Abstract
Inonotus hispidus mushroom is a popular edible and medicinal mushroom with a long history of use. It is well known as a medicinal fungus with various health benefits for its significant anticancer and immunomodulatory activities. Over the last 60 years, secondary metabolites derived from I. hispidus and their biological activities have been discovered and investigated. Structurally, these compounds are mainly polyphenols and triterpenoids, which have anticancer, anti-inflammatory, antioxidant, antimicrobial, and enzyme inhibitor activities. Here, the secondary metabolites derived from I. hispidus and their activities were systematically and comprehensively classified and summarized, and the biosynthetic pathway of stylylpyrones was deduced and analyzed further. This review contributes to our understanding of I. hispidus and will help with research into natural product chemistry, pharmacology, and the biosynthesis of I. hispidus metabolites. According to this review, I. hispidus could be a promising source of bioactive compounds for health promotion and the development of functional foods.
Keywords: Inonotus hispidus, natural product, biological activity, medicinal fungi
1. Introduction
The fungus Inonotus hispidus (Bull.: Fr.) Karst. is a facultative saprophytic (brown-rot Basidiomycete), which has been found to be parasitic on various broadleaf trees in China and Europe including mulberry (Morus alba L.), ash (Fraxinus mandshurica), Populus euphratica, Ulmus campestris, Sorbus aucuparia, and Acer saccharum [1,2]. According to modern classification systems, I. hispidus belongs to the Inonotus genus in Hymenochaetaceae (Hymenochaetales, Agaricomycetes, Basidiomycota) [3], whose obsolete synonyms include Polyporus hispidus (Bull.) Fr., Boletus hispidus (Bull.), Xanthochrous hispidus (Bull.) Pat. The fruit bodies of I. hispidus are annual, sessile, woody mushroom. The fruiting bodies are bright yellow at the initial growth stage (edible) (Figure 1A), and the color gradually changes from light brown to brown as they grow, becoming dark brown when fully matured with hairy surfaces (Figure 1B), and eventually turns to black [4].
Figure 1.
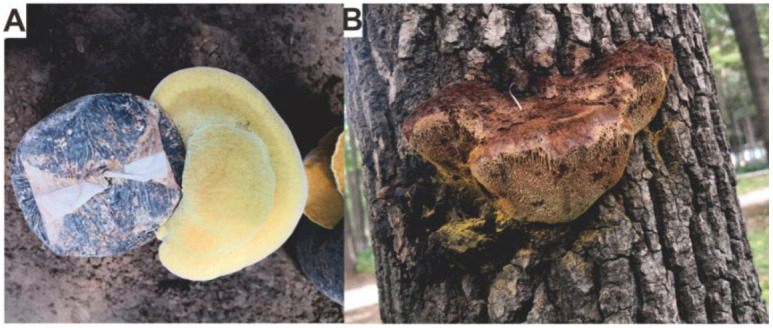
Morphological photographs of fruiting bodies of I. hispidus at the initial growth stage in artificial cultivation (A) and at the mature stage in the wild (B). The photo of mature fruiting body taken on ash on the campus of Northeast Forestry University in August 2021.
Since I. hispidus are frequently parasitic on the mulberry tree (pronounced “sang” in Chinese), their fruit bodies are yellow (pronounced “huang” in Chinese) in color, highly similar in morphology to the modern taxonomic definition of the genus Sanghuangporus [5], and relatively close in herbal activity; I. hispidus were described as “Sanghuang” in the ancient Chinese medical classics. In the Aksu region of Xinjiang and Xiajin County of Shandong in China, I. hispidus parasites on mulberry trees has been used as a traditional indigenous medicine “Sanghuang” for the treatment of dyspepsia, cancer, diabetes, and stomach problems [6]. In fact, “Sanghuang” is a traditional collective name including I. hispidus, which has a long history of medicinal use and high economic value in the Southeast Asia region (“Meshimakobu” in Japan, and Sanghwang in South Korea) [7]. In China, the earliest medicinal records on “Sanghuang” can be traced back to Shen Nong’s Materia Medica in the Qin and Han Dynasties. The “Shen Nong’s Materia Medica” and “Compendium of Materia Medica” have detailed descriptions of the efficacy of “Sanghuang”. After the Second World War, the number of cancer cases in Nagasaki, Japan, increased dramatically. Danjo-gunto in Nagasaki is rich in “Sanghuang” due to the cultivation of mulberries and silkworms, and the residents were more healthy and had fewer cancer cases due to taking “Sanghuang”. It was later found that it was the anti-tumor properties of mulberry that played a key role [8]. Recent studies in natural product chemistry and pharmacology have shown that I. hispidus contains a diverse spectrum of monomeric compounds and exhibits a diversity of physiological actions in in vitro and in vivo assays.
This review summarizes English and Chinese articles published in international academic journals after 1961, based on retrieving electronic databases PubMed, Wiley Online Library, Science Direct, and Web of Science. Totals of 64 compounds, including polyphenols, terpenoids, fatty acids and other types, were classified and sorted. These metabolites with outstanding medicinal value, and crude extracts of the fruit bodies were then inventoried and summarized according to their antitumor, anti-inflammatory, antioxidant, antineurodegenerative, and antimicrobial biological activities. The biosynthetic pathways of styrylpyrones derived from I. hispidus and related fungi were deduced and summarized. Related studies on phenols and sesquiterpenes, and food properties of I. hispidus are discussed. This work will provide a reference for subsequent studies on the natural product chemistry, pharmacological activities, and biosynthesis of secondary metabolites of I. hispidus.
2. Compounds with Diverse Structures
2.1. Polyphenol Compounds
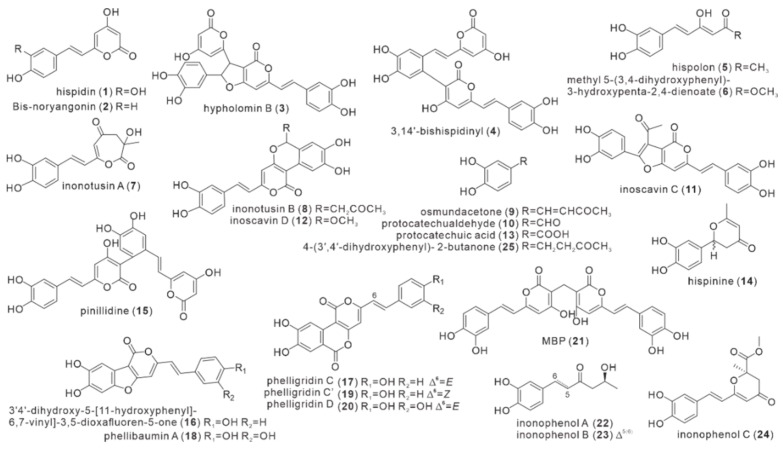
Polyphenols (Figure 2) are the main pigment components present in the fruiting bodies of I. hispidus. Zopf first isolated a series of yellow substances from the fruiting bodies of I. hispidus in 1889, and Edwards et al. isolated the yellow substances to obtain a yellow crystalline hispidin (1) in 1961 [9]. Hispidin was the first reported monomeric compound from I. hispidus, and its structure was identified as 6-(3,4-dihydroxystryl)-4-hydroxy-2-pyrone by infrared techniques and derivatization reactions. Bis-noryangonin (2) was found in the fermentation broth of P. hispidus (synonym of I. hispidus) in liquid medium with glucose as the main carbon source. Its structure was determined as 4-bydroxy-6-(4-hydroxystylyl)-2-pyrone by isotopic label feeding, spectral characterization, and thin-layer chromatography (TLC) [10]. Fiasson and Jean-Louis isolated hispidin (1) from the fruiting bodies of I. hispidus in 1982, along with its two dimer derivatives, hypholomin B (3) and 3,14’-bishispidinyl (4) [11]. In 1996, Nasser Ali et al. obtained two pigment polyphenols, hispolon (5) and known hispidin (1), from the crude ethanolic extract of fruiting bodies under the guidance of immunomodulatory and antiviral activity. The structure of compound 5 was identified as 6-(9,10-dihydroxyphenyl)-3,5(E)-hexadien-4-ol-2-one by combined spectroscopic, mass spectrometry (MS) and nuclear magnetic resonance (NMR) techniques [12]. In 2009, Yousfi et al. extracted and isolated two other known polyphenols (1 and 5) and a new compound methyl 5-(3,4-dihydroxyphenyl)-3-hydroxypenta-2,4-dienoate (6) from the antioxidant components of the fruiting bodies parasitic on Pistacia atlantica, and its structure was determined by NMR and EI-MS [13]. In the experiment to determine the trace element composition of anti-influenza virus mushrooms, compounds 1 and 5 were once more isolated and extracted from the fruiting bodies of I. hispidus in 2011 [14]. In 2011 and 2012, Zan et al. isolated ten phenolic compounds from methanol extracts of fruiting bodies living on ash, among which inonotusins A (7) and B (8) were new natural products. The other known compounds were hispidin (1), hispolon (5), osmundacetone (9), protocatechualdehyde (10), inoscavin C (11), and inoscavin D (12), as well as protocatechuic acid (13) [15]. Compound 7 contains a highly oxidized functional group with a 2,3,4,5-tetrahydrooxepine backbone, which is the only phenolic compound containing a heptameric ring from I. hispidus. A combined approach of MS, spectroscopy and NMR methods clarified the structures of 7 and 8 are 6-(11,12-dihydroxystyryl)-2-hydroxy-2-methyl-5,6-dihydro-oxepine-1,4-dione and 5-(10,11-dihydroxystyryl)-3,4-dihydroxy7-(2-oxopropyl) pyrano-isochromen-1-one [16]. Compounds 9–13 were isolated from this strain for the first time [15]. A content comparison revealed that the fruit bodies contained higher concentrations of hispidin (1), hispolon (5), and osmundacetone (9), which were isolated for the first time from this strain [15]. Gruendemann et al. isolated known compounds hispidin (1) and hispolon (5) from methanol extracts of their fruiting bodies with the help of bioactivity-guided fractionation and analyzed their immunomodulatory effects in 2016 [17]. In 2017, Ren et al. isolated a new compound hispinine (14) and five known polyphenols (1, 4, 5, 9, and 15) from the methanol extract of fruiting bodies of Xinjiang (China) indigenous medicinal fungus I. hispidus [18]. Despite the fact that the structure of compound 14 was determined by spectroscopic analysis to be 2-(3,4-dihydroxyphenyl)-6-methyl-2H-pyran-4(3H)-one [18], its absolute conformation has not yet been verified. Compound 15, pinidine, isolated from I. hispidus in 2017, was first isolated from Phellinus pini [19]. Phellinus pini is a fungus that is morphologically similar to the fungi of the genus Sanghuangporus defined by the latest taxonomy [5]. Five polyphenols (16–20) were isolated and identified by Li et al. from the ethanolic extract of mature yellow fruiting bodies in 2017. Two compounds 3′4′-dihydroxy-5-[11-hydroxyphenyl]-6,7-vinyl]-3,5-dioxafluoren-5-one (16) and phelligridin C (17) were undescribed previously, while phellibaumin A (18), phelligridin C’ (19) and phelligridin D (20) were isolated for the first time from I. hispidus [2]. In 2019, Yang et al. isolated the compound 3,3′-methylene-bis [6-(3,4-dihydroxystyryl)-4-hydroxy-2H-pyran-2-one (MBP, 21) from a methanol extract of the fruiting body, together with four reported polyphenols (9, 10, 13, and 18) derived from I. hispidus [20]. From a methanol extract of the fruiting body, Kou et al. isolated six polyphenols in 2021, including three compounds inonophenols A-C (22–24), as well as 5, 9, and 4-(3′,4′-dihydroxyphenyl)-2-butanone (25) [21]. The structures of compounds 22–24 were identified by NMR, HRMS, and/or computational circular dichroism data [21]. In the work on the analysis of antitumor metabolites in the mycelial fermentation broth of I. hispidus, hispolon (5) was isolated again, which was the first reported isolation from the liquid fermentation [22]. Bis-noryangonin (2), isolated from the mycelial broth of I. hispidus, is the only phenolic metabolite derived solely from the mycelial broth [10]. Structurally, compound 21, MBP, is the symmetrical isomer of compound 1, while compounds 3, 4, and 15 are its non-symmetrical isomers.
Figure 2.
Structures of polyphenol compounds (1–25).
2.2. Triterpenoids
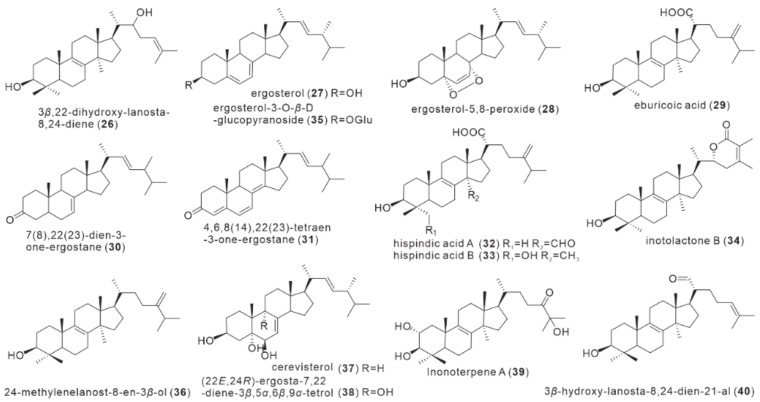
Triterpenoids are another important group of secondary metabolites found in I. hispidus, with a total of 15 triterpene compounds isolated (Figure 3). Yang et al. first reported the isolation and identification of four triterpenoid monomers from the ethanolic extract of fruiting bodies in 2008, which included 3,22-dihydroxy-lanosta-8,24-diene (26), ergosterol (27), ergosterol-5,8-peroxide (28), and eburicoic acid (29), with ergosterol (27) being the main chemical constituent isolated from 95% ethanolic extract of I. hispidus [23]. Zan et al. isolated five triterpenoids from the methanolic extract of fruiting bodies in 2012, including 7(8),22(23)-dien-3-one-ergostane (30), 4,6,8(14),22(23)-tetraen-3-one-ergostane (31) for the first time from this fungus, along with known compounds 27–29 [15]. Compound 28 is a high-content component in the fruiting bodies of I. hispidus [15].
Figure 3.
Structures of triterpenoids (26–40).
Ren et al. isolated six triterpenoids, including hispindic acids A and B (32–33), as well as the known compounds 27, 29, inotolactone B (34), and ergosterol-3-O-β-D-glucopyranoside (35) from the methanolic extract of fruiting bodies in 2017. The two new triterpenoids 32 and 33 were structurally identified as 24-exomethylene-3β-hydroxy-30-oxo-lanost-8-en-21-oic acid and 24-exomethylene-3β, 28-dihydroxy-lanost-8-en-21 oic acid by a combination of mass spectrometry and NMR techniques [18]. Seven triterpenoids were isolated from the methanolic extract of the fruiting bodies of I. hispidus by Kou et al. in 2021, including compound 28, 35, 24-methylenelanost-8-en-3β-ol (36), cerevisterol (37), (22E, 24R)-ergosta-7,22-diene-3β,5α,6β,9α-tetrol (38), and Inonoterpene A (39), and 3β-hydroxy-lanosta-8,24-dien-21-al (40). Among them, Inonoterpene A (39) is a new Lanostane triterpenoid, and compound 40 was isolated from the fungus for the first time. Interestingly, the above 15 triterpenoids are all lanosterol-type triterpenoids, all derived from the fruiting bodies of I. hispidus. Only three triterpenes 32, 33, and 39, have been isolated as new compounds for the first time from I. hispidus. Compounds 32 and 40 contain formyl groups at C30 and C21, respectively, while compound 35 possesses glycosylated modifications. It was discovered that specific combinations of four compounds, methyl jasmonate, salicylic acid, oleic acid, and Cu2+, and their combinations, can increase the content of total triterpenoids in an orthogonal experiment for liquid fermentation of I. hispidus [24].
