Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infections pose a global health threat, especially with the continuous development of antibiotic resistance. As an opportunistic pathogen, MRSA infections have a high mortality rate worldwide. Although classically described as an extracellular pathogen, many studies have shown over the past decades that MRSA also has an intracellular aspect to its infectious cycle, which has been observed in vitro in both non-professional as well as professional phagocytes. In vivo, MRSA has been shown to establish an intracellular niche in liver Kupffer cells upon bloodstream infection. The staphylococci have evolved various evasion strategies to survive the antimicrobial environment of phagolysosomes and use these compartments to hide from immune cells and antibiotics. Ultimately, the host cells get overwhelmed by replicating bacteria, leading to cell lysis and bacterial dissemination. In this review, we describe the different intracellular aspects of MRSA infection and briefly mention S. aureus evasion strategies. We discuss how this intracellular niche of bacteria may assist in antibiotic tolerance development, and lastly, we describe various new antibacterial strategies that target the intracellular bacterial niche.
Keywords: Staphylococcus aureus, MRSA, intracellular infection, antibiotic resistance, antimicrobial therapy
1. Introduction
As the COVID-19 pandemic continues, it represents the leading cause of morbidity and mortality worldwide, causing over 6 million deaths to date. In addition to the human toll, this pandemic represents the single largest burden on healthcare and the world economy. In the lee of this global pandemic, bacterial infections continue in the shadows. Bacterial antimicrobial resistance (AMR) occurs when changes in microbes cause the antimicrobials used to treat infections to become ineffective, and this has emerged as one of the leading public health threats of the 21st century. A recent systematic review showed an estimated 4.95 million annual infections associated with bacterial AMR in 2019, which caused 1.27 million deaths [1]. By far the most dangerous drug-resistant pathogen in 2019 was methicillin-resistant Staphylococcus aureus (MRSA) causing over 100,000 deaths alone. This is almost double compared to mortality caused by other ESKAPE pathogens, such as Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa [1]. What sets MRSA apart is its tremendous reservoir of virulence factors and immune evasion molecules that in combination with AMR make it a particularly deadly pathogen.
Staphylococcus aureus (S. aureus) is part of the normal skin and nasal microbiota, with approximately 30% of the healthy adult population colonized mainly in the nasopharyngeal cavity. While colonization is usually asymptomatic, a symptomatic infection can occur if there is a breach in the mucosal barrier or skin. The severity of symptomatic infections ranges from superficial skin and soft tissue infections [2], to devastating complications, such as necrotizing pneumonia, endocarditis, toxic shock syndrome, and sepsis [3,4]. In the pre-antibiotic era, S. aureus bacteremia mortality rates were astonishingly high, ranging between 75% and 83% [5]. Even though antibiotics have reduced this number significantly, S. aureus bloodstream infections still account for over 19,000 deaths annually in the United States [6,7]. With every new antibiotic that is developed, S. aureus resistance has been quickly observed [8]. MRSA strains that are resistant to all penicillin-like β-lactam antibiotics pose a particularly serious threat to the community [9]. Two types of MRSA exist: hospital-acquired (HA-)MRSA and community-acquired (CA-)MRSA [10]. CA-MRSA strains are typically regarded as more virulent and can cause infections in otherwise healthy individuals. This notion is further supported by experimental animal studies, whereas HA-MRSA strains are less virulent than CA-MRSA and cause fewer disseminating diseases. Although in animal models, mice are typically not treated with antibiotics, which is unlike a hospital setting [11].
The standard treatment for systemic MRSA infections in most countries is 2–6 weeks of intravenous vancomycin administration, which is one of the last remaining treatment options. Vancomycin-resistant S. aureus (VRSA) strains have been reported; however, these infections are still unlikely to occur [12]. Hence, most MRSA infections remain susceptible to vancomycin. Nonetheless, relapse of MRSA infections is quite common, even after prolonged antibiotic treatment of patients [13]. These data suggest the existence of a staphylococcal reservoir that is tolerant to vancomycin treatment. This phenomenon was also observed in experimental mouse studies in which infections with vancomycin-susceptible MRSA strains could still cause disease even in the presence of vancomycin [14]. State-of-the-art in vivo imaging revealed that this may be due to S. aureus adapting to intracellular habitation in macrophages, where bacteria are sheltered from the harmful effects of antibiotics. Upon termination of the vancomycin treatment, the escaped bacteria could survive in the extracellular microenvironment and cause dissemination to other organs and sepsis [15].
Most microbiology and infectious disease textbooks state that S. aureus is solely an extracellular pathogen, however, over the last decades, there has been a significant body of evidence showing that S. aureus can have a pronounced intracellular component in its pathogenic lifecycle [16]. During infection, S. aureus actively employs several invasion strategies to hide inside non-immune cells and expresses an abundance of virulence factors that combat antimicrobial effector functions of phagocytes [17]. Despite S. aureus’ extensive immune evasion repertoire, which includes many molecules to evade uptake by phagocytes, the bacteria are inevitably phagocytosed by professional phagocytes such as macrophages or neutrophils [18]. Within these professional phagocytes, S. aureus ends up in phagolysosomes, which are bactericidal compartments with the main purpose of eradicating bacteria. However, it has become clear that S. aureus can withstand the killing mechanisms of professional phagocytes and survive intracellularly [15,19,20,21,22]. Evidence suggests that the intracellular survival of S. aureus plays a critical role in the antibiotic tolerance of these bacteria. In this review, we will discuss the virulence mechanisms that mediate the intracellular survival of S. aureus, together with the current development of antibiotic therapies that specifically target the intracellular S. aureus reservoir.
2. The Infectious Cycle of S. aureus in Non-Professional Phagocytes
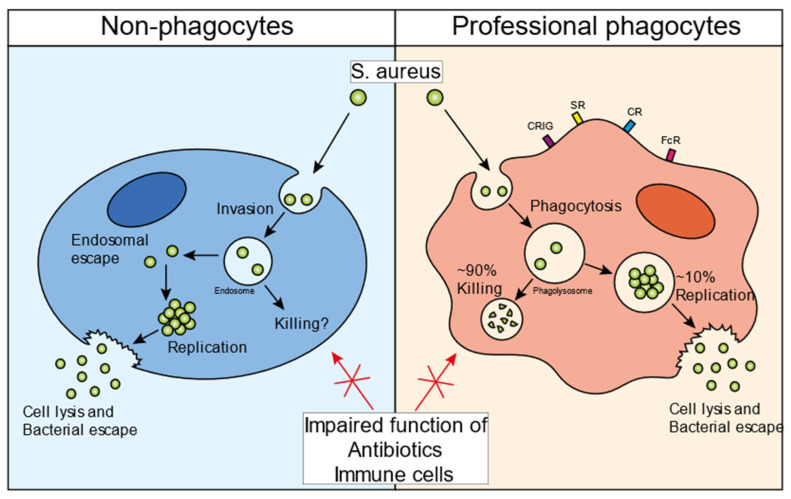
Intracellular survival of S. aureus has been shown in vitro in almost every cell type, including epithelial cells, endothelial cells, osteoclasts, keratinocytes, and fibroblasts [23,24,25,26,27]. However, the infectious cycle caused by the bacteria depends on the cell type that is infected (Figure 1). In general, for the invasion of non-professional phagocytes, S. aureus employs a zipper-like mechanism that utilizes fibronectin-binding proteins A and B. These proteins attract fibronectin to the bacterial surface and this bacterial-protein complex is subsequently recognized by integrin α5β1, leading to the internalization of staphylococci in non-professional phagocytes [28]. Several additional staphylococcal surface proteins have been shown to promote internalization in mammalian cells, such as clumping factor A (ClfA), iron-regulated surface determinant B (IsdB), and efflux pump tet38 [28]. S. aureus can escape from the endosome into the cytosol of the host cell [29]. The bacteria can replicate inside the cytosol, eventually causing cell lysis and escaping into the extracellular environment (Figure 1, left panel). Various bacterial mediators have been suggested to play a role in this escape and survival mechanism. The bacterial two-component system (TCS) accessory gene regulator (Agr), a quorum-sensing regulatory system, has been found to be a key mediator in the process of the endosomal escape of bacteria [30,31,32]. In HeLa cells, Agr is required for autophagy-mediated cytotoxicity and is essential for bacterial escape into the cytoplasm, intracellular replication, and host cell killing [32]. Importantly, this intracellular S. aureus survival mechanism was not observed with an Agr mutant strain. The Agr system is a strict regulator for phenol-soluble modulin (PSM)-α, and -β production [33]. Using an elegant method of S. aureus detection in the cytosol, this study showed that PSM-α is crucial for the destruction of the endosomal membrane in epithelial and endothelial cells [34]. An intracellular role of PSMs is supported by the fact that serum lipoproteins can protect against PSM-induced host cell lysis, both in vitro and in vivo [35,36]. Thus, Agr and PSMs play important roles in the intracellular survival of S. aureus in non-professional phagocytes. However, evidence for intracellular survival in these cell types has only been shown in vitro and there has rarely been any direct visualization of staphylococci persisting in non-professional phagocytes in animal models of infection or in tissues dissected from infected patients.
Figure 1.
S. aureus infectious cycle. Schematic overview of the staphylococcal infectious life cycle in non-professional phagocytes (left panel) and professional phagocytes (right panel). S. aureus invades non-professional phagocytes and ends up inside the endosome. Various bacterial factor regulators, such as Agr and SigmaB, are important for the endosomal escape of the bacteria into the cytosol. Once inside the cytosol, bacteria can persist inside the host cells, maintaining an intracellular bacterial reservoir. Additionally, bacteria can start replicating, eventually lysing the host cell, and escaping into the extracellular environment. Professional phagocytes recognize bacteria with different receptors and take up bacteria by means of phagocytosis (right panel). Once inside the phagolysosome, ~90% of the bacteria are successfully killed. However, the remaining ~10% can evade the intraphagolysosomal killing strategies and then use the cells as a niche to start replicating. Similar to non-professional phagocytes, the bacteria can eventually lyse the host cell and escape into the extracellular environment. The intracellular bacteria in all cell types are protected from immune cells as well as antibiotic treatment. Abbreviations: CRIg, complement receptor of immunoglobulin superfamily; SR, scavenger receptor; CR, complement receptor; FcR, Fc receptor.
