Abstract
Dysphagia is a common symptom with significant impact on quality of life. Our diagnostic armamentarium was primarily limited to endoscopy and barium esophagram until the advent of manometric techniques in the 1970s, which provided the first reliable tool for assessment of esophageal motor function. Since that time, significant advances have been made over the last 3 decades in our understanding of various esophageal motility disorders due to improvement in diagnostics with high resolution esophageal manometry (HRM). HRM has improved the sensitivity for detecting achalasia and has also enhanced our understanding of spastic and hypomotility disorders of the esophageal body. In this review, we discuss the current approach to diagnosis and therapeutics of various esophageal motility disorders.
INTRODUCTION
The esophagus is a complex muscular tube that uses coordinated peristalsis and deglutitive relaxation of the upper and lower esophageal sphincter to transport bolus from the pharynx into the stomach. Disruption in either the peristalsis of esophageal body or deglutitive relaxation of the lower esophageal sphincter can lead to obstructive symptoms, which include dysphagia, non-cardiac chest pain, and regurgitation. Based on population-based surveys, nearly half of the US population might have esophageal symptoms including heartburn, regurgitation, or dysphagia.1, 2 Classically, dysphagia to solids raises suspicion for a primary mechanical pathology, while dysphagia to liquids with or without dysphagia to solids is more suggestive of an esophageal motility disorder. Upper endoscopy with biopsy is recommended first line diagnostic test for esophageal dysphagia, with esophageal motility testing as the next step in evaluation if a mechanical or mucosal source of dysphagia (such as infectious, pill, reflux, or eosinophilic esophagitis) have been ruled out.3
Chest pain is frequently attributed to “esophageal spasm” by medical providers in clinical practice, but it is important to recognize that only 1–2% of patients undergoing esophageal manometry have findings of a spastic disorder.4, 5 On the other hand, one person dies every 36 seconds in the United States from coronary artery disease leading to overall, 1 in every 4 deaths.6 The prevalence of gastroesophageal reflux disease is also significantly higher compared to spastic motor disorders affecting 1 in 3 persons in the United States.1 Hence, in patients with chest pain as the solo symptom, thorough work-up for cardiac etiology followed by reflux should precede any consideration of esophageal dysmotility.
Our diagnostic armamentarium was primarily limited to endoscopy and barium esophagram until the advent of manometric techniques in the 1970s, which provided the first reliable tool for assessment of esophageal motor function.7 Subsequently, in the 1990s, Ray Clouse and his colleagues developed high-resolution manometry (HRM), which revolutionized motility testing by giving us a complete spatial and temporal depiction of esophageal motor function.8, 9 Over time, HRM testing has become the gold standard in evaluation of patients with suspected motility disorders of the esophagus. In this review, we discuss the current approach to diagnosis and therapeutics of various esophageal motility disorders. We start with review of the diagnostic tools followed by evaluation and management of disorders of esophagogastric junction and disorders of esophageal peristalsis. Lastly, we briefly discuss impact of esophageal mucosal diseases, systemic disease, medications, and altered foregut anatomy on esophageal motility.
DIAGNOSTIC TOOLS
Endoscopy
Esophagogastroduodenoscopy (EGD) is an requisite in the evaluation of patients with suspected esophageal dysmotility as it can enable direct mucosal visualization and exclude benign (peptic stricture, hiatal hernia, Schatzki’s ring, eosinophilic esophagitis, altered foregut anatomy) or malignant conditions that can lead to secondary motility abnormalities. Endoscopy also allows delivery of therapeutics with esophageal dilation when a stricture is encountered. Findings of retained saliva or liquid in the esophageal lumen along with puckered lower esophageal sphincter and longitudinal superficial wrinkles of esophageal mucosa (pinstripe mucosa) are suggestive of a major esophageal motility disorder and found in 41–94% of patients with achalasia.10 In this scenario, careful evaluation of gastroesophageal junction, gastric fundus, and gastric cardia should be performed to exclude malignancy that can cause pseudo-achalasia. Table 1 shows the various diagnostic tests and how they can provide adjunctive evidence to esophageal manometry testing in evaluation of patients with suspected esophageal motility disorder. It is important to recognize that a clinical diagnosis depends on information obtained from various tests combined with patient symptom presentations which provide complimentary data in completing the diagnostic puzzle.
Table 1:
Adjunctive diagnostic tests to conventional HRM available for evaluation of esophageal motility disorder.
| Diagnostic test | Protocol | Supportive Evidence |
|---|---|---|
| Endoscopy | -Careful attention to esophageal diameter, LES tone, retention of liquid/saliva on insertion | -Puckered GE junction with resistance to passage of scope with retained liquid in the esophagus is supportive of obstructive process such as achalasia. -Presence of “foam” in the esophagus suggest dysmotility but is not specific for a manometric diagnosis. |
| FLIP | -Placed transorally with balloon traversing the EGJ -Volumetric distention of the balloon to pre-determined volumes based on catheter type |
-EGJ distensibility index of < 2.0 mm2/mmHg is abnormal -EGJ diameter of < 13mm is likely abnormal -Role in various manometric diagnoses is evolving. |
| Timed barium esophagram with barium tablet (13mm) | -Perform in upright position using 8 oz or 236mL of barium. Evaluate barium height at 1, 2, and 5 minutes |
-Barium column height to support outflow obstruction (such as achalasia) >5 cm at 1 minute >2 cm at 5 minutes -13mm barium tablet retention supports obstructive process -3% improvement (decrease) in pre- to post-treatment barium height at 5 minutes might predict long term clinical remission in achalasia -Corkscrew appearance may be suggestive of distal esophageal spasm |
| Multiple rapid swallows (HRM) | -Five swallows of 2-mL liquid at 2–3 second intervals | Absence of esophageal body contractility (DCI <100 mmHg•s•cm) with complete deglutitive inhibition of LES during MRS. - Augmentation (peristaltic reserve) present if post-MRS esophageal body peristaltic contraction is normal (DCI >450 mmHg•s•cm) and any of three post contractions with increased contractile vigor (DCI > single swallow mean DCI) |
| Rapid drinking challenge (HRM) | -Rapid drink of 200ml of liquid | Absence of esophageal body contractility (DCI <100 mmHg•s•cm) with complete deglutitive inhibition of LES during RDC. - IRP >12mmHg (Medtronic software) and pan-esophageal pressurization (>20mmHg) are supportive of outflow obstruction |
Functional Lumen Imaging Probe (FLIP)
Functional Lumen Imaging Probe (FLIP) is the newest FDA approved diagnostic tool to assess esophageal physiology and is still in early clinical phases. The FLIP catheter includes a distensible balloon encasing multiple pairs of impedance sensors and a single distal pressure sensor. During a sedated upper GI endoscopy, the FLIP catheter is placed transorally such that the balloon traverses the EGJ. Then the balloon is distended with a conductive fluid through a mechanical pump. The FLIP system converts the readings into a 3D rendering of the esophageal lumen in real time and measures the relationship between the cross-sectional area (mm2) over the distensive pressure to generate a luminal distensibility.11 12 The most recent FLIP version 2.0 or FLIP topography converts the readings to color-coded luminal diameter plots enabling evaluation of distensibility across the EGJ as well as contractile response to distension in the esophageal body. Data from healthy volunteers suggests that a normal EGJ distensibility index is > 2.8mm2/mmHg and normal EGJ diameter is > 13mm.13 Further, the presence of repetitive antegrade contractions is considered a normal response to distension.14 On the other hand, reduced EGJ distensibility index and/or diameter is often seen in patients with disorders of EGJ outflow such as achalasia or EGJOO. EGJ-DI of <2 mm2/mmHg is considered to definitely abnormal, while EGJ diameter of <13mm is likely abnormal and can serve as a supportive measure when EGJ-DI is indeterminate (2–3 mm2/mmHg).13
While HRM remains the gold standard for motility testing, FLIP is recommended in recent guidelines as a complimentary tool to HRM in instances of manometric EGJOO or other inconclusive patterns.3 FLIP has been shown to predict treatment outcomes and have a role in tailoring foregut interventions such as POEM or LHM.15, 16 In a study of 143 patients undergoing POEM, intraoperative use of FLIP during POEM resulted in additional real-time myotomy in 65% of cases and improved clinical outcomes compared to scenarios when intraoperative FLIP was not used.17 Studies have also identified that FLIP performs superiorly to HRM in evaluation of bolus emptying against the gold standard of barium esophagram.18 Therefore, while the role of a FLIP as a first-line tool for clinical evaluation of esophageal motility is evolving, it’s role as a supportive test to HRM as well as monitoring post-treatment outcomes is increasingly appreciated.
High resolution esophageal manometry
High-resolution manometry (HRM) is the current gold-standard to assess for esophageal motor dysfunction. HRM combined with impedance sensors is recommended if available given the added ability to assess intrabolus pressure and for bolus transit in relation to manometric properties. While commercially available HRM systems provide similar information, technological differences can influence measurements and normative values. Figure 1 depicts manometric classification of various esophageal motility disorders based on Chicago Classification version 4.0 (CCv4.0).19
Figure 1:
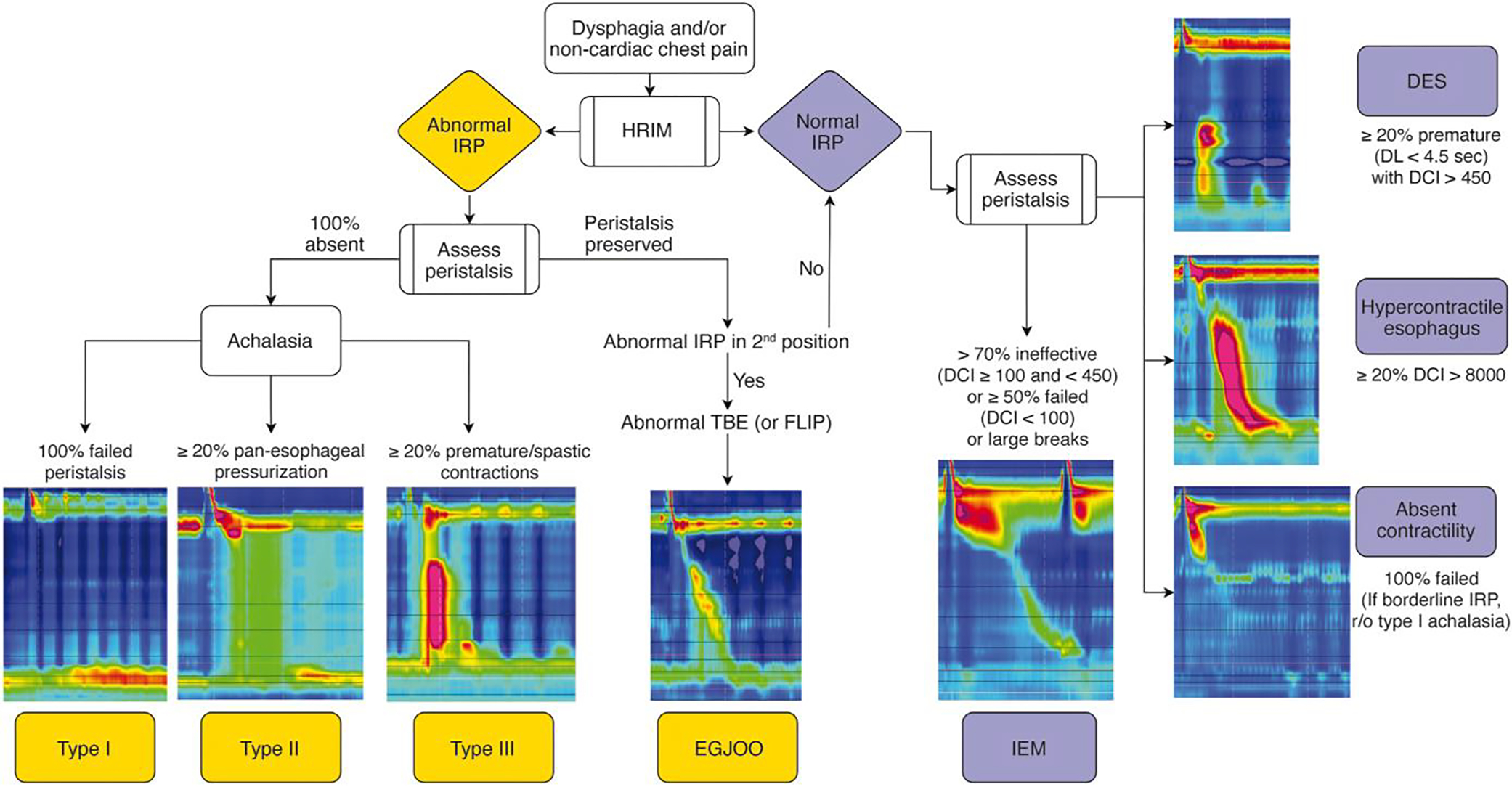
Manometric classification of various esophageal motility disorders based on Chicago Classification v4.0. HRIM (High resolution impedance manometry); IRP (Integrated relaxation pressure); DCI (distal contractile integral); DES (distal esophageal spasm); IEM (ineffective esophageal motility); TBE (timed barium esophagram); FLIP (functional luminal imaging probe).