2.3. Fatty Acid Compounds
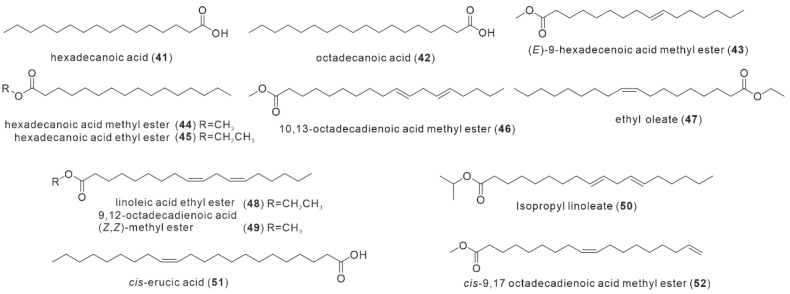
The fatty acid analogues of I. hispidus were first reported by Yang et al. in 2008, and two monomer compounds hexadecanoic acid (41) and octadecanoic acid (42) were obtained in the ethanolic extract of its fruit bodies [23] (Figure 4). The structure of the former was identified by EI-MS and TLC comparison of the standard, and the identification of the latter was done with additional infrared techniques [23]. Zan et al. published 2012 the detection results of gas mass spectrometry of the yellow oily substance obtained from the petroleum ether extraction part of the methanol extract of fruit bodies. A total of 12 fatty acid compounds were identified by consulting the Wiley Online Library. These compounds are hexadecanoic acid (41), (E)-9-hexadecenoic acid methyl ester (43), hexadecanoic acid methyl ester (44), hexadecanoic acid ethyl ester (45), 10,13-octadecadienoic acid methyl ester (46), ethyl oleate (47), linoleic acid ethyl ester (48), 9,12-octadecadienoic acid (Z, Z)-methyl ester (49), Isopropyl linoleate (50), cis-erucic acid (51), and cis-9,17 octadecadienoic acid methyl ester (52) [15]. Due to their low polarity and insignificant drug properties, fatty acid compounds are not preferred subjects in natural product chemistry studies of fungi. Only two cases of I. hispidus have been reported in this regard.
Figure 4.
Structures of fatty acid compounds (41–52).
2.4. Miscellaneous Compounds
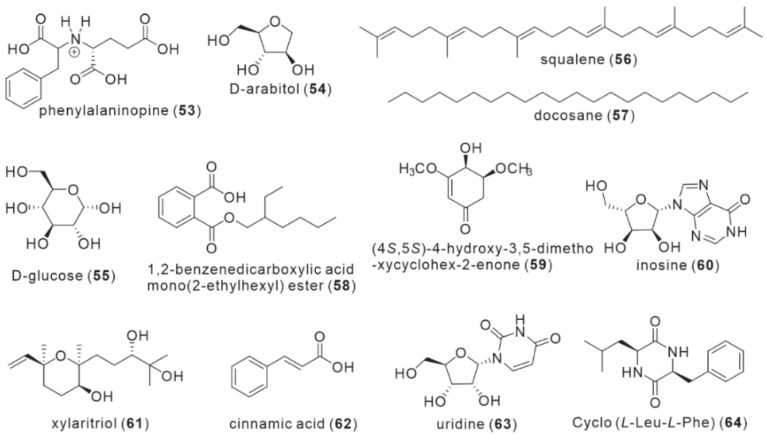
During the constituent analysis of dried fruiting bodies of I. hispidus in 2007, Politi et al. identified a known compound, phenylalaninopine (53), by spectral and mass spectrometric characterization collected by LC-DAD-MS and comparing with the literature, while attempting to develop a phytochemical extraction method for mushrooms using hot water as a solvent [25] (Figure 5). The compound was isolated initially from Clitocybe acromelalga, a poisonous mushroom [26]. Yang et al. obtained two monosaccharides, D-arabitol (54) and D-glucose (55), along with the isolation of four triterpenoids [23]. In their experiments to examine the fatty acid composition of fruiting bodies, Zan et al. discovered two hydrocarbons, squalene (56) and docosane (57), and one phthalate, 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester (58) [15]. In the separation of anticancer active components, Yang et al. isolated a new compound from the methanol extract of fruiting bodies in 2019 and confirmed its structure as (4S,5S)-4-Hydroxy-3,5-dimethoxycyclohex-2-enone (HDE, 59) by LC-MS and NMR [27]. When analyzing the methanol extract composition of I. hispidus fruiting bodies, Yang et al. identified a nucleoside compound, inosine (60), based on a comparison of UV absorption characteristics and retention time with a standard [20]. Kou et al. isolated an unsaturated sesquiterpene, xylaritriol (61) [21], from the methanol extract of fruiting bodies, which had previously been from the metabolites of endophytic fungus Xylaria cubensis (Ascomycete) [28]. Tang et al. isolated compounds, cinnamic acid (62), uridine (63), and Cyclo (L-Leu-L-Phe) (64) from the fermentation broth of I. hispidus mycelium in 2021 [22]. Among these 12 compounds with no shared structural features, compound 59 is the only new compound isolated for the first time from I. hispidus, and compound 61 was the only sesquiterpene isolated from I. hispidus. Cyclic dipeptide compounds are uncommon in mushrooms (Basidomycota), but compound 64 was discovered in I. hispidus. This could be related to the fact that compound 64 is derived from mycelial fermentation broth.
Figure 5.
Structures of miscellaneous compounds (53–64).
3. Biological and Pharmacological Activities
Inonotus hispidus has long been used to treat dyspepsia, cancer, diabetes, and stomach problems in Xinjiang residents [6]. Several studies have been published on the pharmacological activities and mechanisms of major compounds from I. hispidus (Table 1). These studies have been summarized here, with a special emphasis on polyphenols with medicinal potential (Figure 6).
Table 1.
Occurrence of compounds from Inonotus hispidus.
| No. | Name | Resource | Method | Activity | Reference |
|---|---|---|---|---|---|
| 1 | hispidin | fruiting body, mycelium |
EtOH, MeOH |
Antitumor, ABTS, DPPH, influenza A and B viruses, Enzyme-inhibitory, antineurodegenerative | Edwards et al. [9]. Zan et al. [15]. Ali, N.A.A. et al. [29]. Zan et al. [16]. Zan et al. [30]. Benarous, K. et al. [31]. Benarous, K. et al. [32]. Gruendemann, C. et al. [17]. |
| 2 | Bis-noryangonin | mycelium | NA | NA | Perrin, P.W.et al. [10]. |
| 3 | hypholomin B | fruiting body | NA | NA | Fiasson et al. [11]. |
| 4 | 3,14’-bishispidinyl | fruiting body | MeOH | NA | Fiasson et al. [11]. Ren et al. [18]. |
| 5 | hispolon | fruiting body mycelium |
EtOH, MeOH EAC |
DPPH, influenza A and B viruses, PC-12 cell, BV-2 microglia, ntineurodegenerative | Nasser Ali et al. [12]. Zan et al. [15]. Tang et al. [22]. Kou et al. [21]. Ali, N.A.A. et al. [29]. Gruendemann, C. et al. [17]. |
| 6 | methyl 5-(3,4-dihydroxyphenyl)-3-hydroxypenta-2,4-dienoate | fruiting body | MeOH | NA | Yousfi et al. [13]. |
| 7 | inonotusin A | fruiting body | MeOH | MCF-7, ABTS | Zan et al. [15]. Zan et al. [16]. |
| 8 | inonotusin B | fruiting body | MeOH | Antitumor, ABTS | Zan et al. [15]. Zan et al. [16]. |
| 9 | osmundacetone | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Zan et al. [15]. Kou et al. [21]. |
| 10 | protocatechualdehyde | fruiting body | MeOH | NA | Zan et al. [15]. |
| 11 | inoscavin C | fruiting body | MeOH | NA | Zan et al. [15]. |
| 12 | inoscavin D | fruiting body | MeOH | NA | Zan et al. [15]. |
| 13 | protocatechuic acid | fruiting body | MeOH | NA | Zan et al. [15]. |
| 14 | hispinine | fruiting body | MeOH | NA | Ren et al. [18]. |
| 15 | pinillidine | fruiting body | MeOH | NA | Ren et al. [18]. |
| 16 | 3’4’-dihydroxy-5-[11-hydroxyphenyl]-6,7-vinyl]-3,5-dioxafluoren-5-one | fruiting body | EtOH | mouse macrophage cell | Li et al. [2]. |
| 17 | phelligridin C | fruiting body | EtOH | NA | Li et al. [2]. |
| 18 | phellibaumin A | fruiting body | EtOH MeOH |
mouse macrophage cell | Li et al. [2]. Yang et al. [20]. |
| 19 | phelligridin C’ | fruiting body | EtOH | NA | Li et al. [2]. |
| 20 | phelligridin D | fruiting body | EtOH | mouse macrophage cell | Li et al. [2]. |
| 21 | MBP | fruiting body | MeOH | HepG2, MCF-7, Hela and A549 cells | Yang et al. [20]. |
