Abstract
For decades, research in cancer biology has been focused on the protein-coding fraction of the human genome. However, with the discovery of non-coding RNAs (ncRNAs), it has become known that these entities not only function in numerous fundamental life processes such as growth, differentiation, and development, but also play critical roles in a wide spectrum of human diseases, including cancer. Dysregulated ncRNA expression is found to affect cancer initiation, progression, and therapy resistance, through transcriptional, post-transcriptional, or epigenetic processes in the cell. In this review, we focus on the recent development and advances in ncRNA biology that are pertinent to their role in glioma tumorigenesis and therapy response. Gliomas are common, and are the most aggressive type of primary tumors, which account for ~30% of central nervous system (CNS) tumors. Of these, glioblastoma (GBM), which are grade IV tumors, are the most lethal brain tumors. Only 5% of GBM patients survive beyond five years upon diagnosis. Hence, a deeper understanding of the cellular non-coding transcriptome might help identify biomarkers and therapeutic agents for a better treatment of glioma. Here, we delve into the functional roles of microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) in glioma tumorigenesis, discuss the function of their extracellular counterparts, and highlight their potential as biomarkers and therapeutic agents in glioma.
Keywords: non-coding RNA, glioma, microRNA, long non-coding RNA, circular RNA
1. Introduction
RNA was once thought of as just a messenger molecule for relaying the code from DNA by ribosomes to synthesize proteins. However, in the past three decades, different types of RNA molecules have been discovered, the most important of which are the non-coding RNAs (ncRNAs). ncRNAs account for 90% of the RNA species transcribed from the human genome [1]. The discovery of tens of thousands of ncRNAs has augmented their importance in physiology and disease [1,2,3]. For decades, it was believed that only 2% of the human genome that codes for proteins was functional and the remaining 98% was non-functional, or ‘junk’ [4,5]. Only after the ENCODE project was completed, did it become appreciable that the non-protein coding region of the genome is transcribed into thousands of RNA molecules, which are not only involved in numerous fundamental life processes such as growth, differentiation, and developmental processes, but also play critical roles in a wide spectrum of human diseases, including cancer [6,7,8].
The discovery of ncRNAs has added a new dimension to the diagnosis and treatment of cancer. Dysregulated ncRNA expression is found to affect cancer initiation, progression, and therapy resistance, and occurs through transcriptional, post-transcriptional, or epigenetic processes in the cell [1,9]. Genetic alterations in genes to code for ncRNAs are also associated with cancer. For example, deletion of 13q14.3 in chronic lymphocytic leukemia (CLL) results in deletion of miR-15/16 tumor suppressors [10]. Conversely, amplification of chromosomal regions has led to an increase of oncogenic long non-coding RNAs (lncRNAs) FAL1 and PVT1 [11,12]. Moreover, recurrent driver mutations in the promoters of lncRNAs NEAT1 and RMRP can lead to altered expression and breast cancer progression [13].
Gliomas are common, and are the most aggressive type of primary tumors, which account for ~30% of central nervous system (CNS) tumors. Gliomas are constituted of pilocytic astrocytoma, oligodendroglioma, astrocytoma, oligoastrocytoma, ependymoma, and glioblastoma, which are classified by the World Health Organization (WHO) into grade I to IV tumors [14]. Despite the standard of care including surgical resection, radiotherapy, and chemotherapy, the prognosis of glioma patients remains poor [15]. Of these, glioblastoma (GBM), which are grade IV tumors, are common, and are the most lethal brain tumors. Only 5% of GBM patients survive beyond 5 years upon diagnosis [16]. The current treatment regimen had only increased the median survival to 14.6 months in adults [17,18]. Though recent clinical trials have shown that the addition of Tumor-treating fields (TTFields) increased the overall median survival by 4.9 months [19]. Another Grade IV astrocytoma, called diffuse midline glioma is a rare type of glioma and shows poor prognosis [20]. Thus, a deeper understanding of the cellular non-coding transcriptome might help identify biomarkers and therapeutic agents for a better treatment of glioma.
NcRNAs had been broadly classified into housekeeping RNAs and regulatory RNAs. Housekeeping RNAs include transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), and small nucleolar RNA (snoRNA), which are involved in maintaining the regular proteome components of the cell. All other ncRNAs are involved in regulating gene expression in the cell and are categorized based on their size. The small ncRNA include microRNA (miRNA), PIWI-interacting RNAs (piRNAs), and tRNA-derived small RNAs (tsRNAs), while those greater than 200 nucleotides (nts) in length include lncRNAs, which include circular non-coding RNAs (circRNAs) and pseudogenes [21].
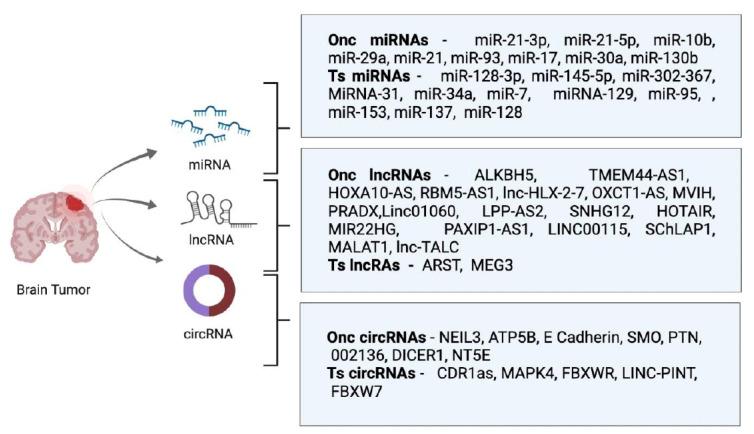
This review focuses on the recent developments and advances in ncRNA biology pertinent to their role in glioma tumorigenesis and therapy response. Since studies of tsRNA in glioma are scarce, here, we delve into the functional roles of miRNA, lncRNA, circRNAs, and briefly, piRNA, in glioma development, progression, and therapy resistance, thus highlighting the potential and clinical implications of the ncRNAs in glioma (Figure 1).
Figure 1.
Oncogenic (Onc) and Tumor-suppressor (Ts) ncRNAs-MicroRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) expressed in Glioma.
2. MicroRNAs: Biogenesis and Function
miRNAs are natural RNA interference (RNAi) molecules produced in cells. They are transcribed in the cell from the nuclear genome (pri-miRNA), processed, and exported out of the nucleus as stem loop precursor pre-miRNAs. In the cytoplasm, these pre-miRNAs are trimmed to form duplex miRNAs, which are loaded on to Argonaut proteins, either individual strand or both, to form the RNA-induced silencing complex (RISC). The miRNA-guided RISC binds to target mRNAs to regulate their expression majorly by mRNA destabilization (~80%) or by repressing translation (~20%) [22]. miRNA-RISC complexes also function in the nucleus and mitochondria [23,24]. These RNAi agents are special because a single miRNA can regulate the expression of multiple genes involved in different cellular functions, hence, these serve as attractive candidates for biomarkers and therapeutic targets in diseases, including cancer [25].
2.1. miRNA Deregulation in Glioma
The role of miRNAs in gliomagenesis has been extensively studied over the past several years, the functions of miRNAs being described as oncogenes or tumor suppressors in glioma.
The diverse action of a single miRNA on multiple targets is well represented in glioma tumorigenesis. For example, enhancing the expression of the tumor suppressor miRNA miR-128-3p or miR-145-5p in GBM results in a plethora of anti-tumorigenic phenotypes, such as increase in apoptosis or senescence, repression in invasive, metastatic, or angiogenesis potential, and alleviation of drug resistance in GBM by regulation of multiple targets by miRNAs [26,27,28,29]. Inhibiting the expression of oncogenic miRNAs, such as miR-21-3p or miR-21-5p, can lead to tumor regression in cancer cells or even lead to tumor eradication, as in the case of miR-10b in GBM [30,31,32]. Furthermore, primary glioma cells stably expressing the miR-302-367 repressed the stemness, proliferation, and tumorigenicity of neighboring GBM cells in a paracrine manner [33]. MiRNA-31 encoded by the MIR31HG gene, positioned adjacent to CDKN2A/B at Chromosome 9, is compromised in >72% of GBM, and its reintroduction reduces tumor burden by inhibiting NF-κB signaling, independent of CDKN2A/B status [34]. An extensive list of GBM-associated miRNAs can be found at Shea at al [35] and a list of the recent miRNA studies in GBM is provided in Table 1.
Table 1.
Non-coding RNAs associated with Glioma.
| Type of ncRNA | Name | Functional Mechanism | Reference |
|---|---|---|---|
| Oncogenic miRNAs | miR-10b | Essential for viability of GBM cells; controls cell proliferation, survival, migration, invasion, and epithelial-to-mesenchymal transition | [123] |
| miR-1246 | Drives the differentiation and activation of myeloid-derived suppressor cells | [124] | |
| miR-30e* | Promotes invasiveness by disrupting the NF-κB/IκBα loop | [125] | |
| miR-637 | Drives the malignant phenotype of glioma by suppressing HMGA1 in vitro and in vivo | [126] | |
| miR-30b-3p | EV-packaged miR-30b-3p (EV-miR-30b-3p) directly targeted ras homolog family member B (RHOB), increases TMZ resistance | [127] | |
| miR-155 and the miR-23a cluster | Down regulate the tumor suppressor MAX interactor 1 (MXI1) | [128] | |
| Tumor suppressive miRNAs | miR-3189 | Controls cancer cell metabolism by inhibiting GLUT3 expression | [129] |
| miR-124 | miR-124-extracellular vesicles (EVs) inhibits M2 microglial polarization | [130] | |
| miR-593 | Regulates the Eph receptor tyrosine kinase Eph receptor B2 (EphB2) levels | [131] | |
| miR-205 | ERBB3 overexpression is sustained by epigenetic inactivation of the oncosuppressor miR-205 | [132] | |
| miR-185-5p | Regulates the expression of tumor promoting HOXB5 expression | [133] | |
| miR-195 | Regulates expression of the cell division cycle 25A (CDC25A) phosphatase, a key regulator of cell cycle progression | [123] | |
| miR-198 | Reduces cellular MGMT levels through binding to the 3′-UTR of MGMT to enhance TMZ sensitivity | [134] | |
| miR-432-5p | Regulates expression of RNA editing enzyme adenosine deaminases acting on RNA (ADAR) | [135] | |
| miR-375 | Selective exosome exclusion of miR-375 by glioma cells inhibited the CTGF-epidermal growth factor receptor (EGFR) signaling pathway | [136] | |
| miR-342-3p | Regulates the expression of the transcription factor ISL2 which transcriptionally regulated VEGFA expression in GSCs | [137] | |
| miR-137 | Has roles in cell proliferation, apoptosis, invasion, and angiogenesis and in glioma treatment | [138] | |
| miR-199a-3p | Regulates expression of the transcription factor EGR1 (early growth response 1) involved in GSC proliferation and self-renewal | [139] | |
| Oncogenic lncRNAs | ALKBH5 | Facilitates IL8 Secretion to Generate an Immunosuppressive TME | [104] |
| TMEM44-AS1 | Sequentially activated Myc and EGR1/IL-6 signaling | [140] | |
| HOXA10-AS | Regulates contact inhibition, cell proliferation, invasion, Hippo signaling, and mitotic and neuro-developmental pathways | [141] | |
| RBM5-AS1 | Interacted with and stabilized sirtuin 6 (SIRT6) protein | [142] | |
| lnc-HLX-2-7 | Modulates oxidative phosphorylation, mitochondrial dysfunction, and sirtuin signaling pathways | [143] | |
| OXCT1-AS | Acts as a ceRNA of miR-195 to enhance CDC25A expression | [90] | |
| lnc PRADX | Suppressed UBXN1 expression through promoter methylation and promoted NF-κB activity | [103] | |
| Linc 01060 | Directly interacts with the transcription factor myeloid zinc finger 1 (MZF1), promotes its nuclear translocation and MZF1-mediated c-Myc transcriptional activities | [110] | |
| LPP-AS2 | Functions as a ceRNA to regulate EGFR expression by sponging miR-7-5p | [144] | |
| SNHG12 | Acts as s sponge for miR-129-5p, leading to upregulation of MAPK1 and E2F7 | [95] | |
| HOTAIR | GBM-serum-EV-enclosed HOTAIR competitively binds to miR-526b-3p to increase EVA1 expression | [145] | |
| MIR22HG | Induces the Wnt/β-catenin signaling pathway | [146] | |
| PAXIP1-AS1 | Upregulates the KIF14 promoter activity by recruiting transcription factor ETS1 | [102] | |
| LINC00115 | Upregulates ZEB1 and promotes ZNF596 transcription by acting as a miRNA sponge for miR-200s | [93] | |
| SChLAP1 | Binds to heterogeneous nuclear ribonucleoprotein L (HNRNPL) to stabilize actinin alpha 4 (ACTN4) through suppression of proteasomal degradation | [98] | |
| MALAT1 | Regulated by NF-κB and p53. MALAT1 is a potential target for the chemo sensitization of GBM | [147] | |
| lnc-TALC | Competitively binds miR-20b-3p to facilitate c-Met expression | [97] | |
| Tumor suppressive lncRNAs | ARST | Inhibits ALDOA-mediated actin cytoskeleton integrity | [148] |
| lncRNA MEG3 | Regulating cell adhesion, EMT, and cell proliferation | [149] | |
| Oncogenic circRNAs | circNEIL3 | Drives macrophage infiltration and immunosuppression by stabilizing IGF2BP3 (insulin-like growth factor 2 mRNA binding protein 3) | [150] |
| circATP5B | Acts as miR-185-5p sponge to upregulate HOXB5 expression | [133] | |
| ASAP1 | Increases the expression of NRAS via sponging miR-502-5p. | [151] | |
| circ E Cadherin | Stimulates EGFR signaling independent of EGF | [152] | |
| circ SMO | Encodes SMO-193a.a., required for sonic hedgehog (Shh) induced G protein-coupled-like receptor smoothened (SMO) activation | [153] | |
| circ ATXN1 | Targeted miR-526b-3p to upregulate MMP2/VEGFA expression | [154] | |
| circ FOXO3 | Sponges both miR-138-5p and miR-432-5p to increase expression of nuclear factor of activated T cells 5 (NFAT5) | [155] | |
| circPTN | Sponges miR-145-5p and miR-330-5p to rescue downregulation of SOX9/ITGA5 in glioma cells | [156] | |
| circ 002136 | Sponges miR-138-5p to enhance SOX13 mediated angiogenesis | [157] | |
| circ DICER1 | Acts as a molecular sponge to adsorb miR-103a-3p/miR-382-5p and enhance ZIC4 in glioma-exposed endothelial cells (GECs) | [158] | |
| circ NT5E | Sponges miR-422a to inhibit the miRNA’s activity | [159] | |
| Tumor suppressive circRNA | CDR1as | Stabilizes p53 protein by preventing it from ubiquitination by MDM2 | [160] |
| circ MAPK4 | Modulates miR-125a-3p to enhance the activity of the p38/MAPK signaling pathway | [161] | |
| circ LINC-PINT | Encodes a 87-aa peptide that interacts with polymerase associated factor complex (PAF1c) and inhibits the transcriptional elongation of multiple oncogenes | [162] | |
| circ FBXW7 | Antagonizing USP28-induced c-Myc stabilization, thus reducing the half-life of c-Myc | [163] |
miRNAs interact with various cancer-associated signaling pathways in GBM [36,37]. In PTEN-deficient GBM, miR-29a promoted growth and invasion, by activating Akt, repressing Sox4 transcription factor, and upregulating the invasion-promoting protein, HIC5 [38]. miR-34a shows tumor suppressive roles in GBM by targeting c-Met and Notch signaling pathways [39]. Also, miR-34a could modulate EGFR levels. In one study, by targeting the Yin Yang-1 (YY1) transcription factor, which stimulated EGFR levels, miR-34a deletion and EGFR amplification were associated with decreased survival in GBM patients [40]. The EGFR pathway in GBM is regulated by another miRNA, miR-7, which is a major regulator of cancer pathways, and its forced expression decreases viability and invasiveness in primary GBM cells [41]. Moreover, miRNA expression regulates the heterogeneity in GBM. miR-125b/miR-20b are expressed only in the proneural (PN) subtype of GBM and not the mesenchymal subtype, and they regulate the Wnt signaling in the PN subtype by repressing the transmembrane protein FDZ6, thus contributing to the heterogeneity in GBM [42].
