Abstract
Previously, we identified a novel component of Bacillus subtilis spores, called TasA, which possesses antibacterial activity. TasA is made early in spore formation, as cells enter stationary phase, and is secreted into the medium as well as deposited into the spore. Here, we show that tasA expression can occur as cells enter stationary phase even under sporulation-repressing conditions, indicating that TasA is a transition-phase protein. tasA and two upstream genes, yqxM and sipW, likely form an operon, transcription of which is under positive control by the transition-phase regulatory genes spo0A and spo0H and negative control by the transition phase regulatory gene abrB. These results are consistent with the suggestion that yqxM, sipW, and tasA constitute a transition phase operon that could play a protective role in a variety of cellular responses to stress during late-exponential-phase and early-stationary-phase growth in B. subtilis.
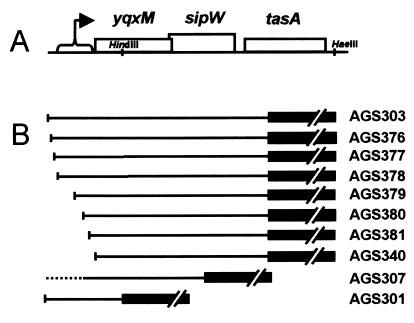
When confronted with nutrient deprivation, B. subtilis can choose from a large set of alternative responses such as the production of antibiotics and proteases, the uptake of exogenous DNA (the state of competence), and the formation of a dormant cell type called the spore (20). Previously, we identified a novel spore-associated protein, called TasA, which possesses an antibacterial activity and is also secreted into the culture supernatant at the beginning of sporulation (19). tasA is immediately downstream of sipW (Fig. 1A) (9), encoding a signal peptidase (22, 23) that is required for the maturation and secretion of TasA (19). sipW is downstream of and partially overlaps yqxM, whose function is unknown. The proximity of these three genes and the functional relationship between SipW and TasA raise the possibility of coordinate regulation of this gene cluster.
FIG. 1.
The yqxM sipW tasA locus and DNA constructs. (A) The yqxM sipW tasA locus. Open boxes represent genes, and the region likely to contain the promoter(s) is indicated by a bracket. Restriction sites are indicated. (B) Transcriptional reporter fusion constructs. Black boxes represent the lacZ gene of E. coli. Names of strains bearing these fusions are indicated. Fusions were inserted by marker replacement at the amyE locus except for sipW-lacZ (a gift from J. M. van Dijl), which was integrated by single-reciprocal integration, indicated by the dotted line (to generate AGS307).
Previously, we found that the production of TasA requires Spo0A and ςH, molecules that guide regulation at the juncture between exponential and stationary phases (5) (Fig. 2A), consistent with TasA synthesis early in sporulation (19). To examine the possibility that TasA is a transition-phase protein and to deepen our understanding of the regulatory controls on the expression of tasA and the adjacent genes, we have studied the regulation of TasA synthesis during a variety of growth conditions, defined 5′ sequences required for tasA expression, and identified some of the relevant regulatory factors.
FIG. 2.
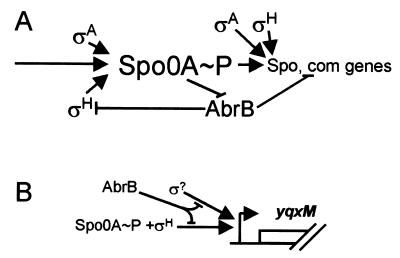
Regulation of transition-phase gene expression and a model for the possible regulation of the yqxM sipW tasA operon. (A) Summary of the roles of Spo0A, ςH, and AbrB on transition-phase gene expression. The long arrow on the left indicates the influence of factors controlling the cellular concentration of Spo0A∼P, which in turn determines whether the cell will express sporulation (Spo) genes, competence (com) genes, or genes associated with another transition phase choice (on the right). Not all transition-phase genes are controlled directly by Spo0A. (B) A speculative model for transcriptional control of the operon. Spo0A and ςH direct expression. Separately, an as yet unidentified additional sigma factor also directs expression. AbrB can repress both events. Although a single promoter is illustrated, multiple promoters may be present.
MATERIALS AND METHODS
Strains, media, and recombinant DNA procedures.