3. The Infectious Cycle of S. aureus in Professional Phagocytes
Intracellular survival has also been observed in professional phagocytes such as neutrophils and macrophages [37,38]. There are important differences between these immune cells. Neutrophils are bone marrow-derived, short-lived, and mostly found in the bloodstream, although very limited numbers can be found extravasated in the peripheral tissues. Neutrophils are typically the first cells to arrive at the site of infection and have an incredible capacity for killing bacteria [39]. Macrophages, on the other hand, populate many different tissues and body cavities in large numbers. In homeostasis, macrophages can be derived from yolk-sac or fetal liver progenitors and are self-maintained by local proliferation or by bone marrow-derived monocytes. Upon inflammation, the turnover of macrophages is primarily monocyte-dependent, as monocytes are recruited and differentiate into macrophages. Depending on the tissue they populate, macrophages have very different gene expression profiles and can exert specific cellular functions such as clearance of dead cells, antigen presentation, and coordination of responses to infection [40].
In 1956, a seminal study by David Rogers showed that upon bloodstream infection in rabbits, a small number of bacteria was not removed from the blood by macrophages in the liver, but was internalized by neutrophils [41]. These intracellular bacteria were predicted to be responsible for the large wave of dissemination later in infection. Since then, others have shown that live S. aureus can survive in both mouse and human neutrophils and have proposed the “Trojan Horse” theory of dissemination by these immune cells [42]. Intravenous infection of S. aureus in mice leads to the uptake of bacteria by neutrophils that carry them in the blood for hours [43]. Isolated neutrophils from an infected animal were sufficient to cause full-blown disease in healthy animals [37]. Furthermore, patients with normal neutrophil counts may be more susceptible to bacterial dissemination compared to patients with reduced neutrophil counts [44]. Although neutrophils have been demonstrated to enable the persistence of S. aureus during infection, eventually leading to bacterial escape, there is limited experimental in vivo evidence that replication occurs inside these cells [37].
In contrast to neutrophils, there is an increasing body of in vivo evidence that intracellular replication occurs in macrophages. During bloodstream infection, the vast majority of staphylococci are taken up in the liver by the largest population of resident macrophages in the body, called Kupffer cells (KCs). KCs are a self-sustaining population of macrophages strategically situated within the liver sinusoids and in direct contact with the blood circulation [45]. In contrast to many other tissue macrophages and neutrophils, KCs are able to catch bacteria directly out of the bloodstream and overcome the high shear forces of blood flow [46]. KCs are crucial cells during bloodstream S. aureus infections, and KC depletion leads to massive, sustained bacteremia, and a greatly increased mortality due to S. aureus infections. Since KCs can kill ~90% of the injected inoculum, they are regarded as an important initial immune bottleneck during staphylococcal bloodstream infections, whereas neutrophils are essential at later time points [47]. Various studies have shown that not all staphylococci succumb to the intracellular bactericidal phagolysosome of KCs, and high-resolution intravital microscopy in combination with replication-reporter bacteria showed that staphylococci can replicate within these cells over time [15,19,20,21,22].
Using intravital microscopy in living animals, it was shown that S. aureus replicates in approximately 10% of infected KCs. Microcolonies of approximately 50–70 bacteria can be observed to lead to the destruction of the KC, which is followed by dissemination to various other internal organs, primarily the kidney (Figure 1, right panel). Mechanistically, S. aureus was found to replicate in phagolysosomes from KCs that did not produce sufficient reactive oxygen species (ROS) to kill the intracellular bacteria [15]. Interestingly, these replicating staphylococci induced α-toxin expression, which is released back into the circulation, thereby causing platelet aggregation, microthrombus formation, vascular occlusion, and organ dysfunction [48]. Even though α-toxin was upregulated by S. aureus in KCs, it remains to be determined which staphylococcal toxin is responsible for the lysis of Kupffer cells. Jorch et al. showed that after S. aureus escapes from the KCs, they relocate from the liver into the peritoneal cavity, where they get phagocytosed by Gata6+ peritoneal macrophages [49]. Similar to KC infections, S. aureus can persist inside the peritoneal macrophages and disseminate to organs in the peritoneal cavity, including the kidneys. Collectively, these data show that in the murine bloodstream infection model, S. aureus can infect and escape from multiple macrophage subtypes, ultimately seeding into the kidney, where it establishes classical abscesses 3–4 days post-infection. In a murine airway infection model, macrophages were also essential for the clearance of S. aureus since the loss of alveolar macrophages inhibited the killing of bacteria and significantly enhanced mortality [50]. Some studies have shown that S. aureus is actively taken up by alveolar macrophages, although it is currently unclear whether S. aureus replication occurs in this population of resident macrophages.
4. Intraphagolysosomal Evasion Strategies of S. aureus
Unlike bacterial invasion observed upon the infection of non-professional phagocytes, professional phagocytes engage in a variety of phagocytic receptors to actively engulf and internalize bacteria via phagocytosis (Figure 1, right panel). During phagocytosis, the microbe is enclosed in a bacteria-containing phagosome within the phagocytic cell. These phagosomes undergo several maturation steps to ultimately become phagolysosomes, a process in which the interior of the vesicle progressively increases its acidity. Phagolysosomes contain a variety of antimicrobial peptides and degradative lysosomal enzymes, and are bombarded with ROS, all of which contribute to the killing of ingested bacteria [51]. The microbicidal activity of the phagolysosome is incredibly disruptive to most bacteria, and in order to establish an infection, many pathogens have evolved various strategies to neutralize or resist the effector components of the phagolysosome. Nonetheless, for S. aureus, there is some controversy on how these bacteria can overcome the phagosomal effector functions. Some literature suggests that the phagosomal maturation pathway is hijacked by S. aureus [22,52], while most papers found normal phagosomal maturation suggesting that S. aureus can withstand the harmful environment of the phagolysosome [3,15,21,34,53,54,55]. S. aureus, therefore, must overcome these defensive mechanisms in order to enable intracellular replication. Unsurprisingly, the bacteria express a plethora of evasion molecules inside the phagolysosome (Figure 2; Table 1). In this section, we will briefly highlight the most important mechanisms and some novel findings. For more information on this topic, there are some excellent reviews that have been previously published [17,28,56].
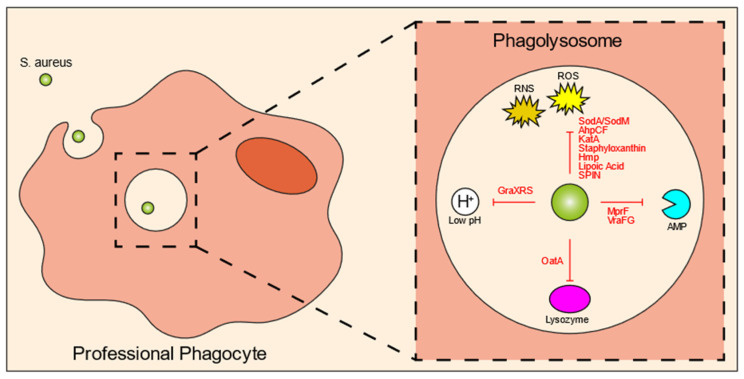
Figure 2.
S. aureus intraphagolysosomal evasion strategies. Once inside the phagolysosome of professional phagocytes, the bacteria get exposed to various antimicrobial molecules designed to kill the bacteria. Co-evolution of host and pathogen has led to a variety of evasion molecules produced by S. aureus to counteract these phagolysosomal killing mechanisms. These mechanisms include evasion molecules against ROS and RNS, AMPs, lysozyme, and acidic pH. Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species; Sod, superoxide dismutase; AhpCF, alkyl hydroperoxidase reductase; KatA, catalase; Hmp, flavohemoglobin; SPIN, staphylococcal peroxidase inhibitor; AMP, antimicrobial peptide; MprF, multiple peptide resistance factor; OatA, O-acetyltransferase.
Table 1.
S. aureus intraphagolysosomal evasion strategies. Bacterial evasion molecules against reactive oxygen species, acidic pH, enzymes, and antimicrobial peptides present in the host phagolysosomal environment. Abbreviations: ROS, reactive oxygen species; Sod, superoxide dismutase; AhpCF, alkyl hydroperoxidase reductase; KatA, catalase; Hmp, flavohemoglobin; RNS, reactive nitrogen species; SPIN, staphylococcal peroxidase inhibitor; MPO, myeloperoxidase; OatA, O-acetyltransferase; MprF, multiple peptide resistance factor; AMPs, antimicrobial peptides.
| Intracellular Killing Mechanisms | S. aureus Evasion Molecule | Mechanism/Explanation | References |
|---|---|---|---|
| ROS | SodA and SodM | Incapacitate superoxide radicals | Karavolos et al., 2003; Das, Saha and Bishayi 2008 [57,58] |
| AhpCF | Resists peroxides | Cosgrove et al., 2007; Mashruwala and Boyd 2017 [59,60] | |
| KatA | Resists H2O2 | Cosgrove et al., 2007; Mashruwala and Boyd 2017 [59,60] | |
| Staphyloxanthin | Antioxidant | Pandey, Sahukhal and Elasri 2019 [61] | |
| Hmp | Resistance to nitric oxide | Nobre, Gonçalves and Saraiva 2008 [62] | |
| lipoic acid | Restricts ROS and RNS production | Grayczyk et al., 2019 [63] | |
| SPIN | Inhibits MPO | de Jong et al., 2017 [64] | |
| Acidification | GraXRS | Senses low pH and allows resistance to acidic environment | Flannagan et al., 2018 [67] |
| Enzymes | OatA | Modifies lysozymal target | Bera et al., 2006; Shimada et al., 2010 [70,71] |
| Antimicrobial peptides | MprF | Resistance to defensins and protegrins | Kristian et al., 2003; Peschel et al., 2001; Peschel et al., 1999 [72,73,74] |
| VraFG | Promotes resistance to cationic AMPs | Li et al., 2007 [75] |
One of the most important killing mechanisms of the host cells is ROS, which is produced by NADPH oxidase (NOX2) via catalyzation of superoxide (O2−). Oxygen radicals have great antimicrobial activity, targeting various bacterial components, such as DNA, proteins, and the bacterial cell membrane. Nitrogen radicals can also be created by immune cells, termed reactive nitrogen species (RNS). It is not surprising that most S. aureus evasion molecules are directed against these molecules (Figure 2; Table 1). SodA and SodM are staphylococcal superoxide dismutases that directly eliminate ROS [57,58]. Similarly, alkyl hydroperoxidase reductase (AhpCF) and catalase (KatA) directly incapacitate peroxides and H2O2, respectively [59,60]. Staphyloxanthin is a carotenoid pigment with strong antioxidant properties [61]. For nitric oxide resistance, S. aureus can produce flavohemoglobin (Hmp), which acts like a denitrosylase [62]. ROS and RNS production can be inhibited by lipoic acid [63], and staphylococcal peroxidase inhibitor (SPIN) can bind to and block myeloperoxidase (MPO), which is the enzyme that converts H2O2 and chloride into hypochlorous acid [64].
