Abstract
This study developed a predictive model for cognitive degeneration in patients with Parkinson’s disease (PD) using a machine learning method. The clinical data, plasma biomarkers, and neuropsychological test results of patients with PD were collected and utilized as model predictors. Machine learning methods comprising support vector machines (SVMs) and principal component analysis (PCA) were applied to obtain a cognitive classification model. Using 32 comprehensive predictive parameters, the PCA-SVM classifier reached 92.3% accuracy and 0.929 area under the receiver operating characteristic curve (AUC). Furthermore, the accuracy could be increased to 100% and the AUC to 1.0 in a PCA-SVM model using only 13 carefully chosen features.
Keywords: Parkinson’s disease, machine learning, neuropsychological test, biomarker
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease. Clinical motor dysfunctions, such as resting tremors, rigidity, bradykinesia, postural instability, and inability to initiate motion, are commonly seen in patients with PD. In addition to motor dysfunction, patients with PD also tend to have cognitive impairments, such as mild cognitive impairment (MCI) and dementia. MCI and dementia may also affect motor dysfunction in PD patients, and there is a complicated relationship between motor function and cognition in patients with PD [1].
According to previous research, there is a high probability that patients with PD develop cognitive impairment that may affect their quality of life; this impairment predominantly involves the cognitive domains of attention, executive function, and visuospatial skills [2,3,4]. Biomarkers obtained mainly from neuroimaging data were extensively discussed for finding predictors of cognitive dysfunction in Parkinson’s disease in a literature survey [5,6]. Indeed, it is crucial to identify the factors influencing cognitive decline that affect clinical prognosis and require early intervention [7].
Machine learning in artificial intelligence is popular in constructing a predictive model. In a study with 45 subjects, four machine learning models were developed to assess the ability to discriminate between PD patients with cognitive integrity (PDCI), mild cognitive impairment (PDMCI), and dementia (PDD). In an SVM model for classifying PDD and PDCI, the most relevant variables related to PD dementia were white matter, lateral ventricle, and hippocampus volume, and the prediction accuracy could reach 96.67% [8]. In another study with a cohort of 75 PD patients, a set of five biomarkers (cerebrospinal fluid (CSF) total tau levels, CSF phosphorylated tau levels, CSF Aβ42 levels, APOE genotype, and SPARE-AD imaging score) was adopted as the predictor of a logistic regression classifier, and 80% accuracy was achieved in discriminating PD patients with normal cognition from PD patients with dementia [9].
In this preliminary study, a cross-sectional investigation of clinical variables, neuropsychological test results, and plasma biomarkers [10,11,12] in patients with PD was conducted to identify features related to cognitive impairment. More specifically, machine learning was applied to obtain a predictive cognitive degeneration model and ascertain key predictors that help medical experts quickly identify a patient’s cognitive condition and provide treatment.
2. Methods
2.1. Participants
This cross-sectional study recruited patients with PD from October 2019 to November 2019 and from July 2020 to November 2020. The patients were recruited at the Neurology Department of the MacKay Memorial Hospital (Taiwan).
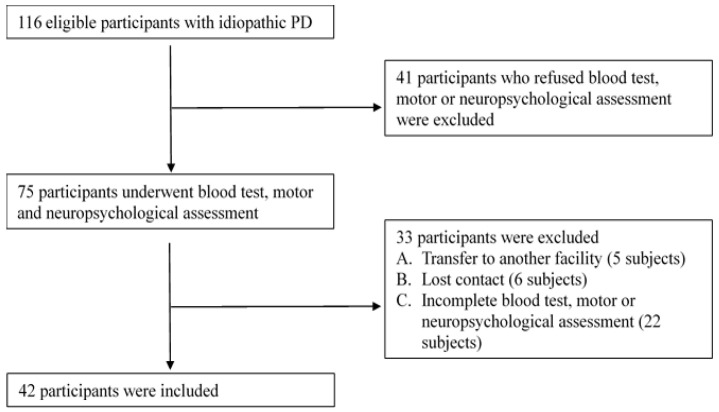
The study was performed following the Declaration of Helsinki and was approved by the Institutional Review Board of Mackay Memorial Hospital in Taiwan (IRB Number: 18MMHIS152). Informed consent was obtained from all participants. A consecutive series of patients with PD were recruited in the Neurology outpatient clinics of a tertiary medical center in northern Taiwan from October 2019 to November 2020. All participants met the following criteria: (a) age > 30 years, (b) diagnosed with idiopathic PD according to the PD clinical diagnostic criteria of the Movement Disorder Association [13,14], and (c) no diagnosis of dementia (for those who have received more than six years of education, the Mini-Mental State Evaluation [MMSE] score must be >23 points; for those who have less than six years of education, the MMSE must be >13 points). Participants were excluded if (a) they had more than two incomplete tests or (b) uncontrolled medical conditions that cause severe physical and cognitive disabilities. A physician evaluated the presence of the exclusion criteria, and the process was shown in Figure 1.