Strains, plasmids, and primers for PCR are listed in Tables 1 and 2. All recombinant DNA procedures were performed by using standard procedures (16), and manipulations in B. subtilis were carried out according to procedures in reference 2. Cells were grown in Difco sporulation medium (DSM) (2) unless otherwise indicated. 2×YT, Luria-Bertani (LB), and Terrific broth (TB) media (16) and King’s B medium (8) were prepared as described elsewhere.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Genotype or description | Reference, source, or construction |
|---|---|---|
| B. subtilis | ||
| PY79 | Prototrophic | 26 |
| JH642 | trpC2 pheA1 | Lab stock |
| HB4035 | sigDΔ::Kanr | J. Helmann |
| RL102 | spo0HΔHindIII-EcoRI::cat trpC2 | R. Losick |
| PM126 | sigBΔ::cat | R. Losick |
| AG518 | abr::Tn917 trpC2 pheA1 | A. D. Grossman |
| AG839 | Δabr::cat trpC2 pheA1 | A. D. Grossman |
| AG1242 | spo0AΔPs trpC2 pheA1 | A. D. Grossman |
| AGS301 | amyE::yqxM-lacZ | PY79 × pAGS43 |
| AGS303 | amyE::tasA-lacZ | PY79 × pAGS42 |
| AGS305 | spo0HΔHindIII-EcoRI::cat::SpecramyE::tasA-lacZ | AGS232 × pAGS42 |
| AGS306 | sigDΔ::Kanr | PY79 × DNA HB4035 |
| AGS307 | sipWΩpLGW201 (sipW-lacZ) | PY79 × 8G5::pLGW201 (22) |
| AGS313 | spo0AΔ::ErmramyE::tasA-lacZ | AGS303 × DNA RL891 (10) |
| AGS340 | amyE::pAGS51 | PY79 × pAGS51 |
| AGS347 | abrB::Tn917 | PY79 × DNA AG518 |
| AGS348 | ΔabrB::cat | PY79 × DNA AG839 |
| AGS349 | spo0HΔHindIII-EcoRI::cat::SpecrabrB::Tn917 | AGS232 × DNA AG518 |
| AGS350 | spo0HΔHindIII-EcoRI::cat::Specr ΔabrB::cat | AGS232 × DNA AG839 |
| AGS351 | abrB::Tn917 amyE::tasA-lacZ | AGS347 × DNA AGS303 |
| AGS352 | abrB::Tn917 spo0HΔHindIII-EcoRI::cat::SpecramyE::tasA-lacZ | AGS349 × DNA AGS303 |
| AGS361 | spo0A::pSPC101 amyE::tasA-lacZ | AGS303 × DNA JKH75 (4) |
| AGS362 | spo0A::pSPC101-S233P amyE::tasA-lacZ | AGS303 × DNA JKH104 (4) |
| AGS363 | spo0A::pSPC101-G227R amyE::tasA-lacZ | AGS303 × DNA JKH74 (4) |
| AGS364 | spo0A::pSPC101-F236S amyE::tasA-lacZ | AGS303 × DNA JKH105 (4) |
| AGS365 | spo0A::pSPC101-V240A amyE::tasA-lacZ | AGS303 × DNA JKH106 (4) |
| AGS366 | spo0A::pSPC101-V240G K265R amyE::tasA-lacZ | AGS303 × DNA JKH107 (4) |
| AGS367 | spo0AΔPs amyE::tasA-lacZ trpC2 pheA | AG1242 × DNA AGS303 |
| AGS368 | ΔsigB::cat | PY79 × DNA PM126 |
| AGS376 | amyE::pAGS53 | PY79 × pAGS53 |
| AGS377 | amyE::pAGS54 | PY79 × pAGS54 |
| AGS378 | amyE::pAGS55 | PY79 × pAGS55 |
| AGS379 | amyE::pAGS56 | PY79 × pAGS56 |
| AGS380 | amyE::pAGS57 | PY79 × pAGS57 |
| AGS381 | amyE::pAGS58 | PY79 × pAGS58 |
| AGS387 | amyE::tasA-lacZ trpC2 pheA1 | AGS351 × DNA AGS303 |
| AGS392 | abrB::Tn917 spo0AΔ::pSPC101 amyE::tasA-lacZ | AGS351 × DNA JKH75 (4) |
| E. coli DH5α | Cloning host | Lab stock |
| Plasmid | ||
| pAGS42 | 351 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS43 | yqxM-lacZ | This study |
| pAGS51 | Promoterless yqxM sipW tasA-lacZ | This study |
| pAGS53 | 281 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS54 | 264 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS55 | 233 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS56 | 114 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS57 | 40 bp upstream of yqxM sipW tasA-lacZ | This study |
| pAGS58 | 351 bp upstream of yqxM sipW tasA-lacZ | This study |
| pDG268 | Permits marker replacement of lacZ fusion at amy | 6 |
| pDG364 | Permits marker replacement at amy | 7 |
| pSL1180 | Harbors extensive multiple cloning site | Pharmacia |
TABLE 2.
