Abstract
Simple Summary
The IMbrave150 trial led to the approval of atezolizumab and bevacizumab for the treatment of unresectable hepatocellular carcinoma (HCC). We performed a retrospective multicenter study including 115 patients with unresectable HCC treated with atezolizumab and bevacizumab, revealing that the combination of atezolizumab and bevacizumab is equally effective for patients meeting the IMbrave150 trial eligibility criteria and for patients not meeting these criteria, generally due to a history of systemic therapy, platelet counts < 75 × 109/L, Child-Pugh B, and 2+ proteinuria. However, liver functional reserve should be carefully monitored in patients not meeting the IMbrave150 trial eligibility criteria.
Abstract
The IMbrave150 trial demonstrated the high efficacy and safety of atezolizumab and bevacizumab for unresectable hepatocellular carcinoma (HCC). In this multicenter study, the efficacy of this combination and its effect on liver functional reserve were evaluated in patients not meeting the eligibility criteria of IMbrave150. Of 115 patients with unresectable HCC treated with atezolizumab and bevacizumab between October 2020 and January 2022, 72 did not meet the eligibility criteria of IMbrave150, most frequently due to a history of systemic therapy (60/72), platelet counts < 75 × 109/L (7/72), Child-Pugh B (9/72), and 2+ proteinuria (8/72). Atezolizumab and bevacizumab therapy was equally effective for patients who did or did not meet the eligibility criteria (PFS, 6.5 vs. 6.9 months, p = 0.765), consistent with subgroup analyses of histories of systemic therapy, platelet counts, Child-Pugh, and proteinuria. Baseline ALBI scores were worse in patients who did not meet the criteria than in those who did and significantly worsened after treatment initiation in patients not meeting the criteria (baseline vs. 12 weeks; 2.35 ± 0.43 vs. −2.18 ± 0.54; p = 0.007). Accordingly, atezolizumab plus bevacizumab was effective for patients not meeting the eligibility criteria of IMbrave150, although careful monitoring for changes in liver functional reserve is needed.
Keywords: atezolizumab, bevacizumab, eligibility criteria of IMbrave150, hepatocellular carcinoma
1. Introduction
Recent advances in systemic therapy have dramatically changed the treatment landscape for unresectable hepatocellular carcinoma (HCC), with prolonged overall survival (OS) [1,2,3,4]. Various systemic therapies have been developed for unresectable HCC, including the multikinase inhibitors sorafenib [1], regorafenib [5], cabozantinib [6], and lenvatinib [2], an antibody against VEGFR2, ramucirumab [7], and the combination of the programmed death ligand 1 (PD-L1) inhibitor atezolizumab and the VEGF inhibitor bevacizumab. Atezolizumab and bevacizumab combination therapy for patients with unresectable HCC significantly prolonged OS over that of patients treated with sorafenib in the phase 3 clinical trial IMbrave150 [3]. Therefore, recent guidelines recommend the combination of atezolizumab and bevacizumab as a first-line systemic therapy for patients with unresectable HCC [8,9].
However, in real-world settings, many patients do not meet eligibility criteria of IMbrave150 due to a history of systematic therapy for unresectable HCC, protein uremia, anemia, or low platelet counts [3]. Thus, the accumulation of real-world data for the efficacy of atezolizumab and bevacizumab in unresectable HCC, especially in patients who do not meet eligibility criteria of IMbrave150, is required. We have recently reported the high efficacy and safety of atezolizumab and bevacizumab for unresectable HCC in patients in the early phase of treatment who do not meet the eligibility criteria of IMbrave150 [10]. However, Tiago de Castro et al. have recently reported that OS and progression-free survival (PFS) were significantly longer in patients who met the eligibility criteria of IMbrave150 than in patients who did not meet the eligibility criteria of IMbrave150 [OS: 15.0 months vs. 6.0 months; PFS: 8.7 months vs. 3.7 months, respectively] [11]. Thus, additional data for the efficacy of atezolizumab and bevacizumab in patients who do not meet IMbrave150 eligibility criteria are urgently needed.
In the treatment of HCC, liver functional reserve is one of the most important factors for treatment decision-making. Given the availability of various therapeutic options for unresectable HCC, the maintenance of hepatic functional reserve during systemic therapy is crucial for subsequent salvage therapy after progressive disease. Terashima et al. have reported that in patients with unresectable HCC treated with tyrosine kinase inhibitor (TKI), a longer OS is highly associated with post-progression survival, not PFS [12]. Thus, determining the factors associated with changes in liver functional reserve during atezolizumab and bevacizumab combination therapy is a clinically important issue.
In this real-world multicenter study, we compared the efficacy of bevacizumab and atezolizumab and its effect on liver functional reserve in patients who did and did not meet the eligibility criteria for IMbrave150.
2. Methods
2.1. Patients and Study Design
This was a retrospective multicenter study. Consecutive unresectable HCC patients who were treated with atezolizumab and bevacizumab were recruited between October 2020 and February 2022 at the institutes participating in the NORTE Study Group [13,14,15,16,17]. Patients were included if they were treated with atezolizumab and bevacizumab between October 2020 and February 2022 and if sufficient clinical information was available. Clinical data were collected, including age, gender, blood tests, tumor markers, Child–Pugh score, albumin-bilirubin (ALBI) grade [18], modified ALBI (mALBI) grade [19], etiology of HCC, and Barcelona Clinic Liver Cancer (BCLC) stage. Patients were excluded if they had insufficient clinical data, had decompensated liver cirrhosis, or declined to participate in this study. All included patients were evaluated, using endoscopy, for the presence of varices before initiation of atezolizumab and bevacizumab, and, when necessary, the varices were properly treated.
Each attending physician typically evaluated the patients every 3 weeks by laboratory data and physical findings, and evaluated the treatment response every 6 to 12 weeks by dynamic CT or MRI according to Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) and modified Response Evaluation Criteria in Solid Tumors (mRECIST) [20]. Adverse events (AEs) were evaluated by the attending physician every 3 weeks. Atezolizumab and/or bevacizumab was interrupted if grade 3 or higher AEs or unacceptable AEs were observed until AEs resolved. Atezolizumab and/or bevacizumab were resumed according to the package inserts. Atezolizumab and bevacizumab was discontinued when progressive disease (PD) was observed or when unacceptable AEs were observed.
AE grades were defined referring to the American Society of Clinical Oncology Clinical Practice Guidelines [21] and the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.0).
PFS, treatment responses, and changes in liver functional reserve were evaluated in patients with unresectable HCC treated with atezolizumab and bevacizumab stratified according to IMbrave150 eligibility and clinical factors.
The study conformed to the ethical guidelines of the Declaration of Helsinki and all participating patients provided informed consent. This study was approved by the ethics committee of Hokkaido University Hospital (020-0267) and by the ethical committee of each participating institution.
2.2. Treatment Protocol
Every 3 weeks, patients were treated with 1200 mg of atezolizumab (Chugai Co. Ltd., Tokyo, Japan) and 15 mg/kg bevacizumab (Chugai Co. Ltd., Tokyo, Japan).
2.3. Evaluation of Liver Functional Reserve during Treatment
Changes in liver functional reserve were evaluated based on the ALBI score at baseline and 3, 6, 9, and 12 weeks after treatment initiation. The changes in mALBI grade between baseline and 12 weeks after treatment initiation were also evaluated. Referring to previous studies, the modified ALBI (mALBI) grade was evaluated by dividing ALBI grade 2 into 2a and 2b, using an ALBI score cut-off value of −2.270 [19].
2.4. Statistical Analysis
We analyzed continuous variables by Mann–Whitney U-test or the paired t-test. We analyzed categorical data using the Fisher’s exact test or chi-squared test. Survival curves for PFS were calculated by the Kaplan–Meier analysis and compared using the log-rank test. In this study, we did not analyze the overall survival due to insufficient follow-up duration.
In this study, we set p < 0.05 as statistically significant. We utilized SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA) in all analyses.
3. Results
3.1. Overview of Patient Characteristics According to Eligibility Criteria of IMbrave150
Between October 2020 and January 2022, 115 patients with unresectable HCC treated with atezolizumab and bevacizumab at the institutes of the NORTE study group were included in this study. Baseline patient characteristics are shown in Table 1. The median age was 72 years (range, 31–89 years). The majority of patients were male (95 males (82.6%) and 20 females (17.4%)). A total of 80 (69.6%) patients had BCLC stage C, and HBV, HCV and non-B non-C were identified in 35 (30.4%), 21 (18.3%), and 59 patients (51.3%), respectively. A total of 77 patients (67.0%) had ALBI grade 2, and 106 (92.2%) and 9 (7.8%) had a baseline Child-Pugh grade of A and B, respectively. All patients with Child-Pugh grade B at the initiation of treatment had Child-Pugh grade A at the decision-making point for the treatment of unresectable HCC.
Table 1.