Figure 1.
Exclusion flow chart.
2.2. Clinical Data
We collected clinical information from patients, including sex, age, course of the disease, education level, levodopa dose, Barthel index, Hoehn and Yahr stage, and Unified Parkinson’s Disease Rating Scale (UPDRS) parts I–III subscale scores [15,16,17,18,19].
Trained nurses performed a comprehensive neuropsychological assessment of all patients. The assessment includes general cognition and specific cognitive domains involving the following examinations: (1) global cognition (MMSE and Clinical Dementia Rating-Sum of Boxes [CDR-SB]); (2) processing speed and working memory (Digits Recall Forward and Backward); (3) verbal learning and memory (California Verbal Language Test-II Short Form [CVLT-SF]); (4) semantic verbal fluency (animal naming); (5) language (Boston Naming Test); (6) attention and visuospatial processing (Trail Making Test A and B [TMT-A and TMT-B]); and (7) visuoperceptual and visuospatial processing (Benton Judgement of Line Orientation) [20,21,22,23,24,25,26,27].
2.3. Neurobiological Indicator
A blood sample of 10 mL was collected from each subject and centrifuged within one hour of collection. The plasma was separated and immediately frozen in test tubes at −80 °C. We then delivered frozen plasma on dry ice to MagQu Co., Ltd. (New Taipei City, Taiwan) and measured the levels of plasma α-syn, Aβ42, and t-tau using an immunomagnetic reduction assay.
2.4. Data Analysis
This study collected 29 clinical data and the three plasma biomarkers for each participant, as shown in Table 1. In addition, 42 patients with these complete data were included to build a classification model using support vector machine (SVM) and principal component analysis (PCA) in the Python Sklearn package. It was previously shown that the PCA–SVM method effectively classified PD–MCI from non-PD–MCI patients with high accuracy, provided good predictors were used [28].
Table 1.
Twenty-nine clinical data and the three plasma biomarkers.
| Hoehn–Yahr Stage | UPDRS I | UPDRS II | UPDRS III |
|---|---|---|---|
| LED (mg/day) | Gender | Age of visits | Age of onset |
| Disease duration | Education (years) |
Barthel Index | MMSE |
| IADL | JLO | PSQI | EQ-5D index |
| EQ-5D VAS | GDS−15 | GAD−7 | TMT-A |
| TMT-B | Verbal fluency | Digits Forwards | Digits Backwards |
| CVLT-SF total recall |
CVLT-SF Immediate |
CVLT-SF delay |
CVLT-SF recognition |
| BNT | α-syn (pg/mL) | Aβ42 (pg/mL) | t-tau (pg/mL) |
Abbreviation: Aβ42, amyloid-β 42; BNT, Boston Naming Test; CVLT-SF, California Verbal Learning Test-Short Form; EQ-5D, EuroQol-5 dimensions; GAD-7, Generalized anxiety disorder scale 7-item; GDS-15, Geriatric depression scale 15-item; IADL, Instrumental activities of daily living; JLO, Judgment of Line Orientation; LED, Levodopa equivalent dose; MMSE, Mini-Mental State Examination; PSQI, Pittsburgh sleep quality index; SD, Standard Deviation; TMT, Trail Making Test; UPDRS, Unified Parkinson’s Disease Rating Scale; VAS, visual analog scale; t-tau, total tau; α-syn, α-synuclein.
2.5. Data Normalization
Before using the SVM prediction model, it is necessary to preprocess the collected data to obtain a better data structure for training and avoid differences in the data distribution area, which affects the convergence speed and accuracy of the prediction model. Normalization is a standard preprocessing technique [29]. Min-max normalization is used in the data preprocessing. The data are scaled to between 0 and 1 through normalization without changing the distribution of the data [30] using the following transformation:
| (1) |
where is the maximum value, is the minimum value, and is the normalized value between 0 and 1 for the dataset, x.
