Abstract
ZmaR is a resistance determinant of unusual abundance in the environment and confers on gram-positive and gram-negative bacteria resistance to zwittermicin A, a novel broad-spectrum antibiotic produced by species of Bacillus. The ZmaR protein has no sequence similarity to proteins of known function; thus, the purpose of the present study was to determine the function of ZmaR in vitro. Cell extracts of E. coli containing zmaR inactivated zwittermicin A by covalent modification. Chemical analysis of inactivated zwittermicin A by 1H NMR, 13C NMR, and high- and low-resolution mass spectrometry demonstrated that the inactivated zwittermicin A was acetylated. Purified ZmaR protein inactivated zwittermicin A, and biochemical assays for acetyltransferase activity with [14C]acetyl coenzyme A demonstrated that ZmaR catalyzes the acetylation of zwittermicin A with acetyl coenzyme A as a donor group, suggesting that ZmaR may constitute a new class of acetyltransferases. Our results allow us to assign a biochemical function to a resistance protein that has no sequence similarity to proteins of known function, contributing fundamental knowledge to the fields of antibiotic resistance and protein function.
The rise in resistance to antibiotics threatens their effectiveness in agriculture and medicine. The development of resistance is driven by both the frequency of resistance determinants in a given environment and selection by the antibiotics. Therefore, the current challenge is to understand the complex interplay of ecological and genetic factors that dictate the appearance and spread of resistance in pathogens of plants and animals. Although soil has long been recognized as a rich source of antibiotics, it has not been the focus of study as a source of resistance determinants. Soil may provide insight into the ecology of resistance for three reasons. First, antibiotic-producing organisms themselves may be a key source of resistance genes (2, 7), and the soil is populated with producers of many antibiotics in use in agriculture and medicine. Second, soil is the most microbiologically rich environment on earth (36), providing both dense populations and tremendous genetic diversity. Furthermore, transfer of resistance determinants across wide phylogenetic distances is common in nature generally (26), but it may be more likely to be detected in soil microbes because of the presence of antibiotic producers, which may provide selection pressure, the large population sizes, and the physical proximity of highly divergent species.
Of the culturable microbes from soil, Bacillus spp. are among the most abundant. A recent study showed that approximately one-half of 16S rRNA clones from a grassland soil environment were Bacillus spp. (8). In a survey of soils from several continents (32), B. cereus was routinely found at levels of 105 CFU/g of soil (30a). We previously identified a zwittermicin A self-resistance gene, zmaR, from B. cereus UW85, which is functional in both gram-negative and gram-positive bacteria (19). zmaR is ubiquitous in soil: approximately 25% of soil isolates of B. cereus contain zmaR and produce zwittermicin A (24, 32), indicating that there may be 25,000 copies of zmaR in every gram of soil. Additionally, certain strains of the insecticidal toxin-producing species of B. thuringiensis, which is the most widely used biopesticide in the world, carry zmaR and produce zwittermicin A (24, 32). The zwittermicin A exposure from naturally occurring zwittermicin A production by B. cereus soil isolates and from the heavy use of zwittermicin A-producing B. thuringiensis in insect control may provide strong selection pressure for acquisition of zwittermicin A resistance, potentially zmaR, in soil-dwelling microorganisms. This is particularly troubling since strains of B. cereus are currently being developed for use as biological control agents to suppress several plant diseases caused by the pathogenic Phytophthora and Pythium oomycetes (11, 22).
Zwittermicin A is a novel antibiotic that does not belong to any previously described class of antibiotics (13, 29). It is a linear aminopolyol that has a broad target range, inhibiting many eukaryotes and prokaryotes, particularly certain plant pathogenic oomycetes belonging to the genera Phytophthora and Pythium (30). Zwittermicin A contributes to the ability of B. cereus to suppress certain plant diseases and acts synergistically with Bt toxin to enhance the insecticidal activity of B. thuringiensis (4a, 18). Spontaneous zwittermicin A-resistant mutants of Escherichia coli are affected in genes encoding subunits of RNA polymerase; however, zwittermicin A does not appear to inhibit RNA transcription in vivo, suggesting that zwittermicin A has an unusual mode of action (31). Therefore, investigation of zwittermicin A resistance in a producing organism will help us predict mechanisms of resistance that may develop in target pathogens and begin designing strategies to slow the development of resistance, will add to the understanding of this novel antibiotic, and may aid in determining the mode of action of zwittermicin A.
zmaR encodes a 43.5-kDa protein with no sequence similarity to proteins of known function. Recent genome sequencing efforts for many bacteria (3, 16) have revealed that approximately 40% of open reading frames are completely uncharacterized. Since modern biochemical characterization of proteins relies heavily on the predicted amino acid coding sequence having sequence similarity to proteins of known function, this represents an immense gap in our understanding of fundamental biological processes that can only be filled by assigning functions to these proteins. One of the goals of this work was to assign a function to ZmaR, a protein with no homology to proteins of known function. In this work we show that ZmaR inactivates zwittermicin A by acetylation, suggesting that ZmaR constitutes a novel acetyltransferase that is abundant in the soil environment.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli DH5α (10), DH5αF′IQ (Stratagene, La Jolla, Calif.), and BL21(DE3) (Stratagene) were grown on Luria-Bertani (LB) broth or agar unless otherwise indicated (27). Mueller-Hinton agar was prepared to either full strength (100MH8.1) or to one-tenth the strength (10MH8.1) as directed by the manufacturer (Difco Laboratories, Detroit, Mich.) with 40 mM Tris and 40 mM 3-(morpholino)propanesulfonic acid (MOPS) buffers added, and the pH adjusted to 8.1 with 5 N NaOH. Plate media contained 15 g of agar per liter, and soft agar contained 7 g of agar per liter. Antibiotics were added as follows: ampicillin at 50 mg/liter and kanamycin at 10 mg/liter. Plasmids pGEM-3Zf(+) (Promega, Madison, Wis.) and pZMG4 (19), pGEM containing zmaR, were used in cell extract experiments. pCAL-n-EK (Stratagene), a protein overexpression vector, was used to make pCAL-ZmaR for the overexpression of the ZmaR protein.
