Abstract
Oxidative stress has been implicated in many diseases, including reproductive and pregnancy disorders, from subfertility to maternal vascular disease or preterm labour. There is, however, discrepancy within the standardized markers of oxidative stress in obstetrics and gynaecology in clinical studies. This review aims to present the scope of markers used between 2012 and 2022 to describe oxidative stress with regard to reproduction, pregnancy, and pregnancy-related issues. Despite the abundance of evidence, there is no consensus on the set of standardised markers of oxidative stress which poses a challenge to achieve universal consensus in order to appropriately triangulate the results.
Keywords: pregnancy, oxidative stress, reproduction, fertility, antioxidants, metabolism
1. Introduction
Oxidative stress (OS) is defined as a state of imbalance between pro-oxidant molecules, including reactive oxygen and nitrogen species, and antioxidant defenses. ROS (reactive oxygen species) and RNS (reactive nitrogen species) have a significant role in human bodies’ oxidative balance. Those molecules are recognised as important factors in redox signaling, growth regulation and initiating, mediating, or regulating the cellular and biochemical complexity of oxidative stress [1]. Lack of balance in that field can cause serious implications, such as oxidative damage and tissue dysfunction [2]. That process leads to various consequences for the organism such as cancer [3], heart disorders, cardiovascular disease, atherosclerosis, hypertension, reperfusion injury, diabetes mellitus, or neurodegenerative diseases [4]. Furthermore, it can especially affect pregnant patients as ROS and RNS are identified as factors causing preeclampsia, placental diseases, and premature birth [5].
The excess of reactive oxygen species can lead to cellular damage of lipids, DNA, and proteins. The consequence of disturbed haemostasis is also the damage of mitochondrial and nuclear DNA as well as lipid peroxidation. Unsaturated fatty acids and other lipids undergo oxidation by becoming peroxides. These compounds, such as MDA (malondialdehyde), impair functioning cells through disorders of structure and breaking cell membranes and also changing functions of receptors. Total antioxidant status (TAS) can determine quantitatively the influence of oxidative stress in a human body and degree of protection against its activity. TAS is a parameter coming from evaluation of blood plasma that finds expression mainly in a number of thiol groups, proteins of blood plasma, and concentration of uric acid [6].
The aim of antioxidants is to protect cells from damage and support, maintaining the integrity of the cell membrane as well as peroxidation reactions. Most commonly used antioxidants—such as vitamins (A, E, C) and elements such as zinc, iron or selenium—have potential protective functions for disease prevention. However, despite overwhelming evidence that the oxidative stress affects reproduction and pregnancy, there is so far limited evidence that antioxidants supplementation is significant with regard to its effects on combating oxidative stress or reversing pathological processes. Some studies suggest the positive effect of antioxidants such as N-acetylcysteine [7], vitamins C and E, L-arginine, and resveratrol on pregnancy-related medical conditions such as preeclampsia [8], intrauterine growth restriction, as well as on pregnancy outcomes in women with polycystic ovarian syndrome [9]. Nonetheless, further studies are needed to draw any conclusions regarding the aforementioned antioxidants’ effectiveness as the currently available data are insufficient [10,11].
The lack of balance between pro-oxidant and antioxidant agents might cause multiple negative reproductive health outcomes, such as polycystic ovary syndrome (PCOS), subfertility, or endometriosis. Pregnancy complications—such as miscarriages, gestational diabetes and preeclampsia, fetal growth restriction, and preterm labour—can also develop in response to oxidative stress. Studies have shown that both being underweight and overweight—as well as certain risk behaviors such as recreational alcohol use, smoking, or illicit drug use—can increase production of excess free radicals, which has a known effect on reproductive and perinatal health. Moreover, being exposed to pollution in the environment or known “endocrine disruptors” present in domestic products can lead to imbalance towards pro-oxidative stress and contribute to struggles with fertility [12].
There have been multiple attempts to define oxidative stress [13,14,15,16,17,18]. Costantini [13] in his commentary proposes biochemical and biological definitions of oxidative stress. Some of the definitions focus on the damage created at the biochemical level and imbalance towards pro-oxidants causing stress at the cellular level [14]; Other definitions look into the biomolecular damage caused by reactive species attacking the constituents of living organisms [15,16]. However, biochemical definitions of oxidative stress can also focus on the effects on cellular signaling and its disruptions [17,18]. Moreover, many authors are not only using different approaches to the definition of oxidative stress but also different parameters to assess oxidative stress. There is no unity in tests and markers—some assess reactive oxygen species (ROS), TAC, antioxidants potentials, or even inflammatory markers as proxies of oxidative stress. Given this discrepancy, our research team decided to look into the definitions and the oxidative stress markers used in literature with regard to obstetrics and gynaecology.
2. Materials and Methods
Two independent reviewers have searched medical and public databases—including Cochrane, PubMed, Google Scholar, and MEDLINE—using the search terms and MeSH terms such as: “oxidative stress”, “antioxidant*”, “pregnancy”, “gyn(a)ecology”, “obstetrics”, “reproduction”, and “fertility”. We were searching for papers which presented the parameters used to describe oxidative stress and its markers and discussed female reproductive tract disorders, subfertility as well as pregnancy and pregnancy-related issues.
The inclusion criterion was for the paper to be published in the peer-reviewed journal in the last 10 years (2012–2022). No limitation to language of the publication or type of the study were made. Papers discussing male infertility and reproductive issues were excluded.
The papers were then vetted by the review team against inclusion criteria and the final list of papers was presented in a table looking at population, materials used to assess oxidative stress, parameters assessed, which reproductive or pregnancy-related issue, which intervention (if any) was introduced, and what the outcomes were of each study.
3. Results
3.1. Study Characteristics
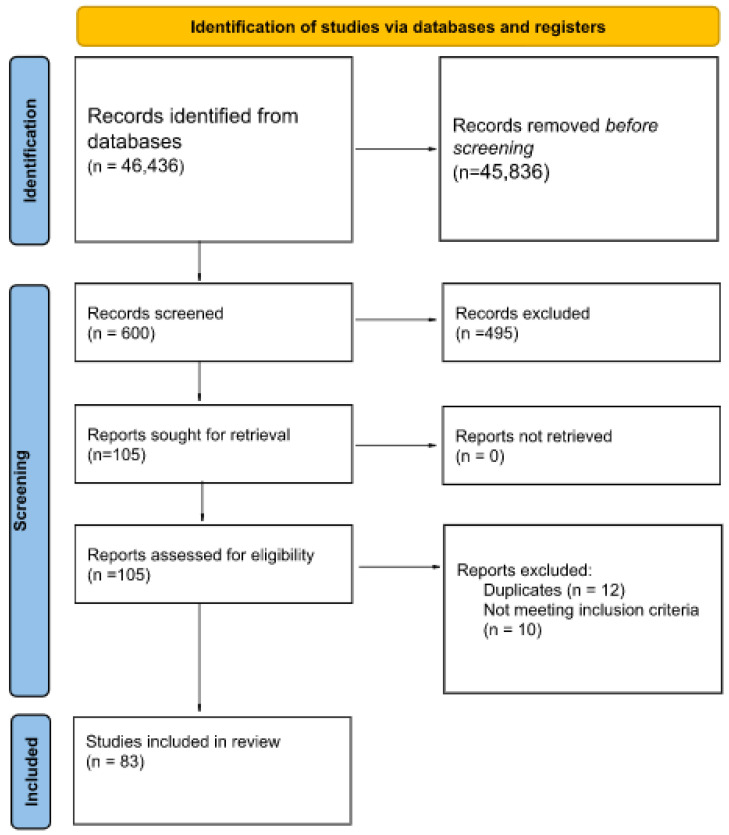
The team of reviewers have identified 46,436 records, 600 of which were then screened. Then, 105 were retrieved and assessed for eligibility and ultimately 83 papers were included into final review. Two reviewers independently screened databases, assessed against the inclusion criteria and eligibility.