2.2. miRNA and Blood-Brain Barrier
The blood-brain barrier (BBB) is a highly complex structural and functional barrier characterized by low permeability, low pinocytosis, and lack of fenestration, thus providing a compartmental resistance across the blood and brain. It facilitates the passing of nutrients and oxygen from the blood and restricts accumulation of neurotoxins in the CNS. The BBB is constituted of different types of cells. Endothelial cells that line the brain capillaries are connected by tight junctions that interact with other supporting cells, including astrocytes, mast cells, pericytes, and microglia, to form a neuro-vascular unit [43]. It is known that, in GBM, morphological changes occur in the BBB, due to which, it shows enhanced vascular permeability and drug uptake. In pathological conditions, miRNA can pass from the brain tissue to the blood, thus serving as biomarkers for CNS diseases [44,45,46]. Moreover, extracellular vesicles from breast cancer cells, containing miR-181c have been found to disrupt the BBB and promote brain metastasis by downregulating 3-phosphoinositide-dependent protein kinase 1 (PDPK1) and altering the actin filaments [47]. Thus, miRNA-based therapeutics are attractive in GBM. One study has shown that miRNA-27a-3p mimics target the membrane protein, Aquaporin-11 (AQP11), located in the endothelial cells of brain capillaries, and has protective effects on BBB integrity in rats with intracerebral hemorrhage [48]. Thus, miRNAs have the potential to serve as potential therapeutics and biomarkers in GBM and other CNS diseases.
2.3. miRNA-Based Therapeutics in Glioma
miRNA levels can be modulated with various tools either at the level of their biogenesis or by affecting their stability. These include gene therapy to sponge miRNAs, oligonucleotide therapy, and RNA delivery systems using nanoparticles [49].
2.3.1. Targeting miRNA Biogenesis by Using Small Molecule Inhibitors
Hypoxia in tumors is known to downregulate Drosha and Dicer, thus reducing miRNA biogenesis and increasing tumor progression mediated through the ETS1/ELK1 transcription factors. Downregulating these factors can rescue miRNA biogenesis and reduce tumor progression in ovarian cancer [50]. Targeting oncogenic miRNAs by knock down of argonaute 2 (AGO2) has shown to induce apoptosis in myeloid leukemia [51]. However, these strategies can target the entire miRNA spectrum in the body and could lead to severe toxicities. Specific drugs that selectively inhibit certain miRNAs have been used in glioma. Phenformin, an antidiabetic drug, reduces stemness and growth in GBM by increasing the expression of the miRNAs, let-7, miR-124, and miR-137 [52]. Erismodegib, a phase III drug for medulloblastoma, induced apoptosis in glioma stem-like cells (GSCs) by suppressing the expression of miR-21 and suppressed EMT by upregulating miR-128 in GSCs [53]. Further, curcumin, a polyphenolic compound blocks the action of a lncRNA lncRNA-ROR, which acts as a competing endogenous RNA (ceRNA) to inhibit miR-145 activity in prostate cancer stem cells [53] and has been suggested to be used in clinical trials for the treatment of human brain tumors owing to its low toxicity [54]. Thus, small molecule inhibitors modulating miRNA biogenesis or expression have been used for combating cancer, including in glioma.
2.3.2. Oligonucleotide-Mediated Therapy
miRNA mimics have been used to increase the expression of a specific miRNA particularly for the restoration of tumor suppressor miRNAs in the cell. These are synthetic double stranded (ds)RNA molecules that have the same sequence as natural miRNAs and can integrate into the RISC to perform the anti-tumorigenic functions of the missing natural miRNA [49]. Chemically modified single strand RNA (ssRNA) mimetic therapy has also shown success in the brain of Huntington disease (HD) mouse model. ssRNA, when stereo tactically injected into the brain, could bind to Ago2, and inhibits several miRNA targets without any carrier [55]. The levels of tumor suppressor miRNAs could also be increased in the brain by reactivating their transcription, by activating silenced hypermethylated miRNA promoter sites, by replenishing missing miRNA genomic regions by CRISPR/Cas9, or by inhibiting miRNA sponges such as lncRNAs and circRNAs, which form complex regulatory networks in the brain [56,57].
Anti-miRNA therapy in glioma is directed against the oncogenic miRNAs expressed in the brain. This is facilitated by antisense oligonucleotides (ASOs) that bind to the mature miRNA and block their functions. These are referred to as anti-miRs, antagomiRs, anti-miRNA oligonucleotides (AMOs), or locked nucleic acids (LNAs) [49]. Other types of ASOs include miRNA masks, which bind to miRNA-recognizing elements (MREs) on mRNA and inhibit mRNA activity; also, small RNA zippers [58], which are ss DNA LNA molecules that block miRNA by connecting mature target miRNAs, end to end through a complementary interaction and forming a DNA-RNA duplex [59].
AMOs are ss RNA molecules, ~20 nts in length, that are chemically modified using 2′-O-methoxyethyl and phosphorothioate and selectively bind to the mature miRNA to form a miRNA-RNA duplex that is sensitive to degradation by the endonuclease RNase H [44]. In one study, delivery of anti-miR-21 antisense oligodeoxynucleotides (antisense-ODN) in a GBM xenograft model led to apoptosis of tumor cells [60]. AntagomiRs are cholesterol-conjugated ssRNA designed to inhibit miRNA molecules in vivo. Delivery of antagomiR-27a in U87 GBM tumor xenografts in mice resulted in reduced proliferation and invasiveness [61,62]. LNAs are anti-miRs which have a more rigid conformation due to the presence of a methylene bridge between the 2′-O and 4′-C atoms of their ribose ring, and are thus more nuclease resistant and have enhanced binding to their target miRNA [44]. Delivery of anti-miR-21 LNA sequences using RNA nanoparticles (RNP) reduced the expression of oncogenic miR-21 and resulted in tumor regression in GBM [63]. miRNA sponges are another mode of miRNA modulation, wherein artificially synthesized ncRNAs, lncRNA, or circRNAs with multiple MREs are delivered into the cell to sponge and modulate miRNA function. In one study, a lentiviral-based miRNA sponge targeting miR-23b delivered in GBM cells and orthotopic mouse model could reduce tumor malignancy [64].
2.3.3. RNA Delivery Systems
The complexity of the CNS and other physiological barriers pose challenges to the delivery of drugs and RNA-based therapeutics in neurological disorders and cancers such as glioma. Thus, efficient, biocompatible carrier systems are required for delivery of nucleic acid loads to a precise location in the brain without being subjected to degradation. The nanotechnology-based carriers, metallic nanoparticles, polymeric systems, liposomes, and lipid-based carriers have shown efficiency in delivering miRNA therapeutics in glioma [36]. Spherical gold (Au) nanoparticles have a densely packed surface with oligonucleotides through a thiolate-Au interaction. These nanoparticles have low toxicity, have high transfection efficiency, and can cross the BBB. However, the RNA loaded onto the surface are chemically modified and can also elicit immune reactions due to constant exposure [49]. As discussed above, delivery of LNA against miR-21 via multi-valent folate (FA)-conjugated three-way-junction (3WJ)-based RNA nanoparticles (RNP) specifically targeted to miR-21, rescued tumor suppressors including PTEN and program cell death 4 (PDCD4), and induce apoptosis in GBM cells in vitro and in vivo [63]. Moreover, delivery of anti-miR-21 ASOs using lipid-polymer nanoparticles (LPNs) has shown efficient targeting in GBM [65]. Cell-penetrating peptides, in which cationic peptides are linked to nucleic acids, are another efficient form of delivery. The delivery of an anti-miR 221 peptide-nucleic acid (PNA) along with temozolomide (TMZ), mesoporous silica nanoparticles (MSNPs), could induce apoptosis in TMZ-resistant GBM cells [66]. The first-in-human phase I clinical trial was conducted for MRX34, a liposomal miR-34a in patients with advanced refractory solid tumors, and showed signs of antitumor activity in a subset of patients [67]. Thus, strategies that allow efficient internalization and delivery of nucleic acids into specific tumor regions can been used capably for miRNA-based therapeutics in GBM.
