Abstract
Nuclear factor (erythroid-derived 2)-like 2 (NRF2) is a redox-sensitive transcription factor that binds to the antioxidant response element consensus sequence, decreasing reactive oxygen species and regulating the transcription of a wide array of genes, including antioxidant and detoxifying enzymes, regulating genes involved in mitochondrial function and biogenesis. Moreover, NRF2 has been shown to directly regulate the expression of anti-inflammatory mediators reducing the expression of pro-inflammatory cytokines. In recent years, attention has turned to the role NRF2 plays in the brain in different diseases such Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and others. This review focused on the evidence, derived in vitro, in vivo and from clinical trials, supporting a role for NRF2 activation in maintaining and improving cognitive function and how its activation can be used to elicit neuroprotection and lead to cognitive enhancement. The review also brings a critical discussion concerning the possible prophylactic and/or therapeutic use of NRF2 activators in treating cognitive impairment-related conditions.
Keywords: NRF2 signaling pathway, cognitive decline, cognition improvement, neurodegenerative diseases
1. Introduction
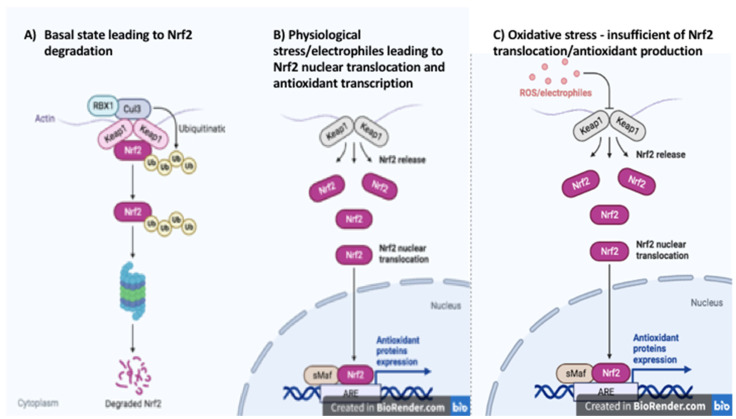
Nuclear factor (erythroid-derived 2)-like 2 (NRF2, also called NFE2L2) is a member of the cap‘n’collar subclass of the basic leucine zipper region containing the protein family. NRF2 is a redox-sensitive transcription factor that binds to the antioxidant response element (ARE) consensus sequence, regulating the transcription of a wide array of genes. The activity of NRF2 is tightly controlled by proteasomal degradation. Under normal conditions, NRF2 is sequestered in the cytosol bound to its cellular chaperone protein Kelch-like ECH-association protein 1 (KEAP1). When bound to KEAP1, NRF2 is targeted for degradation by the proteasome. However, in the presence of electrophiles or oxidative stress the nucleophilic cysteine sulfhydryl groups on KEAP1 are modified resulting in an allosteric conformational change that diminishes the KEAP-dependent degradation of NRF2 and allows the transcription factor to accumulate in the nucleus [1] (Figure 1). Nuclear translocation can also result from the phosphorylation of NRF2 at serine 40. Many kinases have been shown to phosphorylate this site on NRF2 in various tissue types including phosphoinositide-3 kinase)/protein kinase B (PI3K/AKT), mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 1/2 (ERK1/2), glycogen synthase kinase 3 (GSK-3β) and protein kinase C (PKC) [2,3,4,5]. p62 and p21 can increase NRF2 transcriptional activity by decreasing the binding of NRF2 to KEAP1. p62 has been shown to physically block NRF2-Keap1 binding by itself binding to Keap1 in a location that overlaps the binding pocket for NRF2 [6]. In contrast, p21 directly interacts with the binding motifs of NRF2 and competitively inhibits KEAP1 binding [6].
Figure 1.
Keap1–Nrf2 Pathway. (A) basal state leading to Nrf2 degradation, (B) physiological stress/electrophiles leading to Nrf2 nuclear translocation and antioxidant transcription, (C) oxidative stress—insufficient of Nrf2 translocation/antioxidant production (created in BioRender.com, accessed on 20 July 2022).
The wide variety of ARE-containing genes include antioxidant and detoxifying enzymes such as gamma-glutamylcysteine synthetase, superoxide dismutase, catalase, glutathione reductase, thioredoxin reductase, peroxiredoxins, glutathione S-transferase and others [7]. NRF2 has also been implicated in regulating genes involved in both mitochondrial function and biogenesis [8,9]. NRF2 activation modulates the expression of ATP synthase subunit α and NDUFA4, two components of the electron transport chain (ETC) [10,11] as well as other enzymes important for proper bioenergetic function including malic enzyme 1, isocitrate dehydrogenase 1, glucose-6-phosphate dehydrogenase and 6-phosphogluconate-dehydrogenase [12,13]. The antioxidant effects of NRF2 also result in improved mitochondrial function, as decreasing the reactive oxygen species reduces oxidative damage to mitochondria. Additionally, NRF2 affects the expression of several regulators of mitochondrial biogenesis including sirtuin 1 (Sirt1), peroxisome proliferator-activated receptor γ (PPARγ) and PPARy co-activator 1 α (Pgc1α), considered to be the best regulator of biogenesis [14,15,16,17,18].
Significant cross talk also exists between antioxidant and anti-inflammatory pathways. For example, the classical pro-inflammatory transcription factor NF-κB is activated by oxidative stress which can be blocked by the NRF2-dependent induction of antioxidant target genes and thus the transcription of pro-inflammatory cytokines can be decreased [19,20,21]. However, NRF2 has been shown to directly regulate the expression of anti-inflammatory mediators such as IL17D, CD36, the macrophage receptor with collagenous structure (MARCO) and G-protein coupled receptor kinase (GRK) [22,23,24,25]. PPARγ is also known to have anti-inflammatory properties [26]. Moreover, NRF2 has been implicated in reducing the expression of the pro-inflammatory cytokines IL6 and IL1β through a binding site nearby the promoter that prevents the recruitment of RNA Polymerase II [27].
NRF2 is expressed in all tissue but in recent years attention has turned to its role in the brain and how its activation can be used to elicit neuroprotection and lead to cognitive enhancement [28,29]. Although experimental evidence points to an indisputable role of NRF2 activation in mitigating neurodegeneration and improving cognition, the clinical use of NRF2 activators for treating cognitive impairment related conditions represents an incipient and controversial research topic. The initial purpose of this review is to provide a comprehensive presentation and interpretation of the scientific literature supporting a role for NRF2 in maintaining and improving cognitive function. A second purpose of this review is to provide a critical analysis concerning the possible prophylactic and/or therapeutic use of NRF2 activators in treating cognitive impairment related conditions.
2. In Vitro Evidence
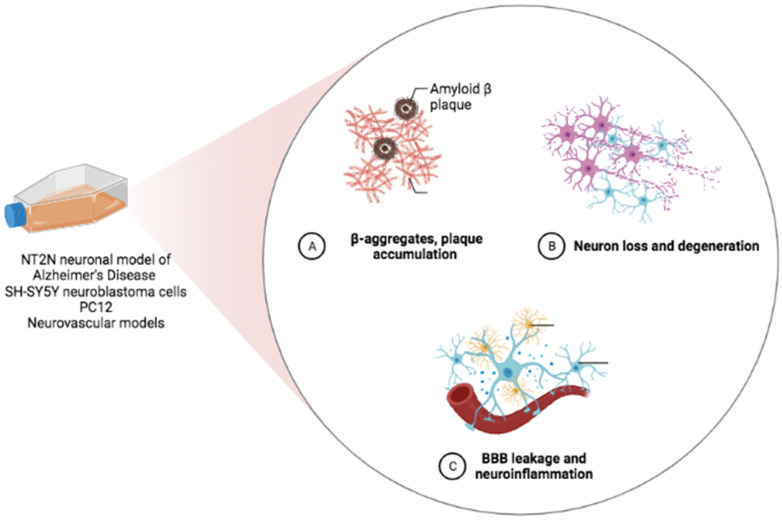
While it is not possible to test cognition per se in an in vitro system, cellular models can recapitulate the neuronal dysfunction as well as the synapse and dendrite loss that form the anatomic basis for cognitive decline [30] (Figure 2). There is a great deal of evidence from cellular models suggesting that NRF2 activation can ameliorate those endpoints.
Figure 2.
In vitro models recapitulating the neuronal dysfunction, synapse and dendrite loss that form the anatomic basis for cognitive decline (created in BioRender.com, accessed on 20 July 2022).
Some models mimic the accumulation of pathogenetic amyloid beta (Aβ) observed in vivo that induces the accumulation of neurofibrillary tangles (NFTs), neuronal cell death and dementia [31]. 2,3′-dihydroxy-4′,6′-dimethoxychalcone (DCD), a member of the flavonoid chalcones group, as well as quercetin and rutin (present for example in citrus plants), syringin and eleutheroside B, have all been shown to be protective against Aβ-induced neuronal death through the activation of the NRF2/ARE pathway [32,33,34]. Similarly, tetra-butyl hydroquinone (tBHQ), a synthetic food preservative and NRF2 activator, and edaravone, a medication used to treat patients with amyotrophic lateral sclerosis, were likewise demonstrated to reduce oxidative stress, attenuate neuronal toxicity and reduce Aβ formation in an NT2N neuronal model of Alzheimer’s Disease [35] and SH-SY5Y neuroblastoma cells [36].
The water extract of Centella asiatica (CWE) has been reported to activate NRF2 in mouse primary neurons and protect against Aβ toxicity [37]. In neurons isolated from the Tg2576 mouse model of Aβ accumulation, CWE treatment was shown to reverse deficits in dendritic arborization and spine density [38]. A similar effect was observed in wild-type neurons and in MC65 neuroblastoma cells where CWE induced the expression of the antioxidant response gene NFE2L2 and its target genes to protect against Aβ-induced cytotoxicity [39].
CWE is phytochemically characterized by the presence of isoprenoids (sesquiterpenes, plant sterols, pentacyclic triterpenoids and saponins) and phenylpropanoid derivatives (eugenol derivatives, caffeoylquinic acids, and flavonoids) [40]. Several of these constituent compounds, including asiatic acid [41], madecassoside [42] and the 1,5-dicaffeoylquinic acid [43] have been shown to be neuroprotective and activate NRF2 in vitro as well.