Different types of studies were included in the analysis: 45 case-control studies, 24 randomized controlled clinical trials, 9 cohort studies, and 5 cross-sectional studies.
The process is illustrated in Figure 1 below. The list and paper characteristics are included in Appendix A, Table A1 at the end of the manuscript.
Figure 1.
PRISMA diagram of the systematic literature review (n—number of records).
3.2. Markers of Oxidative Stress
We found that a plethora of different markers of oxidative stress were used. This includes malondialdehyde (MDA), nitrous oxide (NO), reactive oxygen species (ROS), total antioxidant capacity (TAC), total antioxidant activity (TAA), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione peroxidase (4 GPx), glutathione reductase (GR), lipid peroxidation (LPO), 8-hydroxydeoxyguanosine (8-OHdG), oxidised glutathione (GSSG), catalase (CAT), superoxide (O2−), Paraoxonase (PON-1), oxidative stress index (OSI), hs-CRP, 8-iso-prostaglandin F2α (8-iso-PGF2α), prostaglandin F2α (PGF2α), gluthatione (GSH), and glutathione transferase (GST).
3.3. Materials
Materials used for examination of the markers are characterized by high diversity. Researchers used mostly blood (serum or plasma) (n = 68), placenta (n = 8), urine (n = 6), Wharton’s jelly mesenchymal stem cells from umbilical cord (n = 1), or saliva (n = 4). Ovarian follicular fluid (n = 9), peritoneal fluid (n = 2), and granulosa cells (n = 3) were used when examining reproductive health issues such as polycystic ovarian syndrome and endometriosis.
3.4. Pregnancy-Related Conditions
The team divided emerging themes into pregnancy related and reproduction related conditions. Among pregnancy related conditions, the team distinguished pre-eclampsia, gestational diabetes mellitus, preterm birth, as well as issues with regard to general antenatal care such as association with birth weight or iron supplementation. Neonatal outcomes were not analyzed for the purpose of this study.
3.4.1. Pre-Eclampsia
We retrieved 10 articles about the role of oxidative stress in pre-eclampsia. In total, 17 biomarkers of OS were measured with the number of studies that they were identified in put in brackets (n = X): MDA (n = 5), TAS (n = 4), GSH (n = 3), CAT (n = 2), TOS (n = 2), GSSG (n = 1), TAC (n = 1), OSI (n = 1), SOD (n = 1), GPx (n = 1), NO (n = 1), carbonic anhydrase IX (n = 1), peroxynitrite (ONOO−) (n = 1), paraoxonase (PON-1) (n = 1), O2− (n = 1), 8-OHdG (n = 1), and 8-isoprostane (n = 1) [11,12,13,14,15,16,17,18,19,20].
3.4.2. Gestational Diabetes Mellitus (GDM)
There is great diversity of markers in papers researching correlation between OS and GDM. In 30 studies, 43 biomarkers were measured. The markers that were most frequently measured were: MDA (n = 17), TAC (n = 12), GSH (n = 9), GPx (n = 6), SOD (n = 6), CAT (n = 4), NO (n = 4), and 8-isoprostane (n = 4).
The rest of parameters were oxidative stress index-OSI (n = 3), GST (n = 2), GR (n = 2), uric acid (n = 2), xanthine oxidase (n = 2), TOS (n = 1), TNF-α (n = 1), IL-10 (n = 1), paraoxonase (PON-1) (n = 1), inactivation of aldehyde dehydrogenase (n = 1), irisin (n = 1), bilirubin (n = 1), 8-OHdG (n = 1), sulfhydryl groups (n = 1), plasma and erythrocyte carbonyl proteins (n = 1), heme oxygenase 1 (n = 1), nuclear factor erythroid 2-related factor-2 (n = 1), quinone oxidoreductase (NQO1) (n = 1), aldo-keto reductase family 1 member c1 (AKR1C1) (n = 1), 8-iso-prostaglandin F2α (1), ceruloplasmin (1), hs-CRP (n = 1), transferrin (n = 1), advanced oxidative protein products (AOPPs) (n = 1), protein carbonyl (PCO) (n = 1), GPx3 (n = 1), protein (P-SH) (n = 1), total nitrite (n = 1), non-protein thiol (NP-SH) (n = 1), total thiol (n = 1), non-protein thiol (NP-SH) (n = 1), P66Shc mRNA (n = 1), Drp1 mRNA (n = 1), protein ROS (n = 1), antioxidant enzymes and gene expression for mitochondrial function: ND2, TFAM, PGC1α, and NDUFB9 (n = 1) [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].
3.4.3. Preterm Birth
Four articles about the role of oxidative stress in preterm birth were analyzed. All studies used a different set of OS biomarkers, none appeared in more than one of the studies. In total, 11 markers were measured, including 8-OHdG (n = 1), 8-isoprostane (n = 1), ROS (n = 1), GPx (n = 1), CAT (n = 1), NO (n = 1), O2− (n = 1), peroxynitrite (OONO) (n = 1), hydroxyl radical (OH) (n = 1), 8-iso-prostaglandin F2α (n = 1) and prostaglandin F2α (n = 1) [51,52,53,54].
3.4.4. General Pregnancy and Antenatal Care
Sixteen articles retrieved looked at pregnancy and general antenatal care. In total, 27 markers of OS were investigated in these studies. Parameters that were most frequently used were TAC (n = 7), GPx (n = 4), MDA (n = 4) and SOD (n = 3).
The rest of the markers were researched in either one or two studies: 8-isoprostane (n = 1), 8-OHdG (n = 2), total peroxide (n = 1), nitrotyrosine (n = 1), 8-iso-prostaglandin F2α (n = 2), 8-epiprostaglandin F2-α (n = 1), prostaglandin F2α (n = 1), thiol (n = 1), disulphide (n = 1), TOS (n = 1), TAS (n = 1), DNA damage in blood leukocytes (n = 1), CAT (n = 2), γ-glutamyl transferase (n = 1), hs-CRP (n = 1), GSH (n = 1), NO (n = 1), carbonyl proteins (n = 1), superoxide anion expressed as reduced nitroblue tetrazolium (n = 1), aldehyde dehydrogenase (n = 1), GST (n = 1), soluble fms-like tyrosine kinase-1 (n = 1), and placental growth factor (n = 1) [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70].
3.5. Reproduction and Gynaecological Conditions
Twenty-three articles on reproduction and gynaecological conditions. Most conditions in which the association with oxidative stress was found are polycystic ovarian syndrome, endometriosis, and subfertility.
In total, 26 markers of oxidative stress were identified with particular emphasis on five markers: MDA (n = 11), TAC (n = 11), SOD (n = 10), ROS (n = 6), and GPx (n = 6).
The rest of the markers were: CAT (n = 4), GSH (n = 3), GR (n = 3), 8-Isoprostane (n = 3), 8-OHdG (n = 2), thiol (n = 2), LPO (n = 1), PON-1 (n = 1), advanced oxidation protein products (n = 1), TOC (n = 1), TOS (n = 1), TAA (n = 1), uric acid (n = 1), CRP (n = 1), IL-6 (n = 1), protein carbonyls (n = 1), TNF-α (n = 1), nitrates (n = 1), cortisol (n = 1), OSI (n = 1), and NO (n = 1) [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
4. Discussion
We observed a huge diversity of markers used to describe oxidative stress. Almost every paper used a different set of markers, which made it challenging to compare and triangulate the results or perform a meta-analysis with cohesive conclusions. In the papers we reviewed, oxidative stress has been mentioned both as the exposure or the outcome. Certain papers described the use of antioxidants as a protective factor to prevent the aforementioned diseases. Therefore, there is a need for a cohesive and unified approach to be able to appropriately assess and define oxidative stress. Moreover, different abbreviations are used to describe the same parameter; in some cases, the abbreviation in the brackets stands for the laboratory technique rather than the acronym of the phrase.