2.4. miRNAs as Biomarkers in Glioma
A biomarker is a biological indicator that can be measured objectively to understand the presence of prognosis of a disease. The importance of biomarkers in brain tumor diagnostics has grown over the past decades, as a liquid biopsy from blood or the cerebrospinal fluid (CSF) would spare surgical intervention. Though obtaining CSF is more invasive, it is more useful due to enrichment of miRNAs in the CSF [44].
miR-21 has been pointed out to be the most powerful miRNA in brain cancer diagnostics, as per a meta-analysis from 2015 [68]. In one study, levels of miR-21 expression could differentiate between glioma patients and healthy controls, although not between glioma and other brain tumors [69]. In another study, circulating miRNA biomarkers were used for the diagnosis of glioma. Combined expression analysis could distinguish between glioma patients and healthy controls, and miR-16 expression levels could differentiate between GBM (grade IV) and lower grades of gliomas [70]. Moreover, the expression levels of miR-222 and miR-124-3p along with miR-21 in the serum have been shown to help in the differential diagnosis of gliomas at onset, in the grading of gliomas, and in assessing post-surgical recovery [71].
miRNAs that are secreted into the circulation either circulate freely or are packaged into exosomes, in which these are protected from ribonuclease-mediated degradation by the lipid-bilayer of the exosomes [72]. One study has shown that miR-21 levels in extracellular vesicles (EVs) derived from CSF of GBM patients was around 10 times higher than that from normal controls, thus highlighting its importance as potential biomarker for GBM [73]. Similarly, Yang et al. showed that miR-221 levels in serum exosomes was higher in GBM patients, and its levels increased in GBM (grade IV) comparing to lower grade gliomas [74]. Further, exosomal miRNAs could also serve as independent prognostic markers in glioma. miR-301a levels were found to be associated with a longer overall survival (OS) [75]. Thus, exosomal miRNAs in body fluids have enormous potential for biomarker development in the diagnosis and prognosis of glioma.
3. Long Non-Coding RNAs
In the past several years, lncRNAs have been found to affect gene expression in various biological and physiopathological conditions involving neurodegeneration, cellular stress response, and cancer [76,77,78,79]. These RNA molecules which are >200 nt in length, generally lack protein-coding capacity and mostly contain the 5′m7G caps and 3′ poly(A) tails structures like mRNAs; however, they are more dynamic and tissue-specific compared to mRNAs, thus suggesting they play multiple functional roles [80].
3.1. Classification of LncRNAs
LncRNAs can be characterized based on their genomic location, subcellular localization, or presence of unique structures. Based on their genomic location relative to protein-coding genes, these are characterized as sense, antisense, bidirectional, intronic, or intergenic (linc) RNAs. Antisense (AS) lncRNAs are transcribed by the AS RNA strand of the protein-coding gene while sense lncRNAs overlap with exons or introns of different protein-coding genes in the sense RNA direction. Bidirectional lncRNAs are transcribed from the promoter of non-coding genes in the opposite direction, intronic lncRNAs are transcribed completely from introns whereas intergenic lncRNAs are gnomically located between two protein-coding genes [76,81]. Furthermore, lncRNAs are also classified as nuclear or cytoplasmic based on their localization, which also determines their functions. Nuclear lncRNAs are involved in gene regulation through transcriptional regulation or chromatin remodeling, while cytoplasmic lncRNAs function in processes such as maintaining mRNA processing, stability, or protein regulation [82,83,84].
3.2. Functional Mechanisms of LncRNAs in Glioma
LncRNAs are involved in glioma initiation, progression, and infiltration/invasion processes [80,85,86]. These molecules are implicated in glioma progression by regulating the cellular proliferation, apoptosis, differentiation, stem-cell self-renewal, and response to hypoxic stress. At the molecular level, lncRNAs regulate glioma progression by epigenetic regulation, transcriptional and post-transcriptional modulation, and translational regulation [87]. An extensive list of recent studies on oncogenic and tumor suppressive lncRNAs associated with glioma and their underlying functional mechanisms has been provided in Table 1.
3.2.1. Post-Transcriptional Regulation of LncRNAs as a miRNA-Sponge in Glioma Oncogenesis and Therapy Resistance
Many of the glioma-associated lncRNAs function as miRNA sponges, to diminish the availability of the transcribed miRNAs, resulting in elevation of their downstream targeting genes [88]. LncRNAs function as ceRNA to regulate the miRNA/mRNA axis in various human diseases [89]. One such lncRNA OXCT1 antisense RNA 1 (OXCT1-AS1) is found to be upregulated in GBM patients, functions as a ceRNA of miR-195 to promote the upregulation of CDC25A expression, and in turn, glioma progression [90]. Other examples of the ceRNA-miRNA-mRNA axis in glioma include the lncRNA HOXA-AS3/miR-455-5p/USP3 axis, which promotes malignancy in GBM, and the Linc00152/miR-103a-3p/FEZF1 axis known to enhance malignancy in glioma stem cells (GSCs) [91,92]. Moreover, LINC00115 enhances GSC self-renewal by competitively binding to miR-200s, thereby enhancing ZEB1 signaling, GSC self-renewal, and tumorigenicity [93]. NEAT1 lncRNA is upregulated in glioma to inhibit the expression of miR-132 by removing miR-132′s negative regulation on SOX2, thus promoting gliomagenesis [94]. SNHG12 is an oncogenic lncRNA involved in the acquired resistance to TMZ therapy in GBM. Loss of promoter methylation led to high expression of SNHG12 by the transcription factor SP1. SNHG12 further served as a miRNA sponge in the cytoplasm leading to activation of MAPK1 and E2F7 and conferring TMZ resistance to GBM cells [95]. In another study, SNHG12 functions in GBM by inhibiting the tumor suppressor, miR-627-5p, resulting in the activation of two oncogenic targets, CDK6 and SOX-2. Treatment with anti-CDK6 inhibitor palbociclib in TMZ-resistant GBM PDX mouse model led to anti-tumorigenic phenotypes including reduced SNHG12 transcript levels [96]. Another lncRNA lnc-TALC (temozolomide-associated lncRNA in glioblastoma recurrence) found to be upregulated in TMZ-resistant GBM cells, would competitively bind miR-20b-3p to increase c-Met expression; suggesting the contribution of lnc-TALC contributes to TMZ resistance and GBM recurrence in clinical patients [97].
3.2.2. LncRNAs Regulate Gliomagenesis through Protein Anchoring and Modulation
LncRNAs bind directly to proteins to regulate their signaling or binding with interacting partners, thus regulating their function. A lncRNA SWI/SNF complex antagonist associated with prostate cancer 1 (SChLAP1), which is known to promote prostate cancer has now been shown to have role in the development of GBM. High level of SChLAP1 in primary GBM tumors binds to the heterogeneous nuclear ribonucleoprotein L (HNRNPL), which led to enhanced binding between HNRNPL and α-actinin-4 (ACTN4), and suppression of ACTN4 degradation. This would, in turn, lead to enhanced nuclear factor kB (NF-κB)-signaling activity, which is associated with cancer development [98]. NEAT1 lncRNA is induced by the EGFR pathway in glioma which then sequesters the chromosome modification enzyme EZH2 to facilitate repression of certain target genes and to promote nuclear translocation of β-catenin, thus activating the WNT/β-catenin oncogenic pathway in GBM [99]. Binding of another lncRNA HOTAIR to EZH2 of the polycomb repressive complex (PRC) 2 leads to transcriptional silencing of tumor suppressor genes in glioma. Treatment with AQB (AC1Q3QWB), which is a HOTAIR-EZH2 inhibitor, blocks PRC2 recruitment in PDX models of GBM, thus combating the tumor phenotype [100].
3.2.3. Transcriptional Regulation by LncRNAs in Glioma
Dysregulated lncRNAs in glioma have been found to alter the promoter activity of oncogenic genes by facilitating their interactions with transcription factors. LncRNA PAX-interacting protein 1- antisense RNA1 (lncRNA PAXIP1-AS1) is overexpressed in glioma and is involved in glioma cell invasion and angiogenesis. PAXIP1-AS1 could enhance the promoter activity of kinesin family member 14 (KIF14) involved in cell cycle and mitotic progression by recruiting the transcription factor ETS1 [101,102]. Another lncRNA called PRC2 and dead-box helicase 5 (DDX5) associated lncRNA (PRADX) could enhance tumorigenesis in GBM by suppressing the expression of the UBX domain protein 1 (UBXN1) protein which is a negative regulator of NF-κB signaling, thus enhancing NF-κB signaling leading to GBM cell proliferation. PRADX does so by increasing trimethylation of H3K27 in the UBXN1 gene promoter by recruiting the PRC2/DDX5 complex, thus transcriptionally regulating GBM tumorigenesis [103].
Epi-transcriptomic changes lead to lncRNA regulation in GBM. The m6A demethylase AlkB homolog H5 (ALKBH5), which is induced under hypoxic conditions in GBM erases the m6A deposition from the lncRNA NEAT1, thus stabilizing NEAT1 and enhancing the formation of NEAT1-associated paraspeckle assembly. These paraspeckles induce the relocation of the transcriptional repressor splicing factor proline and glutamine rich (SFPQ) to the paraspeckles from the chemokine C-X-C motif chemokine ligand 8 (CXCL8) promoter leading to upregulation of CXCL8/interleukin-8 (IL-8) expression. Expression and secretion of CXCL8/IL-8 results in an immunosuppressive tumor microenvironment (TME) by recruitment of tumor-associated macrophages (TAM) leading to tumor progression [104].
3.2.4. Role of LncRNAs in Epigenetic Regulation in Glioma
LncRNAs regulate gene expression through epigenetic regulation in glioma by recruiting chromatin modifiers to a particular genomic location, as a scaffold, thus keeping away chromatin modifiers from their regulatory targets as decoys, and also act as modulators of the 3D organization of genomes [80]. The lncRNA HOTAIRM1 regulates the expression of the HOXA1 gene by sequestering the epigenetic modifiers and demethylases G9a and EZH2 away from the transcription start site of HOXA1 gene, thus mediating demethylation of histone H3K9 and H3K27, and thus, reducing the DNA methylation of the HOZA1 gene, resulting in GBM cell proliferation, migration, invasion, and inhibiting apoptosis [105]. Furthermore, the lncRNA SNHG6 (small nucleolar RNA host gene 6) stabilized by its binding partner, NCBP3 (Nuclear cap-binding subunit 3), inhibits GBX2 (gastrulation brain homeobox 2) by mediating H3K27me3 modification induced by PRC2 (polycomb repressive complex 2) to facilitate the malignant progression in glioma [106]. In another study, lncRNA ZFAT-AS1, promotes the transcription of CDX2 (caudal type homeobox 2) by mediating histone H3 methylation on lysine 27 in a PRC2 dependent manner to facilitate the malignant biological behavior of glioma cells [107].
3.3. Exosomal LncRNAs in Glioma
Exosomes are a means of intercellular communication in the tumor microenvironment that transport lipids, nucleic acids, proteins, and bioactive compounds between cells, and participate in tumor initiation and progression by creating a tumor-permissive microenvironment. Exosomes can cross the BBB and are found to be present in almost all body fluids, thus rendering them as attractive biocompatible molecules for delivering therapeutic targets and serving as biomarkers in glioma [72]. Different types of ncRNAs including lncRNA, circRNA, miRNA, piRNA, and tsRNA are packaged into the double-layered exosomes, and are thus protected from the extracellular proteases and nucleases, and are considered attractive therapeutic targets in cancers including glioma.
Chronic inflammation in the brain due to the presence of glioma cells lead to infiltration of reactive astrocytes, which pose an invasive phenotype at the interface with glioma cells [108]. One of the underlying mechanisms by which glioma cells lead to activation of these astrocytes is by the secretion of exosomes. Glioma-derived exosomes shuttle a lncRNA called lncRNA activated by TGF-β (lncRNA-ATB) to astrocytes that activate astrocytes through the suppression of miR-204-3p in an Ago-dependent manner, and activated astrocytes could, in turn, promote the migration and invasion of glioma cells [108]. Another lncRNA antisense transcript of hypoxia-inducible factor-1α (AHIF) is found to be upregulated in cancerous tissues and exosomes derived from AHIF-knockdown GBM cells inhibited cell growth and invasiveness in GBM cells through regulation of factors associated with angiogenesis and migration in exosomes [109]. Further, Linc01060-containing exosomes derived from hypoxic GSCs (H-GSCs) drive disease progression in glioma. Linc01060 transferred to glioma cells through exosomes binds to and stabilizes the transcription factor myeloid zinc finger 1 (MZF1), which then translocate to the nucleus and promotes c-Myc transcriptional activities including accumulation of HIF1α, which binds to Linc01060 promoter and upregulates the lncRNA, leading to glioma progression [110]. Another study reported that high amounts of lncRNA SBF2 antisense RNA 1 (lncRNA SBF2-AS1) were present in exosomes derived from TMZ-resistant GBM cells and it could lead to TMZ resistance in chemo-responsive GBM cells. SBF2-AS1 acts as a ceRNA for miR-151a-3p leading to activation of the miRNA’s target, X-ray repair cross complementing 4 (XRCC4) which repairs double stranded breaks (DSB) caused by TMZ-induced DNA damage, thus resulting in TMZ resistance [111]. In a study by Ma et al., it was shown that the HOTAIR lncRNA-containing exosomes are secreted by glioma cells to endothelial cells where the lncRNA increases angiogenesis by regulating vascular endothelial growth factor (VEGF)-A levels [112]. Similarly, linc-CCAT2 could be transferred to endothelial cells to increase angiogenesis by activating VEGF-A and TGFβ [113].