A recent study by Leliefeld et al. using an in vitro fibrinogen gel model to increase the lifespan of neutrophils, showed that neutrophils limit staphylococcal growth in a pH-dependent manner [65]. Contrarily, in macrophages, it was shown that the intraphagolysosomal acidic pH is important for S. aureus to enable replication. This was orchestrated by GraXRS, which has been shown both in vitro as well as in vivo [66,67]. Similar to survival inside non-professional phagocytes, SigmaB could play a role in intraphagosomal survival, because a wild-type S. aureus strain showed increased resistance to acidic pH and hydrogen peroxide, when compared to a SigmaB-inactivated mutant in vitro [68]. In line with this observation, the SigmaB activating protein RsbU has been shown to be necessary for the intracellular growth of S. aureus within the phagolysosomes of THP-1 macrophages and HUVEC cells [69]. Various host cell-derived enzymes are released into the phagolysosome, among which are proteases. Lysozyme, which can cleave bacterial peptidoglycan and subsequently induce bacterial cell lysis, is particularly harmful. In response, S. aureus produces O-acetyltransferase (OatA) that acetylates peptidoglycan so it can no longer be targeted by lysozyme [70,71]. Finally, there are other antimicrobial peptides released into the phagolysosomal environment, many of which are positively charged. Multiple peptide resistance factors (MprF) of S. aureus catalyze a reaction that attaches a positively-charged lysine to the negatively-charged lipids of the bacterial membrane, thereby reducing interaction with antimicrobial peptides, defensins and protegrins in particular [72,73,74]. VraFG is a transporter of antimicrobial peptides and helps with resistance against cationic antimicrobial peptides [75].
Not all studies suggest that S. aureus replicates within the phagolysosome. Kubica et al. reported that S. aureus escaped into the cytosol of macrophages using α-toxin, where they replicated for a few days followed by host cell lysis. Several S. aureus factors were seen to be important for this survival, including SigmaB and Agr [76]. Another study suggested that the S. aureus USA300 strain can disturb normal phagolysosome formation and can sense the intraphagolysosomal pH in order to escape into the cytosol and commence intracellular replication [21]. Staphylococcus-produced PSMs have been shown to be important for killing neutrophils after phagocytosis. These toxins are actively upregulated by the bacteria within the phagolysosome by Agr and the stringent response system, and both systems appear to be crucial for the intracellular production of PSMs and escape from neutrophils [77,78].
5. Bacterial Specificity of the Intracellular Reservoir
Collectively, research has shown that S. aureus can persist and replicate in multiple cell types, maintaining the bacterial population during infection, and leading to the dissemination of bacteria throughout the body. Commensal bacteria are typically cleared by host phagocytes after infection, whereas non-aureus staphylococci can persist in macrophages but do not replicate [79]. Co-infection of S. aureus and commensal bacteria only led to the proliferation of S. aureus within KCs [80]. The commensal bacteria augment S. aureus infection by acting as a sink for ROS production, thereby increasing the chance that S. aureus can initiate replication inside KCs [81]. Many other bacterial, fungal, or parasitic species have been shown to use circulating phagocytes as an opportunity for dissemination, such as Mycobacterium tuberculosis, Listeria monocytogenes, Leishmania, and Cryptococcus neoformans [82,83,84,85].
The ability to persist and replicate intracellularly might be S. aureus strain specific. For example, where the Newman strain has been shown to be acid sensitive and is cleared in an intracellular infection [86], the more virulent USA300 strain can survive in phagocytes and has even been suggested to require acidification of the phagolysosome for persistence [67]. In a study where different S. aureus strains (Cowan I, 6850, Novel, Wood 46, BS 507, BS 513, BS 800, BS 890, BS 891, and RN4220) were tested in vitro with different cell lines (HEK-293, HeLa, and EA.hy926 cells), it was observed that all strains survived inside fully acidified vesicles except for S. aureus 6850, which was present in vesicles with reduced acidification [55]. Xiong et al. compared genotypes of persistent S. aureus strains with strains associated with rapid clearance in the blood and showed that persistence was associated with overexpression of Agr, staphylococcal cassette chromosome mec type II, clonal complex 30, and spa 16 [87]. Hence, only some S. aureus strains have the unique ability to persist and grow inside host phagocytes. As outlined above, various virulence mechanisms have implicated the ability of S. aureus to withstand the innate defenses of neutrophils and macrophages, even though not all studies are in agreement. The discrepancy between studies could be due to staphylococcal strain variation, use of distinct cells and or cell lines, and methodological differences. It should also be noted that in vitro generated data might be far from the actual in vivo survival mechanisms of S. aureus within macrophages. Nevertheless, it is safe to assume that there is a redundancy in evasion strategies that provides the bacteria time to adapt to the intraphagolysosomal environment to facilitate the intracellular reservoir.
6. Intracellular Persistence Expedites Antibiotic Tolerance
Antibiotic resistance is a continuous process, and indeed, there have been MRSA species identified in the population that are macrolide-resistant [88] or vancomycin-resistant [89]. The intracellular aspect of S. aureus infections certainly impedes antibiotic efficiency and may even assist in acquiring antibiotic tolerance. The antibiotic must reach the bacteria within the cell, and depending on where the bacteria reside, this requires passing the cell membrane once if the bacteria are in the cytosol, or twice if the bacteria remain in the phagolysosome. The fact that antibiotics cannot reach intracellular bacteria has already been described decades ago in vitro [90], and is shown to be an issue for antibiotic treatment of S. aureus infections. Indeed, treatment with antibiotics is far from ideal, with failure of treatment in up to 45% of patients [91]. Some classes of antibiotics are known to poorly penetrate cells, such as β-lactams [92] and aminoglycosides [93]. It is, therefore, not surprising that in vitro studies showed that β-lactams kill intracellular bacteria less efficiently than extracellular bacteria in human THP-1 macrophages [94]. 16 different types of antibiotics from 7 pharmacological classes were used in this study and showed that all molecules except for macrolides decreased the bacterial burden inside cells in a concentration-dependent manner. However, the intracellular activities of all antibiotics tested decreased compared to the extracellular activity, and only a few of the tested compounds had an intracellular bactericidal effect. As for vancomycin, its activity inside cells was greatly impaired when compared to extracellular killings, regardless of the concentration used [95]. Not only is passing through membranes important for killing intracellular bacteria, but the antibiotic must also be able to withstand acidic pH to kill bacteria inside the phagolysosome. It has been observed that an acidic environment decreases the intracellular concentration of gentamicin, macrolides, and quinolones [93]. Interestingly, the cellular concentration of β-lactams, and more prominently, rifampicin, were enhanced in an acidic medium, although this did not improve the intracellular activity of these antibiotics. Notably, acidic pH also affects antibiotic susceptibility to bacteria, as it has been shown that intracellular MRSA, which resists the activity of β-lactams extracellularly, regains sensitivity to β-lactams and vancomycin within the intracellular compartment [96]. Nevertheless, intracellular antibiotic activity remains to be a challenge. An in vitro study by Krut, Sommer, and Kronke showed that only rifampicin was able to eradicate intracellular S. aureus from murine keratinocyte (PAM212) and fibroblast (mKSA) cell lines [97]. Some tested antibiotics, including azithromycin, clindamycin, and linezolid, merely controlled extracellular bacteria, while S. aureus persisted intracellularly. Once the antibiotics were removed from the medium, S. aureus was able to regain its cytotoxicity and escape from these cells. Taken together, antibiotics need to be able to penetrate sufficiently into cells and withstand the bactericidal phagolysosome in order to kill intracellular bacteria. Even though the bacteria might regain susceptibility to certain antibiotics, the intracellular niche by and large creates opportunities for developing antibiotic resistance, especially when the antibiotic concentration within cells remains below minimum inhibitory concentration (MIC) levels [30].
S. aureus can change its phenotype to grow subpopulations that are considered to be more antibiotic-tolerant. These small colony variants (SCVs) are characterized as non-pigmented and non-hemolytic, are slow-growing, and have a colony size of approximately 1/10 of the normal S. aureus colonies [98]. SCVs can be auxotrophs, which is primarily due to mutations in electron transport as shown for menadione- and/or hemin-auxotrophs, or mutations in thymidylate biosynthesis, all of which may be a consequence of the downregulation of citric acid cycle activity [99]. SCV infections have been linked to relapsing or persistent infections in various diseases, such as cystic fibrosis and osteomyelitis [100,101]. SCVs have been demonstrated to be a highly antibiotic tolerant subpopulation of S. aureus, particularly because of their slow growth rate. As bacteriostatic antibiotics depend on obstructing bacterial cell growth for their function, this type of treatment is useless for SCV treatment, because the bacteria are already arrested at their cell growth. Bactericidal antibiotics on the other hand aim to cause cell death by interrupting active processes, such as peptidoglycan synthesis for β-lactams [102], translation for aminoglycosides [103], and DNA replication for fluoroquinolones [104]. However, because these processes are greatly reduced in SCVs, the bacteria can tolerate the antibiotics in their environment. Typically, SCVs only arise on a culture plate after 72 h, which is explained by mutations often observed in the Agr system to facilitate a non-hemolytic phenotype [105]. While other studies show that SCVs occur randomly without the involvement of Agr or Sae [106]. These mutations are reversible however, restoring SCVs to a fully cytotoxic wild-type phenotype, as has been shown in vivo where SCV-infected mice showed dissemination of bacteria in the spleen and kidney that were of wild-type phenotype [98].
Recently, Rowe et al. demonstrated that S. aureus remaining inside macrophages can acquire antibiotic tolerance to multiple antibiotics in the presence of rifampicin in a ROS-dependent manner [107]. The bacterial tricarboxylic acid (TCA) cycle was inhibited by ROS, leading to decreased respiration and ATP generation by the bacteria. Similar to SCVs of S. aureus, these persister cells have a significantly reduced metabolism, which is not compatible with antibiotics and allows the bacteria to actively exploit this antibiotic tolerance. The same group demonstrated that a variety of clinical S. aureus variants all acquired antibiotic tolerance to a similar extent when they were phagocytosed by activated macrophages, even though the ability of these strains to form a persister phenotype in vitro varied greatly [108]. Nevertheless, it was demonstrated that antibiotic tolerance was generated in a ROS-dependent manner in the spleen during a murine systemic infection model [107]. Host-derived peroxynitrite, the reaction product of hydrogen peroxide and nitrite, were found to be the main mediators for inducing this tolerance. In this study, the authors hypothesized that the bacteria reached a persister phenotype in a ROS-dependent manner. The influence of ROS on antibiotic tolerance is supported by studies showing that reduced phagocytic burst in macrophages increased antibiotic efficacy against S. aureus and that improved antibiotic function was observed when bacteria were engulfed in polymorphonuclear (PMN) cells of patients suffering from granulomatous disease [109]. In KCs, ROS production varies greatly between individual cells, and even between phagolysosomes within a single KC [15]. It is tempting to speculate that intracellular S. aureus may start replicating in phagolysosomes with low ROS activity and persister induction could occur in phagolysosomes with high ROS production. However, it remains to be investigated what percentage of staphylococci residing in the phagolysosomes of KCs develop into persisters and induce antibiotic tolerance.