DNA manipulations and analysis.
Plasmid DNA was purified from E. coli by using the Qiagen plasmid isolation kit (Qiagen, Chatsworth, Calif.). Plasmid DNA was introduced into E. coli by calcium chloride transformation (27). Sequencing reactions were performed with the AmpliTaq dye-terminator Cycle Sequencing kit (Perkin-Elmer Corp., Foster City, Calif.) with primers 11114 and 11115; primer 11346 (5′-GGTTGTCGAGGAACAATTGC-3′; nucleotide sequence 362 to 381); and primers 1721, 1720, 1641, 1737 (19), and 677 (24). Partial DNA sequences were aligned and compiled with SeqMan and EditSeq software (DNASTAR Inc., Madison, Wis.). Primer synthesis and sequencing were done at the University of Wisconsin Biotechnology Center (Madison, Wis.). Sequencing was conducted on an ABI model 373A automated DNA sequencer. Protein comparison searches were conducted by using the BLAST algorithm (1) via the NCBI BLAST electronic mail server.
Preparation of cell-free extracts.
E. coli DH5αF′IQ carrying plasmid pZMG4 or pGEM (Promega) was grown to an optical density at 600 nm of 0.5 to 0.6 in LB broth supplemented with ampicillin and kanamycin (50 and 10 mg/liter, respectively) at 37°C. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM to induce expression of genes under control of the lac promoter, and the culture was allowed to grow for an additional 2 h. Cells were harvested from a 500-ml culture by centrifugation (11,000 × g, 15 min), washed with 250 ml of 10 mM sodium phosphate buffer (pH 7.8), and recentrifuged (11,000 × g, 15 min). The cell pellet was resuspended in 5 ml of the same buffer. Cells were disrupted by sonication on ice for three 1-min pulses, with 1 min of cooling between pulses, with a Vibra-Cell probe sonicator at an output setting of 4 and a 50% duty cycle (Sonics & Materials, Inc., Danbury, Conn.). Cell debris and insoluble proteins were removed by centrifugation (14,000 × g, 20 min), and the resulting supernatant was stored in small aliquots at −20°C. Total protein concentration of cell extracts was determined by dye-binding assay (4) with bovine serum albumin for calibration (Bio-Rad Laboratories, Hercules, Calif.).
Interaction of E. coli cell extracts with zwittermicin A.
A total of 10 μl of E. coli cell extract (60 mg of total protein per ml) was incubated with 50 μg of zwittermicin A made up to a final volume of 20 μl with 10 mM sodium phosphate (pH 7.8) for 16 h at 28°C. The antimicrobial activity of zwittermicin A was tested by bioassay on 100MH8.1 plates. To inactivate protein components of the cell extracts, mixtures were either heated to 95°C for 15 min or a 10-μl cell extract was treated with 1 μl of proteinase K (20 mg/ml) (Sigma, St. Louis, Mo.) at 37°C for 30 min.
Bioassay for activity of zwittermicin A.
Antibacterial activity of zwittermicin A was tested against the sensitive target organism E. coli DH5α (Gibco-BRL) in a soft agar overlay on Mueller-Hinton plates. For bioassays performed on 100MH8.1 plates, 25 μl of an overnight bacterial culture was mixed with 3.0 ml of 100MH8.1 soft agar and plated. For bioassays performed on 10MH8.1 plates, 100 μl of a 10−2 dilution of an overnight culture was mixed with 3.0 ml of 10MH8.1 soft agar and plated. Reaction mixtures were spotted on sterile filter disks on top of the soft agar overlay. Plates were incubated for 18 to 24 h at 28°C, and the zones of inhibition were measured.
Chemical analysis of zwittermicin A exposed to E. coli cell extracts.
Zwittermicin A exposed to cell extracts was partially purified by ultrafiltration with an Ultrafree-MC 10,000-Da molecular-mass cutoff column (Millipore Corp., Bedford, Mass.) by mixing the reaction with 300 μl of water and then centrifuging it (5,000 × g, 60 min). The filtrate was dried in a Speed-Vac Concentrator (Savant Instruments, Inc., Farmington, N.Y.) and resuspended to a final concentration of 10 μg of zwittermicin A per ml, and then 30 to 50 μg of zwittermicin A was analyzed by high-voltage paper electrophoresis (HVPE) (29) or thin-layer chromatography (TLC). For analysis by TLC, 30 to 50 μg of zwittermicin A was spotted on silica gel 60 plates (EM Separations Technology, Darmstadt, Germany) and developed by using a solvent system consisting of n-butanol, acetic acid, and water (2:1:1, by volume). HVPE papers were stained with either silver nitrate or ninhydrin (29). TLC plates were stained similarly with either silver nitrate or ninhydrin applied by aerosol propellant (Sigma).
Large-scale inactivation and purification of zwittermicin A.