HRM Protocol
The recent publication of CC v4.0 introduced a recommended HRM protocol to standardize the HRM procedure across motility labs.19 Historically, HRM protocols varied with regards to patient position, number of test swallows, and inclusion of provocative maneuvers. Standardization of the protocol was needed in order to optimize generalizability and reliability of HRM interpretation. According to the CC v4.0 protocol, following HRM catheter placement, the study can begin in either the supine or upright position.20 A minimum of 60 seconds of quiet rest allows for an adaptation period, followed by a minimum of three deep inspirations to confirm catheter placement, and subsequently a baseline period of at least 30 seconds to enable identification of anatomic landmarks including the upper esophageal sphincter (UES), lower esophageal sphincter (LES), respiratory inversion point (RIP), and basal EGJ pressure. A series of ten 5ml single wet swallows are then performed. In equivocal cases, the patient is then transitioned from supine to upright or upright to supine position, again with a 60 second adaptation period, three deep inspirations, and a 30 second baseline period, followed by a series of five single wet swallows. The recommendation for data acquisition in both supine and upright position has been shown to increase diagnostic yield of esophageal motility disorders, as well as uncover borderline or discordant findings that require further investigation.21, 22 Thus, unless the diagnosis is clear cut, positional changes may help in identifying any underlying motility disorders.
HRM metrics
The key HRM metrics that help with interpretation include assessment of deglutitive relaxation across the LES using integrated relaxation pressure (IRP) and metrics of esophageal body peristalsis based on contraction vigor (distal contractile integral; DCI) and latency of deglutitive inhibition (distal latency; DL).
Integrated relaxation pressure (IRP):
IRP assesses the adequacy of swallow-induced LES relaxation and is defined by the median 4-second nadir of esophagogastric junction pressure in the 10-second post swallow period.23 Normal median IRP suggest adequate relaxation of the lower esophageal sphincter to deglutition and rules out disorders of esophagogastric junction. Normative values for IRP differ based on patient position and type of software system used. Median supine IRP of <15 mmHg and upright of <12 mmHg is considered normal for Medtronic systems.24–26 On the other hand, median supine IRP of <22 mmHg and upright of <15 mmHg is considered normal for Laborie/Diversatek systems.19, 22, 25
Distal contractile integral (DCI):
DCI is a three-dimensional metric to assess the contraction vigor of esophageal smooth muscle, taking length, amplitude, and duration of contraction into consideration. It can help differentiate between normal (DCI of 450 mmHg•s•cm to 8000 mmHg•s•cm), hypercontractile swallow (DCI >8000 mmHg•s•cm), weak contraction (DCI 100 mmHg•s•cm and <450 mmHg•s•cm), and failed peristalsis (DCI <100 mmHg•s•cm).
Distal latency (DL):
DL measures timing of peristalsis based on the interval between upper esophageal sphincter relaxation to contractile deceleration point of the peristaltic wave in the distal esophagus. It can help differentiate between a premature (DL <4.5 seconds) versus peristaltic contraction.
Provocative maneuvers such as multiple rapid swallow (MRS) sequences in the supine position and a rapid drink challenge (RDC) in the upright position might increase diagnostic sensitivity and specificity of HRM studies. The recently updated CC v 4.0 includes these maneuvers in the standardized protocol for all patients undergoing HRM study as they can provide supportive data regarding outflow obstruction across the EGJ and provide an assessment of peristaltic reserve in the setting of IEM. Of note, if time and resources constrain the ability to conduct the recommended HRM protocol, clinicians can modify the protocol as long as normative values are applied and tests used appropriately. It is important to highlight that the goal of HRM protocol is to acquire sufficient information to provide a conclusive diagnosis that explains patient symptoms and guide management. Hence, applicability of these tests should be individualized to every patient and further studies are needed to determine if universal application of these maneuvers lead to change in clinician outcomes.
Multiple Rapid Swallows (MRS)
During the MRS, if needed, sequence 2ml of fluid in sequence of five swallows, each separated by a few seconds, is administered. An intact response to MRS is defined as absence of esophageal body contractility (DCI < 100 mmHg•s•cm) with complete deglutitive inhibition of the LES during the repetitive swallows, with an augmented post-MRS contraction. Augmentation is present if the DCI is in the normal range (i.e., DCI >450 mmHg•s•cm) and any of three post-contractions have increased contractile vigor compared to the mean DCI from 10 single water swallows in the same position (ratio >1). In clinical practice, this provocative test might help assess peristaltic reserve in patients with ineffective esophageal motility who are undergoing evaluation for anti-reflux surgery.27, 28 The absence of an augmented contraction following MRS in esophageal studies performed is associated with increased likelihood of post-operative dysphagia in patients referred for consideration of anti-reflux surgery.28–30
Rapid Drink Challenge (RDC)
The RDC is administered, if needed, in the upright position with intake of 200ml waters in a series of rapid water swallows without stopping. An intact response to RDC is defined as absence of esophageal body contractility (DCI < 100 mmHg•s•cm) with complete deglutitive inhibition of the LES during the RDC. When assessing response to RDC, IRP >12mmHg (using Medtronic software) and panesophageal pressurization >20mmHg are criteria for outflow obstruction.31–35 Similar to MRS, RDC is not necessary in majority of cases, but might help in patients with suspected EGJ outflow obstruction and achalasia with inconclusive or discordant findings with single wet swallows. Lastly, additional manometric maneuvers such as solid test meals and post-prandial high-resolution impedance manometry are currently being studied and do not currently have a routine role outside of specialized motility centers.21, 36–39
Barium Esophagram
Radiographic imaging with ingested barium contrast to assess bolus transport through the esophagus and into the gastric lumen has remained an important adjunctive diagnostic modality in patients with dysphagia. It can identify any structural lesions such as strictures, neoplasms, or hiatal hernia, but can also identify some major motility disorders such as achalasia and distal esophageal spasm. However, the overall sensitivity of barium esophagram for motility disorders is fairly limited ranging from 56 to 69%.40, 41 Addition of 13mm barium tablet to the esophagram protocol along with evaluation of esophageal emptying (timed barium esophagram/TBE) at 1, 2, and 5 minutes can increase sensitivity and also be used to monitor treatment response in disorders of esophagogastric junction outflow obstruction.42, 43 It can also show classic findings of dilated esophagus with a “bird’s beak” narrowing distally due to hypertonic lower esophageal sphincter in patients with achalasia. A barium column empties completely in most normal subjects at 1 minute and in all by 5 minutes.44 Hence, barium column height of >5cm at 1 minute and >2 cm at 5 minutes should be suggestive of esophagogastric junction outflow obstruction and can provide valuable complimentary information in patients with manometric diagnosis of EGJOO.45 TBE can also help with pre-to post-achalasia treatment monitoring as a 3% improvement in pre- and post-treatment barium height at 5 minutes rather than absolute cutoff value of <5cm on post-treatment TBS might be a better indicator of long term clinical remission.46
DISORDERS OF ESOPHAGOGASTRIC JUNCTION OUTFLOW
Achalasia
Achalasia represents the classic esophageal motility disorder thought to arise from a selective loss of inhibitory neurons in the myenteric plexus of the distal esophagus and LES resulting in a neuronal imbalance with unopposed excitatory activity and localized decrease of inhibitory activity, resulting in failure of LES relaxation and disrupted esophageal peristalsis. 47–49. Typical symptoms of achalasia include progressive dysphagia to solids and liquids, regurgitation, chest pain, heartburn, and weight loss 50, 51. A first step in evaluation of suspected achalasia is upper GI endoscopy to rule out mechanical source of symptoms such as esophageal strictures or pseudoachalasia. Endoscopic findings in achalasia include retained saliva with puckered gastro-esophageal junction as well as esophagitis from effects of stasis and/or candidiasis. In type 1 or type 2 achalasia absence of peristalsis and/or dilation of esophageal lumen is often appreciated, and in type 3 achalasia an increased presence of peristalsis and/or corkscrewing of esophageal lumen is seen.52
HRM is the gold standard to diagnose and sub-type achalasia. All three sub-types require impaired LES relaxation demonstrated by an elevated median IRP. There are three manometric sub-types of achalasia. Type I achalasia is considered the classic presentation of achalasia, and typically a later state of disease progression.53 On HRM type I achalasia is diagnosed when the median IRP across the LES is elevated and 100% of single wet swallows are failed (DCI < 100mmHg•s•cm), and with less than 20% of swallows exhibiting panesophageal pressurization. Type II achalasia is considered an earlier stage of disease and carries the most favorable prognosis to treatments.54 On HRM type II achalasia is diagnosed when the median IRP is elevated, 100% of single wet swallows are failed, and 20% or more of swallows exhibit panesophageal pressurization.53 Type III achalasia is akin to spastic achalasia, and may reflect a different pathophysiologic consequence than the other subtypes, with less evidence of progressive neuronal cell loss of the myenteric ganglion cells of the distal esophagus and LES.53, 55 On HRM the median IRP is elevated and at least 20% of swallows are premature or spastic, defined as a distal latency (DL) <4.5 seconds in setting of a DCI ≥450 mmHg•s•cm, with all other swallows failed.56, 57
At times, the HRM findings and patient symptoms may or may not align in which case complimentary tests such as timed barium esophagram could serve as an important complimentary diagnostic tool for achalasia, where retained barium at 1-, 2-, and 5 minutes post barium ingestion and birds beaking are supportive of achalasia. If available, FLIP may also be another complimentary diagnostic tool for achalasia, where a reduced distensibility index and/or diameter across the EGJ is supportive of the diagnosis. Endoscopic ultrasound or cross-sectional imaging may be considered in patients with potential for pseudoachalasia, and particularly in elderly patients with a significant short-term weight loss and suspicion for achalasia.