Baseline patient characteristics.
| Clinical Characteristics | Overall Cohort (n = 115) |
Met the IMbrave150 Criteria (n = 43) |
Did Not Meet the IMbrave150 Criteria (n = 72) | p-Value |
|---|---|---|---|---|
| Age, years (range) | 72 (31–89) | 73 (31–84) | 72 (37–89) | 0.744 |
| Sex | ||||
| Male/Female | 95 (82.6%)/20 (17.4%) | 38 (88.4%)/5 (11.6%) | 57 (79.2%)/15 (20.8%) | 0.309 |
| Etiology | ||||
| HBV | 35 (30.4%) | 11 (25.6%) | 24 (33.3%) | 0.411 |
| HCV | 21 (18.3%) | 10 (23.3%) | 11 (15.3%) | 0.324 |
| Non-viral | 59 (51.3%) | 22 (51.2%) | 37 (51.4%) | 1.000 |
| ECOG PS | ||||
| 0/1–2 | 92 (80.0%)/23 (20.0%) | 39 (90.7%)/4 (9.3%) | 53 (73.6%)/19 (26.4%) | 0.031 |
| BMI, kg/m2 | 23.6 (15.9–37.7) | 24.0 (18.7–37.7) | 23.3 (15.9–33.0) | 0.152 |
| Proteinuria | ||||
| 0–1+/2+ | 98 (85.2%)/8 (7.0%) | 41 (89.1%)/0 (0.0%) | 57 (79.2%)/8 (11.1%) | 0.022 |
| White blood cell, mm3 | 4920 (1970–12780) | 5300 (2950–12,780) | 4900 (1970–11,800) | 0.043 |
| Neutrophil count, mm3 | 3203 (1185–9971) | 3380 (1503–9204) | 2978 (1185–9971) | 0.098 |
| Lymphocyte count, mm3 | 1168 (140–2881) | 1207 (559–2657) | 1100 (140–2881) | 0.056 |
| Neutrophil/Lymphocyte ratio | 2.70 (0.83–18.68) | 3.04 (0.83–7.92) | 2.57 (0.98–18.68) | 0.967 |
| Platelet, ×109/L | 162 (36–586) | 154 (77–558) | 167 (36–586) | 0.773 |
| Prothrombin time, % | 92.9 (35.3–150.0) | 90.0 (42.6–116.9) | 94.6 (35.3–150.0) | 0.317 |
| NH3, µg/dL | 42 (8–136) | 42 (17–116) | 42 (8–136) | 0.538 |
| Albumin, g/dL | 3.7 (2.6–4.8) | 3.8 (2.9–4.8) | 3.7 (2.6–4.8) | 0.030 |
| Total bilirubin, mg/dL | 0.8 (0.2–3.8) | 0.8 (0.2–2.9) | 0.8 (0.3–3.8) | 0.979 |
| mALBI grade | ||||
| ½ | 38 (33.0%)/77 (67.0%) | 17 (39.5%)/26 (60.5%) | 21 (29.2%)/51 (70.8%) | 0.307 |
| 1 | 38 (33.0%) | 17 (39.5%) | 21 (29.2%) | 0.307 |
| 2a | 37 (32.2%) | 15 (34.8%) | 22 (30.6%) | 0.683 |
| 2b | 40 (34.8%) | 11 (25.6%) | 29 (40.3%) | 0.156 |
| AST, IU/L | 42 (14–672) | 35 (16–128) | 47 (14–672) | 0.164 |
| ALT, IU/L | 28 (7–278) | 25 (8–122) | 33 (7–289) | 0.256 |
| Child-Pugh Grade | ||||
| A/B | 106 (92.2%)/9 (7.8%) | 43 (100.0%)/0 (0.0%) | 63 (87.5%)/9 (12.5%) | 0.025 |
| Child-Pugh Score | ||||
| 5 | 62 (53.9%) | 24 (55.8%) | 38 (52.8%) | 0.847 |
| 6 | 44 (38.3%) | 19 (44.9%) | 25 (34.7%) | 0.329 |
| 7 | 6 (5.2%) | 0 (0.0%) | 6 (8.3%) | 0.082 |
| 8 | 3 (2.6%) | 0 (0.0%) | 3 (4.2%) | 0.292 |
| AFP, ng/mL * | 74.2 (0.8–1,450,000.0) | 51.6 (0.8–591,315.4) | 77.2 (1.1–14,500,000.0) | 0.817 |
| AFP > 400 | 40 (34.8%) | 15 (34.9%) | 25 (34.7%) | 1.000 |
| DCP, mAU/mL * | 924 (11–245,000) | 509 (21–213,066) | 1787 (11–245,000) | 0.035 |
| Maximum intrahepatic tumor size, mm | 36 (0–220) | 30 (0–167) | 38 (0–220) | 0.307 |
| More than 50% liver involvement | 16 (17.7%) | 4 (9.3%) | 12 (16.7%) | 0.405 |
| Diffuse type | 15 (15.6%) | 5 (11.6%) | 10 (13.9%) | 0.784 |
| Number of intrahepatic tumors | ||||
| None | 14 (12.2%) | 5 (11.6%) | 9 (12.5%) | 1.000 |
| 1 | 11 (9.6%) | 5 (11.6%) | 6 (8.3%) | 0.745 |
| Multiple | 90 (78.3%) | 33 (76.7%) | 57 (79.2%) | 0.817 |
| BCLC stage | ||||
| B/C | 35 (30.4%)/80 (69.6%) | 13 (30.2%)/30 (69.8%) | 22 (30.6%)/50 (69.4%) | 1.000 |
| Up-to-7 in/out | 30 (26.1%)/85 (73.9%) | 10 (23.3%)/33 (76.7%) | 20 (27.8%)/52 (72.2%) | 0.665 |
| Positive for Vp | 23 (20.0%) | 11 (25.6%) | 12 (16.7%) | 0.335 |
| Vp4 | 4 (3.5%) | 2 (4.7%) | 2 (2.8%) | 0.629 |
| Positive for Vv | 5 (4.3%) | 1 (2.3%) | 4 (5.6%) | 0.649 |
| Positive for bile duct invasion | 4 (3.5%) | 1 (2.3%) | 3 (4.2%) | 1.000 |
| Positive for LN metastasis | 20 (17.4%) | 7 (16.8%) | 13 (18.1%) | 1.000 |
| Positive for EHM | 46 (40.0%) | 17 (39.5%) | 29 (40.3%) | 1.000 |
| History of varices treatment | 8 (7.0%) | 1 (2.3%) | 7 (9.7%) | 0.255 |
| History of hypertension | 69 (60.0%) | 27 (62.8%) | 42 (58.3%) | 0.697 |
| Naïve/recurrence | 14 (20.9%)/91 (79.1%) | 15 (34.9%)/28 (65.1%) | 9 (12.5%)/63 (87.5%) | 0.008 |
| History of operation | 59 (51.3%) | 21 (48.8%) | 38 (52.8%) | 0.707 |
| History of RFA | 41 (35.7%) | 14 (32.6%) | 25 (34.7%) | 0.842 |
| History of TACE | 58 (50.4%) | 14 (32.6%) | 44 (61.1%) | 0.004 |
| 1st line systemic chemotherapy | 55 (47.8%) | 43 (100.0%) | 12 (16.7%) | <0.001 |
| 2nd line | 41 (35.7%) | 0 (0.0%) | 41 (56.9%) | |
| 3rd line | 19 (16.5%) | 0 (0.0%) | 19 (26.4%) | |
| History of TKI | 60 (52.2%) | 0 (0.0%) | 60 (83.3%) | |
| Sorafenib | 19 (16.5%) | 0 (0.0%) | 19 (27.5%) | |
| Regorafenib | 8 (7.0%) | 0 (0.0%) | 8 (11.1%) | |
| Lenvatinib | 59 (51.3%) | 0 (0.0%) | 59 (81.9%) | |
| Observation period, months * | 6.8 (0.1–15.4) | 5.6 (0.1–14.9) | 7.2 (0.3–15.4) | 0.140 |
* Data are presented as median (range) or n. Abbreviations: HCV: hepatitis C virus, HBV: hepatitis B virus, ECOG PS: Eastern Cooperative Oncology Group performance status, BMI: body mass index, AST: aspartate transaminase, ALT: alanine aminotransferase, mALBI grade: modified albumin–bilirubin grade, AFP: alpha-fetoprotein, EHM: extrahepatic metastasis, TKI: tyrosine kinase inhibitor, DCP: des-gamma-carboxy prothrombin, BCLC: The Barcelona Clinic Liver Cancer, Vp: portal vein invasion, Vv: hepatic vein invasion, LN: lymph node, RFA: Radiofrequency ablation, TACE: Transcatheter arterial chemoenbolzation.
Of 115 patients, 72 (62.6%) did not meet the eligibility criteria of IMbrave150. Patients did not meet the IMbrave150 eligibility criteria due to a history of TKI treatment (83.3% 60/72), platelet counts < 75 × 109/L (9.7% 7/72); AST or ALT values exceeding 5 times the upper limit of normal (ULN), (5.6% 4/72 and 2.8% 2/72), Child-Pugh B (12.5% 9/72); serum creatinine > 1.5 times the ULN (4.2% 3/72), 2+ or 2+ < proteinuria (11.1% 8/72), and neutrophil count < 1500/mm3 (8.3% 6/72). A comparison of baseline characteristics between patients who did or did not meet the IMbrave150 eligibility criteria is shown in Table 1.
