Abstract
A combination of a three-dimensional conformal radiation therapy (3D-CRT) plan with a dose gradient of the chest wall area and a volumetric modulated arc therapy (VMAT) plan of the supraclavicular area might improve the dose distribution robustness in the junction. To investigate the impact of patient motion on the dose distribution, hybrid 3D-CRT and VMAT plans were recalculated by shifting the isocenter of the VMAT plan. Compared to the nominal plan, the target D98% for high- vs low-dose gradients decreased by 24% vs 12%. Hybrid VMAT with a low-dose gradient 3D-CRT plan was found to be robust towards patient motion.
Keywords: Breast cancer, Hybrid-VMAT, Junction region, Patient motion, Plan robustness
1. Introduction
Breast cancer accounts for a significant proportion of the cancer-related cases among female patients treated with radiation therapy (RT). Adjuvant RT is performed after a lumpectomy or mastectomy to reduce the locoregional recurrence and improve the survival rate [1], [2], [3]. Traditionally, two opposing tangential fields and a mono-isocentric half-beam-blocked anterior field or two opposing anterior–posterior fields have been employed to treat the chest wall area and supraclavicular nodes, respectively [4], [5], [6], [7]. Intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) have been proposed for the treatment of locoregional breast cancer to improve dose homogeneity and conformity, and base-tangential hybrid techniques have been reported to optimize the balance between the target coverage and organ-at-risk (OAR) sparing [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. We use a combination of three-dimensional conformal radiation therapy (3D-CRT) plan with a dose gradient of chest wall area and the VMAT plan of supraclavicular area to provide better target coverage and dose homogeneity [17]. Field edges with steep dose gradients and patient motion may have a significant impact on the dose distribution in the junction region [5], [6], [7]. A robust planning technique for patient motion at the junction of adjacent treatment fields should be considered to avoid the emergence of hot and cold spots.
In this study, the robustness of the hybrid VMAT treatment plans for postoperative breast cancer patients was improved by considering the patient motion with a dose gradient using different jaw settings for the 3D-CRT field on the cranial side.
2. Material and methods
2.1. Patient
All patients provided written informed consent for obtaining clinical data. This study was approved by the institutional review board of Hiroshima University (E-947). Ten post-mastectomy breast cancer patients (left, n = 5; right, n = 5) with supraclavicular nodes, who received adjuvant locoregional RT between August 2020 and September 2020, were selected for this study. The median age of the patients was 61 years (range: 48–84 years). For the reproducibility of patient setup in the treatment position, patients were immobilized with customized vacuum bags in the supine position with their arms raised above their head. A radiopaque wire was placed on the patient’s midline and mid-axillary line by a radiation oncologist during the simulation to help the clinical target volume (CTV). Computed tomography (CT) images for treatment planning were acquired under free respiration for a slice thickness of 2.5 mm (580 W, GE Healthcare, Milwaukee, WI, USA). Subsequently, the CT data were exported to the Eclipse (version 13.5, Varian Medical Systems, Palo Alto, CA, USA) treatment planning system for the target and OAR delineation and treatment planning.
2.2. Treatment planning
The CTV encompassed the entire ipsilateral chest wall area with supraclavicular nodes on the basis of the breast cancer atlas established by the Radiation Therapy Oncology Group (RTOG) Breast Cancer Atlas [21] and clinical data provided by a radiation oncologist. The planning target volume (PTV) was created by adding a uniform margin of 5 mm around the CTV. The CTV was 376 ± 201 cm3 (range: 98–768 cm3), and the PTV was 647 ± 200 cm3 (range: 299–979 cm3). The “modified PTV” was devised by subtracting the PTV by a 2-mm margin corresponding to the skin surface and lungs around the PTV to evaluate the treatment plan. The ipsilateral and contralateral lungs, heart, ipsilateral humeral head, and esophagus were contoured as the OARs. The prescription dose was 50 Gy, which was administered in 25 fractions to the chest wall and regional lymph nodes via 6-MV photons with a maximum available dose rate of 600 MU/min (TrueBeam, Varian Medical Systems). For planning purposes, the Acuros XB (AXB) dose calculation algorithm with a dose grid matrix of 2.5 mm was considered. A hybrid VMAT treatment plan was created through the following three procedures in succession:
First, the isocenter was placed 2 cm caudal to the upper sternum. The gantry angles and field settings of the mediolateral (ML) field were determined while considering the doses to the lung and heart. The opposing lateromedial (LM) field was created such that the beam divergence matched the ML field at the side of the lung. The multileaf collimator (MLC) leaves were opened anterolaterally by at least 2 cm to ensure coverage of the breast during respiration and the subsequent swelling. MLCs were adjusted to shield the heart and lungs. The field-in-field technique employs three individual fields with 5-mm jaw shifts to create a low-dose gradient on the cranial side, which was denoted as “low-dose gradient.” To compare the impact of dose gradient on the dose distribution, a 3D-CRT plan was created without moving the jaw, which was defined as the “high-dose gradient” (Fig. 1-a). The weights of the three individual fields were adjusted to be approximately equivalent, and the 3D-CRT plan for the chest wall area was calculated. The 3D-CRT plan was normalized to the maximum dose (Dmax) to the chest wall area. A marginal zone with an insufficient dose (≤95% of the prescription dose) in the 3D-CRT plan was extracted and utilized for the VMAT-optimized structure to cover the PTV dose.