Treatment options:
First-Line Treatment Options for Achalasia
Three first-line treatment options are available for patients with achalasia that are appropriate candidates. These include pneumatic dilation (PD), surgical laparoscopic Heller myotomy (LHM), and per-oral endoscopic myotomy (POEM).
POEM is one of the more recent therapeutic option available for patients with achalasia with very high clinical success rates of up to 90% response at 5 to 7 years.58 Due to ability of POEM to endoscopically extend the myotomy in the proximal esophagus and target areas of esophageal spasticity, POEM is the preferred first-line treatment option in patients with type III achalasia, with superior response rates (93%) compared to LHM (71%).59 Overall rates of reflux esophagitis are higher in POEM (44%) compared to 29% with LHM (with partial fundoplication) at 2 years, but rates of severe esophagitis (Los Angeles Classification grade C or D) are comparable between POEM and LHM as demonstrated in a head-to-head randomized controlled trial.60 Since POEM is not classically performed with an anti-reflux intervention, it is important to discuss higher potential risk of reflux with POEM and potential need for lifelong acid suppression with patients.52, 61
Pneumatic dilation (PD) is performed with a nonradiopaque graded size polyethylene balloon (RigiFlex), which comes in 3 sizes (3.0, 3.5, and 4.0 cm) and are often used in graded fashion. This procedure can be performed with or without fluoroscopy.62 There is also a novel 30-mm hydrostatic balloon dilator (EsoFLIP) that is available and uses impedance planimetry to provide live real-time feedback during endoscopy on esophageal luminal diameter without the need for fluoroscopy, but use is limited due to availability of only 30mm size balloon.63 PD is overall safe with risk of perforation being 1.0% with 30mm balloon.64 The risk of perforation is higher at 3.2% when 35mm balloon is used for initial dilation, whereas risk of perforation is lower at 0.97% when graded dilation is utilized with long term success rate of 90% at 6 months and 44% at 6 years.64, 65 Predictors of favorable clinical response to PD include older age (>45 years), female sex, narrow (nondilated) esophagus, and type II achalasia.65 Hence, most patients undergoing PD should undergo initial dilation using the 30mm balloon, followed by symptomatic and objective assessment in 4–6 weeks.52 Endoscopic visualization of the post dilation tear should be performed to rule out a perforation and patients should be monitored for any signs or symptoms in the recovery area. There is no role for routine esophagram after PD unless there is clinical suspicion for perforation.52 In those who continue to be symptomatic, graded PD with the next size dilator should be performed and/or POEM or LHM can be offered.
Laparoscopic heller myotomy (LHM) performed with or without partial fundoplication (Dor or Toupet) also provides excellent symptom relief, with efficacy rates ranging from 88% to 95% and lasting 6 to 10 years.66, 67 The most common side effect after surgical myotomy is the development of GERD and partial fundoplication can reduce the incidence from 41.5% to 14.5%.68 Hence, most recent ACG guidelines recommend doing either Dor or Toupet fundoplication to control esophageal acid exposure in patients with achalasia undergoing surgical myotomy.52 Predictors of favorable outcomes with LHM including younger men, tortuous esophagus, and an esophageal diverticulum. Furthermore, similar to PD, patients with type II achalasia respond better (93%) than patients with type I (81%) or III (86%).69 When comparing outcomes of POEM versus LHM, clinical success rates were similar at 2 years (83% with POEM compared to 81.7% with LHM), but rates of reflux esophagitis was higher in patients who underwent POEM (44%) compared to LHM with Dor fundoplication (29%).60
Pharmacologic options
Therapeutic options for achalasia have evolved significantly over the last decade. Oral pharmacologic therapy is the least effective treatment option and currently, has a minimal role in management of patients with achalasia. Two of the most common medications that have been previously studied in achalasia include calcium channel blocker (sub-lingual nifedipine 10–30 mg before meals)70 and nitrates (sublingual isosorbide dinitrate 5mg before meals).71 However, effectiveness varies and patients often develop significant side effects including headaches, hypotension, peripheral edema, and tachyphylaxis, which results in loss of response to these medications after short-term use.72, 73 Due to availability of significantly more effective and durable treatment options, oral pharmacologic therapy should only be considered in very few select patients with achalasia as a bridge to more effective therapy or those who have failed botulinum toxin injections and are not candidates for any modes of myotomy.
Endoscopic botulinum toxin injection should be considered as the primary treatment option in patients who are not candidate for pneumatic dilation or myotomy due to significant comorbidities. During an upper endoscopy, 100 units of botulinum toxin is injected into the lower esophageal sphincter in four quadrants. Symptom relief is reported in 79% of patients at 30 days, 70% at 3 months, 53% at 6 months, and 41% at 12 months.68 Hence, botulinum toxin can provide effective initial treatment results, but the benefit dissipates over time and nearly half of the patients might need repeat injections for symptom relief at 6 months.
Choosing the correct type of intervention
We suggest shared decision making with patients regarding treatment options available for achalasia and reviewing potential risks and benefits. Graded pneumatic dilation, LHM, and POEM are the first line treatment options with all having equal efficacy for type I or type II achalasia, but POEM and LHM are both more invasive procedures with higher rates of post-myotomy GERD.52 In patients with achalasia and a medium to large hiatal hernia, LHM with partial fundoplication is preferred to correct the hiatus defect. In patients with type III achalasia, POEM is preferred over LHM due to ability to perform a tailored longer myotomy. In patients who are not candidates for either of the three definitive treatment options, endoscopic botulinum toxin injection is preferred. Figure 2 shows the various treatment options for achalasia.
Figure 2:
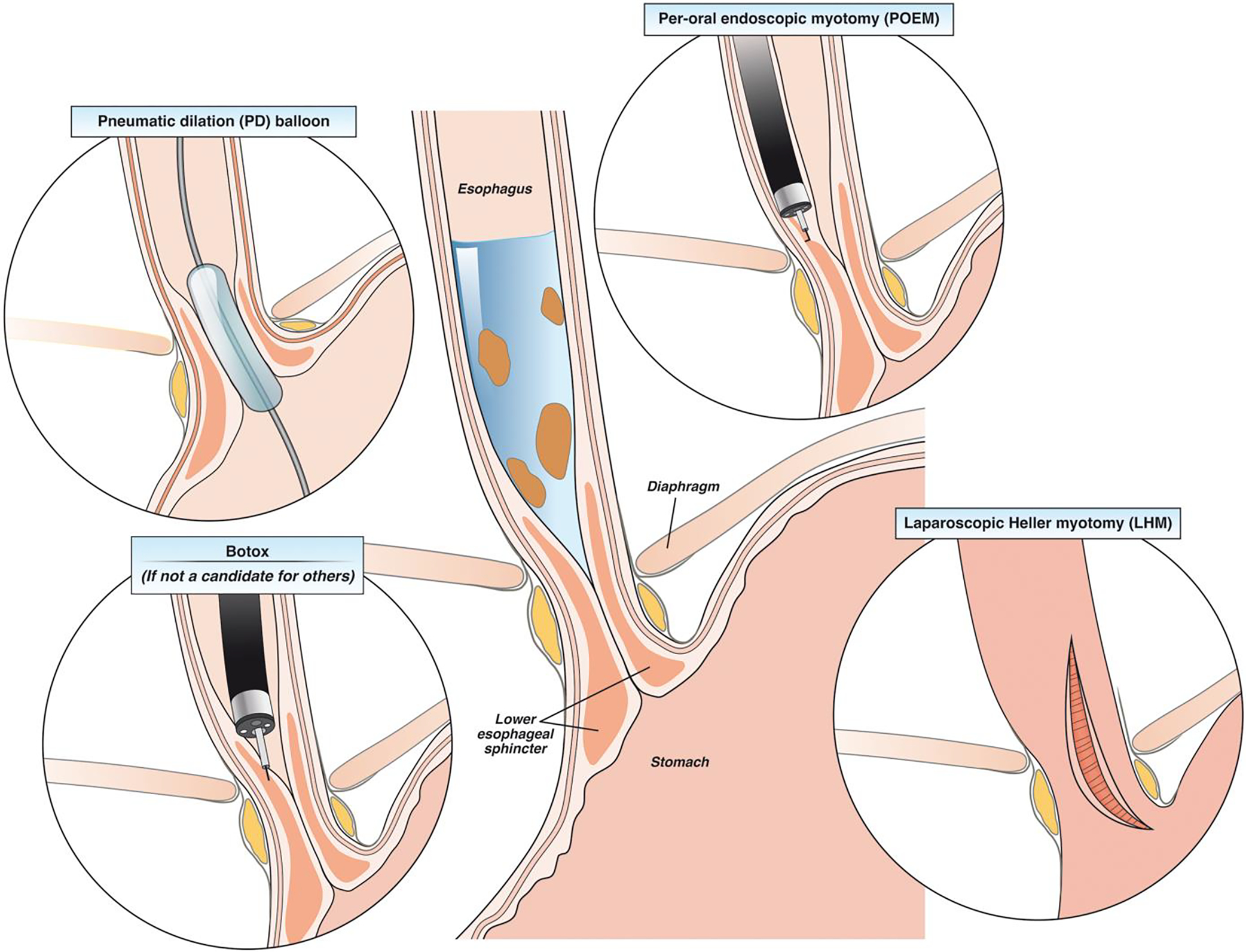
Treatment options in patients with achalasia.
Esophagogastric Junction (EGJ) Outflow Obstruction (EGJOO)
In the setting of non-mechanical obstructive symptoms, such as dysphagia and/or non-cardiac chest pain, and absence of achalasia on HRM, a diagnosis of EGJOO should be considered in those with high IRP and normal esophageal peristalsis. Prior iterations of the Chicago Classification defined EGJOO as a median elevated IRP in the supine position with presence of preserved peristalsis in the esophageal body. 74 According to these criteria, the prevalence of manometric EGJOO ranged from 5 to 24% of patients undergoing HRM.75–92. While some of these patients may represent an incomplete or variant form of achalasia, majority of the patients with manometric EGJOO are not related to a primary functional EGJOO and due to falsely elevated IRP. An estimated 21–28% of elevated IRP cases have been attributed to structural abnormalities76–79 including postoperative anatomic distortion related to fundoplication or bariatric surgery, cancer or other infiltrative processes, luminal stricture or extraluminal compression due to paraesophageal hernia (or cardiovascular compression, etc.), or an artifactual rise of the IRP from catheter effect.76, 77, 79, 90 Opioid analgesics have also been associated with impaired LES relaxation resulting in functional EGJ outflow obstruction 85, 93–95. The overdiagnosis of EGJOO on HRM without any clinical implications has led to concerns of unneeded treatments and was a driving reason to update the Chicago Classification. Therefore, a priority in CC v4.0 was to increase the stringency of manometric criteria for EGJOO and provide context as to which clinical scenarios are relevant to EGJOO and may merit therapeutic action. As such, EGJOO is diagnosed on HRM when the median IRP is elevated in both the supine and upright positions. For instance, in patients with a small hiatal hernia or central obesity an IRP that is elevated in the supine position normalizes in the upright position. Further, intrabolus pressurization must be present in at least 20% or more of swallows, and preserved peristalsis is present such that a diagnosis of achalasia is not met. A clinically conclusive diagnosis of EGJOO requires the presence of obstructive symptoms (dysphagia and/or non-cardiac chest pain) as well as corroboration of obstructive findings on a second diagnostic study (such as timed barium esophagram preferably with a barium tablet or FLIP). Thus, a clinically conclusive diagnosis of EGJOO requires 1) conclusive manometric diagnosis, 2) appropriate symptom presentation, and 3) confirmation of findings on supportive testing.