To inactivate large quantities of zwittermicin A, reaction mixtures were scaled up to include 600 μl of cell extract (60 mg of total protein per ml) and 3 mg of zwittermicin A in a final volume of 960 μl for 16 h at 28°C. The reaction was divided, and 110 μl of the reaction was mixed with 290 μl water and spun in a 10,000-Da molecular-mass cutoff column as described above. Samples were dried in a Speed-Vac, resuspended in 800 μl of water, and centrifuged to remove insoluble debris. The soluble portion was passed through a 0.2-μm-pore-size filter and dried. The sample was resuspended in 150 μl of water, and the equivalent of 1 mg of zwittermicin A was loaded on a Beckman model 125 High-Performance Liquid Chromatograph with a Beckman Ultrasphere Cyano Bonded-Phase Column (10 mm by 25 cm; Beckman Instruments, Inc., Fullerton, Calif.). The mobile phase flow rate was 2 ml/min and consisted of 1 mM ammonium acetate for the first 5 min, a 1 to 19 mM gradient of ammonium acetate (pH 6.5) for 40 min, and 19 mM ammonium acetate (pH 6.5) for 30 min (30). Fractions were monitored at A218, collected every 2 min, dried down, and analyzed by HVPE for the presence of modified zwittermicin A. Fractions containing modified zwittermicin were dried and combined for analysis by nuclear magnetic resonance (NMR).
NMR spectrum determination.
13C NMR data were acquired with a Varian Unity 400 spectrometer. 1H, 1H-1H RelayH, 1H-13C heteronuclear multiple quantum correlation (HMQC) and 1H-13C heteronuclear multiple bond correlation (HMBC) NMR data were collected with a Varian Unity 500 spectrometer. HMQC and HMBC experiments were run by using pulsed-field gradients. All experiments were run in D2O (100.0 Atom %D; Aldrich). 1H spectra were referenced at δ 4.8 by using the residual partially protonated water present in the sample. 13C spectra were referenced with dioxane (δ 67.4) as an internal standard. A high-resolution fast atom bombardment mass spectrum (HRFABMS) was acquired by the University of Illinois (Urbana) Mass Spectrometry Facility.
Construction of ZmaR overexpression vector.
The ligation independent cloning cloning kit was used to clone and overexpress recombinant ZmaR protein. Plasmid pCAL-n-EK (Stratagene) was used to clone PCR products containing the zmaR coding region with 12- and 13-nucleotide vector-specific sequences introduced at either end of the coding sequence by PCR. DNA was amplified in 50-μl PCR reactions, with a final concentration of the following components: 1× Pfu polymerase reaction buffer (Stratagene), 200 μM concentrations of each deoxynucleoside triphosphate (Boehringer Mannheim), 0.2 μM concentrations of each primer (primer 11115 [5′-GACGACGACAAGATGATTTATGAATTGGTAAA-3′] and primer 11114 [5′-GGAACAAGACCCGTTCATCTTAAGCTATCTTCAA-3′]), 0.2 ng of plasmid pZMG4 DNA (16), and 1.25 U of Pfu polymerase (Stratagene). Amplification was performed with a Thermocycler (Robocycler, Stratagene) as follows: one cycle at 94°C for 30 s; 20 cycles of 94°C for 45 s, 45°C for 45 s, and 72°C for 2 min; and a final extension of 72°C for 10 min. PCR products were purified with a QIAQuick-Spin PCR purification kit (Qiagen) and single-strand overhangs of 12 and 13 nucleotides were generated by incubating 50 ng of purified product with 1 U of Pfu polymerase and 1 mM dATP at 72°C for 10 min. The prepared insert and 20 ng of pCAL-n-EK vector were allowed to anneal overnight at room temperature. Mixture was transformed into XL1-Blue (Stratagene) and plated on LB agar containing 50 mg of ampicillin per liter; one resulting pCAL-ZmaR clone was sequenced to confirm that no mutations had been introduced into the zmaR coding region by PCR amplification. This construct was transformed into E. coli BL21(DE3) (Stratagene) for protein purification. Interestingly, the pCAL-ZmaR construct conferred zwittermicin A resistance on E. coli, demonstrating that the recombinant CBP-ZmaR protein retained activity despite the presence of the 4-kDa calmodulin binding peptide (CBP) affinity tag fused to the N terminus of ZmaR (data not shown).
Overexpression and purification of recombinant CBP-ZmaR.
Next, 5.0 ml of an overnight culture grown in LB broth with 50 mg of ampicillin per liter was added to 500 ml of LB-ampicillin in a Fernbach flask, and the mixture was incubated with shaking at 28°C until an optical density at 600 nm of 0.6 to 0.8 was reached. IPTG (1 mM) was added to induce expression of CBP-ZmaR, and the culture was grown at room temperature for an additional 3 h before cells were harvested. Cells were harvested by centrifugation and resuspended in 20 ml of CaCl2 binding buffer consisting of 50 mM Tris-HCl (pH 8.0), 300 mM NaCl, and 2 mM CaCl2. Then, 8 mg of lysozyme was added, and the cells were incubated at 28°C for 30 min. β-Mercaptoethanol (β-ME) was added to a final concentration of 10 mM, and the cells were disrupted by sonication and separated from cell debris and insoluble proteins as described above. By using the lower temperatures of 28°C and room temperature, respectively, for cell growth and induction of CBP-ZmaR expression, nearly 50% of the CBP-ZmaR remained in the soluble fraction of BL21(DE3) cell lysate, which was used for affinity purification of CBP-ZmaR. The soluble fraction was incubated with 10 ml of calmodulin affinity resin (Stratagene) with mechanical rocking for 16 h at 4°C. The mixture was poured into a 10-ml syringe barrel (Becton Dickinson, Franklin Lakes, N.J.), creating the column used for subsequent purification of CBP-ZmaR. The column was washed with 100 ml of CaCl2 binding buffer (5 to 10 column volumes) containing 10 mM β-ME to remove contaminating proteins, and CBP-ZmaR was eluted with 50 ml of elution buffer containing 2 mM EGTA, 50 mM Tris-HCl, 10 mM β-ME, and 0.05% Triton X-100. Then, 8- to 10-ml fractions were collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (17) as described previously (19). We were unable to cleave the 4-kDa affinity tag from the N terminus of the recombinant CBP-ZmaR protein due to proteolytic degradation of the protein (9a). However, since the CBP-ZmaR protein construct conferred resistance on E. coli, it seemed likely that the protein would still be active if the tag were to remain on. Next, 20% glycerol (vol/vol) was added to fractions containing CBP-ZmaR, and aliquots were flash frozen in a dry ice-ethanol bath and stored at −80°C. The protein concentration was determined by dye binding as described above (4).
