Abstract
Flow diverters (FD) have become the mainstay for the endovascular treatment of unruptured intracranial aneurysms (UIA). Most FD procedures are performed under general anesthesia, and the influence of local anesthesia (LA) on outcomes remains unknown. This study evaluated the results of FD placement under LA. Data of patients treated for FD under LA between August 2016 and January 2022 were analyzed retrospectively. A good outcome was defined as a modified Rankin scale score of 0–2. Major stroke, steno-occlusive events of FD, mortality, and satisfactory aneurysm occlusion were also evaluated. In total, 169 patients undertook 182 treatments (139 [82%) female, mean age 61 ± 11 years). The median maximum aneurysm size was 9.5 mm (interquartile range 6.1-14 mm). A flow re-directed endoluminal device and pipeline embolization device were used in 103 (57%) and 78 (43%) treatments. One patient (0.59%) experienced major stroke, and steno-occlusive events were observed in four patients (2.4%). A good outcome at 90 days was obtained in 164 patients (98%), and one patient died (0.59% mortality). During the median follow-up period of 345 days (interquartile range 176–366 days), satisfactory aneurysm occlusion was observed in 126 of 160 aneurysms (79%). Our results suggest that FD placement under LA is a safe and effective treatment for UIA.
Keywords: anesthesia, cerebral aneurysm, flow diverter, outcome
1. Introduction
Endoluminal flow diverters (FDs) such as the pipeline embolization device (PED [CovidieneEv3, Plymouth, MN, USA]) or Flow Re-Direction Endoluminal Device (FRED [MicroVention, Tustin, CA, USA]) have gained widespread acceptance for the treatment of intracranial aneurysms [1,2,3]. Most endovascular procedures, including FD placement, are performed under general anesthesia (GA) for patient comfort, control of hemodynamics and respiration, and better image quality due to decreased motion artifacts in immobile patients [4]. However, GA can carry a potential risk of respiratory and hemodynamic instability during induction, interfere with neurologic examination during the procedure, and have a longer total procedure time [5]. Patients undergoing FD deployment are placed on antiplatelet therapy to avoid thromboembolic complications. Few reports have also shown the risk of esophageal submucosal hemorrhage during the extubation of a tracheal tube as the patient is placed on antiplatelet therapy [6].
FD deployment can potentially cause vessel perforation, arterial thrombosis, dissection or stenosis, vasospasm, and perforator occlusion [7,8]. These complications can be preceded by neurological changes and, in GA patients, may go undetected until the procedure is completed, and the opportunity to intervene would have already passed.
Endovascular procedures are commonly performed in awake patients at our institution after the administration of local anesthetic agents and mild sedatives when required. To date, the influence of local anesthesia (LA) on clinical outcomes in the aneurysmal treatment of FD remains unknown. This study aimed to evaluate the clinical outcomes of FD placement under LA in patients with unruptured intracranial aneurysms (UIAs).
2. Materials and Methods
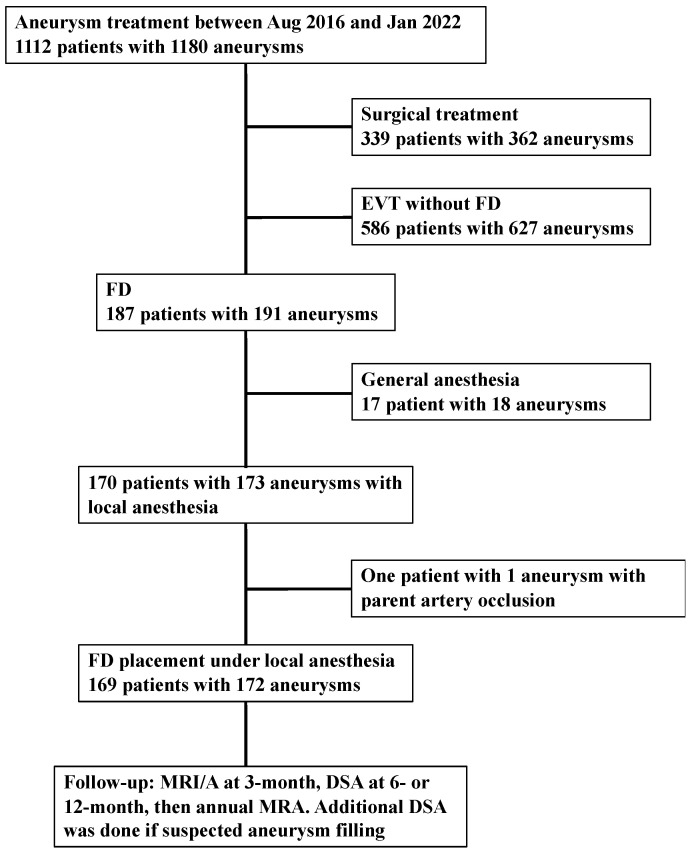