Treatment Options for EGJOO
Given that EGJOO may be an artifact of HRM testing, therapy should only be considered in the setting of other collaborative data. Therapeutic management of conclusive and clinically relevant EGJOO is highly variable and should be based on predominant symptom and severity of symptoms as 52% to 92% of patients with mild symptoms might have spontaneous resolution.75, 88, 90 Standard endoscopic dilation might provide symptomatic relief with pooled response rate of 69.6%, while botulinum toxin injection to the LES has a pooled response rate of 63.6%.96 In a cohort of 33 patients with EGJOO, PD had response rate of 66.7% with an average follow-up of 1.7 years.97 POEM has been evaluated in very small case series showing high symptom resolution ranging from 82% to 93% with median follow-up of 195 days.98, 99 We recommend a conservative approach with botulinum toxin injection and/or endoscopic dilation as the first option. Consideration of more aggressive therapy such as PD or myotomy must be based on other collaborative data as response to these therapies have only been studied in small uncontrolled studies with highly variable response rates. Hence, patients should be educated about potential risks of perforation, variable response rates, and long-term risks of reflux. In patients with non-cardiac chest pain as the dominant symptom, medications (smooth muscle relaxers or tricyclic antidepressants) should be considered initially with symptom relief in 41% at 3 to 6 months.90, 100 If patients have concomitant reflux symptoms (heartburn and/or regurgitation), reflux testing followed by trial of acid suppressive therapy with proton pump inhibitors might also be effective.96 Overall, a diagnosis of EGJOO does not always warrant therapy and treatment options should only be considered in patients with moderate to severe symptoms.
DISORDERS OF ESOPHAGEAL PERISTALSIS
Spastic Motor Disorders
Disordered esophageal motor function can be due to hyperactivity such that peristalsis is premature or contractile vigor is increased. Primary symptoms of such spastic esophageal motor disorders include dysphagia and non-cardiac chest pain and are often accompanied by heartburn and regurgitation. On HRM two groups of spastic esophageal disorders include distal esophageal spasm (DES) and hypercontractile esophagus.101 In the recent CC v4.0 update the manometric criteria for both DES and hypercontractile esophagus remains unchanged, however clinically conclusive diagnoses of DES and hypercontractile esophagus require the presence of clinically relevant obstructive symptoms (dysphagia and/or non-cardiac chest pain)
Distal Esophageal Spasm
Distal Esophageal Spasm is characterized by premature contractions in 20% or more of swallows on manometry in the setting of intact deglutitive relaxation (normal median IRP) (Figure 1). Premature contractions are defined by a distal latency of less than 4.5 seconds in the setting of a DCI of 450 mmHg•s•cm or greater.102 The greater the number of premature swallows on HRM, the greater the confidence in the DES diagnosis. A manometric diagnosis of DES can be supported by lack of peristaltic inhibition during multiple rapid swallows (MRS) or abnormal contractions with stress of a bolus load. Importantly, true primary DES is rare, and when identified considered along the spectrum of type 3 achalasia. TBE can be very helpful as supportive testing in this group of patients as it can identify tertiary contractions, rosary bead, or corkscrew esophagus associated with DES. Retention of barium during TBE is an important distinction between DES and achalasia diagnosis.
Hypercontractile Esophagus
Hypercontractile esophagus is defined on HRM by 20% or more swallows with hypercontractility (DCI> 8000 mmHg•s•cm) with a normal IRP (Figure 1). The term “jackhammer esophagus” is no longer synonymous with hypercontractile esophagus.103 To make a conclusive manometric diagnosis of hypercontractile esophagus, criteria for achalasia and DES must not be met and underlying EGJOO excluded. Supportive findings for manometric hypercontractile esophagus include intrabolus pressurization, abnormal patterns with rapid drink challenge, or absence of peristaltic reserve on MRS.103 A multitude of manometric patterns can meet criteria for hypercontractile esophagus, more commonly single peak hypercontractile peristalsis over jackhammer esophagus with repetitive prolonged contractions, the latter considered to portend greater pathologic significance. Recently, a new entity has been described involving LES hypercontraction and, along with these other phenotypes, is under active investigation.103 103
Treatment of Spastic Disorders
Spastic esophageal disorders can be due to a primary motor dysfunction, opioid-induced, or a reactive clearance mechanism to gastro-esophageal refluxate. Evaluation for underlying reflux should be the initial step in determining best treatment option for patients with symptomatic spastic disorders of the esophageal body (Figure 3). In one study of 108 patients with DES, 34% of patients had pathologic acid exposure on pH testing or endoscopy.104 When gastro-esophageal reflux is driving the spasticity, treatment should focus on anti-reflux management as anti-spasmodic therapy has potential to augment gastro-esophageal reflux physiology and worsen symptoms. In patients with heartburn and/or regurgitation a regimen of proton pump inhibitor for 6 to 8 weeks can be trailed and/or ambulatory reflux monitoring OFF acid suppression can be pursued. Furthermore, careful inspection for any esophageal strictures should also be performed during endoscopy and empiric esophageal dilation can be considered as a treatment modality given spastic contractions of esophageal body can also be a result of a subtle distal esophageal stricture. In most patients with mild symptoms, monitoring is also a reasonable alternative given that these disorders do tend to have a benign long term course with one study looking at natural history over 3–10 years showing that symptoms of dysphagia and non-cardiac chest pain improved significantly over time.105
Figure 3:
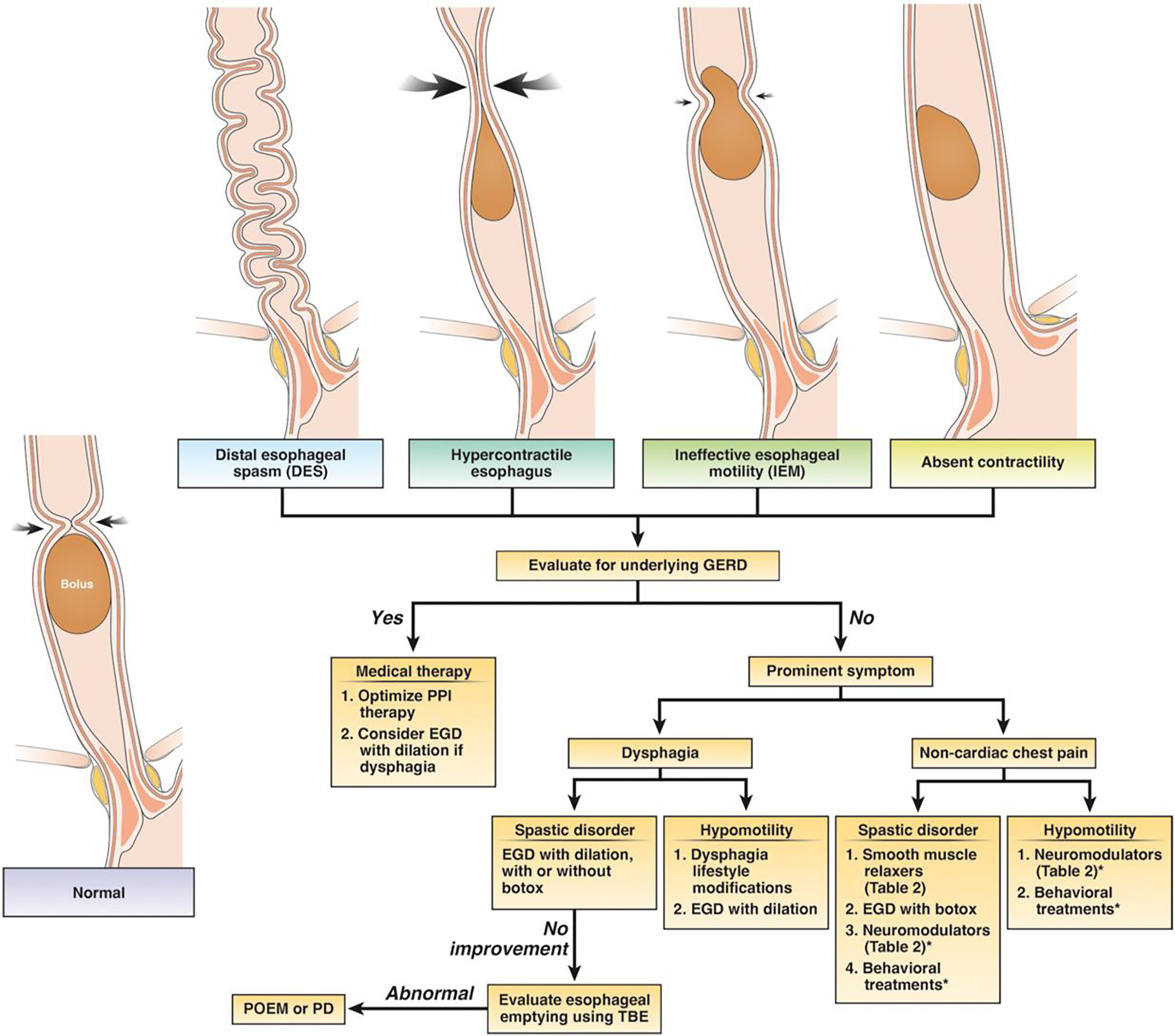
Treatment options in patients with disorders of esophageal peristalsis after a careful endoscopy is performed to rule out a mechanical or mucosal disease (such as eosinophilic esophagitis). *In patients with chest pain as the primary symptom, overlapping esophageal hypersensitivity might play a major role in symptom generation and neuromodulators or behavioral treatments might be beneficial based on studies on non-cardiac chest pain.
Pharmacotherapy
In patients with a spastic esophageal disorder and dysphagia and non-cardiac chest pain, smooth muscle relaxants can be trialed such as calcium channel blockers106, nitrates107, and phosphodiesterase-5 inhibitors108, but their use may be limited due to side effects (headaches, hypotension, and dizziness). Some of the authors prefer a trial of sub-lingual hyoscyamine 30 minutes prior to meals based on anecdotal successes and ease of availability and favorable side effect profile, but this has not been studied for spastic disorders. In patients with dysphagia as the predominant symptom and lack of response to esophageal dilation or smooth muscle relaxant, esophageal botulinum toxin injection at the lower esophageal sphincter, 2cm, and 7 cm above the LES might result in symptom improvement in 50% of patients at 1 month and 30% at 1 year.109 Although botulinum toxin injection can be offered to patients with non-cardiac chest pain as the predominant symptom without dysphagia, limited open label studies suggest that the improvement might be transient and only last for 6 months.110, 111 In patients with chest pain as the predominant symptom with suspicion of overlapping visceral hypersensitivity, neuromodulation with low dose tricyclic antidepressants (imipramine or amitriptyline) or trazodone may be more effective than targeting the esophageal dysmotility.112, 113 Table 2 shows a list of smooth muscle relaxers and neuromodulators with level of evidence, dosage, side effects, and potential special considerations that might allow clinicians to personalize selection based on patient profile and concomitant symptoms. However, it should be noted that most of the data is from patients with non-cardiac chest pain and the level of evidence is limited by very small sample sized studies.