Inactivation of zwittermicin by CBP-ZmaR.
Reaction mixtures made up to a final volume of 18 μl with 10 mM sodium phosphate buffer (pH 7.8) contained 4 μg of zwittermicin A and 100 ng of CBP-ZmaR or a corresponding volume of protein elution buffer containing 20% glycerol. Cofactors were added to a final concentration of 5 mM acetyl coenzyme A (acetyl-CoA; sodium salt; Sigma), 4 mM ATP (pH 7.5), or 4 mM acetyl phosphate (Sigma). Reaction mixtures were incubated at 28°C for 16 h and assayed for antimicrobial activity by bioassay on 10MH8.1 plates.
Assay for acetyltransferase activity.
Reaction mixtures made up to a final volume of 20 μl with 10 mM sodium phosphate buffer (pH 7.8) containing 5 μl of either CBP-ZmaR (0.73 mg/ml) or protein elution buffer with 4 μl of zwittermicin A (1 μg/μl), and 0.1 μl of [1-14C]acetyl-CoA (50 μCi/ml; 50 to 62 mCi/mmol; Amersham Life Science, Inc., Arlington Heights, Ill.) were incubated at 28°C for 2 h and subsequently dried in a Speed-Vac. Then, 2 μl of water was added to the sample, and the entire sample was separated by TLC as described above. The plate was exposed to a PhosphorImager plate (Molecular Dynamics) for 24 h and analyzed. Because free acetyl-CoA and acetylated zwittermicin A have similar Rf values on TLC, in order to achieve the most interpretable results it was necessary to work under conditions where acetyl-CoA was limiting to ensure the transfer of all radioactive label to zwittermicin A. Similar results were obtained under conditions where acetyl-CoA was present in excess or was limiting.
RESULTS
zmaR-containing cell extracts inactivate zwittermicin A.
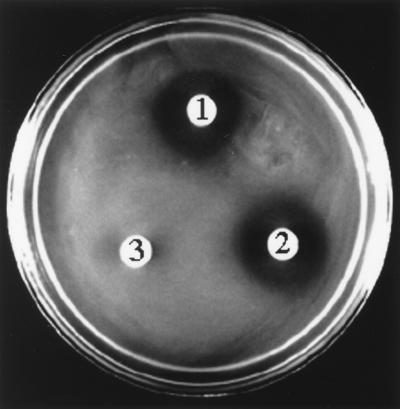
We previously demonstrated that E. coli DH5α strains carrying zmaR express the ZmaR protein and are resistant to zwittermicin A (19). To determine whether ZmaR mediates the inactivation of zwittermicin A, we incubated E. coli DH5αF′IQ cell extracts carrying vector alone (pGEM) or zmaR (pZMG4) with zwittermicin A and assayed for antimicrobial activity of the reaction mixture against E. coli DH5α (Fig. 1). The antibiotic activity of 50 μg of zwittermicin A was abolished when it was mixed with zmaR-containing cell extracts, but no loss of antibiotic activity was observed when it was mixed with pGEM-containing cell extracts. The ability of zmaR-containing cell extracts to inactivate zwittermicin A was abolished by heating the cell extract to 95°C or treating it with proteinase K prior to the addition of zwittermicin A, suggesting dependence on a protein (data not shown).
FIG. 1.
zmaR-containing cell extracts inactivate zwittermicin A. A zone of inhibition indicates activity of zwittermicin A against E. coli; the assay was initiated with 50 μg of zwittermicin A. Zones: 1, zwittermicin A alone; 2, zwittermicin A incubated with vector-containing (pGEM) cell extract; 3, zwittermicin A incubated with zmaR-containing (pZMG4) cell extract.
Inactivated zwittermicin A differs chemically from active zwittermicin A.
A total of 30 to 50 μg of zwittermicin A that had been incubated with pGEM- or zmaR-containing cell extracts and partially purified was analyzed by HVPE and TLC (Table 1). In each of these cases, the zwittermicin A that had been incubated with the pGEM-containing cell extract exhibited a different mobility than the zwittermicin A that had been incubated with the zmaR-containing cell extract. Furthermore, the high-pressure liquid chromatography (HPLC) retention time of zwittermicin A exposed to zmaR-containing cell extract differed from that of zwittermicin A not exposed to cell extract (Table 1). These data demonstrate that zwittermicin A inactivated by zmaR-containing cell extracts differs chemically from active zwittermicin A, suggesting that it has been covalently modified.
TABLE 1.
