Abstract
Breast cancer (BC) with overexpression of human epidermal growth factor receptor 2 (HER2) is closely associated with an elevated risk of multiple distant metastases and unfavorable prognosis. Disitamab Vedotin (RC48) is a newly developed antibody-drug conjugate targeting HER2, which is comprised of hertuzumab coupled to monomethyl auristatin E via a cleavable linker. Pre-clinical studies indicated its strong anti-tumor activity in HER2-positive and low HER2 expression models of BC. The present study reported on the case of a 60-year-old postmenopausal female who suffered from fatigue and was diagnosed with a right-sided BC tumor. The diagnosis was stage IV (cT4N3M1) hormone receptor (HR)-positive and HER2-positive invasive ductal carcinoma with systemic metastases (brain included). The patient initially responded well to 26 cycles of the first-line anti-HER2 targeted therapy plus chemotherapy (trastuzumab+pertuzumab+nab-paclitaxel) combined with whole-brain radiotherapy. However, both extracranial and intracranial lesions achieved progressive disease (PD), which eventually occurred during 5 sequential cycles of maintenance therapy. Subsequently, 4 cycles of second-line treatment (trastuzumab + pyrotinib + capecitabin) were continued until the levels of blood tumor markers CEA, CA15-3 and CA125 were elevated, and systemic PD was able to be attained (the brain metastases were rated as stable disease). Finally, the patient received RC48 as the third-line therapy and achieved a durable and effective clinical response. To date, the patient has benefited from 12 cycles of RC48 without any severe adverse effects. The overall survival was >3 years. The present study showcased that RC48 was effective and tolerable for a patient with HR- and HER2-positive BMBC.
Keywords: RC48, ADC, metastatic breast cancer, HER2-positive breast cancer, anti-HER2 target therapy
Introduction
Human epidermal growth factor receptor 2 (HER2) is amplified in ~15–20% of breast cancer (BC) cases (1), which is associated with a more aggressive disease behavior, unfavorable overall survival (OS) and shorter time to relapse (2). Among patients with HER2-positive BC, approximately half are also hormone receptor (HR)-positive (3). Previous studies have demonstrated that patients with metastatic BC (MBC) with HER2-positive/HR-positive status benefit from HER2-targeted therapy (1–3).
The number of patients with brain MBC (BMBC) with HER2-positive status has increased, and this is associated with a lower quality of life, unfavorable prognosis and less responsiveness to systemic therapies (4–6). In recent decades, the survival time of patients with BMBC was <6 months and even whole-brain radiotherapy (WBRT) and effective systemic treatment have not been established for metastases to the central nervous system (7,8). With the development of anti-HER2 therapies for patients with BMBC with HER2-positive status, the survival rate in advanced stages was significantly improved. The combination of pertuzumab, trastuzumab and docetaxel was recommended as a standard therapeutic option for patients with advanced disease in 2020 (9). While the second-line therapy included the combination of Ado-trastuzumab (T-DM1) and a tyrosine kinase inhibitor pyrotinib-based regimen (10), there is still no recognized single-standard third-line therapy for patients with HER2-positive BMBC (11), and new therapeutic strategies are urgently required.
Antibody-drug conjugates (ADCs), comprised of an antibody against the antigen of interest, a linker and a payload cytotoxic agent, were designed for specific delivery of cytotoxic agents to malignant cells (12). Disitamab Vedotin (RC48) contains the novel humanized anti-HER2 antibody (hertuzumab) conjugated to monomethyl auristatin E (MMAE) via a cleavable linker (12,13), which is the first ADC drug that was independently developed by Rongchang Biology and was approved in China in June 2021 for patients with locally advanced or metastatic gastric cancer (including gastroesophageal junction adenocarcinoma) overexpressing HER-2 who have received at least 2 systemic chemotherapies based on successful trials (13). In a xenograft tumor model of human BC resistant to trastuzumab and lapatinib, the efficacy of RC48 was significantly higher than that of lapatinib, trastuzumab or an equal dose of T-DM1 (11). According to the clinical trials performed to date (NCT03052634 and NCT03500380), RC48 demonstrated promising efficacy for patients with BMBC with high or low expression level of HER2 (14). Furthermore, treatment-related adverse events (AEs) were commonly grade 1–2. Thus, patients with BMBC may consistently benefit from RC48 with promising efficacy and safety. The present study reported the case of a 60-year-old female patient with BMBC with HER2-positive status who responded well to single RC48 therapy as the third-line treatment. The results showcased that RC48 may provide a significant clinical benefit for patients with BMBC with HER2-positive/HR-positive status with tolerable AEs.