Other phenolic compounds have also been reported to have beneficial effects in vitro that are linked to NRF2 activation. In PC12 cells, phenolic compounds induced neuronal differentiation and elicited neuroprotective effects through the binding of NRF2 [44].
The same effect was reported with curcumin, a natural polyphenol with multiple biological activities, including antioxidant and anti-inflammatory properties [45], and gypenoside XVII (a phytoestrogen isolated from Gynostemma pentaphyllum, belong to Cucurbitaceae family) [46,47]. To gain insights relevant for cognitive impairment and dementia, neurovascular models (composed of endothelial cells, myocytes, neurons, astrocytes, perivascular cells, microglia and oligodendroglia) have also been utilized [48]. The NRF2 activator sulforaphane, a metabolite of glucoraphanin (present in Brassica oleracea), reduced neuronal and endothelial death in primary brain endothelial cultures and maintained the integrity of the brain blood barrier (BBB) [49,50,51].
Another good correlate of cognitive function in vitro is synaptic density [52]. Different natural substances have been shown to induce overgrowth and neuroplasticity in vitro [53] and some of these are also known to activate the NRF2 pathway [54]. Carnosic acid, found in rosemary, which has been shown to have memory enhancing effects, has also been shown to induce neurite extension and neural differentiation through NRF2 activation in PC12h cells and the knockdown of NRF2 reduced this effect [55,56]. Similarly, in PC12 cells the flavonoid Luteolin stimulated neurite outgrowth (maximal neurite length and percentage of neurite bearing cells) in a dose-dependent manner [57].
3. In Vivo Evidence
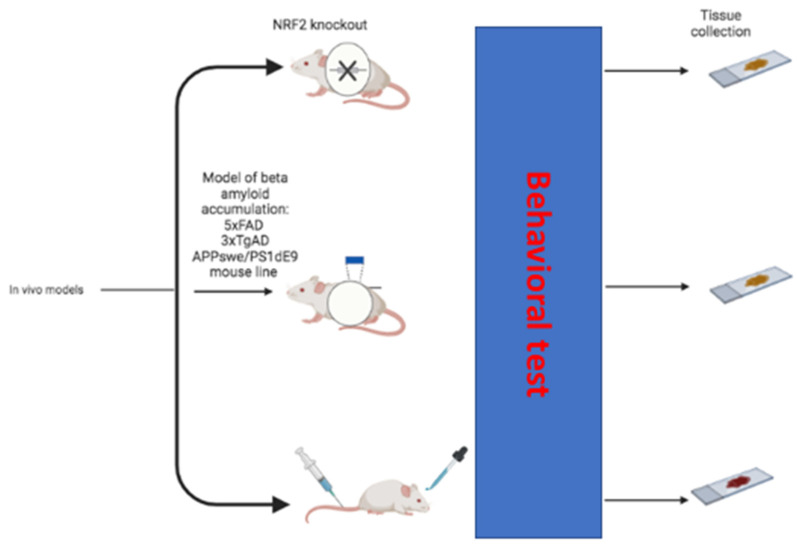
There is ample evidence supporting the role of NRF2 in maintaining cognitive function in a variety of rodent model systems. This comes from the combination of studies demonstrating the deleterious consequences of loss of NRF2 and those detailing the cognitive enhancing effects of NRF2 activation (Figure 3).
Figure 3.
In vivo models used to demonstrate the deleterious consequences of loss of NRF2 and the cognitive enhancing effects of NRF2 activation (created in BioRender.com, accessed on 20 July 2022).
3.1. Cognitive Impairing Effects of Loss of NRF2
Across a broad range of conditions, a reduction in NRF2 expression resulted in worsened cognitive outcomes. Mouse models of healthy aging have shown that NRF2 knockout (NRF2KO) mice experience accelerated cognitive decline between 6 and 18 months relative to wild-type (WT) and exacerbated cognitive impairments at older ages (17–24 months) [58,59,60].
Similarly, intensified cognitive impairment has been reported in mouse models of Alzheimer’s disease (AD) in which NFR2 has been knocked out. When NRF2 was deleted from the 5xFAD mouse model of Aβ accumulation, the expression of Aβ processing enzymes was enhanced leading to increased plaque pathology which was accompanied by worsened cognitive impairment as compared to 5xFAD animals that did express NRF2 [61]. This is consistent with earlier reports in mice that over express double mutations in amyloid precursor protein and presenilin 1 (APP/PS1 mice) in which NRF2 was ablated. In these animals, there was a significant exacerbation of deficits in spatial learning and memory along with working and associative memory relative to NRF2 expressing APP/PS1 mice [62,63]. Similarly, knocking out NRF2 in a mouse line modeling a combination of amyloidopathy and tauopathy together likewise worsened deficits in spatial learning and memory and also reduced long term potentiation in the perforant pathway [64].
The negative effects of the loss of NRF2 on cognitive function can also be seen in other neurodegenerative conditions. In a lipopolysaccharide (LPS) mouse model of multiple sclerosis, NRF2 deficiency resulted in more pronounced impairments in recognition memory [65] and for NRF2KO mice that were subjected to ischemic stroke showed an increased lesion volume and poorer neurocognitive performance [66].
3.2. Cognitive Enhancing Effects of NRF2 Activating Compounds
3.2.1. Plant-Derived Compounds
A host of NRF2 activating compounds have demonstrated potent cognitive enhancing effects across a variety of models of cognitive impairment. The CWE has been shown to increase the expression of NRF2 and its regulated antioxidant target genes in vivo and to improve learning, memory and executive function in mouse models of both healthy aging as well as Aβ accumulation [67,68,69]. A number of other botanically derived compounds demonstrated similar NRF2-activating and cognitive enhancing effects. Pterostilbene (3′,5′-dimethoxy-4-stilbenol), found in berries and grapes, and curcumin have both been shown to activate NRF2 and improve cognitive function in mouse models of Aβ accumulation [70,71]. Sulforaphane has also been shown to reduce Aβ accumulation and ameliorate cognitive deficits in the 5xFAD mouse line as well as the 3xTgAD model of concurrent Aβ and tau accumulation [61]. It similarly improved cognitive impairment in mouse models of traumatic brain injury, diabetes and vascular cognitive impairment [51,72,73].
Incense, the resin of Boswellia genus plants, is used in religious ceremonies, as well as in medicine. The Boswellia extract contains triterpenoids that are pharmacologically active such as 3-O-acetyl-11-keto--boswellic acid (AKBA) and Boswellic acid (BA) that interact with NRF2 [74,75,76]. In APPswe/PS1dE9 mice, AKBA treatment improved performance in the Morris Water maze test of spatial and long-term memory, as well as in the passive avoidance task of fear-associated learning and memory in rodent models of central nervous system disorders [74,75,76]. Moreover, in APPswe/PS1dE9 mice treated with AKBA, the expression of nuclear NRF2 and total HO-1 were noticeably upregulated in the cerebral cortex and hippocampus while the NF-κB pathway was suppressed [76].
Medicinal mushrooms have also been shown to activate NRF2 and improve cognitive function. A recent study found that oral treatment with the medicinal mushrooms Hericium erinaceus and Coriolus versicolor reduced oxidative damage, neuroinflammation, and cognitive impairments in a mouse model of experimental traumatic brain injury. Male CD1 mice were subjected to a control cortical impact injury and then treated with 200 mg/kg of Hericium erinaceus and Coriolus versicolor for 30 days resulting in a potent induction of the expression of NRF2 and its antioxidant target genes in the brains of treated animals. This was accompanied by a reduction in NF-κB signaling as well as reduced markers of astrocytic, microglial activation and improved spatial learning and memory [77]. This study was in line with previous research which also showed that Hericium erinaceus and Coriolus versicolor activate NRF2, increase the expression of its downstream target genes [78,79] and improve cognitive function in mouse models of a high fat diet and Alzheimer’s disease [78,80].
Ginsenoside from ginseng (the root of plant Panax ginseng) and bilobalide and gingkolides from gingko (leaves of Ginkgo biloba) have likewise been reported to enhance cognitive performance in models of stress- and ischemia-induced cognitive impairment, respectively [81,82].
Lycopene, epigallocatechin gallate (EGCG) and resveratrol are also examples of the botanically derived NRF2 activating compound with reported cognitive enhancing effects in animal models of a wide range of conditions. Supplementation with lycopene (a carotenoid found in tomatoes, red fruits and vegetables) improved the cognitive function in rodent models of healthy aging and tauopathy as well as both high fat diet- and oxidative stress-induced cognitive impairment [83,84,85,86,87]. EGCG (a polyphenol highly abundant in green tea, with anti-oxidative and neuroprotective properties [88]) was similarly shown to improve high-fat diet induced cognitive deficits as well as those resulting from high-fructose consumption, Aβ overexpression and healthy aging [89,90,91,92]. Resveratrol is a plant-derived antioxidant polyphenolic compound that exhibits positive effects in animal models of neuropathological conditions [93,94]. Resveratrol attenuated cognitive impairment in a mouse model of traumatic brain injury and prevented type 2 diabetes-induced cognitive deficits [95,96].
3.2.2. Synthetic NRF2 Activating Compounds
A number of synthetic compounds have also been identified as potent NRF2 activators with cognitive enhancing effects. The two synthetic compounds that have been most studied for their effects on cognitive performance are probably dimethyl fumarate (DMF) and tBHQ.