Primers used
| Primer | Sequencea | Enzyme(s) cut | Region boundb |
|---|---|---|---|
| OL99 | 5′AAAAACTCGAGCATATGTTTCGATTGTTTCAC3′ | XhoI and NdeI | 1 to 18 |
| OL109 | 5′AAAAAAGCTAGCAGCGGTGTTTCTTCTGCGTG3′ | NheI | −332 to −351 |
| OL110 | 5′AAAAAAGCGGCCGCCCTCCAACTAAAGCTAATCCTAGTG3′ | NotI | 1424 to 1448 |
| OL116 | 5′AAAAAAGCTAGCATGTTTCGATTGTTTCAC3′ | NheI | 1 to 18 |
| OL131 | 5′AAAAAAAGCTAGCGATCATCATGCTGTCACCCT3′ | NheI | −261 to −280 |
| OL132 | 5′AAAAAAAGCTAGCCTTTCTTTGTTTATTATTACC3′ | NheI | −242 to −262 |
| OL133 | 5′AAAAAAAGCTAGCGGGATATGCATTTAAATTCTCAC3′ | NheI | −209 to −233 |
| OL134 | 5′AAAAAAAGCTAGCATGAGCGATTTCGGTGTTTTT3′ | NheI | −94 to −114 |
| OL135 | 5′AAAAAAAGCTAGCATTGTCATATCAAGT3′ | NheI | −26 to −42 |
| OL136 | 5′AAAAAAAGCTAGCTCAAGTTACAGTGTTTTACAGGAG3′ | NheI | −7 to −32 |
The restriction endonuclease site(s) in each oligonucleotide is underlined. For OL99, the XhoI site is singly underlined and the NdeI site is doubly underlined.
Region of the chromosome bound by the oligonucleotide, indicated as nucleotide positions relative to the start of the yqxM open reading frame.
Transcriptional fusions to lacZ.
First, we digested the amyE replacement vector pDG268 (6) with EcoRI and HindIII, purified the larger resulting fragment, and ligated it to the EcoRI-HindIII fragment of pSL1180 containing the multiple cloning site, creating pAGS38. Next, we amplified the yqxM sipW tasA locus by PCR using primers OL109 and OL110 and digested the fragment with NheI and NotI. We then cloned the fragment between the NheI and NotI sites of pAGS38, creating the tasA-lacZ transcriptional fusion plasmid pAGS42. To make the yqxM-lacZ transcriptional fusion (in pAGS43), we digested pAGS42 with NheI and HindIII and ligated the smaller resulting DNA fragment into similarly digested pAGS38. pAGS42 and pAGS43 were linearized with KpnI and used to transform a variety of strains to chloramphenicol resistance (Table 1). We made pAGS51, pAGS53, pAGS54, pAGS55, pAGS56, pAGS57, and pAGS58 by essentially the same procedure but used oligonucleotides OL116 and OL110, OL131 and OL110, OL132 and OL110, OL133 and OL110, OL134 and OL110, OL135 and OL110, and OL136 and OL110, resulting in strains AGS340, AGS376, AGS377, AGS378, AGS379, AGS380, and AGS381, respectively. Strain 8G5::pLGW201, bearing sipW-lacZ as a single reciprocal (Campbell-type) integration at sipW (22), was a kind gift from J. M. van Dijl. We moved this fusion into PY79 by transformation, resulting in strain AGS307.
Determination of relative TasA levels and β-galactosidase assays.