2.6. SVM
SVMs are a type of supervised learning method. It is to find a hyperplane between two-class categories. The SVMs try to find the decision boundary in the training data set to maximize the margin between the two classes to reduce the generalization error of the classifier. The maximum boundary hyperplane can be determined through various kernels to build a linear or nonlinear classification [31,32,33]. This study uses different kernel functions, including linear, RBF (radial basis function), and Poly (polynomial) functions to compare which model is better for predicting cognitive impairment.
2.7. PCA
PCA is an unsupervised learning method for feature extraction. Using the first few principal components (PCs) of the covariance matrix, normalized high-dimensional data can be projected into a lower-dimension space using orthogonal transformation while preserving the essential features [34,35,36]. More specifically, the dimensionality of the original dataset X ∈ (i.e., n samples and p features) can be reduced to X′ ∈ by PCA with s < p. That is, X′ with less dimension presents the data more concisely while retaining most of the key features (the cumulative energy of the first s eigenvalues of the covariance matrix is above a certain threshold, for example, 90%, of the total energy). The new features are then provided to the SVM with a lower dimension for predictive classification; hence, the training model can accelerate the calculation and improve the accuracy.
2.8. Area under the Receiver Operating Curve
The receiver operating characteristic curve (ROC curve) is drawn as a plot with the false positive rate (FPR) as the X-axis, and the true positive rate (TPR) as the Y-axis that illustrates the diagnostic ability of a classifier as its discrimination threshold is varied. The area under the ROC curve (AUC) measures the power of a classifier to distinguish between classes and is used as a summary of the ROC curve [37]. The higher the AUC, the better the model’s performance at distinguishing between the positive and negative classes. When the AUC is equal to 0.5, the classifier cannot differentiate between positive and negative categories. Therefore, an AUC between 0.9 and 1 indicates that the predictive classifier has an excellent discriminatory ability.
3. Results
After one year of data collection, there were 116 patients with idiopathic PD. Of those, 41 patients refused blood and neuropsychological tests, five were transferred to another hospital, six lost contact, and 22 had incomplete data. Ultimately, only 42 patients had complete data. In this study, we used CDR-SB scores for the two classifications. The score interval for the patients without cognitive impairment (from normal to MCI) was ≤0.5, and the score interval for those with moderate and severe cognitive impairment was >0.5. After judging and categorizing, 16 patients were classified as not having cognitive impairment, and 26 patients had moderate to severe cognitive impairment. The demographic and collected data for these two groups were presented in Table 2.
Table 2.
The demographic and data comparisons of the participants.
| N = 42 | Without Cognitive Impairment (N = 16) | Moderate and Severe Cognitive Impairment (N = 26) | p Value |
|---|---|---|---|
| Hoehn–Yahr stage | 1.78 (0.73) | 2.37 (0.61) | 0.291 |
| UPDRS I | 2.38 (1.147) | 4.15 (1.78) | 0.078 |
| UPDRS II | 5.63 (2.391) | 11.23 (5.88) | 0.002 |