The study was reported based on criteria from the Strengthening the Reporting of Observational Study in Epidemiology statement [9]. The study protocol was approved by the Institutional Ethics Committee (No. 3229). Consent was obtained from all patients. Between August 2016 and January 2022, 170 patients with 173 UIAs underwent FD placement under LA at our institution. Overall, 17 patients with 18 aneurysms underwent the procedure under general anesthesia due to patient preference to undertake the procedure under GA because of restlessness, a difficult-to-access route (especially cervical portion), and initial FD introduction. Because one patient with one aneurysm underwent parent artery occlusion, this patient was excluded (Figure 1). Follow-up was conducted with magnetic resonance imaging/angiography (MRI/A) at 3 months, digital subtraction angiography (DSA) at 6- or 12 months, followed by annual MRA. Additional DSA was undertaken in case of suspected aneurysm filling. However, the routine follow-up was affected by the COVID-19 pandemic.
Figure 1.
Flow chart of the selection process. Abbreviations: EVT = endovascular treatment; FD = flow diverter; MRI = magnetic resonance imaging; MRA = magnetic resonance angiography; DSA = digital subtraction angiography.
The clinical data were obtained through a review of the patients’ medical records and included demographic information, such as age, sex, body mass index, smoking history, daily alcohol consumption, and preoperative modified Rankin scale (mRS) [10]. The following data were also collected: duration of stay, previous treatment of aneurysm, medical history (hypertension, dyslipidemia, and diabetes mellitus), medications (statins and antiplatelet agents), platelet aggregability, P2Y12 reaction units (PRU), aspirin reaction units (ARU), aneurysm morphology (location, size, shape, and whether the aneurysm was thrombosed), type of FD, adjunctive coiling, and balloon angioplasty. Platelet aggregability was measured and classified as being from 1 to 9 using a PRP313M platelet aggregation analyzer (TAIYO Instruments, Osaka, Japan) 2 days prior to treatment, and ARU and PRU were measured using a Verify system (Accriva Diagnostics, San Diego, CA, USA) on the day of the procedure.
If platelet aggregability could not be suppressed sufficiently (ADP value 7–9), 20 mg of prasugrel or 200 mg of cilostazole was administered.
2.1. Outcomes
Major stroke (≥4 points on the National Institutes of Health Stroke Scale), steno-occlusive events of FD, mortality, and satisfactory aneurysm occlusion were evaluated. Satisfactory aneurysm occlusion was defined using the O’Kelly-Marotta grading scale C and D based on digital subtraction angiography, and no apparent aneurysm filling on magnetic resonance angiography [11,12]. A good clinical outcome was defined as an mRS score of 0–2. The mRS was evaluated at 90 days by telephone interviews with the patient or family members or during a physical examination in those who were able to visit our hospital.
2.2. Statistics
Statistical analyses were performed using JMP Pro (version 15.2; SAS Institute, Cary, NC, USA). The variables were expressed as mean ± standard deviation (SD), median (interquartile range [IQR], 25th–75th percentile), or the number of cases (%).
3. Results
In total, 169 patients with 172 UIAs who underwent 182 treatments were included in this study. The mean age of the patients was 61 ± 11 years, and 139 patients (83%) were female. The mean body mass index was 23 ± 3.9 kg/m2. Dual antiplatelet agents were prescribed in 153 patients (91%) (aspirin and clopidogrel: 125 (74%), aspirin and prasugrel: 28 (17%), clopidogrel and cilostazol: 1 (0.59%)), and 15 patients (8.9%) took triple antiplatelet agents (aspirin, clopidogrel, and cilostazol: 10 (5.9%), aspirin, prasugrel, and cilostazol: 5 (2.9%)). Forty-six patients (27%) received statins. Other patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of 169 patients with cerebral aneurysms treated by FD under local anesthesia.