Cells were harvested 5 h after the end of exponential phase, pelleted, and then lysed by resuspension in 25 mM Tris-HCl (pH 7.5)–50 mM glucose–10 mM EDTA–100 μg of lysozyme per ml followed by a 10-s burst of sonication (using a Fisher Dismembrator). We determined the concentration of total protein in each sample with a bicinchoninic acid protein assay reagent kit (Pierce) and then carried out electrophoresis on 12% polyacrylamide gels and Western blot analysis (19). We used a Hewlett-Packard ScanJet 6200C and AlphaImager 2000 version 4.03 software (Alpha Innotech Corporation) to determine the amounts of TasA relative to total protein in each strain and divided these ratios by the ratio of TasA to total protein in wild-type cells. We carried out β-galactosidase assays as described elsewhere (2) except that the reaction was performed at 37°C instead of 30°C.
RESULTS AND DISCUSSION
Medium requirements for TasA synthesis.
We previously found that TasA synthesis began as cells entered stationary phase during growth in sporulation medium (DSM). To determine whether TasA could be made independently of the decision to sporulate, we used Western blot analysis to follow the steady-state accumulation of TasA in 2×YT medium (in which sporulation was decreased to 0.01% [data not shown]). We first detected TasA at the beginning of stationary phase and found that it was present for at least 7 h (Fig. 3 and data not shown). We also detected TasA in cells grown in King’s B, LB, or TB medium, in which sporulation occurred in less than 0.01% of the cells (data not shown). Therefore, TasA is a transition-phase protein that first appears at the beginning of stationary phase.
FIG. 3.
Western blot analysis of TasA during growth in 2×YT. Extracts of cells grown in 2×YT were prepared at the indicated times before and after the onset of stationary phase (T0). After fractionation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransfer to polyvinylidene difluoride membranes, TasA was detected with an anti-TasA antibody. Molecular mass is indicated in kilodaltons on the left.
Sequences required for tasA expression.
The arrangement of yqxM, sipW, and tasA suggested they form an operon (Fig. 1A). As an initial test of this possibility, we compared the level of tasA-lacZ expression in a strain missing all of the sequences upstream of the yqxM open reading frame (AGS340) with the level in a strain in which 351 bp upstream of yqxM were retained (AGS303). In strain AGS303, tasA-lacZ expression began at about 15 min before the beginning of stationary phase and maintained a significant level for at least 7 h (Fig. 4A), similarly to the timing of appearance of TasA (Fig. 3 and reference 19). Consistent with this, we were able to readily detect TasA by Western blot analysis when only 351 bp of upstream sequence were present (data not shown). The removal of the upstream sequence reduced tasA expression almost to background levels (Fig. 4A). Likewise, we did not detect TasA by Western blot analysis when the upstream sequence was removed (data not shown).
FIG. 4.
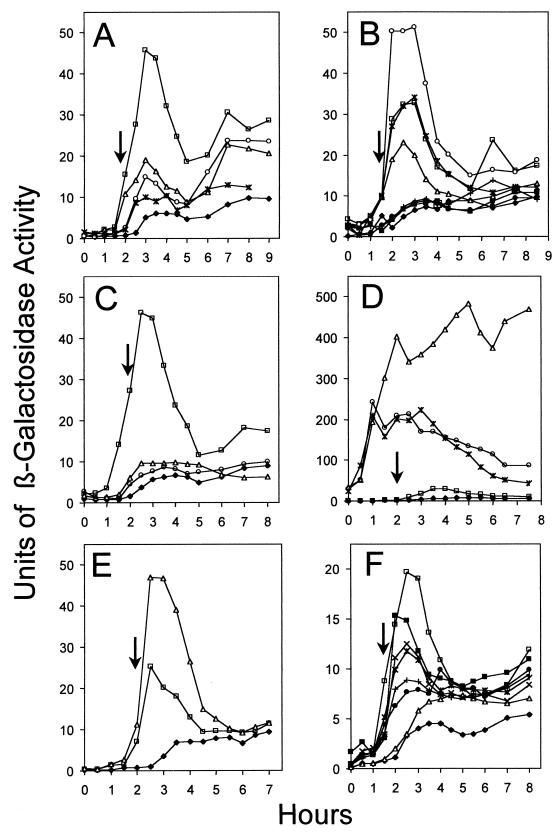