Case report
A 60-year-old postmenopausal female initially presented to Minhang Branch of Zhongshan Hospital, Fudan University (Shanghai, China) in April 2019 with a mass in the right breast, complaining of fatigue and lethargy. Physical examination revealed a 3.0-cm hard irregular mass in the central area of the right breast with inversion of the nipple and several swollen lymph nodes in the right axilla. Analysis of blood samples indicated markedly low hemoglobin level (48 g/l; normal range: 110–150 g/l) and platelet count (39×109/l; normal range: 100–300×109/l) with high levels of carcinoembryonic antigen (CEA; 203.70 ng/ml; normal range: 0–5 ng/ml), carbohydrate antigen 125 (CA125; 2,267 U/ml; normal range: 0–35 U/ml) and CA15-3 (4,754 U/ml; normal range: 0–25 U/ml). Contrast-enhanced breast magnetic resonance imaging (MRI) and computed tomography (CT) revealed right-sided breast malignant tumor with breast imaging-reporting and data system score of 5 (15) and the possibility of multiple axillary lymph nodes and multiple systemic bone metastases (Fig. 1). Pathology of the right breast performed based on the core needle biopsy indicated grade II invasive ductal carcinoma with the following immunohistochemistry (IHC) results: estrogen receptor (ER) (80%), progesterone receptor (PR) (−), HER2 (3+) and Ki-67 proliferation marker (Ki-67) (20%) (Fig. 2A). Furthermore, histology and IHC of bone marrow biopsy from the posterior superior iliac spine detected metastatic tumor cells (adenocarcinoma) with ER (+), PR (−), Ki-67 (<5%) and cytokeratin pan (CK-pan) (+) (Fig. 2B). Tissue samples derived from core needle and bone marrow biopsy specimens were fixed in 10% formalin at room temperature for 24 h, paraffin embedded and subjected to histological or immunohistochemical analysis. Sections (4 µm) were heated at 58°C for 2 h and then deparaffinized in xylene and hydrated with a series of graded alcohols, including anhydrous ethanol for 5 min, 95% ethanol for 2 min, 90% ethanol for 2 min, 80% ethanol for 2 min and 70% ethanol for 2 min. H&E staining was used for histological analysis. Antigen recovery was performed by heating and immersing the slides in citrate buffer (0.01 M, pH 9.0; cat. no., P0020; Beijing Noble Technology Co., Ltd.) in a microwave oven (121°C) for 10 min twice. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 30 min at 20°C, and the sections were incubated with anti-ER (1:1 dilution; 37°C; 24 min; cat. no. 05278406001), anti-PR (1:1 dilution; 37°C; 24 min; cat. no. 05277990001), HER2 (1:1 dilution; 37°C; 20 min; cat. no. 05999570001; all from Roche Diagnostics), Ki-67 (1:1,000 dilution; 4°C overnight; cat. no. ARG11083) and CK-pan (1:500 dilution; 4°C overnight; cat. no. ARG56128; all from Arigo). Subsequently, sections were washed and incubated with a biotinylated anti-mouse/rabbit secondary antibody (1:500 dilution; cat. no. D0486 and D0487; Dako; Agilent Technologies, Inc.) at 37°C for 15 min. Cells with positive immunostaining were counted and imaged under a light microscope (Olympus BX43; Olympus Corporation) with a magnification of ×100, ×200 and ×400. The patient was a postmenopausal female and therefore, no further hormonal blood tests were performed. The positron emission tomography-CT (PET-CT) confirmed the right-sided breast malignant tumor [30.1×25.3 mm; maximum standardized uptake value (SUVmax) of 5.2 (UniSyn™ Image Fusion software; version 2019; Convergent Imaging Solutions, Inc.)] with multiple lymph nodes, systemic bone metastases and possible metastases to the pleura and liver (Fig. 1). Besides, the contrast-enhanced brain MRI indicated nodules in the bilateral frontal lobe and right cerebellar with meningeal enhancement (Fig. 1). However, neurological physical examination revealed no positive signs, which indicated asymptomatic meningeal or brain metastases. Therefore, the tumor-node-metastasis (TNM) classification (16) was designated as cT4N3M1, stage IV.