| Variable | Value |
|---|---|
| Mean age, years (SD) | 61 (11) |
| Female sex, n (%) | 139 (82) |
| Smoking history, n (%) | 64 (38) |
| Alcohol consumption, n (%) | 32 (19) |
| Preoperative mRS 0–2, n (%) | 165 (98) |
| Past medical history, n (%) | |
| Hypertension | 68 (40) |
| Dyslipidemia | 41 (24) |
| Diabetes mellitus | 10 (5.9) |
| Verify Now, median (IQR) | |
| P2Y12 reaction units | 184 (148–217) |
| Base | 248 (219–286) |
| Inhibition | 26 (8–41) |
| Aspirin reaction units | 476 (410–523) |
| Platelet aggregability, median (IQR) | |
| Adenosine diphosphate | 5 (4–5) |
| Collagen | 4 (3–5) |
Abbreviations: FD, flow diverter; IQR, interquartile range; mRS, modified Rankin scale; SD, standard deviation. Data expressed as number of cases (%), unless otherwise indicated.
The median maximum aneurysm size and neck length were 9.5 mm (IQR 6.1–14 mm) and 6.2 mm (IQR 4.4–8.0 mm), respectively. There were 94 aneurysms (52%) greater than or equal to 10 mm in diameter, and 88 (48%) were less than 10 mm. The aneurysms were located in the internal carotid artery (n = 144, 84%), vertebral artery (n = 14, 8.1%), basilar artery (n = 7, 4.1%), posterior communicating artery (n = 4, 2.3%), and middle cerebral artery (n = 3, 1.7%). Among the 145 saccular aneurysms, 97 were a side-wall type, and 48 were bifurcation type aneurysms. The median procedure time was 70 (IQR 60–84 min). Sixty-six aneurysms (38%) underwent balloon angioplasty in order to achieve better FD wall apposition. Other aneurysm characteristics are shown in Table 2.
Table 2.
Clinical and radiological characteristics of 172 aneurysms treated by FD under local anesthesia.
| Variables | Value |
|---|---|
| Aneurysm shape, n (%) | |
| Saccular | 145 (84) |
| Blister | 3 (1.7) |
| Fusiform | 24 (14) |
| Median maximum aneurysm size, mm (Q1–Q3) | 9.5 (6.1–14) |
| Number of aneurysm measuring ≥10 mm (%) | 94 (52%) |
| Median aneurysm neck, mm (Q1–Q3) | 6.2 (4.4–8.0) |
| Growing aneurysm | 47 (27) |
| Branch from aneurysm sac | 7 (4.1) |
| Thrombosed aneurysm | 15 (8.7) |
| Previous treatment of the same aneurysm, n (%) | 26 (15%) |
| coiling | 11 (6.4) |
| Stent assisted coiling | 3 (1.7) |
| FD | 7 (4.1) |
| Direct surgery | 5 (2.9) |
| Number of FD treatment, n (%) | |
| 1 | 162 (94) |
| 2 | 8 (4.7) |
| 3 | 1 (0.58) |
| Type of FD, n (%) | n = 181 |
| FRED | 103 (57) |
| PED | 78 (43) |
| Balloon angioplasty, n (%) | 66 (38%) |
Abbreviations: FD = flow diverter; mRS = modified Rankin scale; FRED = Flow Re-Direction Endoluminal Device; PED = pipeline embolization device. Data expressed as number of cases (%), unless otherwise indicated.
Outcome
One patient died due to an intraoperative aneurysm rupture (0.59%). An mRS of 0–2 at 90 days was obtained in 164 of the 168 patients (98%). Steno-occlusive events of FD were observed in four patients (2.4%), and none of them had an increase in NIHSS score (Table 3). Among these four patients, two underwent medical treatment, one underwent mechanical thrombectomy, and on underwent stent placement. Other evaluated outcomes are shown in Table 3.
Table 3.