3.4. LncRNAs as Potential Biomarkers in Glioma
Glioblastoma microvesicles or exosomes that are secreted by GBM tumor cells have been shown to be clinically relevant biomarkers in the serum of GBM patients [114], providing diagnostic information through a blood test (liquid biopsy) in cancer patients. The tissue-specific expression of lncRNAs in different cancer types and their correlation with tumor-grade makes them attractive prognostic and biomarker targets. For example, the association of HOXA11-AS with the mesenchymal subtype of GBM helps in profiling of glioma subtypes [115]. Additionally, data analysis shows that the lncRNA HOXA-AS3 is associated with tumor grade and shows a poor prognosis in glioma [116], suggesting the potential of lncRNAs as biomarkers in cancer diagnostics.
3.5. Therapeutic Targeting of LncRNAs in Glioma
Antisense oligonucleotide (ASO) therapy to correct aberrant lncRNA functions in cancers is upcoming and promising [117]. Short (21–28 nt) chemically stabilized ASOs targeting the binding sequence of lncRNA to its binding partner have been shown to alter dysregulated lncRNA functions preclinically. A 2′-deoxy-2′-fluoro-D-arabinonucleic acid (2′-FANA) modification protects oligonucleotides from an RNase H-mediated degradation [118]. In a study in melanoma, it was shown that 2′-FANA modified oligonucleotides either antisense to lncRNA SLNCR1 androgen receptor (AR)-binding sequence or mimicking the AR binding site of SLNCR1, inhibits the interaction between the two, suppressing melanoma invasion [119]. In glioma, ASOs targeting the lncRNA taurine up-regulated gene 1 (TUG1) coupled with drug delivery led to GSC differentiation and repressed cell growth in vivo [120]. In a preclinical study, matastasis associated lung adenocarcinoma transcript 1 (Malat1)-specific ASO along with nucleus-targeting peptide gold nanoparticles, reduced Malat1 expression and suppressed metastatic tumor nodule formation in vivo [121]. Moreover, lncRNA Malat1 has been targeted using ASOs in several cancer types preclinically to reduce tumor growth in vitro and in vivo [121,122].
4. Circular RNAs
circRNAs are single-stranded ncRNA molecules with a covalently closed loop structure and lack the 5′-cap and 3′-polyA tail structures [164]. They are discovered to be present in large numbers in the brain and in other tissue types including the lung, liver, heart, stomach, kidney, and colon, with an enrichment in neuronal tissues. CircRNAs were initially considered as ‘splicing noise,’ but with the advent of high throughput RNA sequencing and circRNA detection algorithms, large numbers of circRNAs have been detected in normal as well as cancer tissues. Due to their presence in the serum and other body fluids, circRNAs hold high prognostic and therapeutic value in cancer and their high levels of expression in brain tissues make them an attractive target in glioma [165]. Please refer to Table 1 for a comprehensive list of circRNAs associated with glioma.
4.1. Biogenesis and Classification of CircRNAs
CircRNAs are primarily synthesized by back splicing of pre-mRNAs wherein a downstream 5′-donor splice site is connected to an upstream 3′-acceptor splice site [164,166,167]. The vast majority of circRNAs (>80%) are synthesized in the cytoplasm mostly from the exon of protein-coding genes, while single gene loci can also generate circularization patterns [167,168,169].
CircRNAs are classified based on their biogenesis from different genomic locations [164]. Exonic circRNA (ecircRNAs) are derived from one or more exons through alternative splicing and are localized in the cytoplasm [164]. ecircRNAs are formed either by direct base pairing of flanking introns mediated by reverse complementary Alu sequences, or by lariat formation of flanking introns that facilitates back splicing and circularization of exons, or through binding of RNA binding proteins (RBPs)/splicing factors, to specific sequence motifs in flanking introns. Intronic circRNAs are generated by presence of 7nt GU-rich elements at the 5′-splice site and an 11nt C-rich sequence at the branch point site that is sensitive to debranching enzymes and are localized in the nucleus [164,170]. Intergenic lncRNAs are generated from the region in-between genes. Additionally, circRNAs are generated from certain lncRNAs that contain short open reading frames (ORFs) such as the lncRNA LINC-PINT [171].
4.2. Expression of CircRNAs in Glioma
The enrichment of circRNAs in neuronal tissues suggests their association with various brain disorders, including cancer. Furthermore, mounting evidence of circRNA expression in glioma emphasizes their function in glioma development and tumorigenesis. In an RNA sequencing differential expression analysis using Illumina Hi-Seq, between five GBM tumors and five normal brain tissues, a total of 1411 differentially expressed circRNAs were analyzed, with 206 upregulated and 1205 downregulated circRNAs [172]. In another study to find differentially expressed circRNAs in glioma tissue, the authors used five different methods to screen for circRNAs between three glioma tissues and paired normal tissues—CIRCexplorer2, circRNA-finder, CIRI, find-circ and MapSplice2 and discovered twelve differentially expressed circRNAs in glioma tissues [173]. In a recent study, N Vo et al., have used the technique of exome capture RNA sequencing to detect and characterize circRNAs across >2000 cancer samples including glioma. Using capture, the authors have built a comprehensive catalog of circRNAs directly detected in cancer tissues—called MiOncoCirc. They have also identified a novel class of circRNAs called read-through circRNAs, which contained exons originating from different genes [174].
4.3. Functions of CircRNAs in Glioma
4.3.1. CircRNAs Act as a miRNA Sponge
circATP5B acts a miRNA sponge and promotes proliferation of GSCs. circATP5B sponges miR-185-5p to upregulate homeobox 5 (HOXB5) protein, which regulates transcriptional increase of IL-6 and promotes GSC proliferation through the JAK2/STAT3 signaling pathway [133]. Further, in recurrent GBM tissues, (circ)RNA ADP-ribosylation factor GTPase activating proteins with Src homology 3 domain, ankyrin repeat and Pleckstrin homology domain 1 (circASAP1) was found to be upregulated and conferred TMZ resistance in GBM cells. circASAP1 is a miRNA sponge for miR-502-5p and enhanced the expression of oncogene NRAS, thus causing therapy resistance in GBM [151]. circMMP9 targeted miR-124 and increased GBM migration and invasion through activation of the cyclin-dependent kinase 4 (CDK4) and aurora kinase A (AURKA) signaling axis [175].
4.3.2. Role of CircRNAs and Its Encoded Peptides in Glioma Development
Through circRNA sequencing analysis in glioma and normal tissues, circNEIL3 was identified to be involved in glioma development in vitro and in vivo. circNEIL3, which was cyclized by the EWS RNA-binding protein 1 (EWSR1) RNA binding protein, stabilizes the oncogenic protein insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) by preventing its degradation mediated by HECTD4 ubiquitin ligase [150].
Another study has identified a circular EGFR RNA called circ-EGFR, which is aberrantly activated in more than 50% of adult GBM cases. Circ-EGFR encodes a novel EGFR variant termed rolling-translated EGFR (rtEGFR) which interacts with EGFR, maintains its membrane localization, and thus leads to enhanced downstream signaling and cancer progression. circ-EGFR correlated with poor prognosis in GBM patients and repression of rtEGFR in GSCs inhibited GBM tumorigenesis [176]. The EGFR pathway activity in glioma is regulated by another circRNA circular E-cadherin (circ-E-Cad) RNA, which encodes a secretory E-cadherin protein variant (C-E-Cad) synthesized through multiple-round open reading frame translation. Inhibition of C-E-Cad enhances the anti-tumor effect of anti-EGFR therapies [152]. Similarly, a variant of the Hedgehog signaling component, G protein-coupled-like receptor smoothened (SMO), called SMO-193a.a was encoded by the circRNA circ-SMO. Expression of SMO-193a.a that is regulated by a Shh/Gli1/FUS/SMO-193a.a. signaling axis, correlates with poor prognosis in GBM patients [153].
CircRNAs with a tumor suppressive role have also been identified in GBM. Circ-FBXW7 was identified through circRNA deep sequencing using ten GBM patient tissues and paired normal tissues. Circ-FBXW7-encoded protein FBXW7-185aa had low levels in GBM samples and its overexpression could inhibit proliferation and altered cell cycle in GBM cells [163].
4.3.3. Exosomal CircRNAs Serve as Potential Biomarkers
CircRNAs are found to be enriched and stable in exosomes (exo-circRNA) [177]. Due to the enrichment of exosomes in CSF and serum, exo-circRNA can be used as potential biomarkers for GBM prognosis. In one study, differential circRNA expression was analyzed from EVs isolated from glioma U251 cells (Nor-EVs) and EVs isolated from radiation-resistant RR-U251 cells (RR-EVs). Through RNA-seq, a total of 1235 circRNAs were detected, 63 upregulated and 48 downregulated in RR-U251 compared to Nor-EVs. A highly expressed candidate circATP8B4 in RR-U251 could be transferred to normal U251 glioma cells and cause radioresistance by acting as miRNA sponge [178]. Overexpression of circNEIL3 in glioma cells lead to TAM infiltration into the TME. CircNEIL3 is packaged into the exosomes by hnRNPA2B1 and is delivered to TAMs from glioma cells, afflicting an immunosuppressive phenotype by stabilizing the IGF2BP3 protein, thereby promoting glioma progression [150]. Thus, exo-circRNAs modulate the glioma TME and serve as attractive biomarkers for GBM prognosis and diagnostic purposes.
5. PIWI-Interacting RNAs (piRNAs)
piRNAs are animal-specific small (26–32 nt) ncRNAs which are derived from long intergenic transcripts, ncRNAs, and 3′ UTRs of protein coding RNAs [179,180]. piRNAs have conserved function in the protection of the germline tissues from destabilizing transposable elements [181]. piRNAs function in sequence-specific gene regulation and are present in the human genome in a large number (>30,000 piRNAs) with more piRNAs being identified with the advance in sequencing technologies [182]. These ncRNAs form ribonucleoprotein complexes with PIWI proteins to recruit chromatin modifiers to targets of transposable elements creating heritable epigenetic modifications, and piRNAs have also been found to function in miRNA silencing [182]. piRNAs regulate diverse functional processes including epigenetic reprogramming, transcription, translation, development, and regulation of mRNA stability [183,184].
5.1. piRNA Biogenesis
piRNA biogenies involves two major mechanisms, namely primary and secondary biogenesis [185]. pre-piRNAs are formed by 3′-5′ movement of RNA polymerase on the DNA heterochromatic strand. The pre-piRNA is exported out of the nucleus where it bounds to Yb bodies located around the mitochondria and first discovered in Drosophila. Yb bodies allow the interaction of PIWI proteins and piRNAs to form the piRISC by recognition of the 5′ end of pre-piRNAs [186,187].
5.2. piRNA Functional Mechanisms in Glioma
piRNAs were believed to be restricted to the germline for a long time. However, recent evidence suggests expression of piRNAs in somatic tissues and their dysregulated expression has been observed in 8 different cancer types [188]. Gene expression analysis of tissues other than germline from TCGA has revealed that piRNAs are expressed in both normal and malignant tissues of all 11 distinct anatomical cancer tissues and their expression in cancer tissues is found to be clinically relevant and tumor specific [189].
piRNAs have been studied to have functional role through epigenetic mechanisms in different cancers including glioma [190]. One study has found through array-based piRNA expression profiling, the expression of ~350 piRNAs in both normal and cancer tissues and a subset of piRNAs were dysregulated in cancer. Overexpression or pretreatment of a piRNA piR-8041 restores tumor suppressive properties and reduced GBM tumorigenesis in GBM cell line and mouse xenograft tumors [182]. Moreover, the piRNA interacting protein Piwil1 is enriched in GSCs and maintains GSC self-renewal and Piwil1 knockdown in GBM animal model suppresses tumor growth and promotes mouse survival [191]. Further, a study has found that the PIWIL1/piRNA-DQ593109 (piR-DQ593109) complex is a major regulator of blood-tumor barrier (BTB) permeability in glioma through the MEG3/miR-330-5p/RUNX3 axis, and their inhibitions could enhance efficient delivery of anti-glioma drugs to glioma tissues [192].
6. Concluding Remarks
In recent years, with the availability of only a small fraction of information on transcription of the non-coding genome, remarkable success has been achieved in the field of ncRNA biology, which has helped us understand the complex regulatory network and functions of these molecules. miRNAs function in virtually every cellular process and are functional in the development, differentiation, and homeostasis of normal physiology and of disease [193]. LncRNAs function is, in cis, to regulate the function of nearby genes by modulating their transcription or chromatin dynamics, and in trans, through structural or regulatory roles to affect mRNA stability, splicing, translation, and even signaling [76]. The upcoming circRNAs are now known to be involved in neurogenesis and dysregulated in several human diseases, including neurological disorders and cancer, thus affecting the severity of disease [194].