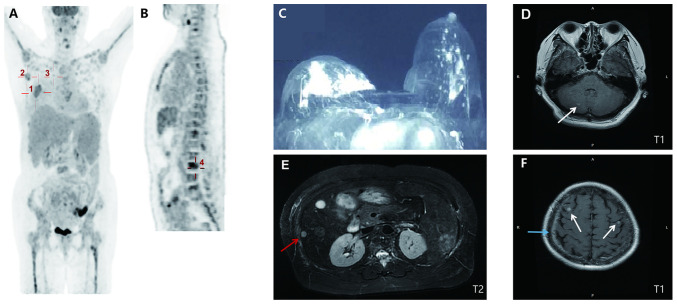
Figure 1.
Baseline imaging presentation. (A) PET/CT (10 days after initial presentation; coronal section) indicated a malignant tumor in the right breast (30.1×25.3 mm2; SUVmax, 5.2; marked by cross 1) with right-sided axillary (20.7×11.2 mm2; SUVmax, 3.2; and 17.4×9.8 mm; SUVmax, 6.0; marked by cross 2) and internal mammary (19.1×11.5 mm2; SUVmax, 2.6; marked by cross 3) metastatic lymph nodes. (B) PET/CT (median sagittal section) indicated systemic bone metastases in spine (marked by cross 4), bilateral ribs and sternum. (C) Contrast-enhanced breast CT (at the initial presentation) indicated an irregular mass with an unclear margin in the areola area of the right breast (31×15×22 mm3; breast imaging-reporting and data system score of 5). (D) Cranial T1 weighted MRI (15 days after initial presentation) revealed nodular lesions in the right cerebellum (marked by the arrow). (E) Abdominal T2-weighted MRI (14 days after initial presentation) suggested metastases in the liver with multiple abnormal round signaling shadows (one is 8 mm in diameter, marked by the red arrow). (F) Cranial T1 weighted MRI revealed nodular lesions in bilateral frontal lobes (marked by white arrows) with leptomeningeal enhancement (marked by the blue arrow). SUVmax, maximum standardized uptake value; PET, positron emission tomography.
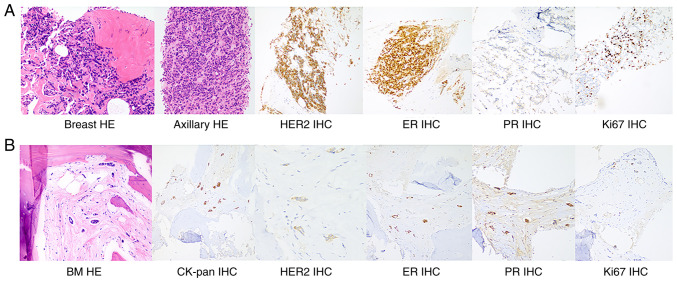
Figure 2.
H&E staining and IHC staining of the breast mass, axillary lymph nodes and bone marrow metastases. (A) H&E staining of breast tissue and axillary lymph nodes suggested grade II invasive ductal carcinoma with the following IHC staining results: HER2 (+++), ER (++), PR (−) and Ki67 (20%). (B) Histology of bone marrow biopsy indicated atypical cells with the following IHC staining results: CK-pan (+), HER2 (+), ER (+), PR (+) and Ki67 (<5%), which are consistent with bone marrow metastasis (magnification, ×200). IHC, immunohistochemistry; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor; CK, cytokeratin; BM, bone marrow.
In order to enhance the patient's ability to tolerate chemotherapy, the patient received transfusion of red blood cells to improve anemia, as well as recombinant human thrombopoietin injection (RHTI; Sansheng Pharmaceutical Co., Ltd.) to elevate the platelet count. Furthermore, zoledronic acid was prescribed for the patient's bone metastasis. The patient received the PPH regimen [trastuzumab (Genentech Inc.; 6 mg/kg) on day 0 (the first dose, 8 mg/kg) + pertuzumab (Genentech Inc.; 420 mg) on day 0 (the first dose, 840 mg) + paclitaxel (Bristol-Myers Squibb Co.) 120 mg on day 1, 150 mg on day 8, q3w] as the first-line therapy for 3 cycles. As for the progressively enlarged brain metastatic lesions, the patient received helical tomotherapy (intensity, DT 4,000 cGy/20 fx for whole-brain and DT 6,000 cGy/20 fx for the metastatic area). As the glucocorticoid pretreatment of paclitaxel caused severe hypertension, the PPH regimen was replaced with the PHA regimen (trastuzumab + pertuzumab + nab-paclitaxel (Abraxis BioScience, Inc.) 200 mg on days 1 and 8, q3w) for an additional 23 cycles. Contrast-enhanced brain MRI then indicated reduction of brain metastases and PET-CT displayed that the right breast mass had shrunk to 18 mm in diameter. Thus, the treatment efficacy was rated as partial response (PR) according to the Response Evaluation Criteria in Solid Tumors version 1.1 (17). Afterwards, trastuzumab and pertuzumab were recommended as maintenance therapy for intolerable grade III neurotoxicity resulting from nab-paclitaxel.