Outcomes.
| Variables | Value |
|---|---|
| Patient outcome | n = 169 |
| mRS 0 to 2 at 90 days (n = 168) | 164 (98) |
| Mortality at discharge | 1 (0.59) |
| Major stroke | 1 (0.59) |
| Steno-occlusive events | 4 (2.4) |
| Aneurysm outcome | n = 172 |
| Satisfactory aneurysm occlusion (n = 160) | 126 (79) |
| Re-treatment | 9 (5.2) |
| Post-treatment rupture | 2 (1.2) |
mRS = modified Rankin scale. Data expressed as number of cases (%), unless otherwise indicated.
Conventional angiography, magnetic resonance angiography, and computed tomography angiography were used for 105 (61%), 64 (37%), and 3 (1.7%) aneurysms, respectively.
4. Discussion
Our study demonstrated that FD placement under LA is a safe and effective treatment for UIAs, with satisfactory occlusion and comparably low rates of permanent neurological morbidity and mortality. Furthermore, the procedure time was shorter than that in previous studies performed under GA, with a median procedure time of 70 (IQR 60-84) min (Table 4) [13,14,15,16,17,18,19,20,21,22,23,24,25]. Additionally, thromboembolic and hemorrhagic complications were comparable to the results of studies performed under GA (Table 4) [13,14,15,16,17,18,19,20,21,22,23,24,25].
Table 4.
Summary of results of the present study and previous studies.
| Study | Year | Number of Aneurysm (Patient) | Anesthesia | Procedure Time (min) | Hemorrhagic Events | Ischemic Events | Aneurysm Occlusion | Neurologic Morbidity and Mortality |
|---|---|---|---|---|---|---|---|---|
| Current study | 2022 | 172 (169) | LA without CS | 70 (IQR 60–84) |
0.59% | 2.4% | 79% (at median 345 days) |
Mortality: 1.2% |
| PITA Nelson et al. [15] |
2011 | 31 (31) | GA | NA | 0% | 6.7% (2/30) | 93.3% (at 6 M) | Morbidity 6.7% |
| PUFS Becske et al. [13,16] |
2013, 2017 | 109 (108) | GA | 124 | 1.9% (2/107) | 6.7% (2/30) | 86.8 % (at 1Y) 95.2% (at 5 Y) | Morbidity 4.7% Mortality 2.8% |
| ASPIRe Kallmes et al. [17] |
2014 | 207 (191) | NA | 112.9 ± 54.9 | 3.7% (7/191) | 1.6% (3/191) | 74.8% (at 7.8 M) | Morbidity 7.4% Mortality 1.6% |
| IntePED Kallmes et al. [18] |
2015 | 906 (793) | NA | 101 ± 50 | 2.4% (19/793) | 4.7% (37/793) | NA | Morbidity 7.4% Mortality 3.8% |
| Australian Registry Chiu et al. [19] |
2015 | 119 (98) | NA | NA | 2.4% (19/793) | 4.7% (37/793) | 93.2% (at 2 Y) | Mortality 0.8% |
| PREMIER Hanel et al. [20] |
2019 | 141 (141) | GA or CS | 78.4 ± 40.3 | 1.4% (2/141) | 0.7% (1/141) | 81.9% (at 1Y) | 2.1% |
| Wakhloo et al. [21] | 2015 | 190 (165) | GA (except in 4: CS) |
79.8 (Average) |
2.5% | 3.7% | 75% (at median, 6 M; range, 1–38) | Morbidity 6% Mortality 2.7% |
| Berge et al. [22] | 2012 | 77 (65) | GA | NA | 6.2% | 4.7% | 84.5% (at 12 M) | Morbidity 7.7% Mortality 3% |
| SAFE Pierot et al. [14] |
2019 | 103 (103) | NA | NA | 1% | 6.8% (4/103) | 73.3% (at 1 Y) | Morbidity 2.9% Mortality 1.9% |
| SCENT Myers et al. [23] |
2019 | 180 (180) | NA | NA | 2.8% | 6.1% (11/180) | 66.1% (at 1 Y) | Total 8.3% Mortality 2.8% |
| Griessenauer et al. [24] | 2017 | CS (199) GA (149) |
CS (70 patients); GA (70 patients) | CS (64) GA (83) |
5.7% (CS); 1.4% (GA) |
5.7% (CS); 2.9% (GA) |
75% (CS at 14 M); 82.4% (GA at 7 M) |
Morbidity: 1.4% (CS); 3.2% (GA) Mortality: 1.4% (CS); 0% (GA) |
| Rangel-Castilla et al. [25]. | 2015 | 139 (130) | CS | 85 | NA | 3% | NA | Morbidity 3% |
Abbreviations: CS, conscious sedation; GA, general anesthesia; LA, local anesthesia; M, month; NA, not applicable; Y, year.