| UPDRS III | 12.63 (5.35) | 20.65 (10.35) | 0.013 |
| LED (mg/day) | 428.56 (229.13) | 440.77 (241.8) | 0.617 |
| Gender | Male 8/50% | Male 10/38.46% | 0.463 |
| Age of visits | 68.38 (8.57) | 76.65 (7.27) | 0.417 |
| Age of onset | 65.81 (8.72) | 71.92 (8.19) | 0.753 |
| Disease duration | 2.56 (2.39) | 4.73 (3.52) | 0.022 |
| Education (years) |
7.69 (3.22) | 7.04 (4.96) | 0.114 |
| Barthel Index | 156.25 (225) | 88.27 (16.31) | 0.019 |
| MMSE | 26.94 (2.24) | 22.96 (3.96) | 0.015 |
| IADL | 23.38 (1.26) | 17.38 (6.76) | 0.000 |
| JLO | 14.5 (4) | 12.23 (4.86) | 0.366 |
| PSQI | 5.38 (2.39) | 7 (2.79) | 0.71 |
| EQ-5D index | 0.77 (0.17) | 0.75 (0.21) | 0.78 |
| EQ-5D VAS | 68.88 (10.78) | 66.54 (16.54) | 0.335 |
| GDS−15 | 2.5 (3.16) | 3.54 (4.71) | 0.067 |
| GAD−7 | 1 (1.86) | 2.08 (3.5) | 0.068 |
| TMT-A | 27.19 (10.88) | 36.62 (12.74) | 0.494 |
| TMT-B | 72.06 (28.19) | 87.96 (33.74) | 0.15 |
| Verbal fluency | 11.56 (4.56) | 9.27 (3.76) | 0.426 |
| Digits Forwards | 7.38 (1.31) | 6.12 (1.58) | 0.21 |
| Digits Backwards | 5.19 (1.56) | 3.58 (1.53) | 0.897 |
| CVLT-SF total recall |
19.94 (5.89) | 17.54 (4.42) | 0.440 |
| CVLT-SF immediate |
6 (1.75) | 4.96 (1.8) | 0.784 |
| CVLT-SF delay |
4.69 (2.06) | 3.81 (1.96) | 0.696 |
| CVLT-SF recognition |
5.69 (2.44) | 4.65 (2.45) | 0.461 |
| BNT | 23.88 (2.99) | 19.08 (6.46) | 0.006 |
| α-syn (pg/mL) | 0.1 (0.05) | 0.12 (0.05) | 0.793 |
| Aβ42 (pg/mL) | 16.66 (0.45) | 16.7 (0.59) | 0.669 |
| t-tau (pg/mL) | 22.75 (2.63) | 23.62 (3.63) | 0.162 |
It was worth mentioning why CDR-SB scores were used for the dichotomy of cognitive degeneration. A more quantitative representation of the CDR is provided by the sum of the severity ratings for the six cognitive and functional domains. CDR-SB provides a more quantitative measure of dementia severity than the global CDR. The CDR-SB frequently assesses Alzheimer’s disease progression in clinical research [38,39] and has been used in patients with Parkinson’s disease [40]. Owing to the increased range of values, the CDR-SB offers several advantages over the global score, including increased utility in tracking changes within and between stages of dementia severity. Unlike the other global cognitive testing (i.e., MMSE) in this study, CDR is not influenced by age, education, and gender.
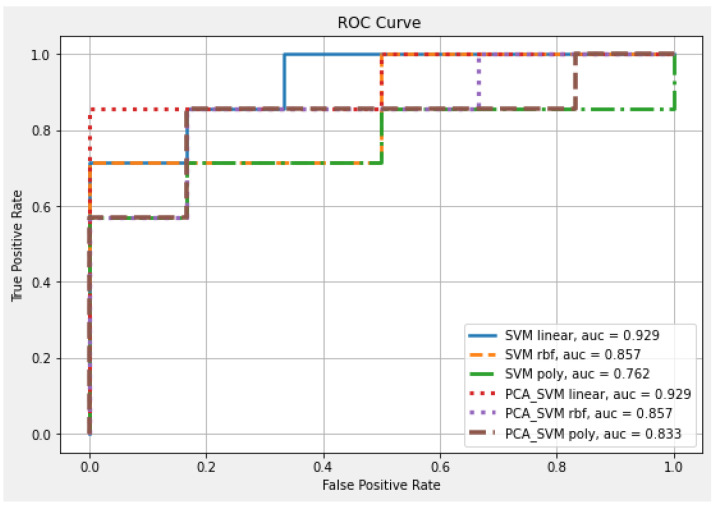
First, all variables (p = 32) were included as feature inputs; 70% of the 42 patients were randomly selected as the training set and 30% as the verification set. Different kernel functions were used to train the SVM and PCA–SVM classification models. The validation accuracy under the full-parameter linear function reached 84.6%, and the AUC was 92.9%. After reducing the dimensionality of the original 32 features using PCA to six features, the accuracy increased to 92.3% for the same AUC rate. After PCA’s dimensionality reduction, the overall forecast confidence improved, as shown in Table 3 and Figure 2.
Table 3.
Thirty-two parameter set to predict CDR-SB deterioration.
| Classifier | Kernel | Feature Number | Accuracy | AUC |
|---|---|---|---|---|
| SVM | Linear | 32 | 0.846 | 0.929 |
| RBF | 0.769 | 0.857 | ||
| Poly | 0.615 | 0.762 | ||
| PCA-SVM | Linear | 6 | 0.923 | 0.929 |
| RBF | 0.769 | 0.857 | ||
| Poly | 0.615 | 0.833 |
Figure 2.
ROC curve and AUC results for each 32-parameter classifier of CDR-SB deterioration.