However, after 5 cycles of maintenance therapy, splenic parenchyma exhibiting nodule formation with a high glucose metabolism rate was identified by PET-CT. It is noteworthy that only a number of the bone metastatic lesions were detected with high uptake of 16α-[18F] fluoro-17β-estradiol (18F-FES) by ER imaging, which demonstrated inter-tumoral and temporal heterogeneity of ER expression levels. At the same time, CA125 was markedly increased from 237.8 (December 2020) to 713.3 (January 2021) U/ml. The breast mass was enlarged to 33×25×18 mm3 in size and the contrast-enhanced brain MRI also suggested an increase in the size of cerebral nodules with peripheral edema. Therefore, the final evaluation of therapeutic efficacy indicated progressive disease (PD) and the patient received the second-line treatment [trastuzumab (6 mg/kg) on day 1 + pyrotinib (Hengrui Pharmaceutical Co., Ltd.; 400 mg) po daily + capecitabine (1,250 mg/m2) bid from day 1 to day 14, q3w] for 4 cycles. Although loperamide was prescribed to prevent severe diarrhea and the dose of pyrotinib was modulated to 320 mg, the patient still experienced grade III diarrhea, grade I/II vomiting and grade I/II hand-foot syndrome. Furthermore, CA-125 levels continued to rise, accompanied by progressively decreased hemoglobin levels, and PET-CT was repeated and indicated enlarged splenic metastases with a higher level of glucose metabolism, again indicating PD.
As a result, the third-line treatment, including anti-HER-2 ADC RC48 (Rongchang Biopharmaceutical Co., Ltd.; 120 mg, q2w) was prescribed for 12 cycles. It is noteworthy that the patient demonstrated a satisfactory stable response with acceptable tolerability to this regimen, which was more obvious when the patient's current and previous statuses were compared and there were previously several BC-related complications (anemia and low platelet level) and development of trastuzumab resistance. Cranial and abdominal MRI scans were performed again, which indicated stable disease (SD) (Fig. 3), and breast ultrasonography suggested a reduction in the size of the mass (25×12×20 mm3). Of note, the levels of the three tumor markers (CEA, CA-125 and CA15-3) were progressively reduced, while hemoglobin levels increased to a normal level within two months (Fig. 4). Furthermore, RC48 demonstrated promising safety and no obvious drug toxicity was recorded during the treatment. AEs, such as grade I liver injury and grade I fatigue, were able to be controlled by symptomatic treatment, which not only prolonged the patient's survival time (>3 years so far) but also markedly improved the patient's quality of life as self-reported by the patient. In the future, the patient will be closely followed up by blood test and imaging examinations. If the treatment effect is rated as PD or the patient is not able to tolerate RC48 in the future, a combination of hormone therapy may be considered or RC48 may be replaced with another ADC drug.
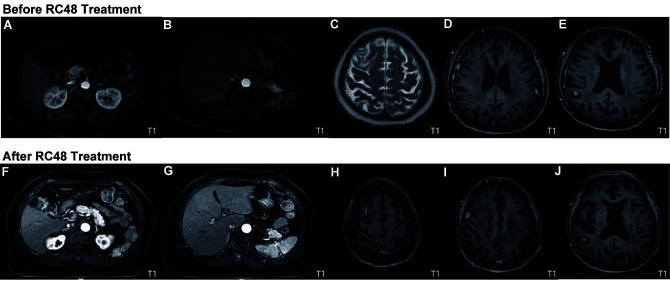
Figure 3.