Fumaric acid esters, such as DMF and its primary metabolite monomethylfumarate (MMF), represent a class of molecules that have been shown to exhibit both antioxidant and anti-inflammatory properties [97]. Of note, there are studies showing the beneficial effects of DMF in counteracting oxidative stress and inflammation in neurodegenerative conditions through the activation of NRF2, although additional transcription factors (i.e., NF-κB) also modulated this compound [98]. DMF treatment was shown to improve spatial learning and memory in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis [99]. Spatial memory was likewise enhanced in aged rats treated with streptozotocin [100] and in a mouse model of subarachnoid hemorrhage [101]. Reference and working memory were also improved in those same mice [101]. DMF was reported to have similar cognitive enhancing properties in a model of sepsis [102], a double transgenic model of Aβ and tau accumulation [103] and a model of traumatic brain injury [104].
tBHQ is another small molecule that has shown promising cognitive enhancing effects. Rats treated with tBHQ showed reduced deficits in spatial learning and memory following subarachnoid hemorrhage [105]. tBHQ also improved spatial memory in rats, and spatial, associative and recognition memory in mice subjected to a mild traumatic brain injury [106,107].
3.3. Biochemical Pathways Associated with Cognitive Enhancement by NRF2 Activating Compounds
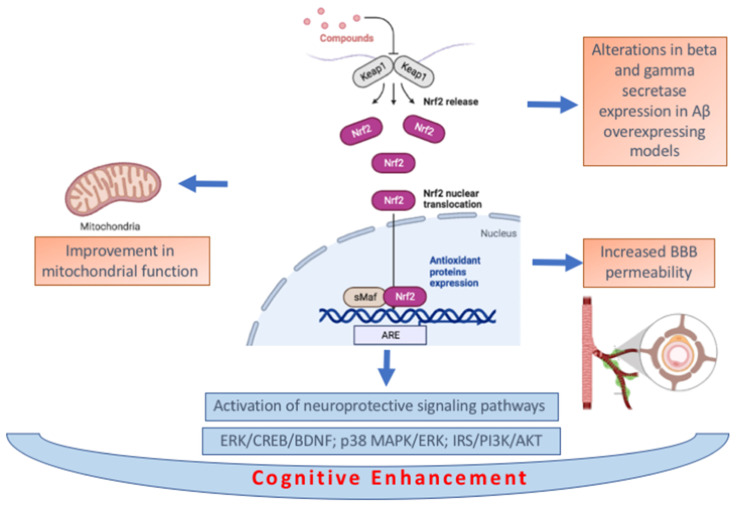
The interconnected and wide-ranging cellular consequences of NRF2 activation makes it difficult to identify a single mechanism of action underlying the cognitive enhancing effects of NRF2. In vivo effects on oxidative stress have frequently been reported alongside cognitive enhancement following treatment with NRF2-activating compounds as has improvement in mitochondrial function [37,59,63,69,70,76,85,86]. Activation of neuroprotective signaling pathways including ERK/CREB/BDNF, p38 MAPK/ERK and IRS/PI3K/AKT has also been implicated in mediating the cognitive effects of NRF2 activation [81,90,95].
Increased BBB permeability may also contribute to the beneficial effects of NRF2 activation on cognitive function although this has to our knowledge so far only been reported in the context of subarachnoid hemorrhage [92]. Another context-specific mechanism that may mediate additional cognitive enhancing effects could be alterations in beta and gamma secretase expression in Aβ overexpressing models [61,90].
The anti-inflammatory effects of NRF2 may also play an important role as neuroinflammation is a common feature of many conditions associated with cognitive impairment. Decreases in NF-κB signaling, reactive microgliosis, the overactivation of astrocytes and other markers of neuroinflammation have been reported where NRF2 activating compounds resulted in cognitive enhancement [63,65,76,83,86,87,90,103,104,107]. In Figure 4 a summary of the described biochemical pathways is provided.
Figure 4.
Summary of biochemical pathways associated with cognitive. enhancement by NRF2 activating compounds (created in BioRender.com, accessed on 20 July 2022).
4. Clinical Evidence for the Effects of NRF2 Activation on Cognitive Function
In line with the experimental studies supporting the role of NRF2 in maintaining cognitive function in animal models (discussed in the previous item), there is a growing body of evidence pointing to a potential relationship between NRF2 and cognition in humans. Particularly, there is mounting evidence indicating that NRF2 activators are able to improve (or at least prevent the decline of) cognition in humans. Nevertheless, although experimental studies with NRF2KO animals allow us to investigate the direct involvement of NRF2 in cognition, such a direct association is not as easy to determine in human studies. In the following sections, we provide an overview of the most frequently reported NRF2 activators with modulatory effects in cognition in humans.
4.1. Curcumin
Curcumin has been reported to display a favorable safety profile although recently a number of cases of acute non-infectious cholestatic hepatitis related to the consumption of curcumin dietary supplements were reported [108,109,110]. Curcumin is able to cross the BBB [111,112] without neurotoxicity, even at a high dose [113]. Curcumin activates NRF2 by alkylating a protein thiol on the Keap-1-NRF2 binding complex [114,115]. Concerning the effects of curcumin in human cognition, Ng et al. [116] observed that elderly subjects with occasional or frequent consumption of curry performed better than those reporting rare consumption.
Three years later, a randomized double-blind, placebo-controlled trial (ClinicalTrials.gov, accessed on 20 July 2022; Identifier NCT00164749) investigated the effect of curcumin in subjects affected by AD [117]. Thirty-four subjects were treated with different doses of oral curcumin (up to 4 g a day) or with placebo during 6 months. The authors found no significant effects of curcumin on cognition; however, they argued that the lack of cognitive decline in the placebo group may have precluded any ability to detect a relative protective effect of curcumin [117]. A second clinical trial (ClinicalTrials.gov, accessed on 20 July 2022; Identifier NCT00099710) evaluated the effects of Curcumin C3 Complex® in 30 subjects with mild to moderate AD [118]. After 24-weeks of treatment, the authors were likewise unable to demonstrate clinical or biochemical evidence of the efficacy of the Curcumin C3 Complex(®).
Cox et al. [119] developed a study on the short-term effects of curcumin in a healthy, elderly population. After treatment with curcumin (solid lipid emulsion of curcumin named Longvida® [120]), the authors found that both short term and chronic treatment with curcumin improved working memory and digit vigilance. In a similar study, Rainey-Smith et al. [121] performed a 12-month, randomized, placebo-controlled, double-blind study that investigated the ability of a curcumin formulation to prevent cognitive decline in a population of community-dwelling older adults. After six months of treatment, the placebo group manifested signs of cognitive decline that were not present in the group treated with curcumin.
More recently, Small et al. [122] studied the effect of curcumin (Theracurmin® containing 90 mg of curcumin twice daily or placebo for 18 months) on memory in non-demented adults and explored its impact on brain amyloid and tau accumulation.
Cognitive skills (assessed by the Buschke Selective Reminding Test) were improved after six months and was maintained for the entire eighteen months of the trial. In a parallel group of subjects, curcumin induced a significant reduction in plaque and tangle deposition.
In 2020, Cox et al. [123] conducted a double-blind, placebo-controlled, parallel-groups trial in order to partially replicate their previous study [119]. Eighty aged participants (mean = 68.1 years) were randomized to receive Longvida© (400 mg daily containing 80 mg curcumin) or a matching placebo. The participants were assessed at baseline, and 4- and 12-weeks treatment. Compared with placebo, curcumin was associated with better working memory performance at 12-weeks, and lower fatigue scores at both 4- and 12-weeks; lower tension, anger, confusion and total mood disturbance were also observed in the curcumin group at 4-weeks only. The pattern of results is consistent with improvements in hippocampal function.
In summary, these clinical studies on the effects of curcumin in cognition/dementia do not provide a definitive response. As noted, there are studies reporting either beneficial or not significant results. It seems that the bioavailability of curcumin has a great influence in the achieved results. In addition, it is important to mention that the diverse protocols (measured cognitive skills) and characteristics of the enrolled subjects (especially age and health condition) in each of the aforementioned studies limits our ability to compare their results. Nevertheless, the reported beneficial effects of curcumin [122,123] indicate that this polyphenol represents a potential strategy to prevent the decline of cognition mainly in elderly individuals.
4.2. Centella asiatica
Centella asiatica has also been evaluated for its effects on human cognition. A double-blind trial developed more than four decades ago [124] showed significant increases in the general mental ability, attention and concentration of mentally retarded children treated with Centella asiatica (0.5 g/day during 6 months). Studies also reported improvements in cognitive function after Centella asiatica treatment in adult humans after stroke [125], as well as in healthy middle aged [126] and elderly individuals [127,128,129].
We found one clinical trial on the effects of Centella asiatica in humans with mild cognitive impairment (MCI) (ClinicalTrials.gov, accessed on 20 July 2022; Identifier NCT03937908). In summary, the study aimed to measure the oral bioavailability and pharmacokinetics of known bioactive compounds from a standardized Centella asiatica water extract product in mildly demented elders on cholinesterase inhibitor therapy. However, the trial was terminated before its conclusion.
4.3. Resveratrol
There are a significant number of clinical studies investigating the beneficial effects of resveratrol in human cognition. More than a decade ago, Kennedy et al. assessed the effects of oral resveratrol on cognitive performance and localized cerebral blood flow variables in healthy human adults [130]. In short, resveratrol administration resulted in dose-dependent increases in cerebral blood flow during task performance; however, cognitive function was not affected.
Evans et al. [131] tested whether chronic supplementation with resveratrol could improve cerebrovascular function, cognition and mood in post-menopausal women (aged 45–85 years).
Compared to placebo, resveratrol elicited improvements in the performance of cognitive tasks in the domain of verbal memory and in overall cognitive performance.
More recently, these same authors [132] developed a larger, longer term study to confirm the benefits of resveratrol in post-menopausal women. Compared to placebo, resveratrol improved overall cognitive performance and attenuated the decline in cerebrovascular responsiveness to cognitive stimuli.
Huhn et al. [133] performed a randomized controlled trial to determine the effects of resveratrol on memory performance and to identify potential related mechanisms using blood-based biomarkers, hippocampus connectivity and microstructure assessed with magnetic resonance imaging. Sixty elderly participants (60–79 years) were randomized to receive either resveratrol (200 mg/day) or placebo for 26 weeks (ClinicalTrials.gov, accessed on 20 July 2022: NCT02621554). This interventional study failed to show significant improvements in verbal memory after 6 months of resveratrol in healthy older adults. Similarly, in a study with sedentary and overweight older adults treated with placebo, 300 mg/day resveratrol, or 1000 mg/day resveratrol, Anton et al. [134] observed that 90 days of resveratrol supplementation at a high dose (1000/mg per day) selectively improved psychomotor speed but did not significantly affect other domains of cognitive function in older adults.