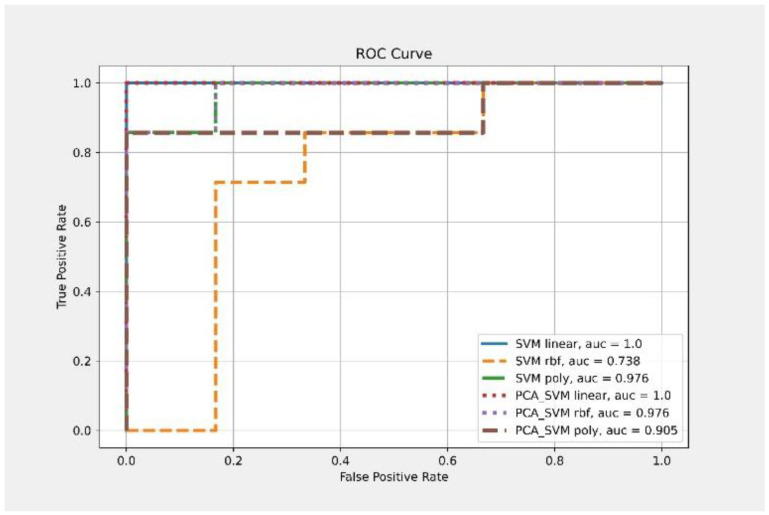
Second, from the above results, it suggested that a set of more concise predictors was possible. The six items (Hoehn–Yahr stage, IADL, Barthel Index, UPDRS I, II, and III) are related to essential motor and non-motor functions in PD patients. They are commonly used as clinical tools to assess PD patients. For the advanced neuropsychological tests, we selected four tests on executive functioning (TMT-B, Verbal fluency, Digits Forwards and Backwards) based on previous research [41,42,43] showing that executive dysfunction was joint in PD, especially early PD. The three biomarkers (α-syn, Aβ42, and t-tau), typically pathognomonic for the pathology of PD and AD, were also included which could predict executive dysfunction and cognitive decline in PD [12,44]. Therefore, a total of condensed 13 parameters were chosen as feature inputs as shown in Table 4. A randomly selected set of 70% of the 42 patients was used to train the prediction model, and the remaining 30% were used to verify the model performance. Different kernel functions were used to train the SVM and PCA–SVM classification models. The validation accuracy under the linear function in the SVM classification model reached 84.6%, and the AUC reached 100%. When reducing the dimensionality of the 13 features using PCA to three features, the accuracy under the linear function significantly improved to 100%. The AUC was maintained at 100%, as shown in Table 5 and Figure 3.
Table 4.
Condensed thirteen parameters as the model predictors.
| Hoehn–Yahr Stage | IADL | Barthel Index |
|---|---|---|
| UPDRS I | UPDRS II | UPDRS III |
| Verbal fluency | Digits Forwards | Digits Backwards |
| TMT-B | α-syn | Aβ42 |
| t-tau |
Table 5.
Thirteen selected parameters to predict CDR-SB deterioration.
| Classifier | Kernel | Feature Number | Accuracy | AUC |
|---|---|---|---|---|
| SVM | Linear | 13 | 0.846 | 1 |
| RBF | 0.538 | 0.738 | ||
| Poly | 0.846 | 0.976 | ||
| PCA-SVM | Linear | 3 | 1 | 1 |
| RBF | 0.923 | 0.976 | ||
| Poly | 0.692 | 0.905 |
Figure 3.
ROC curve and AUC result for each 13-parameter classifier of CDR-SB deterioration.
4. Discussion
In this study, machine learning was used to accurately classify the presence or absence of cognitive disorders in terms of CDR-SB scores in patients with idiopathic PD. In particular, we selected ten parameters related to clinical data and dynamic execution in neuropsychological tests and the three plasma biological indicators shown in Table 4 as the predictors that led to an accuracy rate and AUC for the PCA-SVM model as high as 100%. Therefore, dynamic execution and plasma biometrics are highly relevant for assessing the cognitive ability of PD patients. Compared to the two previously mentioned machine learning models for predicting cognitive degeneration [8,9], the developed PCA-SVM model produced the best prediction accuracy. In addition, literature on the use of the standard clinical assessment tools including neuropsychological tests for PD patients as cognitive predictors in a machine learning was limited and was even rarely seen using plasma biomarkers.