| 22 | inonophenol A | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 23 | inonophenol B | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 24 | inonophenol C | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 25 | 4-(3′,4′-dihydroxyphenyl)-2-butanone | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 26 | 3β, 22-dihydroxy-lanosta-8,24-diene | fruiting body | EtOH | U937, Hela, QRH-7701 | Yang et al. [23]. |
| 27 | ergosterol | fruiting body mycelium |
EtOH, MeOH EAC |
NA | Yang et al. [23]. Zan et al. [15]. Tang et al. [22]. |
| 28 | ergosterol-5,8-peroxide | fruiting body | EtOH, MeOH | BV-2 microglia | Yang et al. [23]. Zan et al. [15]. Kou et al. [21]. |
| 29 | eburicoic acid | fruiting body | EtOH, MeOH | NA | Yang et al. [23]. Zan et al. [15]. |
| 30 | 7(8),22(23)-dien-3-one-ergostane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 31 | 4,6,8(14),22(23)-tetraen-3-one-ergostane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 32 | hispindic acid A | fruiting body | MeOH | NA | Ren et al. [18]. |
| 33 | hispindic acid B | fruiting body | MeOH | NA | Ren et al. [18]. |
| 34 | inotolactone B | fruiting body | MeOH | B16 melanoma cell | Ren et al. [18]. |
| 35 | ergosterol-3-O-β-D-glucopyranoside | fruiting body | MeOH | BV-2 microglia | Ren et al. [18]. Kou et al. [21]. |
| 36 | 24-methylenelanost-8-en-3β-ol | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 37 | cerevisterol | fruiting body | MeOH | NA | Kou et al. [21]. |
| 38 | (22E, 24R)-ergosta-7,22-diene-3β,5α,6β,9α-tetrol | fruiting body | MeOH | NA | Kou et al. [21]. |
| 39 | Inonoterpene A | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 40 | 3β-hydroxy-lanosta-8,24-dien-21-al | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 41 | hexadecanoic acid | fruiting body | EtOH, MeOH | NA | Yang et al. [23]. Zan et al. [15]. |
| 42 | octadecanoic acid | fruiting body | EtOH | NA | Yang et al. [23]. |
| 43 | (E)-9-hexadecenoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 44 | hexadecanoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 45 | hexadecanoic acid ethyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 46 | 10,13-octadecadienoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 47 | ethyl oleate | fruiting body | MeOH | NA | Zan et al. [15]. |
| 48 | linoleic acid ethyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 49 | 9,12-octadecadienoic acid (Z, Z)-methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 50 | Isopropyl linoleate | fruiting body | MeOH | NA | Zan et al. [15]. |
| 51 | cis-erucic acid | fruiting body | MeOH | NA | Zan et al. [15]. |
| 52 | cis-9,17 octadecadienoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 53 | phenylalaninopine | fruiting body | Water | NA | Politi et al. [25]. |
| 54 | D-arabitol | fruiting body | EtOH | NA | Yang et al. [23]. |
| 55 | D-glucose | fruiting body | EtOH | NA | Yang et al. [23]. |
| 56 | squalene | fruiting body | MeOH | NA | Zan et al. [15]. |
| 57 | docosane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 58 | 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 59 | HDE | fruiting body | MeOH | HepG2, McF-7, Hela, A549 and H22 cells | Yang et al. [27]. |
| 60 | inosine | fruiting body | MeOH | NA | Yang et al. [20]. |
| 61 | xylaritriol | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 62 | cinnamic acid | mycelium | NA | NA | Tang et al. [22]. |
| 63 | uridine | mycelium | NA | NA | Tang et al. [22]. |
| 64 | Cyclo (L-Leu-L-Phe) | mycelium | NA | NA | Tang et al. [22]. |
NA indicated the data is not available.
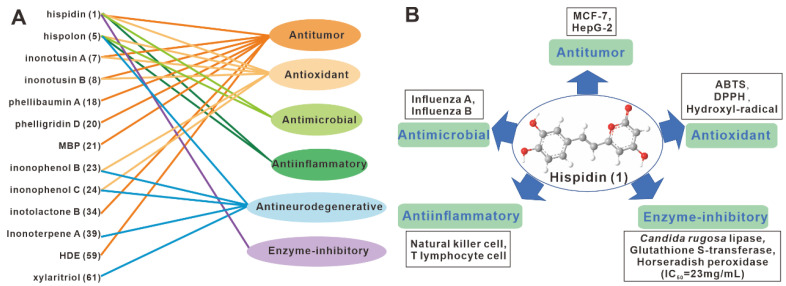
Figure 6.
The bioactivities of representative compounds (A) and hispidin (1) (B) from I. hispidus compounds.
3.1. Antitumor Activity
As a traditional medicinal fungus with a long-applied history, anti-cancer activity is the principal medicinal value of I. hispidus. Multiple monomers with anticancer activity from I. hispidus have been discovered and preliminarily resolved in modern chemical chemistry and pharmacological investigations. In vitro screening of five compounds including hispidin (1), inonotusin A (7) and inonotusin B (8) against breast cancer cells MCF-7 using the SRB method revealed that inonotusin A (7) had moderate activity against MCF-7 with an IC50 value of 19.6 μM [16]. The anti-tumor screening study of MBP (21) was carried out by MTT method. The results showed that MBP (21) had a dosage-dependent inhibitory effect on the proliferation of HepG2, MCF-7, Hela and A549 cells. Among them, HepG2 showed the best inhibitory effect, with an IC50 of 2.3 μg/mL.
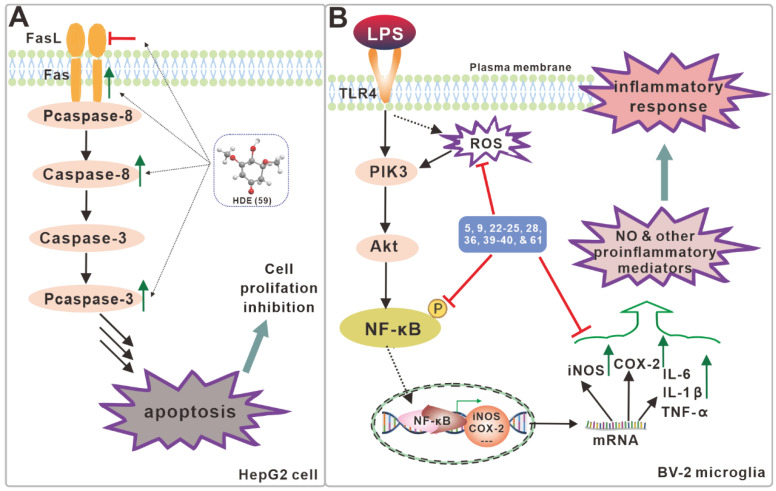
According to the results of the apoptosis analysis, it is hypothesized that the compound (21) achieves its anti-tumor activity by inducing apoptosis [20]. A study of compound 26’s antitumor activity discovered that it inhibited the growth of lymphoma cells U937, cervical tumor cells Hela, and liver cancer cells QRH-7701 [23]. In vitro screening showed that HDE (59) inhibited the proliferation of HepG2, McF-7, Hela, A549 and H22 cells, with a greater effect on HepG2 cells (IC50 = 7.9μg/mL) [27]. Not only did HDE (59) increase the relative activities of Caspase-3 and Caspase-8 and induce HepG2 apoptosis, but it also promoted HepG2 apoptosis by up-regulating Fas expression and down-regulating FasL expression [27] (Figure 7A). In vivo mouse experiments revealed that HDE (59) does not cross the blood–brain barrier and is rapidly metabolized with no adverse effects on organs. Overall, HDE (59) has the potential to be an effective antineoplastic agent [27]. At 10 mmol/L, the compound inotolactone B (34) still showed activity in activating melanogenesis and tyrosinase in B16 melanoma cells, outperforming 8-MOP (a drug used clinically in the treatment of vitiligo). This indicates that inotolactone B (34) has the potential to be developed as an anti-vitiligo agent [18]. The evaluation of the cellular activity of mouse macrophage cells RAW264.7 revealed that compounds 16, 18 and 20 all had anti-inflammatory activity, with compound 16 being the most active. Cytotoxicity studies showed that all three compounds have a low impact on cell proliferation [2]. This result suggests that compound 16 has the potential to be an anti-leukemic agent. In the supernatant of fermented mycelium of a suspected new subspecies of I. hispidus, IH3, a single fraction with high purity of anti-tumor activity, WIH3, was obtained. WIH3 had strong inhibitory against melanoma cells B16 (IC50 29.32 µg/mL), liver cancer cells Hep-3B (37.39 µg/mL), human cervical cancer cells Hela (47.03 µg/mL), and human breast cancer cells MCF-7 (58.01 µg/mL) [33].
Figure 7.
Schematic diagram of the proposed inhibitory mechanism of compound 59 on HepG2 cell (A) and compounds including 24 on inflammatory BV-2 microglia induced by LPS (B).