Moreover, we discovered that different materials are being used to measure the markers of oxidative stress. For instance, in papers on polycystic ovarian syndrome we had markers retrieved from serum, blood, follicular fluid, or granulosa cells which all have different reference ranges and therefore it poses immense challenges of unifying and triangulating the results in order to make appropriate recommendations or conclusions.
Types of studies included in the final analysis varied in design. In many cases, the authors used different nomenclature to describe similar study designs, for example randomized controlled clinical trials and case-control studies often had similar methodology but authors used to describe them differently.
Additionally, in some studies we could observe a lack of disaggregation of the populations included in the study based on age and BMI—two known factors affecting oxidative status and stress. In light of the increasing number of non-communicable diseases deriving from obesity and its increased role in metabolic balance, it would be important to disaggregate specific populations in order to be able to avoid confounding results.
Finally, there is a clear need to differentiate between inflammation and oxidative stress markers. In many studies, the line between inflammatory and oxidative stress markers is not clearly stated and division is not well explained. For instance, C-reactive protein (CRP) is being used in many studies as a proxy for inflammation process; however, this might pose unnecessary confusion of comparing inflammation and oxidative stress markers as this division is not well explained, leading to potential interpretation errors.
Oxidative stress and antioxidants are becoming more popular in social media with regard to healthy diet culture as well as vitamin and other supplements intake. It is therefore extremely important to have unified definitions and markers of oxidative stress given that it might be the source of manipulation in the public discourse. Many pharmaceuticals and supplements are being advertised as antioxidants and gatekeeping them with the use of appropriate definitions and markers would allow validation and reliability, as well as replicability of the studies.
Finally, we would recommend creating a common, basic panel of oxidative stress markers that could be used in all studies on oxidative stress in obstetrics and gynaecology. This way, we could achieve reproducible results that could be further analyzed for oxidative stress to be better understood. The most commonly used markers of oxidative stress that we would recommend adding to the basic set are: reactive oxygen species (ROS)—as a direct marker of oxidative stress; 8-hydroxydeoxyguanosine (8-OHdG)—as a marker of DNA/RNA damage; and malondialdehyde (MDA)—as a marker of lipid peroxidation. Additionally, we would like to suggest adding two antioxidants parameters that are often used in studies—total antioxidant capacity (TAC) and gluthatione (GSH). Using the same basic set of oxidative stress markers would enable researchers to investigate and understand their actual clinical significance in order to create an even more adequate and reliable set of oxidative stress markers in the future. Moreover, we would like to recommend that the researchers use the basic set of proposed markers in order to standardize the studies on oxidative stress. However, the choice of additional markers should be made independently, depending on the studied disease and material.
5. Conclusions
There are no universal parameters assessing oxidative stress in human reproduction and pregnancy-related issues. In order to be able to appropriately derive conclusions, a unified set of parameters and definitions would be of use.
Appendix A
Table A1.
Characteristics of the studies.
| Paper | Country | Population | Oxidative Stress Markers | Materials | Type of Study | |
|---|---|---|---|---|---|---|
| Pregnancy-Related Conditions | ||||||
| Preeclampsia | ||||||
| 1 | Samimi et al. (2016) [19] | Iran | 60 pregnant women at risk for pre-eclampsia | GSH | blood | randomised controlled clinical trial |
| 2 | Asemi et al. (2012) [20] | Iran | 42 pregnant women | TAC, GSH | blood | randomised controlled clinical trial |
| 3 | Mentese et al. (2018) [21] | Turkey | 53 pregnant women; 23 with HELLP syndrome, 30 controls | TOS, TAS, OSI, MDA, carbonic anhydrase IX | serum | case-control study |
| 4 | Bharadwaj et al. (2018) [22] | India | 143 pregnant women; 71 with pre-eclampsia and 72 controls | TAS, MDA | maternal and cord blood | cohort study |
| 5 | Sahay et al. (2015) [23] | India | 60 pregnant women; 5 normotensive; 11 with pre-eclampsia delivered at term; 14 with pre-eclampsia, delivered preterm | MDA, CAT, GPx | placenta | cross-sectional study |
| 6 | Al-Kuraishy et al. (2018) [24] | Iraq | 68 pregnant women; 40 with pre-eclampsia, 28 controls | MDA, NO, peroxynitrite (ONOO−), paraoxonase (PON-1) | serum | case-control study |
| 7 | Can et al. (2014) [25] | Turkey | 63 pregnant women; 32 with pre-eclampsia, 31 controls | MDA, TAS | placenta | case-control study |
| 8 | Ahmad et al. (2019) [26] | USA | 114 pregnant women; 23 with pre-eclampsia, 91 controls | O2−, SOD, CAT, GSH, GSSG | blood | case-control study |
| 9 | Mert et al. (2012) [27] | USA | 81 pregnant women; 24 with pre-eclampsia, 20 with intrauterine growth restriction, 37 controls | TOS, TAS | plasma | case-control study |
| 10 | Ferguson et al. (2017) [28] | USA | 441 pregnant women; 50 with preeclampsia, 391 controls | 8-OHdG, 8-isoprostane | urine and plasma | cohort study |
| Gestational diabetes mellitus (GDM) | ||||||
| 1 | Zhang et al. (2019) [29] | China | 175 pregnant women; 93 patients with GDM, 82 controls | MDA, GSH, SOD, heme oxygenase 1, nuclear factor erythroid 2-related factor-2, quinone oxidoreductase (NQO1), aldo-keto reductase family 1 member c1 (AKR1C1) | serum, placenta | randomised controlled clinical trial |
| 2 | Murthy et al. (2018) [30] | India | 60 pregnant women; 30 with GDM, 30 controls | GPx, SOD, uric acid, bilirubin | serum | case-control study |
| 3 | Razavi et al. (2017) [31] | Iran | 120 pregnant women with GDM | NO, TAC, GSH, MDA | serum | randomised controlled clinical trial |
| 4 | Jamilian et al. (2019) [32] | Iran | 87 pregnant women with GDM | TAC, GSH, MDA | serum | randomised controlled clinical trial |
| 5 | Badehnoosh et al. (2018) [33] | Iran | 60 pregnant women with GDM | MDA, TAC, OSI | serum | randomised controlled clinical trial |
| 6 | Zhu et al. (2015) [34] | China | 72 women: 36 with GDM, 36 control | ceruloplasmin, hs-CRP, transferrin, 3-nitrotyrosin | blood | case-control study |
| 7 | Jamilian et al. (2019) [35] | Iran | 60 pregnant women at risk of GDM | total nitrite, MDA, TAC, GSH | blood | randomised controlled clinical trial |
| 8 | Rueangdetnarong et al. (2018) [36] | Thailand | 62 pregnant women; 30 GDM and 32 control | 8-Isoprostane | blood | case-control study |
| 9 | López-Tinoco et al. (2013) [37] | Spain | 78 pregnant women; 53 with GDM, 25 controls | lipoperoxides, CAT, SOD, GPx, GSH, GST | blood | case-control study |