3.2. Progression-Free Survival and Associated Factors in Patients Who Did or Did Not Meet the IMbrave150 Eligibility Criteria
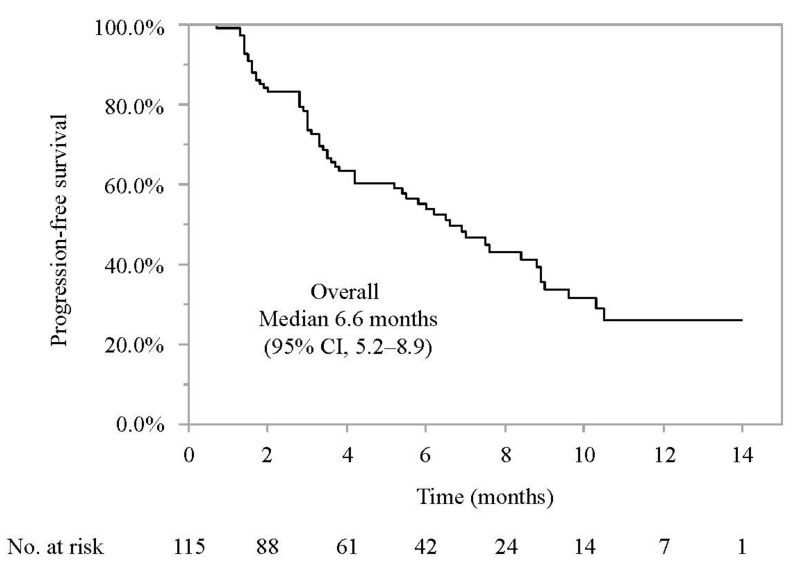
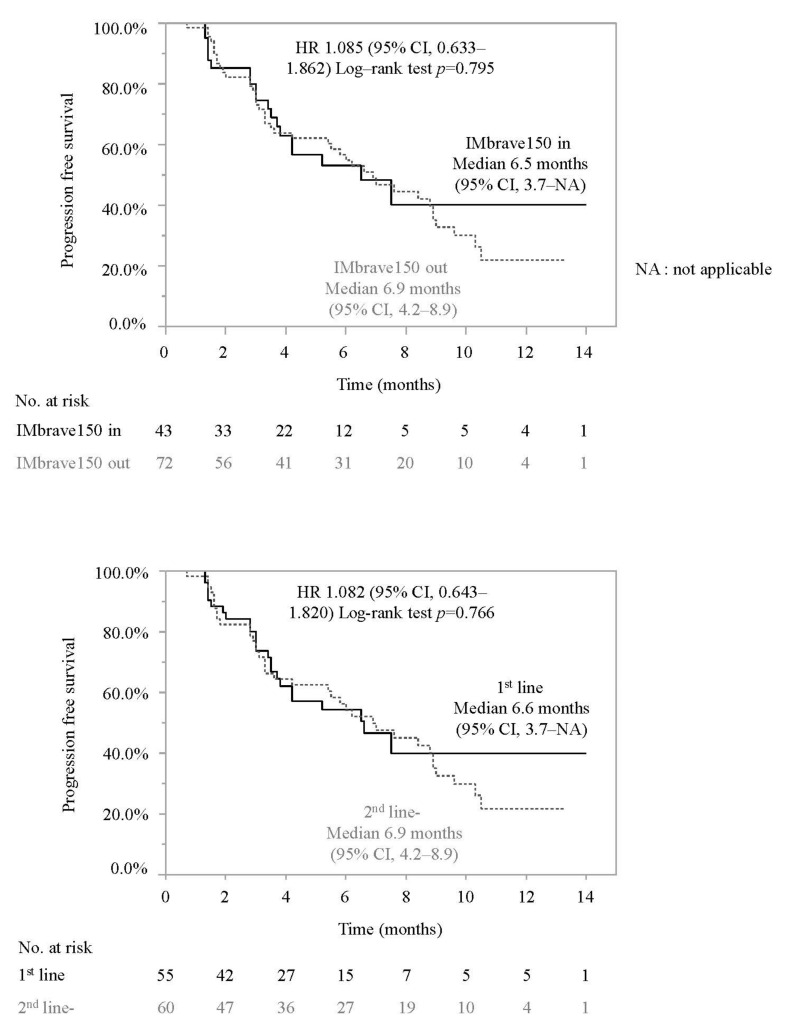
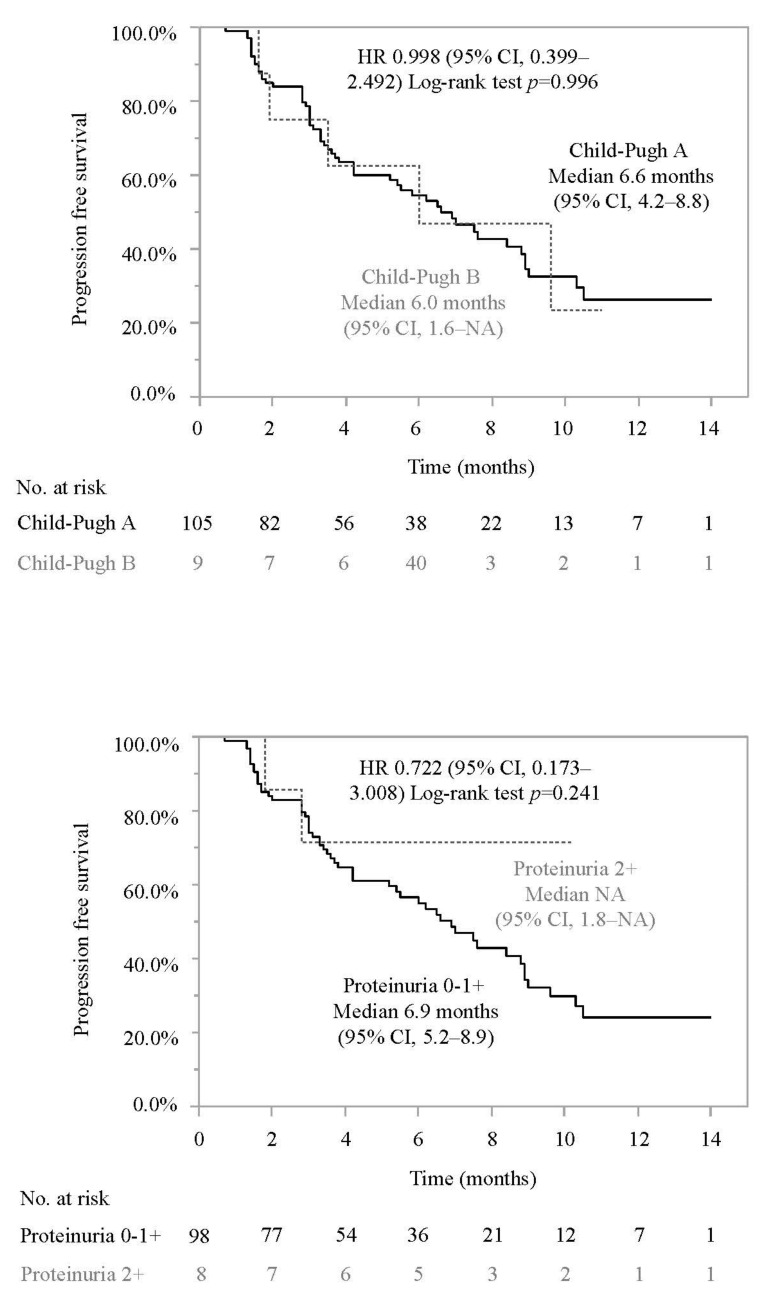
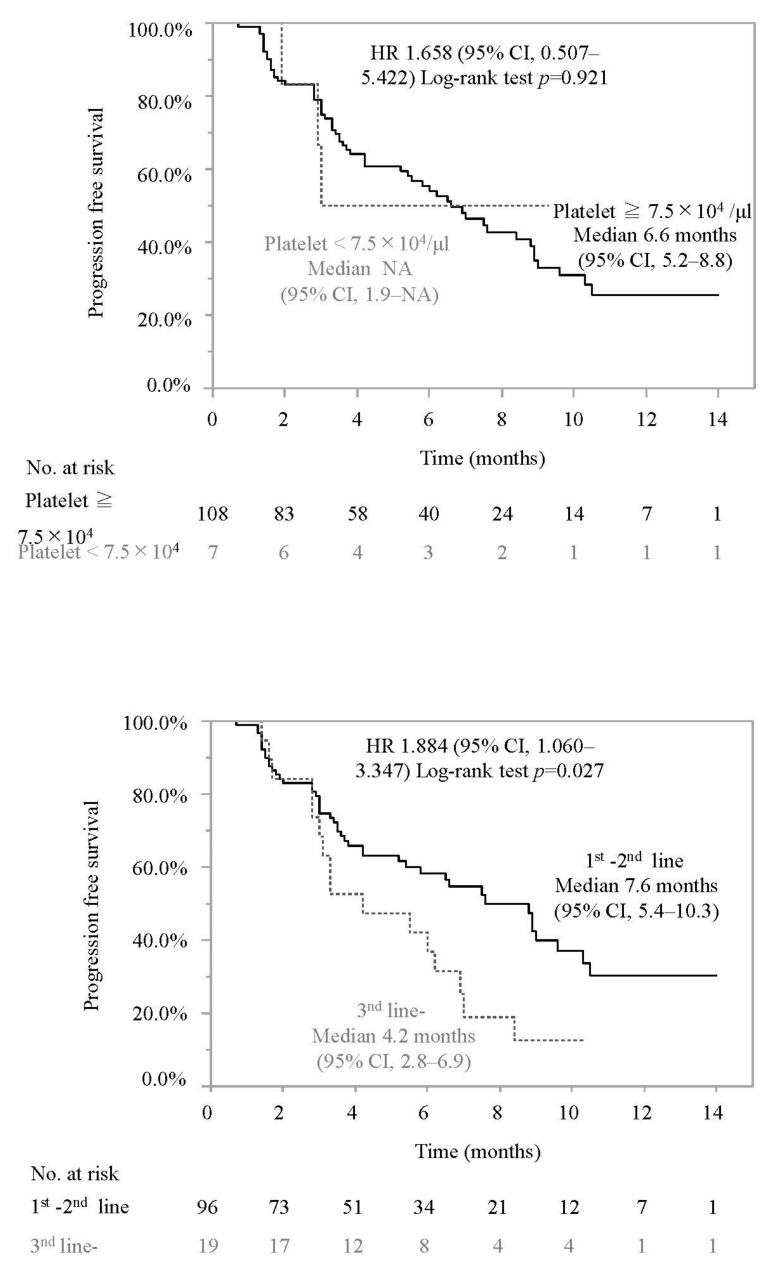
As shown in Figure 1, the median PFS was 6.6 months (95% confidence interval (CI) 5.2–8.9 months). As shown in Figure 2, the median PFS was similar in patients who did and did not meet the eligibility criteria of IMbrave150 (median PFS 6.5 months (95% CI; 3.7–NE) vs. 6.9 months (95% CI; 4.2–8.9), HR 1.085 (95% CI 0.633–1.862); p = 0.795). Among the main factors preventing IMbrave150 eligibility, the median PFS were similar in patients with and without a history of systemic therapy (median PFS 6.6 months (95% CI; 3.7–NA months) vs. 6.9 months (95% CI; 4.2–8.9 months), HR 1.082 (95% CI 0.643–1.820); p = 0.766), patients with or without Child-Pugh B (6.0 months (95% CI, 1.6–NA months) vs. 6.6 months (95% CI, 4.2–8.8 months), HR 0.998 (95% CI, 0.399–2.492); p = 0.996), patients with or without 2+ proteinuria or more than 2+ proteinuria (NA (95% CI, 1.8–NA) vs. 6.9 months (95% CI, 5.2–8.9 months), HR 0.722 (95% CI, 0.173–3.008); p = 0.241), and patients with or without platelet < 7.5 × 104/μL (median PFS NA (95% CI, 1.9–NA months) vs. 6.6 months (95% CI, 5.2–8.8 months), HR 1.658 (95% CI, 0.507–5.422); p = 0.921).