Despite the data indicating that resveratrol supplementation might improve select measures of cognitive performance [130,131,132], the current literature seems to be inconsistent and limited [135]. In a meta-analysis of 225 patients concerning the effect of resveratrol on cognitive and memory, Farzaei et al. [136] concluded that resveratrol has no significant impact on factors related to memory and cognitive performance. More randomized controlled trials are needed to achieve more conclusive results.
4.4. Sulforaphane
We identified two recent studies concerning the effects of sulforaphane in human cognition. Liu et al. [137] are developing a double-blind randomized controlled clinical trial to assess the efficacy of sulforaphane for improving cognitive function in patients with frontal brain damage. In this trial (ClinicalTrials.gov, accessed on 20 July 2022: NCT04252261), ninety eligible patients (having cognitive deficits after frontal brain damage) will be randomly allocated to sulforaphane treatment or placebo; they will undergo a series of cognitive and neuropsychiatric tests at baseline at different time points to determine the effect of sulforaphane on cognition. This study will also evaluate brain metabolites markers (including N-acetyl aspartate, glutamate, glutathione and γ-aminobutyric acid) and long-term outcomes of brain trauma, brain tumors and cerebrovascular disease via exploratory analyses. In July 2022, the recruitment status was “Not yet recruiting”.
A very recent clinical trial by Nouchi et al. [138] examined whether combined brain-training (BT) and sulforaphane intake intervention has beneficial effects on cognitive function in older adults.
The authors observed that the BT and sulforaphane treatments separately led to improvements in cognitive functions; the BT group showed a significant improvement in processing speed compared to the active intervention group and the sulforaphane intake groups revealed significant improvements in processing and working memory performance compared to the placebo supplement intake group.
Moreover, sulforaphane administered to 10 patients with schizophrenia induced an improvement in cognitive function, which was evaluated using the Japanese version of CogState battery at the beginning of the study and after 8-weeks of treatment [139]. We also found one ongoing clinical trial on the effects of sulforaphane on cognition in prodromal to mild AD (ClinicalTrials.gov, accessed on 20 July 2022; Identifier NCT04213391). The investigators will evaluate the efficacy, safety and related mechanism of sulforaphane in the treatment of AD patients. The study will recruit 160 AD patients, and then these patients will be randomized to a sulforaphane group or placebo group (80 patients per arm) for a 24-weeks clinic trial. The Alzheimer’s Disease Assessment Scale (ADAS-cog) will be performed at screen/baseline, 4-weeks, 12-weeks and 24-weeks to test the cognition of patients. As of July 2022, the recruitment status was “Recruiting”.
4.5. Epigallocatechin Gallate
We found three clinical studies on the effects of EGCG on human cognition. In 2012, Scholey et al. [140] investigated whether EGCG modulates brain activity and self-reported mood in a double-blind, placebo controlled crossover study. In short, at baseline assessments or at 120 min following the administration of 300 mg EGCG or matched placebo, cognitive and cardiovascular functioning, mood and a resting state electroencephalogram (EEG) were evaluated. The authors observed that EGCG administration significantly increased alpha, beta and theta activity, which was also reflected in overall EEG activity. In comparison to the placebo, the EGCG treatment also increased self-rated calmness and reduced self-rated stress, suggesting that participants receiving EGCG may have been in a more relaxed and attentive state after consuming the compound. It is important to stress that this attentive state was detected after acute EGCG treatment, suggesting that EGCG may act as a potential cognitive enhancer.
Approximately 5 years ago, de la Torre et al. [141] developed a double-blind, randomized, placebo-controlled, phase 2 trial to evaluate whether the administration of a green tea extract containing EGCG would improve the effects of non-pharmacological cognitive rehabilitation in young adults with Down’s syndrome (ClinicalTrials.gov, accessed on 20 July 2022; NCT01699711). After 12 months, EGCG and cognitive training was significantly more effective than placebo and cognitive training at improving visual recognition memory, inhibitory control, and adaptive behavior. No differences were noted in adverse effects between the two treatment groups. The authors state that Phase 3 trials with a larger population of individuals with Down’s syndrome will be needed to assess and confirm the long-term efficacy of EGCG and cognitive training.
Liu et al. [142] performed a single-blind, placebo-controlled, crossover study to examine the effects of green tea extract on working memory in healthy younger (21–29 years) and older (50–63 years) women. The study was characterized by a small number of subjects, whereby twenty non-smoking Caucasian women were recruited in the younger (10) and older (10) age group. Subjects received 5.4 g of green tea extract (at least 45% EGCG) or placebo within a 24-h period. Green tea extract significantly improved reading span performance in older women (higher absolute and partial scores of reading span), although no significant changes were observed in the younger group.
Among the three aforementioned studies concerning the effects of EGCG in human cognition, two of them suggested that short-term EGCG treatment can display significant effects in cognition, leading to an attentive state [140] and improving reading span performance [142]. Concerning the study by de la Torre et al. [141], the assumed beneficial effects of EGCG (combined with cognitive training) in visual recognition memory, inhibitory control, and adaptive behavior of young adults with Down’s syndrome seemed to open a new therapeutic possibility, although additional trials with a larger population of individuals with Down’s syndrome will be needed to better assess this issue.
4.6. Dimethylfumarate
In 2013, DMF was approved by FDA to treat patients with relapsing forms of multiple sclerosis (MS) [143]. Despite the significant amount of data showing that DMF is able to improve (or prevent the decline of) cognition in experimental animal models, we found only one clinical study concerning DMF and cognitive function in humans.
Amato et al. [144] performed a clinical study to evaluate the effect of 2-year treatment with oral DMF on cognition in relapsing remitting MS (RRMS).
In short, the authors concluded that the 2-years treatment with DMF was associated with the slowing of cognitive impairment and with significant improvements in quality of life and psychosocial function. Based on the mechanism of action of DMF, as well as on the fact that early and progressive cognitive decline in patients with MS represents a consequence of an immune-mediated inflammatory condition leading to demyelination and synaptic loss [145], the results of Amato et al. [144] strongly suggest that DMF was able to mitigate the predicted cognitive decline resulting from neuroinflammatory and neurodegenerative events that occur in MS, rather than acting as a cognitive enhancer.
4.7. Extract of Boswellia Species
MS patients were recruited in a randomized, double-blinded, and placebo-controlled study (IRCT2013070813911N1), to evaluate the effects of Boswellia papyrifera administration (300 mg, twice daily per os) on visuospatial and verbal memory and also information processing speed. After 2 months, only visuospatial memory was improved in patients treated in comparison to placebo [146]. However, the trial suffered from several weaknesses including that it involved only 38 patients and neither the chemical composition of Boswellia papyrifera with information on the percentage of the AKBA and BA nor a description of capsule production were reported.
5. Potential Use of NRF2 Activators to Treat Cognitive Impairment Related Conditions: Drawbacks and Perspectives
In this review, we summarized the substances used in attempts to stimulate NRF2 in different in vitro and in vivo models. Although promising, only some of the beneficial effects of these agents were detected in clinical testing (Table 1).
Table 1.
Evidence from substances treatment supporting a role for NRF2 in maintaining and improving cognitive function. In this table are reported a summary of results from substance tested in vitro and/or in vivo and in clinical trials.
| Compound | In Vitro | In Vivo | Clinical Trial |
|---|---|---|---|
| Curcumin | Induction of neuronal differentiation and neuroprotective effects in PC12 cells [45]. |
Improvement of cognitive function in mouse models of Aβ accumulation [70,71]. |
Conflicting results [116,117,118,119,120,121,122,123]. |
| Centella asiatica | Reverse deficits in dendritic arborization and spine density in neurons isolated from the Tg2576 mouse model of Aβ accumulation, in wild-type neurons and in MC65 neuroblastoma cells [38,39]. |
Improvement of learning, memory and executive function in mouse models of both healthy aging and Aβ accumulation [67,68,69]. |
Increase in the general mental ability, attention and concentration of mentally retarded children. Improvement in adult humans after stroke, in healthy middle age and elderly individuals [124,125,126,127,128,129]. ClinicalTrials.gov, accessed on 20 July 2022: NCT03937908. |
| Resveratrol | None | Attenuation of cognitive impairment in a mouse models of traumatic brain injury [95,96]. | Data are inconsistent and limited [130,131,132,133,134,135,136]. |
| Sulforaphane | Neuronal and endothelial death reduction in primary brain endothelial cultures [49,50,51]. | Reduction of Aβ accumulation and amelioration of cognitive deficits in the 5xFAD and the 3xTgAD mouse line [51,61,72,73]. |
Data are inconsistent and limited [137,138,139]. |
| Epigallocatechin gallate | None | Improvement of cognitive deficits induced from Aβ overexpression mouse model, high-fat diet or high-fructose consumption. Improvement of cognitive deficits also in healthy aging [89,90,91,92]. |
Three clinical studies. Short-term treatment improved cognition. Beneficial effects in visual recognition memory, inhibitory control, and adaptive behavior of young adults with Down’s syndrome [140,141,142]. |
| Dimethylfumarate | None | Improvement of cognitive performances in autoimmune encephalomyelitis mouse model of multiple sclerosis, in aged rats treated with streptozotocin, in a mouse model of subarachnoid hemorrhage, in a model of sepsis, in a double transgenic model of Aβ and tau accumulation and in a model of traumatic brain injury [99,100,101,102,103,104]. |
Only one clinical study. The 2-year treatment was associated with slowing of cognitive impairment [144]. |
| Extract Boswellia | None | Alleviation of cognitive deficiencies in APPswe/PS1dE9 mice (AKBA treatment) [76]. |
One clinical study [146]. Data are inconsistent and limited. |
Cognitive deficits resulting from neurodegenerative conditions (commonly characterized by atrophy and/or loss of neuronal cell function) are unlikely to recover unless significant neuroplasticity or neurogenesis occurs. Considering that NRF2 is a transcription factor that orchestrates the expression of cytoprotective genes [147] and that mRNA and protein synthesis represent events dependent on adequate cellular metabolic homeostasis, which is compromised in neurodegenerating neurons, the potential therapeutic use of NRF2 activators to treat cognitive impairment resulting from neurodegenerative conditions appears to be unlikely. In this regard, although there is a growing body of experimental evidence pointing to NRF2 activation as a strategy to combat neurodegeneration and improve cognition (see Section 3), it appears that the reported beneficial effects of NRF2 activators in these studies primarily result from the prevention of neurotoxicity, neuroinflammation and/or neurodegeneration. According to this perspective, to the best of our knowledge, there is no scientific evidence pointing to the beneficial clinical effects of NRF2 activators in improving cognitive deficits in patients with neurodegenerative diseases.