Comparison of MRI prior to (June, 2021) and after (December, 2021) third-line treatment based on the newest evidence. On contrast-enhanced abdominal T1-weighted MRI, abnormal small nodular signals were observed in (A and F) segment II of the left lobe and in segment VI of the right lobe of the liver, and (B and G) a wedge-shaped abnormal signal area was observed in the subcapsular of the spleen (10×13 mm2). Contrast-enhanced brain MRI indicated (Cand H) multiple brain metastases in the bilateral frontal lobes, (D and I) leptomeninges and (E and J) right parietal lobe. The results indicated that there was no obvious progression after administration of RC48 and the imaging findings were rated as stable disease.
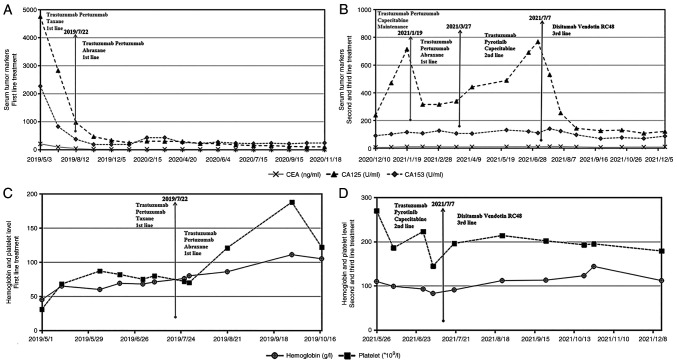
Figure 4.
Levels of three serum tumor markers measured (A) after the first-line therapy and (B) after the other treatment strategies had been applied. (A) The patient responded well to the initial 3 and 14 cycles of the first-line therapy, as the levels of all three tumor markers significantly decreased. (B) CA-125 levels continuously increased with the maintenance and second-line therapies. Single-dose RC48 significantly reduced the levels of the three tumor markers. Measurement of the hemoglobin level and platelet count (C) after the first-line treatment and (D) during the second- and third-line therapy. (C) After transfusion of red blood cells and injection of RHTI, first-line chemotherapy was initiated. Both hemoglobin levels and platelet count increased after 3 cycles of the PPH regimen and 5 cycles of the PHA regimen. (D) The hemoglobin level and platelet count of the patient of the present study remained stable during administration of RC48, which indicated that RC48 hardly caused damage to the hematopoietic function. Regimens: RHTI, recombinant human thrombopoietin injection; PPH, trastuzumab (6 mg/kg) on day 0 (the first dose, 8 mg/kg) + pertuzumab (420 mg) on day 0 (the first dose, 840 mg) + paclitaxel 120 mg on day 1, 150 mg on day 8, q3w; PHA, trastuzumab + pertuzumab + nab-paclitaxel 200 mg on days 1 and 8, q3w; RC48, Disitamab Vedotin.
Discussion
The selection of the first-line treatment for a patient with BMBC initially diagnosed with multiple metastases, and even accompanied by severe anemia and thrombocytopenia caused by bone marrow involvement, asymptomatic meningeal or brain metastasis, liver metastasis and poor performance status posed a significant challenge. The present study indicated that the expression of Ki-67 in the primary mass of the breast (20%) and the bone marrow involvement (<5%) was different, and it was initially deduced that the metastatic lesions were relatively indolent. Furthermore, the decalcification of the bone marrow specimen may have impaired the IHC results for Ki-67. For patients with HER-2 positive and HR-positive BMBC, the National Comprehensive Cancer Network guidelines (18) recommend trastuzumab + pertuzumab + paclitaxel as the first-line treatment, and dual HER2 blockade and hormonal therapy are only prescribed for those who were not able to tolerate chemotherapy. For the present case, chemotherapy was the first choice due to visceral metastasis. However, the common AE of paclitaxel is myelo-suppression, which generally occurs 8–10 days after treatment (19). A previous study demonstrated that myelosuppression occurred faster and more severe in patients with bone metastasis after chemotherapy or radiotherapy (20). Endocrine therapy is tolerable, while it requires a longer time to reach its highest efficacy. Finally, the patient chose the standard regimen with dual anti-HER2 therapy combined with paclitaxel after diligently correcting anemia and thrombocytopenia. A weekly regimen rather than 3-week administration of paclitaxel was used to reduce the incidence of AEs. Furthermore, more effective therapeutic schemes have been developed to avert bone marrow suppression and other AEs. The metronomic paclitaxel therapy, which was defined as low-dose oral administration of metronomic paclitaxel, previously demonstrated a stronger anti-angiogenesis activity, prolonged survival and a decreased incidence of AEs in patients with BC in clinical practice (21,22). Of note, in the present case, there was no obvious recurrent myelosuppression during the treatment.