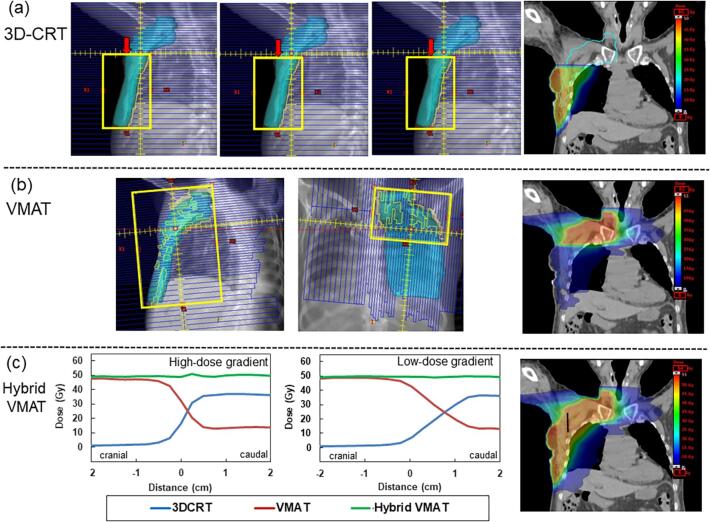
Fig. 1.
Typical field setup for the (a) three-dimensional conformal radiation therapy (3D-CRT) plan of chest wall area and (b) the volumetric modulated arc therapy (VMAT) plan of supraclavicular area. The dose gradient in the junction region is created by shifting the jaw on the cranial side. The planning target volume (PTV) is expressed in cyan, and the jaw is indicated in yellow. The shifted jaw is indicated by the red arrow. In segment 1, the tangential field is half-beam blocked at the isocenter level. In segment 2, the cranial jaw is shifted by 5 mm in the caudal direction. In segment 3, the jaw moves an additional 5 mm toward the closure. (c) Typical dose profile for hybrid VMAT plan with (a) high- or (b) low-dose gradient on 3D-CRT plan along the black line between the same two points at the junction region. Dose profiles of the 3D-CRT plan, VMAT plan, and total sum dose are indicated by the blue, red, and green lines, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Second, the VMAT plan was formulated by using two coplanar arcs with gantry rotation angles of 240° (range: from 60 to 181° and from 181 to 60° for the right side; from 179 to 300° and from 300 to 179° for the left side), and the collimator angle of each arc was set to 10° or 80° to avoid a tongue-and-groove effect (Fig. 1-b). Notably, a single arc with a collimator angle of 80° covered the supraclavicular area owing to the maximum travel of a leaf. The VMAT plan for the supraclavicular area and marginal zone was optimized for the entire PTV based on the calculation results of the 3D-CRT plan for the chest wall area. The planning objectives for the VMAT plan were identical to those for the comparison plans.
Finally, the hybrid plans were created by summing the 3D-CRT and VMAT plans (Fig. 1-c). Cumulative dose-volume histograms (DVHs) were evaluated to assess the targets and OARs. The corresponding OAR dose constraints are as follows: esophagus: D1cc (Gy) ≦20 Gy; esophagus: D5cc (Gy) ≦15 Gy; lungs, V5Gy (%) ≦30%; and lungs, mean dose (%) ≤8 Gy. In this study, we defined the treatment plan as a “nominal plan.” The dose distributions and DVHs of the hybrid VMAT plans with high- or low-dose gradients were compared to investigate the plan quality.