When reviewing some reflections related to the available clinical literature on the effects of NRF2 activators in human cognition, it is important to mention that cognitive decline, which represents an expected consequence even among relatively healthy “successful agers”, is associated with structural, functional, and metabolic brain changes whose mechanisms involve oxidative stress and neuroinflammation [148]. Moreover, cognitive decline is commonly more intense in dementia frontotemporal and with Lewy bodies but also in individuals with specific diseases, such Alzheimer’s disease [149], Parkinson’s disease [150,151], type 2 diabetes mellitus [152], and Huntington’s disease [153], among others. Based on the well-known antioxidant and anti-inflammatory events resulting from NRF2 activation [154], some of the aforementioned clinical evidence on the beneficial effects of NRF2 in human cognition strongly suggest that the described NRF2-based interventions were able to prevent cognitive decline rather than directly improve cognition.
In this context, it is important to note the clear distinction between cognitive enhancers and disease modifying strategies [155]. Particularly in the clinical studies involving patients (MS, mental retardation, Down’s syndrome) [124,141,144] or elderly subjects [116,119,122,126,127,128,129,131,132,138], it is likely that, due to the antioxidant and anti-inflammatory effects resulting from NRF2-related downstream proteins, the upregulation of this pathway prevented cognitive decline directly by preventing neurodegeneration, thus displaying an indirect effect in promoting cognition.
On the other hand, some clinical studies observed improvements in cognition even after acute treatments with NRF2 activators (particularly, EGCG) [140,142], pointing to the direct effects of this compound in human cognition (not necessarily linked to the prevention of neurodegenerative events). Considering the acute effect of EGCG on cognitive ability, one may posit that this compound directly enhances cognition in humans. Even though this hypothesis seems reasonable, the available studies [140,142] do not allow for the conclusion that such improvements in cognition were specifically the result of NRF2-mediating events. In fact, the direct acute effect of EGCG in modulating the neurotransmitter/synaptic homeostasis on cognition via NRF2-independent mechanisms cannot be ruled out [156]. In conclusion, the summarized in vivo and clinical studies suggest that some NRF2 activators (described in Section 3 and Section 4) can improve cognition, especially under specific neuropathological conditions, due to their ability to prevent neurodegeneration. However, the potential clinical use of NRF2 activators to treat cognitive impairment after neurodegeneration has occurred appears to be unlikely.
Author Contributions
Conceptualization, N.E.G., M.F., L.S. and P.T.; methodology and literature search, N.E.G., L.S., M.F. and P.T.; writing—original draft preparation, N.E.G., L.S., M.F. and P.T.; writing—review and editing, N.E.G., L.S., M.F. and P.T. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seminotti B., Grings M., Tucci P., Leipnitz G., Saso L. Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell. Neurosci. 2021;15:785057. doi: 10.3389/fncel.2021.785057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X., Xi Z., Liang H., Sun Y., Zhong Z., Wang B., Bian L., Sun Q. Melatonin Prevents Mice Cortical Astrocytes from Hemin-Induced Toxicity Through Activating PKCalpha/Nrf2/HO-1 Signaling in vitro. Front. Neurosci. 2019;13:760. doi: 10.3389/fnins.2019.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X., Song D., Cheng Y., Hu Y., Wang F., Lu Z., Wang Y. Biogenic nanoselenium particles activate Nrf2-ARE pathway by phosphorylating p38, ERK1/2, and AKT on IPEC-J2 cells. J. Cell. Physiol. 2019;234:11227–11234. doi: 10.1002/jcp.27773. [DOI] [PubMed] [Google Scholar]
- 5.Yang T., Sun Y., Mao L., Zhang M., Li Q., Zhang L., Shi Y., Leak R.K., Chen J., Zhang F. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018;17:323–337. doi: 10.1016/j.redox.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 7.Zolnourian A., Galea I., Bulters D. Neuroprotective Role of the Nrf2 Pathway in Subarachnoid Haemorrhage and Its Therapeutic Potential. Oxidative Med. Cell. Longev. 2019;2019:6218239. doi: 10.1155/2019/6218239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinkova-Kostova A., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi G., Jasoliya M., Sahdeo S., Saccà F., Pane C., Filla A., Marsili A., Puorro G., Lanzillo R., Brescia Morra V., et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017;26:2864–2873. doi: 10.1093/hmg/ddx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdullah A., Kitteringham N.R., Jenkins R.E., Goldring C., Higgins L., Yamamoto M., Hayes J., Park B.K. Analysis of the role of Nrf2 in the expression of liver proteins in mice using two-dimensional gel-based proteomics. Pharmacol. Rep. 2012;64:680–697. doi: 10.1016/S1734-1140(12)70863-0. [DOI] [PubMed] [Google Scholar]
- 11.Agyeman A.S., Chaerkady R., Shaw P.G., Davidson N.E., Visvanathan K., Pandey A., Kensler T.W. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012;132:175–187. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku H.J., Park J.W. Downregulation of IDH2 exacerbates H2O2-mediated cell death and hypertrophy. Redox Rep. 2017;22:35–41. doi: 10.1080/13510002.2015.1135581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan B., Ezerina D., Amoako T.N., Riemer J., Seedorf M., Dick T.P. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat. Chem. Biol. 2013;9:119–125. doi: 10.1038/nchembio.1142. [DOI] [PubMed] [Google Scholar]
- 14.Cho H.Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S.P., Kleeberger S.R. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2010;182:170–182. doi: 10.1164/rccm.200907-1047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K., Gao X., Wei W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017;361:63–72. doi: 10.1016/j.yexcr.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Lai L., Wang M., Martin O.J., Leone T.C., Vega R.B., Han X., Kelly D.P. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J. Biol. Chem. 2014;289:2250–2259. doi: 10.1074/jbc.M113.523654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping Z., Zhang L.F., Cui Y.J., Chang Y.M., Jiang C.W., Meng Z.Z., Xu P., Liu H.Y., Wang D.Y., Cao X.B. The Protective Effects of Salidroside from Exhaustive Exercise-Induced Heart Injury by Enhancing the PGC-1 alpha -NRF1/NRF2 Pathway and Mitochondrial Respiratory Function in Rats. Oxidative Med. Cell. Longev. 2015;2015:876825. doi: 10.1155/2015/876825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song N.Y., Lee Y.H., Na H.K., Baek J.H., Surh Y.J. Leptin induces SIRT1 expression through activation of NF-E2-related factor 2: Implications for obesity-associated colon carcinogenesis. Biochem. Pharmacol. 2018;153:282–291. doi: 10.1016/j.bcp.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Suzen S., Tucci P., Profumo E., Buttari B., Saso L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals. 2022;15:692. doi: 10.3390/ph15060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellezza I., Tucci A., Galli F., Grottelli S., Mierla A.L., Pilolli F., Minelli A. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012;23:1583–1591. doi: 10.1016/j.jnutbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.F., Kuo H.P., Liu M., Chou C.K., Xia W., Du Y., Shen J., Chen C.T., Huo L., Hsu M.C., et al. KEAP1 E3 ligase-mediated downregulation of NF-κB signaling by targeting IKKbeta. Mol. Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii T., Itoh K., Ruiz E., Leake D.S., Unoki H., Yamamoto M., Mann G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 23.Saddawi-Konefka R., Seelige R., Gross E.T., Levy E., Searles S.C., Washington A., Jr., Santosa E.K., Liu B., O’Sullivan T.E., Harismendy O., et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 25.Wu M., Gibbons J.G., DeLoid G.M., Bedugnis A.S., Thimmulappa R.K., Biswal S., Kobzik L. Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L138–L153. doi: 10.1152/ajplung.00075.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. 2010;690:57–63. doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deus C.M., Teixeira J., Raimundo N., Tucci P., Borges F., Saso L., Oliveira P.J. Modulation of cellular redox environment as a novel therapeutic strategy for Parkinson’s disease. Eur. J. Clin. Investig. 2022:e13820. doi: 10.1111/eci.13820. [DOI] [PubMed] [Google Scholar]
- 29.Saha S., Buttari B., Profumo E., Tucci P., Saso L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2021;15:787258. doi: 10.3389/fncel.2021.787258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terry R.D., Katzman R. Life span and synapses: Will there be a primary senile dementia? Neurobiol. Aging. 2001;22:347–348; discussion 353–354. doi: 10.1016/S0197-4580(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 31.Montine T.J., Bukhari S.A., White L.R. Cognitive Impairment in Older Adults and Therapeutic Strategies. Pharmacol. Rev. 2021;73:152–162. doi: 10.1124/pharmrev.120.000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki M., Izuo N., Izumi Y., Takada-Takatori Y., Akaike A., Kume T. Protective Effect of Green Perilla-Derived Chalcone Derivative DDC on Amyloid β Protein-Induced Neurotoxicity in Primary Cortical Neurons. Biol. Pharm. Bull. 2019;42:1942–1946. doi: 10.1248/bpb.b19-00657. [DOI] [PubMed] [Google Scholar]