β-galactosidase activity in cells bearing yqxM-, sipW-, or tasA-lacZ. All strains harbor either sipW-lacZ (AGS301), yqxM-lacZ (AGS307), no fusion (PY79 and JH642), or tasA-lacZ (all remaining strains). In each plot, the onset of stationary phase (time zero) is indicated by an arrow. (A) Fusions to tasA, sipW, or yqxM and a tasA-lacZ fusion in which the 351 bp upstream of yqxM have been deleted. Strains tested: AGS301 (yqxM-lacZ) (▵), AGS307 (sipW-lacZ) (○), AGS303 (tasA-lacZ) (□), AGS340 (yqxM upstream sequences deleted, tasA-lacZ) (✻), and PY79 (⧫). (B) tasA-lacZ fusions retaining various regions upstream of yqxM. Strains tested: AGS303 (retaining 351 bp) (□), AGS376 (281 bp) (▵), AGS377 (264 bp) (○), AGS378 (233 bp) (✻), AGS379 (114 bp) (●), AGS380 (40 bp) (+), AGS381 (30 bp) (■), and PY79 (⧫). (C) Effects of spo0A and spo0H. Strains tested: AGS303 (wild type) (□), AGS305 (spo0HΔHindIII-EcoRI::cat::Specr) (▵), AGS313 (spo0AΔ::Ermr) (○), and PY79 (⧫). (D) Effects of abrB. Strains tested: AGS303 (wild type) (□), AGS351 (ΔabrB::Tn917) (▵), AGS352 (ΔabrB::cat spo0HΔHindIII-EcoRI::cat::Specr) (○), AGS392 (abrB::Tn917::pSpc101 spo0AΔ::Ermr) (✻), and PY79 (⧫). (E) Effect of deletion of the ςH-driven promoter of spo0A. Strains tested: AGS387 (wild type) (□), AGS367 (spo0AΔPs) (▵), and JH642 (⧫). (F) Effects of alleles of spo0A deficient in ςA-dependent gene expression. Strains tested: AGS303 (wild type) (□), AGS361 (spo0A::pSPC101) (▵), AGS362 (spo0A::pSPC101-S233P) (×), AGS363 (spo0A::pSPC101-G227R) (✻), AGS364 (spo0A::pSPC101-F236S) (●), AGS365 (spo0A::pSPC101-V240A) (+), AGS366 (spo0A::pSPC101-V240G K265R) (■), and PY79 (⧫). Units of β-galactosidase activity are defined in Materials and Methods. Scales along the y axis vary from panel to panel; in particular, the scale in panel D is compressed.
To study the timing of expression of the genes in this locus, we measured β-galactosidase activities of strains harboring transcriptional fusions of yqxM, sipW, and tasA to lacZ of E. coli (strains AGS301, AGS307, and AGS303, respectively [Fig. 1B]). We found that expression of all three genes initiated at the beginning of stationary-phase growth (Fig. 4A), consistent with the timing of TasA production (Fig. 3 and reference 19). The wild-type strain, without the tasA-lacZ fusion (PY79), showed background levels of β-galactosidase activity (and a white color on solid indicator medium [data not shown]). For unknown reasons, the levels of expression of the yqxM-lacZ and sipW-lacZ fusions were consistently lower than that of tasA-lacZ. To further define the upstream region needed for tasA-lacZ transcription, we generated a set of fusions missing progressively more of the 351-bp region (Fig. 1B). We found significant levels of tasA-lacZ expression when 233 bp or more upstream of yqxM were retained but detected no expression when 114 or fewer bp of upstream sequence were present (Fig. 4B). These results suggest that yqxM, sipW, and tasA constitute an operon and that the sequences required for tasA expression under these conditions of growth are confined to a region 233 bp upstream of yqxM. They also argue against the presence of strong promoters between the beginning of the yqxM open reading frame and the tasA open reading frame.
Roles of Spo0A, ςH, and AbrB in tasA transcription.
We found background levels of β-galactosidase activity in strains bearing mutations in spo0A (AGS313) and spo0H (AGS305) (Fig. 4C), suggesting that Spo0A and ςH are required for tasA expression. We detected about 10-fold-higher levels of tasA-lacZ expression in a strain bearing an abrB mutation (AGS351) (Fig. 4D), indicating that AbrB represses tasA expression. We also found that TasA steady-state levels in two strains bearing null mutations in abrB (AGS347 and AGS348) were approximately sixfold higher than in the wild type (Table 3).
TABLE 3.