Table 2:
Smooth muscle relaxers and neuromodulators that might be used in patients with disorder of esophageal peristalsis and non-cardiac chest pain (NCCP) as the primary symptom. Smooth muscle relaxers should only be used in patients with distal esophageal spasm or hypercontractile esophagus.
| Dosage | Level of evidence | Side effects | Special considerations | ||
|---|---|---|---|---|---|
| Smooth muscle relaxers | Peppermint oil | −5 drops in 10mL of water (or 2 Altoid mints sublingual before every meal) | Case series148 | Might worsen reflux symptoms | -Decreases simultaneous contractions and amplitude, but less effect on pain |
| Calcium channel blockers | -Diltiazem 60 to 90 mg four times a day -Nifedipine 10mg 30 mins prior to meals |
RCT in nutcracker esophagus106 | Hypotension, peripheral edema, headaches, dizziness, and tachyphylaxis | -Improves chest pain and decreases esophageal amplitude | |
| Phosphodiesterase-5 inhibitor | -Sildenafil 25 to 50 mg twice a day | RCT in spastic motor disorder149 | Headache, hypotension, and dizziness | -Might improve manometric findings, but less effect on symptoms | |
| Tricyclic anti-depressants (TCAs) | Imipramine Amitriptyline | −50 mg daily −10 to 25mg at night | RCT for NCCP112, 150 RCT for NCCP151 | QT prolongation, dry mouth, excessive sleeping, dizziness, and constipation | -Might use nortriptyline (less anti-cholinergic effects in hypomotility disorder and NCCP) |
| Serotonin-norepinephrine re-uptake inhibitor (SNRI) | Venlafaxine | 75 mg daily | RCT for NCCP152 | Sleep disturbances, nausea, hypertension | -Less constipation compared to TCA, but similar pain relief |
| Selective serotonin reuptake inhibitor (SSRI) | Sertraline | 50 to 200 mg daily | RCT for NCCP153, 154 | Nausea, restlessness, dry mouth, diarrhea | -Might be beneficial if concomitant anxiety disorder or symptom hypervigilance |
| Paroxetine | 10 to 50 mg daily | RCT for NCCP155 | -More drug-drug interactions due to inhibition of P450 isoenzymes -Short half-life, risk of discontinuation syndrome if stopped abruptly -Fluoxetine preferred in patients who may miss doses (due to longer half-life) |
||
| Miscellaneous | Trazadone | −100 to 150 mg daily | RCT in spastic motor disorder113 | Nausea, dizziness, and drowsiness | -Less effect on manometric abnormalities, but significant improvement in chest pain |
Myotomy
In patients who fail pharmacotherapy, we recommend performing a TBE and/or FLIP to evaluate esophageal emptying and see if there is significant retention of barium or obstruction at the esophagogastric junction. If consistent with an obstructive physiology, myotomy might be considered. In a meta-analysis of 8 observational studies (total of 18 patients with DES and 37 with jackhammer esophagus), POEM had clinical success rates of 88% and 72%, respectively.114 Another retrospective study involving 11 centers that included 17 patients with DES and 18 with jackhammer esophagus found that POEM improved chest pain in 87% with clinical success (based on Eckardt score) of 84.9% with median follow-up of 272 days.99 Surgical myotomy has also been studied in a small prospective study of 20 patients with extended myotomy for DES and found that both dysphagia and chest pain improved in 100% and 90%, respectively, over 50 month follow-up.115 Due to lack of well controlled long term randomized controlled trials, myotomy for treatment of spastic esophageal body disorders should be reserved for highly selected patients as long term prognosis even without treatment seems to be good for these disorders.
Hypomotility Disorders
Ineffective esophageal motility
Manometric diagnosis of ineffective esophageal motility (IEM) has evolved over time and now require more than 70% ineffective swallows (DCI ≥ 100 mmHg•s•cm and < 450 mmHg•s•cm) or at least 50% failed peristalsis (DCI < 100 mmHg•s•cm) (Figure 1).19 Clinical relevance of IEM has been under debate116 as several studies have shown no correlation between IEM and esophageal symptoms.117, 118 but it has been associated with higher esophageal reflux burden.
Findings of IEM might affect decision making prior to anti-reflux surgery due to risk of post-operative dysphagia although some studies show similar outcomes after a partial (Dor or Toupet) or Nissen fundoplication.119–122 Provocative maneuvers such as multiple rapid swallows (MRS) might identify contractile reserve in this group of patients and predict risk of postoperative dysphagia with sensitivity and specificity of 67% and 64%, respectively.28 Hence, presence of IEM and findings on MRS might alter the type of fundoplication offered to the patient. Minimum of three MRS sequences should be performed to demonstrate adequate contraction reserve.123 Contrary to surgical fundoplication, it should be noted that IEM is a relative contraindication for magnetic sphincter augmentation as esophageal peristalsis is necessary to distend the magnetic band and allow the bolus to enter the stomach.124, 125
Absent contractility
Absent contractility is defined as 100% failed peristalsis (DCI <100 mmHg•s•cm) with a normal median IRP in the supine and upright position (Figure 1).19 This is also commonly characterized in clinical practice as “scleroderma like esophagus,” due to higher prevalence in patients with connective tissue disease. However, if dysphagia is a prominent symptom and manometric evaluation shows a borderline median IRP in supine position of 10 mmHg to 15 mmHg (using the Medtronic software), type I achalasia should be considered in the differential. In this setting, correlating the manometric findings with TBE, FLIP or endoscopy to ensure that LES is open is essential in avoiding misdiagnosis.
Treatment of hypomotility disorders
Management of IEM and absent contractility is very challenging given lack of clear association with symptoms. Given there is no effective pharmacotherapy to improve esophageal contractile vigor, management should be focused towards improving predominant symptoms and treating any concurrent reflux if present (Figure 3). In patients with dysphagia as the predominant symptom, endoscopy to evaluate for a mechanical stricture (such as Schatzki’s ring or peptic stricture) should be performed and even in cases of normal exam, empiric esophageal dilation might be considered in this group due to lack of other reliable options. Lifestyle modifications such as chewing carefully, maintaining positional gravity and chasing solid bolus with liquids can be helpful. Newer agents such as prucalopride may increase the amplitude of primary esophageal contractions in patients with GERD126 and might have a role in patients with either concomitant gastroparesis or chronic idiopathic constipation, though is not a primary treatment option for esophageal hypomotility. Buspirone, a mixed dopamine D2 receptor antagonist and partial 5HT-1A agonist has been shown to increase esophageal contraction amplitude in patients with scleroderma127, 128, but was not better than placebo in a small placebo-controlled crossover trial of 10 patients with IEM in improving dysphagia.129 Overall, it is important to recognize that in most cases, natural history of IEM suggest that it does not progress over time and quality of life is not impacted.130 In patients with chest pain as the primary symptom, esophageal hypersensitivity might be the major driving factor and neuromodulators or behavioral therapies might be beneficial based on studies in patients with non-cardiac chest pain (Table 2).
IMPACT OF ESOPHAGEAL MUCOSAL DISEASE, SYSTEMIC DISEASE, AND MEDICATIONS ON MOTILITY
Recent advances in diagnostics have improved our knowledge on how various inflammatory esophageal disorders, rheumatologic disorders, and medications cause secondary motility abnormalities (Figure 4). Previously, GERD has been commonly associated with both hypomotility and spastic disorders of the esophageal body, but recent studies show that certain phenotypes of other disorders such as eosinophilic esophagitis (EoE) might also affect motility. Hence, careful endoscopic examination and clinical history are paramount in distinguishing primary (idiopathic) motility disorders from secondary diseases, which can significantly alter treatment options and long-term clinical outcomes.
Figure 4:
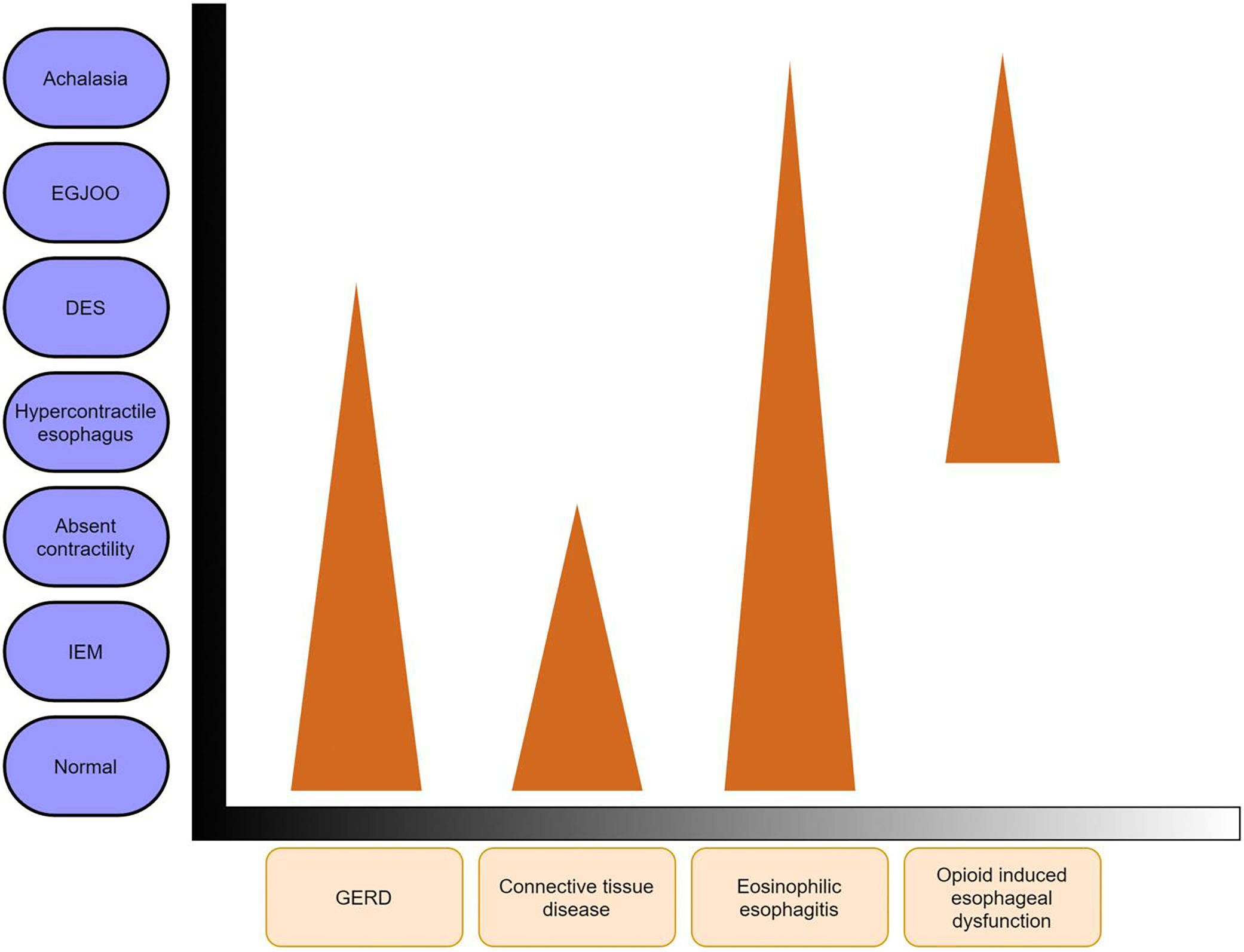
Impact of mucosal diseases, opioids, and connective tissue disease on esophageal motility. Figure shows presence of various secondary motility abnormalities, and the triangle shape indicates visual approximations of the prevalence of those motility abnormalities with each disease state. Most patients will have a normal motility testing. For instance, in opioid induced esophageal dysfunction, there is higher prevalence of hypercontractile esophagus and distal esophageal compared to achalasia. On the other hand, majority of patients with GERD will have normal esophageal motility followed by hypomotility disorders and lower prevalence of distal esophageal spasm. DES (distal esophageal spasm); IEM (ineffective esophageal motility).