In summary, there are several factors contributing to the improved antibiotic resistance of intracellular S. aureus. First, antibiotics might not be able to reach the bacteria through the cell membrane. Second, antibiotic activity may be altered because of the intraphagolysosomal environment within macrophages. Third, over time, bacteria inside macrophages may change their phenotype to an SCV-like state, which is tolerant to antibiotic action. Finally, ROS inside phagolysosomes may induce a cell metabolism arrested state that, similar to the SCV phenotype, is antibiotic tolerant. Finding ways to reach the intracellular bacteria and kill them when they are in an inactive state is extremely important to counteract S. aureus infections.
7. Treatment Options to Manage Intracellular S. aureus Infections
In the last decade, a large number of new studies have identified treatment options that show promising activity against intracellular S. aureus. However, many of these studies have only been performed in vitro, so there is not much indication of their success in a clinical context. There are some recent reviews that give an overview of new compounds that have been developed over the last years [110,111,112]. In this section of the review, we summarize the most promising novel antibiotic strategies that specifically target the intracellular MRSA reservoir, which have shown in vivo effectivity or are currently being tested in clinical trials. We have divided the new treatment options into three different classes: delivery systems, conjugated bioproteins, and indirect killing mechanisms (Figure 3).
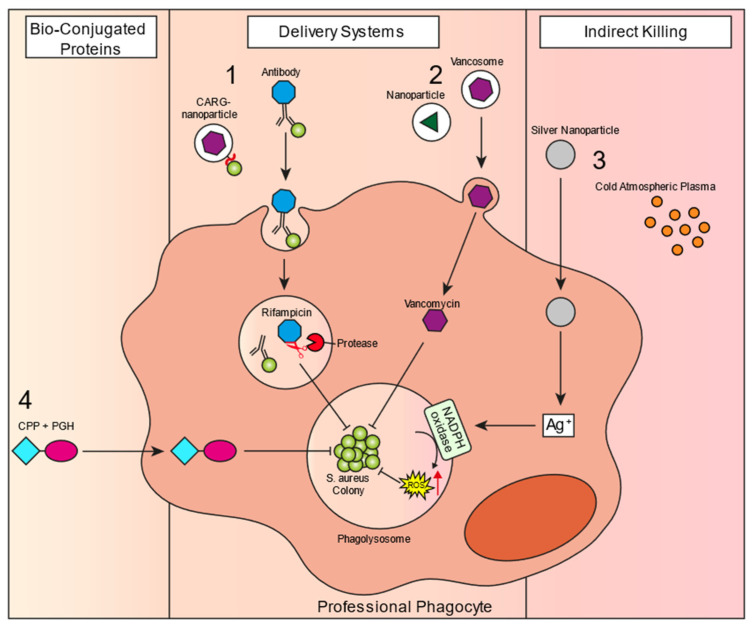
Figure 3.
Novel treatment strategies to reach the intracellular reservoir of S. aureus. Schematic of different therapeutics that can penetrate host cells. 1. S. aureus-targeted delivery: antibody conjugated to antibiotic—a specific S. aureus antibody binds extracellular bacteria and is taken up into the phagolysosome with the bacteria. Inside the phagolysosome, host-derived proteases can cleave off the rifalogue conjugated to the antibody, activating it, which can then attack the intracellular bacteria; CARG-nanoparticles—a specific peptide based on phage-displayed peptide libraries conjugated to vancomycin-containing nanoparticles. Similar to the antibody approach, it targets S. aureus and can reach the intracellular reservoir. 2. Nanoparticle/liposome delivery system: antibiotics can be taken up inside nanoparticles or liposomes, which can fuse with the cellular membrane to deliver the encapsulated drug intracellularly. 3. Indirect killing: silver nanoparticles deliver silver into the host cell cytoplasm, which in turn activates NADPH oxidase to induce ROS production, leading to improved killing of the intracellular reservoir. Cold atmospheric plasma can directly act upon NADPH oxidase to stimulate ROS production. 4. Bio-conjugated proteins: cell penetrating peptides (CPPs) are molecules that can penetrate the host cell to deliver the attached peptidoglycan hydrolase (PGH).
7.1. Delivery Systems
To reach the intracellular reservoir of S. aureus, various delivery formulations have been developed that induce phagocytic uptake of the particle and, in turn, deliver the encapsulated antibiotic to kill the intracellular bacteria. These systems include various nanoparticles and liposomes that have been developed to deliver antibiotics non-specifically to the intracellular compartment of the host cells or have S. aureus specificity to reach the intracellular niche.
7.1.1. Intracellular Delivery
Numerous studies have reported the development of nanoparticles that show intracellular effectivity against bacterial infections. Nanoparticles have great potential to encapsulate and carry drugs to intracellular bacteria and were already demonstrated to have treatment efficacy against well-known intracellular bacteria, such as Listeria monocytogenes and Salmonella spp. 30 years ago [113,114]. Like nanoparticles, antibiotic-loaded liposomes are another promising class of antimicrobial formulations that enhance intracellular antimicrobial efficacy against S. aureus infections. The efficacy of liposomes has been shown over a decade ago in a study that used gold nanoparticle-stabilized liposomes containing vancomycin as a compound against MRSA and showed improved inhibition of bacterial growth compared to free vancomycin [115]. As the bacteria reside in the acidic phagolysosomal environment, a promising approach was shown to be pH-responsive lipid-dendrimer hybrid nanoparticles (LDH-NPs), which were used to deliver vancomycin [116]. These LDH-NPs showed significantly improved killing of intracellular MRSA in HEK 293 cells in vitro. Complete eradication of MRSA was found when cells were treated with LDH-NPs, whereas millions of bacteria remained when treated with vancomycin alone. Importantly, the nanoparticles showed no toxicity towards HEK 293 cells, as none of these cells stained positive for live cells, indicating Hoechst 33342 staining. Additionally, another study had a similar approach with pH-responsive liposomes, which contained quaternary lipid (QL) and oleic acid (OA) in the membrane, which together formed a complex structure [117]. These QL-OA-complexes on the liposomal surface change the composition of acidic pH, creating pores and leading to drug release. Treatment with QL-OA-liposomes had a significantly improved antibacterial effect compared to vancomycin alone, both in TPH-1 macrophage and HEK293 cell lines in vitro. A similar effect was observed in a skin infection mouse model in vivo. Together, these results show that pH-responsive nanoparticles might be a promising new strategy to treat intracellular infections. To deplete KCs, clodronate-loaded liposomes were the method of choice for many years before ingenious genetic tools became available. Furthermore, it has been shown that upon intravenous injection, 70% to 80% of liposomes concentrate in KCs [118]. This propensity for KCs to take up liposomes inspired us to use liposomes containing vancomycin or vancosomes to precision-target the intracellular reservoir of S. aureus within KCs [15]. The efficacy of vancosomes was 30-fold higher than treatment with vancomycin alone, which was not able to reach the intracellular staphylococci. Intravital microscopy showed that vancosomes co-localized with MRSA-containing KCs and increased intracellular killing was observed. This was sufficient to prevent liver damage and bacterial dissemination to the kidneys. Importantly, upon the termination of the antibiotic treatment, the staphylococci escaped from the intracellular niche and caused dissemination to the kidney in the vancomycin control group, which was not seen when the intracellular reservoir was eradicated by vancosomes. As vancosomes have shown their efficacy in vivo, this might be an exciting strategy to target the intracellular bacterial reservoir in patients. Finally, a study by Li et al. generated membrane-encapsulated, antimicrobial-conjugated nanoparticles (Me-ANPs) consisting of self-assembling triclosan, and ciprofloxacin conjugates within membranes isolated from J774.A1 macrophages [119]. These Me-ANPs were found to specifically be taken up by S. aureus-infected macrophages, as the negative charge of the nanoparticles could bind to positive patches of infected macrophages that were lysozyme-rich. Furthermore, Me-ANPs showed significantly improved killing capacity of intracellular S. aureus in J774.A1 macrophages in vitro compared to standard NPs or free ciprofloxacin. Me-ANP treatment was also significantly more effective in vivo, both in an acute peritoneal infection model as well as in a mouse organ infection model. This shows that coating nanoparticles with macrophage membranes could enhance the delivery potential of antibiotics.
7.1.2. S. aureus-Targeted Delivery
Investigators from Genentech took a completely novel approach to target the intracellular reservoirs. They developed an antibody-antibiotic conjugate (AAC) where an anti-staphylococcal wall techochoic acid (WTA) monoclonal antibody was conjugated to a rifalogue [14]. The antibiotic rifalogue can be liberated from the antibody when it is cleaved off by intracellular cathepsins and does not have an antibiotic function when still attached to the antibody. The antibody is S. aureus specific, and binds extracellular bacteria, leading to internalization while still attached to the bacteria. When the antibody-antibiotic-bacteria complex is phagocytosed, intraphagolysosomal cathepsin liberates the antibiotic that can subsequently kill the bacteria. This is a very promising method to kill hard-to-treat intracellular infections as it showed great efficacy in staphylococcal bacteremia models [14], and this AAC is currently in a phase-one clinical trial [120]. In line with this, another more recent study used phage-displayed peptide libraries to select peptides with a high binding capacity to S. aureus [121]. In vivo screening confirmed the binding of the peptides to bacteria in S. aureus infected lungs. Next, an identified peptide of interest, CARGGLKSC (CARG), was conjugated to vancomycin-loaded nanoparticles. In a murine lung infection model, the nanoparticles were shown to specifically co-localize in S. aureus infected lungs, whereas no nanoparticle localization of Pseudomonas infected lungs was observed. Furthermore, 100% survival of CARG-nanoparticle treated mice was observed compared to 33% survival after an equivalent dose of vancomycin. One great advantage of these targeted delivery systems is that only the specific microbe is targeted, while broad-spectrum antibiotics indiscriminately kill both pathogens and commensals. The latter could result in dysbiosis of the gut microbiota, which has been associated with Clostridium difficile infections and diseases such as inflammatory bowel disease, obesity, and atherosclerosis. However, the intracellular specificity of these approaches could also be considered a disadvantage as it does not kill MRSA extracellularly, nor does it eliminate biofilm-associated infections. Additionally, in patients with low phagocyte levels, such as cancer patients that receive chemotherapy, which is a known risk factor for staphylococcal infections, this approach may be ineffective.