The potential for of ncRNAs as biomarkers and therapeutics in glioma and cancer at large is enormous and holds great promise. The fact that ncRNAs use nucleotide hybridization to bind to their targets makes them easy-to-use tools as compared to small molecule inhibitors where structure-based designing is required. Instead, for ncRNAs, finding the correct binding sequence and testing the candidate therapeutics can help prepare ncRNA-based inhibitors. Also, the detection of cancer-associated ncRNAs in blood and urine could serve as excellent biomarkers and could spare cancer patients from more invasive tissue collection procedures. Although, as pointed out above, constant exposure to chemically modified RNA molecules can elicit immune reactions, which has been a challenge for RNA medicine, and more research is being pursued to combat this issue. Research to improve the oligonucleotide delivery methods and refine their chemistry is ongoing [2,3,195,196].
miRNA therapeutics have shown promising results in preclinical studies in glioma. Thus, the identification of the right targets and a better understanding of their mechanisms of action can help to build novel treatments to overcome the therapy resistance and tumor recurrence in glioma [18]. The nanotherapeutic strategy has been an attractive delivery strategy in vitro and vivo, however, it is less successful in human applications due to issues of toxicity, stability, efficacy, and targeting, and thus, demands more research. Alternatively, patient-derived exosomes are more compatible and safer clinically. Exosomal ncRNAs are selectively packaged, secreted, and transferred between cells, and can thus modulate the TME, which can be leveraged therapeutically. Also, their ability to cross the BBB and their enrichment in body fluids make them excellent diagnostic and therapeutic agents.
The understanding of the mechanisms of lncRNAs and circRNAs is still relatively low compared to that of miRNAs, and thus, is less developed clinically; however, promising results have been shown in preclinical studies. In glioma, lncRNAs have been deeply studied for their roles as ceRNA, and hence, a better understanding of their lncRNA functions is required. Additionally, since lncRNAs function by binding to other molecules, an understanding of the structure of lncRNA binding motifs would be important. Finally, circRNA research is still in its nascent stage. With the implementation of more sequencing and other research strategies, the roles of circRNAs in glioma tumorigenesis and therapy responses will be revealed. Lastly, circRNAs and piRNAs could prove to be excellent therapeutic and biomarker targets, especially in glioma research, due to their enrichment and stability in the brain.
Acknowledgments
This work was supported by United States National Institutes of Health (NIH) grants NS115403, NS122375, NS126810, NS125318, and Lou and Jean Malnati Brain Tumor Institute at Northwestern Medicine (S.Y.C.); NIH CA234799, United States Army Medical Research Acquisition Activity W81XWH-22-10373 (D.M.T.) and W81XWH-22-1-0374 (X.S.). Fundação de Amparo à Pesquisa do Estado de São Paulo 2020/03714-8 (R.P.I.).
Author Contributions
Conceptualization: A.G. and S.-Y.C., writing—original draft: A.G. and S.-Y.C., review and editing: A.G., S.-Y.C., D.M.T., X.S., R.P.I., M.L. and B.H. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The text is the sole product of the author(s) and that no third party had input or gave support to its writing. The authors have no financial or competing interests to declare.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slack F.J., Chinnaiyan A.M. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams B.D., Parsons C., Walker L., Zhang W.C., Slack F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 4.Mattick J.S., Makunin I.V. Non-coding RNA. Hum. Mol. Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 5.Slack F.J. Regulatory RNAs and the demise of ‘junk’ DNA. Genome Biol. 2006;7:328. doi: 10.1186/gb-2006-7-9-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein M.B., Kundaje A., Hariharan M., Landt S.G., Yan K.K., Cheng C., Mu X.J., Khurana E., Rozowsky J., Alexander R., et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489:91–100. doi: 10.1038/nature11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveson I.W., Hardwick S.A., Mercer T.R., Mattick J.S. The Dimensions, Dynamics, and Relevance of the Mammalian Noncoding Transcriptome. Trends Genet. 2017;33:464–478. doi: 10.1016/j.tig.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Anastasiadou E., Faggioni A., Trivedi P., Slack F.J. The Nefarious Nexus of Noncoding RNAs in Cancer. Int. J. Mol. Sci. 2018;19:2072. doi: 10.3390/ijms19072072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X., Feng Y., Zhang D., Zhao S.D., Hu Z., Greshock J., Zhang Y., Yang L., Zhong X., Wang L.P., et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26:344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng Y.Y., Moriarity B.S., Gong W., Akiyama R., Tiwari A., Kawakami H., Ronning P., Reuland B., Guenther K., Beadnell T.C., et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rheinbay E., Parasuraman P., Grimsby J., Tiao G., Engreitz J.M., Kim J., Lawrence M.S., Taylor-Weiner A., Rodriguez-Cuevas S., Rosenberg M., et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547:55–60. doi: 10.1038/nature22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldape K., Brindle K.M., Chesler L., Chopra R., Gajjar A., Gilbert M.R., Gottardo N., Gutmann D.H., Hargrave D., Holland E.C., et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019;16:509–520. doi: 10.1038/s41571-019-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrom Q.T., Patil N., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22:iv1–iv96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 18.Goenka A., Tiek D., Song X., Huang T., Hu B., Cheng S.Y. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells. 2021;10:484. doi: 10.3390/cells10030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rynkeviciene R., Simiene J., Strainiene E., Stankevicius V., Usinskiene J., Miseikyte Kaubriene E., Meskinyte I., Cicenas J., Suziedelis K. Non-Coding RNAs in Glioma. Cancers. 2018;11:17. doi: 10.3390/cancers11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Nishi K., Nishi A., Nagasawa T., Ui-Tei K. Human TNRC6A is an Argonaute-navigator protein for microRNA-mediated gene silencing in the nucleus. RNA. 2013;19:17–35. doi: 10.1261/rna.034769.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarova J.A., Shkurnikov M.U., Wicklein D., Lange T., Samatov T.R., Turchinovich A.A., Tonevitsky A.G. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 2016;51:33–49. doi: 10.1016/j.proghi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Rooj A.K., Ricklefs F., Mineo M., Nakano I., Chiocca E.A., Bronisz A., Godlewski J. MicroRNA-Mediated Dynamic Bidirectional Shift between the Subclasses of Glioblastoma Stem-like Cells. Cell Rep. 2017;19:2026–2032. doi: 10.1016/j.celrep.2017.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godlewski J., Nowicki M.O., Bronisz A., Williams S., Otsuki A., Nuovo G., Raychaudhury A., Newton H.B., Chiocca E.A., Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- 27.Alvarado A.G., Turaga S.M., Sathyan P., Mulkearns-Hubert E.E., Otvos B., Silver D.J., Hale J.S., Flavahan W.A., Zinn P.O., Sinyuk M., et al. Coordination of self-renewal in glioblastoma by integration of adhesion and microRNA signaling. Neuro Oncol. 2016;18:656–666. doi: 10.1093/neuonc/nov196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H., Liu Z., Liu T., Cai Y., Wang Y., Lin S., Chen J., Wang J., Wang Z., Jiang B. Fas signaling promotes chemoresistance in gastrointestinal cancer by up-regulating P-glycoprotein. Oncotarget. 2014;5:10763–10777. doi: 10.18632/oncotarget.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Q., Liu L.Z., Qian X., Chen Q., Jiang Y., Li D., Lai L., Jiang B.H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang C., Guo Y., Hong Y., Liu Y.H., Xue Y.X. MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Mol. Biol. Rep. 2015;42:721–727. doi: 10.1007/s11033-014-3820-3. [DOI] [PubMed] [Google Scholar]
- 31.Ge Y., Zhang L., Nikolova M., Reva B., Fuchs E. Strand-specific in vivo screen of cancer-associated miRNAs unveils a role for miR-21* in SCC progression. Nat. Cell Biol. 2016;18:111–121. doi: 10.1038/ncb3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Fatimy R., Subramanian S., Uhlmann E.J., Krichevsky A.M. Genome Editing Reveals Glioblastoma Addiction to MicroRNA-10b. Mol. Ther. 2017;25:368–378. doi: 10.1016/j.ymthe.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fareh M., Almairac F., Turchi L., Burel-Vandenbos F., Paquis P., Fontaine D., Lacas-Gervais S., Junier M.P., Chneiweiss H., Virolle T. Cell-based therapy using miR-302-367 expressing cells represses glioblastoma growth. Cell Death Dis. 2017;8:e2713. doi: 10.1038/cddis.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajbhandari R., McFarland B.C., Patel A., Gerigk M., Gray G.K., Fehling S.C., Bredel M., Berbari N.F., Kim H., Marks M.P., et al. Loss of tumor suppressive microRNA-31 enhances TRADD/NF-kappaB signaling in glioblastoma. Oncotarget. 2015;6:17805–17816. doi: 10.18632/oncotarget.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shea A., Harish V., Afzal Z., Chijioke J., Kedir H., Dusmatova S., Roy A., Ramalinga M., Harris B., Blancato J., et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016;5:1917–1946. doi: 10.1002/cam4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pottoo F.H., Javed M.N., Rahman J.U., Abu-Izneid T., Khan F.A. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin. Cancer Biol. 2021;69:391–398. doi: 10.1016/j.semcancer.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Hassan A., Mosley J., Singh S., Zinn P.O. A Comprehensive Review of Genomics and Noncoding RNA in Gliomas. Top. Magn. Reson. Imaging. 2017;26:3–14. doi: 10.1097/RMR.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Huang W., Kim T.M., Jung Y., Menon L.G., Xing H., Li H., Carroll R.S., Park P.J., Yang H.W., et al. MicroRNA-29a activates a multi-component growth and invasion program in glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:36. doi: 10.1186/s13046-019-1026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Guessous F., Zhang Y., Dipierro C., Kefas B., Johnson E., Marcinkiewicz L., Jiang J., Yang Y., Schmittgen T.D., et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin D., Ogawa S., Kawamata N., Leiter A., Ham M., Li D., Doan N.B., Said J.W., Black K.L., Phillip Koeffler H. miR-34a functions as a tumor suppressor modulating EGFR in glioblastoma multiforme. Oncogene. 2013;32:1155–1163. doi: 10.1038/onc.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kefas B., Godlewski J., Comeau L., Li Y., Abounader R., Hawkinson M., Lee J., Fine H., Chiocca E.A., Lawler S., et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 42.Huang T., Alvarez A.A., Pangeni R.P., Horbinski C.M., Lu S., Kim S.H., James C.D., Raizer J.J., Kessler J.A., Brenann C.W., et al. A regulatory circuit of miR-125b/miR-20b and Wnt signalling controls glioblastoma phenotypes through FZD6-modulated pathways. Nat. Commun. 2016;7:12885. doi: 10.1038/ncomms12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terstappen G.C., Meyer A.H., Bell R.D., Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021;20:362–383. doi: 10.1038/s41573-021-00139-y. [DOI] [PubMed] [Google Scholar]
- 44.Petrescu G.E.D., Sabo A.A., Torsin L.I., Calin G.A., Dragomir M.P. MicroRNA based theranostics for brain cancer: Basic principles. J. Exp. Clin. Cancer Res. 2019;38:231. doi: 10.1186/s13046-019-1180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P., Singh A., Shah S., Vataliya J., Mittal A., Chitkara D. RNA Interference Nanotherapeutics for Treatment of Glioblastoma Multiforme. Mol. Pharm. 2020;17:4040–4066. doi: 10.1021/acs.molpharmaceut.0c00709. [DOI] [PubMed] [Google Scholar]