Biochemical properties of active and inactivated zwittermicin A from cell extracts
| Zwittermicin A | Biochemical properties as determined by:
|
||
|---|---|---|---|
| HVPEa (Rm) | TLCb (Rf) | HPLCc (RT [min]) | |
| Active | 0.14 | 0.08 | 34–40 |
| Inactive | 0.10 | 0.15 | 24–28 |
HVPE was carried out as described in the text. The relative mobility (Rm) of zwittermicin A was determined in relation to Orange G.
TLC was carried out on silica gel 60 plates developed by using a solvent system of n-butanol, acetic acid, and water (2:1:1) by volume.
Retention time (RT) on HPLC was determined on a cyano-bonded-phase column with a gradient of ammonium acetate.
Structure of modified zwittermicin A.
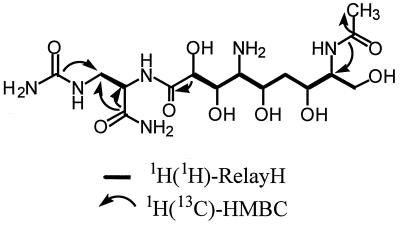
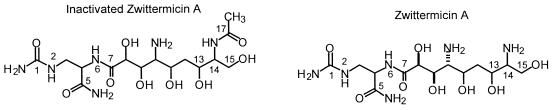
1H-1H RelayH, 1H-13C HMQC, and 1H-13C HMBC experiments confirm that the inactivated zwittermicin skeleton (C-1 to C-15) is identical to that of native zwittermicin A1 (Fig. 2). 13C chemical shifts assigned by 1H-13C HMQC confirm that C-3, C-10, and C-14 are N-substituted carbons, while C-8, C-9, C-11, C-13, and C-15 are O-substituted carbons as seen in zwittermicin A. Two spin systems, C-3 to C-4 and C-8 to C-15, are defined by 1H-1H RelayH experiments. The C-1 to C-5 partial structure of the native zwittermicin A skeleton is confirmed by HMBC correlations from the C-5 carbonyl and C-1 urea to both H-3 methylene protons and an HMBC correlation from the C-5 carbonyl to the H-4 methine of the C-3 to C-4 spin system. The amide bond that links C-4 to the second spin system (C-8 to C-15), which completes the native zwittermicin skeleton, is confirmed by an HMBC correlation between the C-7 carbonyl and H-8 methine proton.
FIG. 2.
Important correlations used to define the structure of inactivated zwittermicin A.
The molecular formula of inactivated zwittermicin A C15H30N6O9 (HRFABMS, m/z = 439.2149 [MH+]; calculated, 439.2152) differs from native zwittermicin A (C13H28N6O8) by C2H2O. This two-carbon unit is established as an acetate by an HMBC correlation between the additional C-17 (δ 174.4) carbonyl carbon and the H-18 (δ 2.01) methyl singlet observed in the inactivated zwittermicin A 13C and 1H NMR spectra. Deshielding of H-14 (Δ +0.47 δ) and shielding of H-13 (Δ −0.30 δ) and H-15 (Δ −0.16, Δ −0.10 δ) define inactivated zwittermicin A as the C-14 N-acetylation product of zwittermicin A. N-Acetylation at C-14 is further confirmed by the presence of a weak HMBC correlation between the C-17 carbonyl and H-14 methine proton (Table 2).
TABLE 2.
NMR spectral data of inactivated zwittermicin A and native zwittermicin A
| Atom | Chemical shifts
|
|||
|---|---|---|---|---|
| Inactivated zwittermicin A
|
Zwittermicin Aa
|
|||
| 13Cb (ppm) | 1H (ppm)c | 13C (ppm) | 1H (ppm) | |
| 1 | 162.5 | 164.6 | ||
| 2 | ||||
| 3 | 41.5 | 3.60 (dd, 15, 4.5) | 43.5 | 3.62 (dd, 14.5, 3.5) |
| 3.47 (dd, 15, 7) | 3.49 (dd, 14.5, 7) | |||
| 4 | 55.3 | 4.43 (dd, 7, 4.5) | 57.3 | 4.45 (dd, 7, 3.5) |
| 5 | 175.1 | 177.1 | ||
| 6 | ||||
| 7 | 175.6 | 177.9 | ||
| 8 | 72.8 | 4.52 (d, 2) | 74.7 | 4.55 (d, 2) |
| 9 | 68.6 | 4.34 (dd, 6, 2) | 72.1 | 4.35 (dd, 4.5, 2) |
| 10 | 58.8 | 3.49 (t, 6) | 60.6 | 3.56 (dd, 6, 4.5) |
| 11 | 66.2 | 4.23 (ddd, 9, 6, 3) | 68.4 | 4.28 (m) |
| 12 | 36.4 | 1.67 (m, 2H) | 37.3 | 1.80 (m) |
| 1.76 (m) | ||||
| 13 | 67.6 | 3.89 (m)d | 68.8 | 4.19 (br d, 10) |
| 14 | 56.6 | 3.90 (m)d | 59.7 | 3.43 (ddd, 8.5, 4, 4) |
| 15 | 61.2 | 3.78 (dd, 11.5, 3.5) | 60.1 | 3.94 (dd, 12.5, 4) |
| 3.65 (dd, 11.5, 6.5) | 3.78 (dd, 12.5, 8.5) | |||
| 16 | ||||
| 17 | 175.3 | |||
| 18 | 22.9 | 2.01 (s, 3H) | ||
Chemical shifts for zwittermicin are from He et al. (12).
Assignments determined by HMQC.
Abbreviations: dd, double doublet; d, doublet; t, triplet; ddd, double double doublet; m, multiplet; s, singlet; br d, broad doublet. Numbers in parentheses refer to coupling constants (in hertz).