FD has been shown to be effective in treating large, wide-necked, and anatomically challenging aneurysms [13,14,18]. Similar to other neuroendovascular procedures, FD placement is performed under GA for patient comfort, control of hemodynamics and respiration, and better image quality because of the decreased motion artifacts in immobile patients [4]. However, GA using an endotracheal tube is not risk-free and can cause a range of airway injuries, including hoarseness, vocal cord paralysis, tracheomalacia, and esophageal submucosal hematoma [26,27]. The incidence of airway complications caused by endotracheal intubation ranges from 0.5 to 7% [26]. Inhaled anesthetic agents have been shown to create significant vasodilation in the cerebral artery, which would give an inaccurate dimension of the vessel, causing the selection of inappropriately sized devices [28]. This can be avoided by performing the procedure under LA. Moreover, recent studies have shown technical success as well as a reduction in endovascular-related complications in patients undergoing carotid angioplasty and stenting and thrombectomy treated with LA [5,29,30].
Moreover, Rangel-Castilla et al. demonstrated the safety and feasibility of FD placement under conscious sedation (CS) for the treatment of intracranial aneurysms [25]. In addition, a retrospective study using a matched-cohort study design conducted by Griessenauer et al. found that CS was equivalent to GA in terms of occlusion rate, functional outcome, and complications [24].
The procedure under LA also allows for the serial monitoring of neurological function, especially at the time of microcatheterization or FD deployment, enabling the early detection of embolization or branch occlusion. Potential neurological changes precede any angiographic evidence of thromboembolism or hemorrhagic episodes [25]. These possible neurological complications would go undetected if the patient were treated with GA. Although intraprocedural neuromonitoring under GA is helpful, it is not as effective and accurate as direct neurological monitoring under LA. Therefore, the early detection of neurological deficits when the procedure is performed under LA could aid in rapid and accurate management and complete recovery, which would likely have been delayed under GA [25]. It is also important to carefully monitor the patient undergoing the procedure under LA who may require sedation as there might be difficulties in the neurological assessment of these patients.
A reduction in procedure time in FD is also an important advantage of LA over GA. Griessenauer et al. demonstrated a significantly shorter procedure time in patients treated under conscious sedation than in those treated under GA (CS: 64 min [16–353 min]; GA: 83 min [25–147 min]; p = 0.03) [24]. Our study also demonstrated a median procedure time of 70 (60–84) min. This is shorter than that of most procedures performed under GA (Table 4). In addition, FD deployment under LA not only shortens the procedure time but also minimizes fluoroscopy time, resulting in less radiation exposure [25]. Moreover, the experience of surgeons and improvement in the deliverability of FD would further reduce the procedure length, fluoroscopy time, and radiation exposure.
LA can be beneficial in patients with comorbidities in which GA is contraindicated or associated with a high risk of mortality and morbidity. Other benefits of FD deployment under LA include cost-effectiveness owing to shorter hospital stays and higher patient satisfaction. Zanaty et al. demonstrated the safety and feasibility of PED treatment under monitored anesthesia care, where patients were discharged home 6 h after the procedure [31]. Additionally, there is a debate that LA carries a greater risk of complications (such as wire perforation causing hemorrhage or dissection) due to patient agitation and movements that impede catheter navigation and degrade image quality. However, this has not been proven in previous studies [32]. Moreover, one study has shown that the use of a modified head holder could minimize excessive head movements during the procedure, producing an image quality comparable to those treated under GA [25].
Limitations
This study had several limitations. First, it is a single-institutional retrospective experience, and as such, the results may not be generalizable. Second, a direct comparison between patients treated for similar lesions using GA and LA was not performed. Third, the study lacked data on patients who were converted to conscious sedation from LA. This is particularly important when patients treated with LA may experience fatigue and discomfort, as well as when there is an urgent need to induce CS because the patient cannot cooperate. Finally, data on fluoroscopy time and radiation exposure were unavailable. Nevertheless, our results support the treatment of intracranial aneurysms with FD deployment under LA.