CDR is generally used to determine the severity of a patient’s overall cognitive status, which is time-consuming and requires professional judgment. A patient’s cognitive ability cannot be determined by questionnaires alone. However, only ten questionnaire items from clinical and neuropsychological tests and three plasma biological indicators were needed to train the predictive model through this training model. It is noted that questionnaires can be readily implemented after suitable personnel training and do not necessarily require professional medical persons; thus, it can reduce the time and burden on medical persons.
This study has some limitations. First, this was a cross-sectional study. Longitudinal studies are needed to identify the key indicators that can predict cognitive degeneration in a future time in PD patients, trace these predictors in the different disease stages, and clarify their roles in other cognition domains. Second, the sample size of this study was relatively small because of the need for neuropsychological evaluations and blood tests. Third, there was a lack of a control group of healthy subjects to compare the levels of these indicators. Finally, although all our participants fulfilled the diagnostic criteria of clinically established or probable PD, the possibility of overlapping clinical manifestation and misdiagnosis of progressive supranuclear palsy-parkinsonism predominant type (PSP-P) and postural instability and gait difficulty subtype of PD should be emphasized [45].
For future perspectives, increasing the sample size and conducting a longitudinal study are among the priorities. Specifically, increasing the sample size can further validate and support the developed model’s performance in view of the small sample size in this study. Conducting longitudinal study can identify the key indicators and help develop a prediction model that can predict cognitive degeneration in a future time in PD patients, which is extremely important for medical experts to quickly identify a patient’s cognitive condition and provide treatment in advance.
Acknowledgments
The authors would like to thank all the participants and the research assistants, Shu-Ju Shiao, Wen-Chun Wu, and Ya-Yuan Yang for participating in the assessment.
Author Contributions
Study conception and design: P.-H.C., F.-Y.C. and J.-S.S. Material preparation, data collection and analysis: P.-H.C., F.-Y.C. and T.-Y.H. Reviewing and supervision: P.-H.C. and J.-S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Mackay Memorial Hospital in Taiwan (IRB Number: 18MMHIS152).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to the privacy of participants.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Taipei University of Technology, Mackay Medical College, and MacKay Memorial Hospital (NTUT-MMH-109-07, MMH-MM-10811, MMH-TT-10905).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B., Pillon B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1997;244:2–8. doi: 10.1007/PL00007725. [DOI] [PubMed] [Google Scholar]
- 3.Caballol N., Marti M.J., Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov. Disord. 2007;22((Suppl. 17)):S358–S366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- 4.Saredakis D., Collins-Praino L.E., Gutteridge D.S., Stephan B.C.M., Keage H.A.D. Conversion to MCI and dementia in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2019;65:20–31. doi: 10.1016/j.parkreldis.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Kalia L.V. Biomarkers for cognitive dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2018;46:S19–S23. doi: 10.1016/j.parkreldis.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Vitale A., Villa R., Ugga L., Romeo V., Stanzione A., Cuocolo R. Artificial intelligence applied to neuroimaging data in Parkinsonian syndromes: Actuality and expectations. Math. Biosci. Eng. 2021;18:1753–1773. doi: 10.3934/mbe.2021091. [DOI] [PubMed] [Google Scholar]