Meningeal metastasis in brain parenchyma was another clinical challenge for the patient of the present study. In previous studies, the median OS in the brain metastasis group with the best prognostic survival score was generally no more than 13 months (23), while the median OS in the meningeal metastasis group was only 6 months (24,25). Expert consensus and guidelines have demonstrated that systematic treatment should be used as the first treatment for patients with BMBC with rapid progression of extracranial lesions, followed by local treatments of intracranial lesions, such as stereotactic radiosurgery and WBRT, until the disease is controlled. Trastuzumab combined with pertuzumab was able to significantly prolong OS of patients with cancer compared with anti-HER2 therapy (44 vs. 17 months, P<0.001) (26,27). The present case was diagnosed with BMBC at the first visit and defined as HER2-positive. The patient received dual anti-HER2 therapy plus chemotherapy and one month later, SD was confirmed with a slightly enlarged mass. The patient then underwent WBRT and both the intracranial and extracranial lesions achieved PR. Thus, even for patients with HER2 overexpression undergoing relatively effective systematic treatment, the local treatment is also important, particularly for those with symptoms.
Although HR positivity predicts the efficacy of endocrine agents, preclinical and clinical data suggested that HER2 overexpression may indicate intrinsic resistance to hormonal therapy. The strategy of using anti-estrogen treatment was considered for the patient of the present study. Several meta-analyses have demonstrated that 16α-18F-fluoro-17β-estradiol (18F-FES)-PET imaging noninvasively assesses the ER status in BC, which is consistent with the results of IHC (28,29). The sensitivity and specificity of 18F-FES-PET in a meta-analysis were good, i.e. 0.82-0.84 and 0.93-0.98, respectively (29). To indicate the heterogeneous expression of ER in patients with MBC prior to and after treatment (29), patients underwent PET-CT and 18F-FES-PET during the course of the disease, in which the former assessed the extent of malignant lesions and the latter sensitively quantified the distribution and biological activity of functional ER. However, only a number of the bone metastatic lesions with high uptake of 18F-FES were detected using ER imaging and all the other masses were negative, which demonstrated tumor heterogeneity in terms of ER expression. As IHC indicated negativity for PR (although ER was positive) and the visceral and brain metastases were life-threatening, no anti-estrogen treatment was selected. However, anti-estrogen therapy combined with cyclin-dependent kinase 4/6 inhibitors and anti-HER2 drugs may be prescribed for the patient as the next treatments.
In June 2021, the new indication for RC48 was identified by the Center for Drug Evaluation in China as a breakthrough therapy for HER2-positive patients with MBC with advanced liver metastases that had been treated with trastuzumab and paclitaxel (Table I). A pooled analysis of two studies published by the American Society of Clinical Oncology indicated that of 118 patients with MBC treated with RC48, 59.3% were HER2-positive, 40.7% had low expression of HER2 and 39.8% received no less than three chemotherapy-dependent regimens. Subgroup analysis indicated that patients benefited from treatment even if they were not driven by HER2 gene mutation or fusion (30). Thus, RC48 demonstrated consistent efficacy in MBC with high or low expression of HER2. Nearly 40% of patients were still able to achieve remission in the later-line treatment. Furthermore, the RC48 monotherapy demonstrated a promising efficacy for the patient of the present study in the third-line treatment and the progression-free survival reached 8 months.
Table I.
Completed/ongoing clinical trials of RC48 in HER2 breast cancer treatment.
| A, Completed trials | |||||
|---|---|---|---|---|---|
|
| |||||
| Study phase | Registration no. | Arms and interventions | Condition | Subjects | Outcomes/status |
| I | NCT02881138 | RC48-ADC | HER2-positive metastatic breast cancer | 23 | ORR: 72.7% in 11 trastumab pretreated pts; SD: 28.6% in 14 pts at dose ≥1.5 mg/kg; PR: 57.1%. |
| I | NCT03052634 | RC48-ADC | HER2-positive metastatic breast cancer | 30 | ORR: 36.7%; SD: 60%; CBR: 46.7%. |
|
| |||||
| B, Ongoing studies | |||||
|
| |||||
| Study phase | Registration no. | Arms and interventions | Condition | Subjects | Outcomes/status |
|
| |||||
| II/III | NCT03500380 | RC48-ADC vs. Lapatinib + Capecitabine | HER2-positive metastatic breast cancer with or without liver metastases | 301 | Recruiting |
| II | NCT03052634 | RC48-ADC | Advanced breast cancer with HER2 positive or HER2 low expression | 112 | Active, not recruiting |
| III | NCT04400695 | RC48-ADC vs. Paclitaxel or Docetaxel or Vinorelbine Tartrate or Capecitabine | HER2 low breast cancer with recurrence/metastasis | 366 | Recruiting |
| II | NCT05134519 | RC48-ADC | HER2-positive breast cancer | 20 | Not yet recruiting |
ORR, overall response rate; SD, stable disease; PR, partial response; CBR, clinical benefit rate; pts, patients; HER2, human epidermal growth factor receptor 2; ADC, antibody-drug conjugates; RC48, Disitamab Vedotin.