- 46.Dalkara T., Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015;1623:3–17. doi: 10.1016/j.brainres.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 47.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., Lotvall J., Nakagama H., Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi T., Jin F., Zhu Y., Wang J., Tang L., Wang Y., Liebeskind D.S., Scalzo F., He Z. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J. Biol. Chem. 2018;293:20041–20050. doi: 10.1074/jbc.RA118.001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anthiya S., Griveau A., Loussouarn C., Baril P., Garnett M., Issartel J.P., Garcion E. MicroRNA-Based Drugs for Brain Tumors. Trends Cancer. 2018;4:222–238. doi: 10.1016/j.trecan.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Rupaimoole R., Wu S.Y., Pradeep S., Ivan C., Pecot C.V., Gharpure K.M., Nagaraja A.S., Armaiz-Pena G.N., McGuire M., Zand B., et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat. Commun. 2014;5:5202. doi: 10.1038/ncomms6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naoghare P.K., Tak Y.K., Kim M.J., Han E., Song J.M. Knock-down of argonaute 2 (AGO2) induces apoptosis in myeloid leukaemia cells and inhibits siRNA-mediated silencing of transfected oncogenes in HEK-293 cells. Basic Clin. Pharmacol. Toxicol. 2011;109:274–282. doi: 10.1111/j.1742-7843.2011.00716.x. [DOI] [PubMed] [Google Scholar]
- 52.Jiang W., Finniss S., Cazacu S., Xiang C., Brodie Z., Mikkelsen T., Poisson L., Shackelford D.B., Brodie C. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget. 2016;7:56456–56470. doi: 10.18632/oncotarget.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J., Rodova M., Nanta R., Meeker D., Van Veldhuizen P.J., Srivastava R.K., Shankar S. NPV-LDE-225 (Erismodegib) inhibits epithelial mesenchymal transition and self-renewal of glioblastoma initiating cells by regulating miR-21, miR-128, and miR-200. Neuro Oncol. 2013;15:691–706. doi: 10.1093/neuonc/not011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klinger N.V., Mittal S. Therapeutic Potential of Curcumin for the Treatment of Brain Tumors. Oxid Med. Cell. Longev. 2016;2016:9324085. doi: 10.1155/2016/9324085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu D., Pendergraff H., Liu J., Kordasiewicz H.B., Cleveland D.W., Swayze E.E., Lima W.F., Crooke S.T., Prakash T.P., Corey D.R. Single-stranded RNAs use RNAi to potently and allele-selectively inhibit mutant huntingtin expression. Cell. 2012;150:895–908. doi: 10.1016/j.cell.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira H.J., Esteller M. Non-coding RNAs, epigenetics, and cancer: Tying it all together. Cancer Metastasis Rev. 2018;37:55–73. doi: 10.1007/s10555-017-9715-8. [DOI] [PubMed] [Google Scholar]
- 57.Kleaveland B., Shi C.Y., Stefano J., Bartel D.P. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018;174:350–362.e17. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z. The principles of MiRNA-masking antisense oligonucleotides technology. Methods Mol. Biol. 2011;676:43–49. doi: 10.1007/978-1-60761-863-8_3. [DOI] [PubMed] [Google Scholar]
- 59.Meng L., Liu C., Lu J., Zhao Q., Deng S., Wang G., Qiao J., Zhang C., Zhen L., Lu Y., et al. Small RNA zippers lock miRNA molecules and block miRNA function in mammalian cells. Nat. Commun. 2017;8:13964. doi: 10.1038/ncomms13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh B., Song H., Lee D., Oh J., Kim G., Ihm S.H., Lee M. Anti-cancer effect of R3V6 peptide-mediated delivery of an anti-microRNA-21 antisense-oligodeoxynucleotide in a glioblastoma animal model. J. Drug Target. 2017;25:132–139. doi: 10.1080/1061186X.2016.1207648. [DOI] [PubMed] [Google Scholar]
- 61.Krutzfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M., Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 62.Ge Y.F., Sun J., Jin C.J., Cao B.Q., Jiang Z.F., Shao J.F. AntagomiR-27a targets FOXO3a in glioblastoma and suppresses U87 cell growth in vitro and in vivo. Asian Pac. J. Cancer Prev. 2013;14:963–968. doi: 10.7314/APJCP.2013.14.2.963. [DOI] [PubMed] [Google Scholar]
- 63.Lee T.J., Yoo J.Y., Shu D., Li H., Zhang J., Yu J.G., Jaime-Ramirez A.C., Acunzo M., Romano G., Cui R., et al. RNA Nanoparticle-Based Targeted Therapy for Glioblastoma through Inhibition of Oncogenic miR-21. Mol. Ther. 2017;25:1544–1555. doi: 10.1016/j.ymthe.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., Zhang K., Shi Z., Zhang A., Jia Z., Wang G., Pu P., Kang C., Han L. A lentivirus-mediated miR-23b sponge diminishes the malignant phenotype of glioma cells in vitro and in vivo. Oncol. Rep. 2014;31:1573–1580. doi: 10.3892/or.2014.3012. [DOI] [PubMed] [Google Scholar]
- 65.Kucukturkmen B., Devrim B., Saka O.M., Yilmaz S., Arsoy T., Bozkir A. Co-delivery of pemetrexed and miR-21 antisense oligonucleotide by lipid-polymer hybrid nanoparticles and effects on glioblastoma cells. Drug Dev. Ind. Pharm. 2017;43:12–21. doi: 10.1080/03639045.2016.1200069. [DOI] [PubMed] [Google Scholar]
- 66.Bertucci A., Prasetyanto E.A., Septiadi D., Manicardi A., Brognara E., Gambari R., Corradini R., De Cola L. Combined Delivery of Temozolomide and Anti-miR221 PNA Using Mesoporous Silica Nanoparticles Induces Apoptosis in Resistant Glioma Cells. Small. 2015;11:5687–5695. doi: 10.1002/smll.201500540. [DOI] [PubMed] [Google Scholar]
- 67.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu S., Guan J., Liu Y. Identification of microRNAs as novel biomarkers for glioma detection: A meta-analysis based on 11 articles. J. Neurol. Sci. 2015;348:181–187. doi: 10.1016/j.jns.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q., Li P., Li A., Jiang W., Wang H., Wang J., Xie K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012;31:97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ivo D’Urso P., Fernando D’Urso O., Damiano Gianfreda C., Mezzolla V., Storelli C., Marsigliante S. miR-15b and miR-21 as Circulating Biomarkers for Diagnosis of Glioma. Curr. Genom. 2015;16:304–311. doi: 10.2174/1389202916666150707155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santangelo A., Imbruce P., Gardenghi B., Belli L., Agushi R., Tamanini A., Munari S., Bossi A.M., Scambi I., Benati D., et al. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J. Neurooncol. 2018;136:51–62. doi: 10.1007/s11060-017-2639-x. [DOI] [PubMed] [Google Scholar]
- 72.Cheng J., Meng J., Zhu L., Peng Y. Exosomal noncoding RNAs in Glioma: Biological functions and potential clinical applications. Mol. Cancer. 2020;19:66. doi: 10.1186/s12943-020-01189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akers J.C., Ramakrishnan V., Kim R., Skog J., Nakano I., Pingle S., Kalinina J., Hua W., Kesari S., Mao Y., et al. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): A platform for glioblastoma biomarker development. PLoS ONE. 2013;8:e78115. doi: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang J.K., Yang J.P., Tong J., Jing S.Y., Fan B., Wang F., Sun G.Z., Jiao B.H. Exosomal miR-221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J. Neurooncol. 2017;131:255–265. doi: 10.1007/s11060-016-2308-5. [DOI] [PubMed] [Google Scholar]
- 75.Lan F., Qing Q., Pan Q., Hu M., Yu H., Yue X. Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell. Oncol. 2018;41:25–33. doi: 10.1007/s13402-017-0355-3. [DOI] [PubMed] [Google Scholar]
- 76.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell. Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goenka A., Sengupta S., Pandey R., Parihar R., Mohanta G.C., Mukerji M., Ganesh S. Human satellite-III non-coding RNAs modulate heat-shock-induced transcriptional repression. J. Cell Sci. 2016;129:3541–3552. doi: 10.1242/jcs.189803. [DOI] [PubMed] [Google Scholar]
- 78.Goenka A., Ganesh S. Role of Long Non-coding RNAs in Cellular Stress Response. Proc. Indian Natl. Sci. Acad. 2018;84:513–520. doi: 10.16943/ptinsa/2018/49333. [DOI] [Google Scholar]
- 79.Goenka A., Parihar R., Ganesh S. Heat Shock-Induced Transcriptional and Translational Arrest in Mammalian Cells. In: Asea A.A.A., Kaur P., editors. Heat Shock Proteins and Stress. Springer International Publishing; Cham, Switzerland: 2018. pp. 267–280. [Google Scholar]
- 80.Peng Z., Liu C., Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol. Cancer. 2018;17:61. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balas M.M., Johnson A.M. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018;3:108–117. doi: 10.1016/j.ncrna.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain A.K., Xi Y., McCarthy R., Allton K., Akdemir K.C., Patel L.R., Aronow B., Lin C., Li W., Yang L., et al. LncPRESS1 Is a p53-Regulated LncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol. Cell. 2016;64:967–981. doi: 10.1016/j.molcel.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li W., Notani D., Ma Q., Tanasa B., Nunez E., Chen A.Y., Merkurjev D., Zhang J., Ohgi K., Song X., et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin A., Li C., Xing Z., Hu Q., Liang K., Han L., Wang C., Hawke D.H., Wang S., Zhang Y., et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat. Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X., Yang L., Wang J., Hao Y., Wang C., Lu Z. The Involvement of Long Non-Coding RNAs in Glioma: From Early Detection to Immunotherapy. Front. Immunol. 2022;13:897754. doi: 10.3389/fimmu.2022.897754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stackhouse C.T., Gillespie G.Y., Willey C.D. Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells. 2020;9:2369. doi: 10.3390/cells9112369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun W., Yang Y., Xu C., Guo J. Regulatory mechanisms of long noncoding RNAs on gene expression in cancers. Cancer Genet. 2017;216–217:105–110. doi: 10.1016/j.cancergen.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Cen L., Liu R., Liu W., Li Q., Cui H. Competing Endogenous RNA Networks in Glioma. Front. Genet. 2021;12:675498. doi: 10.3389/fgene.2021.675498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong C., Yu Q., Peng Y., Zhou S., Liu Z., Deng Y., Guo L., Zhao S., Chen G. Novel LncRNA OXCT1-AS1 indicates poor prognosis and contributes to tumorigenesis by regulating miR-195/CDC25A axis in glioblastoma. J. Exp. Clin. Cancer Res. 2021;40:123. doi: 10.1186/s13046-021-01928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen W., Li Q., Zhang G., Wang H., Zhu Z., Chen L. LncRNA HOXA-AS3 promotes the malignancy of glioblastoma through regulating miR-455-5p/USP3 axis. J. Cell. Mol. Med. 2020;24:11755–11767. doi: 10.1111/jcmm.15788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu M., Xue Y., Zheng J., Liu X., Yu H., Liu L., Li Z., Liu Y. Linc00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol. Cancer. 2017;16:110. doi: 10.1186/s12943-017-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Tang J., Yu B., Li Y., Zhang W., Alvarez A.A., Hu B., Cheng S.Y., Feng H. TGF-beta-activated lncRNA LINC00115 is a critical regulator of glioma stem-like cell tumorigenicity. EMBO Rep. 2019;20:e48170. doi: 10.15252/embr.201948170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou K., Zhang C., Yao H., Zhang X., Zhou Y., Che Y., Huang Y. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol. Cancer. 2018;17:105. doi: 10.1186/s12943-018-0849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu C., Wei Y., Wang X., Zhang Z., Yin J., Li W., Chen L., Lyu X., Shi Z., Yan W., et al. DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma. Mol. Cancer. 2020;19:28. doi: 10.1186/s12943-020-1137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z., Zhang J., Zheng H., Li C., Xiong J., Wang W., Bao H., Jin H., Liang P. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2019;38:380. doi: 10.1186/s13046-019-1371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu P., Cai J., Chen Q., Han B., Meng X., Li Y., Li Z., Wang R., Lin L., Duan C., et al. Lnc-TALC promotes O(6)-methylguanine-DNA methyltransferase expression via regulating the c-Met pathway by competitively binding with miR-20b-3p. Nat. Commun. 2019;10:2045. doi: 10.1038/s41467-019-10025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ji J., Xu R., Ding K., Bao G., Zhang X., Huang B., Wang X., Martinez A., Wang X., Li G., et al. Long Noncoding RNA SChLAP1 Forms a Growth-Promoting Complex with HNRNPL in Human Glioblastoma through Stabilization of ACTN4 and Activation of NF-kappaB Signaling. Clin. Cancer Res. 2019;25:6868–6881. doi: 10.1158/1078-0432.CCR-19-0747. [DOI] [PubMed] [Google Scholar]