- 7.Jones J.D., Kuhna T.P., Szymkowiczb S.M. Reverters from PD-MCI to cognitively intact are at risk for future cognitive impairment: Analysis of the PPMI cohort. Parkinsonism Relat. Disord. 2018;47:3–7. doi: 10.1016/j.parkreldis.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales D.A., Vives-Gilabert Y., Gómez-Ansón B., Bengoetxea E., Larrañaga P., Bielza C., Pagonabarraga J., Kulisevsky J., Corcuera-Solano I., Delfino M. Predicting dementia development in Parkinson’s disease using Bayesian network classifiers. Psychiatry Res. 2013;213:92–98. doi: 10.1016/j.pscychresns.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Berlyand Y., Weintraub D., Xie S.X., Mellis I.A., Doshi J., Rick J., McBride J., Davatzikos C., Shaw L.M., Hurtig H., et al. An Alzheimer’s disease-derived biomarker signature identifies Parkinson’s disease patients with dementia. PLoS ONE. 2016;11:e0147319. doi: 10.1371/journal.pone.0147319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C.W., Yang S.Y., Yang C.C., Chang C.W., Wu Y.R. Plasma and serum alpha-synuclein as a biomarker of diagnosis in patients with Parkinson’s disease. Front. Neurol. 2019;10:1388. doi: 10.3389/fneur.2019.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C.H., Lee B.C., Lin C.H. Integrated plasma and neuroimaging biomarkers associated with motor and cognition severity in Parkinson’s disease. J. Parkinsons Dis. 2020;10:77–88. doi: 10.3233/JPD-191766. [DOI] [PubMed] [Google Scholar]
- 12.Lin W.T., Shaw J.S., Cheng F.Y., Chen P.H. Plasma total tau predicts executive dysfunction in Parkinson’s disease. Acta Neurol. Scand. 2022;145:30–37. doi: 10.1111/ane.13517. [DOI] [PubMed] [Google Scholar]
- 13.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 14.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn L., Mathkour M., Lee S.X., Gouveia E.E., Hanna J.A., Garces J., Scullen T., McCormack E., Riffle J., Glynn R., et al. Long-term outcomes of deep brain stimulation in severe Parkinson’s disease utilizing UPDRS III and modified Hoehn and Yahr as a severity scale. Clin. Neurol. Neurosurg. 2019;179:67–73. doi: 10.1016/j.clineuro.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Siciliano M., Micco R.D., Trojano L., Stefano M.D., Baiano C., Passaniti C., Mase A.D., Russo A., Tedeschi G., Tessitore A. Cognitive impairment is associated with Hoehn and Yahr stages in early, de novo Parkinson disease patients. Parkinsonism Relat. Disord. 2017;41:86–91. doi: 10.1016/j.parkreldis.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Winser S.J., Kannan P., Bello U.M., Whitney S. Measures of balance and falls risk prediction in people with Parkinson’s disease: A systematic review of psychometric properties. Clin. Rehabil. 2019;33:1949–1962. doi: 10.1177/0269215519877498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangarapillai K., Norman B.M., Almeida Q.J. Boxing vs sensory exercise for Parkinson’s disease: A double-blinded randomized controlled trial. Neurorehabil. Neural. Repair. 2021;35:769–777. doi: 10.1177/15459683211023197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polo-Morales A., Alcocer-Salas Á., Rodríguez-Violante M., Pinto-Solís D., Solís-Vivanco R., Cervantes-Arriaga A. Association between somatization and nonmotor symptoms severity in people with Parkinson disease. J. Geriatr. Psychiatry Neurol. 2021;34:60–65. doi: 10.1177/0891988720901787. [DOI] [PubMed] [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Lynch C.A., Walsh C., Blanco A., Moran M., Coen R.F., Walsh J.B., Lawlor B.A. The Clinical Dementia Rating Sum of Box Score in mild dementia. Dement. Geriatr. Cogn. Disord. 2006;21:40–43. doi: 10.1159/000089218. [DOI] [PubMed] [Google Scholar]
- 22.Dennis N., Michael P.A.P., Jane H. Learning nonwords: The Hebb repetition effect as a model of word learning. Memory. 2018;26:852–857. doi: 10.1080/09658211.2017.1416639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods S.P., Delis D.C., Scott J.C., Kramer J.H., Holdnack J.A. The California Verbal Learning Test—Second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch. Clin. Neuropsychol. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Nevado A., Río D.D., Martín-Aragoneses M.T., Prados J.A., López-Higes R. Preserved semantic categorical organization in mild cognitive impairment: A network analysis of verbal fluency. Neuropsychologia. 2021;157:107875-1–107875-8. doi: 10.1016/j.neuropsychologia.2021.107875. [DOI] [PubMed] [Google Scholar]
- 25.Tallberg I.M. The Boston Naming Test in Swedish: Normative data. Brain Lang. 2005;94:19–31. doi: 10.1016/j.bandl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Nascimento Silva R.D., Afonso S.V., Felipe L.R., Oliveira R.A., Martins L.J.P., Souza L.A.P.S.D. Dual-task intervention based on trail making test: Effects on Parkinson’s disease. J. Bodyw. Mov. 2021;27:628–633. doi: 10.1016/j.jbmt.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Qualls C.E., Bliwise N.G., Stringer A.Y. Short forms of The Benton Judgment of Line Orientation Test: Development and psychometric properties. Arch. Clin. Neuropsychol. 2000;1:159–163. [PubMed] [Google Scholar]