Of note, a recent study indicated that combination of RC48 with programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors significantly enhanced tumor suppression and antitumor immunity by massive T-cell infiltration and immune marker activation in a human HER2-expressing syngeneic breast cancer model, and contributed to immune memory formation in animals with tumor eradication to protect them against tumor recurrence (31). Previous studies suggested that ADC drugs have anti-tumor immune activity by exerting a more marked killing effect on the tumor or adjacent tissues during intracellular release of the cytotoxic payload (32,33), such as MMAE (34), which is a potent inhibitor of tubulin polymerization and a major component of RC48 (35). Therefore, the combination of RC48 and anti-PD-1/PD-L1 antibodies is expected to be an emerging treatment for patients with HER2-positive BMBC in future clinical trials.
In recent years, treatments for patients with HR- and HER2-positive advanced BMBC have noticeably attracted clinicians' attention, particularly due to multiple organ involvement and poor physical status. Of note, the increasing application of RC48 will improve the prognosis of such patients.
Acknowledgements
Not applicable.
Funding Statement
This study was financially supported by the National Natural Science Foundation of China (grant nos. 81172522 and 81301858), the Suzhou Science and Technology Project (grant nos. SYS201508 and SYS201308) and the Natural Science Foundation of Jiangsu Province (grant no. BK20181186).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QW, LH and JL wrote the manuscript and contributed to the data analysis and interpretation. YX, CW and WJ aquired, evaluated and analyzed the clinical data, contributed to the manuscript drafting and critical revisions. LH and QW contributed to generating the figures and table. All authors read and approved the final manuscript. YX and CW checked and confirmed the authenticity of the raw data.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Shanghai, China) and the Ethics Committee of Minhang Branch, Zhongshan Hospital Affiliated to Fudan University (Shanghai, China).
Patient consent for publication
Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 4.Klos KJ, O'Neill BP. Brain metastases. Neurologist. 2004;10:31–46. doi: 10.1097/01.nrl.0000106922.83090.71. [DOI] [PubMed] [Google Scholar]
- 5.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 6.Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: A multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147:103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 7.Fokstuen T, Wilking N, Rutqvist LE, Wolke J, Liedberg A, Signomklao T, Fernberg JO. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res Treat. 2000;62:211–216. doi: 10.1023/A:1006486423827. [DOI] [PubMed] [Google Scholar]
- 8.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: Old challenge, new frontiers. Clin Cancer Res. 2013;19:6404–6418. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 10.Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, Danese S, Vietti-Ramus G, Durando A, Venturini M, Aglietta M. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 11.Cesca MG, Vian L, Cristóvão-Ferreira S, Pondé N, de Azambuja E. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev. 2020;88:102033. doi: 10.1016/j.ctrv.2020.102033. [DOI] [PubMed] [Google Scholar]
- 12.Yao X, Jiang J, Wang X, Huang C, Li D, Xie K, Xu Q, Li H, Li Z, Lou L, Fang J. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res Treat. 2015;153:123–133. doi: 10.1007/s10549-015-3503-3. [DOI] [PubMed] [Google Scholar]
- 13.Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, Zhang X, Fan N, Luo S, Li Z, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: A single-arm phase II study. Cancer Commun (Lond) 2021;41:1173–1182. doi: 10.1002/cac2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Xu B, Wang W, Fang J. An open-label, dose-escalation phase I study to evaluate RC48-ADC, a novel antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2018;36:1030–1030. doi: 10.1200/JCO.2018.36.15_suppl.1030. [DOI] [Google Scholar]
- 15.Arian A, Dinas K, Pratilas GC, Alipour S. The breast imaging-reporting and data system (BI-RADS) Made Easy. Iranian J Radiol. 2022;19:e121155. doi: 10.5812/iranjradiol-121155. [DOI] [Google Scholar]
