Short abstract
Content available: Author Interview and Audio Recording
Watch the interview with the author.
Listen to an audio presentation of this article.
INTRODUCTION
The most common cause of chronic liver disease in the United States is nonalcoholic fatty liver disease (NAFLD), which is closely associated with excess body fat as a result of obesity. 1 Within NAFLD lies nonalcoholic steatohepatitis (NASH). 2 The latter includes steatosis and inflammation of the liver, and increased risks for liver failure, hepatocellular carcinoma, and cirrhosis. Present in approximately 2%–4% of the US population, NASH predominantly affects those with diabetes or obesity, and the prevalence rate in those populations may reach 20%. 3
Obesity is classified as having a body mass index (BMI) ≥30, and it is a chronic disease that poses further health, social, and financial issues. Individuals with obesity are at risk for coronary heart disease, diabetes mellitus, cancer, stroke, and other comorbidities. Financially, by 2030, 16%–18% of health care costs will be attributed to obesity management. 4 Links between obesity and mental health have been explored in recent literature, and it has been found that those with a mental health disorder are two to three times more likely to suffer from obesity. With an estimated 18.6% of the United States suffering from mental health disorders, it is important to understand the connections, which may be attributed to a number of biological and psychosocial causal factors.
TYPES OF BEHAVIORAL PROBLEMS LINKED TO NASH
Mood disorders, attention deficit hyperactivity disorder, and schizophrenia are some mental health disorders most commonly associated with obesity. 4 More specific to NASH are higher incidences of major depressive disorder (MDD), generalized anxiety disorder, bipolar disorder, and schizophrenia (Table 1). 5 Insulin resistance, a common causal factor of NASH, is often present in patients with depression and anxiety. 6 Lack of treatment of patients with these risk factors may lead to inflammatory states, oxidative stress, aggravation of genetic predispositions, and eventually NASH. 7 The psychopathology associated with obesity and NAFLD, which can escalate to NASH, may not only contribute to behavior that promotes disease development and progression but may also impact the ability to adopt and maintain lifestyle changes needed to reverse the course of the disease. One study showed that patients with NASH were largely in a “pre‐contemplation” state and did not consider they had a weight‐related disorder despite being in a clinic with regular weight counseling being provided; such patients are unlikely to adopt behavior change to lose weight. 1 There is thus a need to better understand the psychopathology underlying NASH and integrate specific behavioral interventions based on the individual patient’s determinants of health‐related behavior in the treatment plan. TREATMENTS FOR OBESITY IN THE CONTEXT OF NASH AND MENTAL HEALTH.
TABLE 1.
Mental health disorders associated with NASH
| Mental health disorder | Mechanisms for association with NASH |
|---|---|
| Depression | Insulin resistance, 6 , 22 inflammatory state 7 , 23 |
| Anxiety | Insulin resistance, 24 inflammatory state 7 , 25 |
| Bipolar disorder | PNPLA3, 26 HPA axis, 27 insulin resistance, 5 , 28 inflammation, 5 , 27 , 28 oxidative stress 5 , 27 |
| Schizophrenia | Inflammation, 29 , 30 metabolic dysfunction, 30 , 31 oxidative stress, 32 mitochondrial dysregulation 33 |
| Autism spectrum disorders | Metabolic dysregulation 34 |
MECHANISMS FOR THE LINK BETWEEN OBESITY, NASH, AND MENTAL HEALTH
Psychosocial links
Food choice can be influenced by one’s mood, and mental disorders such as depression or anxiety may exacerbate overeating in an effort to dispel negative emotions (Table 2). Emotional eating–mediated weight gain has been noted in patients with depressive symptoms, and adoption of unhealthy eating behaviors as a result of undesirable emotional states is considered a coping mechanism. 8 , 9 “Coping foods” or calorie‐dense meals are often preferred under these circumstances, leading to increased BMI 10 (Figure 1). Depressive symptoms may also be associated with a decline in cognitive restraint, a behavior that is generally responsible for weight maintenance or loss. Thus, those with depression often report changes in appetite, which can compel them to increase intake of coping foods. Moreover, it is believed that depression contributes to the dampening of self‐efficacy, the belief in one’s self to be able to accomplish a task. 8 Consequently, behaviors such as physical activity and controlled eating are diminished, leading to weight gain and, potentially, obesity. 8 Emotional eating in response to stress has also been described, with college students attributing it as a motivator to turn to comfort foods, thus leading to potential weight gain. 11 Similarly, excessive eating in patients with posttraumatic stress disorder (PTSD) is a likely response to stress. 4 In contrast, stopping a long‐duration high‐fat diet has been shown to lead to depressive symptoms in patients, suggesting that the diet itself may have hedonistic effects, with its withdrawal unmasking or precipitating depressive symptoms. All of these promote cycles of weight gain and depressive moods. 9
TABLE 2.