5. Conclusions
Our study suggests that FD placement under LA is a safe and effective treatment for cerebral aneurysms with satisfactory occlusion and comparably low rates of permanent neurological morbidity and mortality. This is associated with a shorter procedure length. It is not associated with additional thromboembolic or hemorrhagic complications compared with FD under GA. Continuous neurological examination throughout the procedure may aid in the rapid recognition of neurological changes related to procedural complications and prompt appropriate management.
Author Contributions
Conceptualization, S.Y.; methodology, H.M. and K.U.; formal analysis, H.M.; investigation, S.R. and H.M.; writing—original draft preparation, S.R. and H.M.; writing—review and editing, K.U., M.S. and S.Y.; and supervision, S.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Hyogo College of Medicine (No. 3229).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Conflicts of Interest
H. Matsukawa received a non-related lecture fee from Daiichi-Sankyo and consulting services fee from B. Braun. K. Uchida received a non-related lecture fee from Daiichi-Sankyo, Bristol-Myers Squibb, Stryker, and Medtronic. S. Yoshimura received a non-related lecture fee from Stryker, Medtronic, Johnson & Johnson, Kaneka Medics. The remaining authors have no conflicts of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Briganti F., Napoli M., Tortora F., Solari D., Bergui M., Boccardi E., Cagliari E., Castellan L., Causin F., Ciceri E., et al. Italian Multicenter Experience with Flow-Diverter Devices for Intracranial Unruptured Aneurysm Treatment with Periprocedural Complications—A Retrospective Data Analysis. Neuroradiology. 2012;54:1145–1152. doi: 10.1007/s00234-012-1047-3. [DOI] [PubMed] [Google Scholar]
- 2.Arrese I., Sarabia R., Pintado R., Delgado-Rodriguez M. Flow-Diverter Devices for Intracranial Aneurysms: Systematic Review and Meta-Analysis. Neurosurgery. 2013;73:193–199. doi: 10.1227/01.neu.0000430297.17961.f1. [DOI] [PubMed] [Google Scholar]
- 3.Brinjikji W., Murad M.H., Lanzino G., Cloft H.J., Kallmes D.F. Endovascular Treatment of Intracranial Aneurysms with Flow Diverters: A Meta-Analysis. Stroke. 2013;44:442–447. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Chebl A., Lin R., Shazam Hussain M., Jovin T.G., Levy E.I., Liebeskind D.S., Yoo A.J., Hsu D.P., Rymer M.M., Tayal A.H., et al. Conscious Sedation versus General Anesthesia during Endovascular Therapy for Acute Anterior Circulation Stroke: Preliminary Results from a Retrospective, Multicenter Study. Stroke. 2010;41:1175–1179. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 5.Brinjikji W., Murad M.H., Rabinstein A.A., Cloft H.J., Lanzino G., Kallmes D.F. Conscious Sedation versus General Anesthesia during Endovascular Acute Ischemic Stroke Treatment: A Systematic Review and Meta-Analysis. AJNR. Am. J. Neuroradiol. 2015;36:525–529. doi: 10.3174/ajnr.A4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito S., Iwata S., Kondo I., Iwade M., Ozaki M., Ishikawa T., Kawamata T. Esophageal Submucosal Hematoma Developed after Endovascular Surgery for Unruptured Cerebral Aneurysm under General Anesthesia: A Case Report. JA Clin. Rep. 2017;3:54. doi: 10.1186/s40981-017-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui A.H., Kan P., Abla A.A., Hopkins L.N., Levy E.I. Complications after Treatment with Pipeline Embolization for Giant Distal Intracranial Aneurysms with or without Coil Embolization. Neurosurgery. 2012;71:E509–E513. doi: 10.1227/NEU.0b013e318258e1f8. [DOI] [PubMed] [Google Scholar]