Figure 1.
Progression-free survival in patients with unresectable HCC who were treated with atezolizumab and bevacizumab. Median progression-free survival was 6.6 months (95% confidence interval 5.2–8.9 months). 95% CI: 95% confidence interval.
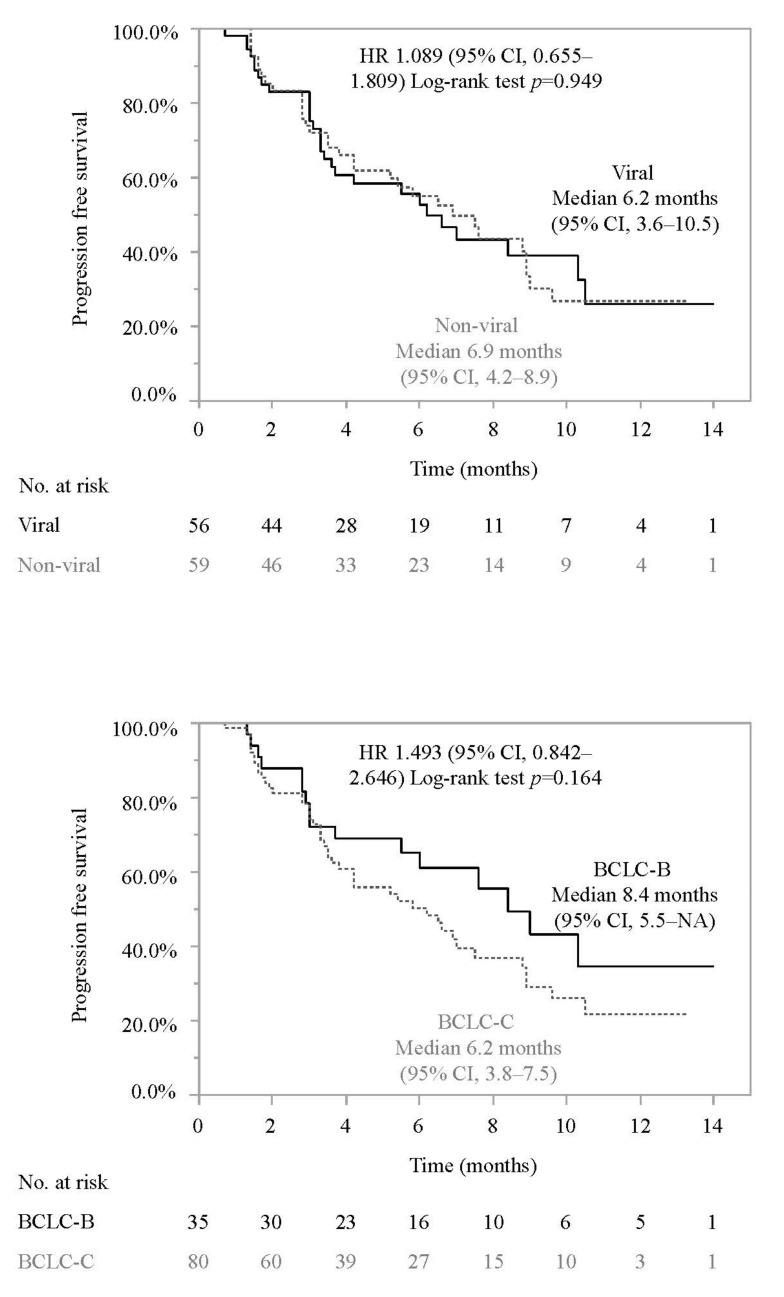
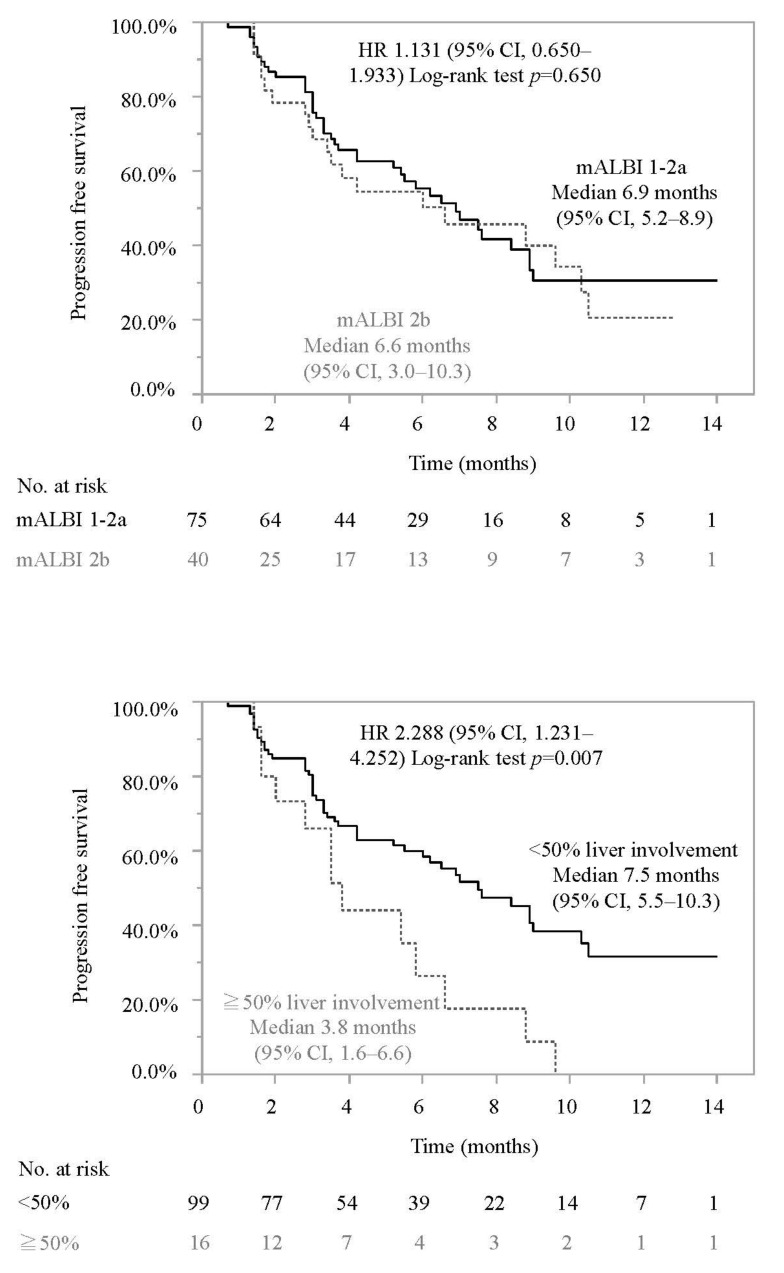
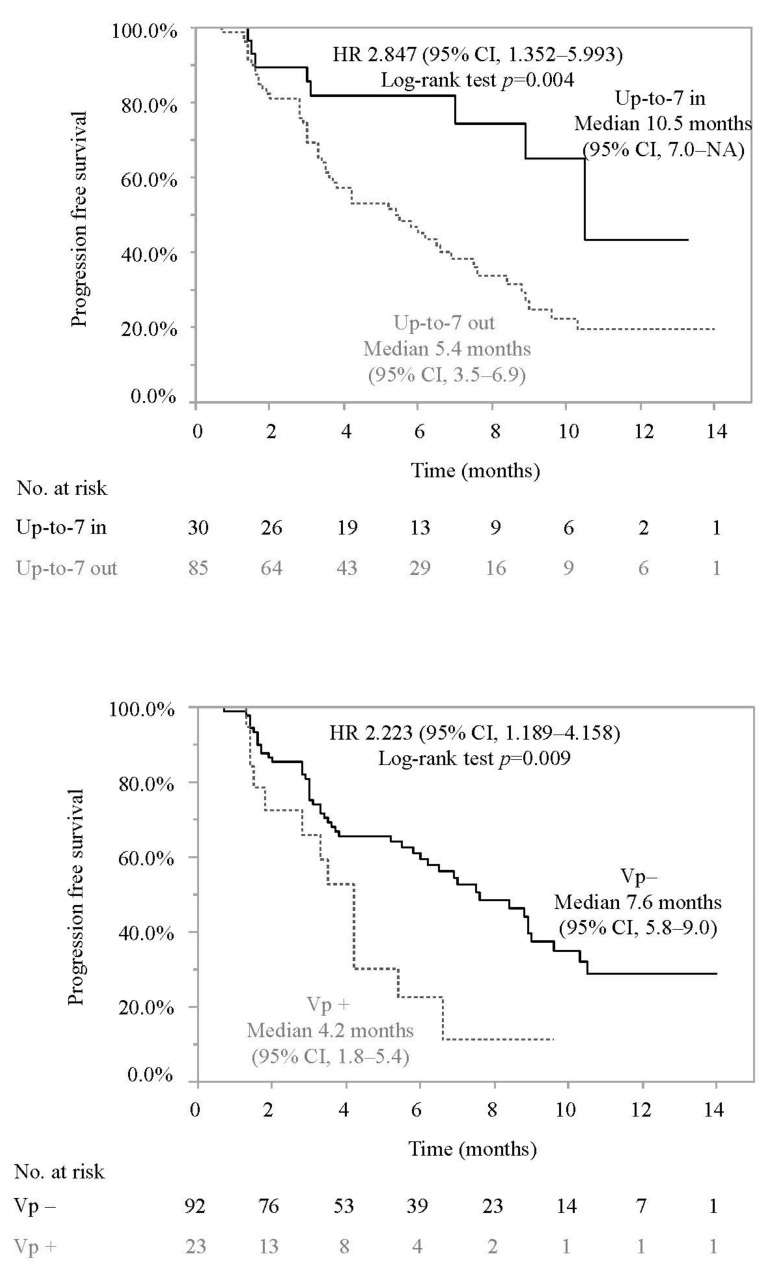
Figure 2.
Comparison of progression-free survival in each subgroup. Survival curves for PFS were calculated by the Kaplan-Meier method and compared using the log-rank test. HR: Hazard Ratio, 95% CI: 95% confidence interval, NA: not applicable, BCLC: Barcelona Clinic Liver Cancer, mALBI: modified albumin-bilirubin grade, Vp: portal vein invasion.
Subsequently, we conducted subgroup analyses. As shown in Figure 2, median PFS was similar between patients with an etiology of viral hepatitis and non-viral hepatitis, BCLC B and C, and mALBI grade 1–2a and 2b.
Patients treated with atezolizumab and bevacizumab as a third-line or further lines therapy had a significantly shorter PFS than that of patients treated with atezolizumab and bevacizumab as a first- or second-line therapy (4.2 months (95% CI 2.8–6.9 months) vs. 7.6 months (95% CI 5.4–10.3 months), HR 1.884 (95% CI 1.060–3.347); p = 0.027). Patients with HCC with >50% liver involvement had a significantly shorter PFS than that of patients with <50% liver involvement (3.8 months (95% CI 1.6–6.6 months) vs. 7.5 months (95% CI 5.5–10.3 months), HR 2.288 (95% CI 1.231–4.252); p = 0.007). Patients who did not meet up to seven criteria had a significantly shorter PFS than that of patients did meet up to seven criteria (5.4 months (95% CI 3.5–6.9 months) vs. 10.5 months (95% CI 7.0–NE months), HR 2.847 (1.352–5.993); p = 0.004). Patients with portal vein invasion (Vp) had a significantly shorter PFS than that of patients without portal vein invasion (4.2 months (95% CI 1.8–5.4 months) vs. 7.6 months (95% CI; 5.8–9.0 months HR 2.223 (1.189–4.158); p = 0.009).
Treatment Response
