Figure 1.
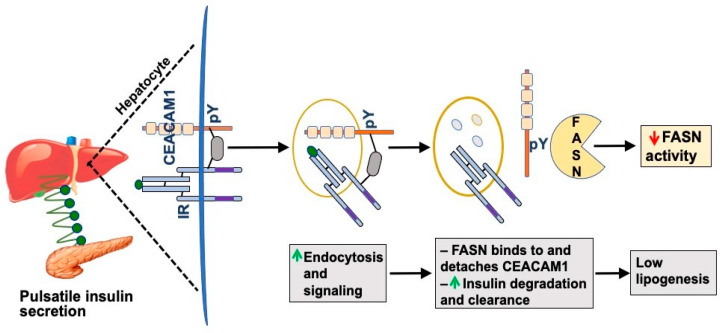
CEACAM1 phosphorylation induces hepatic insulin clearance. In response to pulses of secreted insulin from pancreatic β-cells (green circles), the insulin receptor (IR) tyrosine kinase on the surface membrane of hepatocytes is activated. This phosphorylates CEACAM1 on the species conserved tyrosine (pY) 488 residue, an event that mediates the formation of a stable insulin–IR–CEACAM1 complex to increase the rate of insulin endocytosis and targeting to its degradation process (green upward arrow). Fatty acid synthase (FASN) binds to pY488 of CEACAM1 to cause its detachment and facilitate the separation of insulin from its receptor to undergo degradation in the acidic milieu of late endosomes (grey circles). FASN binding to phosphorylated CEACAM1 also causes repression of FASN activity (red downward arrow), thus maintaining low lipogenesis in the liver despite the higher level of insulin in the portal vein than systemic circulation. Not shown in this schematic diagram, IR recycles back to the surface membrane.