- 99.Chen Q., Cai J., Wang Q., Wang Y., Liu M., Yang J., Zhou J., Kang C., Li M., Jiang C. Long Noncoding RNA NEAT1, Regulated by the EGFR Pathway, Contributes to Glioblastoma Progression Through the WNT/beta-Catenin Pathway by Scaffolding EZH2. Clin. Cancer Res. 2018;24:684–695. doi: 10.1158/1078-0432.CCR-17-0605. [DOI] [PubMed] [Google Scholar]
- 100.Li Y., Ren Y., Wang Y., Tan Y., Wang Q., Cai J., Zhou J., Yang C., Zhao K., Yi K., et al. A Compound AC1Q3QWB Selectively Disrupts HOTAIR-Mediated Recruitment of PRC2 and Enhances Cancer Therapy of DZNep. Theranostics. 2019;9:4608–4623. doi: 10.7150/thno.35188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q., Wang L., Li D., Deng J., Zhao Z., He S., Zhang Y., Tu Y. Kinesin family member 14 is a candidate prognostic marker for outcome of glioma patients. Cancer Epidemiol. 2013;37:79–84. doi: 10.1016/j.canep.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Xu H., Zhao G., Zhang Y., Jiang H., Wang W., Zhao D., Yu H., Qi L. Long non-coding RNA PAXIP1-AS1 facilitates cell invasion and angiogenesis of glioma by recruiting transcription factor ETS1 to upregulate KIF14 expression. J. Exp. Clin. Cancer Res. 2019;38:486. doi: 10.1186/s13046-019-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Liu X., Cui X., Tan Y., Wang Q., Wang Y., Xu C., Fang C., Kang C. LncRNA PRADX-mediated recruitment of PRC2/DDX5 complex suppresses UBXN1 expression and activates NF-kappaB activity, promoting tumorigenesis. Theranostics. 2021;11:4516–4530. doi: 10.7150/thno.54549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong F., Qin X., Wang B., Li Q., Hu J., Cheng X., Guo D., Cheng F., Fang C., Tan Y., et al. ALKBH5 Facilitates Hypoxia-Induced Paraspeckle Assembly and IL8 Secretion to Generate an Immunosuppressive Tumor Microenvironment. Cancer Res. 2021;81:5876–5888. doi: 10.1158/0008-5472.CAN-21-1456. [DOI] [PubMed] [Google Scholar]
- 105.Li Q., Dong C., Cui J., Wang Y., Hong X. Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2018;37:265. doi: 10.1186/s13046-018-0941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X., Zhang F., Ma J., Ruan X., Liu X., Zheng J., Liu Y., Cao S., Shen S., Shao L., et al. NCBP3/SNHG6 inhibits GBX2 transcription in a histone modification manner to facilitate the malignant biological behaviour of glioma cells. RNA Biol. 2021;18:47–63. doi: 10.1080/15476286.2020.1790140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang F., Ruan X., Ma J., Liu X., Zheng J., Liu Y., Liu L., Shen S., Shao L., Wang D., et al. DGCR8/ZFAT-AS1 Promotes CDX2 Transcription in a PRC2 Complex-Dependent Manner to Facilitate the Malignant Biological Behavior of Glioma Cells. Mol. Ther. 2020;28:613–630. doi: 10.1016/j.ymthe.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bian E.B., Chen E.F., Xu Y.D., Yang Z.H., Tang F., Ma C.C., Wang H.L., Zhao B. Exosomal lncRNAATB activates astrocytes that promote glioma cell invasion. Int. J. Oncol. 2019;54:713–721. doi: 10.3892/ijo.2018.4644. [DOI] [PubMed] [Google Scholar]
- 109.Dai X., Liao K., Zhuang Z., Chen B., Zhou Z., Zhou S., Lin G., Zhang F., Lin Y., Miao Y., et al. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int. J. Oncol. 2019;54:261–270. doi: 10.3892/ijo.2018.4621. [DOI] [PubMed] [Google Scholar]
- 110.Li J., Liao T., Liu H., Yuan H., Ouyang T., Wang J., Chai S., Li J., Chen J., Li X., et al. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1alpha Axis. Cancer Res. 2021;81:114–128. doi: 10.1158/0008-5472.CAN-20-2270. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Z., Yin J., Lu C., Wei Y., Zeng A., You Y. Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:166. doi: 10.1186/s13046-019-1139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma X., Li Z., Li T., Zhu L., Li Z., Tian N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am. J. Transl. Res. 2017;9:5012–5021. [PMC free article] [PubMed] [Google Scholar]
- 113.Lang H.L., Hu G.W., Zhang B., Kuang W., Chen Y., Wu L., Xu G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017;38:785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Q., Zhang J., Liu Y., Zhang W., Zhou J., Duan R., Pu P., Kang C., Han L. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. doi: 10.1016/j.canlet.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 116.Wu F., Zhang C., Cai J., Yang F., Liang T., Yan X., Wang H., Wang W., Chen J., Jiang T. Upregulation of long noncoding RNA HOXA-AS3 promotes tumor progression and predicts poor prognosis in glioma. Oncotarget. 2017;8:53110–53123. doi: 10.18632/oncotarget.18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y., Li Z., Chen X., Zhang S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B. 2021;11:340–354. doi: 10.1016/j.apsb.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt K., Carroll J.S., Yee E., Thomas D.D., Wert-Lamas L., Neier S.C., Sheynkman G., Ritz J., Novina C.D. The lncRNA SLNCR Recruits the Androgen Receptor to EGR1-Bound Genes in Melanoma and Inhibits Expression of Tumor Suppressor p21. Cell Rep. 2019;27:2493–2507.e4. doi: 10.1016/j.celrep.2019.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmidt K., Weidmann C.A., Hilimire T.A., Yee E., Hatfield B.M., Schneekloth J.S., Jr., Weeks K.M., Novina C.D. Targeting the Oncogenic Long Non-coding RNA SLNCR1 by Blocking Its Sequence-Specific Binding to the Androgen Receptor. Cell Rep. 2020;30:541–554.e5. doi: 10.1016/j.celrep.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Katsushima K., Natsume A., Ohka F., Shinjo K., Hatanaka A., Ichimura N., Sato S., Takahashi S., Kimura H., Totoki Y., et al. Targeting the Notch-regulated non-coding RNA TUG1 for glioma treatment. Nat. Commun. 2016;7:13616. doi: 10.1038/ncomms13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong N., Teng X., Li J., Liang X.J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl. Mater. Interfaces. 2019;11:37–42. doi: 10.1021/acsami.8b18288. [DOI] [PubMed] [Google Scholar]
- 122.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S., Kim Y., MacLeod A.R., Krainer A.R., Norton L., et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deforzh E., Uhlmann E.J., Das E., Galitsyna A., Arora R., Saravanan H., Rabinovsky R., Wirawan A.D., Teplyuk N.M., El Fatimy R., et al. Promoter and enhancer RNAs regulate chromatin reorganization and activation of miR-10b/HOXD locus, and neoplastic transformation in glioma. Mol. Cell. 2022;82:1894–1908.e5. doi: 10.1016/j.molcel.2022.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiu W., Guo X., Li B., Wang J., Qi Y., Chen Z., Zhao R., Deng L., Qian M., Wang S., et al. Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells. Mol. Ther. 2021;29:3449–3464. doi: 10.1016/j.ymthe.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang L., Lin C., Song L., Wu J., Chen B., Ying Z., Fang L., Yan X., He M., Li J., et al. MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-kappaB/IkappaBalpha negative feedback loop. J. Clin. Investig. 2021;131:e155916. doi: 10.1172/JCI155916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng Y., Que T., Lin J., Zhan Z., Xu A., Wu Z., Xie C., Luo J., Ding S., Long H., et al. Oncogenic ZEB2/miR-637/HMGA1 signaling axis targeting vimentin promotes the malignant phenotype of glioma. Mol. Ther. Nucleic Acids. 2021;23:769–782. doi: 10.1016/j.omtn.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin J., Ge X., Shi Z., Yu C., Lu C., Wei Y., Zeng A., Wang X., Yan W., Zhang J., et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics. 2021;11:1763–1779. doi: 10.7150/thno.47057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao L., Li X., Mu Z., Zhou J., Zhou P., Xie C., Jiang S. FTO Inhibition Enhances the Antitumor Effect of Temozolomide by Targeting MYC-miR-155/23a Cluster-MXI1 Feedback Circuit in Glioma. Cancer Res. 2020;80:3945–3958. doi: 10.1158/0008-5472.CAN-20-0132. [DOI] [PubMed] [Google Scholar]
- 129.Kwak S., Park S.H., Kim S.H., Sung G.J., Song J.H., Jeong J.H., Kim H., Ha C.H., Kim S.W., Choi K.C. miR-3189-targeted GLUT3 repression by HDAC2 knockdown inhibits glioblastoma tumorigenesis through regulating glucose metabolism and proliferation. J. Exp. Clin. Cancer Res. 2022;41:87. doi: 10.1186/s13046-022-02305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hong S., You J.Y., Paek K., Park J., Kang S.J., Han E.H., Choi N., Chung S., Rhee W.J., Kim J.A. Inhibition of tumor progression and M2 microglial polarization by extracellular vesicle-mediated microRNA-124 in a 3D microfluidic glioblastoma microenvironment. Theranostics. 2021;11:9687–9704. doi: 10.7150/thno.60851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou F., Wang B., Wang H., Hu L., Zhang J., Yu T., Xu X., Tian W., Zhao C., Zhu H., et al. circMELK promotes glioblastoma multiforme cell tumorigenesis through the miR-593/EphB2 axis. Mol. Ther. Nucleic Acids. 2021;25:25–36. doi: 10.1016/j.omtn.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Bacco F., Orzan F., Erriquez J., Casanova E., Barault L., Albano R., D’Ambrosio A., Bigatto V., Reato G., Patane M., et al. ERBB3 overexpression due to miR-205 inactivation confers sensitivity to FGF, metabolic activation, and liability to ERBB3 targeting in glioblastoma. Cell Rep. 2021;36:109455. doi: 10.1016/j.celrep.2021.109455. [DOI] [PubMed] [Google Scholar]
- 133.Zhao J., Jiang Y., Zhang H., Zhou J., Chen L., Li H., Xu J., Zhang G., Jing Z. The SRSF1/circATP5B/miR-185-5p/HOXB5 feedback loop regulates the proliferation of glioma stem cells via the IL6-mediated JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2021;40:134. doi: 10.1186/s13046-021-01931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nie E., Jin X., Miao F., Yu T., Zhi T., Shi Z., Wang Y., Zhang J., Xie M., You Y. TGF-beta1 modulates temozolomide resistance in glioblastoma via altered microRNA processing and elevated MGMT. Neuro Oncol. 2021;23:435–446. doi: 10.1093/neuonc/noaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen P., Yang T., Chen Q., Yuan H., Wu P., Cai B., Meng L., Huang X., Liu J., Zhang Y., et al. CircNEIL3 regulatory loop promotes pancreatic ductal adenocarcinoma progression via miRNA sponging and A-to-I RNA-editing. Mol. Cancer. 2021;20:51. doi: 10.1186/s12943-021-01333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu X., Liu Y., Li Y., Chen H., Zhang Y., Liu J., Deng S., Zheng Y., Sun X., Wang J., et al. Selective exosome exclusion of miR-375 by glioma cells promotes glioma progression by activating the CTGF-EGFR pathway. J. Exp. Clin. Cancer Res. 2021;40:16. doi: 10.1186/s13046-020-01810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang Y., Zhou J., Zhao J., Zhang H., Li L., Li H., Chen L., Hu J., Zheng W., Jing Z. The U2AF2 /circRNA ARF1/miR-342-3p/ISL2 feedback loop regulates angiogenesis in glioma stem cells. J. Exp. Clin. Cancer Res. 2020;39:182. doi: 10.1186/s13046-020-01691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Chen R., Zhou X., Guo R., Yin J., Li Y., Ma G. miR-137: A Novel Therapeutic Target for Human Glioma. Mol. Ther. Nucleic Acids. 2020;21:614–622. doi: 10.1016/j.omtn.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Almairac F., Turchi L., Sakakini N., Debruyne D.N., Elkeurti S., Gjernes E., Polo B., Bianchini L., Fontaine D., Paquis P., et al. ERK-Mediated Loss of miR-199a-3p and Induction of EGR1 Act as a “Toggle Switch” of GBM Cell Dedifferentiation into NANOG- and OCT4-Positive Cells. Cancer Res. 2020;80:3236–3250. doi: 10.1158/0008-5472.CAN-19-0855. [DOI] [PubMed] [Google Scholar]
- 140.Bian E., Chen X., Cheng L., Cheng M., Chen Z., Yue X., Zhang Z., Chen J., Sun L., Huang K., et al. Super-enhancer-associated TMEM44-AS1 aggravated glioma progression by forming a positive feedback loop with Myc. J. Exp. Clin. Cancer Res. 2021;40:337. doi: 10.1186/s13046-021-02129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Isaev K., Jiang L., Wu S., Lee C.A., Watters V., Fort V., Tsai R., Coutinho F.J., Hussein S.M.I., Zhang J., et al. Pan-cancer analysis of non-coding transcripts reveals the prognostic onco-lncRNA HOXA10-AS in gliomas. Cell Rep. 2021;37:109873. doi: 10.1016/j.celrep.2021.109873. [DOI] [PubMed] [Google Scholar]
