. J Agric Food Chem 64, 1326–1334 [DOI] [PubMed] [Google Scholar]
- 25.Makkar HP, and Becker K (1993) Vanillin-HCl method for condensed tannins: Effect of organic solvents used for extraction of tannins. J Chem Ecol 19, 613–621 [DOI] [PubMed] [Google Scholar]
- 26.Jarboe CH, Zirvi KA, Nicholson JA, and Schmidt CM (1967) Scopoletin, an Antispasmodic Component of Viburnum opulus and prunifolium. J Med Chem 10, 488–489 [DOI] [PubMed] [Google Scholar]
- 27.Redford KE, and Abbott GW (2020) The ubiquitous flavonoid quercetin is an atypical KCNQ potassium channel activator. Commun Biol 3, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brueggemann LI, Haick JM, Cribbs LL, and Byron KL (2014) Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol 86, 330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, and Byron KL (2014) Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 289, 2099–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brueggemann LI, Mackie AR, Martin JL, Cribbs LL, and Byron KL (2011) Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol Pharmacol 79, 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, and Greenwood IA (2014) Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34, 887–893 [DOI] [PubMed] [Google Scholar]
- 32.Knuth S, Abdelsalam RM, Khayyal MT, Schweda F, Heilmann J, Kees MG, Mair G, Kees F, and Jurgenliemk G (2013) Catechol conjugates are in vivo metabolites of Salicis cortex. Planta Med 79, 1489–1494 [DOI] [PubMed] [Google Scholar]
- 33.Vlachojannis J, Magora F, and Chrubasik S (2011) Willow species and aspirin: different mechanism of actions. Phytother Res 25, 1102–1104 [DOI] [PubMed] [Google Scholar]
- 34.Hamel PB, and Chiltoskey MU (1975) Cherokee Plants and Their Uses - A 400 Year History, Herald Publishing Co., Sylva, North Carolina, USA [Google Scholar]
- 35.Tantaquidgeon G (1972) Folk Medicine of the Delaware and Related Algonkian Indians. Pennsylvania Historial Commision Anthrological Papers 3, 28, 78 [Google Scholar]
- 36.Herrick JW (1977) Iroquois Medical Botany. Vol. Ph.D., State University of New York, Albany [Google Scholar]
- 37.Chandler RF, Freeman L, and Hooper SN (1979) Herbal remedies of the Maritime Indians. J Ethnopharmacol 1, 49–68 [DOI] [PubMed] [Google Scholar]
- 38.Smith HH (1932) Ethnobotany of the Ojibwe Indians. In Bulletin of the Public Museum of Milwaukee Vol. 4 pp. 327–525 [Google Scholar]
- 39.Nicholson JA, Darby TD, and Jarboe CH (1972) Viopudial, a hypotensive and smooth muscle antispasmodic from Viburnum opulus. Proc Soc Exp Biol Med 140, 457–461 [DOI] [PubMed] [Google Scholar]
- 40.Gilmore MR (1933) Some Chippewa Uses of Plants, University of Michigan Press, Ann Arbor [Google Scholar]
- 41.Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, and Steinmeyer K (2000) Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem 275, 22395–22400 [DOI] [PubMed] [Google Scholar]
- 42.Jensen HS, Callo K, Jespersen T, Jensen BS, and Olesen SP (2005) The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH, and volume changes. Brain Res Mol Brain Res 139, 52–62 [DOI] [PubMed] [Google Scholar]
- 43.McCallum LA, Greenwood IA, and Tribe RM (2009) Expression and function of K(v)7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch 457, 1111–1120 [DOI] [PubMed] [Google Scholar]
- 44.Mistry HD, McCallum LA, Kurlak LO, Greenwood IA, Broughton Pipkin F, and Tribe RM (2011) Novel expression and regulation of voltage-dependent potassium channels in placentas from women with preeclampsia. Hypertension 58, 497–504 [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Yang D, and Hughes BA (2011) KCNQ5/Kv5 Potassium Channel Expression and Subcellular Localization in Primate Retinal Pigment Epithelium and Neural Retina. Am J Physiol Cell Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, and Jentsch TJ (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96, 437–446 [DOI] [PubMed] [Google Scholar]
- 47.Taylor LA (1940) Plants Used As Curatives by Certain Southeastern Tribes, Botanical Museum of Harvward University, Cambridge, MA, USA [Google Scholar]
- 48.Densmore F (1928) Uses of Plants by the Chippewa Indians. In SI-BAE Annual Report Vol. 44 pp. 273–379 [Google Scholar]
- 49.Oh KS, Ryu SY, Oh BK, Seo HW, Kim YS, and Lee BH (2008) Antihypertensive, vasorelaxant, and antioxidant effect of root bark of Ulmus macrocarpa. Biol Pharm Bull 31, 2090–2096 [DOI] [PubMed] [Google Scholar]
- 50.Syed AA, Lahiri S, Mohan D, Valicherla GR, Gupta AP, Riyazuddin M, Kumar S, Maurya R, Hanif K, and Gayen JR (2016) Evaluation of antihypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt- and L-NAME-induced hypertensive rats. J Ethnopharmacol 193, 555–565 [DOI] [PubMed] [Google Scholar]
- 51.Panchal SK, and Brown L (2013) Cardioprotective and hepatoprotective effects of ellagitannins from European oak bark (Quercus petraea L.) extract in rats. Eur J Nutr 52, 397–408 [DOI] [PubMed] [Google Scholar]
- 52.Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, and Greenwood IA (2011) Downregulation of kv7.4 channel activity in primary and secondary hypertension. Circulation 124, 602–611 [DOI] [PubMed] [Google Scholar]
- 53.Joshi S, Sedivy V, Hodyc D, Herget J, and Gurney AM (2009) KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329, 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Horst J, Greenwood IA, and Jepps TA (2020) Cyclic AMP-Dependent Regulation of Kv7 Voltage-Gated Potassium Channels. Front Physiol 11, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldwin SN, Sandow SL, Mondejar-Parreno G, Stott JB, and Greenwood IA (2020) KV7 Channel Expression and Function Within Rat Mesenteric Endothelial Cells. Front Physiol 11, 598779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Chu X, Liu L, Zhang N, Guo H, Yang F, Liu Z, Dong Y, Bao Y, Zhang X, and Zhang J (2016) Tannic acid activates the Kv7.4 and Kv7.3/7.5 K(+) channels expressed in HEK293 cells and reduces tension in the rat mesenteric arteries. J Pharm Pharmacol 68, 494–502 [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Zhang H, Zhou N, Xu J, Si M, Jia Z, Du X, and Zhang H (2015) Tannic acid modulates excitability of sensory neurons and nociceptive behavior and the Ionic mechanism. Eur J Pharmacol 764, 633–642 [DOI] [PubMed] [Google Scholar]
- 58.Abbott GW, Redford KE, Yoshimura RF, Manville RW, Moreira L, Tran K, Arena G, Kookootsedes A, Lasky E, and Gunnison E (2021) KCNQ and KCNE Isoform-Dependent Pharmacology Rationalizes Native American Dual Use of Specific Plants as Both Analgesics and Gastrointestinal Therapeutics. Front Physiol 12, 777057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets are available upon reasonable request.