| 10 | Li et al. (2016) [38] | China | 52 pregnant women; 22 with GDM, 30 controls | 8-iso-prostaglandin F2α, advanced oxidative protein products (AOPPs), protein carbonyl (PCO), GPx3, PON-1 | plasma | case-control study |
| 11 | Usluoğullari et al. (2017) [39] | Turkey | 94 pregnant women; 48 with GDM, 46 controls | TOS, irisin, OSI | serum | case-control study |
| 12 | Shang et al. (2018) [40] | China | 208 pregnant women; 105 with GDM, 103 controls | MDA, 8-isoprostane, xanthine oxidase | maternal plasma, cord plasma, placenta | case-control study |
| 13 | Shang et al. (2015) [41] | China | 68 pregnant women; 28 with GDM, 40 controls | MDA, 8-isoprostane, xanthine oxidase, lipid peroxides, SOD, GPx, TAC | maternal and cord plasma and placenta | case-control study |
| 14 | Jamilian et al. (2017) [42] | Iran | 60 pregnant women with PCOS | TAC, NO, MDA | blood | randomised controlled clinical trial |
| 15 | Asemi et al. (2013) [43] | Iran | 32 pregnant women with GDM | TAC, GSH | plasma | randomised controlled clinical trial |
| 16 | Hajifaraji et al. (2018) [44] | Iran | 64 pregnant women with GDM | MDA, GR, GPx | serum | randomised controlled clinical trial |
| 17 | Toljic et al. (2017) [45] | Serbia | 86 pregnant women; 37 patients who developed GDM, 21 patients with gestational hypertension and 28 healthy pregnant women | malondialdehyde equivalents (TBARS), 8-OHdG | blood | case-control study |
| 18 | Asemi et al. (2015) [46] | Iran | 70 pregnant women with GDM | NO, TAC, MDA, GSH | plasma | randomised controlled clinical trial |
| 19 | Zygula et al. (2019) [47] | Poland | 89 pregnant women; 59 with GDM and 30 controls | MDA, TAC, inactivation of aldehyde dehydrogenase, GPx, GST | plasma, saliva | case-control study |
| 20 | Saifi et al. (2020) [48] | Algeria | 180 pregnant women; 120 with GDM, 60 healthy | CAT, SOD, GPx, GR, plasma and erythrocyte carbonyl proteins, MDA | plasma | case-control study |
| 21 | Jatavan et al. (2020) [49] | Thailand | 80 pregnant women; 43 with GDM, 37 controls | 8-isoprostane, TNF-α, IL-10 | serum | cross-sectional study |
| 22 | Jamilian et al. (2018) [50] | Iran | 60 pregnant women at risk of GDM | TAC, MDA, NO | plasma | randomised controlled clinical trial |
| 23 | Rodrigues et al. (2018) [51] | Brazil | 78 pregnant women; 48 with GDM, 30 controls | thiobarbituric acid reactive substances (TBARS), protein (P-SH) and non-protein thiol (NP-SH), CAT | blood | case-control study |
| 24 | Li et al. (2019) [52] | China | 152 pregnant women; 72 with GDM, 80 control | MDA | blood | case-control study |
| 25 | Bulut et al. (2021) [53] | Cyprus, Turkey | 51 pregnant women; 22 with GDM, 29 controls | MDA, NO, sulfhydryl | blood, saliva | case-control study |
| 26 | Gunasegaran et al. (2021) [54] | India | 70 pregnant women with GDM | GSH | serum | randomised controlled clinical trial |
| 27 | Ahmadi-Motamayel et al. (2021) [55] | Iran | 40 pregnant women; 20 with GDM, 20 healthy | TAC, MDA, CAT, uric acid, total thiol | saliva | case-control study |
| 28 | Huang et al. (2021) [56] | China | 30 pregnant women; 15 with GDM, 15 controls | P66Shc mRNA, Drp1 mRNA, protein ROS | serum, placenta | case-control study |
| 29 | Ma et al. (2021) [57] | China | 230 pregnant women; 104 with GDM, 126 controls | TAC, MDA, GSH, SOD | blood | case-control study |
| 30 | Kong et al. (2019) [58] | Singapore | 9 pregnant women; 3 mothers without GDM, 3 insulin-controlled GDM mothers, 3 diet-controlled GDM mothers | LPO, antioxidant enzymes and gene expression for mitochondrial function: ND2, TFAM, PGC1α, NDUFB9 | Wharton’s jelly mesenchymal stem cells from umbilical cord | case-control study |
| Preterm birth | ||||||
| 1 | Ferguson et al. (2015) [59] | USA | 482 pregnant women; 130 with preterm birth, 352 controls | 8-OHdG, 8-isoprostane | urine | case-control study |
| 2 | Moore et al. (2020) [60] | USA | 140 pregnant women at risk of preterm birth | ROS, O2−, peroxynitrite (OONO), hydroxyl radical (OH) | blood | cohort study |
| 3 | Eick et al. (2020) [61] | Puerto Rico | 460 pregnant women at risk of preterm birth | 8-iso-prostaglandin F2α, prostaglandin F2α | urine | cohort study |
| 4 | Abiaka et al. (2012) [62] | Oman | 74 pregnant women; 37 with preterm birth, 37 with term birth | NO, CAT, GPx | blood | case-control study |
| General pregnancy and antenatal care | ||||||
| 1 | Hsieh et al. (2012) [63] | Taiwan | 503 pregnant women | plasma: TAC, 8-isoprostane, erythrocyte GPx and SOD; urine: 8-OHdG | plasma, urine | cohort study |
| 2 | Gerszi et al. (2021) [64] | Hungary | 61 pregnant women | total peroxide, TAC, nitrotyrosine | plasma | case-control study |
| 3 | Arogbokun et al. (2021) [65] | USA | 736 pregnant women | 8-iso-prostaglandin F2α and its primary metabolite, prostaglandin F2α | urine | cohort study |
| 4 | Lindström et al. (2012) [66] | Bangladesh | 374 pregnant women | free 8-iso-prostaglandin F(2α), 8-OHdG | urine, blood | cohort study |
| 5 | Sanhal et al. (2018) [67] | Turkey | 107 pregnant women; 57 with intrahepatic cholestasis, 50 controls | thiol, disulphide | plasma | case-control study |
| 6 | Yilmaz et al. (2015) [68] | Turkey | 80 pregnant women; 41 with hyperemesis gravidarum, 39 healthy | TOS, TAS | blood | case-control study |
| 7 | Jiang et al. (2012) [69] | USA | 47 women; 26 pregnant, 21 non-pregnant | DNA damage in blood leukocytes | blood | randomised controlled clinical trial |
| 8 | Motamed et al. (2020) [70] | Iran | 84 pregnant women | MDA, TAC | serum, cord blood serum | randomised controlled clinical trial |
| 9 | Lymperaki et al. (2015) [71] | Greece | 75 women; 50 pregnant, 25 non-pregnant | TAC | serum | case-control study |
| 10 | Kajarabille et al. (2017) [72] | Spain | 110 pregnant women | GPx, SOD, CAT | blood | randomised controlled clinical trial |
| 11 | Korkmaz et al. (2014) [73] | Turkey | 108 healthy pregnant women | γ-glutamyl transferase | serum | randomised controlled clinical trial |
| 12 | Aalami-Harandi et al. (2015) [74] | Iran | 44 pregnant women at risk of pre-eclampsia | hs-CRP, GSH | blood | randomised controlled clinical trial |
| 13 | Malti et al. (2014) [75] | Algeria | 90 pregnant women; 40 with obesity, 50 healthy controls | MDA, NO, SOD, CAT, GSH, carbonyl proteins, superoxide anion expressed as reduced Nitroblue Tetrazolium | Maternal, cord blood, placenta samples | case-control study |
| 14 | Ballesteros-Guzmán et al. (2019) [76] | Mexico | 33 pregnant women; 18 with pre-pregnancy body mass index (pBMI) within normal range; 15 with pBMI ≥ 30 kg/m2 | TAC, MDA, placental expression of GPx4 | maternal and cord serum, placenta | cross-sectional study |
| 15 | Zygula et al. (2020) [77] | Poland | 104 pregnant women; 27 with pregnancy-induced hypertension, 30 with intrauterine growth restriction, 47 controls | MDA, TAC, aldehyde dehydrogenase, GPx, GST | saliva and plasma | case-control study |
| 16 | Odame et al. (2018) [78] | Ghana | 175 pregnant women | TAC, soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor, 8-epiprostaglandin F2-α | blood | cohort study |
| Reproduction and gynaecological conditions | ||||||
| 1 | Panti et al. (2018) [79] | Nigeria | 200 women with PCOS | GPx, SOD, CAT, MDA | serum | randomised controlled clinical trial |
| 2 | Liu et al. (2021) [80] | China | 146 women; 86 with PCOS, 60 controls | TAC, MDA, GSH, SOD, TOC | follicular fluid and serum | case-control study |