Effects of transcription factor gene mutations on TasA levels
| Strain | Relevant genotype | Fold change in TasA level |
|---|---|---|
| AG1242 | spo0AΔPs | 4.3 |
| AGS347 | abrB | 6.3 |
| AGS348 | abrB | 6.4 |
| AGS349 | spo0H abrB | 1.7 |
| AGS350 | spo0H abrB | 1.6 |
| AGS362 | spo0A S233P | 0.31 |
| AGS363 | spo0A G227R | 0.31 |
| AGS364 | spo0A F236S | 0.11 |
| AGS365 | spo0A V240A | 0.13 |
| AGS366 | spo0A V240G K265R | 0.46 |
The effect of AbrB on tasA expression could be indirect, via repression of spo0H (25) (Fig. 2A). If this is true, then tasA-lacZ expression should be at background levels in an abrB spo0H double mutant. In such a strain (AGS352), expression of tasA-lacZ occurred about 2 h earlier than in the wild type and ultimately was three- to fivefold higher than in the wild type, similarly to an abrB strain (AGS351) (Fig. 4D). TasA levels in strains bearing abrB and spo0H mutations (AGS349 and AGS350) were somewhat higher than in the wild type (Table 3). From these data, we infer that AbrB does not repress tasA expression solely by inhibition of spo0H expression. These results also suggest that AbrB represses a ςH-independent mechanism of tasA expression that acts prior to the onset of stationary phase. In contrast to the abrB mutant, in the double mutant tasA expression did not remain high but rather decreased over time. The difference in tasA expression between the two strains further argues that AbrB also represses a post-exponential ςH-dependent phase of expression. Apparently, when abrB is intact, this phase of ςH-directed expression is not completely repressed, accounting for the wild-type pattern of tasA expression.
One interpretation of these findings is the presence of two promoters upstream of yqxM, one of which is ςH and Spo0A dependent and other of which utilizes a second sigma factor and is Spo0A independent. To determine whether a ςH-independent promoter might reside within the already identified 351-bp region upstream of the yqxM open reading frame, we measured β-galactosidase activities in spo0H abrB tasA-lacZ strains missing various portions of this region. We detected tasA-lacZ expression when 233 bp or more were present but not when only 114 bp or fewer remained (data not shown). Therefore, if a second promoter exists, its activity requires sequences within the same 233-bp region required by the ςH-dependent promoter.
Spo0A could affect tasA expression indirectly, via repression of abrB (Fig. 2A). If this is so, then tasA expression in the abrB spo0A strain would appear similar to what was seen in the strain bearing only the abrB mutation. If, on the other hand, Spo0A directly activated tasA expression, then tasA expression in the abrB spo0A strain would resemble expression in the abrB spo0H strain. We addressed this issue by measuring tasA-lacZ expression in an abrB spo0A strain (AGS392). We found that the pattern of expression resembled that of the abrB spo0H strain (AGS352) (Fig. 4D). These data indicate that Spo0A does not act exclusively through AbrB to activate tasA expression and are consistent with the possibility that Spo0A binds the tasA promoter. A possible Spo0A-binding site is present between nucleotides −32 and −38 relative to the beginning of the yqxM open reading frame.
The finding that tasA expression depends on ςH raised the possibility that tasA expression requires ςH-directed expression of spo0A (1, 15). To test this, we measured tasA-lacZ expression and TasA steady-state levels in a strain in which spo0A is missing its ςH-dependent promoter (spo0AΔPs), placing spo0A expression under the control solely of ςA (18). We compared β-galactosidase activity in a spo0AΔPs tasA-lacZ-bearing strain (AGS367) to the activity in congenic strains harboring either the tasA-lacZ fusion (AGS387) or no fusion (JH642). We found that the levels of tasA-lacZ expression (Fig. 4E) and TasA synthesis (Table 3) were significantly above background, suggesting that the absolute requirement for ςH is not due to its action at the spo0A promoter. Because the spo0AΔPs strain (AG1242) cannot sporulate (18), this result also supports our view that TasA is not exclusively a sporulation protein.
Role of ςA in tasA transcription.