2.3. Perturbed dose evaluation
To investigate the impact of patient motion in the cranial–caudal (CC) direction on the dose distribution, we simulated the robustness of the hybrid VMAT plan with high- or low-dose gradients in the 3D-CRT plan. To assess the potential risk associated with patient motion, the dose distribution of the VMAT plan was shifted by 1, 2, and 3 mm in the cranial (separation) and caudal (overlap) directions. The summed dose distributions for the simulated patient motions were compared with the nominal dose distribution (i.e., absence of patient motion error). The shifted plans were recalculated with the same monitor units, gantry/collimator angles, and MLC shape as those for the nominal plan. The D2% and D98% of the CTV were assessed as indicators of hot and cold spots, which are relevant for the overlap- and separation-simulated plans, respectively.
2.4. Statistical analysis
Data were analyzed using the Wilcoxon signed-rank test with statistical significance set at p < 0.05, using a free software: R Version 3.5.2 (www.r-project.org).
3. Results
3.1. Treatment planning
The beam arrangement and an example of the dose distribution for the 3D-CRT plan on the chest wall area, VMAT plan on supraclavicular nodes, and hybrid plan on the summed plans are illustrated in Fig. 1. The dose profile in the junction region is depicted in Fig. 1(c) revealing a homogenous and smooth dose-gradient for hybrid VMAT plans both with high- or low-dose gradients. Table S1 in the supplementary materials shows the DVH statistics of the hybrid VMAT plans with high- and low-dose gradients. The doses administered to the target and OARs of the hybrid VMAT plan with high- or low-dose gradients in the 3D-CRT plan exhibited no significant differences (p > 0.05).
3.2. Perturbed dose evaluation
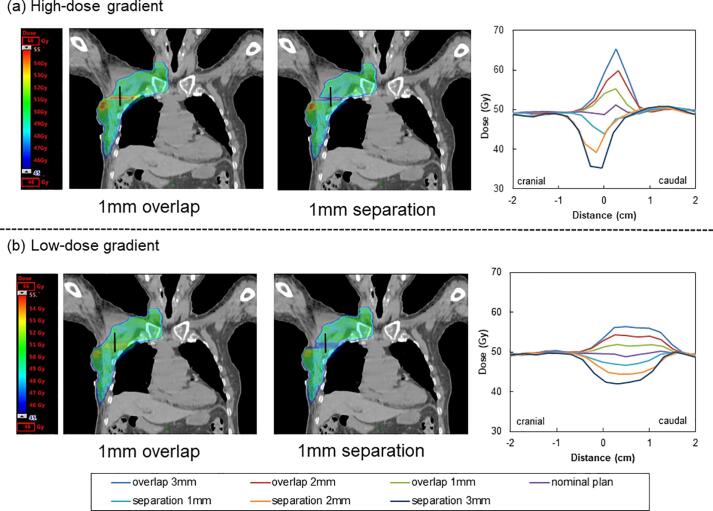
An example of the summed dose distributions with simulated patient motions of 1 mm in the CC direction for the hybrid VMAT plan with high- or low-dose gradients in the 3D-CRT plan is presented in Fig. 2. Compared to the hybrid VMAT plan with a high-dose gradient (Fig. 2-a), the hybrid VMAT plan with a low-dose gradient resulted in a reduction of hot and cold spots (Fig. 2-b). An example of the dose profiles acquired in the junction region is presented in Fig. 2. The low-dose gradient plan mitigated the dose differences against patient motion. Tables S2 and S3 in the supplementary materials shows the D2% and D98% doses to the CTV for the hybrid VMAT plan with patient motion, respectively. Compared to the nominal plan, the D2% to the CTV for the hybrid VMAT plan with high- or low-dose gradients increased by 5% versus 2%, 13% versus 7%, and 21% versus 11% for each 1 mm, 2 mm, and 3 mm overlapped shift, respectively. The D98% to the CTV for the hybrid VMAT plan with high- or low-dose gradients decreased by 6% versus 2%, 15% versus 7%, and 24% versus 12% for each 1 mm, 2 mm, and 3 mm separated shift, respectively. The D2% (overlap) and D98% (separation) of the CTV for the hybrid VMAT plan with high- or low-dose gradients exhibited significant differences (p < 0.05).