- 28.Chen P.H., Lien C.W., Wu W.C., Lee L.S., Shaw J.S. Gait-based machine learning for classifying patients with different types of mild cognitive impairment. J. Med. Syst. 2020;44:1–6. doi: 10.1007/s10916-020-01578-7. [DOI] [PubMed] [Google Scholar]
- 29.Jain S., Shukla S., Wadhvani R. Dynamic selection of normalization techniques using data complexity measures. Expert Syst. Appl. 2018;106:252–262. doi: 10.1016/j.eswa.2018.04.008. [DOI] [Google Scholar]
- 30.Singh D., Singh B. Investigating the impact of data normalization on classification performance. Appl. Soft Comput. 2020;97:105524-1–105524-23. doi: 10.1016/j.asoc.2019.105524. [DOI] [Google Scholar]
- 31.Liu Z., Xu H. Kernel parameter selection for support vector machine classification. J. Algorithm Comput. Technol. 2014;8:163–177. doi: 10.1260/1748-3018.8.2.163. [DOI] [Google Scholar]
- 32.Gu X., Chen C., Yang Y., Miao X., Yao B. Reliability prediction of further transit service based on support vector machine. Meas Control. 2020;54:845–855. doi: 10.1177/0020294019858101. [DOI] [Google Scholar]
- 33.Yu Y., Shao M., Jiang L., Ke Y., Wei D., Zhang D., Jiang M., Yang Y. Quantitative analysis of multiple components based on support vector machine (SVM) Optik. 2021;237:166759-1–166759-6. doi: 10.1016/j.ijleo.2021.166759. [DOI] [Google Scholar]
- 34.Ali A.K., Erçelebi E. Automatic modulation classification using different neural network and PCA combinations. Expert Syst. Appl. 2021;178:114931-1–114931-17. doi: 10.1016/j.eswa.2021.114931. [DOI] [Google Scholar]
- 35.Qaraei M., Abbaasi S., Ghiasi-Shirazi K. Randomized nonlinear PCA networks. Inf. Sci. 2021;545:241–253. doi: 10.1016/j.ins.2020.08.005. [DOI] [Google Scholar]
- 36.Zhang J., Zhou D., Chen M. Monitoring multimode processes: A modified PCA algorithm with continual learning ability. J. Process. Control. 2021;103:76–86. doi: 10.1016/j.jprocont.2021.05.007. [DOI] [Google Scholar]
- 37.Mandrekar J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 38.Williams M.M., Storandt M., Roe C.M., Morris J.C. Progression of Alzheimer’s disease as measured by Clinical Dementing Rating Sum of Boxes scores. Alzheimers Dement. 2013;9:S39–S44. doi: 10.1016/j.jalz.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samtani M.N., Raghavan N., Novak G., Nandy P., Narayan V.A. Disease progression model for Clinical Dementia Rating-Sum of Boxes in mild cognitive impairment and Alzheimer’s subjects from the Alzheimer’s Disease Neuroimaging Initiative. Neuropsychiatr. Dis. Treat. 2014;10:929–952. doi: 10.2147/NDT.S62323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyman-Chick K.A., Scott B.J. Development of clinical dementia rating scale cut-off scores for patients with parkinson’s disease. Mov. Disord. Clin. Pract. 2015;2:243–248. doi: 10.1002/mdc3.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy G., Jacobs D.M., Tang M.X., Côté L.J., Louis E.D., Alfaro B., Mejia H., Stern Y., Marder K. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov. Disord. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 42.Kudlicka A., Clare L., Hindle J.V. Executive functions in Parkinson’s disease: Systematic review and meta-analysis. Mov. Disord. 2011;26:2305–2315. doi: 10.1002/mds.23868. [DOI] [PubMed] [Google Scholar]
- 43.Lange F., Brückner C., Knebel A., Seer C., Kopp B. Executive dysfunction in Parkinson’s disease: A meta-analysis on the Wisconsin Card Sorting Test literature. Neurosci. Biobehav. Rev. 2018;93:38–56. doi: 10.1016/j.neubiorev.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Cholerton B., Shi M., Ginghina C., Cain K.C., Auinger P., Parkinson Study Group DATATOP Investigators. Zhang J. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat. Disord. 2015;21:271–276. doi: 10.1016/j.parkreldis.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alster P., Madetko N., Koziorowski D., Frieman A. Progressive Supranuclear Palsy—Parkinsonism Predominant (PSP-P)—A clinical challenge at the boundaries of PSP and Parkinson’s Disease (PD) Front. Neurol. 2020;11:180. doi: 10.3389/fneur.2020.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to the privacy of participants.