In vivo antitumor activity experiments with extracts of fruit bodies from different growth stages of I. hispidus revealed that both petroleum ether extracts (IPE) and aqueous extracts at the mature stage had the best tumor suppressive effect on H22-bearing mice [15]. Using non-targeted metabolomics techniques, it was found that the antitumor effects of IPE on H22 tumor-bearing mice were mainly mediated by energy modulation and regulation of biosynthetic pathways including amino acids and corticosteroids [34]. A series of key regulators of the antitumor activity of IPE were identified in H22 tumor suppressor model mice through transcriptomic and proteomic approaches, and the results provide a useful reference for the pharmacological study of the antitumor activity of IPE [35]. The feeding experiment of solid fermentation powder of I. hispidus on H22-bearing mice demonstrated that it has antitumor effect, whose mechanism may be related to antioxidation, improving immunity and inhibiting tumor tissue angiogenesis [36].
3.2. Antioxidant Activity
One of the important properties of mushrooms is their antioxidant function, and antioxidant active molecules derived from I. hispidus have been investigated and studied. Antioxidant assays revealed that compounds 1, 7, and 8 have significant oxidative protective activity against 2,2′-azino-bis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) [16]. Compounds 5, 9, 22–25 have remarkable inhibitory activity against 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH) with IC50 values of 9.82−21.43 μM, with compound 24 being more effective than the positive control [21]. Preliminary structure–activity relationship analysis revealed that catechol unit, double bond on the side-chain, and pyran ring may be important pharmacophores for increasing activity [21]. A study of the antioxidant activity of the compound hispidin (1) to scavenge free radicals discovered that at a low concentration (12.5 mg/L), the scavenging rate of 1 to free radical DPPH was 84.70%. At low concentrations, the DPPH scavenging rate was higher than that of BHA, a commercial antioxidant, and compound 1 has natural antioxidant value [30]. The antioxidant activity of compound 1 for scavenging hydroxyl radicals was found to be superior to that of BHA at concentrations as low as 25 mg/kg [30]. DPPH, ABTS and phosphomolybdenum were used as indicators to determine the antioxidant activity of the ethyl acetate extract of I. hispidus. The extract was found to be more powerful than the commonly tested antioxidant compounds gallic acid and quercetin [31]. The scavenging effect of hydroxyl radical, DPPH radical and superoxide anion of the volatile oil fragments (H-2) obtained by the diethyl ether reflux extraction of the fruiting bodies of I. hispidus was significantly better than that of the volatile oil fragments (H-1) obtained by the steam distillation extraction of the fruiting bodies of I. hispidus [37].
3.3. Antimicrobial Activity
The antibacterial activity tests performed on hispidin (1) revealed that compound 1 lacked adequate inhibitory activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis [15]. The inhibition activity of a 70% ethanolic extract of fruit bodies was tested, and it was discovered that the ethanolic extract inhibited only E. coli, with the high dosage extract (40.96 mg/mL) achieving a significant level of inhibition. S. aureus and B. subtilis were not inhibited significantly [15]. At a concentration of 1 × 10−5 g/mL, both the H-1 (water extract) and H-2 (ether extract) fractions of fruit bodies inhibited S. aureus, Candida albicans, Aspergillus niger, and Pseudomonas aeruginosa, with a positive correlation with concentration, but neither inhibited E. coli [15]. The disc diffusion assay of ethanolic extracts of I. hispidus fruit bodies revealed a minor antimicrobial effect against S. aureus, E. coli, and P. aeruginosa [38]. Hispolon (5) and hispidin (1) were discovered to have antiviral activity, inhibiting the growth of influenza A and B viruses [29]. After feeding mice I. hispidus mushroom containing compounds 1 and 5, the anti-influenza virus ability of mice was improved, as was the content of trace elements zinc (194 ± 16.9 mg/kg), selenium, and iron in mouse serum. These metal elements are thought to enhance the antiviral ability of the I. hispidus mushroom [14].
3.4. Neurotrophic and Neuroprotective Activity
Compounds 5, 9, and 22–25 exhibited a growth-promoting effect on PC-12 cells. Of these, compounds 23 (33.21 ± 0.8%) and 24 (33.34 ± 1.0%) were the most significant in promoting growth at a concentration of 10 μM [21]. In screening assays for anti-neuroinflammatory activity, compounds 5, 9, 22–25, 28, 36, 39–40, and 61 all inhibited of LPS-induced NO production in BV-2 microglia, with compound 24 showing significant activity with an IC50 value of 11.56 μM. None of the compounds tested toxic [21]. Additional molecular immunological experiments revealed that compounds 5, 9, and 22–25 inhibited the expression of TLR-4 to down-regulate the NF-κB signaling pathway, and then inhibited the expression of COX-2 and iNOS to reduce inflammation (Figure 7B) [21]. These findings show that these polyphenols and triterpenoids have anti-neurodegenerative activity.
3.5. Enzyme Inhibitory Activity
Yousfi et al. discovered in 2013 that the polyphenol-rich ethyl acetate extract of I. hispidus fruit bodies was very effective in inhibiting Candida rugosa lipase, but the specific inhibitory compounds were unknown [39]. In 2015, they isolated hispidin (1) from the fruit bodies and demonstrated that hispidin (1) strongly inhibited C. rugosa lipase, proposing the use of hispidin (1) in the treatment of candidiasis [31]. Hispidin (1) was discovered to have anti-peroxidase activity, with an IC50 of 23 mg/mL showing a strong competitive inhibition of horseradish peroxidase (HRP) activity. The inhibition mechanism of compound 1 against peroxidases (horseradish and thyroid) was studied using molecular docking simulations in terms of hispidin interaction with amino acid residues, and its drug-forming potential was predicted using ADEMT and Lipinski filtering analyses [40]. According to the combined evaluation, hispidin (1) is a more potent irreversible thyroperoxidase inhibitor than the anti-thyroid drug 6-hropylthiouracil [40]. The discovery of this result, to some extent, revealed the mechanism of the antioxidant activity of hispidin (1). Furthermore, low doses of ethanolic extracts of I. hispidus fruiting bodies increased GST enzyme activity [38]. Compound 34 (IC50 = 0.24 mM) has excellent α-glycosidase inhibitory activity and is more potent than the clinically used acarbose (IC50 = 0.46 mM) [40]. At the same concentration, the ethyl acetate extract of fruiting bodies had higher α-glucosidase inhibitory activity than the n-butanol, methanol, and petroleum ether extracts [41].
3.6. Immunomodulatory Effects and Other Biological activities
Cellular activity assays on NK cells showed that compounds 1 and 5 both inhibited the activation and proliferation of activated lymphocytes, but the combination of the two did not enhance this effect [17]. This result demonstrates the immunomodulatory activity of these phenolic compounds. In addition, the fermentation broth exopolysaccharide of I. hispidus was shown to attenuate inflammatory responses in mice with acute alcoholic liver disease [42].
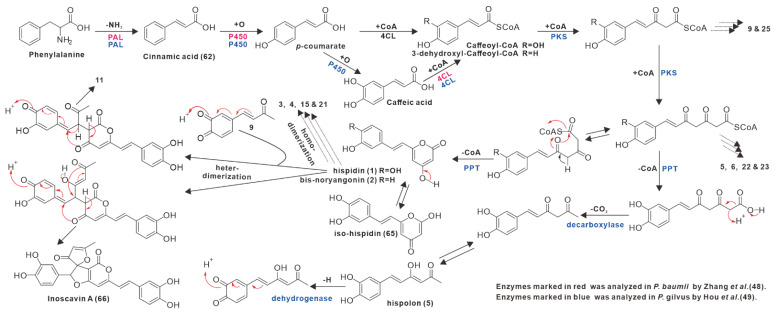
4. Biosynthetic Progresses on Styrylpyrones
Styrylpyrone compounds are a specific class of polyphenols with a wide range of structures and high yields. The majority of I. hispidus-derived yellow polyphenol pigments have a styrylpyrone backbone, and 10 styrylpyrone compounds (1, 3–4, 8, 12, 15–16, 20–21, 24) have been identified from the secondary metabolites of I. hispidus. Nearly hundreds of styrylpyrone pigments have been discovered in a variety of fungi dominated by the Hymenochaetaceae family since the discovery of hispidin (1) as the first naturally occurring styrylpyrone metabolite [43,44,45]. Experiments on light-regulated enzymatic activity revealed that the cinnamic acid (62) pathway involved in phenylalanine metabolism is linked to hispidin synthesis in P. hispidus. Cinnamic acid (62) is thought to be a key intermediate in the biosynthesis of hispidin and other phenylpropanoids [46]. Isotope labeling traces revealed that the styrene unit was incorporated with phenylalanine, tyrosine, cinnamic acid (62), p-coumarate, and caffeic acids, while the pyrone ring was incorporated with acetate and malonate [10]. The isolation of five phenylpropanoids from the fruiting bodies of Inonotus sp., including iso-hispidin (65), inonotic acid methyl ester, and inotilone, provided clues to the biosynthesis of styrylpyrones. The phenylpropanoid polyketide is an important intermediate that is thought to be a side chain elongation of caffeic acid catalyzed by PKS [47]. Integrating metabolomic and proteomic findings that the up-regulation of phenylpropanoid biosynthesis leads to increased levels of hispidin and other polyphenols in P. baumii, genes involved in the up-regulation of expression include PAL, C4H, and 4- coumarate-CoA ligase (4CL) (Figure 8) [48]. PAL, 4CL, C4H, polyketide synthase (PKS), palmitoyl protein thioesterase (PPT), decarboxylase, and dehydrogenase genes were identified in a combined investigation of molecular docking simulations and RNA-seq-based analysis of differentially expressed genes in P. gilvus (Figure 8) [49]. Based on biosynthetic research and the analysis of existing styrylpyrone compounds derived from the Phellinus and Inonotus fungi, the comprehensive biosynthetic pathways of styrylpyrone compounds, including hispidin (1), hispolon (5) and their dimers, as well as inoscavin A (66), have been deduced in this review (Figure 8) [47,48,49]. The natural structural diversity of styrylpyrones suggests that their biosynthetic gene clusters are active and contain multiple post-modification genes. It is speculated that the study of its biosynthetic pathway and related synthetic gene clusters may aid in understanding the biosynthetic network of styrylpyrones and their derivatives in I. hispidus.