We analyzed the response during treatment and at 6 weeks after atezolizumab and bevacizumab initiation by RECIST 1.1. and mRECIST (Table 2). As shown in Table 2, in the best response, 3 (2.9%), 17 (16.3%), 63 (60.6%), and 21 (20.2%) patients showed a complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), respectively, based on RESICT v1.1. Thus, the objective response rate (ORR) and disease control rate (DCR) were 19.2% (20/104) and 79.8% (83/104), respectively. Similarly, in the mRECIST evaluation, 9 (8.7%), 20 (19.2%), 51 (49.0%), and 16 (15.4%) patients showed a CR, PR, SD, and PD, respectively, and the data for 8 (7%) patients were not obtained. Thus, the ORR and DCR were 27.9% (29/104) and 79.8% (83/104), respectively. The treatment responses at 6 weeks after atezolizumab and bevacizumab initiation are also shown in Table 2.
Table 2.
Comparison of progression-free survival in each subgroup.
| RECIST v1.1 | mRECIST | |||
|---|---|---|---|---|
| 6 weeks | Best response | 6 weeks | Best response | |
| CR, n (%) | 0 (0.0) | 3 (2.9) | 2 (1.9) | 9 (8.7) |
| PR, n (%) | 9 (8.7) | 17 (16.3) | 18 (17.3) | 20 (19.2) |
| SD, n (%) | 74 (71.2) | 63 (60.6) | 61 (58.7) | 51 (49.0) |
| PD, n (%) | 18 (17.3) | 21 (20.2) | 12 (12.5) | 16 (15.4) |
| NE, n (%) | 3 (2.9) | 0 (0.0) | 10 (9.6) | 8 (7.7) |
| ORR, n (%) | 9 (8.7) | 20 (19.2) | 20 (19.2) | 29 (27.9) |
| DCR, n (%) | 83 (79.8) | 83 (79.8) | 81 (77.9) | 80 (76.9) |
Abbreviations: CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, NE: not evaluable, ORR: objective response rate, DCR: disease control rate.
Subsequently, we compared the ORR and DCR between patients who did or did not meet the eligibility criteria of IMbrave150. As shown in Table 3, the ORR and DCR were similar between patients who did or did not meet the eligibility criteria of IMbrave150 in RECIST (p = 0.804, 18.4% vs. 19.7% and p = 1.000, 81.6% vs. 78.8%). Regarding the main factors causing patients to not meet the eligibility criteria of IMbrave150, ORR and DCR were similar between patients with or without history of systemic therapy, patients with or without platelet < 7.5 × 104/μL, patients with or without 2+ proteinuria or more than 2+ proteinuria, and patients with or without Child-Pugh B. A subgroup analysis revealed that in RECIST v1.1, ORR was significantly higher in patients with up to 7-IN than in patients with up to 7—Out (40.7% vs. 11.7%, p = 0.003) (Table 3).
Table 3.
Comparison of clinical responses in patients who were treated with atezolizumab and bevacizumab in each subgroup.