7.2. Bio-Conjugated Proteins
Similar to nanoparticles and liposomes, bio-conjugation provides a delivery strategy for existing antibiotics into the host cells to kill the intracellular staphylococcal reservoir. A few bio-conjugated proteins have been described in the literature, of which one has shown in vivo effectivity. In this study, the authors looked into peptidoglycan hydrolases (PGHs), such as lysostaphin, for bio-conjugation [122]. Peptidoglycan hydrolases are enzymes that can cleave the peptidoglycan of the bacterial cell wall, inducing lysis. After screening for various cell-penetrating peptides (CPPs) and PGHs, bio-conjugation of lysostaphin (LST) to either trans-activator of transcription (TAT) or penetratin showed the most promise, as improved intracellular killing of different S. aureus strains (Newman, Cowan, and USA300 JE2) was observed in vitro in three eukaryotic cell lines (A549, MG-63, and 3T3-L1). No detectable CFU counts were observed for these bio-conjugated proteins after 4 h of treatment compared to considerable viable CFUs (~104 CFU/mL) after treatment with LST alone. The authors also showed that a variety of different CPG-PGH combinations worked in synergy to kill bacteria, with decreased intracellular bacterial counts, weight loss, and abscess volume in a murine abscess model compared to the individual CPG-PGH molecules. These CPG-PGH cocktail therapies are a promising treatment option for intracellular infections.
7.3. Indirect Killing Mechanisms
Rather than delivering an antibiotic into the cells, indirect killing mechanisms are strategies to boost host-cell killing capacities against S. aureus. Two approaches discussed below activate ROS production by macrophages. Other approaches led to the activation of intracellular receptors of innate immunity NOD2, which detected fragments of peptidoglycans-muramyl peptides, including those from Staphylococcus aureus. Inducing ROS production could be a very valuable antibacterial strategy, as the literature has shown that phagolysosomes with a reduced ROS capacity are most vulnerable to harboring the intracellular staphylococcal reservoir. Therefore, these approaches could prove to be useful tools for antibiotic treatment, either on their own or in combination with bacterial-targeted approaches.
7.3.1. Silver Nanoparticles
Metal nanoparticles have multiple antimicrobial properties that could be useful for intracellular infections, especially because these combined properties decrease antibiotic resistance opportunities for the bacteria. Silver nanoparticles (AgNPs) release Ag+ ions that can both disrupt electron transport and induce ROS production, which in turn harms the bacteria [123]. These AgNPs have been shown to kill 100% of extracellular bacteria as well as 76% of intracellular S. aureus within osteoblasts. The intracellular killing was at similar rates to the rifampicin treatment, whereas no intracellular killing was shown with gentamicin treatment alone. A clinical trial (NCT04431440) took sputum and blood samples of 75 critically ill patients and found 55.3% occurrence of MRSA and 12.7% occurrence of VRSA in the samples [124]. S. aureus bacteria were isolated from the samples and tested on culture plates with AgNP-discs, which showed effective inhibition of growth for both MRSA and VRSA variants. If efficacy is shown in vivo, AgNPs may provide a useful tool to combat S. aureus infections.
7.3.2. Cold Atmospheric Plasma
Cold atmospheric plasma (CAP), electrical discharged gas, has antimicrobial properties, which a study by Duchesne et al. investigated [125]. It was found that CAP treatment increased intracellular ROS in RAW 264.7 macrophages irrespective of whether the cells were infected with S. aureus. CAP significantly reduced intracellular MRSA USA300 infection in macrophages over 7 h of infection compared to an untreated control. However, bacterial killing by CAP was only improved by approximately 45% compared to the untreated bacteria, and treatment was initiated prior to bacterial infection, which is not relevant in a clinical situation. The authors concluded that CAP increased ROS production by macrophages, leading to an increased killing capacity. In a recent clinical pilot study, CAP was tested on patients with mild to moderate atopic dermatitis [126]. CAP-treated patients showed significant improvement in the clinical score for the severity of the disease. Additionally, a microbiome analysis was performed with 16s-RNA sequencing before and after treatment, which showed a significant decrease in S. aureus prevalence in the skin microbiome, compared to sham patients. Together, these studies show that CAP is safe to use on patients, but the intracellular effect of this compound must be examined further. This compound might prove to be useful in combination with other therapeutic strategies.
7.3.3. Muramyl Peptides
Activation of intracellular NOD receptors to potentiate the killing of intracellular S. aureus is another important avenue of research. Muramyl peptides present in the staphylococcal cell walls have been shown to increase the phagocytic and microbicidal activity of neutrophils, including ROS production [127]. The synergism of NOD2 receptor activation together with anti-staphylococcal immunoglobulin in patients with chronic sluggish furunculosis with identified antibiotic-resistant strains of staphylococcus made it possible to achieve remission in 99% of patients while using only anti-staphylococcal immunoglobulin. Remission was achieved in 40% of patients during six months of observation [127]. An explanation for the therapeutic effect of muramyl peptides in the treatment of diseases caused by antibiotic-resistant strains of staphylococcus may be an increase in the production of cyclooxygenase-2 (COX-2) [128] and the recruitment of neutrophils when exposed to muramyl peptides together with staphylococcus aureus lipoteichoic acid at the site of inflammation [129].
In summary, innovative targeting approaches are continuously in development, many of which show promising results for treating intracellular S. aureus infections.
8. Conclusions
It has become clear over the past decades that S. aureus has an intracellular aspect to its infectious cycle, leading to infections that are difficult to treat. Persistence in both non-professional as well as professional phagocytes has been suggested in an abundance of studies. Moreover, it has been shown in vivo that S. aureus can use liver KCs as a niche to replicate upon bloodstream infection, while hiding from other host immune cells as well as antibiotic therapy. This intracellular aspect of the bacterial infectious cycle could in turn stimulate adaptations in S. aureus that favor antibiotic tolerance. Thus, it is crucial to find alternate strategies to kill the intracellular staphylococcal reservoir. So far, several new therapeutic strategies have been suggested with variable, yet promising results. Initially, during bloodstream infection, 10% survival of the bacteria inside KCs is enough to enable the preservation of an intracellular reservoir; any potential antibiotic strategy must further reduce that survival rate. Likely, some combination of currently available antibiotics plus a novel therapeutic strategy will have optimal efficacy, as not only the intracellular, but also the extracellular bacteria need to be targeted. Finding the ideal possible combination is of high importance, as AMR remains a global health problem. The herein described strategies illustrate major steps in the right direction towards finding a suitable approach to deal with the intracellular staphylococcal reservoir.
Acknowledgments
We thank Bruna Araujo David, Marie Siwicki, and Lindsey Orthner for their thoughtful comments on the manuscript.
Author Contributions
Conceptualization, J.W.H. and B.G.J.S.; writing—original draft preparation, J.W.H.; writing—review and editing, B.G.J.S.; visualization, J.W.H.; funding acquisition, B.G.J.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the University of Calgary, Department of Microbiology, Immunology, and Infectious Disease, the International Microbiome Centre, and the Snyder Institute for Chronic Diseases; University of Calgary Start-up funds.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray C.J., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim H.F.L., Melles D.C., Vos M.C., Van Leeuwen W., Van Belkum A., Verbrugh H.A., Nouwen J.L. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect. Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Lowy F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Foster T.J. Colonization and Infection of the Human Host by Staphylococci: Adhesion, Survival and Immune Evasion. Vet. Dermatol. 2009;20:456–470. doi: 10.1111/j.1365-3164.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 5.Macneal W.J., Frisbee F.C. One Hundred Patients with Staphylococcus Septicemia Receiving Bacteriophage Service. Am. J. Med. Sci. 1936;191:179–195. doi: 10.1097/00000441-193602000-00004. [DOI] [Google Scholar]
- 6.Wyllie D.H., Crook D.W., Peto T.E.A. Mortality after Staphylococcus Aureus Bacteraemia in Two Hospitals in Oxfordshire, 1997–2003: Cohort Study. BMJ. 2006;333:281. doi: 10.1136/bmj.38834.421713.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kourtis A.P., Hatfield K., Baggs J., Mu Y., See I., Epson E., Nadle J., Kainer M.A., Dumyati G., Petit S., et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019;68:214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell K.M.G., Hodgkinson J.T., Sore H.F., Welch M., Salmond G.P.C., Spring D.R. Combating Multidrug-Resistant Bacteria: Current Strategies for the Discovery of Novel Antibacterials. Angew. Chem. Int. Ed. 2013;52:10706–10733. doi: 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- 9.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 10.Furuya E.Y., Lowy F.D. Antimicrobial-Resistant Bacteria in the Community Setting. Nat. Rev. Microbiol. 2006;4:36–45. doi: 10.1038/nrmicro1325. [DOI] [PubMed] [Google Scholar]