It is possible that tasA expression requires Spo0A-dependent ςA-directed gene expression. To test this, we made use of strains carrying special alleles of spo0A (spo0A::pSPC101-G227R, spo0A::pSPC101-S233P, spo0A::pSPC101-F236S, spo0A::pSPC101-V240A, or spo0A::pSPC101-V240G K265R [4]), encoding versions of Spo0A that are unable to activate the ςA-dependent genes spoIIE and spoIIG but are able to direct expression of at least one ςH-dependent gene (spoIIA) to different degrees. We compared tasA-lacZ expression and TasA steady-state levels in the wild-type strain (AGS303) and the spo0A null mutant strain (AGS361) with strains bearing these altered spo0A alleles (AGS362, AGS363, AGS364, AGS365, and AGS366). The levels of tasA-lacZ expression (Fig. 4F) and of TasA (Table 3) varied in strains bearing the altered spo0A alleles but were significantly above background in all cases. These data are consistent with the possibility that tasA expression largely relies on Spo0A acting together with ςH.
To determine whether the specialized sigma factors ςB or ςD (3) are needed for TasA synthesis, we used Western blot analysis to determine if TasA was present in extracts of a sigBΔ::cat strain (AGS368) or a sigDΔ::Kanr strain (AGS306). In both strains, TasA levels were similar to those in wild type (data not shown), indicating that neither ςB nor ςD is absolutely required for TasA production.
Our results suggest that Spo0A and ςH, directly or indirectly, activate expression of the operon (Fig. 2B). The involvement of ςH in tasA expression is supported by the results of Serrano et al. (17), who showed that tasA was expressed during vegetative growth when spo0H expression was activated prematurely, using an inducible promoter. The expression of tasA in a strain missing the ςH-directed spo0A promoter (spo0AΔPs) argues against the possibility that the major role of ςH in tasA expression is at the level of spo0A expression and further implies that high levels of Spo0A are not required for tasA expression (18). AbrB is likely to repress both ςH-dependent and ςH-independent phases of tasA expression, possibly originating from two promoters. Therefore, AbrB appears to serve at least two roles in tasA expression. First, AbrB prevents inappropriate tasA expression during exponential phase, as it does for many other genes (20). Second, it reduces tasA expression during post-exponential-phase growth.
We have not yet identified the start site(s) of transcription of the operon. As yet, primer extension and 5′RACE (rapid amplification of 5′ cDNA ends) methods have yielded ambiguous results. This could be due to a low level of message and/or secondary structure at the 5′ end of the message.
We do not know the significance of the coordinate regulation of yqxM, sipW, and tasA. However, the clustering and coregulation of a specialized signal peptidase gene with one or more substrate-encoding genes may occur more widely than is usually appreciated. Interesting examples of this occur in Bacillus subtilis (natto), which is well known to harbor a variety of cryptic plasmids (21, 24). Two such plasmids, pTA1015 and pTA1040, encode proteins very similar to the chromosomally encoded B. subtilis signal peptidase SipS (11, 12). Intriguingly, in each of these plasmids, an open reading frame of unknown function, encoding a plausible signal peptide, sits immediately upstream of the signal peptidase gene. Apparently, these pairs of genes form operons. The potentially secreted gene products could be substrates for the encoded signal peptidases. The additional presence of genes encoding response regulator aspartate phosphatases (13, 14) in some of these plasmids suggests a role in post-exponential-phase responses. Possibly, the coordinate expression of a signal peptidase gene with one or more genes encoding substrates provides an important level of control over secretion during early-stationary-phase growth.
ACKNOWLEDGMENTS
We thank Jean Greenberg, John Helmann, Shawn Little, Mark Strauch, and Phil Youngman for critically reading the manuscript and for helpful discussions. We thank Janet Hatt, John Helmann, Alan Grossman, Phil Youngman, and Jan Maarten van Dijl for gifts of strains. We especially acknowledge the advice of John Lopes and the editor. We also acknowledge the excellent technical assistance of Dong Chae.
This work was supported by Public Health Service grant GM539898 from the National Institutes of Health and the Schweppe Foundation. A.G.S. was funded, in part, by a Schmitt dissertation fellowship.