- 8.Chalouhi N., Chitale R., Starke R.M., Jabbour P., Tjoumakaris S., Dumont A.S., Rosenwasser R.H., Gonzalez L.F. Treatment of Recurrent Intracranial Aneurysms with the Pipeline Embolization Device. J. Neurointerv. Surg. 2014;6:19–23. doi: 10.1136/neurintsurg-2012-010612. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Van Swieten J.C., Koudstaal P.J., Visser M.C., Schouten H., Van Gijn J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke. 1988;19:604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 11.O’Kelly C.J., Krings T., Fiorella D., Marotta T.R. A Novel Grading Scale for the Angiographic Assessment of Intracranial Aneurysms Treated Using Flow Diverting Stents. Interv. Neuroradiol. 2010;16:133. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang S., Fan F., Hu P., Yang K., Zhai X., Geng J., Ji Z., Lu J., Zhang H. The Sensitivity and Specificity of TOF-MRA Compared with DSA in the Follow-up of Treated Intracranial Aneurysms. J. Neurointerv. Surg. 2021;13:1172–1179. doi: 10.1136/neurintsurg-2020-016788. [DOI] [PubMed] [Google Scholar]
- 13.Becske T., Kallmes D.F., Saatci I., McDougall C.G., Szikora I., Lanzino G., Moran C.J., Woo H.H., Lopes D.K., Berez A.L., et al. Pipeline for Uncoilable or Failed Aneurysms: Results from a Multicenter Clinical Trial. Radiology. 2013;267:858–868. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 14.Pierot L., Spelle L., Berge J., Januel A.C., Herbreteau D., Aggour M., Piotin M., Biondi A., Barreau X., Mounayer C., et al. SAFE Study (Safety and Efficacy Analysis of FRED Embolic Device in Aneurysm Treatment): 1-Year Clinical and Anatomical Results. J. Neurointerv. Surg. 2019;11:184–189. doi: 10.1136/neurintsurg-2018-014261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson P.K., Lylyk P., Szikora I., Wetzel S.G., Wanke I., Fiorella D. The Pipeline Embolization Device for the Intracranial Treatment of Aneurysms Trial. AJNR. Am. J. Neuroradiol. 2011;32:34–40. doi: 10.3174/ajnr.A2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becske T., Potts M.B., Shapiro M., Kallmes D.F., Brinjikji W., Saatci I., McDougall C.G., Szikora I., Lanzino G., Moran C.J., et al. Pipeline for Uncoilable or Failed Aneurysms: 3-Year Follow-up Results. J. Neurosurg. 2017;127:81–88. doi: 10.3171/2015.6.JNS15311. [DOI] [PubMed] [Google Scholar]
- 17.Kallmes D.F., Brinjikji W., Boccardi E., Ciceri E., Diaz O., Tawk R., Woo H., Jabbour P., Albuquerque F., Chapot R., et al. Aneurysm Study of Pipeline in an Observational Registry (ASPIRe) Interv. Neurol. 2016;5:89–99. doi: 10.1159/000446503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallmes D.F., Hanel R., Lopes D., Boccardi E., Bonafé A., Cekirge S., Fiorella D., Jabbour P., Levy E., McDougall C., et al. International Retrospective Study of the Pipeline Embolization Device: A Multicenter Aneurysm Treatment Study. Am. J. Neuroradiol. 2015;36:108–115. doi: 10.3174/ajnr.A4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu A.H.Y., Cheung A.K., Wenderoth J.D., De Villiers L., Rice H., Phatouros C.C., Singh T.P., Phillips T.J., McAuliffe W. Long-Term Follow-Up Results Following Elective Treatment of Unruptured Intracranial Aneurysms with the Pipeline Embolization Device. AJNR. Am. J. Neuroradiol. 2015;36:1728–1734. doi: 10.3174/ajnr.A4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanel R.A., Kallmes D.F., Lopes D.K., Nelson P.K., Siddiqui A., Jabbour P., Pereira V.M., Szikora István I., Zaidat O.O., Bettegowda C., et al. Prospective Study on Embolization of Intracranial Aneurysms with the Pipeline Device: The PREMIER Study 1 Year Results. J. Neurointerv. Surg. 2020;12:62–66. doi: 10.1136/neurintsurg-2019-015091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakhloo A.K., Lylyk P., De Vries J., Taschner C., Lundquist J., Biondi A., Hartmann M., Szikora I., Pierot L., Sakai N., et al. Surpass Flow Diverter in the Treatment of Intracranial Aneurysms: A Prospective Multicenter Study. AJNR. Am. J. Neuroradiol. 