Chemical shift determined from HMQC.
Zwittermicin A inactivation by recombinant CBP-ZmaR.
Based on the structure of the modified antibiotic, we postulated that ZmaR is an acetyltransferase acting directly on zwittermicin A. Furthermore, the addition of the potential cofactors acetyl-CoA and ATP to zmaR-containing cell extracts increased the inactivation of zwittermicin A, as determined by bioassay and TLC; the addition of the potential cofactors malonyl-CoA, propionyl-CoA, and butyryl-CoA did not increase the inactivation of zwittermicin A (data not shown). Since the addition of either acetyl-CoA or ATP to cell extract would have the net effect of increasing the amount of acetyl-CoA in cell extract, this suggested that the preferred donor group for the inactivation of zwittermicin A is an acetyl group. We incubated CBP-ZmaR and zwittermicin A individually with ATP, acetyl-CoA, and acetyl phosphate (the latter two cofactors are potential donors of the acetyl group) and assayed for antimicrobial activity of zwittermicin A by bioassay (Table 3). Protein elution buffer provided a control treatment to show dependence of the reaction on CBP-ZmaR. Zwittermicin A that had been incubated with CBP-ZmaR and acetyl-CoA, but not the other combinations, showed a loss of antimicrobial activity (Table 3), suggesting that CBP-ZmaR acts directly on zwittermicin A as an acetyltransferase and requires acetyl-CoA as a cofactor.
TABLE 3.
Inactivation of zwittermicin A by CBP-ZmaR
| Treatmenta | Zone size (mm) |
|---|---|
| Elution buffer + zwittermicin A + acetyl-CoA | 3.0 |
| CBP-ZmaR + zwittermicin A + acetyl-CoA | 0.0 |
| Elution buffer + zwittermicin A + acetyl phosphate | 2.5 |
| CBP-ZmaR + zwittermicin A + acetyl phosphate | 2.5 |
| Elution buffer + zwittermicin A + ATP | 3.0 |
| CBP-ZmaR + zwittermicin A + ATP | 3.0 |
Treatments contained 4 μg of zwittermicin A, 100 ng of CBP-ZmaR, and either acetyl-CoA (5 mM), acetyl phosphate (4 mM), or ATP (4 mM). Reactions were incubated at 28°C for 16 h. Protein elution buffer (50 mM Tris-Cl [pH 8.0], 10 mM β-ME, 2 mM EGTA, 0.05% Triton X-100, 20% glycerol) was included in place of CBP-ZmaR as a mock treatment. Reaction mixtures were spotted onto 10MH8.1 agar against a lawn of E. coli and scored for zones of inhibition after 24 h. Data reported are representative of three experiments.
Acetyltransferase activity of CBP-ZmaR.
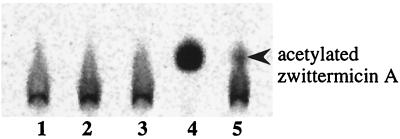
To provide direct evidence that the inactivation of zwittermicin A by CBP-ZmaR is due to the transfer of an acetyl group from acetyl-CoA to zwittermicin A, we incubated CBP-ZmaR with zwittermicin A and radiolabeled acetyl-CoA and then analyzed the products by TLC (Fig. 3). We observed a distinct shift in both the mobility and the shape of the radioactive spot only when zwittermicin A, CBP-ZmaR, and acetyl-CoA were supplied together in a reaction and not when any of these components were omitted. A TLC plate containing both radioactive acetyltransferase reactions and HPLC-purified acetylated zwittermicin A as a standard, which was stained with silver nitrate subsequent to radioactive detection, verified that the radioactive spot attributed to acetylated zwittermicin A had the same Rf as the purified acetylated zwittermicin A (data not shown). Together, these data demonstrate that zwittermicin A is the substrate of the acetyltransferase reaction catalyzed by the enzyme CBP-ZmaR and that acetyl-CoA is the donor group. Therefore, given the lack of homology of ZmaR with known acetyltransferases, ZmaR may constitute a novel class of acetyltransferase.
FIG. 3.
CBP-ZmaR acetyltransferase reactions. Acetyltransferase reaction mixtures contained CBP-ZmaR or protein elution buffer, zwittermicin A, and [1-14C]acetyl-CoA. Samples were separated by TLC on silica 60 gel plates with n-butanol–acetic acid–water (2:1:1) as solvent. TLC plate was visualized on a PhosphorImager. Lanes: 1, free acetyl-CoA; 2, acetyltransferase reaction with zwittermicin A omitted; 3, acetyltransferase reaction with CBP-ZmaR omitted; 4, complete acetyltransferase reaction; 5, complete acetyltransferase reaction with one-tenth the amount of CBP-ZmaR. Acetylated zwittermicin A was identified by comigration with authentic HPLC-purified acetylated zwittermicin A (not shown).
DISCUSSION
We have shown that zmaR, a zwittermicin A resistance gene from B. cereus UW85, encodes an acetyltransferase that inactivates zwittermicin A in vitro. E. coli cell extracts containing zmaR inactivated zwittermicin A by acetylation, and purified ZmaR protein inactivated zwittermicin A only in the presence of the cofactor acetyl-CoA. By radiochemical assay we demonstrated directly that ZmaR is an acetyltransferase. This work is particularly significant since ZmaR, by its lack of sequence similarity to proteins of known function, appears to constitute a novel antibiotic resistance determinant. In this era of increasing antibiotic resistance, the characterization of new resistance determinants is vital. Moreover, since the zmaR gene is widespread among Bacillus spp., which are abundant in soils worldwide, the possibility of horizontal transfer to target pathogens is great.