| RECIST v1.1 | mRECIST | |||||
|---|---|---|---|---|---|---|
| IMbrave150 in (n = 38) | IMbrave150 out (n = 66) | p-Value | IMbrave150 in (n = 38) | IMbrave150 out (n = 66) | p-Value | |
| ORR (%) | 18.4% | 19.7% | 1.000 | 29.0% | 27.3% | 1.000 |
| DCR (%) | 81.6% | 78.8% | 0.804 | 73.7% | 78.8% | 0.631 |
| 1st line (n = 48) | 2nd line- (n = 56) | p-value | 1st line (n = 48) | 2nd line- (n = 56) | p-value | |
| ORR (%) | 18.8% | 19.6% | 1.000 | 31.3% | 25.0% | 0.517 |
| DCR (%) | 81.3% | 78.6% | 0.809 | 75.0% | 78.6% | 0.816 |
| Child-Pugh A (n = 96) | Child-Pugh B (n = 8) | p-value | Child-Pugh A (n = 96) | Child-Pugh B (n = 8) | p-value | |
| ORR (%) | 20.8% | 0.0% | 0.349 | 28.1% | 25.0% | 1.000 |
| DCR (%) | 79.2% | 87.5% | 1.000 | 77.1% | 75.0% | 1.000 |
| Proteinuria 0-1+ (n = 91) | Proteinuria 2+ (n = 7) | p-value | Proteinuria 0-1+ (n = 91) | Proteinuria 2+ (n = 7) | p-value | |
| ORR (%) | 18.7% | 28.6% | 0.618 | 27.5% | 28.6% | 1.000 |
| DCR (%) | 82.4% | 85.7% | 1.000 | 79.1% | 85.7% | 1.000 |
| Platelet ≧ 7.5 × 104/μL (n = 98) | Platelet < 7.5 × 104/μL (n = 6) | p-value | Platelet ≧ 7.5 × 104/μL (n = 98) | Platelet < 7.5 × 104/μL (n = 6) | p-value | |
| ORR (%) | 18.4% | 33.3% | 0.325 | 26.5% | 50.0% | 0.345 |
| DCR (%) | 79.6% | 83.3% | 1.000 | 77.6% | 66.7% | 0.620 |
| 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | |
| ORR (%) | 21.2% | 10.5% | 0.355 | 31.8% | 10.5% | 0.089 |
| DCR (%) | 78.8% | 84.2% | 0.758 | 74.1% | 89.5% | 0.230 |
| Viral (n = 51) | Non-viral (n = 53) | p-value | Viral (n = 51) | Non-viral (n = 53) | p-value | |
| ORR (%) | 17.7% | 20.8% | 0.805 | 23.5% | 32.1% | 0.386 |
| DCR (%) | 80.4% | 79.3% | 1.000 | 72.6% | 81.1% | 0.356 |
| BCLC-B (n = 41) | BCLC-C (n = 63) | p-value | BCLC-B (n = 41) | BCLC-C (n = 63) | p-value | |
| ORR (%) | 19.5% | 19.0% | 1.000 | 34.2% | 23.8% | 0.271 |
| DCR (%) | 87.5% | 76.4% | 0.290 | 87.5% | 72.2% | 0.130 |
| mALBI 1-2a (n = 73) | mALBI 2b (n = 31) | p-value | mALBI 1-2a (n = 73) | mALBI 2b (n = 31) | p-value | |
| ORR (%) | 21.9% | 12.9% | 0.416 | 31.5% | 19.4% | 0.240 |
| DCR (%) | 78.1% | 83.9% | 0.600 | 76.7% | 77.4% | 1.000 |
| <50% liver involvement (n = 90) | ≧50% liver involvement (n = 14) | p-value | <50% liver involvement (n = 90) | ≧50% liver involvement (n = 14) | p-value | |
| ORR (%) | 20.0% | 14.3% | 1.000 | 26.0% | 14.3% | 0.340 |
| DCR (%) | 80.0% | 78.6% | 1.000 | 76.7% | 78.6% | 1.000 |
| Up-to-7 in (n = 27) | Up-to-7 out (n = 77) | p-value | Up-to-7 in (n = 27) | Up-to-7 out (n = 77) | p-value | |
| ORR (%) | 40.7% | 11.7% | 0.003 | 48.2% | 20.8% | 0.002 |
| DCR (%) | 88.9% | 76.6% | 0.265 | 85.2% | 74.0% | 0.296 |
| Vp − (n = 88) | Vp + (n = 16) | p-value | Vp − (n = 88) | Vp + (n = 16) | p-value | |
| ORR (%) | 21.6% | 6.3% | 0.298 | 31.8% | 6.3% | 0.037 |
| DCR (%) | 83.0% | 62.5% | 0.087 | 79.6% | 62.5% | 0.194 |
Abbreviations: ORR: objective response rate, DCR: disease control rate, BCLC: Barcelona Clinic Liver Cancer, mALBI: modified albumin-bilirubin grade, Vp: portal vein invasion.
3.3. Changes in ALBI Score, mALBI Grade, and the Rate of Treatment Interruption
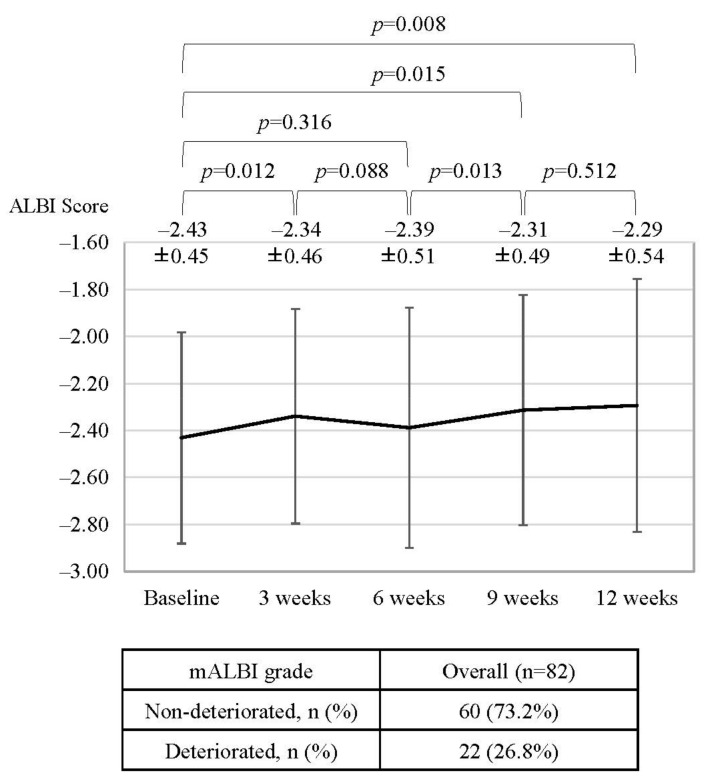
Subsequently, we evaluated the ALBI score and mALBI grade at baseline, 3, 6, 9, and 12 weeks after atezolizumab and bevacizumab initiation. Data for sequential changes in the ALBI score were available for 82 patients. As shown in Figure 3, similar to previous reports [22], the ALBI score deteriorated significantly at 3 weeks after treatment initiation (baseline vs. 3 weeks; 2.43 ± 0.45 vs. −2.34 ± 0.46; p = 0.012) but was restored to baseline levels at 6 weeks (baseline vs. 6 weeks; −2.43 ± 0.45 vs. −2.39 ± 0.51; p = 0.316). Thereafter, the ALBI score deteriorated gradually but significantly (baseline vs. 12 weeks; −2.43 ± 0.45 vs. −2.29 ± 0.54; p = 0.008). Between baseline and 12 weeks after treatment initiation, 26.8% of patients (22/82) showed a worsening in mALBI grade (Figure 3).
Figure 3.
Changes in the ALBI score and mALBI grade during atezolizumab and bevacizumab treatment for unresectable HCC in all cohorts. mALBI: modified albumin-bilirubin grade.
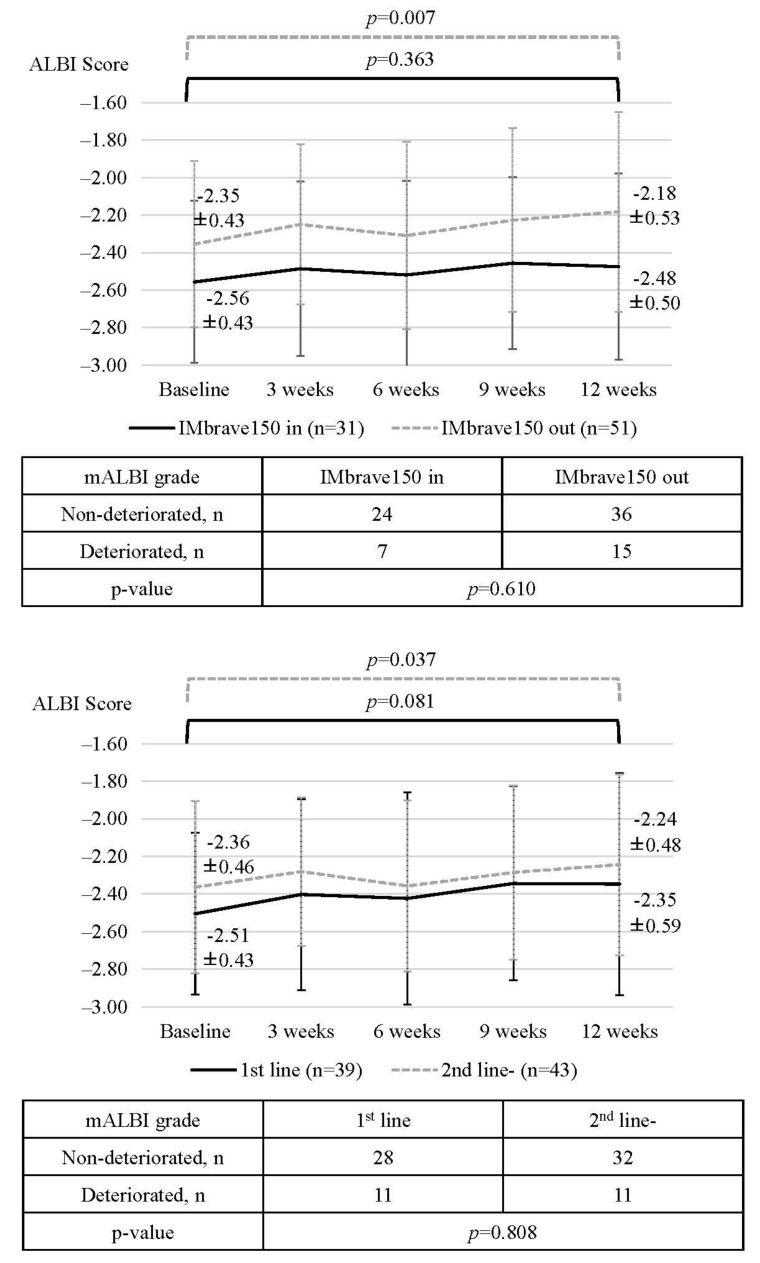
A subgroup analysis revealed that in patients who did not meet the IMbrave150 criteria, ALBI scores worsened significantly at 12 weeks after treatment initiation (baseline vs. 12 weeks; 2.35 ± 0.43 vs. −2.18 ± 0.53; p = 0.007), while in patients who did meet the IMbrave150 criteria, ALBI scores were similar between baseline and 12 weeks after treatment initiation (baseline vs. 12 weeks; −2.56 ± 0.43 vs. −2.48 ± 0.50; p = 0.363) (Figure 4). In addition, baseline ALBI scores tended to be deteriorated in patients who did not meet the eligibility criteria of IMbrave150 compared to patients who did meet the eligibility criteria of IMbrave150 (−2.35 ± 0.43 vs −2.56 ± 0.43 p = 0.051).