Eosinophilic Esophagitis
One study of 109 patients with EoE found that 38% of the cohort had abnormal findings on HRM with majority (59%) of those being minor disorders (23 with IEM, 1 with fragmented peristalsis), but 41% had major motor disorders (8 with achalasia, 1 DES, 2 jackhammer esophagus, 5 EGJOO, and 1 absent contractility).131 Another study with 20 symptomatic EoE patients evaluated manometric changes before and after 8 weeks of topical steroid treatment with budesonide. They found that minor motility abnormalities of early pan-esophageal pressurizations and weak peristalsis were found in 35% of patients, but 86% of them had resolution of the abnormalities after budesonide therapy and achieving histologic remission.132 Hence, eosinophils might cause reversible esophageal motility disturbances, but presence of mucosal eosinophilia in major motility disorder such as achalasia might need to be interpreted with caution as this can also be seen as a consequence of prolonged esophageal stasis of retained food/saliva that causes mucosal irritation.
Connective tissue disorders
Sjogren’s syndrome (SS) and systemic sclerosis (SSc) are the two most common connective tissue disorders associated with esophageal motility disorders. Dysphagia is a common symptom reported by 26–65% of patients with these disorders.133, 134 Most common manometric findings include hypotensive lower esophageal sphincter pressure along with esophageal dysmotility with up to 68.5% with SSc having absent contractility and 35% of patients with SS having dysmotility.134–136 However, it should be noted that severity of dysphagia in this population has not been associated with any specific motility pattern.137 Furthermore, there is also high incidence of reflux symptoms and erosive esophagitis in up to 32% of patients, which is also associated with hypomotility disorders.138 Thus, management of esophageal dysmotility in patients with connective tissue disorders should be focused towards optimization of reflux management (acid suppressive therapy) and potential consideration of esophageal dilation in patients with dysphagia since they are at higher risk of developing peptic stricture.
Opioid induced esophageal dysfunction (OIED)
Opioid medications have recently been associated with spastic disorders of the esophagus. In a recent retrospective study of 2,342 patients (224 on chronic daily opioids), 62% of patients on opioids reported dysphagia and they were also more likely to have following manometric abnormalities: type III achalasia (13% vs. 1%, p <0.01), EGJOO (13% vs. 3%, p <0.01), and DES (3% vs. 0.5%, p <0.01).139 Given there is no gold standard test to determine idiopathic vs medication effect on esophageal motility, withdrawal of the opioid if feasible and repeating the manometry study is ideal to see if the abnormality resolves.140 Provocative testing evaluating amyl nitrate induced rebound contraction of the lower esophageal sphincter and paradoxical esophageal contraction during the first phase of cholecystokinin response might be able to differentiate opioid effect from idiopathic type III achalasia.141 However, these are available in only few centers across United States. Given OIED has just been recently described with very few studies in the literature, there is currently lack of evidence on optimal management strategy.142 Given withdrawal of opioids might reverse the motility abnormality, invasive treatment options such as POEM or surgical myotomy should be reserved in highly selected cases given lack of data on long term outcomes. If opioid withdrawal is not an option, consideration should be made towards changing to a partial agonist since they are not associated with motility abnormalities.95 If persistent symptoms despite above, we prefer to use endoscopic dilation with botulinum toxin injection targeting spasticity of the esophagus as the next step prior to proceeding with myotomy interventions that have higher risks.142
POST-SURGICAL ESOPHAGEAL MOTILITY DISORDERS
Fundoplication
Postoperative dysphagia after anti-reflux surgery is present in 6% to 26% of patients.26, 143 Esophageal dysmotility can be found in up to 7% of patients after Nissen fundoplication with majority having non-specific motility abnormalities followed by secondary achalasia and distal esophageal spasm.144 Evaluation should include EGD, with FLIP if available, to assess the fundoplication wrap and barium esophagram to evaluate esophageal emptying. Symptom of dysphagia in most of these patients is generated due to inability of esophageal propulsive force to distend the wrap for bolus transport into the stomach. Lack of contraction reserve on MRS has been independently associated with late post-fundoplication dysphagia.30 Studies show that conventional endoscopic dilation of the wrap leads to symptom resolution in majority of these patients with very few needing wrap takedown.26, 30, 143 In patients with lack of response to conventional esophageal dilation (up to 20mm), pneumatic dilation can be considered in patients with abnormal esophageal emptying, but might not be effective in patients with dysphagia and no signs of obstruction from the wrap.145, 146
Bariatric surgery
The rising incidence of obesity and utilization of bariatric surgery has brought impact of altered foregut anatomy on esophageal motility to the forefront of clinical care. Dysphagia has been reported in 51% of patients after laparoscopic sleeve gastrectomy (LSG) and 46% of patients after Roux-en-Y gastric bypass (RYGB).147 In a recent study of 97 patients who under HRIM after bariatric surgery, 61.5% had manometric abnormalities per CC v3.0 with 29.9% of them being major motility disorders (achalasia, EGJOO, DES, and jackhammer).147 The remaining had ineffective esophageal motility. They also found that 5.2% had unique achalasia-like pattern defined by aperistalsis and increased intragastric pressure, which was defined as postobesity surgery esophageal dysfunction (POSED). Hence, secondary esophageal dysmotility may be an under-recognized complication of bariatric surgery as up to 13.7% of patients continued to have dysphagia at a mean of 3.9 years after surgery.147 Treatment in this group of patients should be focused towards assessment of the gastric pouch, sleeve, and/or anastomosis and improving any source of mechanical or functional outflow obstruction.
Conclusion
Significant advances have been made over the last 3 decades in our understanding of various esophageal motility disorders due to improvement in diagnostics with high resolution esophageal manometry. Barium esophagram and possibly FLIP may serve as complementary tools in assessment of patients with suspected motility disorders, but ultimately it is the overall clinical picture along with diagnostic tests that complete the clinical assessment for patients. Achalasia remains as the most studied motility disorder with highly effective therapeutic options for palliation of symptoms. However, there has been a lack of advancement in studying therapeutics for non-achalasia motility disorders, which should be a priority for the next decade.
Acknowledgments
RY: Institutional Consulting Agreement: Medtronic, Ironwood Pharmaceuticals, Diversatek; Consultant: Phantom Pharmaceuticals; Research Support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix. RY is supported by NIH K23 DK125266 (PI: Yadlapati).
Footnotes
Disclosures and Conflicts of Interest: All authors (DP and MV) have no conflicts of interest.
REFERENCES
- 1.Delshad SD, Almario CV, Chey WD, et al. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology 2020;158:1250–1261 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins C, Takakura W, Spiegel BMR, et al. Prevalence and Characteristics of Dysphagia Based on a Population-Based Survey. Clin Gastroenterol Hepatol 2020;18:1970–1979 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyawali CP, Carlson DA, Chen JW, et al. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Am J Gastroenterol 2020;115:1412–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology 2011;141:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103:27–37. [DOI] [PubMed] [Google Scholar]
- 6.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 7.Arndorfer RC, Stef JJ, Dodds WJ, et al. Improved infusion system for intraluminal esophageal manometry. Gastroenterology 1977;73:23–7. [PubMed] [Google Scholar]
- 8.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol 1991;261:G677–84. [DOI] [PubMed] [Google Scholar]
- 9.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol 2000;95:2720–30. [DOI] [PubMed] [Google Scholar]
- 10.Minami H, Isomoto H, Miuma S, et al. New endoscopic indicator of esophageal achalasia: “pinstripe pattern”. PLoS One 2015;10:e0101833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol 2016;111:1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savarino E, di Pietro M, Bredenoord AJ, et al. Use of the Functional Lumen Imaging Probe in Clinical Esophagology. Am J Gastroenterol 2020;115:1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson DA, Kou W, Masihi M, et al. Repetitive Antegrade Contractions: A novel response to sustained esophageal distension is modulated by cholinergic influence. Am J Physiol Gastrointest Liver Physiol 2020. [DOI] [PubMed] [Google Scholar]
- 15.Attaar M, Su B, Wong HJ, et al. Intraoperative impedance planimetry (EndoFLIP) results and development of esophagitis in patients undergoing peroral endoscopic myotomy (POEM). Surg Endosc 2021;35:4555–4562. [DOI] [PubMed] [Google Scholar]
- 16.Su B, Callahan ZM, Novak S, et al. Using Impedance Planimetry (EndoFLIP) to Evaluate Myotomy and Predict Outcomes After Surgery for Achalasia. J Gastrointest Surg 2020;24:964–971. [DOI] [PubMed] [Google Scholar]
- 17.Holmstrom AL, Campagna RAJ, Cirera A, et al. Intraoperative use of FLIP is associated with clinical success following POEM for achalasia. Surg Endosc 2021;35:3090–3096. [DOI] [PubMed] [Google Scholar]
- 18.Jain AS, Carlson DA, Triggs J, et al. Esophagogastric Junction Distensibility on Functional Lumen Imaging Probe Topography Predicts Treatment Response in Achalasia-Anatomy Matters! Am J Gastroenterol 2019;114:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil 2021;33:e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MR, Sweis R, Yadlapati R, et al. Chicago classification version 4.0((c)) technical review: Update on standard high-resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterol Motil 2021;33:e14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misselwitz B, Hollenstein M, Butikofer S, et al. Prospective serial diagnostic study: the effects of position and provocative tests on the diagnosis of oesophageal motility disorders by high-resolution manometry. Aliment Pharmacol Ther 2020;51:706–718. [DOI] [PubMed] [Google Scholar]
- 22.Triggs JR, Carlson DA, Beveridge C, et al. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019;17:2218–2226 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol 2007;293:G878–85. [DOI] [PubMed] [Google Scholar]
- 24.Herregods TV, Roman S, Kahrilas PJ, et al. Normative values in esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:175–87. [DOI] [PubMed] [Google Scholar]
- 25.Rengarajan A, Rogers BD, Wong Z, et al. High-Resolution Manometry Thresholds and Motor Patterns Among Asymptomatic Individuals. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 26.Athanasiadis DI, Selzer D, Stefanidis D, et al. Postoperative Dysphagia Following Esophagogastric Fundoplication: Does the Timing to First Dilation Matter? J Gastrointest Surg 2021. [DOI] [PubMed] [Google Scholar]
- 27.Elvevi A, Mauro A, Pugliese D, et al. Usefulness of low- and high-volume multiple rapid swallowing during high-resolution manometry. Dig Liver Dis 2015;47:103–7. [DOI] [PubMed] [Google Scholar]