Figure 8.
Proposed biosynthesis pathways for styrylpyrones in Inonotus and Phellinus fungi.
5. Discussions and Perspectives
Triterpenes are characteristic pharmacological components of lignified traditional medicinal fungi, such as Ganoderic acid from Ganoderma species [50], and Eburicoic acid and its derivatives from polypore species [51]; however, they are not monolithic. Phenols, a class of high-value natural products with medicinal potential [44,52], are prominent bioactive components of the highly lignified and well-known medicinal mushrooms of the genus Phellinus and Inonotus belonging to the order of Hymenochaetales [43]. The diversely modified phenolic derivatives yielded by these mushrooms have promising applications in drug discovery and development [43,44]. Hispidin (1) is the first compound isolated and identified from I. hispidus, and these phenolic compounds with styryl and pyrone moieties are gathered here with diverse activities such as antitumor, antioxidant, antimicrobial, and anti-inflammatory activities (Figure 6). These diverse activities of the natural product reflect the biological activities of its producer. Despite the fact that 5 was initially isolated from I. hispidus in 1996 [12], it is more of a characteristic component of fungi belonging to the genus Phellinus, as it has also been isolated from various species of the genus Phellinus, including P. linteus, P. igniarius, P. lonicerinus and P. merrillii [43]. A summary of the anticancer, antidiabetic, antioxidant, antiviral, and anti-inflammatory properties of compound 5 revealed its potential development value as a complementary and alternative medicine [43].
As one of the most abundant secondary metabolites found in mushrooms, numerous mushroom-derived sesquiterpenes have been isolated and identified [53]. Sesquiterpene synthases are one of the core gene types involved in the biosynthesis of secondary metabolites. Mushroom genomes contain typically at least ten sesquiterpene synthases. I. obliquus is a congener species of I. hispidus. Although the genome of I. obliquus contains at least 4 types of 20 sesquiterpene synthases [54], only 8 sesquiterpenoids of the drimane-type have been reported [55]. So far, only one sesquiterpenoid (61) has been reported for I. obliquus [21], and the type and number of sesquiterpene synthases are also unknown. The identification of sesquiterpene cyclases derived from lots of mushrooms including Coprinus cinereus [56], Omphalotus olearius [57], Stereum hirsutum [58,59], Flammulina velutipes [60], and Agrocybe aegerita [61] have led to the discovery of numerous novel sesquiterpenoids, which are not found in their natural producer instead. As a result, we speculate that in most mushrooms, only one or a few types of sesquiterpene synthases can be expressed to produce specific sesquiterpenoids, while the majority of sesquiterpene synthases are silent and have no corresponding products.
Although I. hispidus has been widely considered a traditional medicinal fungus due to its secondary metabolites with remarkable significant pharmacological value, the food properties of I. hispidus that are rich in health and nutritional functional factors should not be overlooked. The edible fruiting body of I. hispidus is a scarce ingredient. I. hispidus possess a good selenium-enriching function [62], and the antiviral ability of I. hispidus rich in selenium has also been enhanced. The dihydroxy phenylalanine-melanin contained in I. hispidus could effectively remove DPPH radicals and can therefore be used in health food or as a food additive [63]. Furthermore, the extracellular exopolysaccharide of I. hispidus was investigated and found to have antioxidant activity [64] and hepatoprotective functions [42].
Mushrooms are often perceived more for their anticancer or immunomodulatory activity than for their antibacterial activity. In fact, mushrooms with antimicrobial activity are uncommon [65,66], with Pleurotus mutilus and I. hispidus being the few examples. Pleuromutilin was found to have good antifungal inhibitory activity and was used as the lead compound in the development of the human antibiotic retapamulin, which was approved by the FDA in 2007 [67]. Both aqueous and ether extracts of I. hispidus mushrooms inhibit pathogenic microorganisms such as S. aureus [15]; however, monomeric compounds with antibacterial activity have yet to be identified. As a result, the discovery and development of antimicrobial compounds derived from I. hispidus is worth pursuing.
Herein, a total of 64 compounds derived from I. hispidus were collected and classified according to their structural features. Biological and pharmacological activities including antitumor, antioxidant, as well as anti-inflammatory and neurotrophic and neuroprotective activities were summarized. These studies, however, were limited to the isolation, identification, and activity evaluation of compounds. Current research has not paid enough attention to I. hispidus, despite its significant medicinal efficacy and functional food value. Although the genomes of multiple rare medicinal fungi including I. obliquus [54] have been sequenced, the genome of I. hispidus has not been sequenced. The lack of genomic information of I. hispidus severely hampered the biosynthetic pathway elucidation of its active compounds. As a result, it is critical to complete the genome sequencing of I. hispidus [54] using rapidly developing sequencing technology, which will lay a firm foundation for biosynthesis research and synthetic biology production of valuable compounds to meet the needs of the drug development and functional food industries.