| 3 | Özer et al. (2016) [81] | Turkey | 124 women; 71 with PCOS, 53 controls | MDA, GPx, CAT | follicular fluid and serum | case-control study |
| 4 | Wang et al. (2019) [82] | China | 270 women; 205 with PCOS, 65 controls | MDA, SOD, TAA | blood | cross-sectional study |
| 5 | Heshmati et al. (2020) [83] | Iran | 72 women with PCOS | GPx, SOD | serum | randomised controlled clinical trial |
| 6 | Desai et al. (2014) [84] | India | 50 women; 25 with PCOS, 25 controls | MDA, TAC, uric acid | serum | case-control study |
| 7 | Kazemi et al. (2021) [85] | Iran | 60 women with PCOS | TAC, MDA, CRP, TNF-α | serum | randomised controlled clinical trial |
| 8 | Turan et al. (2015) [86] | Turkey | 90 women; 33 with PCOS without insulin resistance, 27 with PCOS and insulin resistance, 30 healthy controls | MDA, thiol, CAT, SOD | blood | case-control study |
| 9 | Sulaiman et al. (2018) [87] | Oman | 96 women; 51 with PCOS, 45 controls | GPx, GR, GSH, TAC | serum | case-control study |
| 10 | Lai et al. (2018) [88] | China | 47 women; 22 with PCOS, 25 with tubal factor infertility | ROS | granulosa cells | case-control study |
| 11 | Yilmaz et al. (2016) [89] | Turkey | 63 women; 22 with PCOS, 41 controls | TAC | follicular fluid | case-control study |
| 12 | Fatemi et al. (2017) [90] | Iran | 105 women with PCOS and infertility | MDA, TAC | serum | randomised controlled clinical trial |
| 13 | Gongadashetti et al. (2021) [91] | India | 100 women; 43 with PCOS, 57 with tubal factor infertility | ROS, TAC, 8-isoprostane | follicular fluid | cross-sectional study |
| 14 | Nishihara et al. (2018) [92] | Japan | 117 women with infertility | TAC, GSH, 8-OHdG | follicular fluid | cohort study |
| 15 | Alam et al. (2019) [93] | Pakistan | 328 women; 164 with infertility, 164 controls | cortisol, GR | serum | case-control study |
| 16 | Gong et al. (2020) [94] | China | 163 women; 105 with subfertility and poor ovarian response, 58 controls | MDA, TOS, OSI, ROS, SOD, TAC | follicular fluid | randomised controlled clinical trial |
| 17 | Younis et al. (2012) [95] | USA | 15 women; Group-1 was baseline blood collected on day-2–3 of the menstrual cycle. Group-2 is blood collected at the end of FSH/hMG injection. | PON-1, SOD, IL-6, GPx, 8-isoprostane | serum | case-control study |
| 18 | Singh et al. (2013) [96] | India | 340 women; 200 with endometriosis, 140 with tubal infertility | ROS, NO, TAC, SOD, GPx, GR, CAT, LPO | follicular fluid | case-control study |
| 19 | Prieto et al. (2013) [97] | Spain | 91 women; 23 with endometriosis, 68 controls | MDA, SOD | follicular fluid, plasma | case-control study |
| 20 | Liu et al. (2013) [98] | China | 42 women; 20 with endometriosis, 22 with tubal factor infertility | ROS, SOD | serum, follicular fluid | case-control study |
| 21 | Santulli et al. (2015) [99] | France | 235 women; 150 women with histologically proven endometriosis, 85 endometriosis-free controls | thiols, advanced oxidation protein products (AOPP), protein carbonyls, nitrates/nitrites | peritoneal fluid | case-control study |
| 22 | Polak et al. (2013) [100] | Poland | 229 women; 110 with endometriosis, 119 controls with ovarian cysts | 8-OHdG and 8-isoprostane | peritoneal fluid | case-control study |
| 23 | Amini et al. (2021) [101] | Iran | 60 women with pelvic pain and endometriosis | MDA, ROS, TAC | plasma and serum | randomised controlled clinical trial |
Author Contributions
Conceptualisation and methodology, M.A.D., K.R. and E.M.; Formal analysis, M.A.D., K.R. and E.M.; Resources, M.A.D., K.R. and E.M.; Data curation, M.A.D., K.R. and E.M.; Writing—original draft preparation, M.A.D., K.R., E.M., K.P.-R., G.J.-B., K.P.-M. and K.G.-T.; Writing—review and editing, M.A.D., K.R., E.M., K.P.-R. and G.J.-B.; Supervision, G.J.-B., W.K., M.W., M.M., M.P.-K. and K.P.-R.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zarkovic N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells. 2020;9:767. doi: 10.3390/cells9030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Liu H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Aouache R., Biquard L., Vaiman D., Miralles F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018;19:1496. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motawei S.M., Attalla S.M., Gouda H.E., Harouny M.A., Elmansoury A.M. The effects of N-acetyl cysteine on oxidative stress among patients with pre-eclampsia. Int. J. Gynecol. Obstet. 2016;135:226–227. doi: 10.1016/j.ijgo.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Tenório M.B., Ferreira R.C., Moura F.A., Bueno N.B., Goulart M.O.F., Oliveira A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018;28:865–876. doi: 10.1016/j.numecd.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Sandhu J.K., Waqar A., Jain A., Joseph C., Srivastava K., Ochuba O., Poudel S. Oxidative stress in polycystic ovarian syndrome and the effect of antioxidant N-acetylcysteine on ovulation and pregnancy rate. Cureus. 2021;13:e17887. doi: 10.7759/cureus.17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Showell M.G., Mackenzie-Proctor R., Jordan V., Hart R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020;8 doi: 10.1002/14651858.CD007807.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell L.C., Seed P.T., Cstat, Kelly F.J., Briley A., Hunt B.J., Charnock-Jones D., Mallet A., Poston L. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. Am. J. Obstet. Gynecol. 2002;187:777–784. doi: 10.1067/mob.2002.125735. [DOI] [PubMed] [Google Scholar]
- 12.Duhig K., Chappell L.C., Shennan A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016;9:113–116. doi: 10.1177/1753495X16648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costantini D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019;222:jeb194688. doi: 10.1242/jeb.194688. [DOI] [PubMed] [Google Scholar]
- 14.Sies H. Oxidative stress: Introductory remarks. In: Sies H., editor. Oxidative Stress. Academic Press; London, UK: 1985. pp. 1–8. [DOI] [Google Scholar]
- 15.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costantini D., Verhulst S. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 2009;23:506–509. doi: 10.1111/j.1365-2435.2009.01546.x. [DOI] [Google Scholar]
- 17.Jones D.P. Redefining oxidative stress. Antiox. Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 18.Sies H., Jones D. Oxidative stress. In: Fink G., editor. Encyclopedia of Stress. 2nd ed. Volume 3. Elsevier; Amsterdam, The Netherlands: 2007. pp. 45–48. [DOI] [Google Scholar]
- 19.Samimi M., Kashi M., Foroozanfard F., Karamali M., Bahmani F., Asemi Z., Hamidian Y., Talari H.R., Esmaillzadeh A. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J. Hum. Nutr. Diet. 2016;29:505–515. doi: 10.1111/jhn.12339. [DOI] [PubMed] [Google Scholar]
- 20.Asemi Z., Samimi M., Heidarzadeh Z., Khorrammian H., Tabassi Z. A randomized controlled clinical trial investigating the effect of calcium supplement plus low-dose aspirin on hs-CRP, oxidative stress and insulin resistance in pregnant women at risk for pre-eclampsia. Pak. J. Biol. Sci. 2012;15:469–476. doi: 10.3923/pjbs.2012.469.476. [DOI] [PubMed] [Google Scholar]