- 28.Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoikes N, Drapekin J, Kushnir V, et al. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc 2012;26:3401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasak S, Brunt LM, Wang D, et al. Clinical Characteristics and Outcomes of Patients With Postfundoplication Dysphagia. Clin Gastroenterol Hepatol 2019;17:1982–1990. [DOI] [PubMed] [Google Scholar]
- 31.Ang D, Hollenstein M, Misselwitz B, et al. Rapid Drink Challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 32.Woodland P, Gabieta-Sonmez S, Arguero J, et al. 200 mL Rapid Drink Challenge During High-resolution Manometry Best Predicts Objective Esophagogastric Junction Obstruction and Correlates With Symptom Severity. J Neurogastroenterol Motil 2018;24:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil 2016;28:543–53. [DOI] [PubMed] [Google Scholar]
- 34.Krause AJ, Su H, Triggs JR, et al. Multiple rapid swallows and rapid drink challenge in patients with esophagogastric junction outflow obstruction on high-resolution manometry. Neurogastroenterol Motil 2020:e14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanagapalli S, McGuire J, Leong R, et al. The clinical relevance of manometric esophagogastric junction outflow obstruction can be determined using rapid drink challenge and solid swallows. Am J Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- 36.Wang YT, Tai LF, Yazaki E, et al. Investigation of Dysphagia After Antireflux Surgery by High-resolution Manometry: Impact of Multiple Water Swallows and a Solid Test Meal on Diagnosis, Management, and Clinical Outcome. Clin Gastroenterol Hepatol 2015;13:1575–83. [DOI] [PubMed] [Google Scholar]
- 37.Sanagapalli S, McGuire J, Leong RW, et al. The Clinical Relevance of Manometric Esophagogastric Junction Outflow Obstruction Can Be Determined Using Rapid Drink Challenge and Solid Swallows. Am J Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- 38.Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol 2017;2:654–661. [DOI] [PubMed] [Google Scholar]
- 39.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil 2004;16:533–42. [DOI] [PubMed] [Google Scholar]
- 40.Ott DJ, Richter JE, Chen YM, et al. Esophageal radiography and manometry: correlation in 172 patients with dysphagia. AJR Am J Roentgenol 1987;149:307–11. [DOI] [PubMed] [Google Scholar]
- 41.O’Rourke AK, Lazar A, Murphy B, et al. Utility of Esophagram versus High-Resolution Manometry in the Detection of Esophageal Dysmotility. Otolaryngol Head Neck Surg 2016;154:888–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neyaz Z, Gupta M, Ghoshal UC. How to perform and interpret timed barium esophagogram. J Neurogastroenterol Motil 2013;19:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaezi MF, Baker ME, Achkar E, et al. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002;50:765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol 1999;94:1802–7. [DOI] [PubMed] [Google Scholar]
- 45.Blonski W, Kumar A, Feldman J, et al. Timed Barium Swallow: Diagnostic Role and Predictive Value in Untreated Achalasia, Esophagogastric Junction Outflow Obstruction, and Non-Achalasia Dysphagia. Am J Gastroenterol 2018;113:196–203. [DOI] [PubMed] [Google Scholar]
- 46.Blonski W, Kumar A, Feldman J, et al. Timed barium swallow for assessing long-term treatment response in patients with achalasia: Absolute cutoff versus percent change - A cross-sectional analytic study. Neurogastroenterol Motil 2021;33:e14005. [DOI] [PubMed] [Google Scholar]
- 47.Singaram C, Sengupta A, Sweet MA, et al. Nitrinergic and peptidergic innervation of the human oesophagus. Gut 1994;35:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park W, Vaezi MF. Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol 2005;100:1404–14. [DOI] [PubMed] [Google Scholar]
- 49.Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest 1993;23:724–8. [DOI] [PubMed] [Google Scholar]
- 50.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013;108:1238–49; quiz 1250. [DOI] [PubMed] [Google Scholar]
- 51.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology 2010;139:369–74. [DOI] [PubMed] [Google Scholar]
- 52.Vaezi MF, Pandolfino JE, Yadlapati RH, et al. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol 2020;115:1393–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA 2015;313:1841–52. [DOI] [PubMed] [Google Scholar]
- 54.Zhou MJ, Kamal A, Freedberg DE, et al. Type II Achalasia Is Increasing in Prevalence. Dig Dis Sci 2020. [DOI] [PubMed] [Google Scholar]
- 55.Rieder E, Fernandez-Becker NQ, Sarosiek J, et al. Achalasia: physiology and diagnosis. Ann N Y Acad Sci 2020;1482:85–94. [DOI] [PubMed] [Google Scholar]
- 56.Kim TH, Patel N, Ledgerwood-Lee M, et al. Esophageal contractions in type 3 achalasia esophagus: simultaneous or peristaltic? Am J Physiol Gastrointest Liver Physiol 2016;310:G689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S, Zifan A, Kumar D, et al. Genesis of Esophageal Pressurization and Bolus Flow Patterns in Patients With Achalasia Esophagus. Gastroenterology 2018;155:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modayil RJ, Zhang X, Rothberg B, et al. Peroral endoscopic myotomy: 10-year outcomes from a large, single-center U.S. series with high follow-up completion and comprehensive analysis of long-term efficacy, safety, objective GERD, and endoscopic functional luminal assessment. Gastrointest Endosc 2021. [DOI] [PubMed] [Google Scholar]
- 59.Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332–341. [DOI] [PubMed] [Google Scholar]
- 60.Werner YB, Hakanson B, Martinek J, et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med 2019;381:2219–2229. [DOI] [PubMed] [Google Scholar]
- 61.Kahrilas PJ, Katzka D, Richter JE. Clinical Practice Update: The Use of Per-Oral Endoscopic Myotomy in Achalasia: Expert Review and Best Practice Advice From the AGA Institute. Gastroenterology 2017;153:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel DA, Vaezi MF. Bringing real-time live feedback to esophageal dilations, but not ready for prime time. Gastrointest Endosc 2020;92:1258–1260. [DOI] [PubMed] [Google Scholar]
- 63.Sloan JA, Triggs JR, Pandolfino JE, et al. Treatment experience with a novel 30-mm hydrostatic balloon in esophageal dysmotility: a multicenter retrospective analysis. Gastrointest Endosc 2020;92:1251–1257. [DOI] [PubMed] [Google Scholar]
- 64.van Hoeij FB, Prins LI, Smout A, et al. Efficacy and safety of pneumatic dilation in achalasia: A systematic review and meta-analysis. Neurogastroenterol Motil 2019;31:e13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vela MF, Richter JE, Khandwala F, et al. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol 2006;4:580–7. [DOI] [PubMed] [Google Scholar]
- 66.Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807–16. [DOI] [PubMed] [Google Scholar]
- 67.Persson J, Johnsson E, Kostic S, et al. Treatment of achalasia with laparoscopic myotomy or pneumatic dilatation: long-term results of a prospective, randomized study. World J Surg 2015;39:713–20. [DOI] [PubMed] [Google Scholar]
- 68.Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45–57. [DOI] [PubMed] [Google Scholar]
- 69.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718–25; quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 70.Traube M, Dubovik S, Lange RC, et al. The role of nifedipine therapy in achalasia: results of a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 1989;84:1259–62. [PubMed] [Google Scholar]
- 71.Gelfond M, Rozen P, Gilat T. Isosorbide dinitrate and nifedipine treatment of achalasia: a clinical, manometric and radionuclide evaluation. Gastroenterology 1982;83:963–9. [PubMed] [Google Scholar]
- 72.Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol 1998;27:21–35. [DOI] [PubMed] [Google Scholar]
- 73.Bassotti G, Annese V. Review article: pharmacological options in achalasia. Aliment Pharmacol Ther 1999;13:1391–6. [DOI] [PubMed] [Google Scholar]
- 74.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Fernandez MT, Santander C, Marinero A, et al. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil 2016;28:116–26. [DOI] [PubMed] [Google Scholar]
- 76.Scherer JR, Kwiatek MA, Soper NJ, et al. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg 2009;13:2219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2015;27:1310–6. [DOI] [PubMed] [Google Scholar]
- 78.DeLay K, Austin GL, Menard-Katcher P. Anatomic abnormalities are common potential explanations of manometric esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2016;28:1166–71. [DOI] [PubMed] [Google Scholar]
- 79.Clayton SB, Patel R, Richter JE. Functional and Anatomic Esophagogastic Junction Outflow Obstruction: Manometry, Timed Barium Esophagram Findings, and Treatment Outcomes. Clin Gastroenterol Hepatol 2016;14:907–911. [DOI] [PubMed] [Google Scholar]
- 80.Blais P, Patel A, Sayuk GS, et al. Upper esophageal sphincter (UES) metrics on high-resolution manometry (HRM) differentiate achalasia subtypes. Neurogastroenterol Motil 2017;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okeke FC, Raja S, Lynch KL, et al. What is the clinical significance of esophagogastric junction outflow obstruction? evaluation of 60 patients at a tertiary referral center. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 82.Biasutto D, Mion F, Garros A, et al. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil 2018;30:e13293. [DOI] [PubMed] [Google Scholar]
- 83.Liu A, Woo M, Nasser Y, et al. Esophagogastric junction outflow obstruction on manometry: Outcomes and lack of benefit from CT and EUS. Neurogastroenterol Motil 2019;31:e13712. [DOI] [PubMed] [Google Scholar]
- 84.Song BG, Min YW, Lee H, et al. Clinicomanometric factors associated with clinically relevant esophagogastric junction outflow obstruction from the Sandhill high-resolution manometry system. Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- 85.Babaei A, Shad S, Szabo A, et al. Pharmacologic interrogation of patients with esophagogastric junction outflow obstruction using amyl nitrite. Neurogastroenterol Motil 2019:e13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Triggs JR, Carlson DA, Beveridge C, et al. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beveridge CA, Falk GW, Ahuja NK, et al. Low Yield of Cross-Sectional Imaging in Patients with Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 88.Schupack D, Katzka DA, Geno DM, et al. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil 2017;29:1–9. [DOI] [PubMed] [Google Scholar]
- 89.Song BG, Min YW, Lee H, et al. Combined Multichannel Intraluminal Impedance and High-resolution Manometry Improves Detection of Clinically Relevant Esophagogastric Junction Outflow Obstruction. J Neurogastroenterol Motil 2019;25:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynch KL, Yang YX, Metz DC, et al. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis Esophagus 2017;30:1–6. [DOI] [PubMed] [Google Scholar]
- 91.Zheng E, Gideon RM, Sloan J, et al. Esophagogastric junction outflow obstruction is often associated with coexistent abnormal esophageal body motility and abnormal bolus transit. Dis Esophagus 2017;30:1–4. [DOI] [PubMed] [Google Scholar]
- 92.Su H, Ge H, Liu H, et al. High-resolution manometry in the upright position could improve the manometric evaluation of morbidly obese patients with esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2020;32:e13924. [DOI] [PubMed] [Google Scholar]
- 93.Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol 2015;110:979–84. [DOI] [PubMed] [Google Scholar]
- 94.Babaei A, Szabo A, Shad S, et al. Chronic daily opioid exposure is associated with dysphagia, esophageal outflow obstruction, and disordered peristalsis. Neurogastroenterol Motil 2019:e13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Snyder DL, Crowell MD, Horsley-Silva J, et al. Opioid-Induced Esophageal Dysfunction: Differential Effects of Type and Dose. Am J Gastroenterol 2019;114:1464–1469. [DOI] [PubMed] [Google Scholar]