Mechanisms driving mental health disorders in NASH
|
FIGURE 1.
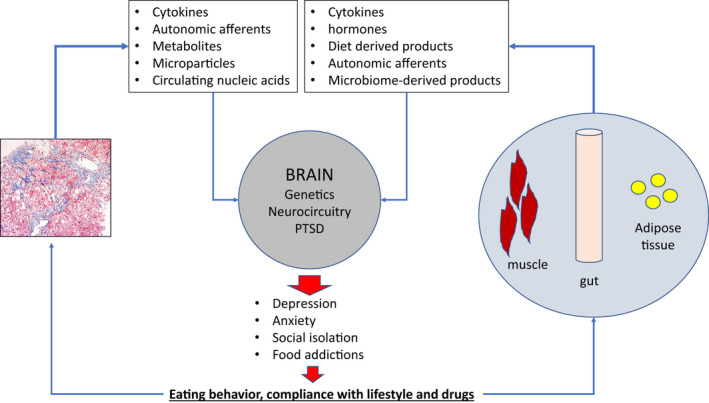
Biological and psychosocial factors linking NASH with psychiatric disorders. A number of biological, behavioral, and psychosocial factors have been implicated in the pathophysiological links between obesity, NASH, and mental health. Biological factors such as genetics, disturbances in the HPA axis, inflammatory pathways, and microbiome and other hormonal imbalances closely interact with psychosocial factors such as eating behaviors, perpetuating a vicious cycle of obesity, NASH, and psychiatric disorders
Neurocircuitry
Neurobiological processes of the brain responsible for drug addiction have been hypothesized to play a role in food addiction. 12 Addiction follows a three‐stage cycle consisting of binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. During the first stage, the patient experiences a rewarding feeling via the mesocorticostriatal dopamine pathways, which release dopamine and opioid peptides. Conditioned reinforcement through repeated drug use may occur through the mesocorticolimbic dopamine system, where neurons begin to fire during a specific stimulus prior to reward delivery. This process, referred to as incentive salience, leads to specific cues that cause cravings and desire for the drug, particularly during stressful situations. During withdrawal, decreased sensitivity to the drug leads to emotional dysregulation that can manifest as stress, irritability, and malaise, in part because of reduced dopamine and serotonin in the nucleus accumbens, in addition to disruption of the hypothalamic‐pituitary‐adrenal (HPA) axis. Aversive emotional states worsened by changes in release of corticotropin‐releasing factor perpetuates negative reinforcement, which leads to drug‐seeking behavior. Finally, relapse after drug avoidance occurs in the preoccupation stage. 12 , 13 This cycle is highlighted in food addiction, where repeat exposure triggers reward mechanisms releasing dopamine into the nucleus accumbens. Over time, dopamine D2 receptors decline so a greater intake is required for the same level of reward response. 13 Most who have food addiction often have a family history of addiction, and a genetic component is possible. Binge eating disorder (BED), a diagnosis of food addiction, is often associated with risk factors present in NASH and NAFLD. Development of type 2 diabetes is 13 times more likely in patients with BED, and obesity is present in more than 40% of patients. 14 , 15 Thus, treatment of BED can help minimize the risk for NASH development.
Biological links
A number of biological factors, such as genetics, disturbances in the HPA axis, inflammatory pathways, and other hormonal imbalances, have been implicated in the genesis of psychopathology related to obesity and NAFLD (Table 2, Figure 1).