Fig. 2.
An example of the 1-mm overlapped and separated dose distribution for the hybrid volumetric modulated arc therapy (VMAT) plan with (a) high- or (b) low-dose gradients on three-dimensional conformal radiation therapy (3D-CRT) plan. Typical dose profiles for hybrid VMAT plan with high- or low-dose gradients on 3D-CRT plan for 1-mm, 2-mm, and 3-mm separated and overlapped shift along the black line at the junction region. Compared to the nominal plan, the simulation of the ± 3 mm cranial–caudal (CC) direction in the hybrid VMAT plan with a high-dose gradient in the 3D-CRT plan resulted in considerable over- (132%) and under-dosing (72%) in the junction region. The hybrid VMAT plan with low-dose gradient in the 3D-CRT plan resulted in slight over- (115%) and under-dosing (85%) in the junction region.
4. Discussion
We investigated the robustness of hybrid VMAT with high- compared to low-dose gradients in the 3D-CRT component regarding robustness against patient motion in the junction region. As shown in the dose profile, patient motion can potentially lead to areas of high and low doses, resulting in a large dose differentiation from the nominal plan. The hybrid VMAT technique with a low-dose gradient could reduce over-and under-dosage in the junction region: a shifting jaw in the 3D-CRT plan resulted in a low-dose gradient from the VMAT that was smooth in the junction region. Compared to those with a high-dose gradient, the near-maximum or near-minimum dose to the CTV for the hybrid VMAT plan with a low-dose gradient was more robust against patient motion along the longitudinal axis.
A combination of two tangential fields and VMAT for treating breast cancer has been reported previously [14]; the reported strategy comprises two tangential open fields with a 2 cm cranial slip zone delivering 85% of the dose to the chest wall area to minimize hot/cold spots in case of intrafractional movemements between breath-holds. In particular, a balance between the junction overlap length and dose step-size should be considered based on the plan quality. 3D-CRT beam weights should be assigned values as large as possible to decrease the probability of radiation-induced tumors in the contralateral breast [9].
In particular, a few limitations of this study have to be considered. Firstly, we only examined one dose gradient with jaw shift: a large jaw shift may contribute to a shallow dose gradient, which improves the robustness of the plan for patient motion. Secondly, this study was performed in ten patients: a larger patient cohort is needed to determine an adequate dose gradient. Finally, the daily setup errors may have resulted in the blurring of the cold spots or hot spots in the junction region. Owing to our image-guided strategy, mathematical computation of the probability distribution should be considered for a number of fractions as the day-to-day setup variations are random [22].
In conclusion, our study demonstrates that the geometric misalignment in the longitudinal direction owing to patient motion significantly affects the dosimetric accuracy of breast cancer treatment in the junction region. To reduce the magnitude of any over- or under-dosing that may occur during treatment when applying a hybrid VMAT technique, a low-dose gradient in the junction region in the 3D-CRT plan is advised, which improves the plan robustness and treatment delivery accuracy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by JSPS KAKENHI Grand Number 21K15846.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2022.08.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A., et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. New Engl J Med. 2002;347:1227–1232. doi: 10.1056/nejmoa020989. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/nejmoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J., Olivotto I.A., Spinelli J.J., Phillips N., Jackson S.M., Wilson K.S., et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Miles E.A., Venables K., Hoskin P.J., Aird E.G., START Trial Management Group Dosimetry and field matching for radiotherapy to the breast and supraclavicular fossa. Radiother Oncol. 2009;91:42–48. doi: 10.1016/j.radonc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Idzes M.H., Holmberg O., Mijnheer B.J., Huizenga H. Effect of set-up uncertainties on the dose distribution in the match region of supraclavicular and tangential breast fields. Radiother Oncol. 1998;46:91–98. doi: 10.1016/s0167-8140(97)00170-9. [DOI] [PubMed] [Google Scholar]