- 96.Zikos TA, Triadafilopoulos G, Clarke JO. Esophagogastric Junction Outflow Obstruction: Current Approach to Diagnosis and Management. Curr Gastroenterol Rep 2020;22:9. [DOI] [PubMed] [Google Scholar]
- 97.Clayton SB, Shin CM, Ewing A, et al. Pneumatic dilation improves esophageal emptying and symptoms in patients with idiopathic esophago-gastric junction outflow obstruction. Neurogastroenterol Motil 2019;31:e13522. [DOI] [PubMed] [Google Scholar]
- 98.Filicori F, Dunst CM, Sharata A, et al. Long-term outcomes following POEM for non-achalasia motility disorders of the esophagus. Surg Endosc 2019;33:1632–1639. [DOI] [PubMed] [Google Scholar]
- 99.Khashab MA, Familiari P, Draganov PV, et al. Peroral endoscopic myotomy is effective and safe in non-achalasia esophageal motility disorders: an international multicenter study. Endosc Int Open 2018;6:E1031–E1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beveridge C, Lynch K. Diagnosis and Management of Esophagogastric Junction Outflow Obstruction. Gastroenterol Hepatol (N Y) 2020;16:131–138. [PMC free article] [PubMed] [Google Scholar]
- 101.Roman S, Kahrilas PJ. Management of spastic disorders of the esophagus. Gastroenterol Clin North Am 2013;42:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roman S, Lin Z, Pandolfino JE, et al. Distal Contraction Latency: A Measure of Propagation Velocity Optimized for Esophageal Pressure Topography Studies. Am J Gastroenterol 2011;106:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen JW, Savarino E, Smout A, et al. Chicago Classification Update (v4.0): Technical review on diagnostic criteria for hypercontractile esophagus. Neurogastroenterol Motil 2021:e14115. [DOI] [PubMed] [Google Scholar]
- 104.Almansa C, Heckman MG, DeVault KR, et al. Esophageal spasm: demographic, clinical, radiographic, and manometric features in 108 patients. Dis Esophagus 2012;25:214–21. [DOI] [PubMed] [Google Scholar]
- 105.Spencer HL, Smith L, Riley SA. A questionnaire study to assess long-term outcome in patients with abnormal esophageal manometry. Dysphagia 2006;21:149–55. [DOI] [PubMed] [Google Scholar]
- 106.Cattau EL Jr., Castell DO, Johnson DA, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol 1991;86:272–6. [PubMed] [Google Scholar]
- 107.Konturek JW, Gillessen A, Domschke W. Diffuse esophageal spasm: a malfunction that involves nitric oxide? Scand J Gastroenterol 1995;30:1041–5. [DOI] [PubMed] [Google Scholar]
- 108.Fox M, Sweis R, Wong T, et al. Sildenafil relieves symptoms and normalizes motility in patients with oesophageal spasm: a report of two cases. Neurogastroenterol Motil 2007;19:798–803. [DOI] [PubMed] [Google Scholar]
- 109.Vanuytsel T, Bisschops R, Farre R, et al. Botulinum toxin reduces Dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 2013;11:1115–1121 e2. [DOI] [PubMed] [Google Scholar]
- 110.Miller LS, Pullela SV, Parkman HP, et al. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol 2002;97:1640–6. [DOI] [PubMed] [Google Scholar]
- 111.Storr M, Allescher HD, Rosch T, et al. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc 2001;54:754–9. [PubMed] [Google Scholar]
- 112.Cannon RO 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994;330:1411–7. [DOI] [PubMed] [Google Scholar]
- 113.Clouse RE, Lustman PJ, Eckert TC, et al. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 1987;92:1027–36. [DOI] [PubMed] [Google Scholar]
- 114.Khan MA, Kumbhari V, Ngamruengphong S, et al. Is POEM the Answer for Management of Spastic Esophageal Disorders? A Systematic Review and Meta-Analysis. Dig Dis Sci 2017;62:35–44. [DOI] [PubMed] [Google Scholar]
- 115.Leconte M, Douard R, Gaudric M, et al. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg 2007;94:1113–8. [DOI] [PubMed] [Google Scholar]
- 116.Gyawali CP, Sifrim D, Carlson DA, et al. Ineffective esophageal motility: Concepts, future directions, and conclusions from the Stanford 2018 symposium. Neurogastroenterol Motil 2019;31:e13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shetler KP, Bikhtii S, Triadafilopoulos G. Ineffective esophageal motility: clinical, manometric, and outcome characteristics in patients with and without abnormal esophageal acid exposure. Dis Esophagus 2017;30:1–8. [DOI] [PubMed] [Google Scholar]
- 118.Xiao Y, Kahrilas PJ, Nicodeme F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol 2014;109:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Booth M, Stratford J, Dehn TC. Preoperative esophageal body motility does not influence the outcome of laparoscopic Nissen fundoplication for gastroesophageal reflux disease. Dis Esophagus 2002;15:57–60. [DOI] [PubMed] [Google Scholar]
- 120.Ravi N, Al-Sarraf N, Moran T, et al. Acid normalization and improved esophageal motility after Nissen fundoplication: equivalent outcomes in patients with normal and ineffective esophageal motility. Am J Surg 2005;190:445–50. [DOI] [PubMed] [Google Scholar]
- 121.Rydberg L, Ruth M, Abrahamsson H, et al. Tailoring antireflux surgery: A randomized clinical trial. World J Surg 1999;23:612–8. [DOI] [PubMed] [Google Scholar]
- 122.Fibbe C, Layer P, Keller J, et al. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology 2001;121:5–14. [DOI] [PubMed] [Google Scholar]
- 123.Mauro A, Savarino E, De Bortoli N, et al. Optimal number of multiple rapid swallows needed during high-resolution esophageal manometry for accurate prediction of contraction reserve. Neurogastroenterol Motil 2018;30:e13253. [DOI] [PubMed] [Google Scholar]
- 124.Smith CD, DeVault KR, Buchanan M. Introduction of mechanical sphincter augmentation for gastroesophageal reflux disease into practice: early clinical outcomes and keys to successful adoption. J Am Coll Surg 2014;218:776–81. [DOI] [PubMed] [Google Scholar]
- 125.Asti E, Bonitta G, Lovece A, et al. Longitudinal comparison of quality of life in patients undergoing laparoscopic Toupet fundoplication versus magnetic sphincter augmentation: Observational cohort study with propensity score analysis. Medicine (Baltimore) 2016;95:e4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lei WY, Hung JS, Liu TT, et al. Influence of prucalopride on esophageal secondary peristalsis in reflux patients with ineffective motility. J Gastroenterol Hepatol 2018;33:650–655. [DOI] [PubMed] [Google Scholar]
- 127.Scheerens C, Tack J, Rommel N. Buspirone, a new drug for the management of patients with ineffective esophageal motility? United European Gastroenterol J 2015;3:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karamanolis GP, Panopoulos S, Denaxas K, et al. The 5-HT1A receptor agonist buspirone improves esophageal motor function and symptoms in systemic sclerosis: a 4-week, open-label trial. Arthritis Res Ther 2016;18:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aggarwal N, Thota PN, Lopez R, et al. A randomized double-blind placebo-controlled crossover-style trial of buspirone in functional dysphagia and ineffective esophageal motility. Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- 130.Ravi K, Friesen L, Issaka R, et al. Long-term Outcomes of Patients With Normal or Minor Motor Function Abnormalities Detected by High-resolution Esophageal Manometry. Clin Gastroenterol Hepatol 2015;13:1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ghisa M, Laserra G, Marabotto E, et al. Achalasia and Obstructive Motor Disorders Are Not Uncommon in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 132.Nennstiel S, Bajbouj M, Becker V, et al. High-resolution manometry in patients with eosinophilic esophagitis under topical steroid therapy-a prospective observational study (HIMEOS-study). Neurogastroenterol Motil 2016;28:599–607. [DOI] [PubMed] [Google Scholar]
- 133.Mandl T, Ekberg O, Wollmer P, et al. Dysphagia and dysmotility of the pharynx and oesophagus in patients with primary Sjogren’s syndrome. Scand J Rheumatol 2007;36:394–401. [DOI] [PubMed] [Google Scholar]
- 134.Anselmino M, Zaninotto G, Costantini M, et al. Esophageal motor function in primary Sjogren’s syndrome: correlation with dysphagia and xerostomia. Dig Dis Sci 1997;42:113–8. [DOI] [PubMed] [Google Scholar]
- 135.Karamanolis GP, Denaxas K, Panopoulos S, et al. Severe oesophageal disease and its associations with systemic sclerosis. Clin Exp Rheumatol 2017;35 Suppl 106:82–85. [PubMed] [Google Scholar]
- 136.Rosztoczy A, Kovacs L, Wittmann T, et al. Manometric assessment of impaired esophageal motor function in primary Sjogren’s syndrome. Clin Exp Rheumatol 2001;19:147–52. [PubMed] [Google Scholar]
- 137.Grande L, Lacima G, Ros E, et al. Esophageal motor function in primary Sjogren’s syndrome. Am J Gastroenterol 1993;88:378–81. [PubMed] [Google Scholar]
- 138.Marie I, Ducrotte P, Denis P, et al. Oesophageal mucosal involvement in patients with systemic sclerosis receiving proton pump inhibitor therapy. Aliment Pharmacol Ther 2006;24:1593–601. [DOI] [PubMed] [Google Scholar]
- 139.Babaei A, Szabo A, Shad S, et al. Chronic daily opioid exposure is associated with dysphagia, esophageal outflow obstruction, and disordered peristalsis. Neurogastroenterol Motil 2019;31:e13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010;31:601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Babaei A, Shad S, Massey BT. Motility Patterns Following Esophageal Pharmacologic Provocation With Amyl Nitrite or Cholecystokinin During High-Resolution Manometry Distinguish Idiopathic vs Opioid-Induced Type 3 Achalasia. Clin Gastroenterol Hepatol 2020;18:813–821 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patel D, Callaway J, Vaezi M. Opioid-Induced Foregut Dysfunction. Am J Gastroenterol 2019;114:1716–1725. [DOI] [PubMed] [Google Scholar]
- 143.Yang H, Meun C, Sun X, et al. Outcome following management of dysphagia after laparoscopic anti-reflux surgery. World J Surg 2012;36:838–43. [DOI] [PubMed] [Google Scholar]
- 144.Wehrli NE, Levine MS, Rubesin SE, et al. Secondary achalasia and other esophageal motility disorders after laparoscopic Nissen fundoplication for gastroesophageal reflux disease. AJR Am J Roentgenol 2007;189:1464–8. [DOI] [PubMed] [Google Scholar]
- 145.Hui JM, Hunt DR, de Carle DJ, et al. Esophageal pneumatic dilation for postfundoplication dysphagia: safety, efficacy, and predictors of outcome. Am J Gastroenterol 2002;97:2986–91. [DOI] [PubMed] [Google Scholar]
- 146.Schuitenmaker JM, van Hoeij FB, Schijven MP, et al. Pneumatic dilation for persistent dysphagia after antireflux surgery, a multicentre single-blind randomised sham-controlled clinical trial. Gut 2021. [DOI] [PubMed] [Google Scholar]
- 147.Miller AT, Matar R, Abu Dayyeh BK, et al. Postobesity Surgery Esophageal Dysfunction: A Combined Cross-Sectional Prevalence Study and Retrospective Analysis. Am J Gastroenterol 2020;115:1669–1680. [DOI] [PubMed] [Google Scholar]
- 148.Pimentel M, Bonorris GG, Chow EJ, et al. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol 2001;33:27–31. [DOI] [PubMed] [Google Scholar]
- 149.Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut 2002;50:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J 1998;19:250–4. [DOI] [PubMed] [Google Scholar]
- 151.Park SW, Lee H, Lee HJ, et al. Low-dose amitriptyline combined with proton pump inhibitor for functional chest pain. World J Gastroenterol 2013;19:4958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee H, Kim JH, Min BH, et al. Efficacy of venlafaxine for symptomatic relief in young adult patients with functional chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol 2010;105:1504–12. [DOI] [PubMed] [Google Scholar]
- 153.Varia I, Logue E, O’Connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J 2000;140:367–72. [DOI] [PubMed] [Google Scholar]
- 154.Keefe FJ, Shelby RA, Somers TJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain 2011;152:730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doraiswamy PM, Varia I, Hellegers C, et al. A randomized controlled trial of paroxetine for noncardiac chest pain. Psychopharmacol Bull 2006;39:15–24. [PubMed] [Google Scholar]