Author Contributions
Z.-x.W. and X.-l.F. collected data and prepared figures, J.Q. wrote the manuscript, and C.L. and J.-m.G. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31800031), the China Postdoctoral Science Foundation (2019M653760), Natural Science Basic Research Plan in Shaanxi Province of China (2019JQ-046).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piątek M. Atlas of the Geographical Distribution of Fungi in Poland, Fascicle 1. Polish Academy of Sciences; Kraków, Poland: 2000. Inonotus hispidus (Bull.: Fr.) Karst; pp. 35–40. [Google Scholar]
- 2.Qing-Jie L. Ph.D. Thesis. Jilin Agricultural University; Changchun, China: 2017. Study on the Active Substances and Quality Standards of Sanghuang. [Google Scholar]
- 3.Kirk P., Cannon P., Stalpers J., Minter D.W. Dictionary of the Fungi. 10th ed. CABI Publishing; Oxfordshire, UK: 2008. [Google Scholar]
- 4.Zhang F., Xue F., Xu H., Yuan Y., Wu X., Zhang J., Fu J. Optimization of Solid-State Fermentation Extraction of Inonotus hispidus Fruiting Body Melanin. Foods. 2021;10:2893. doi: 10.3390/foods10122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S.H., Chang C.C., Wei C.L., Jiang G.Z., Cui B.K. Sanghuangporus toxicodendri sp. nov. (Hymenochaetales, Basidiomycota) from China. MycoKeys. 2019;57:101–111. doi: 10.3897/mycokeys.57.36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zan L.-F., Bao H.-Y. Progress in Inonotus hispidus research. Acta Edulis. Fungi. 2011;18:78–82. doi: 10.16488/j.cnki.1005-9873.2011.01.008. [DOI] [Google Scholar]
- 7.Wu S.-H., Dai Y.-C., Hattori T., Yu T.W., Wang D.-M., Parmasto É.K., Chang H.-Y., Shih S.-Y. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bota. Stu. 2012;53:135–149. [Google Scholar]
- 8.Ikekawa T., Nakanishi M., Uehara N., Chihara G., Fukuoka F. Antitumor action of some Basidiomycetes, especially Phllinus linteus. GaN. 1968;59:155–157. doi: 10.20772/cancersci1959.59.2_155. [DOI] [PubMed] [Google Scholar]
- 9.Edwards R.L., Lewis D.G., Wilson D.V. 983. Constituents of the higher fungi. Part I. Hispidin, a new 4-hydroxy-6-styryl-2-pyrone from polyporus hispidus (Bull.) Fr. J. Chem. Soc. 1961:4995–5002. doi: 10.1039/jr9610004995. [DOI] [Google Scholar]
- 10.Perrin P.W., Towers G.H.N. Hispidin biosynthesis in cultures of Polyporus hispidus. Phytochemistry. 1973;12:589–592. doi: 10.1016/S0031-9422(00)84448-9. [DOI] [Google Scholar]
- 11.Fiasson J.-L. Distribution of styrylpyrones in the basidiocarps of various Hymenochaetaceae. Biochem. Syst. Ecol. 1982;10:289–296. doi: 10.1016/0305-1978(82)90002-3. [DOI] [Google Scholar]
- 12.Ali N.A.A., Jansen R., Pilgrim H., Liberra K., Lindequist U. Hispolon, a yellow pigment from Inonotus hispidus. Phytochemistry. 1996;41:927–929. doi: 10.1016/0031-9422(95)00717-2. [DOI] [Google Scholar]
- 13.Yousfi M., Djeridane A., Bombarda I., Chahrazed H., Duhem B., Gaydou E.M. Isolation and Characterization of a New Hispolone Derivative from Antioxidant Extracts of Pistacia atlantica. Phytother. Res. 2009;23:1237–1242. doi: 10.1002/ptr.2543. [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Hou Y. Determination of trace elements in anti-influenza virus mushrooms. Biol. Trace Elem. Res. 2011;143:1799–1807. doi: 10.1007/s12011-011-8986-0. [DOI] [PubMed] [Google Scholar]
- 15.Lifeng Z. Ph.D. Thesis. Jilin Agricultural University; Changchun, China: Studies on the Chemical Constituents and Pharmacological Activities of Inonotus hispidus and Fomitiporia ellipsoidea. [Google Scholar]
- 16.Zan L.-F., Qin J.-C., Zhang Y.-M., Yao Y.-H., Bao H.-Y., Li X. Antioxidant Hispidin Derivatives from Medicinal Mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011;59:770–772. doi: 10.1248/cpb.59.770. [DOI] [PubMed] [Google Scholar]
- 17.Gruendemann C., Arnhold M., Meier S., Baecker C., Garcia-Kaeufer M., Grunewald F., Steinborn C., Klemd A.M., Wille R., Huber R., et al. Effects of Inonotus hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med. 2016;82:1359–1367. doi: 10.1055/s-0042-111693. [DOI] [PubMed] [Google Scholar]
- 18.Ren Q., Lu X.-Y., Han J.-X., Aisa H.A., Yuan T. Triterpenoids and phenolics from the fruiting bodies of Inonotus hispidus and their activations of melanogenesis and tyrosinase. Chin. Chem. Lett. 2017;28:1052–1056. doi: 10.1016/j.cclet.2016.12.010. [DOI] [Google Scholar]
- 19.Kemami Wangun H.V., Hertweck C. Squarrosidine and Pinillidine: 3,3′-Fused Bis(styrylpyrones) from Pholiota squarrosa and Phellinus pini. Eur. J. Org. Chem. 2007;2007:3292–3295. doi: 10.1002/ejoc.200700090. [DOI] [Google Scholar]
- 20.Yang S., Bao H., Wang H. Chemical components and anti-tumour compounds from Inonotus hispidus. Mycosystema. 2019;38:127–133. doi: 10.13346/j.mycosystema.180133. [DOI] [Google Scholar]
- 21.Kou R.W., Du S.T., Xia B., Zhang Q., Yin X., Gao J.M. Phenolic and Steroidal Metabolites from the Cultivated Edible Inonotus hispidus Mushroom and Their Bioactivities. J. Agric. Food Chem. 2021;69:668–675. doi: 10.1021/acs.jafc.0c06822. [DOI] [PubMed] [Google Scholar]
- 22.Shaojun T., Ping L., Wenhua M., Chenxia S., Shenglian W., Yi Y., Yuelin H., Jun X. Isolation and Identification of Antitumor Metabolites from Fermentation Broth of Inonotus hispidus. Acta Edulis. Fungi. 2021;28:109–116. doi: 10.16488/j.cnki.1005-9873.2021.06.014. [DOI] [Google Scholar]
- 23.Yang X. Master Thesis. Shandong University of Traditional Chinese Medicine; Jinan, China: 2008. Studies on the Chemical Constituents of Xanthochrous hispidus. [Google Scholar]
- 24.Zhou J., Lin X., Liu S., Wang Z., Liu D., Huo Y., Li D. Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes) Molecules. 2022;27:2618. doi: 10.3390/molecules27092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Politi M., Silipo A., Siciliano T., Tebano M., Flamini G., Braca A., Jiménez-Barbero J. Current analytical methods to study plant water extracts: The example of two mushrooms species, Inonotus hispidus and Sparassis crispa. Phytochem. Anal. 2007;18:33–41. doi: 10.1002/pca.949. [DOI] [PubMed] [Google Scholar]
- 26.Fushiya S., Matsuda M., Yamada S., Nozoe S. New opine type amino acids from a poisonous mushroom, Clitocybe acromelalga. Tetrahedron. 1996;52:877–886. doi: 10.1016/0040-4020(95)00929-9. [DOI] [Google Scholar]
- 27.Yang S., Bao H., Wang H., Li Q. Anti-tumour Effect and Pharmacokinetics of an Active Ingredient Isolated from Inonotus hispidus. Biol. Pharm. Bull. 2019;42:10–17. doi: 10.1248/bpb.b18-00343. [DOI] [PubMed] [Google Scholar]
- 28.Fan N.-W., Chang H.-S., Cheng M.-J., Hsieh S.-Y., Liu T.-W., Yuan G.-F., Chen I.-S. Secondary Metabolites from the Endophytic Fungus Xylaria cubensis. Helv. Chim. Acta. 2014;97:1689–1699. doi: 10.1002/hlca.201400091. [DOI] [Google Scholar]
- 29.Ali N.A.A., Mothana R.A.A., Lesnau A., Pilgrim H., Lindequist U. Antiviral activity of Inonotus hispidus. Fitoterapia. 2003;74:483–485. doi: 10.1016/s0367-326x(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 30.Zan L., Liang R., Bao H. Antioxidant and Antimicrobial Activities of Inonotus hispidus Extracts. [(accessed on 10 July 2022)];North. Hortic. 2015 5:151–155. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=BFYY201505047&DbName=CJFQ2015. [Google Scholar]
- 31.Benarous K., Bombarda I., Iriepa I., Moraleda I., Gaetan H., Linani A., Tahri D., Sebaa M., Yousfi M. Harmaline and hispidin from Peganum harmala and Inonotus hispidus with binding affinity to Candida rugosa lipase: In silico and in vitro studies. Bioorg. Chem. 2015;62:1–7. doi: 10.1016/j.bioorg.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Benarous K., Benali F.Z., Bekhaoua I.C., Yousfi M. Novel potent natural peroxidases inhibitors with in vitro assays, inhibition mechanism and molecular docking of phenolic compounds and alkaloids. J. Biomol. Struct Dyn. 2021;39:7168–7180. doi: 10.1080/07391102.2020.1808073. [DOI] [PubMed] [Google Scholar]
- 33.Tang S.J., Shen D.A., Shao C.X., Jun X.U., Yang Y., Jin L., Wu S.-L. Classification and identification of a wild Inonotus hispidus and the antitumor activity of the fermentation supernatant. J. South. Agric. 2019;50:1671–1679. doi: 10.3969/j.issn.2095-1191.2019.08.04. [DOI] [Google Scholar]