Figure 4.
Comparison of changes in the ALBI score and mALBI grade during atezolizumab and bevacizumab treatment for unresectable HCC in subgroups stratified by eligibility criteria of IMbrave150 and history of systemic therapy. mALBI: modified albumin-bilirubin grade.
Among patients not meeting the eligibility criteria of IMbrave150, the ALBI score was significantly worse in patients with a history of systemic therapy than without (baseline vs. 12 weeks; −2.36 ± 0.46 vs. −2.24 ± 0.48, p = 0.037), while significant changes were not observed in patients who met the eligibility criteria of IMbrave150 (Figure 4). Supplementary Figure S1 summarizes the changes in ALBI scores in subgroups of patients with Platelet < 7.5 × 104/μL, 2+ proteinuria or more than 2+ proteinuria, and Child-Pugh B.
Subsequently, we analyzed the rate of treatment interruption in patients who did or did not meet the IMbrave150 inclusion criteria. As shown in Table 4, the rate of atezolizumab interruption was significantly higher in patients who did not meet the inclusion criteria than in patients who met the inclusion criteria (22.2% (16/72) vs. 2.3% (1/46), p = 0.003). In addition, in patients with Child-Pugh grade B, the rate of atezolizumab interruption was significantly higher than that in patients with Child-Pugh A (12.3% (13/106) vs. 44.4% (4/9), p = 0.026). The precise reasoning behind treatment interruption in patients who did or did not meet the IMbrave150 eligibility criteria is demonstrated in Supplementary Table S1.
Table 4.
Comparison of the rate of treatment discontinuation and interruption in patients who were treated with atezolizumab and bevacizumab in each subgroup.
| Overall | |||
|---|---|---|---|
| Discontinuation due to AE n, (%) | 7 (6.1) | ||
| Interruption of Atezo n, (%) | 17 (14.8) | ||
| Interruption of Bev n, (%) | 30 (26.1) | ||
| IMbrave150 in (n = 43) | IMbrave150 out (n = 72) | p-value | |
| Discontinuation due to AE n, (%) | 1 (2.3) | 6 (8.3) | 0.254 |
| Interruption of Atezo n, (%) | 1 (2.3) | 16 (22.2) | 0.003 |
| Interruption of Bev n, (%) | 8 (18.6) | 22 (30.6) | 0.191 |
| 1st line (n = 55) | 2nd line (n = 60) | p-value | |
| Discontinuation due to AE n, (%) | 4 (7.3) | 3 (5.0) | 0.710 |
| Interruption of Atezo n, (%) | 5 (9.1) | 12 (20.0) | 0.120 |
| Interruption of Bev n, (%) | 13 (23.6) | 17 (28.3) | 0.672 |
| Child-Pugh A (n = 106) | Child-Pugh B (n = 9) | p-value | |
| Discontinuation due to AE n, (%) | 6 (5.7) | 1 (11.1) | 0.444 |
| Interruption of Atezo n, (%) | 13 (12.3) | 4 (44.4) | 0.026 |
| Interruption of Bev n, (%) | 26 (24.5) | 4 (44.4) | 0.237 |
| Proteinuria 0-1+ (n = 98) | Proteinuria 2+ (n = 8) | p-value | |
| Discontinuation due to AE n, (%) | 6 (6.1) | 1 (12.5) | 0.423 |
| Interruption of Atezo n, (%) | 14 (14.3) | 2 (25.0) | 0.347 |
| Interruption of Bev n, (%) | 26 (26.5) | 3 (37.5) | 0.681 |
| Platelet ≧ 7.5 × 104/μL (n =108) | Platelet < 7.5 × 104/μL (n = 7) | p-value | |
| Discontinuation due to AE n, (%) | 7 (6.5) | 0 (0.0) | 1.000 |
| Interruption of Atezo n, (%) | 14 (13.0) | 3 (42.9) | 0.065 |
| Interruption of Bev n, (%) | 26 (24.1) | 4 (57.1) | 0.075 |
| 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | |
| Discontinuation due to AE n, (%) | 7 (7.3) | 0 (0.0) | 0.598 |
| Interruption of Atezo n, (%) | 13 (13.5) | 4 (21.1) | 0.478 |
| Interruption of Bev n, (%) | 22 (22.9) | 8 (42.1) | 0.093 |
Abbreviations: AE: adverse event, Atezo: Atezolizumab, Bev: bevacizumab, BCLC: Barcelona Clinic Liver Cancer, mALBI: modified albumin-bilirubin grade, Vp: portal vein invasion.
4. Discussion
In this real-world multicenter study, of 115 patients with unresectable HCC who were treated with atezolizumab and bevacizumab, 62.6% (72/115) did not meet the eligibility criteria of IMbrave150, largely due to a history of TKI treatment (83.3%, 60/72), platelet counts < 75 × 109/L (9.7%, 7/72), Child-Pugh B (12.5%, 9/72); and 2+ proteinuria (11.1%, 8/72). We revealed that atezolizumab and bevacizumab were equally effective for patients who did or did not meet the eligibility criteria of IMbrave150 (median PFS 6.5 months (95% CI; 3.7–NE months) vs. 6.9 months (95% CI; 4.2–8.9 months), respectively, p = 0.794, with no difference in ORR and DCR between groups). Thus, even in patients who did not meet the eligibility criteria of IMbrave150, atezolizumab and bevacizumab treatment was highly effective.
Baseline ALBI scores tended to be worse in patients who did not meet the eligibility criteria of IMbrave150 than in patients who did meet the eligibility criteria of IMbrave150 (−2.35 ± 0.43 vs. −2.56 ± 0.43 p = 0.051). Furthermore, ALBI scores decreased significantly from baseline to 12 weeks after treatment initiation (2.35 ± 0.43 vs. −2.18 ± 0.54, respectively, p = 0.007) in patients who did not meet the IMbrave150 criteria, with no difference over time in patients who did meet the criteria. Maintaining the liver functional reserve is essential for subsequent salvage systematic therapy; accordingly, it may be necessary to carefully monitor changes in liver functional reserve during atezolizumab and bevacizumab therapy in patients who did not meet the IMbrave150 criteria.
A recent real-world study has reported that the median PFS is significantly shorter in patients who did not meet the IMbrave150 eligibility criteria than in patients who met the criteria [PFS: 3.7 months vs. 8.7 months] [11]. Although more than half of patients did not meet the eligibility criteria for IMbrave150 in this study, median PFS was similar between patients who did and those who did not meet the criteria (PFS 6.5 months (95% CI; 3.7–NE months) vs. 6.9 (95% CI; 4.2–8.9 months)). This discrepancy may be attributed to the difference in the number of patients who did not meet the IMbrave150 eligibility criteria, with deteriorated hepatic functional reserve. In the present study, there were 9 and 0 cases of Child-Pugh B and C, respectively, whereas the previous study included 35 (47.9%) Child-Pugh B cases and 6 (8.2%) Child-Pugh C cases not meeting the IMbrave150 eligibility criteria. Thus, the present study could reveal that, if hepatic functional reserve is preserved, atezolizumab and bevacizumab combination therapy is effective even for patients who did not meet the IMbrave150 inclusion criteria.
A recent study has revealed that OS and PFS are significantly shorter in patients with Child-Pugh B and C than in patients with Child-Pugh A [11,23]. In this study, the PFS and treatment response were similar between patients with Child-Pugh A and B. A small number of patients with Child-Pugh B could affect these results. In addition, most patients had a Child-Pugh score of 7. A recent study reports that the median PFS for atezolizumab and bevacizumab is similar in patients with a Child-Pugh score of 6 (5.1 months, 95%CI: 3.8–6.4 months) and 7 (6.3 months, 95% CI: 2.3–7.0 months) [24]. This might explain the better PFS of patients with Child-Pugh B in this study; however, further studies with larger sample sizes are required to confirm this.
In this study, as shown in Figure 2, the median PFS was similar between patients treated with atezolizumab and bevacizumab as a first-line therapy and as a second-line or later therapy. However, in patients who were treated with atezolizumab and bevacizumab as third line or later, the median PFS was significantly shorter than that in patients treated with atezolizumab and bevacizumab as first- or second-line systemic therapy. These results indicate that atezolizumab and bevacizumab should be selected as a first-line therapy or a second-line systemic therapy for unresectable HCC. It is not clear why patients treated with atezolizumab and bevacizumab as third-line or later line systematic therapy showed a shorter median PFS. Almost all drugs used as first- and second-line systematic therapy have anti-VEGF activity; thus, a lack of response to these drugs might be related to resistance to anti-VEGF therapy. A recent report has shown that anti-VEGF therapy could induce CD8+ T cell infiltration in HCC; therefore, immune checkpoint inhibitors combined with bevacizumab (e.g., atezolizumab and bevacizumab) could achieve a good response [25,26,27]. Additionally, interruption of bevacizumab tended to be higher in patients treated with atezolizumab and bevacizumab as third-line or later line systematic therapy, compared with those treated as first or second line (p = 0.09). Thus, HCC with resistance to anti-VEGF therapy might show a poor response to atezolizumab and bevacizumab. Further analyses are required to validate this hypothesis.