- 16.Brierley JD, Gospodarowicz MK, Wittekind C, editors. 8th Edition. Chichester: John Wiley and Sons; 2017. TNM Classification of Malignant Tumours; pp. 151–158. [Google Scholar]
- 17.Nematollahy N. Response evaluation criteria in solid tumors version 1.1. Iranian Soc Radiol. 2019;35:46–46. [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 19.Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6:609–621. doi: 10.1517/14740338.6.5.609. [DOI] [PubMed] [Google Scholar]
- 20.Norman H, Lee KT, Stearns V, Alcorn SR, Mangini NS. Incidence and severity of myelosuppression with palbociclib after palliative bone radiation in advanced breast cancer: A single center experience and review of literature. Clin Br Cancer. 2022;22:e65–e73. doi: 10.1016/j.clbc.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao WY, Liang XS, Liu Y, Wang CY, Pang D. Decrease of let-7f in low-dose metronomic Paclitaxel chemotherapy contributed to upregulation of thrombospondin-1 in breast cancer. Int J Biol Sci. 2015;11:48–58. doi: 10.7150/ijbs.9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Xia R, Zheng W, Zhang L, Li P, Sun X, Shi J. Metronomic paclitaxel improves the efficacy of PD-1 monoclonal antibodies in breast cancer by transforming the tumor immune microenvironment. Am J Transl Res. 2020;12:519–530. [PMC free article] [PubMed] [Google Scholar]
- 23.Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer. 2017;74:17–25. doi: 10.1016/j.ejca.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Assi HI, Mahmoud T, Saadeh FS, El Darsa H. Management of leptomeningeal metastasis in breast cancer. Clin Neurol Neurosurg. 2018;172:151–159. doi: 10.1016/j.clineuro.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis-the role of multimodality treatment. J Neurooncol. 2007;84:57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 26.Bergen ES, Binter A, Starzer AM, Heller G, Kiesel B, Tendl-Schulz K, Bago-Horvath Z, Furtner J, Leitner J, Exner R, et al. Favourable outcome of patients with breast cancer brain metastases treated with dual HER2 blockade of trastuzumab and pertuzumab. Ther Adv Med Oncol. 2021;13:17588359211009002. doi: 10.1177/17588359211009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mounsey LA, Deal AM, Keith KC, Benbow JM, Shachar SS, Zagar T, Dees EC, Carey LA, Ewend MG, Anders CK. Changing Natural History of HER2-positive breast cancer metastatic to the brain in the era of new targeted therapies. Clin Breast Cancer. 2018;18:29–37. doi: 10.1016/j.clbc.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Evangelista L, Guarneri V, Conte PF. 18F-fluoroestradiol positron emission tomography in breast cancer patients: Systematic review of the literature & meta-analysis. Curr Radiopharm. 2016;9:244–257. doi: 10.2174/1874471009666161019144950. [DOI] [PubMed] [Google Scholar]
- 29.Kurland BF, Wiggins JR, Coche A, Fontan C, Bouvet Y, Webner P, Divgi C, Linden HM. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18F-Fluoro-17β-estradiol positron emission tomography: Meta-analysis and recommendations for integration into clinical applications. Oncologist. 2020;25:835–844. doi: 10.1634/theoncologist.2019-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Liu Y, Zhang Q, Feng J, Fang J, Chen X, Han Y, Li Q, Zhang P, Yuan P, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J Clin Oncol. 2021;39:1022–1022. doi: 10.1200/JCO.2021.39.15_suppl.1022. [DOI] [Google Scholar]
- 31.Huang L, Wang R, Xie K, Zhang J, Tao F, Pi C, Feng Y, Gu H, Fang J. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Br Cancer Res Treat. 2022;191:51–61. doi: 10.1007/s10549-021-06384-4. [DOI] [PubMed] [Google Scholar]
- 32.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br J Cancer. 2017;117:1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 34.Müller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, Lardinois D, Heinzelmann-Schwarz VA, Schlaak M, Kvasnicka HM, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–755. doi: 10.1158/2326-6066.CIR-13-0198. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Yu C, Jiang J, Huang C, Yao X, Xu Q, Yu F, Lou L, Fang J. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol Ther. 2016;17:346–354. doi: 10.1364/CANCER.2016.JW3A.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.