- 6.Hedin E., Bäck A., Chakarova R. Jaw position uncertainty and adjacent fields in breast cancer radiotherapy. J Appl Clin Med Phys. 2015;16:240–251. doi: 10.1120/jacmp.v16i6.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homann K.L., Gates B.E., Salehpour M., Followill D.S., Kirsner S.M., White R.A., et al. Use of a matchline dosimetry analysis tool (MDAT) to quantify dose homogeneity in the region between abutting tangential and supraclavicular radiation fields. J Appl Clin Med Phys. 2010;11:3294. doi: 10.1120/jacmp.v11i4.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaji K., Yadav P., BalajiSubramanian S., Anu Radha C., Ramasubramanian V. Hybrid volumetric modulated arc therapy for chest wall irradiation: For a good plan, get the right mixture. Phys Med. 2018;52:86–92. doi: 10.1016/j.ejmp.2018.06.641. [DOI] [PubMed] [Google Scholar]
- 9.Yao W. A two-point scheme for optimal breast IMRT treatment planning. J Appl Clin Med Phys. 2013;14:4525. doi: 10.1120/jacmp.v14i6.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farace P., Zucca S., Solla I., Fadda G., Durzu S., Porru S., et al. Planning hybrid intensity modulated radiation therapy for whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e115–e122. doi: 10.1016/j.ijrobp.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Xie X., Ouyang S., Wang H., Yang W., Jin H., Hu B., et al. Dosimetric comparison of left-sided whole breast irradiation with 3D-CRT, IP-IMRT and hybrid IMRT. Oncol Rep. 2014;31:2195–2205. doi: 10.3892/or.2014.3058. [DOI] [PubMed] [Google Scholar]
- 12.Jeulink M., Dahele M., Meijnen P., Slotman B.J., Verbakel W.F. Is there a preferred IMRT technique for left-breast irradiation? J Appl Clin Med Phys. 2015;16:197–205. doi: 10.1120/jacmp.v16i3.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin J.F., Yeh D.C., Yeh H.L., Chang C.F., Lin J.C. Dosimetric comparison of hybrid volumetric-modulated arc therapy, volumetric-modulated arc therapy and intensity-modulated radiation therapy for left-sided early breast cancer. Med Dosim. 2015;40:262–267. doi: 10.1016/j.meddos.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 14.van Duren-Koopman M.J., Tol J.P., Dahele M., Bucko E., Meijnen P., Slotman B.J., et al. Personalized automated treatment planning for breast plus locoregional lymph nodes using Hybrid RapidArc. Pract Radiat Oncol. 2018;8:332–341. doi: 10.1016/j.prro.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Mayo C.S., Urie M.M., Fitzgerald T.J. Hybrid IMRT plans–concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys. 2005;61:922–932. doi: 10.1016/j.ijrobp.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Popescu C.C., Olivotto I.A., Beckham W.A., Ansbacher W., Zavgorodni S., Shaffer R., et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y., Nakao M., Miura H., Ozawa S., Kenjo M., Nagata Y. Hybrid volumetric-modulated arc therapy for postoperative breast cancer including regional lymph nodes: the advantage of dosimetric data and safety of toxicities. J Radiat Res. 2020;61:747–754. doi: 10.1093/jrr/rraa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venjakob A., Oertel M., Hering D.A., Moustakis C., Haverkamp U., Eich H.T. Hybrid volumetric modulated arc therapy for hypofractionated radiotherapy of breast cancer: a treatment planning study. Strahlenther Onkol. 2021;197:296–307. doi: 10.1007/s00066-020-01696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang K., Loritz B., Schwartz A., Hunzeker A., Lenards N., Culp L., et al. Dosimetric comparison between volumetric-modulated arc therapy and a hybrid volumetric-modulated arc therapy and segmented field-in-field technique for postmastectomy chest wall and regional lymph node irradiation. Med Dosim. 2020;45:121–127. doi: 10.1016/j.meddos.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Redapi L., Rossi L., Marrazzo L., Penninkhof J.J., Pallotta S., Heijmen B. Comparison of volumetric modulated arc therapy and intensity-modulated radiotherapy for left-sided whole-breast irradiation using automated planning. Strahlenther Onkol. 2022;198:236–246. doi: 10.1007/s00066-021-01817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.srobf.cz/downloads/cilove-objemy/breastcanceratlas.pdf [accessed 11 April 2022].
- 22.Sabour S. Effects of multiple breath-hold reproducibility on treatment localization and dosimetric accuracy in radiotherapy of left-sided breast cancer: Methodology and statistical issues in reproducibility and accuracy. Med Dosim. 2018;43:327. doi: 10.1016/j.meddos.2017.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.