In this study, eight patients had 2+ or more than 2+ proteinuria, and most of these patients (7/8) had a history of TKI therapy. Although the sample size was limited, the median PFS, treatment response, and rate of treatment interruption in patients with 2+ or more than 2+ proteinuria were similar to those in patients without it. Thus, atezolizumab and bevacizumab might be effective and safe even in patients with 2+ or more than 2+ proteinuria.
There are several limitations to this retrospective multicenter study. The number of patients, especially patients with Child-Pugh B, 2+ or more than 2+ proteinuria and platelet < 7.5 × 104/μL was limited, and this should be considered when interpreting the results. In addition, the observational period was relatively limited. Thus, a larger prospective study with a longer observational period is required in the near future to validate the results of this study.
5. Conclusions
In conclusion, atezolizumab and bevacizumab is effective even for patients who do not meet the IMbrave150 inclusion criteria. However, these patients showed a worse baseline liver functional reserve and decreases in median ALBI scores during atezolizumab and bevacizumab therapy, emphasizing the need for careful monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14163938/s1, Supplementary Figure S1: Changes in the ALBI score and mALBI grade during atezolizumab and bevacizumab treatment for unresectable HCC in each subgroup. mALBI: modified albumin-bilirubin grade, BCLC: Barcelona Clinic Liver Cancer, mALBI: modified albumin-bilirubin grade, Vp: portal vein invasion. Supplementary Table S1.
Author Contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; T.S., G.S., Y.Y., K.F., M.B., K.O., A.K., Y.T., Q.F., Z.Y., M.K., T.K. (Takashi Kitagataya), O.M., S.O., A.N., R.Y., M.O., N.K., M.N. (Mitsuteru Natsuizaka), M.N. (Masato Nakai), K.S., T.I., T.M. (Takashi Meguro), K.T., T.T., J.I., T.K. (Tomoe Kobayashi) and T.M. (Takuto Miyagishima); Drafting the work or revising it critically for important intellectual content; S.O. and N.S.; Final approval of the version to be published; G.S.; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. G.S. and N.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the ethics committee of Hokkaido University Hospital (020-0267) and the ethical committee of each participating institution at 9 September 2020.
Informed Consent Statement
All participating patients provided informed consent.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Professor Naoya Sakamoto received lecture fees from Chugai Pharmaceutical Co., Ltd., and research grants from Gilead Sciences Inc. and AbbVie GK. Goki Suda received research grants from Gilead Sciences Inc. The other authors have no conflicts of interest to disclose.
Funding Statement
This study was supported in part by grants from the Japan Agency for Medical Research and Development (AMED; grant numbers JP22fk0310501, JP22fk0210072, JP22fk0310518, JP22fk0210103, JP22fk0210113, JP22fk0210064, JP22fk0210066, JP22fk0210104, JP22fk0210111, JP22fk0210112, JP22fk0310524, JP22fk0210067.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 3.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z., Suda G., Maehara O., Ohara M., Yoshida S., Hosoda S., Kimura M., Kubo A., Tokuchi Y., Fu Q., et al. Changes in Serum Growth Factors during Lenvatinib Predict the Post Progressive Survival in Patients with Unresectable Hepatocellular Carcinoma. Cancers. 2022;14:232. doi: 10.3390/cancers14010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M., Tsuchiya K., Kato N., Hagihara A., Numata K., Aikata H., Inaba Y., Kondo S., Motomura K., Furuse J., et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: A phase 2 multicenter study. J. Gastroenterol. 2021;56:181–190. doi: 10.1007/s00535-020-01753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu A.X., Kang Y.K., Yen C.J., Finn R.S., Galle P.R., Llovet J.M., Assenat E., Brandi G., Pracht M., Lim H.Y., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 8.Gordan J.D., Kennedy E.B., Abou-Alfa G.K., Beg M.S., Brower S.T., Gade T.P., Goff L., Gupta S., Guy J., Harris W.P., et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M., Kawamura Y., Hasegawa K., Tateishi R., Kariyama K., Shiina S., Toyoda H., Imai Y., Hiraoka A., Ikeda M., et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sho T., Suda G., Ogawa K., Kimura M., Kubo A., Tokuchi Y., Kitagataya T., Maehara O., Ohnishi S., Shigesawa T., et al. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol. Res. 2021;51:979–989. doi: 10.1111/hepr.13693. [DOI] [PubMed] [Google Scholar]
- 11.de Castro T., Jochheim L.S., Bathon M., Welland S., Scheiner B., Shmanko K., Roessler D., Khaled N.B., Jeschke M., Ludwig J.M., et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: A real-world experience. Ther. Adv. Med. Oncol. 2022;14:17588359221080298. doi: 10.1177/17588359221080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terashima T., Yamashita T., Takata N., Nakagawa H., Toyama T., Arai K., Kitamura K., Yamashita T., Sakai Y., Mizukoshi E., et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol. Res. 2016;46:650–656. doi: 10.1111/hepr.12601. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K., Suda G., Yamamoto Y., Furuya K., Baba M., Nakamura A., Miyoshi H., Kimura M., Maehara O., Yamada R., et al. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J. Gastroenterol. 2021;56:168–180. doi: 10.1007/s00535-020-01750-3. [DOI] [PubMed] [Google Scholar]
- 14.Ohara M., Suda G., Kimura M., Maehara O., Shimazaki T., Shigesawa T., Suzuki K., Nakamura A., Kawagishi N., Nakai M., et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol. Res. 2020;50:715–725. doi: 10.1111/hepr.13499. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa K., Kobayashi T., Furukawa J.I., Hanamatsu H., Nakamura A., Suzuki K., Kawagishi N., Ohara M., Umemura M., Nakai M., et al. Tri-antennary tri-sialylated mono-fucosylated glycan of alpha-1 antitrypsin as a non-invasive biomarker for non-alcoholic steatohepatitis: A novel glycobiomarker for non-alcoholic steatohepatitis. Sci. Rep. 2020;10:321. doi: 10.1038/s41598-019-56947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K., Suda G., Yamamoto Y., Furuya K., Baba M., Kimura M., Maehara O., Shimazaki T., Yamamoto K., Shigesawa T., et al. Entecavir treatment of hepatitis B virus-infected patients with severe renal impairment and those on hemodialysis. Hepatol. Res. 2019;49:1294–1304. doi: 10.1111/hepr.13399. [DOI] [PubMed] [Google Scholar]
- 17.Suda G., Furusyo N., Toyoda H., Kawakami Y., Ikeda H., Suzuki M., Arataki K., Mori N., Tsuji K., Katamura Y., et al. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: A nationwide retrospective study in Japan. J. Gastroenterol. 2018;53:119–128. doi: 10.1007/s00535-017-1353-y. [DOI] [PubMed] [Google Scholar]
- 18.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L., O’Beirne J., Fox R., Skowronska A., Palmer D., et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraoka A., Kumada T., Tsuji K., Takaguchi K., Itobayashi E., Kariyama K., Ochi H., Tajiri K., Hirooka M., Shimada N., et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer. 2019;8:121–129. doi: 10.1159/000488778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 21.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraoka A., Kumada T., Tada T., Hirooka M., Kariyama K., Tani J., Atsukawa M., Takaguchi K., Itobayashi E., Fukunishi S., et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep. 2022;5:e1464. doi: 10.1002/cnr2.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Alessio A., Fulgenzi C.A.M., Nishida N., Schonlein M., von Felden J., Schulze K., Wege H., Gaillard V.E., Saeed A., Wietharn B., et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022 doi: 10.1002/hep.32468. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T., Hiraoka A., Tada T., Hirooka M., Kariyama K., Tani J., Atsukawa M., Takaguchi K., Itobayashi E., Fukunishi S., et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol. Res. 2022 doi: 10.1111/hepr.13797. in press . [DOI] [PubMed] [Google Scholar]
- 25.Ishikura N., Sugimoto M., Yorozu K., Kurasawa M., Kondoh O. AntiVEGF antibody triggers the effect of antiPDL1 antibody in PDL1(low) and immune desertlike mouse tumors. Oncol. Rep. 2022;47:1–10. doi: 10.3892/or.2021.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai T., Sugimoto M., Patil N.S., Bower D., Suzuki M., Kato C., Yorozu K., Kurasawa M., Shames D.S., Kondoh O. Both T cell priming in lymph node and CXCR3-dependent migration are the key events for predicting the response of atezolizumab. Sci. Rep. 2021;11:13912. doi: 10.1038/s41598-021-93113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M. Combination Immunotherapy with Anti-PD-1/PD-L1 Antibody plus Anti-VEGF Antibody May Promote Cytotoxic T Lymphocyte Infiltration in Hepatocellular Carcinoma, Including in the Noninflamed Subclass. Liver Cancer. 2022;11:185–191. doi: 10.1159/000524977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.