- 21.Mentese A., Güven S., Demir S., Sumer A., Yaman S., Alver A., Sönmez M., Karahan S.C. Circulating parameters of oxidative stress and hypoxia in normal pregnancy and HELLP syndrome. Adv. Clin. Exp. Med. 2018;27:1567–1572. doi: 10.17219/acem/74653. [DOI] [PubMed] [Google Scholar]
- 22.Bharadwaj S.K., Vishnu Bhat B., Vickneswaran V., Adhisivam B., Bobby Z., Habeebullah S. Oxidative stress, antioxidant status and neurodevelopmental outcome in neonates born to pre-eclamptic mothers. Indian J. Pediatrics. 2018;85:351–357. doi: 10.1007/s12098-017-2560-5. [DOI] [PubMed] [Google Scholar]
- 23.Sahay A.S., Sundrani D.P., Wagh G.N., Mehendale S.S., Joshi S.R. Regional differences in the placental levels of oxidative stress markers in pre-eclampsia. Int. J. Gynecol. Obstet. 2015;129:213–218. doi: 10.1016/j.ijgo.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Maiahy T.J. Concept and connotation of oxidative stress in preeclampsia. J. Lab. Physicians. 2018;10:276–282. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Can M., Guven B., Bektas S., Arikan I. Oxidative stress and apoptosis in preeclampsia. Tissue Cell. 2014;46:477–481. doi: 10.1016/j.tice.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad I.M., Zimmerman M.C., Moore T.A. Oxidative stress in early pregnancy and the risk of preeclampsia. Pregnancy Hypertens. 2019;18:99–102. doi: 10.1016/j.preghy.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mert I., Sargın Oruc A., Yuksel S., Cakar E.S., Buyukkagnıcı U., Karaer A., Danısman N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012;38:658–664. doi: 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson K.K., Meeker J.D., McElrath T.F., Mukherjee B., Cantonwine D.E. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am. J. Obstet. Gynecol. 2017;216:527.e1–527.e9. doi: 10.1016/j.ajog.2016.12.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., Yang Y., Chen R., Wei Y., Feng Y., Zheng W., Liao H., Zhang Z. Aberrant expression of oxidative stress related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. Am. J. Transl. Res. 2019;11:269–279. [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy K.S., Bhandiwada A., Chandan S.L., Gowda S.L., Sindhusree G. Evaluation of oxidative stress and proinflammatory cytokines in gestational diabetes mellitus and their correlation with pregnancy outcome. Indian J. Endocrinol. Metab. 2018;22:79–84. doi: 10.4103/ijem.IJEM_232_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razavi M., Jamilian M., Samimi M., Afshar Ebrahimi F., Taghizadeh M., Bekhradi R., Hosseini E.S., Kashani H.H., Karamali M., Asemi Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr. Metab. 2017;14:80. doi: 10.1186/s12986-017-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamilian M., Amirani E., Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:2098–2105. doi: 10.1016/j.clnu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Badehnoosh B., Karamali M., Zarrati M., Jamilian M., Bahmani F., Tajabadi-Ebrahimi M., Jafari P., Rahmani E., Asemi Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2018;31:1128–1136. doi: 10.1080/14767058.2017.1310193. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C., Yang H., Geng Q., Ma Q., Long Y., Zhou C., Chen M. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: A case-control study. PLoS ONE. 2015;10:e0126490. doi: 10.1371/journal.pone.0126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamilian M., Mirhosseini N., Eslahi M., Bahmani F., Shokrpour M., Chamani M., Asemi Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19:107. doi: 10.1186/s12884-019-2258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rueangdetnarong H., Sekararithi R., Jaiwongkam T., Kumfu S., Chattipakorn N., Tongsong T., Jatavan P. Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: Cohort study. Endocr. Connect. 2018;7:681–687. doi: 10.1530/EC-18-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Tinoco C., Roca M., García-Valero A., Murri M., Tinahones F.J., Segundo C., Bartha J.L., Aguilar-Diosdado M. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 2013;50:201–208. doi: 10.1007/s00592-011-0264-2. [DOI] [PubMed] [Google Scholar]
- 38.Li H., Yin Q., Li N., Ouyang Z., Zhong M. Plasma markers of oxidative stress in patients with gestational diabetes mellitus in the second and third trimester. Obstet. Gynecol. Int. 2016;2016:3865454. doi: 10.1155/2016/3865454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usluoğullari B., Usluogullari C.A., Balkan F., Orkmez M. Role of serum levels of irisin and oxidative stress markers in pregnant women with and without gestational diabetes. Gynecol. Endocrinol. 2017;33:405–407. doi: 10.1080/09513590.2017.1284789. [DOI] [PubMed] [Google Scholar]
- 40.Shang M., Dong X., Hou L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J. Obstet. Gynaecol. Res. 2018;44:637–646. doi: 10.1111/jog.13586. [DOI] [PubMed] [Google Scholar]
- 41.Shang M., Zhao J., Yang L., Lin L. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res. Clin. Pract. 2015;109:404–410. doi: 10.1016/j.diabres.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Jamilian M., Dizaji S.H., Bahmani F., Taghizadeh M., Memarzadeh M.R., Karamali M., Akbari M., Asemi Z. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can. J. Diabetes. 2017;41:143–149. doi: 10.1016/j.jcjd.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Asemi Z., Samimi M., Tabassi Z., Sabihi S.S., Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. 2013;29:619–624. doi: 10.1016/j.nut.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Hajifaraji M., Jahanjou F., Abbasalizadeh F., Aghamohammadzadeh N., Abbasi M.M., Dolatkhah N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018;27:581–591. doi: 10.6133/apjcn.082017.03. [DOI] [PubMed] [Google Scholar]
- 45.Toljic M., Egic A., Munjas J., Orlic N.K., Milovanovic Z., Radenkovic A., Vuceljic J., Joksic I. Increased oxidative stress and cytokinesis-block micronucleus cytome assay parameters in pregnant women with gestational diabetes mellitus and gestational arterial hypertension. Reprod. Toxicol. 2017;71:55–62. doi: 10.1016/j.reprotox.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Asemi Z., Jamilian M., Mesdaghinia E., Esmaillzadeh A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition. 2015;31:1235–1242. doi: 10.1016/j.nut.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Zygula A., Kosinski P., Zwierzchowska A., Sochacka M., Wroczynski P., Makarewicz-Wujec M., Pietrzak B., Wielgos M., Rzentala M., Giebultowicz J. Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2019;148:72–80. doi: 10.1016/j.diabres.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Saifi H., Mabrouk Y., Saifi R., Benabdelkader M., Saidi M. Influence of selenium supplementation on carbohydrate metabolism and oxidative stress in pregnant women with gestational diabetes mellitus. J. Med. Biochem. 2020;39:191–198. doi: 10.2478/jomb-2019-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jatavan P., Lerthiranwong T., Sekararithi R., Jaiwongkam T., Kumfu S., Chattipakorn N., Tongsong T. The correlation of fetal cardiac function with gestational diabetes mellitus (GDM) and oxidative stress levels. J. Perinat. Med. 2020;48:471–476. doi: 10.1515/jpm-2019-0457. [DOI] [PubMed] [Google Scholar]