Genetics
Approximately 200 genetic loci linked to obesity and weight gain have been described. Many of these genes are expressed in the hypothalamus, pituitary gland, hippocampus, and limbic system, with the former being responsible for appetite and the latter for mood regulation. Of the 50 loci found to be responsible for MDD, genes that contributed the greatest to depressive phenotypes overlapped with genes responsible for BMI determination, such as neuronal growth regulator 1 (NEGR1). NEGR1 has been specifically identified as a modulator for appetite and mood, with higher hypothalamic expression being linked to restricted feeding. 16 Dysfunctions with the gene have demonstrated correlations with both obesity and MDD. The recessive variant of another gene, patatin‐like phospholipase domain‐containing protein 3 (PNPLA3), responsible for glucose and fatty acid regulation, has been found more often in individuals with bipolar disorder and obesity. 5 Genome‐wide association studies have indicated that PNPLA3 has NAFLD predisposing variants that may lead to NASH. 17 Beyond common genetic loci between NAFLD and mental disorders, it is hypothesized that microRNAs have some role in progression of NAFLD and mental disorders. miR‐34a, responsible for lipid secretion and hepatic lipogenesis, is found to be elevated in patients with bipolar disorder, as well as patients with NAFLD. 5
Inflammation
Obesity is a state of inflammation marked by the release of inflammatory markers, such as tumor necrosis factor (TNF) alpha, interleukins, and inflammatory cytokines. The inflammatory markers can disrupt negative feedback suppression of HPA activity by hindering glucocorticoid receptor (GR) function, preventing cortisol binding, and demonstrating shared links between obesity and mental health disorders. 16 In addition, secretion of inflammatory cytokines and adipokines contributes to insulin resistance and drives steatosis and lipotoxicity, a central feature of NASH. 5 The secretory protein, adiponectin, responsible for glucose and lipid homeostasis, shares links with both obesity and MDD. Adiponectin inhibits the secretion of cytokines such as TNF and interleukins, and a decrease in the anti‐inflammatory protein has been found in patients with depression. Lower levels of circulating adiponectin are present in overweight patients, as well as those with NASH. 18 Moreover, under normal conditions, leptin mediates food intake, regulates mood via antidepressive properties, and is responsible for some immune function, such as producing inflammatory cytokines, TNF, and interleukins. However, disruption through loss‐of‐function mutations or leptin resistance may be risk factors for depression and weight gain. 16 , 19 A study investigating the relationship between inflammation and NAFLD, as well as NASH, reported finding elevated levels of TNF and interleukins. 20 Overall, the hepatic lipogenesis present in NAFLD is consistent with the secretion of inflammatory cytokines and adipokines present in obesity and mental health disorders. 10 The links between obesity, NASH, and mental health disorders are evident through inflammation and support a relationship in which one illness can lead to the others.
Microbiome
Buildup of fat in the liver through increased intestinal absorption and development of NAFLD and NASH has been attributed to a change in gut microbiota. Relationships between inflammation of hepatic and adipose tissue, as well as steatosis, and alterations in intestinal flora are continuously being investigated, as are treatments. In addition, there is some evidence of a correlation among variations in the microbiota and mental health disorders. For example, inflammatory dysregulation by bacterial products has been linked to depression, dementia, and autism. 5 Human metabolism is directly impacted by the hundreds of species found in the gut. Bacteroidetes and firmicutes are two phyla of gastrointestinal bacteria responsible for energy production, and imbalances in the Bacteroidetes/firmicutes ratio have been attributed to obesity. 16 Milaneschi et al.16 note that experimental manipulations of the ratio may lead to inflammation through an increase in gut permeability, and they can alter mood through depression‐specific pathways. Specifically, a smaller ratio has been found in patients with MDD, and depression‐like symptoms have been induced in rats via microbiota transplantation from depressed patients.