- 33.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Wang C.-Y., Zhang Q., Xun Z., Yuan L., Li R., Li X., Tian S.-Y., Xin N., Xu Y. Increases of iASPP-Keap1 interaction mediated by syringin enhance synaptic plasticity and rescue cognitive impairments via stabilizing Nrf2 in Alzheimer’s models. Redox Biol. 2020;36:101672. doi: 10.1016/j.redox.2020.101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eftekharzadeh B., Maghsoudi N., Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie. 2010;92:245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Guo Y., Wang H., Zhao L., Ma Z., Li T., Liu J., Sun M., Jian Y., Yao L., et al. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci. 2019;221:259–266. doi: 10.1016/j.lfs.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 37.Zweig J.A., Brandes M.S., Brumbach B.H., Caruso M., Wright K.M., Quinn J.F., Soumyanath A., Gray N.E. Prolonged Treatment with Centella asiatica Improves Memory, Reduces Amyloid-β Pathology, and Activates NRF2-Regulated Antioxidant Response Pathway in 5xFAD Mice. J. Alzheimer’s Dis. 2021;81:1453–1468. doi: 10.3233/JAD-210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray N.E., Zweig J.A., Murchison C., Caruso M., Matthews D.G., Kawamoto C., Harris C.J., Quinn J.F., Soumyanath A. Centella asiatica attenuates Aβ-induced neurodegenerative spine loss and dendritic simplification. Neurosci. Lett. 2017;646:24–29. doi: 10.1016/j.neulet.2017.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray N.E., Sampath H., Zweig J.A., Quinn J.F., Soumyanath A. Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2015;45:933–946. doi: 10.3233/JAD-142217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray N.E., Alcazar Magana A., Lak P., Wright K.M., Quinn J., Stevens J.F., Maier C.S., Soumyanath A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018;17:161–194. doi: 10.1007/s11101-017-9528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi Z., Ci X., Huang J., Liu Q., Yu Q., Zhou J., Deng X. Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed. Pharmacother. 2017;88:252–259. doi: 10.1016/j.biopha.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 42.Wang W., Wu L., Li Q., Zhang Z., Xu L., Lin C., Gao L., Zhao K., Liang F., Zhang Q., et al. Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018;103:1137–1145. doi: 10.1016/j.biopha.2018.04.162. [DOI] [PubMed] [Google Scholar]
- 43.Cao X., Xiao H., Zhang Y., Zou L., Chu Y., Chu X. 1,5-Dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes. Brain Res. 2010;1347:142–148. doi: 10.1016/j.brainres.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 44.Moosavi F., Hosseini R., Saso L., Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2016;10:23–42. doi: 10.2147/DDDT.S96936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal N.B., Jain S., Agarwal N.K., Mediratta P.K., Sharma K.K. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine. 2011;18:756–759. doi: 10.1016/j.phymed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Xu J., Zhou L., Weng Q., Xiao L., Li Q. Curcumin analogues attenuate Aβ25-35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem. Biol. Interact. 2019;305:171–179. doi: 10.1016/j.cbi.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Meng X., Wang M., Sun G., Ye J., Zhou Y., Dong X., Wang T., Lu S., Sun X. Attenuation of Aβ25-35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharmacol. 2014;279:63–75. doi: 10.1016/j.taap.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Helman A.M., Murphy M.P. Vascular cognitive impairment: Modeling a critical neurologic disease in vitro and in vivo. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016;1862:975–982. doi: 10.1016/j.bbadis.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro T.A., Fahey J.W., Wade K.L., Stephenson K.K., Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol. Biomark. Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 50.Atanasov A.G., Zotchev S.B., Dirsch V.M., International Natural Product Sciences Taskforce. Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L., Yang T., Li X., Lei X., Sun Y., Zhao Y., Zhang W., Gao Y., Sun B., Zhang F. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 2019;39:352–366. doi: 10.1177/0271678X18764083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheyne J.E., Montgomery J.M. The cellular and molecular basis of in vivo synaptic plasticity in rodents. Am. J. Physiol.-Cell Physiol. 2020;318:C1264–C1283. doi: 10.1152/ajpcell.00416.2019. [DOI] [PubMed] [Google Scholar]
- 53.Uddin M.S., Mamun A.A., Rahman M.M., Jeandet P., Alexiou A., Behl T., Sarwar M.S., Sobarzo-Sánchez E., Ashraf G.M., Sayed A.A., et al. Natural Products for Neurodegeneration: Regulating Neurotrophic Signals. Oxidative Med. Cell. Longev. 2021;2021:8820406. doi: 10.1155/2021/8820406. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Davies D.A., Adlimoghaddam A., Albensi B.C. Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer’s Disease. Cells. 2021;10:1884. doi: 10.3390/cells10081884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis J.E., Poles J., Shaw D.P., Karhu E., Khan S.A., Lyons A.E., Sacco S.B., McDaniel H.R. The effects of twenty-one nutrients and phytonutrients on cognitive function: A narrative review. J. Clin. Transl. Res. 2021;7:575–620. [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh K., Kosaka K., Mimura J., Satoh T. Role of nrf2 in the neurotrophic action of the electrophiles. Hirosaki Med. J. 2010;61:S63–S69. [Google Scholar]
- 57.Lin C.-W., Wu M.-J., Liu I.Y.C., Su J.-D., Yen J.-H. Neurotrophic and Cytoprotective Action of Luteolin in PC12 Cells through ERK-Dependent Induction of Nrf2-Driven HO-1 Expression. J. Agric. Food Chem. 2010;58:4477–4486. doi: 10.1021/jf904061x. [DOI] [PubMed] [Google Scholar]
- 58.Gergues M.M., Moiseyenko A., Saad S.Z., Kong A.N., Wagner G.C. Nrf2 deletion results in impaired performance in memory tasks and hyperactivity in mature and aged mice. Brain Res. 2018;1701:103–111. doi: 10.1016/j.brainres.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zweig J.A., Brandes M.S., Brumbach B.H., Caruso M., Wright K.M., Quinn J.F., Soumyanath A., Gray N.E. Loss of NRF2 accelerates cognitive decline, exacerbates mitochondrial dysfunction, and is required for the cognitive enhancing effects of Centella asiatica during aging. Neurobiol. Aging. 2021;100:48–58. doi: 10.1016/j.neurobiolaging.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zweig J.A., Caruso M., Brandes M.S., Gray N.E. Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp. Gerontol. 2020;131:110767. doi: 10.1016/j.exger.2019.110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahn G., Park J.S., Yun U.J., Lee Y.J., Choi Y., Park J.S., Baek S.H., Choi B.Y., Cho Y.S., Kim H.K., et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA. 2019;116:12516–12523. doi: 10.1073/pnas.1819541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Branca C., Ferreira E., Nguyen T.V., Doyle K., Caccamo A., Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2017;26:4823–4835. doi: 10.1093/hmg/ddx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren P., Chen J., Li B., Zhang M., Yang B., Guo X., Chen Z., Cheng H., Wang P., Wang S., et al. Nrf2 Ablation Promotes Alzheimer’s Disease-Like Pathology in APP/PS1 Transgenic Mice: The Role of Neuroinflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2020;2020:3050971. doi: 10.1155/2020/3050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rojo A.I., Pajares M., Rada P., Nunez A., Nevado-Holgado A.J., Killik R., Van Leuven F., Ribe E., Lovestone S., Yamamoto M., et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017;13:444–451. doi: 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Kelly M.G., Yang X.R., Fernandez T.G., Wierzbicki E.L., Skrobach A., Dore S. Nrf2 Deficiency Exacerbates Cognitive Impairment and Reactive Microgliosis in a Lipopolysaccharide-Induced Neuroinflammatory Mouse Model. Cell. Mol. Neurobiol. 2020;40:1185–1197. doi: 10.1007/s10571-020-00807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L., Vollmer M.K., Fernandez V.M., Dweik Y., Kim H., Dore S. Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front. Cell. Neurosci. 2018;12:74. doi: 10.3389/fncel.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray N.E., Harris C.J., Quinn J.F., Soumyanath A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol. 2016;180:78–86. doi: 10.1016/j.jep.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray N.E., Zweig J.A., Caruso M., Martin M.D., Zhu J.Y., Quinn J.F., Soumyanath A. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav. 2018;8:e01024. doi: 10.1002/brb3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gray N.E., Zweig J.A., Caruso M., Zhu J.Y., Wright K.M., Quinn J.F., Soumyanath A. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice. Mol. Cell. Neurosci. 2018;93:1–9. doi: 10.1016/j.mcn.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu J., Liu J., Li Q., Mi Y., Zhou D., Meng Q., Chen G., Li N., Hou Y. Pterostilbene Alleviates Abeta1-42 -Induced Cognitive Dysfunction via Inhibition of Oxidative Stress by Activating Nrf2 Signaling Pathway. Mol. Nutr. Food Res. 2021;65:e2000711. doi: 10.1002/mnfr.202000711. [DOI] [PubMed] [Google Scholar]
- 71.Zheng K., Dai X., Xiao N., Wu X., Wei Z., Fang W., Zhu Y., Zhang J., Chen X. Curcumin Ameliorates Memory Decline via Inhibiting BACE1 Expression and beta-Amyloid Pathology in 5xFAD Transgenic Mice. Mol. Neurobiol. 2017;54:1967–1977. doi: 10.1007/s12035-016-9802-9. [DOI] [PubMed] [Google Scholar]