- 50.Jamilian M., Ravanbakhsh N. Effects of Vitamin E plus Omega-3 Supplementation on Inflammatory Factors, Oxidative Stress Biomarkers and Pregnancy Consequences in Women with Gestational Diabetes. J. Arak Univ. Med. Sci. 2018;21:32–41. doi: 10.1371/journal.pone.0240244. [DOI] [Google Scholar]
- 51.Rodrigues F., de Lucca L., Neme W.S., Goncalves T.D.L. Influence of gestational diabetes on the activity of δ-aminolevulinate dehydratase and oxidative stress biomarkers. Redox Rep. 2018;23:63–67. doi: 10.1080/13510002.2017.1402981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H., Dong A., Lv X. Advanced glycation end products and adipocytokines and oxidative stress in placental tissues of pregnant women with gestational diabetes mellitus. Exp. Ther. Med. 2019;18:685–691. doi: 10.3892/etm.2019.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bulut A., Akca G., Aktan A.K., Akbulut K.G., Babül A. The significance of blood and salivary oxidative stress markers and chemerin in gestational diabetes mellitus. Taiwan. J. Obstet. Gynecol. 2021;60:695–699. doi: 10.1016/j.tjog.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Gunasegaran P., Tahmina S., Daniel M., Nanda S.K. Role of vitamin D-calcium supplementation on metabolic profile and oxidative stress in gestational diabetes mellitus: A randomized controlled trial. J. Obstet. Gynaecol. Res. 2021;47:1016–1022. doi: 10.1111/jog.14629. [DOI] [PubMed] [Google Scholar]
- 55.Ahmadi-Motamayel F., Fathi S., Goodarzi M.T., Borzouei S., Poorolajal J., Barakian Y. Comparison of Salivary Antioxidants and Oxidative Stress Status in Gestational Diabetes Mellitus and Healthy Pregnant Women. Endocr. Metab. Immune Disord.-Drug Targets. 2021;21:1485–1490. doi: 10.2174/1568026620666201022151059. [DOI] [PubMed] [Google Scholar]
- 56.Huang T.T., Sun W.J., Liu H.Y., Ma H.L., Cui B.X. p66Shc-mediated oxidative stress is involved in gestational diabetes mellitus. World J. Diabetes. 2021;12:1894–1907. doi: 10.4239/wjd.v12.i11.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma H., Qiao Z., Li N., Zhao Y., Zhang S. The relationship between changes in vitamin A, vitamin E, and oxidative stress levels, and pregnancy outcomes in patients with gestational diabetes mellitus. Ann. Palliat. Med. 2021;10:6630–6636. doi: 10.21037/apm-21-1036. [DOI] [PubMed] [Google Scholar]
- 58.Kong C.M., Subramanian A., Biswas A., Stunkel W., Chong Y.S., Bongso A., Fong C.Y. Changes in stemness properties, differentiation potential, oxidative stress, senescence and mitochondrial function in Wharton’s jelly stem cells of umbilical cords of mothers with gestational diabetes mellitus. Stem Cell Rev. Rep. 2019;15:415–426. doi: 10.1007/s12015-019-9872-y. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson K.K., McElrath T.F., Chen Y.H., Loch-Caruso R., Mukherjee B., Meeker J.D. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am. J. Obstet. Gynecol. 2015;212:208.e1–208.e8. doi: 10.1016/j.ajog.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore T.A., Samson K., Ahmad I.M., Case A.J., Zimmerman M.C. Oxidative Stress in Pregnant Women Between 12 and 20 Weeks of Gestation and Preterm Birth. Nurs. Res. 2020;69:244–248. doi: 10.1097/NNR.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eick S.M., Ferguson K.K., Milne G.L., Rios-McConnell R., Vélez-Vega C., Rosario Z., Alshawabkeh A., Cordero J.F., Meeker J.D. Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico. Free. Radic. Biol. Med. 2020;146:299–305. doi: 10.1016/j.freeradbiomed.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abiaka C., Machado L. Nitric oxide and antioxidant enzymes in venous and cord blood of late preterm and term omani mothers. Sultan Qaboos Univ. Med. J. 2012;12:300–305. doi: 10.12816/0003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh T.S.T.A., Chen S.F., Lo L.M., Li M.J., Yeh Y.L., Hung T.H. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod. Sci. 2012;19:505–512. doi: 10.1177/1933719111426601. [DOI] [PubMed] [Google Scholar]
- 64.Gerszi D., Penyige Á., Mezei Z., Sárai-Szabó B., Benkő R., Bányai B., Demendi C., Ujvári E., Várbíró S., Horvath E.M. Evaluation of oxidative/nitrative stress and uterine artery pulsatility index in early pregnancy. Physiol. Int. 2021;107:479–490. doi: 10.1556/2060.2020.00041. [DOI] [PubMed] [Google Scholar]
- 65.Arogbokun O., Rosen E., Keil A.P., Milne G.L., Barrett E., Nguyen R., Bush N.R., Swan S.H., Sathyanarayana S., Ferguson K.K. Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. J. Clin. Endocrinol. Metab. 2021;106:1427–1436. doi: 10.1210/clinem/dgab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindström E., Persson L.Å., Raqib R., Arifeen S.E., Basu S., Ekström E.C. Associations between oxidative parameters in pregnancy and birth anthropometry in a cohort of women and children in rural Bangladesh: The MINIMat-cohort. Free Radic. Res. 2012;46:253–264. doi: 10.3109/10715762.2011.651467. [DOI] [PubMed] [Google Scholar]
- 67.Sanhal C.Y., Daglar K., Kara O., Yılmaz Z.V., Turkmen G.G., Erel O., Uygur D., Yucel A. An alternative method for measuring oxidative stress in intrahepatic cholestasis of pregnancy: Thiol/disulphide homeostasis. J. Matern.-Fetal Neonatal Med. 2018;31:1477–1482. doi: 10.1080/14767058.2017.1319922. [DOI] [PubMed] [Google Scholar]
- 68.Yilmaz S., Ozgu-Erdinc A.S., Demirtas C., Ozturk G., Erkaya S., Uygur D. The oxidative stress index increases among patients with hyperemesis gravidarum but not in normal pregnancies. Redox Rep. 2015;20:97–102. doi: 10.1179/1351000214Y.0000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang X., Bar H.Y., Yan J., West A.A., Perry C.A., Malysheva O.V., Devapatla S., Pressman E., Vermeylen F.M., Wells M.T., et al. Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women. PLoS ONE. 2012;7:e46736. doi: 10.1371/journal.pone.0046736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motamed S., Nikooyeh B., Kashanian M., Chamani M., Hollis B.W., Neyestani T.R. Evaluation of the efficacy of two doses of vitamin D supplementation on glycemic, lipidemic and oxidative stress biomarkers during pregnancy: A randomized clinical trial. BMC Pregnancy Childbirth. 2020;20:619. doi: 10.1186/s12884-020-03311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lymperaki E., Tsikopoulos A., Makedou K., Paliogianni E., Kiriazi L., Charisi C., Vagdatli E. Impact of iron and folic acid supplementation on oxidative stress during pregnancy. J. Obstet. Gynaecol. 2015;35:803–806. doi: 10.3109/01443615.2015.1011102. [DOI] [PubMed] [Google Scholar]
- 72.Kajarabille N., Hurtado J.A., Peña-Quintana L., Peña M., Ruiz J., Diaz-Castro J., Rodríguez-Santana Y., Martin-Alvarez E., López-Frias M., Soldado O., et al. Omega-3 LCPUFA supplement: A nutritional strategy to prevent maternal and neonatal oxidative stress. Matern. Child Nutr. 2017;13:e12300. doi: 10.1111/mcn.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korkmaz V., Ozkaya E., Seven B.Y., Duzguner S., Karsli M.F., Kucukozkan T. Comparison of oxidative stress in pregnancies with and without first trimester iron supplement: A randomized double-blind controlled trial. J. Matern.-Fetal Neonatal Med. 2014;27:1535–1538. doi: 10.3109/14767058.2013.863869. [DOI] [PubMed] [Google Scholar]