- 11.Voyich J.M., Braughton K.R., Sturdevant D.E., Whitney A.R., Saïd-Salim B., Porcella S.F., Long R.D., Dorward D.W., Gardner D.J., Kreiswirth B.N., et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J. Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 12.Nannini E., Murray B.E., Arias C.A. Resistance or Decreased Susceptibility to Glycopeptides, Daptomycin, and Linezolid in Methicillin-Resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 2010;10:516–521. doi: 10.1016/j.coph.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Kreisel K., Boyd K., Langenberg P., Roghmann M.C. Risk Factors for Recurrence in Patients with Staphylococcus aureus Infections Complicated by Bacteremia. Diagn. Microbiol. Infect. Dis. 2006;55:179–184. doi: 10.1016/j.diagmicrobio.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Lehar S.M., Pillow T., Xu M., Staben L., Kajihara K.K., Vandlen R., DePalatis L., Raab H., Hazenbos W.L., Hiroshi Morisaki J., et al. Novel Antibody-Antibiotic Conjugate Eliminates Intracellular S. Aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 15.Surewaard B.G.J., Deniset J.F., Zemp F.J., Amrein M., Otto M., Conly J., Omri A., Yates R.M., Kubes P. Identification and Treatment of the Staphylococcus aureus Reservoir in Vivo. J. Exp. Med. 2016;213:1141–1151. doi: 10.1084/jem.20160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraunholz M., Sinha B. Intracellular Staphylococcus aureus: Live-in and Let Die. Front. Cell. Infect. Microbiol. 2012;2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pidwill G.R., Gibson J.F., Cole J., Renshaw S.A., Foster S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2021;11:3506. doi: 10.3389/fimmu.2020.620339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn J., Stelzner K., Rudel T., Fraunholz M. Inside Job: Staphylococcus aureus Host-Pathogen Interactions. Int. J. Med. Microbiol. 2018;308:607–624. doi: 10.1016/j.ijmm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Flannagan R.S., Heit B., Heinrichs D.E. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens. 2015;4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacoma A., Cano V., Moranta D., Regueiro V., Domínguez-Villanueva D., Laabei M., González-Nicolau M., Ausina V., Prat C., Bengoechea J.A. Investigating Intracellular Persistence of Staphylococcus aureus within a Murine Alveolar Macrophage Cell Line. Virulence. 2017;8:1761–1775. doi: 10.1080/21505594.2017.1361089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tranchemontagne Z.R., Camire R.B., O’Donnell V.J., Baugh J., Burkholder K.M. Staphylococcus aureus Strain USA300 Perturbs Acquisition of Lysosomal Enzymes and Requires Phagosomal Acidification for Survival inside Macrophages. Infect. Immun. 2015;84:241–253. doi: 10.1128/IAI.00704-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubrail J., Morris P., Bewley M.A., Stoneham S., Johnston S.A., Foster S.J., Peden A.A., Read R.C., Marriott H.M., Dockrell D.H. Inability to Sustain Intraphagolysosomal Killing of Staphylococcus aureus Predisposes to Bacterial Persistence in Macrophages. Cell. Microbiol. 2016;18:80–96. doi: 10.1111/cmi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayles K.W., Wesson C.A., Liou L.E., Fox L.K., Bohach G.A., Trumble W.R. Intracellular Staphylococcus aureus Escapes the Endosome and Induces Apoptosis in Epithelial Cells. Infect. Immun. 1998;66:336–342. doi: 10.1128/IAI.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess D.J., Henry-Stanley M.J., Erickson E.A., Wells C.L. Intracellular Survival of Staphylococcus aureus within Cultured Enterocytes. J. Surg. Res. 2003;114:42–49. doi: 10.1016/S0022-4804(03)00314-7. [DOI] [PubMed] [Google Scholar]
- 25.Menzies B.E., Kourteva I. Staphylococcus aureus α-Toxin Induces Apoptosis in Endothelial Cells. FEMS Immunol. Med. Microbiol. 2000;29:39–45. doi: 10.1016/S0928-8244(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 26.Nair S.P., Bischoff M., Senn M.M., Berger-Bächi B. The ΣB Regulon Influences Internalization of Staphylococcus aureus by Osteoblasts. Infect. Immun. 2003;71:4167–4170. doi: 10.1128/IAI.71.7.4167-4170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers R., Tompsett R. The Survival of Staphylococci within Human Leukocytes. J. Exp. Med. 1952;28:470. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moldovan A., Fraunholz M.J. In or out: Phagosomal Escape of Staphylococcus aureus. Cell. Microbiol. 2019;21:e12997. doi: 10.1111/cmi.12997. [DOI] [PubMed] [Google Scholar]
- 30.Qazi S.N.A., Harrison S.E., Self T., Williams P., Hill P.J. Real-Time Monitoring of Intracellular Staphylococcus aureus Replication. J. Bacteriol. 2004;186:1065–1077. doi: 10.1128/JB.186.4.1065-1077.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shompole S., Henon K.T., Liou L.E., Dziewanowska K., Bohach G.A., Bayles K.W. Biphasic Intracellular Expression of Staphylococcus aureus Virulence Factors and Evidence for Agr-Mediated Diffusion Sensing. Mol. Microbiol. 2003;49:919–927. doi: 10.1046/j.1365-2958.2003.03618.x. [DOI] [PubMed] [Google Scholar]
- 32.Schnaith A., Kashkar H., Leggio S.A., Addicks K., Krönke M., Krut O. Staphylococcus aureus Subvert Autophagy for Induction of Caspase-Independent Host Cell Death. J. Biol. Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 33.Queck S.Y., Jameson-Lee M., Villaruz A.E., Bach T.H.L., Khan B.A., Sturdevant D.E., Ricklefs S.M., Li M., Otto M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosz M., Kolter J., Paprotka K., Winkler A.C., Schäfer D., Chatterjee S.S., Geiger T., Wolz C., Ohlsen K., Otto M., et al. Cytoplasmic Replication of Staphylococcus aureus upon Phagosomal Escape Triggered by Phenol-Soluble Modulin α. Cell. Microbiol. 2014;16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surewaard B.G.J., Nijland R., Spaan A.N., Kruijtzer J.A.W., de Haas C.J.C., van Strijp J.A.G. Inactivation of Staphylococcal Phenol Soluble Modulins by Serum Lipoprotein Particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hommes J.W., Kratofil R.M., Wahlen S., de Haas C.J.C., Hildebrand R.B., Hovingh G.K., Otto M., van Eck M., Hoekstra M., Korporaal S.J.A., et al. High Density Lipoproteins Mediate in Vivo Protection against Staphylococcal Phenol-Soluble Modulins. Sci. Rep. 2021;11:15357. doi: 10.1038/s41598-021-94651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gresham H.D., Lowrance J.H., Caver T.E., Wilson B.S., Cheung A.L., Lindberg F.P. Survival of Staphylococcus aureus Inside Neutrophils Contributes to Infection. J. Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 38.Koziel J., Maciag-Gudowska A., Mikolajczyk T., Bzowska M., Sturdevant D.E., Whitney A.R., Shaw L.N., DeLeo F.R., Potempa J. Phagocytosis of Staphylococcus aureus by Macrophages Exerts Cytoprotective Effects Manifested by the Upregulation of Antiapoptotic Factors. PLoS ONE. 2009;4:e5210. doi: 10.1371/journal.pone.0005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spaan A.N., Surewaard B.G.J., Nijland R., van Strijp J.A.G. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu. Rev. Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 40.Varol C., Mildner A., Jung S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015;33:643–675; 0324141122. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 41.Rogers D.E. Studies on Bacteriemia I. Mechanisms Relating to the Persistence of Bacteriemia in Rabbits Following the Intravenous Injection of Staphylococci. J. Exp. Med. 1956;103:713–742. doi: 10.1084/jem.103.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thwaites G.E., Gant V. Are Bloodstream Leukocytes Trojan Horses for the Metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 2011;9:215–222. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 43.Krezalek M.A., Hyoju S., Zaborin A., Okafor E., Chandrasekar L., Bindokas V., Guyton K., Montgomery C.P., Daum R.S., Zaborina O., et al. Can Methicillin-Resistant Staphylococcus aureus Silently Travel from the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Ann. Surg. 2018;267:749–758. doi: 10.1097/SLA.0000000000002173. [DOI] [PubMed] [Google Scholar]
- 44.Venditti M., Falcone M., Micozzi A., Carfagna P., Taglietti F., Serra P.F., Martino P. Staphylococcus aureus Bacteremia in Patients with Hematologic Malignancies: A Retrospective Case-Control Study. Haematologica. 2003;88:923–930. [PubMed] [Google Scholar]
- 45.Krenkel O., Tacke F. Liver Macrophages in Tissue Homeostasis and Disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 46.Jenne C.N., Kubes P. Immune Surveillance by the Liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 47.Pollitt E.J.G., Szkuta P.T., Burns N., Foster S.J. Staphylococcus aureus Infection Dynamics. PLoS Pathog. 2018;14:e1007112. doi: 10.1371/journal.ppat.1007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surewaard B.G.J., Thanabalasuriar A., Zeng Z., Tkaczyk C., Cohen T.S., Bardoel B.W., Jorch S.K., Deppermann C., Bubeck Wardenburg J., Davis R.P., et al. α-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe. 2018;24:271–284. doi: 10.1016/j.chom.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorch S.K., Surewaard B.G.J., Hossain M., Peiseler M., Deppermann C., Deng J., Bogoslowski A., van der Wal F., Omri A., Hickey M.J., et al. Peritoneal GATA6+ Macrophages Function as a Portal for Staphylococcus aureus Dissemination. J. Clin. Investig. 2019;129:4643–4656. doi: 10.1172/JCI127286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitur K., Parker D., Nieto P., Ahn D.S., Cohen T.S., Chung S., Wachtel S., Bueno S., Prince A. Toxin-Induced Necroptosis Is a Major Mechanism of Staphylococcus aureus Lung Damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flannagan R.S., Cosío G., Grinstein S. Antimicrobial Mechanisms of Phagocytes and Bacterial Evasion Strategies. Nat. Rev. Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 52.Kahl B.C., Goulian M., Van Wamel W., Herrmann M., Simon S.M., Kaplan G., Peters G., Cheung A.L. Staphylococcus aureus RN6390 Replicates and Induces Apoptosis in a Pulmonary Epithelial Cell Line. Infect. Immun. 2000;68:5385–5392. doi: 10.1128/IAI.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flannagan R.S., Heit B., Heinrichs D.E. Intracellular Replication of Staphylococcus aureus in Mature Phagolysosomes in Macrophages Precedes Host Cell Death, and Bacterial Escape and Dissemination. Cell. Microbiol. 2016;18:514–535. doi: 10.1111/cmi.12527. [DOI] [PubMed] [Google Scholar]