- 34.Li Z., Bao H. Anti-tumor effect of Inonotus hispidus petroleum ether extract in H22 tumor-bearing mice and analysis its mechanism by untargeted metabonomic. J. Ethnopharmacol. 2022;285:114898. doi: 10.1016/j.jep.2021.114898. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Bao H. Deciphering key regulators of Inonotus hispidus petroleum ether extract involved in anti-tumor through whole transcriptome and proteome analysis in H22 tumor-bearing mice model. J. Ethnopharmacol. 2022;296:115468. doi: 10.1016/j.jep.2022.115468. [DOI] [PubMed] [Google Scholar]
- 36.Wang T., Bao H.Y., Bau T., Li Y. Antitumor Effect of Solid State Fermentation Powder of Inonotus hispidus on H22 Bearing Mice. Zhong Yao Cai. 2016;39:389–394. doi: 10.13863/j. [DOI] [PubMed] [Google Scholar]
- 37.Yang S., Bao H., Wang H., Li Z., Han C. Bacteriostatic and Antioxidative Effects of Volatile Oil from Inonotus hispidus. J. Jilin Agric. Univ. 2020;42:148–153. doi: 10.13327/j.jjlau.2020.4499. [DOI] [Google Scholar]
- 38.Shomali N., Onar O., Alkan T., Demirtaş N., Akata I., Yildirim Ö. Investigation of the Polyphenol Composition, Biological Activities, and Detoxification Properties of Some Medicinal Mushrooms from Turkey. Turk. J. Pharm. Sci. 2019;16:155–160. doi: 10.4274/tjps.galenos.2018.03274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benarous K., Djeridane A., Kameli A., Yousfi M. Inhibition of Candida rugosa Lipase by Secondary Metabolites Extracts of Three Algerian Plants and their Antioxydant Activities. Curr. Enzym. Inhib. 2013;9:75–82. doi: 10.2174/1573408011309010010. [DOI] [Google Scholar]
- 40.Ying Y.-M., Zhang L.-Y., Zhang X., Bai H.-B., Liang D.-E., Ma L.-F., Shan W.-G., Zhan Z.-J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry. 2014;108:171–176. doi: 10.1016/j.phytochem.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Tang M., Ding Y., Yang Y., Xie M., Wang S., Chen C., Wang H., Li Y. α-glucosidase Inhibition and Antioxidant Activities of Different Polar Extracts of Inonotus hispidus. Edible Fungi China. 2021;40:37–41. doi: 10.13629/j.cnki.53-1054.2021.02.008. [DOI] [Google Scholar]
- 42.Liu X., Hou R., Yan J., Xu K., Wu X., Lin W., Zheng M., Fu J. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019;129:41–49. doi: 10.1016/j.ijbiomac.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Lee I.-K., Yun B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 44.Dos Santos N.C., Menezes R., Stewart D. Polyphenols as New Leads in Drug Discovery: Biological Activity and Mechanisms. Curr. Pharm. Des. 2018;24:2041–2042. doi: 10.2174/138161282419180924094610. [DOI] [PubMed] [Google Scholar]
- 45.Sarfraz A., Rasul A., Sarfraz I., Shah M.A., Hussain G., Shafiq N., Masood M., Adem S., Sarker S.D., Li X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Env. Res. 2020;190:110017. doi: 10.1016/j.envres.2020.110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nambudiri A.M.D., Vance C.P., Towers G.H.N. Effect of light on enzymes of phenylpropanoid metabolism and hispidin biosynthesis in Polyporus hispidus. Biochem. J. 1973;134:891–897. doi: 10.1042/bj1340891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemami Wangun H.V., Härtl A., Tam Kiet T., Hertweck C. Inotilone and related phenylpropanoid polyketides from Inonotus sp. and their identification as potent COX and XO inhibitors. Org. Biom. Chem. 2006;4:2545–2548. doi: 10.1039/B604505G. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H., Chen R., Zhang J., Bu Q., Wang W., Liu Y., Li Q., Guo Y., Zhang L., Yang Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019;9:16172. doi: 10.1038/s41598-019-52711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huo J., Zhong S., Du X., Cao Y., Wang W., Sun Y., Tian Y., Zhu J., Chen J., Xuan L., et al. Whole-genome sequence of Phellinus gilvus (mulberry Sanghuang) reveals its unique medicinal values. J. Adv. Res. 2020;24:325–335. doi: 10.1016/j.jare.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angulo-Sanchez L.T., López-Peña D., Torres-Moreno H., Gutiérrez A., Gaitán-Hernández R., Esqueda M. Biosynthesis, Gene Expression, and Pharmacological Properties of Triterpenoids of Ganoderma Species (Agaricomycetes): A Review. Int. J. Med. Mushrooms. 2022;24:1–17. doi: 10.1615/IntJMedMushrooms.2022044016. [DOI] [PubMed] [Google Scholar]
- 51.Grienke U., Zöll M., Peintner U., Rollinger J.M. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014;154:564–583. doi: 10.1016/j.jep.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Floris B., Galloni P., Conte V., Sabuzi F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules. 2021;11:1325. doi: 10.3390/biom11091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dasgupta A., Acharya K. Chapter 10—Bioactive terpenoids from mushrooms. In: Singh J., Gehlot P., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2021. pp. 145–154. [Google Scholar]
- 54.Duan Y., Han H., Qi J., Gao J.-m., Xu Z., Wang P., Zhang J., Liu C. Genome sequencing of Inonotus obliquus reveals insights into candidate genes involved in secondary metabolite biosynthesis. BMC Genom. 2022;23:314. doi: 10.1186/s12864-022-08511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yingce D., Dan S., Lin W., Xiaoning Z., Chunlei W., Chengwei L. Research Progress of Small Molecule Chemical Components and Pharmacological Values of Inonotus obliquus. J. Fungal Res. 2022;20:1–14. doi: 10.13341/j.jfr.2021.1451. [DOI] [Google Scholar]
- 56.Agger S., Lopez-Gallego F., Schmidt-Dannert C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbiol. 2009;72:1181–1195. doi: 10.1111/j.1365-2958.2009.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wawrzyn G.T., Quin M.B., Choudhary S., López-Gallego F., Schmidt-Dannert C. Draft Genome of Omphalotus olearius Provides a Predictive Framework for Sesquiterpenoid Natural Product Biosynthesis in Basidiomycota. Chem. Biol. 2012;19:772–783. doi: 10.1016/j.chembiol.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quin M.B., Flynn C.M., Wawrzyn G.T., Choudhary S., Schmidt-Dannert C. Mushroom Hunting by Using Bioinformatics: Application of a Predictive Framework Facilitates the Selective Identification of Sesquiterpene Synthases in Basidiomycota. ChemBioChem. 2013;14:2480–2491. doi: 10.1002/cbic.201300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagamine S., Liu C., Nishishita J., Kozaki T., Sogahata K., Sato Y., Minami A., Ozaki T., Schmidt-Dannert C., Maruyama J.-I., et al. Ascomycete Aspergillus oryzae Is an Efficient Expression Host for Production of Basidiomycete Terpenes by Using Genomic DNA Sequences. Appl. Environ. Microbio. 2019;85:e00409–e00419. doi: 10.1128/AEM.00409-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao Q., Ma K., Yang Y., Wang K., Chen B., Huang Y., Han J., Bao L., Liu X.-B., Yang Z., et al. Bioactive Sesquiterpenes from the Edible Mushroom Flammulina velutipes and Their Biosynthetic Pathway Confirmed by Genome Analysis and Chemical Evidence. J. Org. Chem. 2016;81:9867–9877. doi: 10.1021/acs.joc.6b01971. [DOI] [PubMed] [Google Scholar]
- 61.Zhang C., Chen X., Orban A., Shukal S., Birk F., Too H.-P., Rühl M. Agrocybe aegerita Serves as a Gateway for Identifying Sesquiterpene Biosynthetic Enzymes in Higher Fungi. ACS Chem. Biol. 2020;15:1268–1277. doi: 10.1021/acschembio.0c00155. [DOI] [PubMed] [Google Scholar]
- 62.Song F., Su D., Keyhani N.O., Wang C., Shen L., Qiu J. Influence of selenium on the mycelia of the shaggy bracket fungus, Inonotus hispidus. J. Sci. Food Agric. 2022;102:3762–3770. doi: 10.1002/jsfa.11724. [DOI] [PubMed] [Google Scholar]
- 63.Hou R., Liu X., Xiang K., Chen L., Wu X., Lin W., Zheng M., Fu J. Characterization of the physicochemical properties and extraction optimization of natural melanin from Inonotus hispidus mushroom. Food Chem. 2019;277:533–542. doi: 10.1016/j.foodchem.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Liu X., Hou R., Xu K., Chen L., Wu X., Lin W., Zheng M., Fu J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019;123:468–476. doi: 10.1016/j.ijbiomac.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 65.Alves M.J., Ferreira I.C.F.R., Dias J., Teixeira V., Martins A., Pintado M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- 66.Patel D.K., Dutta S.D., Ganguly K., Cho S.-J., Lim K.-T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules. 2021;26:1359. doi: 10.3390/molecules26051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Y.Z., Liu Y.H., Chen J.X. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini Rev. Med. Chem. 2012;12:53–61. doi: 10.2174/138955712798868968. [DOI] [PubMed] [Google Scholar]