- 74.Aalami-Harandi R., Karamali M., Asemi Z. The favorable effects of garlic intake on metabolic profiles, hs-CRP, biomarkers of oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia: Randomized, double-blind, placebo-controlled trial. J. Matern.-Fetal Neonatal Med. 2015;28:2020–2027. doi: 10.3109/14767058.2014.977248. [DOI] [PubMed] [Google Scholar]
- 75.Malti N., Merzouk H., Merzouk S.A., Loukidi B., Karaouzene N., Malti A., Narce M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta. 2014;35:411–416. doi: 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Ballesteros-Guzmán A.K., Carrasco-Legleu C.E., Levario-Carrillo M., Chávez-Corral D.V., Sanchez-Ramirez B., Mariñelarena-Carrillo E.O., Guerrero-Salgado F., Reza-López S.A. Prepregnancy obesity, maternal dietary intake, and oxidative stress biomarkers in the fetomaternal unit. BioMed Res. Int. 2019;2019:5070453. doi: 10.1155/2019/5070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zygula A., Kosinski P., Wroczynski P., Makarewicz-Wujec M., Pietrzak B., Wielgos M., Giebultowicz J. Oxidative stress markers differ in two placental dysfunction pathologies: Pregnancy-induced hypertension and intrauterine growth restriction. Oxid. Med. Cell. Longev. 2020;2020:1323891. doi: 10.1155/2020/1323891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Odame Anto E., Owiredu W.K., Sakyi S.A., Turpin C.A., Ephraim R.K., Fondjo L.A., Obirikorang C., Adua E., Acheampong E. Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: A prospective cohort study in Ghana. PLoS ONE. 2018;13:e0200581. doi: 10.1371/journal.pone.0200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panti A.A., Shehu C.E., Saidu Y., Tunau K.A., Nwobodo E.I., Jimoh A., Bilbis L.S., Umar A.B., Hassan M. Oxidative stress and outcome of antioxidant supplementation in patients with polycystic ovarian syndrome (PCOS) Int. J. Reprod. Contracept. Obs. Gynecol. 2018;7:1667. doi: 10.18203/2320-1770.ijrcog20181892. [DOI] [Google Scholar]
- 80.Liu Y., Yu Z., Zhao S., Cheng L., Man Y., Gao X., Zhao H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 2021;38:471–477. doi: 10.1007/s10815-020-02014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Özer A., Bakacak M., Kıran H., Ercan Ö., Köstü B., Kanat-Pektaş M., Kılınç M., Aslan F. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol. Pol. 2016;87:733–738. doi: 10.5603/GP.2016.0079. [DOI] [PubMed] [Google Scholar]
- 82.Wang H., Ruan X., Li Y., Cheng J., Mueck A.O. Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch. Gynecol. Obstet. 2019;300:1413–1421. doi: 10.1007/s00404-019-05305-7. [DOI] [PubMed] [Google Scholar]
- 83.Heshmati J., Golab F., Morvaridzadeh M., Potter E., Akbari-Fakhrabadi M., Farsi F., Tanbakooei S., Shidfar F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:77–82. doi: 10.1016/j.dsx.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Desai V., Prasad N.R., Manohar S.M., Sachan A., Narasimha S.R.P.V.L., Bitla A.R.R. Oxidative stress in non-obese women with polycystic ovarian syndrome. J. Clin. Diagn. Res. 2014;8:CC01–CC03. doi: 10.7860/JCDR/2014/8125.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kazemi M., Lalooha F., Nooshabadi M.R., Dashti F., Kavianpour M., Haghighian H.K. Randomized double blind clinical trial evaluating the Ellagic acid effects on insulin resistance, oxidative stress and sex hormones levels in women with polycystic ovarian syndrome. J. Ovarian Res. 2021;14:100. doi: 10.1186/s13048-021-00849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turan V., Sezer E.D., Zeybek B., Sendag F. Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J. Pediatric Adolesc. Gynecol. 2015;28:119–123. doi: 10.1016/j.jpag.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Sulaiman M.A., Al-Farsi Y.M., Al-Khaduri M.M., Saleh J., Waly M.I. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int. J. Women’s Health. 2018;10:763–771. doi: 10.2147/IJWH.S166461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai Q., Xiang W., Li Q., Zhang H., Li Y., Zhu G., Xiong C., Jin L. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 2018;12:518–524. doi: 10.1007/s11684-017-0575-y. [DOI] [PubMed] [Google Scholar]
- 89.Yilmaz N., Inal H.A., Gorkem U., Sargin Oruc A., Yilmaz S., Turkkani A. Follicular fluid total antioxidant capacity levels in PCOS. J. Obstet. Gynaecol. 2016;36:654–657. doi: 10.3109/01443615.2016.1148683. [DOI] [PubMed] [Google Scholar]
- 90.Fatemi F., Mohammadzadeh A., Sadeghi M.R., Akhondi M.M., Mohammadmoradi S., Kamali K., Lackpour N., Jouhari S., Zafadoust S., Mokhtar S., et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN. 2017;18:23–30. doi: 10.1016/j.clnesp.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Gongadashetti K., Gupta P., Dada R., Malhotra N. Follicular fluid oxidative stress biomarkers and ART outcomes in PCOS women undergoing in vitro fertilization: A cross-sectional study. Int. J. Reprod. Biomed. 2021;19:449–456. doi: 10.18502/ijrm.v19i5.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishihara T., Matsumoto K., Hosoi Y., Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod. Med. Biol. 2018;17:481–486. doi: 10.1002/rmb2.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alam F., Khan T.A., Amjad S., Rehman R. Association of oxidative stress with female infertility-A case control study. J. Pak. Med. Assoc. 2019;69:627. [PubMed] [Google Scholar]
- 94.Gong Y., Zhang K., Xiong D., Wei J., Tan H., Qin S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: A randomized controlled trial. Reprod. Biol. Endocrinol. 2020;18:91. doi: 10.1186/s12958-020-00648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Younis A., Clower C., Nelsen D., Butler W., Carvalho A., Hok E., Garelnabi M. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J. Assist. Reprod. Genet. 2012;29:1083–1089. doi: 10.1007/s10815-012-9831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh A.K., Chattopadhyay R., Chakravarty B., Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013;42:116–124. doi: 10.1016/j.reprotox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 97.Prieto L., Quesada J.F., Cambero O., Pacheco A., Pellicer A., Codoceo R., Garcia-Velasco J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012;98:126–130. doi: 10.1016/j.fertnstert.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 98.Liu F., He L., Liu Y., Shi Y., Du H. The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clin. Exp. Obstet. Gynecol. 2013;40:372–376. [PubMed] [Google Scholar]
- 99.Santulli P., Chouzenoux S., Fiorese M., Marcellin L., Lemarechal H., Millischer A.E., Batteux F., Borderie D., Chapron C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015;30:49–60. doi: 10.1093/humrep/deu290. [DOI] [PubMed] [Google Scholar]
- 100.Polak G., Wertel I., Barczyński B., Kwaśniewski W., Bednarek W., Kotarski J. Increased levels of oxidative stress markers in the peritoneal fluid of women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;168:187–190. doi: 10.1016/j.ejogrb.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 101.Amini L., Chekini R., Nateghi M.R., Haghani H., Jamialahmadi T., Sathyapalan T., Sahebkar A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021;2021:5529741. doi: 10.1155/2021/5529741. [DOI] [PMC free article] [PubMed] [Google Scholar]