- 54.Jarry T.M., Cheung A.L. Staphylococcus aureus Escapes More Efficiently from the Phagosome of a Cystic Fibrosis Bronchial Epithelial Cell Line than from Its Normal Counterpart. Infect. Immun. 2006;74:2568–2577. doi: 10.1128/IAI.74.5.2568-2577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lâm T.T., Giese B., Chikkaballi D., Kühn A., Wolber W., Pané-Farré J., Schäfer D., Engelmann S., Fraunholz M., Sinha B. Phagolysosomal Integrity Is Generally Maintained after Staphylococcus aureus Invasion of Nonprofessional Phagocytes but Is Modulated by Strain 6850. Infect. Immun. 2010;78:3392–3393. doi: 10.1128/IAI.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis M.L., Surewaard B.G.J. Neutrophil Evasion Strategies by Streptococcus Pneumoniae and Staphylococcus aureus. Cell Tissue Res. 2018;371:489–503. doi: 10.1007/s00441-017-2737-2. [DOI] [PubMed] [Google Scholar]
- 57.Karavolos M.H., Horsburgh M., Ingham E., Foster S.J. Role and Regulation of the Superoxide Dismutases of Staphylococcus aureus. Microbiology. 2003;149:2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 58.Das D., Saha S.S., Bishayi B. Intracellular Survival of Staphylococcus aureus: Correlating Production of Catalase and Superoxide Dismutase with Levels of Inflammatory Cytokines. Inflamm. Res. 2008;57:340–349. doi: 10.1007/s00011-007-7206-z. [DOI] [PubMed] [Google Scholar]
- 59.Cosgrove K., Coutts G., Jonsson I.M., Tarkowski A., Kokai-Kun J.F., Mond J.J., Foster S.J. Catalase (KatA) and Alkyl Hydroperoxide Reductase (AhpC) Have Compensatory Roles in Peroxide Stress Resistance and Are Required for Survival, Persistence, and Nasal Colonization in Staphylococcus aureus. J. Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mashruwala A.A., Boyd J.M. The Staphylococcus aureus SrrAB Regulatory System Modulates Hydrogen Peroxide Resistance Factors, Which Imparts Protection to Aconitase during Aerobic Growth. PLoS ONE. 2017;12:e0170283. doi: 10.1371/journal.pone.0170283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandey S., Sahukhal G.S., Elasri M.O. The MsaABCR Operon Regulates the Response to Oxidative Stress in Staphylococcus aureus. J. Bacteriol. 2019;201:e00417-19. doi: 10.1128/JB.00417-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nobre L.S., Gonçalves V.L., Saraiva L.M. Flavohemoglobin of Staphylococcus aureus. Methods Enzymol. 2008;436:203–216. doi: 10.1016/S0076-6879(08)36011-X. [DOI] [PubMed] [Google Scholar]
- 63.Grayczyk J.P., Alonzo F., III Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production to Promote Survival during Infection. Infect. Immun. 2019;87:e00344-19. doi: 10.1128/IAI.00344-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Jong N.W.M., Ramyar K.X., Guerra F.E., Nijland R., Fevre C., Voyich J.M., McCarthy A.J., Garcia B.L., Van Kessel K.P.M., Van Strijp J.A.G., et al. Immune Evasion by a Staphylococcal Inhibitor of Myeloperoxidase. Proc. Natl. Acad. Sci. USA. 2017;114:9439–9444. doi: 10.1073/pnas.1707032114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leliefeld P.H.C., Pillay J., Vrisekoop N., Heeres M., Tak T., Kox M., Rooijakkers S.H.M., Kuijpers T.W., Pickkers P., Leenen L.P.H., et al. Differential Antibacterial Control by Neutrophil Subsets. Blood Adv. 2018;2:1344–1354. doi: 10.1182/bloodadvances.2017015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villanueva M., García B., Valle J., Rapún B., Ruiz De Los Mozos I., Solano C., Martí M., Penadés J.R., Toledo-Arana A., Lasa I. Sensory Deprivation in Staphylococcus aureus. Nat. Commun. 2018;9:523. doi: 10.1038/s41467-018-02949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flannagan R.S., Kuiack R.C., McGavin M.J., Heinrichs D.E. Staphylococcus aureus Uses the GraXRS Regulatory System To Sense and Adapt to the Acidified Phagolysosome in Macrophages. MBio. 2018;9:e01143-18. doi: 10.1128/mBio.01143-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan P.F., Foster S.J., Ingham E., Clements M.O. The Staphylococcus aureus Alternative Sigma Factor σ(B) Controls the Environmental Stress Response but Not Starvation Survival or Pathogenicity in a Mouse Abscess Model. J. Bacteriol. 1998;180:6082–6089. doi: 10.1128/JB.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olivier A.C., Lemaire S., Van Bambeke F., Tulkens P.M., Oldfield E. Role of RsbU and Staphyloxanthin in Phagocytosis and Intracellular Growth of Staphylococcus aureus in Human Macrophages and Endothelial Cells. J. Infect. Dis. 2009;200:1367–1370. doi: 10.1086/606012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bera A., Biswas R., Herbert S., Götz F. The Presence of Peptidoglycan O-Acetyltransferase in Various Staphylococcal Species Correlates with Lysozyme Resistance and Pathogenicity. Infect. Immun. 2006;74:4598–4604. doi: 10.1128/IAI.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimada T., Park B.G., Wolf A.J., Brikos C., Goodridge H.S., Becker C.A., Reyes C.N., Miao E.A., Aderem A., Götz F., et al. Staphylococcus aureus Evades Lysozyme-Based Peptidoglycan Digestion That Links Phagocytosis, Inflammasome Activation, and IL-1β Secretion. Cell Host Microbe. 2010;7:38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kristian S.A., Dürr M., Van Strijp J.A.G., Neumeister B., Peschel A. MprF-Mediated Lysinylation of Phospholipids in Staphylococcus aureus Leads to Protection against Oxygen-Independent Neutrophil Killing. Infect. Immun. 2003;71:546–549. doi: 10.1128/IAI.71.1.546-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peschel A., Jack R.W., Otto M., Collins L.V., Staubitz P., Nicholson G., Kalbacher H., Nieuwenhuizen W.F., Jung G., Tarkowski A., et al. Staphylococcus aureus Resistance to Human Defensins and Evasion of Neutrophil Killing via the Novel Virulence Factor MprF Is Based on Modification of Membrane Lipids with L-Lysine. J. Exp. Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peschel A., Otto M., Jack R.W., Kalbacher H., Jung G., Götz F. Inactivation of the Dlt Operon in Staphylococcus aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 75.Li M., Cha D.J., Lai Y., Villaruz A.E., Sturdevant D.E., Otto M. The Antimicrobial Peptide-Sensing System Aps of Staphylococcus aureus. Mol. Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 76.Kubica M., Guzik K., Koziel J., Zarebski M., Richter W., Gajkowska B., Golda A., Maciag-Gudowska A., Brix K., Shaw L., et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. Aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surewaard B., de Haas C., Vervoort F., Rigby K., DeLeo F., Otto M., van Strijp J., Nijland R. Staphylococcal Alpha-Phenol Soluble Modulins Contribute to Neutrophil Lysis after Phagocytosis. Cell Microbiol. 2013;15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geiger T., Francois P., Liebeke M., Fraunholz M., Goerke C., Krismer B., Schrenzel J., Lalk M., Wolz C. The Stringent Response of Staphylococcus aureus and Its Impact on Survival after Phagocytosis through the Induction of Intracellular PSMs Expression. PLoS Pathog. 2012;8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flannagan R.S., Watson D.W., Surewaard B.G.J., Kubes P., Heinrichs D.E. The Surreptitious Survival of the Emerging Pathogen Staphylococcus Lugdunensis within Macrophages as an Immune Evasion Strategy. Cell. Microbiol. 2018;20:e12869. doi: 10.1111/cmi.12869. [DOI] [PubMed] [Google Scholar]
- 80.Boldock E., Surewaard B.G.J., Shamarina D., Na M., Fei Y., Ali A., Williams A., Pollitt E.J.G., Szkuta P., Morris P., et al. Human Skin Commensals Augment Staphylococcus aureus Pathogenesis. Nat. Microbiol. 2018;3:881–890. doi: 10.1038/s41564-018-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibson J.F., Pidwill G.R., Carnell O.T., Surewaard B.G.J., Shamarina D., Sutton J.A.F., Jeffery C., Derré-Bobillot A., Archambaud C., Siggins M.K., et al. Commensal Bacteria Augment Staphylococcus aureus Infection by Inactivation of Phagocyte-Derived Reactive Oxygen Species. PLoS Pathog. 2021;17:e1009880. doi: 10.1371/journal.ppat.1009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis J.M., Ramakrishnan L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drevets D.A. Dissemination of Listeria Monocytogenes by Infected Phagocytes. Infect. Immun. 1999;67:3512–3517. doi: 10.1128/IAI.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters N.C., Egen J.G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M.P., Germain R.N., Sacks D. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science. 2008;321:970–975. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi M., Li S.S., Zheng C., Jones G.J., Kim K.S., Zhou H., Kubes P., Mody C.H. Real-Time Imaging of Trapping and Urease-Dependent Transmigration of Cryptococcus Neoformans in Mouse Brain. J. Clin. Investig. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sedlyarov V., Eichner R., Girardi E., Essletzbichler P., Goldmann U., Nunes-Hasler P., Srndic I., Moskovskich A., Heinz L.X., Kartnig F., et al. The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification. Cell Host Microbe. 2018;23:766–774. doi: 10.1016/j.chom.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong Y.Q., Fowler V.G., Yeaman M.R., Perdreau-Remington F., Kreiswirth B.N., Bayer A.S. Phenotypic and Genotypic Characteristics of Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia In Vitro and in an Experimental Endocarditis Model. J. Infect. Dis. 2009;199:201–208. doi: 10.1086/595738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bishr A.S., Abdelaziz S.M., Yahia I.S., Yassien M.A., Hassouna N.A., Aboshanab K.M. Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients. Biology. 2021;10:624. doi: 10.3390/biology10070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klevens R.M., Morrison M.A., Nadle J., Petit S., Gershman K., Ray S., Harrison L.H., Lynfield R., Dumyati G., Townes J.M., et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. J. Am. Med. Assoc. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 90.Holmes B., Quie P.G., Windhorst D.B., Pollara B., Good R.A. Protection of Phagocytized Bacteria from the Killing Action of Antibiotics. Nature. 1966;210:1131–1132. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- 91.Yoon Y.K., Kim J.Y., Park D.W., Sohn J.W., Kim M.J. Predictors of Persistent Methicillin-Resistant Staphylococcus aureus Bacteraemia in Patients Treated with Vancomycin. J. Antimicrob. Chemother. 2010;65:1015–1018. doi: 10.1093/jac/dkq050. [DOI] [PubMed] [Google Scholar]
- 92.Yancey R.J., Sanchez M.S., Ford C.W. Activity of Antibiotics against Staphylococcus aureus within Polymorphonuclear Neutrophils. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:107–113. doi: 10.1007/BF01964421. [DOI] [PubMed] [Google Scholar]