- 142.Zhu C., Li K., Jiang M., Chen S. RBM5-AS1 promotes radioresistance in medulloblastoma through stabilization of SIRT6 protein. Acta Neuropathol. Commun. 2021;9:123. doi: 10.1186/s40478-021-01218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Katsushima K., Lee B., Kunhiraman H., Zhong C., Murad R., Yin J., Liu B., Garancher A., Gonzalez-Gomez I., Monforte H.L., et al. The long noncoding RNA lnc-HLX-2-7 is oncogenic in Group 3 medulloblastomas. Neuro Oncol. 2021;23:572–585. doi: 10.1093/neuonc/noaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X., Niu W., Mu M., Hu S., Niu C. Long non-coding RNA LPP-AS2 promotes glioma tumorigenesis via miR-7-5p/EGFR/PI3K/AKT/c-MYC feedback loop. J. Exp. Clin. Cancer Res. 2020;39:196. doi: 10.1186/s13046-020-01695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X., Yu X., Xu H., Wei K., Wang S., Wang Y., Han J. Serum-derived extracellular vesicles facilitate temozolomide resistance in glioblastoma through a HOTAIR-dependent mechanism. Cell Death Dis. 2022;13:344. doi: 10.1038/s41419-022-04699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han M., Wang S., Fritah S., Wang X., Zhou W., Yang N., Ni S., Huang B., Chen A., Li G., et al. Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/beta-catenin signalling. Brain. 2020;143:512–530. doi: 10.1093/brain/awz406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Voce D.J., Bernal G.M., Wu L., Crawley C.D., Zhang W., Mansour N.M., Cahill K.E., Szymura S.J., Uppal A., Raleigh D.R., et al. Temozolomide Treatment Induces lncRNA MALAT1 in an NF-kappaB and p53 Codependent Manner in Glioblastoma. Cancer Res. 2019;79:2536–2548. doi: 10.1158/0008-5472.CAN-18-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun J., He D., Fu Y., Zhang R., Guo H., Wang Z., Wang Y., Gao T., Wei Y., Guo Y., et al. A novel lncRNA ARST represses glioma progression by inhibiting ALDOA-mediated actin cytoskeleton integrity. J. Exp. Clin. Cancer Res. 2021;40:187. doi: 10.1186/s13046-021-01977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buccarelli M., Lulli V., Giuliani A., Signore M., Martini M., D’Alessandris Q.G., Giannetti S., Novelli A., Ilari R., Giurato G., et al. Deregulated expression of the imprinted DLK1-DIO3 region in glioblastoma stemlike cells: Tumor suppressor role of lncRNA MEG3. Neuro Oncol. 2020;22:1771–1784. doi: 10.1093/neuonc/noaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan Z., Zhao R., Li B., Qi Y., Qiu W., Guo Q., Zhang S., Zhao S., Xu H., Li M., et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol. Cancer. 2022;21:16. doi: 10.1186/s12943-021-01485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wei Y., Lu C., Zhou P., Zhao L., Lyu X., Yin J., Shi Z., You Y. EIF4A3-induced circular RNA ASAP1 promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1-2 signaling. Neuro Oncol. 2021;23:611–624. doi: 10.1093/neuonc/noaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao X., Xia X., Li F., Zhang M., Zhou H., Wu X., Zhong J., Zhao Z., Zhao K., Liu D., et al. Circular RNA-encoded oncogenic E-cadherin variant promotes glioblastoma tumorigenicity through activation of EGFR-STAT3 signalling. Nat. Cell Biol. 2021;23:278–291. doi: 10.1038/s41556-021-00639-4. [DOI] [PubMed] [Google Scholar]
- 153.Wu X., Xiao S., Zhang M., Yang L., Zhong J., Li B., Li F., Xia X., Li X., Zhou H., et al. A novel protein encoded by circular SMO RNA is essential for Hedgehog signaling activation and glioblastoma tumorigenicity. Genome Biol. 2021;22:33. doi: 10.1186/s13059-020-02250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu X., Shen S., Zhu L., Su R., Zheng J., Ruan X., Shao L., Wang D., Yang C., Liu Y. SRSF10 inhibits biogenesis of circ-ATXN1 to regulate glioma angiogenesis via miR-526b-3p/MMP2 pathway. J. Exp. Clin. Cancer Res. 2020;39:121. doi: 10.1186/s13046-020-01625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang S., Liao K., Miao Z., Wang Q., Miao Y., Guo Z., Qiu Y., Chen B., Ren L., Wei Z., et al. CircFOXO3 promotes glioblastoma progression by acting as a competing endogenous RNA for NFAT5. Neuro Oncol. 2019;21:1284–1296. doi: 10.1093/neuonc/noz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen J., Chen T., Zhu Y., Li Y., Zhang Y., Wang Y., Li X., Xie X., Wang J., Huang M., et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J. Exp. Clin. Cancer Res. 2019;38:398. doi: 10.1186/s13046-019-1376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He Z., Ruan X., Liu X., Zheng J., Liu Y., Liu L., Ma J., Shao L., Wang D., Shen S., et al. FUS/circ_002136/miR-138-5p/SOX13 feedback loop regulates angiogenesis in Glioma. J. Exp. Clin. Cancer Res. 2019;38:65. doi: 10.1186/s13046-019-1065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He Q., Zhao L., Liu X., Zheng J., Liu Y., Liu L., Ma J., Cai H., Li Z., Xue Y. MOV10 binding circ-DICER1 regulates the angiogenesis of glioma via miR-103a-3p/miR-382-5p mediated ZIC4 expression change. J. Exp. Clin. Cancer Res. 2019;38:9. doi: 10.1186/s13046-018-0990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. CircNT5E Acts as a Sponge of miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018;78:4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 160.Lou J., Hao Y., Lin K., Lyu Y., Chen M., Wang H., Zou D., Jiang X., Wang R., Jin D., et al. Circular RNA CDR1as disrupts the p53/MDM2 complex to inhibit Gliomagenesis. Mol. Cancer. 2020;19:138. doi: 10.1186/s12943-020-01253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He J., Huang Z., He M., Liao J., Zhang Q., Wang S., Xie L., Ouyang L., Koeffler H.P., Yin D., et al. Circular RNA MAPK4 (circ-MAPK4) inhibits cell apoptosis via MAPK signaling pathway by sponging miR-125a-3p in gliomas. Mol. Cancer. 2020;19:17. doi: 10.1186/s12943-019-1120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang M., Zhao K., Xu X., Yang Y., Yan S., Wei P., Liu H., Xu J., Xiao F., Zhou H., et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat. Commun. 2018;9:4475. doi: 10.1038/s41467-018-06862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., Huang N., Yang X., Zhao K., Zhou H., et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang L., Wilusz J.E., Chen L.L. Biogenesis and Regulatory Roles of Circular RNAs. Annu. Rev. Cell Dev. Biol. 2022 doi: 10.1146/annurev-cellbio-120420-125117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu C.X., Chen L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell. 2022;185:2016–2034. doi: 10.1016/j.cell.2022.04.021. [DOI] [PubMed] [Google Scholar]
- 166.Ebbesen K.K., Kjems J., Hansen T.B. Circular RNAs: Identification, biogenesis and function. Biochim. Biophys. Acta. 2016;1859:163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 167.Wilusz J.E. Circular RNAs: Unexpected outputs of many protein-coding genes. RNA Biol. 2017;14:1007–1017. doi: 10.1080/15476286.2016.1227905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 169.Salzman J., Gawad C., Wang P.L., Lacayo N., Brown P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Santer L., Bar C., Thum T. Circular RNAs: A Novel Class of Functional RNA Molecules with a Therapeutic Perspective. Mol. Ther. 2019;27:1350–1363. doi: 10.1016/j.ymthe.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun J., Li B., Shu C., Ma Q., Wang J. Functions and clinical significance of circular RNAs in glioma. Mol. Cancer. 2020;19:34. doi: 10.1186/s12943-019-1121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu J., Ye J., Zhang L., Xia L., Hu H., Jiang H., Wan Z., Sheng F., Ma Y., Li W., et al. Differential Expression of Circular RNAs in Glioblastoma Multiforme and Its Correlation with Prognosis. Transl. Oncol. 2017;10:271–279. doi: 10.1016/j.tranon.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu H., Zhang Y., Qi L., Ding L., Jiang H., Yu H. NFIX Circular RNA Promotes Glioma Progression by Regulating miR-34a-5p via Notch Signaling Pathway. Front. Mol. Neurosci. 2018;11:225. doi: 10.3389/fnmol.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y., Wu Y.M., Dhanasekaran S.M., Engelke C.G., Cao X., et al. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869–881.e813. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. Correction to: EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol. Cancer. 2020;19:153. doi: 10.1186/s12943-020-01271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Y., Li Z., Zhang M., Zhou H., Wu X., Zhong J., Xiao F., Huang N., Yang X., Zeng R., et al. Rolling-translated EGFR variants sustain EGFR signaling and promote glioblastoma tumorigenicity. Neuro Oncol. 2021;23:743–756. doi: 10.1093/neuonc/noaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao M., Xu J., Zhong S., Liu Y., Xiao H., Geng L., Liu H. Expression profiles and potential functions of circular RNAs in extracellular vesicles isolated from radioresistant glioma cells. Oncol Rep. 2019;41:1893–1900. doi: 10.3892/or.2019.6972. [DOI] [PubMed] [Google Scholar]
- 179.Vagin V.V., Sigova A., Li C., Seitz H., Gvozdev V., Zamore P.D. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–324. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 180.Zhang P., Wu W., Chen Q., Chen M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019;16 doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thomson T., Lin H. The biogenesis and function of PIWI proteins and piRNAs: Progress and prospect. Annu. Rev. Cell Dev. Biol. 2009;25:355–376. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jacobs D.I., Qin Q., Fu A., Chen Z., Zhou J., Zhu Y. piRNA-8041 is downregulated in human glioblastoma and suppresses tumor growth in vitro and in vivo. Oncotarget. 2018;9:37616–37626. doi: 10.18632/oncotarget.26331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mani S.R., Juliano C.E. Untangling the web: The diverse functions of the PIWI/piRNA pathway. Mol. Reprod. Dev. 2013;80:632–664. doi: 10.1002/mrd.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grivna S.T., Beyret E., Wang Z., Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–1714. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gainetdinov I., Colpan C., Arif A., Cecchini K., Zamore P.D. A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol. Cell. 2018;71:775–790.e5. doi: 10.1016/j.molcel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y., Liu W., Li R., Gu J., Wu P., Peng C., Ma J., Wu L., Yu Y., Huang Y. Structural insights into the sequence-specific recognition of Piwi by Drosophila Papi. Proc. Natl. Acad. Sci. USA. 2018;115:3374–3379. doi: 10.1073/pnas.1717116115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tamtaji O.R., Behnam M., Pourattar M.A., Hamblin M.R., Mahjoubin-Tehran M., Mirzaei H., Asemi Z. PIWI-interacting RNAs and PIWI proteins in glioma: Molecular pathogenesis and role as biomarkers. Cell Commun. Signal. 2020;18:168. doi: 10.1186/s12964-020-00657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mei Y., Clark D., Mao L. Novel dimensions of piRNAs in cancer. Cancer Lett. 2013;336:46–52. doi: 10.1016/j.canlet.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martinez V.D., Vucic E.A., Thu K.L., Hubaux R., Enfield K.S., Pikor L.A., Becker-Santos D.D., Brown C.J., Lam S., Lam W.L. Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology. Sci. Rep. 2015;5:10423. doi: 10.1038/srep10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Siddiqi S., Matushansky I. Piwis and piwi-interacting RNAs in the epigenetics of cancer. J. Cell. Biochem. 2012;113:373–380. doi: 10.1002/jcb.23363. [DOI] [PubMed] [Google Scholar]
- 191.Huang H., Yu X., Han X., Hao J., Zhao J., Bebek G., Bao S., Prayson R.A., Khalil A.M., Jankowsky E., et al. Piwil1 Regulates Glioma Stem Cell Maintenance and Glioblastoma Progression. Cell Rep. 2021;34:108522. doi: 10.1016/j.celrep.2020.108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shen S., Yu H., Liu X., Liu Y., Zheng J., Wang P., Gong W., Chen J., Zhao L., Xue Y. PIWIL1/piRNA-DQ593109 Regulates the Permeability of the Blood-Tumor Barrier via the MEG3/miR-330-5p/RUNX3 Axis. Mol. Ther. Nucleic Acids. 2018;10:412–425. doi: 10.1016/j.omtn.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gebert L.F.R., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 195.Levin A.A. Treating Disease at the RNA Level with Oligonucleotides. N. Engl. J. Med. 2019;380:57–70. doi: 10.1056/NEJMra1705346. [DOI] [PubMed] [Google Scholar]
- 196.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug. Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]