Antibiotic resistance via acetylation is common in both antibiotic-producing organisms (6, 23, 33, 34) and target organisms (14, 20, 25, 28), and acetyltransferases are a well-studied group of enzymes. Therefore, it is especially surprising that ZmaR does not have amino acid similarity or motifs common to any known acetyltransferases, as determined by the MOTIFS and PROFILESCAN programs and by PROCITE (19). Two conserved domains present in N-acetyltransferases are believed to be important for acetyl-CoA binding (5, 15). Manual alignment of the ZmaR sequence with these motifs revealed that 6 of 24 amino acids in the consensus amino acid sequence (15) are conserved in the ZmaR sequence. Recent analysis of a superfamily of diverse N-acetyltransferases, with representatives drawn from plants, animals, and prokaryotes, suggests that the sequence of these motifs may diverge from the previously described consensus sequence (21). ZmaR contains five of eight highly conserved residues, but contains very few moderately conserved residues identified in this analysis (21). Thus, ZmaR may have limited similarity to other known acetyltransferases in regions believed to interact with acetyl-CoA. To determine whether the three-dimensional structure of ZmaR resembled other acetyltransferase enzymes, we subjected ZmaR to a fold recognition server (9, 34a), since the amino acid sequence of a protein may not show homology to proteins with which it shares a three-dimensional (3D) structure. The recent elucidation of the crystal structure of an aminoglycoside phosphotransferase enzyme revealed striking similarities in its 3D structure compared to eukaryotic protein kinases, despite no evidence of sequence homology (13). However, ZmaR did not show similarity to any proteins, suggesting that the overall 3D structure of ZmaR may, in fact, be novel. Alternatively, these results may simply reflect the lack of 3D structural analysis of acetyltransferase enzymes.
It is interesting that ZmaR shares significant sequence similarity (24% sequence identity; 43% sequence similarity over 269 amino acids) with a protein of unknown function deduced from the B. subtilis gene ydfB (16). ydfB is located in a region of the B. subtilis genome that encodes many putative antibiotic resistance proteins. It is intriguing that ZmaR may therefore represent the first of a class of proteins, with ydfB possibly being another, that acetylate structurally similar substrates. The range of molecules acetylated by these proteins may include antibiotics that have not yet been discovered, as well as known antibiotics of clinical or agricultural importance.
A worldwide survey showed that B. cereus is present in soil at 105 CFU/g (30a), and 25% of this population contain zmaR (24, 32), indicating that there are likely to be 25,000 copies of zmaR in every gram of soil. Many crops are inoculated with an insecticide-producing strain of B. thuringiensis, HD-1, that contains zmaR and produces zwittermicin A (24, 32). Strains of B. cereus are currently being developed for use as biological control agents to suppress several plant diseases caused by the pathogenic Phytophthora and Pythium oomycetes (11, 22). Is it possible that zmaR could be transferred to these target organisms, short-circuiting the ability of B. cereus to control plant disease? It has long been suggested that antibiotic-producing organisms are the source of the antibiotic resistance genes found in clinical isolates because the biochemical mechanisms of antibiotic resistance from antibiotic-producing organisms and target organisms are similar (2). Moreover, it was recently shown that some antibiotic preparations are contaminated with DNA, including antibiotic resistance genes, from the antibiotic-producing organisms (35). This suggests a source of antibiotic resistance genes for target human pathogens, which are conveniently administered simultaneously with the antibiotic. The prevalence of zmaR in an agricultural setting concomitant with selection pressure from zwittermicin A may represent an analogous situation for plant pathogenic oomycetes and other soil microflora. Further studies of zmaR will contribute to our understanding of zwittermicin A resistance and may aid in the development of strategies to reduce the rate of appearance of resistance in target organisms.
ACKNOWLEDGMENTS
We thank Sandra J. Raffel for purification of the zwittermicin A.
This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grant RR02301 from the Biomedical Research Technology Program, National Center for Research Resources. Equipment in the facility was purchased with funds from the University of Wisconsin, the NSF Biological Instrumentation Program (grant DMB-8415048), the NIH Biomedical Research Technology Program (grant RR02301), the NIH Shared Instrumentation Program (grant RR02781), and the U.S. Department of Agriculture. We thank Robert Steele for initial support of this project. This work was also supported by the University-Industry Research Program and Hatch Project 4038 of the University of Wisconsin-Madison College of Agricultural and Life Sciences.
REFERENCES
- 1.Altshchul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste R, Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci USA. 1973;70:2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett III G, Block C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4a.Broderick, N. A., R. M. Goodman, K. F. Raffa, and J. Handelsman. Synergy between zwittermicin A and Bacillus thuringiensis subsp. kurstaki against gypsy moth. Environ. Entomol., in press.
- 5.Coon S L, Roseboom P H, Baler R, Weller J L, Namboodiri M A A, Koonin E V, Klein D C. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science. 1995;270:1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- 6.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 7.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 8.Felske A, Wolterink A, Van Lis R, Akkermans A D L. Phylogeny of the main bacterial 16S rRNA sequences in Drentse A grassland soils (The Netherlands) Appl Environ Microbiol. 1998;64:871–879. doi: 10.1128/aem.64.3.871-879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer D, Eisenberg D. Assigning folds to the proteins encoded by the genome of Mycoplasma genitalium. Proc Natl Acad Sci USA. 1997;94:11929–11934. doi: 10.1073/pnas.94.22.11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Gross, J. A., et al. Unpublished data.