- 93.Van Bambeke F., Barcia-Macay M., Lemaire S., Tulkens P.M. Cellular Pharmacodynamics and Pharmacokinetics of Antibiotics: Current Views and Perspectives. Curr. Opin. Drug Discov. Dev. 2006;9:218–230. [PubMed] [Google Scholar]
- 94.Barcia-Macay M., Seral C., Mingeot-Leclercq M.P., Tulkens P.M., Van Bambeke F. Pharmacodynamic Evaluation of the Intracellular Activities of Antibiotics against Staphylococcus aureus in a Model of THP-1 Macrophages. Antimicrob. Agents Chemother. 2006;50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Nawas B., Shah P.M. Intracellular Activity of Vancomycin and Ly333328, a New Semisynthetic Glycopeptide, against Methicillin-Resistant Staphylococcus aureus. Infection. 1998;26:165–167. doi: 10.1007/BF02771843. [DOI] [PubMed] [Google Scholar]
- 96.Sabath L.D., Wallace S.J., Gerstein D.A. Suppression of Intrinsic Resistance to Methicillin and Other Penicillins in Staphylococcus aureus. Antimicrob. Agents Chemother. 1972;2:350–355. doi: 10.1128/AAC.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krut O., Sommer H., Krönke M. Antibiotic-Induced Persistence of Cytotoxic Staphylococcus aureus in Non-Phagocytic Cells. J. Antimicrob. Chemother. 2004;53:167–173. doi: 10.1093/jac/dkh076. [DOI] [PubMed] [Google Scholar]
- 98.Sandberg A., Lemaire S., Van Bambeke F., Tulkens P.M., Hughes D., Von Eiff C., Frimodt-Møller N. Intra- and Extracellular Activities of Dicloxacillin and Linezolid against a Clinical Staphylococcus aureus Strain with a Small-Colony-Variant Phenotype in an In Vitro Model of THP-1 Macrophages and an In Vivo Mouse Peritonitis Model. Antimicrob. Agents Chemother. 2011;55:1443–1452. doi: 10.1128/AAC.00205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kriegeskorte A., Grubmüller S., Huber C., Kahl B.C., von Eiff C., Proctor R.A., Peters G., Eisenreich W., Becker K. Staphylococcus aureus Small Colony Variants Show Common Metabolic Features in Central Metabolism Irrespective of the Underlying Auxotrophism. Front. Cell. Infect. Microbiol. 2014;4:141. doi: 10.3389/fcimb.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahl B., Herrmann M., Everding A.S., Koch H.G., Becker K., Harms E., Proctor R.A., Peters G. Persistent Infection with Small Colony Variant Strains of Staphylococcus aureus in Patients with Cystic Fibrosis. J. Infect. Dis. 1998;177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 101.Sendi P., Rohrbach M., Graber P., Frei R., Ochsner P.E., Zimmerli W. Staphylococcus aureus Small Colony Variants in Prosthetic Joint Infection. Clin. Infect. Dis. 2006;43:961–967. doi: 10.1086/507633. [DOI] [PubMed] [Google Scholar]
- 102.Cho H., Uehara T., Bernhardt T.G. Beta-Lactam Antibiotics Induce a Lethal Malfunctioning of the Bacterial Cell Wall Synthesis Machinery. Cell. 2014;159:1300–1311. doi: 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davis B.D., Chen L., Tai P.C. Misread Protein Creates Membrane Channels: An Essential Step in the Bactericidal Action of Aminoglycosides. Proc. Natl. Acad. Sci. USA. 1986;83:6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malik M., Zhao X., Drlica K. Lethal Fragmentation of Bacterial Chromosomes Mediated by DNA Gyrase and Quinolones. Mol. Microbiol. 2006;61:810–825. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 105.Sendi P., Proctor R.A. Staphylococcus aureus as an Intracellular Pathogen: The Role of Small Colony Variants. Trends Microbiol. 2009;17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Edwards A.M. Phenotype Switching Is a Natural Consequence of Staphylococcus aureus Replication. J. Bacteriol. 2012;194:5404–5412. doi: 10.1128/JB.00948-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rowe S.E., Wagner N.J., Li L., Beam J.E., Wilkinson A.D., Radlinski L.C., Zhang Q., Miao E.A., Conlon B.P. Reactive Oxygen Species Induce Antibiotic Tolerance during Systemic Staphylococcus aureus Infection. Nat. Microbiol. 2020;5:282–290. doi: 10.1038/s41564-019-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beam J.E., Wagner N.J., Shook J.C., Bahnson E.S.M., Fowler V.G.J., Rowe S.E., Conlon B.P. Macrophage-Produced Peroxynitrite Induces Antibiotic Tolerance and Supersedes Intrinsic Mechanisms of Persister Formation. Infect. Immun. 2021;89:e00286-21. doi: 10.1128/IAI.00286-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacobs R.F., Wilson C.B. Activity of Antibiotics in Chronic Granulomatous Disease Leukocytes. Pediatr. Res. 1983;17:916–919. doi: 10.1203/00006450-198311000-00016. [DOI] [PubMed] [Google Scholar]
- 110.Hulme J. Application of Nanomaterials in the Prevention, Detection, and Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA) Pharmaceutics. 2022;14:805. doi: 10.3390/pharmaceutics14040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamaruzzaman N.F., Kendall S., Good L. Targeting the Hard to Reach: Challenges and Novel Strategies in the Treatment of Intracellular Bacterial Infections. Br. J. Pharmacol. 2017;174:2225–2236. doi: 10.1111/bph.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanamala K., Tatiparti K., Bhise K., Sau S., Scheetz M.H., Rybak M.J., Andes D., Iyer A.K. Novel Approaches for the Treatment of Methicillin-Resistant Staphylococcus aureus: Using Nanoparticles to Overcome Multidrug Resistance. Drug Discov. Today. 2021;26:31–43. doi: 10.1016/j.drudis.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinto-Alphandary H., Balland O., Laurent M., Andremont A., Puisieux F., Couvreur P. Intracellular Visualization of Ampicillin-Loaded Nanoparticles in Peritoneal Macrophages Infected in Vitro with Salmonella Typhimurium. Pharm. Res. 1994;11:38–46. doi: 10.1023/A:1018985308984. [DOI] [PubMed] [Google Scholar]
- 114.Forestier F., Gerrier P., Chaumard C., Quero A.M., Couvreur P., Labarre C. Effect of Nanoparticle-Bound Ampicillin on the Survival of Listeria Monocytogenes in Mouse Peritoneal Macrophages. J. Antimicrob. Chemother. 1992;30:173–179. doi: 10.1093/jac/30.2.173. [DOI] [PubMed] [Google Scholar]
- 115.Pornpattananangkul D., Zhang L., Olson S., Aryal S., Obonyo M., Vecchio K., Huang C.-M., Zhang L. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maji R., Omolo C.A., Agrawal N., Maduray K., Hassan D., Mokhtar C., Mackhraj I., Govender T. PH-Responsive Lipid-Dendrimer Hybrid Nanoparticles: An Approach to Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019;16:4594–4609. doi: 10.1021/acs.molpharmaceut.9b00713. [DOI] [PubMed] [Google Scholar]
- 117.Omolo C.A., Megrab N.A., Kalhapure R.S., Agrawal N., Jadhav M., Mocktar C., Rambharose S., Maduray K., Nkambule B., Govender T. Liposomes with PH Responsive ‘on and off’ Switches for Targeted and Intracellular Delivery of Antibiotics. J. Liposome Res. 2021;31:45–63. doi: 10.1080/08982104.2019.1686517. [DOI] [PubMed] [Google Scholar]
- 118.Senior J.H. Fate and Behavior of Liposomes in Vivo: A Review of Controlling Factors. Crit. Rev. Ther. Drug Carrier Syst. 1987;3:123–193. [PubMed] [Google Scholar]
- 119.Li Y., Liu Y., Ren Y., Su L., Li A., An Y., Rotello V., Zhang Z., Wang Y., Liu Y., et al. Coating of a Novel Antimicrobial Nanoparticle with a Macrophage Membrane for the Selective Entry into Infected Macrophages and Killing of Intracellular Staphylococci. Adv. Funct. Mater. 2020;30:2004942. doi: 10.1002/adfm.202004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peck M., Rothenberg M.E., Deng R., Lewin-Koh N., She G., Kamath A.V., Carrasco-Tringuero M., Saad O., Castro A., Teufel L., et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2019;63:e02588-18. doi: 10.1128/AAC.02588-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hussain S., Joo J., Kang J., Kim B., Braun G.B., She Z.G., Kim D., Mann A.P., Mölder T., Teesalu T., et al. Antibiotic-Loaded Nanoparticles Targeted to the Site of Infection Enhance Antibacterial Efficacy. Nat. Biomed. Eng. 2018;2:95–103. doi: 10.1038/s41551-017-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Röhrig C., Huemer M., Lorgé D., Luterbacher S., Phothaworn P., Schefer C., Sobieraj A.M., Zinsli L.V., Mairpady Shambat S., Leimer N., et al. Targeting Hidden Pathogens: Cell-Penetrating Enzybiotics Eradicate Intracellular Drug-Resistant Staphylococcus aureus. MBio. 2020;11:e00209-20. doi: 10.1128/mBio.00209-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qayyum S., Oves M., Khan A.U. Obliteration of Bacterial Growth and Biofilm through ROS Generation by Facilely Synthesized Green Silver Nanoparticles. PLoS ONE. 2017;12:e0181363. doi: 10.1371/journal.pone.0181363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elsawy S., Elsherif W.M., Hamed R. Effect of Silver Nanoparticles on Vancomycin Resistant Staphylococcus aureus Infection in Critically Ill Patients. Pathog. Glob. Health. 2021;115:315–324. doi: 10.1080/20477724.2021.1914412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duchesne C., Frescaline N., Blaise O., Lataillade J.-J., Banzet S., Dussurget O., Rousseau A. Cold Atmospheric Plasma Promotes Killing of Staphylococcus aureus by Macrophages. mSphere. 2021;6:e00217-21. doi: 10.1128/mSphere.00217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim Y.J., Lim D.J., Lee M.Y., Lee W.J., Chang S.E., Won C.H. Prospective, Comparative Clinical Pilot Study of Cold Atmospheric Plasma Device in the Treatment of Atopic Dermatitis. Sci. Rep. 2021;11:14461. doi: 10.1038/s41598-021-93941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guryanova S.V., Khaitov R.M. Strategies for Using Muramyl Peptides—Modulators of Innate Immunity of Bacterial Origin—in Medicine. Front. Immunol. 2021;12:607178. doi: 10.3389/fimmu.2021.607178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahn K.B., Jeon J.H., Baik J.E., Park O.-J., Kang S.-S., Yun C.-H., Park J.-H., Han S.H. Muramyl Dipeptide Potentiates Staphylococcal Lipoteichoic Acid Induction of Cyclooxygenase-2 Expression in Macrophages. Microbes Infect. 2014;16:153–160. doi: 10.1016/j.micinf.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Bougarn S., Cunha P., Harmache A., Fromageau A., Gilbert F.B., Rainard P. Muramyl Dipeptide Synergizes with Staphylococcus aureus Lipoteichoic Acid to Recruit Neutrophils in the Mammary Gland and to Stimulate Mammary Epithelial Cells. Clin. Vaccine Immunol. 2010;17:1797–1809. doi: 10.1128/CVI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]