- 72.Dash P.K., Zhao J., Orsi S.A., Zhang M., Moore A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009;460:103–107. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pu D., Zhao Y., Chen J., Sun Y., Lv A., Zhu S., Luo C., Zhao K., Xiao Q. Protective Effects of Sulforaphane on Cognitive Impairments and AD-like Lesions in Diabetic Mice are Associated with the Upregulation of Nrf2 Transcription Activity. Neuroscience. 2018;381:35–45. doi: 10.1016/j.neuroscience.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Ding Y., Chen M., Wang M., Li Y., Wen A. Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 75.Ding Y., Chen M., Wang M., Wang M., Zhang T., Park J., Zhu Y., Guo C., Jia Y., Li Y., et al. Neuroprotection by acetyl-11-keto-beta-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci. Rep. 2014;4:7002. doi: 10.1038/srep07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei C., Fan J., Sun X., Yao J., Guo Y., Zhou B., Shang Y. Acetyl-11-keto-beta-boswellic acid ameliorates cognitive deficits and reduces amyloid-beta levels in APPswe/PS1dE9 mice through antioxidant and anti-inflammatory pathways. Free Radic. Biol. Med. 2020;150:96–108. doi: 10.1016/j.freeradbiomed.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 77.D’Amico R., Trovato Salinaro A., Fusco R., Cordaro M., Impellizzeri D., Scuto M., Ontario M.L., Lo Dico G., Cuzzocrea S., Di Paola R., et al. Hericium erinaceus and Coriolus versicolor Modulate Molecular and Biochemical Changes after Traumatic Brain Injury. Antioxidants. 2021;10:898. doi: 10.3390/antiox10060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang X., Jiang Y., Ji H., Zhao L., Xiao W., Wang Z., Ding G. The Synergistic Beneficial Effects of Ginkgo Flavonoid and Coriolus versicolor Polysaccharide for Memory Improvements in a Mouse Model of Dementia. Evid. Based Complement. Altern. Med. 2015;2015:128394. doi: 10.1155/2015/128394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trovato A., Siracusa R., Di Paola R., Scuto M., Ontario M.L., Bua O., Di Mauro P., Toscano M.A., Petralia C.C.T., Maiolino L., et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing. 2016;13:23. doi: 10.1186/s12979-016-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai Y.C., Lin Y.C., Huang C.C., Villaflores O.B., Wu T.Y., Huang S.M., Chin T.Y. Hericium erinaceus Mycelium and Its Isolated Compound, Erinacine A, Ameliorate High-Fat High-Sucrose Diet-Induced Metabolic Dysfunction and Spatial Learning Deficits in Aging Mice. J. Med. Food. 2019;22:469–478. doi: 10.1089/jmf.2018.4288. [DOI] [PubMed] [Google Scholar]
- 81.Liu Q., Jin Z., Xu Z., Yang H., Li L., Li G., Li F., Gu S., Zong S., Zhou J., et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24:441–452. doi: 10.1007/s12192-019-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Lv J., Jiang N., Huang H., Wang Q., Liu X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother. Res. 2021;35:2523–2535. doi: 10.1002/ptr.6947. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., Wang Z., Li B., Qiang Y., Yuan T., Tan X., Wang Z., Liu Z., Liu X. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut-liver-brain axis. Int. J. Obes. 2019;43:1735–1746. doi: 10.1038/s41366-018-0277-9. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z., Fan J., Wang J., Li Y., Xiao L., Duan D., Wang Q. Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci. Lett. 2016;627:185–191. doi: 10.1016/j.neulet.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Yu L., Wang W., Pang W., Xiao Z., Jiang Y., Hong Y. Dietary Lycopene Supplementation Improves Cognitive Performances in Tau Transgenic Mice Expressing P301L Mutation via Inhibiting Oxidative Stress and Tau Hyperphosphorylation. J. Alzheimer’s Dis. 2017;57:475–482. doi: 10.3233/JAD-161216. [DOI] [PubMed] [Google Scholar]
- 86.Zhao B., Liu H., Wang J., Liu P., Tan X., Ren B., Liu Z., Liu X. Lycopene Supplementation Attenuates Oxidative Stress, Neuroinflammation, and Cognitive Impairment in Aged CD-1 Mice. J. Agric. Food Chem. 2018;66:3127–3136. doi: 10.1021/acs.jafc.7b05770. [DOI] [PubMed] [Google Scholar]
- 87.Zhao B., Ren B., Guo R., Zhang W., Ma S., Yao Y., Yuan T., Liu Z., Liu X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 88.Park D.J., Kang J.B., Shah M.A., Koh P.O. Epigallocatechin Gallate Alleviates Down-Regulation of Thioredoxin in Ischemic Brain Damage and Glutamate-Exposed Neuron. Neurochem. Res. 2021;46:3035–3049. doi: 10.1007/s11064-021-03403-0. [DOI] [PubMed] [Google Scholar]
- 89.Bao J., Liu W., Zhou H.Y., Gui Y.R., Yang Y.H., Wu M.J., Xiao Y.F., Shang J.T., Long G.F., Shu X.J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020;40:18–27. doi: 10.1007/s11596-020-2142-z. [DOI] [PubMed] [Google Scholar]
- 90.Lee J.W., Lee Y.K., Ban J.O., Ha T.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κB pathways in mice. J. Nutr. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 91.Mi Y., Qi G., Fan R., Qiao Q., Sun Y., Gao Y., Liu X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017;31:4998–5011. doi: 10.1096/fj.201700400RR. [DOI] [PubMed] [Google Scholar]
- 92.Wei B.B., Liu M.Y., Zhong X., Yao W.F., Wei M.J. Increased BBB permeability contributes to EGCG-caused cognitive function improvement in natural aging rats: Pharmacokinetic and distribution analyses. Acta Pharmacol. Sin. 2019;40:1490–1500. doi: 10.1038/s41401-019-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J.Y., Zhu Q., Zhang S., OuYang D., Lu J.H. Resveratrol in experimental Alzheimer’s disease models: A systematic review of preclinical studies. Pharmacol. Res. 2019;150:104476. doi: 10.1016/j.phrs.2019.104476. [DOI] [PubMed] [Google Scholar]
- 94.Su C.F., Jiang L., Zhang X.W., Iyaswamy A., Li M. Resveratrol in Rodent Models of Parkinson’s Disease: A Systematic Review of Experimental Studies. Front. Pharmacol. 2021;12:644219. doi: 10.3389/fphar.2021.644219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi Z., Qiu W., Xiao G., Cheng J., Zhang N. Resveratrol Attenuates Cognitive Deficits of Traumatic Brain Injury by Activating p38 Signaling in the Brain. Med. Sci. Monit. 2018;24:1097–1103. doi: 10.12659/MSM.909042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X., Fang H., Xu G., Yang Y., Xu R., Liu Q., Xue X., Liu J., Wang H. Resveratrol Prevents Cognitive Impairment in Type 2 Diabetic Mice by Upregulating Nrf2 Expression and Transcriptional Level. Diabetes Metab. Syndr. Obes. 2020;13:1061–1075. doi: 10.2147/DMSO.S243560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gold R., Kappos L., Arnold D.L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M.T., Yang M., Sheikh S.I., et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 98.Scuderi S.A., Ardizzone A., Paterniti I., Esposito E., Campolo M. Antioxidant and Anti-inflammatory Effect of Nrf2 Inducer Dimethyl Fumarate in Neurodegenerative Diseases. Antioxidants. 2020;9:630. doi: 10.3390/antiox9070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das Neves S.P., Santos G., Barros C., Pereira D.R., Ferreira R., Mota C., Monteiro S., Fernandes A., Marques F., Cerqueira J.J. Enhanced cognitive performance in experimental autoimmune encephalomyelitis mice treated with dimethyl fumarate after the appearance of disease symptoms. J. Neuroimmunol. 2020;340:577163. doi: 10.1016/j.jneuroim.2020.577163. [DOI] [PubMed] [Google Scholar]
- 100.Majkutewicz I., Kurowska E., Podlacha M., Myslinska D., Grembecka B., Rucinski J., Pierzynowska K., Wrona D. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018;1686:19–33. doi: 10.1016/j.brainres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y., Qiu J., Wang Z., You W., Wu L., Ji C., Chen G. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J. Neurosurg. 2015;123:915–923. doi: 10.3171/2014.11.JNS132348. [DOI] [PubMed] [Google Scholar]
- 102.Zarbato G.F., de Souza Goldim M.P., Giustina A.D., Danielski L.G., Mathias K., Florentino D., de Oliveira Junior A.N., da Rosa N., Laurentino A.O., Trombetta T., et al. Dimethyl Fumarate Limits Neuroinflammation and Oxidative Stress and Improves Cognitive Impairment After Polymicrobial Sepsis. Neurotox. Res. 2018;34:418–430. doi: 10.1007/s12640-018-9900-8. [DOI] [PubMed] [Google Scholar]
- 103.Rojo A.I., Pajares M., Garcia-Yague A.J., Buendia I., Van Leuven F., Yamamoto M., Lopez M.G., Cuadrado A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018;18:173–180. doi: 10.1016/j.redox.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casili G., Campolo M., Paterniti I., Lanza M., Filippone A., Cuzzocrea S., Esposito E. Dimethyl Fumarate Attenuates Neuroinflammation and Neurobehavioral Deficits Induced by Experimental Traumatic Brain Injury. J. Neurotrauma. 2018;35:1437–1451. doi: 10.1089/neu.2017.5260. [DOI] [PubMed] [Google Scholar]
- 105.Wang Z., Ji C., Wu L., Qiu J., Li Q., Shao Z., Chen G. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: Role of Keap1/Nrf2/ARE pathway. PLoS ONE. 2014;9:e97685. doi: 10.1371/journal.pone.0097685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saykally J.N., Rachmany L., Hatic H., Shaer A., Rubovitch V., Pick C.G., Citron B.A. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience. 2012;223:305–314. doi: 10.1016/j.neuroscience.2012.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Z.W., Liang J., Yan J.X., Ye Y.C., Wang J.J., Chen C., Sun H.T., Chen F., Tu Y., Li X.H. TBHQ improved neurological recovery after traumatic brain injury by inhibiting the overactivation of astrocytes. Brain Res. 2020;1739:146818. doi: 10.1016/j.brainres.2020.146818. [DOI] [PubMed] [Google Scholar]
- 108.Donelli D., Antonelli M., Firenzuoli F. Considerations about Turmeric-Associated Hepatotoxicity Following a Series of Cases Occurred in Italy: Is Turmeric Really a New Hepatotoxic Substance? Intern. Emerg. Med. 2020;15:725–726. doi: 10.1007/s11739-019-02145-w. [DOI] [PubMed] [Google Scholar]
- 109.Luber R.P., Rentsch C., Lontos S., Pope J.D., Aung A.K., Schneider H.G., Kemp W., Roberts S.K., Majeed A. Turmeric Induced Liver Injury: A Report of Two Cases. Case Rep. Hepatol. 2019;2019:6741213. doi: 10.1155/2019/6741213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nash E., Sabih A.H., Chetwood J., Wood G., Pandya K., Yip T., Majumdar A., McCaughan G.W., Strasser S.I., Liu K. Drug-induced liver injury in Australia, 2009–2020: The increasing proportion of non-paracetamol cases linked with herbal and dietary supplements. Med. J. Aust. 2021;215:261–268. doi: 10.5694/mja2.51173. [DOI] [PubMed] [Google Scholar]