HPA axis
Dysregulation of the HPA axis leads to extended release of cortisol, leading to damage in brain areas responsible for mood and inhibition of lipid‐mobilizing enzymes. 21 This has similar outcomes to Cushing syndrome, an endocrine illness that leads to weight gain via prolonged release of cortisol and shares similarities with defects in the HPA axis as seen in depression, thereby linking obesity with psychiatric disorders. 4 Hypercortisolism is common in obesity and often associated with changes in mood. 18 Excessive exposure to psychological stressors elevates cortisol levels via the initial release of corticotropin‐releasing hormone and arginine vasopressin, followed by the release of adrenocorticotropic hormone. 18 Cortisol may promote obesity by promoting adipogenesis in visceral fat, increasing appetite, or suppressing thermogenesis in brown fat. The inflammatory characteristics of obesity can inhibit the negative feedback of cortisol release by interfering with the GR. 16
CONCLUSION
The relationship between NASH, obesity, and mental health disorders is dependent on various biological elements and psychosocial factors. Elevated stress leading to emotional eating, genetics, dysfunction of the HPA axis, inflammation, and microbiome and hormonal imbalances all play significant roles. Despite the current understanding of the onset of the diseases, more research is required to understand the bidirectional correlation between obesity and mental health, as well as the specific pathways.
CONFLICT OF INTEREST
Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect, Exhalenz, and Hemoshear. He has served as a consultant to Astra Zeneca, Conatus, Coherus, Bristol Myers Squibb, Blade, Tobira, Takeda, Siemens, Merck, Genentech, Tern, Gilead, Lilly, Poxel, Artham, Boehringer Ingelhiem, Novo Nordisk, NGM Bio, Birdrock, Novartis, Pfizer, and Genfit. He has been an unpaid consultant to Intercept, Echosens, Perspectum, Immuron, Galectin, Fractyl, Affimune, Chemomab, and Nordic Bioscience. His institution has received grant support from Gilead, Salix, Tobira, Intercept, Bristol Myers, Shire, Merck, Astra Zeneca, Malinckrodt, Cumberland, and Novartis. He receives royalties from Elsevier and UptoDate.
Sharma A, Albhaisi S, Sanyal AJ. Behavioral health disorders related to nonalcoholic steatohepatitis. Clinical Liver Disease. 2022;20:43–47. 10.1002/cld.1211
REFERENCES
- 1. Stewart KE, Haller DL, Sargeant C, Levenson JL, Puri P, Sanyal AJ. Readiness for behaviour change in non‐alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015;35:936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tesfay M, Goldkamp WJ, Neuschwander‐Tetri BA. NASH: the emerging most common form of chronic liver disease. Mo Med. 2018;115:225–9. [PMC free article] [PubMed] [Google Scholar]
- 3. Charlton M, Kasparova P, Weston S, Lindor K, Maor‐Kendler Y, Wiesner RH, et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–14. [DOI] [PubMed] [Google Scholar]
- 4. Avila C, Holloway AC, Hahn MK, Morrison KM, Restivo M, Anglin R, et al. An overview of links between obesity and mental health. Curr Obes Rep. 2015;4:303–10. [DOI] [PubMed] [Google Scholar]
- 5. Soto‐Angona Ó, Anmella G, Valdés‐Florido MJ, De Uribe‐Viloria N, Carvalho AF, Penninx BWJH, et al. Non‐alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winokur A, Maislin G, Phillips JL, Amsterdam JD. Insulin resistance after oral glucose tolerance testing in patients with major depression. Am J Psychiatry. 1988;145:325–30. [DOI] [PubMed] [Google Scholar]
- 7. Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68:563–9. [DOI] [PubMed] [Google Scholar]
- 8. Clum GA, Rice JC, Broussard M, Johnson CC, Webber LS. Associations between depressive symptoms, self‐efficacy, eating styles, exercise and body mass index in women. J Behav Med. 2014;37:577–86. [DOI] [PubMed] [Google Scholar]
- 9. Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazarevich I, Irigoyen Camacho ME, Velázquez‐Alva MDC, Zepeda ZM. Relationship among obesity, depression, and emotional eating in young adults. Appetite. 2016;107:639–44. [DOI] [PubMed] [Google Scholar]