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Handelsman J, Raffel S, Mester E H, Wunderlich L, Grau C R. Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl Environ Microbiol. 1990;56:713–718. doi: 10.1128/aem.56.3.713-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He H, Silo-Suh L A, Clardy J, Handelsman J. Zwittermicin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett. 1994;35:2499–2502. [Google Scholar]
- 13.Hon W-C, McKay G A, Thompson P R, Sweet R M, Yang D S C, Wright G D, Berghuis A M. Structure of an enzyme required for aminoglycoside antibiotic resistance reveals homology to eukaryotic protein kinases. Cell. 1997;89:887–895. doi: 10.1016/s0092-8674(00)80274-3. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Zhu C-B, Ogata T, Sunada A, Ishikawa J, Mizuno S. Enzymatic 2′-N-acetylation of arbekacin and antibiotic activity of its product. J Antibiot. 1996;49:458–464. doi: 10.7164/antibiotics.49.458. [DOI] [PubMed] [Google Scholar]
- 15.Kimberly L L, Berkey A, Casero R A., Jr RGFGIGS is an amino acid sequence required for acetyl coenzyme A binding and activity of human spermidine/spermine N1 acetyltransferase. J Biol Chem. 1996;271:18920–18924. doi: 10.1074/jbc.271.31.18920. [DOI] [PubMed] [Google Scholar]
- 16.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Manker, D. C., W. D. Lidster, R. L. Starnes, and S. C. MacIntosh. May 1994. Potentiator of Bacillus pesticidal activity. Patent cooperation treaty, WO 94/09630.
- 19.Milner J L, Stohl E A, Handelsman J. Zwittermicin A resistance gene from Bacillus cereus. J Bacteriol. 1996;178:4266–4272. doi: 10.1128/jb.178.14.4266-4272.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray I A, Shaw W V. O-Acetyltransferases for chloramphenicol and other natural products. Antimicrob Agents Chemother. 1997;41:1–6. doi: 10.1128/aac.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuwald A F, Landsman D. GCN5-related histone-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 22.Osburn R M, Milner J L, Oplinger E S, Smith R S, Handelsman J. Effect of Bacillus cereus UW85 on the yield of soybean at two field sites in Wisconsin. Plant Dis. 1995;79:551–556. [Google Scholar]
- 23.Perez-Gonzalez J A, Lopez-Cabrera M, Pardo J M, Jimenez A. Biochemical characterization of two cloned resistance determinants encoding a paromycin acetyltransferase and a paromycin phosphotransferase from Streptomyces rimosus forma paromomycinus. J Bacteriol. 1989;171:329–334. doi: 10.1128/jb.171.1.329-334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raffel S J, Stabb E V, Milner J L, Handelsman J. Genotypic and phenotypic analysis of zwittermicin A-producing strains of Bacillus cereus. Microbiology. 1996;142:3425–3436. doi: 10.1099/13500872-142-12-3425. [DOI] [PubMed] [Google Scholar]
- 25.Rather P N, Orosz E, Shaw K J, Hare R, Miller G. Characterization and transcriptional regulation of the 2′-N-acetyltransferase gene from Providencia stuartii. J Bacteriol. 1993;175:6492–6498. doi: 10.1128/jb.175.20.6492-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salyers A A, Shoemaker N B. Resistance gene transfer in anaerobes: new insights, new problems. Clin Infect Dis. 1996;23(Suppl. 1):36–43. doi: 10.1093/clinids/23.supplement_1.s36. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silo-Suh L A, Lethbridge B J, Raffel S J, He H, Clardy J, Handelsman J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microbiol. 1994;60:2023–2030. doi: 10.1128/aem.60.6.2023-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silo-Suh L A, Stabb E V, Raffel S J, Handelsman J. Target range of zwittermicin A, an aminopolyol antibiotic from Bacillus cereus. Curr Microbiol. 1998;37:6–11. doi: 10.1007/s002849900328. [DOI] [PubMed] [Google Scholar]
- 30a.Stabb, E. V., et al. Unpublished data.
- 31.Stabb E V, Handelsman J. Genetic analysis of zwittermicin A resistance in Escherichia coli: effects on membrane potential and RNA polymerase. Mol Microbiol. 1998;27:311–322. doi: 10.1046/j.1365-2958.1998.00678.x. [DOI] [PubMed] [Google Scholar]
- 32.Stabb E V, Jacobson L M, Handelsman J. Zwittermicin A-producing strains of Bacillus cereus from diverse soils. Appl Environ Microbiol. 1994;60:4404–4412. doi: 10.1128/aem.60.12.4404-4412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama M, Kumagai T, Shionoya M, Kimura E, Davies J E. Inactivation of bleomycin by an N-acetyltransferase in the bleomycin-producing strain Streptomyces verticillus. FEMS Microbiol Lett. 1994;121:81–86. doi: 10.1111/j.1574-6968.1994.tb07079.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama M, Paik S-Y, Nomi R. Mechanism of self-protection in a puromycin-producing micro-organism. J Gen Microbiol. 1985;131:1999–2005. doi: 10.1099/00221287-131-8-1999. [DOI] [PubMed] [Google Scholar]
- 34a.UCLA-DOE Fold Recognition Server. [Online.] http://fold.doe-mbi.ucla.edu/. [27 July 1999, last date accessed.]
- 35.Webb V, Davies J. Antibiotic preparations contain DNA: a source of drug resistance genes? Antimicrob Agents Chemother. 1993;37:2379–2384. doi: 10.1128/aac.37.11.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitman W B, Coleman D C, Wiebe W J. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]