- 111.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 112.Zhu Y., Bu Q., Liu X., Hu W., Wang Y. Neuroprotective effect of TAT-14-3-3epsilon fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS ONE. 2014;9:e93334. doi: 10.1371/journal.pone.0093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Epstein J., Sanderson I., Macdonald T. Curcumin as a Therapeutic Agent: The Evidence from in Vitro, Animal and Human Studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 114.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turpaev K.T. Keap1-Nrf2 signaling pathway: Mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry. 2013;78:111–126. doi: 10.1134/S0006297913020016. [DOI] [PubMed] [Google Scholar]
- 116.Ng T.P., Chiam P.C., Lee T., Chua H.C., Lim L., Kua E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- 117.Baum L., Lam C.W., Cheung S.K., Kwok T., Lui V., Tsoh J., Lam L., Leung V., Hui E., Ng C., et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 118.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., Gylys K.H., Badmaev V., Heath D.D., Apostolova L.G., et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cox K.H., Pipingas A., Scholey A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015;29:642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 120.Gota V.S., Maru G.B., Soni T.G., Gandhi T.R., Kochar N., Agarwal M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010;58:2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- 121.Rainey-Smith S.R., Brown B.M., Sohrabi H.R., Shah T., Goozee K.G., Gupta V.B., Martins R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016;115:2106–2113. doi: 10.1017/S0007114516001203. [DOI] [PubMed] [Google Scholar]
- 122.Small G.W., Siddarth P., Li Z., Miller K.J., Ercoli L., Emerson N.D., Martinez J., Wong K.P., Liu J., Merrill D.A., et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry. 2018;26:266–277. doi: 10.1016/j.jagp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 123.Cox K.H.M., White D.J., Pipingas A., Poorun K., Scholey A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients. 2020;12:1678. doi: 10.3390/nu12061678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Appa Rao V., Srinivasan K., Koteswara Rao T. The Effect of Centella asiatica on The General Mental Ability of Mentally Retarded Children. Indian J. Psychiatry. 1977;19:54–59. [Google Scholar]
- 125.Farhana K.M., Malueka R.G., Wibowo S., Gofir A. Effectiveness of Gotu Kola Extract 750 mg and 1000 mg Compared with Folic Acid 3 mg in Improving Vascular Cognitive Impairment after Stroke. Evid. Based Complement. Altern. Med. 2016;2016:2795915. doi: 10.1155/2016/2795915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dev Omar Dev R., Mohamed S., Hambali Z., Abu Samah B. Comparison on Cognitive Effects of Centella asiatica in Healthy Middle Age Female and Male Volunteers. Eur. J. Sci. Res. 2009;31:553–565. [Google Scholar]
- 127.Roushan R., Tiwari S., Gehlot S., Gambhir I. Response of Centella asiatica in the management of age related problems among elderly with special reference to cognitive problems as per Prakriti. Int. J. Res. Ayurveda Pharm. 2013;4:163–167. doi: 10.7897/2277-4343.04215. [DOI] [Google Scholar]
- 128.Tiwari S., Singha S., Patwardhan K., Gehlot S., Gambhir I. Effect of Centella asiatica on Mild Cognitive Impairment MCI and Other Common Age Related Clinical Problems. Dig. J. Nanomater. Biostruct. 2008;3:215–220. [Google Scholar]
- 129.Wattanathorn J., Mator L., Muchimapura S., Tongun T., Pasuriwong O., Piyawatkul N., Yimtae K., Sripanidkulchai B., Singkhoraard J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008;116:325–332. doi: 10.1016/j.jep.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 130.Kennedy D.O., Wightman E.L., Reay J.L., Lietz G., Okello E.J., Wilde A., Haskell C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010;91:1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 131.Evans H.M., Howe P.R., Wong R.H. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients. 2017;9:27. doi: 10.3390/nu9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thaung Zaw J.J., Howe P.R.C., Wong R.H.X. Sustained Cerebrovascular and Cognitive Benefits of Resveratrol in Postmenopausal Women. Nutrients. 2020;12:828. doi: 10.3390/nu12030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huhn S., Beyer F., Zhang R., Lampe L., Grothe J., Kratzsch J., Willenberg A., Breitfeld J., Kovacs P., Stumvoll M., et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults—A randomized controlled trial. Neuroimage. 2018;174:177–190. doi: 10.1016/j.neuroimage.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 134.Anton S.D., Ebner N., Dzierzewski J.M., Zlatar Z.Z., Gurka M.J., Dotson V.M., Kirton J., Mankowski R.T., Marsiske M., Manini T.M. Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. J. Altern. Complement. Med. 2018;24:725–732. doi: 10.1089/acm.2017.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marx W., Kelly J.T., Marshall S., Cutajar J., Annois B., Pipingas A., Tierney A., Itsiopoulos C. Effect of resveratrol supplementation on cognitive performance and mood in adults: A systematic literature review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018;76:432–443. doi: 10.1093/nutrit/nuy010. [DOI] [PubMed] [Google Scholar]
- 136.Farzaei M.H., Rahimi R., Nikfar S., Abdollahi M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018;128:338–344. doi: 10.1016/j.phrs.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Liu F., Huang J., Hei G., Wu R., Liu Z. Effects of sulforaphane on cognitive function in patients with frontal brain damage: Study protocol for a randomised controlled trial. BMJ Open. 2020;10:e037543. doi: 10.1136/bmjopen-2020-037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nouchi R., Hu Q., Saito T., Kawata N., Nouchi H., Kawashima R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients. 2021;13:352. doi: 10.3390/nu13020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shiina A., Kanahara N., Sasaki T., Oda Y., Hashimoto T., Hasegawa T., Yoshida T., Iyo M., Hashimoto K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015;13:62–67. doi: 10.9758/cpn.2015.13.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scholey A., Downey L.A., Ciorciari J., Pipingas A., Nolidin K., Finn M., Wines M., Catchlove S., Terrens A., Barlow E., et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG) Appetite. 2012;58:767–770. doi: 10.1016/j.appet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 141.de la Torre R., de Sola S., Hernandez G., Farre M., Pujol J., Rodriguez J., Espadaler J.M., Langohr K., Cuenca-Royo A., Principe A., et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:801–810. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y., Fly A.D., Wang Z., Klaunig J.E. The Effects of Green Tea Extract on Working Memory in Healthy Women. J. Nutr. Health Aging. 2018;22:446–450. doi: 10.1007/s12603-017-0962-8. [DOI] [PubMed] [Google Scholar]
- 143.Venci J.V., Gandhi M.A. Dimethyl fumarate (Tecfidera): A new oral agent for multiple sclerosis. Ann. Pharmacother. 2013;47:1697–1702. doi: 10.1177/1060028013509232. [DOI] [PubMed] [Google Scholar]
- 144.Amato M.P., Goretti B., Brescia Morra V., Gallo P., Zaffaroni M., Onofrj M., Cocco E., Borriello G., Zipoli V., Trojano M. Effects of 2-year treatment with dimethyl fumarate on cognition and functional impairment in patients with relapsing remitting multiple sclerosis. Neurol. Sci. 2020;41:3185–3193. doi: 10.1007/s10072-020-04320-w. [DOI] [PubMed] [Google Scholar]
- 145.Geloso M.C., D’Ambrosi N. Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells. 2021;10:686. doi: 10.3390/cells10030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sedighi B., Pardakhty A., Kamali H., Shafiee K., Hasani B.N. Effect of Boswellia papyrifera on cognitive impairment in multiple sclerosis. Iran. J. Neurol. 2014;13:149–153. [PMC free article] [PubMed] [Google Scholar]
- 147.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen R., Marsiske M., Smith G. Handbook of Clinical Neurology. Volume 167. Elsevier; Amsterdam, The Netherlands: 2019. Neurobiology of Aging; pp. 149–180. [DOI] [PubMed] [Google Scholar]
- 149.Dos Santos T., de Carvalho R.L.S., Nogueira M., Baptista M.A.T., Kimura N., Lacerda I.B., Dourado M.C.N. The Relationship between Social Cognition and Executive Functions in Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2020;17:487–497. doi: 10.2174/1567205017666200626205154. [DOI] [PubMed] [Google Scholar]
- 150.Aarsland D., Batzu L., Halliday G.M., Geurtsen G.J., Ballard C., Ray Chaudhuri K., Weintraub D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primer. 2021;7:47. doi: 10.1038/s41572-021-00280-3. [DOI] [PubMed] [Google Scholar]
- 151.Mack J., Marsh L. Parkinson’s Disease: Cognitive Impairment. Focus (Am. Psychiatr. Publ.) 2017;15:42–54. doi: 10.1176/appi.focus.20160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yuan X.Y., Wang X.G. Mild cognitive impairment in type 2 diabetes mellitus and related risk factors: A review. Rev. Neurosci. 2017;28:715–723. doi: 10.1515/revneuro-2017-0016. [DOI] [PubMed] [Google Scholar]
- 153.Rosca E.C., Simu M. Montreal cognitive assessment for evaluating cognitive impairment in Huntington’s disease: A systematic review. CNS Spectr. 2020;27:27–45. doi: 10.1017/S1092852920001868. [DOI] [PubMed] [Google Scholar]
- 154.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., León R., López M.G., Oliva B., et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 155.Franco R., Cedazo-Minguez A. Successful Therapies for Alzheimer’s Disease: Why so Many in Animal Models and None in Humans? Front. Pharmacol. 2014;5:146. doi: 10.3389/fphar.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gilbert N. The science of tea’s mood-altering magic. Nature. 2019;566:S8–S9. doi: 10.1038/d41586-019-00398-1. [DOI] [PubMed] [Google Scholar]