- 11. Bennett J, Greene G, Schwartz‐Barcott D. Perceptions of emotional eating behavior. A qualitative study of college students. Appetite. 2013;60:187–92. [DOI] [PubMed] [Google Scholar]
- 12. Kalon E, Hong JY, Tobin C, Schulte T. Psychological and neurobiological correlates of food addiction. Int Rev Neurobiol. 2016;129:85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raevuori A, Suokas J, Haukka J, Gissler M, Linna M, Grainger M, et al. Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. Int J Eat Disord. 2015;48:555–62. [DOI] [PubMed] [Google Scholar]
- 16. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24:18–33. [DOI] [PubMed] [Google Scholar]
- 17. Gerhard GS, Chu X, Wood GC, Gerhard GM, Benotti P, Petrick AT, et al. Next‐generation sequence analysis of genes associated with obesity and nonalcoholic fatty liver disease‐related cirrhosis in extreme obesity. Hum Hered. 2013;75:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor VH, MacQueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010: 748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ochner CN, Barrios DM, Lee CD, Pi‐Sunyer FX. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav. 2013;120:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Das SK, Balakrishnan V. Role of cytokines in the pathogenesis of non‐alcoholic fatty liver disease. Indian J Clin Biochem. 2011;26:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. [DOI] [PubMed] [Google Scholar]
- 22. Cho IY, Chang Y, Sung E, Kang J‐H, Wild SH, Byrne CD, et al. Depression and increased risk of non‐alcoholic fatty liver disease in individuals with obesity. Epidemiol Psychiatr Sci. 2021;30:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–85. [DOI] [PubMed] [Google Scholar]
- 24. Choi JM, Chung GE, Kang SJ, Kwak M‐S, Yang JI, Park B, et al. Association between anxiety and depression and nonalcoholic fatty liver disease. Front Med. 2021;7:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maes M, Lin A‐H, Delmeire L, Van Gastel A, Kenis G, De Jongh R, et al. Elevated serum interleukin‐6 (IL‐6) and IL‐6 receptor concentrations in posttraumatic stress disorder following accidental man‐made traumatic events. Biol Psychiatry. 1999;45:833–9. [DOI] [PubMed] [Google Scholar]
- 26. Kenneson A, Funderburk JS. Patatin‐like phospholipase domain‐containing protein 3 (PNPLA3): a potential role in the association between liver disease and bipolar disorder. J Affect Disord. 2017;209:93–6. [DOI] [PubMed] [Google Scholar]
- 27. Muneer A. The neurobiology of bipolar disorder: an integrated approach. Chonnam Med J. 2016;52:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu J‐H, Chien I‐C, Lin C‐H. Increased risk of chronic liver disease in patients with bipolar disorder: a population‐based study. Gen Hosp Psychiatry. 2016;42:54–9. [DOI] [PubMed] [Google Scholar]
- 29. Fuller BE, Rodriguez VL, Linke A, Sikirica M, Dirani R, Hauser P. Prevalence of liver disease in veterans with bipolar disorder or schizophrenia. Gen Hosp Psychiatry. 2011;33:232–7. [DOI] [PubMed] [Google Scholar]
- 30. Yan J, Hou C, Liang Y. The prevalence and risk factors of young male schizophrenics with non‐alcoholic fatty liver disease. Neuropsychiatr Dis Treat. 2017;13:1493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morlán‐Coarasa MJ, Arias‐Loste MT, Ortiz‐García de la Foz V, Martínez‐García O, Alonso‐Martín C, Crespo J, et al. Incidence of non‐alcoholic fatty liver disease and metabolic dysfunction in first episode schizophrenia and related psychotic disorders: a 3‐year prospective randomized interventional study. Psychopharmacology. 2016;233:3947–52. [DOI] [PubMed] [Google Scholar]
- 32. Maas DA, Vallès A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry. 2017;7:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry. 2015;14:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shedlock K, Susi A, Gorman GH, Hisle‐Gorman E, Erdie‐Lalena CR, Nylund CM. Autism spectrum disorders and metabolic complications of obesity. J Pediatr. 2016;178:183–7.e1. [DOI] [PubMed] [Google Scholar]