2015;36:98–107. doi: 10.3174/ajnr.A4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berge J., Biondi A., Machi P., Brunel H., Pierot L., Gabrillargues J., Kadziolka K., Barreau X., Dousset V., Bonafée A. Flow-Diverter Silk Stent for the Treatment of Intracranial Aneurysms: 1-Year Follow-up in a Multicenter Study. AJNR. Am. J. Neuroradiol. 2012;33:1150–1155. doi: 10.3174/ajnr.A2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers P.M., Coon A.L., Kan P.T., Wakhloo A.K., Hanel R.A. SCENT Trial. Stroke. 2019;50:1473–1479. doi: 10.1161/STROKEAHA.118.024135. [DOI] [PubMed] [Google Scholar]
- 24.Griessenauer C.J., Shallwani H., Adeeb N., Gupta R., Rangel-Castilla L., Siddiqui A.H., Levy E.I., Boone M.D., Thomas A.J., Ogilvy C.S. Conscious Sedation Versus General Anesthesia for the Treatment of Cerebral Aneurysms with Flow Diversion: A Matched Cohort Study. World Neurosurg. 2017;102:1–5. doi: 10.1016/j.wneu.2017.02.111. [DOI] [PubMed] [Google Scholar]
- 25.Rangel-Castilla L., Cress M.C., Munich S.A., Sonig A., Krishna C., Gu E.Y., Snyder K.V., Hopkins L.N., Siddiqui A.H., Levy E.I. Feasibility, Safety, and Periprocedural Complications of Pipeline Embolization for Intracranial Aneurysm Treatment under Conscious Sedation: University at Buffalo Neurosurgery Experience. Clin. Neurosurg. 2015;11:426–430. doi: 10.1227/NEU.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco-Lopez P.C., Berkow L.C., Hillel A.T., Akst L.M. Complications of Airway ManagementDiscussion. Respir. Care. 2014;59:1006–1021. doi: 10.4187/respcare.02884. [DOI] [PubMed] [Google Scholar]
- 27.Takeyama E., Wada A., Amano E., Shibuya H. Oesophageal Submucosal Hematoma after Flow Diverter Embolization with Favourable Outcome Treated by Discontinuing Postoperative Antiplatelet Therapy for Only Three Days: A Case Report. JA Clin. Rep. 2019;5:2. doi: 10.1186/s40981-018-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arıcan Ş., Bakdık S., Hacıbeyoğlu G., Yılmaz R., Koç O., Tavlan A., Uzun S.T. The Effects of Sevoflurane Anesthesia on Hemodynamics and Cerebral Artery Diameters in Endovascular Treatment of Intracranial Aneurysm: A Pilot Study. Ulusal Travma ve Acil Cerrahi Dergisi. 2021;27:200–206. doi: 10.14744/tjtes.2021.00269. [DOI] [PubMed] [Google Scholar]
- 29.Erickson K.M., Cole D.J. Carotid Artery Disease: Stenting vs Endarterectomy. Br. J. Anaesth. 2010;105((Suppl. S1)):i34–i49. doi: 10.1093/bja/aeq319. [DOI] [PubMed] [Google Scholar]
- 30.Schönenberger S., Uhlmann L., Hacke W., Schieber S., Mundiyanapurath S., Purrucker J.C., Nagel S., Klose C., Pfaff J., Bendszus M., et al. Effect of Conscious Sedation vs General Anesthesia on Early Neurological Improvement Among Patients with Ischemic Stroke Undergoing Endovascular Thrombectomy: A Randomized Clinical Trial. JAMA. 2016;316:1986–1996. doi: 10.1001/jama.2016.16623. [DOI] [PubMed] [Google Scholar]
- 31.Zanaty M., Daou B., Chalouhi N., Starke R.M., Samaniego E., Derdeyn C., Jabbour P., Hasan D. Same-Day Discharge After Treatment with the Pipeline Embolization Device Using Monitored Anesthesia Care. World Neurosurg. 2016;96:31–35. doi: 10.1016/j.wneu.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 32.Hendén P.L., Rentzos A., Karlsson J.E., Rosengren L., Leiram B., Sundeman H., Dunker D., Schnabel K., Wikholm G., Hellström M., et al. General Anesthesia Versus Conscious Sedation for Endovascular Treatment of Acute Ischemic Stroke: The AnStroke Trial (Anesthesia During Stroke) Stroke. 2017;48:1601–1607. doi: 10.1161/STROKEAHA.117.016554. [DOI] [PubMed] [Google Scholar]