REFERENCES
- 1.Chibazakura T, Kawamura F, Takahashi H. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J Bacteriol. 1991;173:2625–2632. doi: 10.1128/jb.173.8.2625-2632.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutting S M, Vander Horn P B. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1990. [Google Scholar]
- 3.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatt J K, Youngman P. Spo0A mutants of Bacillus subtilis with sigma factor-specific defects in transcription activation. J Bacteriol. 1998;180:3584–3591. doi: 10.1128/jb.180.14.3584-3591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 6.Karmazyn-Campelli C, Bonamy C, Savelli B, Stragier P. Tandem genes encoding ς-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989;3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Karmazyn-Campelli C, Fluss L, Leighton T, Stragier P. The spoIIN279(ts) mutation affects the FtsA protein of Bacillus subtilis. Biochimie. 1992;74:689–694. doi: 10.1016/0300-9084(92)90141-z. [DOI] [PubMed] [Google Scholar]
- 8.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 9.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 10.Margolis P S, Driks A, Losick R. Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol. 1993;175:528–540. doi: 10.1128/jb.175.2.528-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer W J, de Jong A, Bea G, Wisman A, Tjalsma H, Venema G, Bron S, van Dijl J M. The endogenous Bacillus subtilis (natto) plasmids pTA1015 and pTA1040 contain signal peptidase-encoding genes: identification of a new structural module on cryptic plasmids. Mol Microbiol. 1995;17:621–631. doi: 10.1111/j.1365-2958.1995.mmi_17040621.x. [DOI] [PubMed] [Google Scholar]
- 12.Meijer W J, Wisman G B, Terpstra P, Thorsted P B, Thomas C M, Holsappel S, Venema G, Bron S. Rolling-circle plasmids from Bacillus subtilis: complete nucleotide sequences and analyses of genes of pTA1015, pTA1040, pTA1050 and pTA1060, and comparisons with related plasmids from gram-positive bacteria. FEMS Microbiol. Rev. 1998. pp. 337–368. [DOI] [PubMed] [Google Scholar]
- 13.Perego M. Kinase-phosphatase competition regulates Bacillus subtilis development. Trends Microbiol. 1998;6:366–370. doi: 10.1016/s0966-842x(98)01350-x. [DOI] [PubMed] [Google Scholar]
- 14.Perego M, Hanstein C, Welsh K M, Djavakhishvili T, Glaser P, Hoch J A. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 15.Predich M, Nair G, Smith I. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing sigma H. J Bacteriol. 1992;174:2771–2778. doi: 10.1128/jb.174.9.2771-2778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 17.Serrano M, Zilhao R, Ricca E, Ozin A J, Moran C P, Jr, Henriques A O. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J Bacteriol. 1999;181:3632–3643. doi: 10.1128/jb.181.12.3632-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siranosian K J, Grossman A D. Activation of spo0A transcription by sigma H is necessary for sporulation but not for competence in Bacillus subtilis. J Bacteriol. 1994;176:3812–3815. doi: 10.1128/jb.176.12.3812-3815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stöver A G, Driks A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J Bacteriol. 1999;181:1664–1672. doi: 10.1128/jb.181.5.1664-1672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauch M A, Hoch J A. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol. 1993;7:337–342. doi: 10.1111/j.1365-2958.1993.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Koshikawa T. Isolation and characterization of four types of plasmids from Bacillus subtilis (natto) J Bacteriol. 1977;131:699–701. doi: 10.1128/jb.131.2.699-701.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjalsma H, Bolhuis A, van Roosmalen M L, Wiegert T, Schumann W, Broekhuizen C P, Quax W J, Venema G, Bron S, van Dijl J M. Functional analysis of the secretory precursor processing machinery of Bacillus subtilis: identification of a eubacterial homolog of archaeal and eukaryotic signal peptidases. Genes Dev. 1998;12:2318–2331. doi: 10.1101/gad.12.15.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjalsma H, Noback M A, Bron S, Venema G, Yamane K, van Dijl J M. Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities. Constitutive and temporally controlled expression of different sip genes. J Biol Chem. 1997;272:25983–25992. doi: 10.1074/jbc.272.41.25983. [DOI] [PubMed] [Google Scholar]
- 24.Uozumi T, Ozaki A, Beppu T, Arima K. New cryptic plasmid of Bacillus subtilis and restriction analysis of other plasmids found by general screening. J Bacteriol. 1980;142:315–318. doi: 10.1128/jb.142.1.315-318.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir J, Predich M, Dubnau E, Nair G, Smith I. Regulation of spo0H, a gene coding for the Bacillus subtilis sigma H factor. J Bacteriol. 1991;173:521–529. doi: 10.1128/jb.173.2.521-529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youngman P, Perkins J B, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]